Abstract
Several countries have in recent years introduced prescribed requirements for treatment and monitoring of outcomes, as well as a licensing or accreditation requirement for in vitro fertilization (IVF) clinics and their laboratories. It is commonplace for Assisted Reproductive Technology (ART) laboratories to be required to have a quality control system. However, more effective Total Quality Management systems are now being implemented by an increasing number of ART clinics. In India, it is now a requirement to have a quality management system in order to be accredited and to help meet customer demand for improved delivery of ART services. This review contains the proceedings a quality management session at the Indian Fertility Experts Meet (IFEM) 2010 and focuses on the creation of a patient-oriented best-in-class IVF laboratory.
KEY WORDS: Assisted reproduction technique, in vitro fertilization, Total Quality Management
INTRODUCTION
In reproductive medicine, a new frontier was opened up and new hope was given to infertile couples when the first baby conceived in vitro was born in 1978.[1] According to the World Health Organization, 80 million couples worldwide are infertile, with 15% of them residing in India.[2,3] On a conservative basis, the market for infertility treatment could be estimated at over Rs. 25,000 crores per year.[2,3] Per started cycle, the success rate is <30% for the Assisted Reproductive Technology (ART) procedures,[4] and the whole exercise could be both expensive and emotionally draining, given the personal and societal pressures on the individual subjects. Nowadays, in vitro fertilization (IVF) has become a routine and commonly accepted practice treatment option for infertility. New IVF-based techniques, such as cryopreservation using vitrification, micromanipulation of gametes and embryos and pre-implantation genetic diagnosis (PGD), have provided prospective parents with a wide range of new reproductive options. Today, ART refers not only to IVF but also to several variations tailored to patients’ unique conditions. The technology transfer in the ART has resulted in the advanced practices being offered in almost all countries.
The growth of the ART industry and the potential health risks to patients and offspring have driven attempts to monitor and regulate the services provided although there are still differences of opinion with regard to what constitutes valid efficacy measures that allow comparisons between clinics. This may seem strange, since it has been possible to reach such agreements in other fields of medicine, namely desired outcome of the intervention per intention to treat. The efficacy measures must, however, be modulated by factors taking into account the human cost of achieving the outcomes, e.g., the proportion of multiple births and the proportion of cycles complicated by ovarian hyperstimulation syndrome. From global monitoring efforts, it is clear that treatment efficacy is increasing in countries that have ART registries.[4,5] Notably, many of the high growth markets lack effective regulation or have difficulties in enforcing the regulations that may be present.
This paper focuses on the recent approaches in setting up and operating a best in class laboratory to improve the efficacy of IVF treatment and discusses the design and use of Quality Management Systems (QMS).
THE CONCEPT OF QUALITY
Quality of care is a multi-dimensional concept, encompassing treatment efficacy and impact on health and welfare of both patients and offspring. In addition, the concept of quality includes the cost in financial and human terms of achieving the desired outcome.
To optimize quality using the Total Quality Management approach, it is necessary to map all processes, to thoroughly describe all procedures involved in the processes, and to define performance targets for each procedure. There should also be an assessment of how the procedures might fail; the impact of the failure and the possible cause(s) of such failure. It is then necessary to ensure that the clinic and its staff have the requisite skills, knowledge and equipment to achieve the performance targets. Finally, the performance must be monitored, both with regard to absolute measures as well as trends. In all instances where performance falls outside the set limits or is trending towards a non-conformance with targets, corrective actions must be taken and documented. As a first step in the corrective action it must be clearly established what has occurred. Then there must be an analysis of possible causes of the failure with a view to identifying deficiencies in the system that allowed the failure to occur [Figure 1]. Quality management is a larger concept than quality assurance and quality control, which are subsets of quality management.[6] There is growing recognition that quality management not only ensures improvement of the clinical aspects of the operations of a clinic, but also leads to improved financial performance and increased staff satisfaction.[7]
Figure 1.
Basic principle of quality management
CREATING A PATIENT-ORIENTED BEST-IN-CLASS IN VITRO FERTILIZATION LABORATORY
Setting up of an ideal IVF laboratory is vital to the success in ART and the critical determinants of the function of the IVF laboratory are people, procedures, equipment and the laboratory design.
