Abstract
OBJECTIVES:
The use of nicotine through smoking remains a serious health problem. It has been associated with reduced fertility, although the mechanism responsible is still unclear. The present study was designed to investigate whether nicotine-induced infertility is associated with altered male reproductive hormones in male albino rats.
MATERIALS AND METHODS:
Forty male rats were divided equally into five groups and treated orally for thirty days. Group I, which served as the control received 0.2 ml/kg normal saline, Group II and III received 0.5 mg/kg (low dose) and 1.0 mg/kg (high dose) body weight of nicotine, respectively. The fourth and fifth groups were gavaged with 0.5 mg/kg and 1.0 mg/kg body weight of nicotine but were left untreated for another 30 days. These groups served as the recovery groups. Serum was analyzed for testosterone, luteinizing hormone (LH), follicle stimulating hormones (FSH), and prolactin using radioimmunoassay.
RESULTS:
Results showed that nicotine administration significantly decreased (P < 0.05) testosterone in the low and high treated groups and FSH in the high dose treated group when compared with the control group. There was a significant increase (P < 0.05) in mean LH and prolactin level in the high dose treated group when compared with the control. However, the values of the recovery groups were comparable with the control.
CONCLUSION:
The findings in this study suggest that nicotine administration is associated with distorted reproductive hormones in male rats although ameliorated by nicotine cessation. It is plausible that the decreased testosterone level is associated with testicular dysfunction rather than a pituitary disorder.
KEY WORDS: Luteinizing hormone, nicotine, prolactin, rat, testosterone
INTRODUCTION
Nicotine abuse through cigarette smoking is a major public health problem. Morbidity and mortality associated with cigarette smoking is estimated to account for approximately 430,000 deaths annually resulting from lungs cancer, chronic obstructive pulmonary diseases and ischemic heart diseases. By 2030, it is estimated that tobacco use will kill more than 8 million people worldwide each year if current trends continue.[1] However, despite worldwide anti-smoking campaigns, cigarette smoking is very common. The highest prevalence of smoking is observed in young male adults during their reproductive period with 46% smokers between 20 and 39 years.[2]
Nicotine is one of the most used licit drugs. It is highly toxic and absorbed quickly through the respiratory tract, mouth mucosa and skin. The liver metabolizes approximately 80-90% of nicotine but the kidney and lungs are involved as well.[3] It is now considered to be one of the most insidiously addicting substances because most users of nicotine develop rapid tolerance for it and also have extremely long-lasting craving for it when trying to stop. Studies have shown that nicotine can induce tolerance and physical dependence.[4]
The drug is widely consumed as cigarette smoking and among the various effect of nicotine on the biological systems is infertility which has earlier been attributed to nicotine in previous study.[5] Infertility is a major health issue among couples of child-bearing age and approximately half of known causes of primary infertility are now attributes to male factor.[6] However, the mechanism by which nicotine causes male infertility is poorly understood. It was also previously demonstrated under experimental condition in rats that exposure to reference cigarette smoke resulted in reduced birth weight[7] and oral administration of nicotine have been associated with testicular degeneration, disorganization of the cytoarchitecture, and decreased serum testosterone levels.[8]
Cigarette smoking has been shown to stimulate the release of several anterior and posterior pituitary hormones. It increases the plasma levels of prolactin, growth hormone (GH), adrenocorticotrophin (ACTH), and arginine vasopressin (AVP) without significant changes in thyroid stimulating hormone (TSH), luteinizing hormone (LH), and follicle-stimulating hormone (FSH).[9] However, there are controversies with the effect of cigarette smoking and reproductive hormones in men.[10,11] Tobacco consumption has recently been documented to act as an endocrine disruptor on the male hormone profile, specifically on LH, testosterone, and prolactin levels.[12] However, these group recommended basic research studies to determine what physiological mechanisms are involved in the endocrine effects of smoking, as well as which of the more than 4000 toxicants contained in tobacco smoke are responsible for these effects.
In spite of the growing knowledge of adverse reproductive effects of smoking on reproduction, it is relatively unclear whether or not; nicotine has the same effects and mechanism of action on male reproductive activities. However, to the best of our knowledge no study has documented the effect of nicotine withdrawal on male reproductive hormones in male rats. The present study was, therefore, designed to investigate if nicotine induced infertility is associated with alter pituitary-gonadal hormones and its effects during treatment and withdrawal periods.
MATERIALS AND METHODS
Nicotine preparation
Nicotine hydrogen tartrate with product number 26140 (95% Nicotine) was purchased from BDH chemical Ltd., Poole, England. The Nicotine dosage freshly prepared in normal saline for each group of animals was delivered at 0.5 mg\kg and 1.0 mg\kg body weight. The working solutions were stored in foil-wrapped glass bottle at 4°C for no longer than 3 days.
