Abstract
AIM:
This study aimed to evaluate the efficacy of ketotifen on sperm motility of asthenospermic infertile men.
SETTING AND DESIGN:
It is a prospective study designed in vivo.
MATERIALS AND METHODS:
In this interventional experimental study, a total of 40 infertile couples with asthenospermic infertility factor undergoing assisted reproductive technology (ART) cycles were enrolled. The couples were randomly assigned to one of two groups at the starting of the cycle. In control group (n = 20), the men did not receive Ketotifen, while in experiment group (n = 20), the men received oraly ketotifen (1 mg Bid) for 2 months. Semen analysis, under optimal circumferences, was obtained prior to initiation of treatment. The second semen analysis was done 2-3 weeks after stopped ketotifen treatment and sperm motility was defined. Clinical pregnancy was identified as the presence of a fetal sac by vaginal ultrasound examination.
STATISTICAL ANALYSIS USED:
All data are expressed as the mean ± standard error of mean (SEM). t test was used for comparing the data of the control and treated groups.
RESULTS:
The mean sperm motility increased significantly (from 16.7% to 21.4%) after ketotifen treatment (P < 0.001). This sperm motility improvement was more pronounced in the primary infertility cases (P < 0.003). The rate of pregnancy was 12.5% in infertile couples that their men receiving 1 mg/twice a day ketotifen. In 52% of infertile men's semen, the percentage of sperm motility was increased from 5% to 35% and this sperm motility improvement was also observed in 33% of necrospermia (0% motility) cases.
CONCLUSION:
These results suggest that ketotifen may represent as a novel therapeutic approach to improve sperm motility in the infertile men with cause of asthenospermia or necrospermia.
KEY WORDS: Infertility, ketotifen, male factor, sperm motility
INTRODUCTION
A male factor is the only identifiable cause of infertility in twenty percent of infertile men and it is a causal factor in as many as 30-40 percent of couples. An equally important parameter in the semen analysis is sperm motility.[1]
The WHO and many laboratories use a cut off of 40% progressive motility as the lower limit of normal, whereas others use less than 40% motility as a criterion for defining poor sperm motility (asthenospermia).[2]
Overall, asthenospermia indicates testicular or epididymal failure that can be caused by numerous causes.
The cause of the asthenospermia is not exactly clear. It may be due to autoimmunity to the sperm, pyospermia, partial obstruction of the ejaculatory ducts in unsuccessful vasectomy reversal operation, longer abstinence intervals, and varicoceles.[3] Also the immotile cilia syndrome (Kartagener syndrome), in which the sperms have tail defects and cannot flagellate, can cause asthenospermia or necrospermia. Hyperviscosity has also been suggested as a possible causes of an artificially decreased the motility of spermatozoa.[4,5]
Ketotifen, a tricyclic benzocycloheptatiophene, is most commonly used for prevention and treatment of allergic reactions of the respiratory and integumentary systems. It has a stabilizing effect on the mast cells (MCs). Additionally, this agent also blocks histamine (H1) receptors and inhibits SRS-A (the slow releasing substance of anaphylaxis) and phosphodiesterase release with increasing intracellular Cyclic adenosine monophosphate (cAMP) concentrations.[6,7]
Ketotifen's androgenic effects on male infertility, associated with oligospermia and asthenospermia, have been reported previously.[8] Studies have also shown that it may counteract the ability of MCs to trigger an inflammatory response. It is worth mentioning that the number of MCs sometimes increases more than normal in the testicular tissue of infertile men.[6,9,10]
Yamamoto et al., revealed that the MC blocker, tranilast, could significantly improve the concentrations and motility of sperm.[11] Moreover, administration of the tranilast has resulted in increasing pregnancy rate comparing non-treated couples.[11] However, the data in support of using ketotifen to improve sperm motility followed pregnancy rate is limited. Therefore, we sought to evaluate the potential efficacy of ketotifen on sperm motility and then pregnancy rate in intrauterine insemination for male factor infertility associated with asthenospermia.
MATERIALS AND METHODS
Study design
Forty couples with male factor infertility were participated in this study during the period from May 2010 to December 2011. A full assessment includes demographic information (including ages of couple, duration of marriage, and infertility) and medical and gynecological histories with physical examination and routine laboratory screening (including Body mass index (BMI), complete blood count (CBC), Pap smear, Thyroid-stimulating hormone (TSH), prolactin (PRL), and viral serology). Also, a Hysterosalpingography (HSG) or a laparoscopy for tubal and peritoneal factors in the women and a semen analysis for evaluation of men were done.
The inclusion criteria were infertile couples with just male factor infertility associated with asthenospermia (less than 40% progressive motile sperm) and absence of female factor infertility. However, the infertile couples associated with a clinical varicocele were excluded.