People, their characteristics and management
An embryologist using his or her personality, knowledge and skills must be capable of taking initiatives and improving the laboratory. Skill is an absolute requirement along with the ability to collate and structure information from the laboratory and literature, so that one can analyze, foresee and solve problems that occur in the daily practice. Also, communication skills are important to connect with staff, patients and society. The embryologist must also have good writing skills in order to author quality management documents. Furthermore, in any organization, relationship skills are important to enable honest and open discussion in the workplace. The knowledge of cell and reproductive biology must be of sufficient depth to allow the embryologist to analyze and solve problems from basic physiological principles. It is important that the embryologist is imbued with a culture of intellectual and scientific rigor that allows independent search for knowledge and solutions instead of unquestioning references to statements of authorities in the field. Trust and integrity are fundamental in any workplace and it is crucial that there is comprehensive and honest reporting of all data. Good working conditions and good equipment should be provided for all staff to help them do a good job. Maintaining openness drives accountability, which in turn drives performance.
Procedures, documentation and data
All processes should be mapped, using appropriate flow chart methodology. The process map then forms the basis of descriptions of procedures and how they should be performed and what outcome is to be expected (performance indictors). These descriptions are often called standard operating procedures (SOPs). The SOPs should be structured in a standardized format and their distribution must be controlled. Finally, they should be based on documented scientific evidence and require regular updating. The performance indicators should be collected in a computer database. Many comprehensive systems are available commercially and provide administrative as well as medical functionalities and allow easy report generation. Some clinics develop their own IT solutions, sometimes based on spreadsheets (e.g., MS Excel) and sometimes built in relational database applications (e.g., MS Access), the latter being preferable. Ideally, the database should provide information on demographics, medical history, investigations, treatments, observations and outcomes. Data should be analyzed regularly, both with regard to absolute levels as well trends in the data.
Procedures should maximize the chance of success and minimize risk. Prior to the implementation of any new method, it needs to be validated and monitored in the current setting. Importantly, the laboratory staff members need to undergo training and prove competence for each procedure performed.
The data on the performance of the clinic as a whole, but also of the individuals should be collected and analyzed regularly. Data should be audited, assessed and structured to discern the input quality, the process quality and the output quality as appropriate. The list of data that is required for collecting and auditing is presented in Table 1. In addition, data on the functioning of equipment and technical systems, e.g., air quality and level of microbial contamination, must be collected and regularly audited.
Table 1.
Collection of data

For laboratory functioning assessment (process quality), the proportion of embryos reaching developmental milestones at pre-defined time points of embryo culture, are often used. The most sensitive indictor of the performance of the culture system is the average cell numbers at e.g., 42-44 h post insemination. The most significant measure of the output quality is the implantation rate rather than clinical pregnancy rates. Obviously, detailed records must be maintained for proper risk assessment in the clinic.
Equipment and consumables and supporting physiological processes
All consumables that come in contact with gametes and embryos must be validated as non-toxic to embryos and supportive of normal embryo development. All new equipment must be validated before use; it should be reliable in function, and be tolerant of non-ideal conditions. The equipment must be acquired on the characteristics of performance, but not on its price. The four key environmental variables; temperature, pH, osmolarity and contamination, must be controlled and carefully monitored from the “Tip-to-Tip” of the culture system (tip of the oocyte aspiration needle to the tip of the embryo transfer catheter). It is critical that the variables are measured at the point where the embryos are handled. For example, it must be ensured that the temperature of the culture medium in the dish is 37°C. The temperature of the heated stage then needs to be set at whatever value is needed to maintain the temperature of the medium in the dish at the desired level.
Work stations and laboratory design
Work stations should provide filtered air and a heated surface to maintain optimum temperature without any contamination. Ergonomically designed equipment is preferred to alleviate unnecessary strain on the embryologist. The laboratory design should be based on clean room technology, using low emission materials with a clearly defined air quality. Work-flow should be planned to ensure a minimal distance between the incubator, the work station and the microscope. This will also to minimize the risk of collisions between laboratory staff members. Before starting operations, the reliability of the laboratory equipment and processes must be checked in dry runs and all problems should be corrected before treatment of patients is commenced.
Quality management standards
There are several quality management standards like International Organization for Standardization (ISO) 9000-2008 for certification of QMS (ascertaining that conditions will allow quality targets to be achieved) and ISO 15189 for accreditation of clinical laboratories (ensuring that the lab is doing what it says it does). Data acquired between the period of 2006 and 2010 from Nurture IVF, UK, [Figure 2], shows that over time, the variations in clinical pregnancy rate were moderate (5% with an upward trend) and the rate of improvement was 0.2% per quarter or 0.9% per year. However, the average improvement rates in the UK and in Sweden were between 0.3% and 0.4% per year and that is the benchmark against which improvement initiatives should be measured. American Society for Reproductive Medicine (ASRM) and European Society for Human Reproduction and Embryology (ESHRE) provide guidelines for good IVF laboratory practice.[8,9] The guidelines give information for support and guidance to the laboratory staff and deals with all aspects required to provide a safe working system for people in an IVF laboratory.