Animals and treatments
Experiments were performed on 40 male Sprague-Dawley rats whose average weight ranged between 150 g and 180 g (2-2.5 month old) were used for this research. Animals were divided into five equal groups with ad libitum access to rat chow and drinking water. Animals were also maintained in a well-ventilated room with a 12:12-hour light-dark at room temperature. The experiment was conducted in accordance with the Guidelines of the U.S. National Institute of Health (NIH) on the care and use of laboratory animals. The male animals were divided into five equal groups: Control group that received 0.2 ml/kg normal saline for 30 days, 0.5 mg/kg nicotine treated for 30 days, 1.0 mg/kg nicotine treated for 30 days, 0.5 mg/kg nicotine treated for 30 days but left untreated for another 30 days, and 1.0 mg/kg nicotine treated for 30 days but left untreated for another 30 days.
Hormonal assay
Blood samples were spun at 2,500 rpm for 10 minutes in a table top centrifuge. The serum samples obtained were analyzed to determine the concentration of testosterone, LH, FSH, and prolactin. In order to minimize the effect of diurnal fluctuation, all samples were obtained in baseline conditions between 8:00 A.M. and 9:30 A.M.
The analysis was carried via the tube-based enzyme immunoassay (EIA) method. The protocol used for the hormone was according to the method described for the kit (Immunometrics Limited, UK) and meet the World Health Organization (WHO) standards in research programme for human reproduction.
Statistical analysis
The results are presented as means ± SEM for each group. Differences among groups were analyzed using one-way analysis of variance (ANOVA) followed by the Duncan's multiple range Post hoc test for pairwise comparisons. All statistical comparisons and tests were performed using Statistical Package for the Social Sciences (SPSS) (SPSS Inc., Chicago, IL., USA) for Windows.
RESULTS
Effect of nicotine on mean hormone levels
Testosterone
The mean serum testosterone level of rats that received 0.5 mg/kg body weight (B.W.) (low dose) and those that received 1.0 mg/kg B.W. (high dose) of nicotine for four weeks was significantly decreased (P < 0.05) when compared with the control group. The observed decrease is dose dependent as shown in Figure 1.
Figure 1.
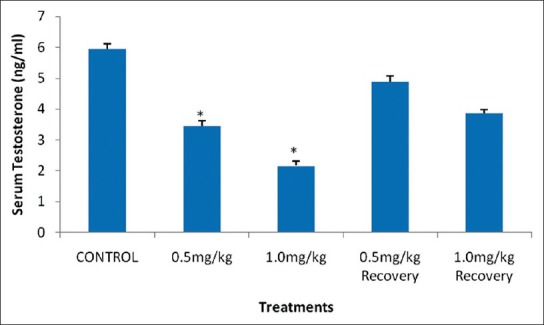
Effect of nicotine on serum testosterone level in male rats. Values are expressed as means ± S.E.M of eight rats per group. Bar carrying the asterisk sign on each parameter are significantly different at P < 0.05
The recovery groups of these treatments also showed an insignificant decrease (P < 0.05) in serum level of testosterone when compared with the control group as shown in Figure 1.
Luteinizing hormone
The results showed that there was a significant increase (P < 0.05) in the mean serum LH level of rats that received 1.0 mg/kg B.W. nicotine daily for four weeks when compared with their control. However, 0.5 mg/kg B.W. and their recovery groups showed an insignificant decrease (P > 0.05) in their mean serum LH level when compared with the control as shown in Figure 2.
Figure 2.
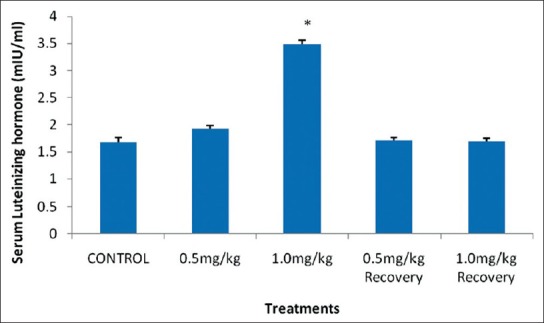
Effect of nicotine on serum luteinizing hormone level in male rats. Values are expressed as means ± S.E.M of eight rats per group. Bar carrying the asterisk sign on each parameter are significantly different at P < 0.05
Follicle stimulating hormone
The mean FSH level of rats that received 1.0 mg/kg BW nicotine daily for four weeks was significantly decreased (P < 0.05) when compared with the control group. However, 0.5 mg/kg BW and the recovery groups showed an insignificant decrease (P > 0.05) in the mean serum FSH level when compared with their control counterpart as shown in Figure 3.
Figure 3.