The semen specimen was collected by masturbation after an abstinence period of 2-3 days.
After a primary semen analysis, ketotifen was administered 1 mg bid for 2 months. Second semen analysis was done 2-3 weeks after ketotifen treatment was stopped and then sperm motility was examined. Clinical pregnancy was identified by the presence of a fetal sac detected by vaginal ultrasound.
End points
The end point of the study was to evaluate the efficacy of ketotifen on sperm motility of asthenospermic infertile men.
Statistical analysis
All analyses were carried out with the Statistical Package for the Social Sciences (SPSS) 16 statistical software. All data are expressed as the mean ± standard error of mean (SEM). t test were used for comparison the data of the non-treated in versus the treated groups. P value less than 0.05 were considered as significant difference.
RESULTS
Outcome data were available for 100% of couples. None of the couples was lost to follow up [Figure 1]. The two groups were similar except for the date of acceptance of the patients (the seasonal changes) for investigate. The females of the infertile couples were non gravid. The men were aged 23-40 years of mean age 32.8 and the mean duration of infertility among participants was at least 2 years. Twenty-eight cases of the infertile couples (70%) had primary infertility and the remaining 12 (30%) had secondary infertility [Table 1].
Figure 1.
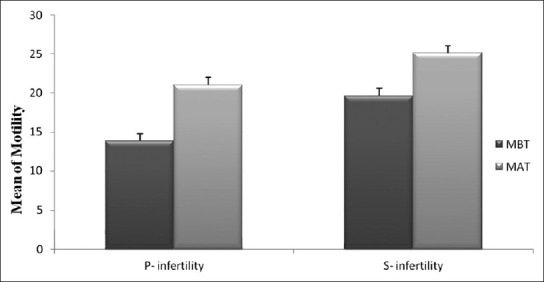
Comparison of sperm motility percent between non-treated (MBT) and treated (MAT) groups in primary and secondary infertility cases
Table 1.
Couples demographic and clinical characteristics

None of the men of the ketotifen treated group reported adverse events that were linked apparently to the treatment. All parameters measured in semen analysis i.e., sperm count, morphology, volume, and pH according to World Health Organization (WHO) were normal except for the motility. Sperm motility in 34 cases was 10-35% and in six cases was 0%.
The mean sperm motility before treatment was 16.7% ± 3.8, whereas after ketotifen treatment was 21.4% ± 4.2 [Table 1] and it was statistically significant difference especially in primary infertility (P = 0.003) [Figure 1]. In 52% of asthenospermia cases, the percent of sperm motility was increased (from 5% to 35%) [Figure 2]. Also in 33% of necrospermia (0% motility), this variable was increased (from 23% to 25%). The cumulative pregnancy rate (12.5%) in the treated couples was more pronounced than the non treated group (4.3%).
Figure 2.
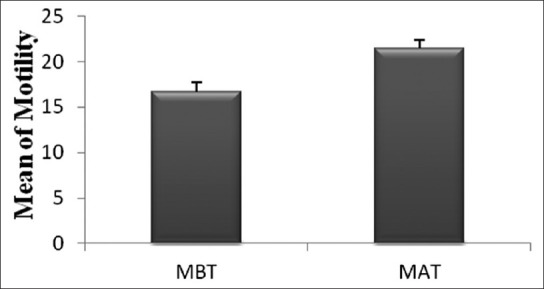
Comparison of sperm motility percent between non-treated (MBT) and treated (MAT) groups in asthenospermic infertile men
DISCUSSION
Some studies have been reported that MCs, which play a key role in the inflammation, hypersensitivity and fibrosis, are as well normally present in the testes.[12,13,14] The previous studies suggest that there is a prominent increase in the number of testicular MCs in the testes of infertile men which may disrupt spermatogenesis.[15] Also it was reported that increased numbers of MCs have been associated with different types of infertility, including asthenospermia.[15,16,17,18,19] Therefore some of male factor infertility problems could possibly diminished if such pathogenesis agent would greatly reduce using drug that block MC mediator release.
In the present study, it was observed that the percent of sperm motility in asthenospermic infertile men and also, surprisingly, in necrospermic cases remarkably increased.
As mentioned in the introduction, ketotifen is a tricyclic benzocycloheptatiophene having a high affinity for blocking histamine (H1) receptors that most commonly used for prevention and treatment of allergic reactions of the respiratory and integumentary systems.[5,6]
Regardless of there being various reports on enhanced fertility following given of MC blockers, there is contrary report about the effect of ketotifen, as a MC blocker, on sperm motility or rate of pregnancy.[15,20] Kondo et al.,[21] and Slater et al.,[22] suggested that ketotifen can block the H1 receptor, which is present in the male reproductive tract; consequently, it may reduce the effectiveness of histamine.