Figure 2.
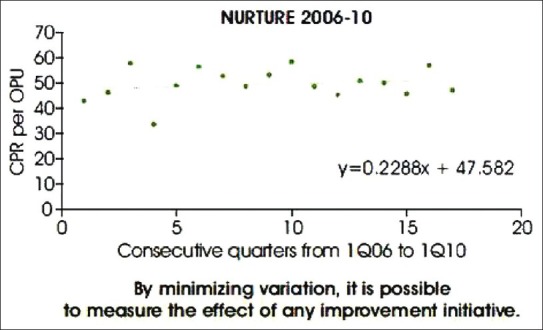
Expected improvement
Future developments in embryology
The future of IVF is postulated to involve the use of microfluidics for culture systems, embryo selection using ‘Omics’ and automatic acquisition of information, including video time-lapse recordings of cultures into electronically stored databases. Microfluidics is an emerging concept, embedded on a chip to provide a stable environment. It allows for better control of conditions, continuous supply of nutrients and removal of waste products. To facilitate the identification of top quality embryos, time-lapse video, metabolomics and other ‘Omics’ might play an important role in future. A broader use of databases will also help in the development of the IVF laboratory.
In vitro fertilization scenario in India and points to consider before starting an IVF clinic in India
Issues surrounding pregnancy, childbirth and motherhood are complex in all societies but particularly so in patriarchal settings such as India where infertility is a life crisis, and its consequences can be manifold.[10]
The Indian public health system does not provide access to adequate preventive and curative services or counseling for infertility. A postal survey of 6000 gynecologists in India revealed that, none of the public sector providers offered ARTs like IVF and only 36% offered intrauterine insemination (IUI). Hence services are available predominantly in the private sector, but the quality and the costs of these services vary considerably.[11]
A major concern in the present context of ART services in India is the quality of care. Services are not regulated and the quality of treatment is variable, ranging from clinics that offer professional, high-quality services to those that are run by unqualified practitioners.[11]
India has been known to play host to visiting foreign IVF infertility practitioners to carry out procedures that have been banned in the home country of patients and/or the visiting practitioners.[12]
The Indian Council of Medical Research (ICMR) reports an average take-home baby rate of 20-30% per IVF cycle,[13] while leading clinics in India claim a success rates varying from 40% to even 75%.[14]
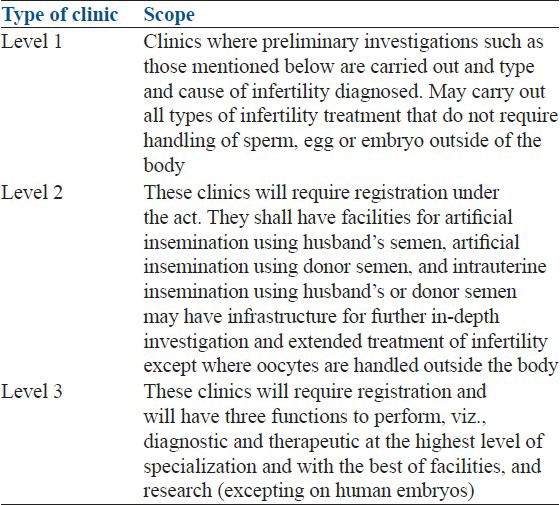
The ICMR recently finalized national guidelines for the regulation of ART clinics.[15] According to ICMR guidelines, Infertility clinics have been categorized in to three levels based on the availability of complexity of ART service. The guidelines provides minimum requirement regarding Staff in infertility clinics as well as physical requirements for an ART clinic [Table 2].
Table 2.
Categorization of in vitro fertilization clinics adapted from schedule-1, Indian council of medical research draft guidelines for assisted reproductive technology clinics 2010
It remains to be seen how these will be implemented in India. Implementing and following any ethical guidelines would mean that doctors would have to look critically at issues of informed consent, screening donors, legal and ethical issues including the misuse of sex-pre-selection technologies like pre-implantation diagnosis.[11]
ESSENTIAL REQUIREMENTS FOR AN ASSISTED REPRODUCTIVE TECHNOLOGY CLINIC
As described in the ICMR Guidelines[16] a well-designed ART clinic should have a non-sterile and a strictly sterile area. The non-sterile area must have a reception area, waiting room(s) for patients, medical procedure rooms (e.g., for blood sampling, injections), doctors’ offices and examination rooms with ultrasound equipment, a general-purpose clinical laboratory, storage rooms for equipment, utensils and pharmaceuticals, nursing quarters, postoperative recovery rooms, records storage and/or IT server room, autoclave room, semen collection room, etc., In addition, the availability of other in-house clinical facilities (e.g., staff meeting rooms, private counseling rooms), and location of the hospital in the city must also be carefully considered. Adequate steps must be taken for vermin proofing and disinfection. The sterile area shall house the operation theatre, a room for intra uterine transfer of sperm or embryos and an adjoining embryology and sperm processing laboratory. Entry to the sterile area must be strictly controlled by an anteroom for changing footwear, in addition to an area for changing into sterile garments and a scrub-station.
The embryology laboratory must have facilities for the control of temperature and humidity and must have filtered air. The infertility clinic need not have in-house facilities to perform all the procedures necessary to diagnose infertility, such as those for complete hormone and other assays. These can be subcontracted to specialty laboratories specializing in delivering such services, as long as they are located in close proximity with short transportation times. Each clinic should maintain in writing, standard-operating manuals for all the different procedures carried out in the clinic. Quality of consumables used in the laboratory must be procured from reliable sources after ensuring that they are non-toxic to the embryos. Special measures and equipment should be installed to secure uninterruptible power supply to critical areas of the clinic, e.g., the operation room and vital equipment in the laboratory such as the incubators, as well as to other essential services in the clinic.
Essential qualifications of an art team
The practice of ART requires a well-orchestrated team work between the five functional areas of an IVF clinic: Clinical, nursing, embryology, counseling and administration. The staff should have formal qualifications in their area of responsibility and their actual performance should be monitored against set standards.
The Australian experience and the effect on the quality management system
In Australia, prior to the legislation being passed in 1988, IVF had been treated as research to which prescriptive guidelines were attached. Clinical and ART laboratories must be accredited, usually by the National Association of Testing Authorities. These requirements have been strengthened over time, and were upgraded to ISO 17025:1999 and are now becoming aligned to ISO 15189:2003. However, IVF laboratories are regulated separately by the Reproductive Technology Accreditation Committee (RTAC)[17] which works with the government to create specific item numbers for IVF; to determine the eligibility rules and to implement a process of accreditation for all IVF units in Australia and New Zealand [Table 3]. All clinics are now required to have a QMS and other new requirements include risk management policies for several procedures and management review. The quality policy is now a requirement[18] The standard ISO 9001:2008 [Table 4] helps an organization build QMS that allows it to achieve its stated quality targets. However, ISO does not prescribe what those targets should be. A more stringent standard is ISO 15189, which specifies requirements for quality and competence in medical laboratories.
Table 3.
Requirements of the quality management systems for accreditation of assisted reproductive technology facilities in Australia and New Zealand

Table 4.
General requirement of ISO 9001:2008

SUMMARY
There has been increased demand for transparency pertaining to the safety aspects, effectiveness and health outcomes of ART treatments both from the general public, patients and regulators. The important components that make a laboratory great are the people, procedure, equipment and the laboratory design. Recruiting the right people is the critical step in setting up the best IVF laboratory. An IVF center is only as good as the staff it employs and there is an absolute need to ensure consistent and continuous education and training. QMS, that have been proven effective by other industries, can be used by ART clinics to meet existing requirements by monitoring and controlling the way in which services are delivered. Thus, by determining and meeting the expectations of patients and other stakeholders, implementation of QMS will also be a vital tool for setting, planning and meeting advancements through documented processes enabling continuous improvement.
ACKNOWLEDGMENTS
Medical writing and editorial assistance was provided by Peter Pothula, PhD, BioQuest.