Anderson et al.,[23] identified that ketotifen also prevents degradation of MCs and accordingly inhibits the release of inflammatory mediators. These findings show a relation between ketotifen and inflammatory mediators and therefore may be useful to inhibit inflammatory mediator's activities of MC in the testes.[15] Therefore, it seems that in this way ketotifen may improve semen parameters in infertile men.
Schill et al.,[22] also suggested that ketotifen can be more helpful to improve the patients’ sperm count and sperm motility with idiopathic oligo/asthenospermia. However, the ongoing pregnancy rate was within the range of spontaneous conception.
Contrary, the current study, more significant in increasing motility in sperm, showed that the cumulative pregnancy in the treated couples significantly improved by 4 months post-treatment compared with the non-treated ones.
Alejandro et al.,[24] carried out a study to assess the efficacy of daily administration of ketotifen, on the semen quality of men with leukocytospermia and unexplained infertility. They noticed that ketotifen diminished the white blood cell count in semen and so the sperm motility have been dramatically improved. Moreover, the number of morphologically normal spermatozoa was more pronounced at 8 weeks of treatment and these changes remained until at least 4 weeks after stopped of ketotifen treatment.
CONCLUSION
Although the present study had a number of restrictions; first, it was not double blind. Sample size and power to detect clinically essential outcomes were small. The trial was not designed with sufficient power to address important basic outcomes. Nevertheless, it was shown that it seems the failure rate of infertility treatments can be decreased if MC blockers such as ketotifen are given to the asthenospermic men before who were enrolled in intrauterine insemination (IUI) or ART cycles of treatment. Indeed, it seems that further research is necessary to identify the precise capacity of ketotifen to improve quality of semen parameters and whether this MC blocker has effects on the cumulative outcome of reproductive.
ACKNOWLEDGEMENT
The authors wish to acknowledge the efforts of all Fertility, Infertility and Perinatology Research Center and Mrs. Mitra Shabab for their generous help in processing the study.
Footnotes
Source of Support: This project was financially supported by the research deputy of Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran
Conflict of Interest: None declared
REFERENCES
- 1.Thonneau P, Marchand S, Tallec A. An incidence and main causes of infertility in a resident population (1.850, 000) of three French regions (1988-1989) Hum Reprod. 1991;6:811–6. doi: 10.1093/oxfordjournals.humrep.a137433. [DOI] [PubMed] [Google Scholar]
- 2.Birmingham, AL: The American Fertility Society; 1994. The American Fertility Society and the Society for Assisted Reproductive Technology: Clinic-Specific Outcome Assessment for the Year 1992. [Google Scholar]
- 3.Jeyendran RS, Van der Ven HH, Perez-Pelaez M, Crabo BG, Zaneveld LJ. Development of an assay to assess the functional integrity of the human sperm membrane and its relationship to other semen characteristics. J Reprod Fertil. 1984;70:219–28. doi: 10.1530/jrf.0.0700219. [DOI] [PubMed] [Google Scholar]
- 4.Siciliano L, Tarantino P, Longobardi F, Rago V, Destofano C, Carpino A. Impaired seminal antioxidant capacity in human semen with hyperviscosity or oligoasthenozoospermia. J Androl. 2001;22:798–803. [PubMed] [Google Scholar]
- 5.Curi SM, Ariagno JI, Chenlo PH, Mendeluk GR, Pugliese MN, Sardi Sogovia LM, et al. Asthenozoospermia: Analysis of a large population. Arch Androl. 2003;49:343–9. doi: 10.1080/01485010390219656. [DOI] [PubMed] [Google Scholar]
- 6.Behrnadt H, Hilscher B, Passia D, Hofman N, Hilsher W. The occurrence of mast cells in the human testis. Acta Anat. 1981;111:14–6. [Google Scholar]
- 7.Schill WB, Schneider J. The use of ketotifen a mast cell blocker, for treatment of oligo-and asthenozoospermia. Andrologia. 1986;18:570–3. doi: 10.1111/j.1439-0272.1986.tb01831.x. [DOI] [PubMed] [Google Scholar]
- 8.Oliva A, Multigner L. Ketotifen improves sperm motility and sperm morphology in male patients with leukocytospermia and unexplained infertility. Fertil Steril. 2006;85:240–3. doi: 10.1016/j.fertnstert.2005.06.047. [DOI] [PubMed] [Google Scholar]
- 9.Moskovtsev SI, Willis J, White J, Mullen JB. Leukocytospermia: Relationship to sperm deoxyribonucleic acid integrity in patients evaluated for male factor infertility. Fertil Steril. 2007;88:737–40. doi: 10.1016/j.fertnstert.2006.11.132. [DOI] [PubMed] [Google Scholar]
- 10.Aziz N, Agarwal A, Lewis-Jones I, Sharma RK, Thomas AJ., Jr Novel associations between specific sperm morphological defects and leukocytospermia. Fertil Steril. 2004;82:621–7. doi: 10.1016/j.fertnstert.2004.02.112. [DOI] [PubMed] [Google Scholar]
- 11.Yamamoto M, Hibi H, Miyaka K. New treatment of idiopathic sever oligozoospermia with mast cell blocker results of a single-blind study. Fert Steril. 1995;64:1221–3. doi: 10.1016/s0015-0282(16)57992-8. [DOI] [PubMed] [Google Scholar]
- 12.Meineke V, Frungieri MB, Jessberger B, Vogt H, Mayerhofer A. Human testicular mast cells contain tryptase: Increased mast cell number and altered distribution in the testes of infertile men. Fertil Steril. 2000;74:239–44. doi: 10.1016/s0015-0282(00)00626-9. [DOI] [PubMed] [Google Scholar]
- 13.Chakraborty S, Bonthu N, Swanson BJ, Batra SK. Role of mucins in the skin during benign and malignant conditions. Cancer Lett. 2011;301:127–41. doi: 10.1016/j.canlet.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pejler G, Rönnberg E, Waern I, Wernersson S. Mast cell proteases: Multifaceted regulators of inflammatory disease. Blood. 2010;115:4981–990. doi: 10.1182/blood-2010-01-257287. [DOI] [PubMed] [Google Scholar]
- 15.Roaiah MM, Khatab H, Mostafa T. Mast cells in testicular biopsies of azoospermic men. Andrologia. 2007;39:185–9. doi: 10.1111/j.1439-0272.2007.00793.x. [DOI] [PubMed] [Google Scholar]
- 16.Haidl G, Duan YG, Chen SJ, Kohn FM, Schuppe HC, Allam JP. The role of mast cells in male infertility. Expert Rev Clin Immunol. 2011;7:627–34. doi: 10.1586/eci.11.57. [DOI] [PubMed] [Google Scholar]
- 17.Allam JP, Langer M, Fathy A, Oltermann I, Bieber T, Novak N, et al. Mast cells in the seminal plasma of infertile men as detected by flow cytometry. Andrologia. 2009;41:1–6. doi: 10.1111/j.1439-0272.2008.00879.x. [DOI] [PubMed] [Google Scholar]
- 18.Welter H, Köhn FM, Mayerhofer A. Mast cells in human testicular biopsies from patients with mixed atrophy: Increased numbers, heterogeneity, and expression of cyclooxygenase 2 and prostaglandin D2 synthase. Fertil Steril. 2011;96:309–13. doi: 10.1016/j.fertnstert.2011.05.035. [DOI] [PubMed] [Google Scholar]
- 19.Azadi L, Abbasi H, Deemeh MR, Tavalaee M, Arbabian M, Pilevarian AA, et al. Zaditen (Ketotifen), as mast cell blocker, improves sperm quality, chromatin integrity and pregnancy rate after varicocelectomy. Int J Androl. 2011;34:446–52. doi: 10.1111/j.1365-2605.2010.01112.x. [DOI] [PubMed] [Google Scholar]
- 20.Weidinger S, Mayerhofer A, Frungieri MB, Meineke V, Ring J, Kohn FM. Mast cell-sperm interaction: Evidence for tryptase and proteinase-activated receptors in the regulation of sperm motility. Hum Reprod. 2003;18:2519–24. doi: 10.1093/humrep/deg476. [DOI] [PubMed] [Google Scholar]
- 21.Kondo N, Fukutomi O, Kameyama T, Nishida T, Li GP, Aagata H, et al. Suppression of proliferative responses of lymphocytes to food antigens by an anti-allergic drug, ketotifen fumarate, in patients with food-sensitive atopic dermatitis. Int Arch Allergy Immunol. 1994;103:234–8. doi: 10.1159/000236633. [DOI] [PubMed] [Google Scholar]
- 22.Slater JW, Zechnich AD, Haxby DG. Second-generation antihistamines: A comparative review. Drugs. 1999;57:31–47. doi: 10.2165/00003495-199957010-00004. [DOI] [PubMed] [Google Scholar]
- 23.Anderson DJ. Cell-mediated immunity and inflammatory process in male infertility. Arch Immunol Therapy Exp (Warsz) 1990;38:79–86. [PubMed] [Google Scholar]
- 24.Olivia A, Multigner L. Ketotifen improves sperm motility and morphology in male patients with leukocytospermia and unexplained infertility. Fertil Steril. 2006;85:240–3. doi: 10.1016/j.fertnstert.2005.06.047. [DOI] [PubMed] [Google Scholar]