Footnotes
Source of Support: Organon India Pvt. Ltd., a subsidiary of Merck & Co., Inc., provided a grant for the creation of this manuscript
Conflict of Interest: Dr. Jan I Oloffson: He has received honoraria from MSD and Merck Serono and the time of creation of the manuscript was the Regional director Medical Affairs of Women's health Asia Pacific region based at Singapore Dr. Manish. R. Banker: Is a board member of ASPIRE (Serono), and has received travel and honorarium from MSD. He has also received consultancy fees from GLG consultants
REFERENCES
- 1.Steptoe PC, Edwards RG. Birth after the reimplantation of a human embryo. Lancet. 1978;2:366. doi: 10.1016/s0140-6736(78)92957-4. [DOI] [PubMed] [Google Scholar]
- 2.Sen N. ICMR spurs public debate on infertility clinics. Curr Sci. 2002;83:1185–6. [Google Scholar]
- 3.Boivin J, Bunting L, Collins JA, Nygren KG. International estimates of infertility prevalence and treatment-seeking: Potential need and demand for infertility medical care. Hum Reprod. 2007;22:1506–12. doi: 10.1093/humrep/dem046. [DOI] [PubMed] [Google Scholar]
- 4.Nygren KG, Sullivan E, Zegers-Hochschild F, Mansour R, Ishihara O, Adamson GD, et al. International Committee for Monitoring Assisted Reproductive Technology (ICMART) world report: Assisted reproductive technology 2003. Fertil Steril. 2011;95:2209–22. doi: 10.1016/j.fertnstert.2011.03.058. [DOI] [PubMed] [Google Scholar]
- 5.Abdalla HI, Bhattacharya S, Khalaf Y. Is meaningful reporting of national IVF outcome data possible? Hum Reprod. 2010;25:9–13. doi: 10.1093/humrep/dep357. [DOI] [PubMed] [Google Scholar]
- 6.Mortimer D, Mortimer ST. Cambridge: Cambridge University Press; 2005. Quality and Risk Management in the IVF Laboratory; pp. 24–44. chapter 3. [Google Scholar]
- 7.van Empel IW, Nelen WL, Hermens RP, Kremer JA. Coming soon to your clinic: High-quality ART. Hum Reprod. 2008;23:1242–5. doi: 10.1093/humrep/den094. [DOI] [PubMed] [Google Scholar]
- 8.Practice Committee of American Society for Reproductive Medicine, Practice Committee of Society for Assisted Reproductive Technology. Revised guidelines for human embryology and andrology laboratories. Fertil Steril. 2008;90:S45–59. doi: 10.1016/j.fertnstert.2008.08.099. [DOI] [PubMed] [Google Scholar]
- 9.Magli MC, Van den Abbeel E, Lundin K, Royere D, Van der Elst J, Gianaroli L, et al. Revised guidelines for good practice in IVF laboratories. Hum Reprod. 2008;23:1253–62. doi: 10.1093/humrep/den068. [DOI] [PubMed] [Google Scholar]
- 10.Widge A. Seeking conception: Experiences of urban Indian women with in vitro fertilisation. Patient Educ Couns. 2005;59:226–33. doi: 10.1016/j.pec.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 11.Widge A, Cleland J. The public sector's role in infertility management in India. Health Policy Plan. 2009;24:108–15. doi: 10.1093/heapol/czn053. [DOI] [PubMed] [Google Scholar]
- 12.Kharab D. Assisted reproductive techniques ethical and legal concerns. The Internet Journal of Law, Healthcare and Ethics. 2007;4:2. [Google Scholar]
- 13.Indian Council for Medical Research, Need and feasibility of providing assisted technologies for infertility management in resource poor settings. Icmr Bull. 2000;30:6–7. [Google Scholar]
- 14.Sarojini N, Marwah V, Shenoi A. Globalisation of birth markets: A case study of assisted reproductive technologies in India. Global Health. 2011;7:27. doi: 10.1186/1744-8603-7-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Indian Council of Medical Research Drafting committee. The Assisted Reproductive Technology (Regulation) Rules - 2010; Draft; New Delhi. [Last accessed 23-5-2012]. available at http://icmr.nic.in/guide/ART%20REGULATION%20Draft%20 Rules%201.pdf .
- 16.Screening of Patients for ART: Selection Criteria and Possible Complication. Guidelines for ART Clinics in India ICMR/NAMS. [Last accessed 24-05-2012]. Available at: http://www.icmr.nic.in/art/Chapter%20_2.pdf .
- 17.The Fertility Society of Australia. RTAC/Code of Practice. [Last accessed 2005 Jun 18-20]. Available at: http://www.fertilitysociety.com.au/rtac .
- 18.Warnes GM, Norman RJ. Quality management systems in ART: Are they really needed? An Australian clinic's experience. Best Pract Res Clin Obstet Gynaecol. 2007;21:41–55. doi: 10.1016/j.bpobgyn.2006.09.005. [DOI] [PubMed] [Google Scholar]