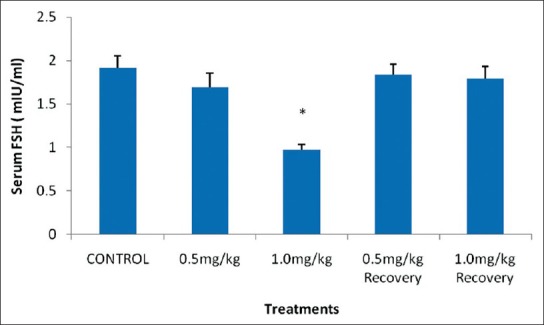
Effect of nicotine on serum follicle stimulating hormone level in male rats. Values are expressed as means ± S.E.M of eight rats per group. Bar carrying the asterisk sign on each parameter are significantly different at P < 0.05
Prolactin
The results showed that there was a significant increase in the mean serum prolactin level in the 1.0 mg/kg B.W. group when values were compared with the control. However, 0.5 mg/kg B.W. group and the recovery groups showed an insignificant increase (P < 0.05) when compared with the recovery group as shown in Figure 4.
Figure 4.
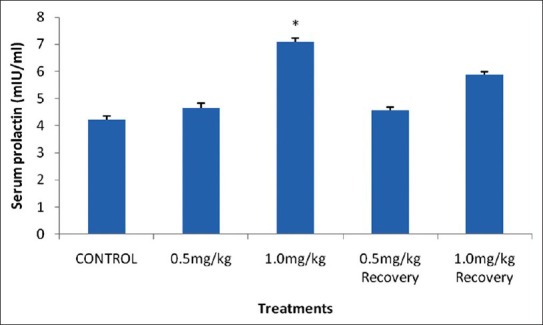
Effect of nicotine on serum prolactin level in male rats. Values are expressed as means ± S.E.M of eight rats per group. Bar carrying the asterisk sign on each parameter are significantly different at P < 0.05
DISCUSSION
Present results show that nicotine significantly decreases serum level of testosterone, FSH and significantly increase the circulating levels of prolactin and LH.
Testosterone, being an important androgen plays a pivotal role in several aspects of sexual maturation, behavior, spermatogenesis, differentiation, and maintenance of accessory sex organs.[13] The synthesis and release of androgens is dependent on the pituitary gonadotrophins, which are FSH and LH. Both FSH and LH are essential for testicular function and spermatogenesis. LH is the main tropic regulator of Leydig cell function without which androgen production is not possible.[14]
The observed decrease in serum testosterone level of rats treated with the two doses of nicotine is not associated with a decrease in LH thus suggesting that the etiology is not associated with a disorder in the pituitary but rather a testicular dysfunction, which is indicated when low serum testosterone levels are accompanied by high levels of serum LH. This might have been caused by the disruption of testicular cytoarchitecture by nicotine also observed in previous studies; consequently, adversely affected Leydig cell number and functioning leading to decrease serum testosterone level since Leydig cells secrete testosterone.[8]
The results obtained from this study is in consonance with earlier studies[15] but contrary to Trummer et al.,[16] who reported an increased free and total serum testosterone. The recovery groups showed comparable values with the control indicating that the effect of nicotine on testosterone can be ameliorated by nicotine cessation.
This study also demonstrate that nicotine treatment cause a significant increase in the mean serum LH in male rats in the high treated group. It is well-known that testosterone production by Leydig cell is primary under the control of LH and stimulation of LH is usually followed by stimulation of testosterone.[14] Increased LH level in serum and decreased testosterone observed negates this notion. Therefore, taking into account the physiology of the hypothalamus pituitary gonadal axis, an increase in the secretion of gonadotropin-releasing hormone (GnRH) by the hypothalamus could be expected, which in turn would increase the secretion of pituitary gonadotropins, specifically LH, which would stimulate the Leydig cells in the testes to increase testosterone secretion. However, our results do not support this hypothesis, since we observed a decrease in total testosterone levels and a rise in serum LH. The increase in LH is consistent with previous studies.[16,17] But contrary to the work of Patterson et al.,[18] The recovery group of the study showed comparable LH value with the control which probably indicates that the effect of nicotine were reversible.
The serum level of FSH of the rats treated with 1.0 mg/kg B.W. of nicotine was significantly decreased when compared with their control counterparts. This decrease in epididymal sperm count observed in the previous study could be connected to the decrease in the serum testosterone and FSH level observed in this present study. Testosterone is associated with FSH, which acts on the seminiferous tubules to initiate and maintain spermatogenesis. Sexual maturation in male rat is related with an increase in FSH secretion since FSH binds within the seminiferous tubules to facilitate spermatogenesis.[13] Decrease in FSH observed with nicotine treatment could be due to increase release of inhibin by the sertoli cell thus inhibiting the release of FSH from the anterior pituitary and possibly also a negative effect on the hypothalamus to inhibit the secretion of GnRH.
The decrease observed in this study is in agreement with earlier study that also reported a decrease in FSH with nicotine administration.[19] Primary testicular failure and hypothalamic causes have been reported to likely result in primary male infertility due to sexually transmitted diseases, trauma, environmental factors and social habits like smoking and consuming alcohol.
There was appreciable increase in the serum FSH level of rats in the recovery group showing that the effect of nicotine on serum FSH level can be ameliorated by nicotine cessation.
The serum level of prolactin of the high treated rats was significantly increased. Of great interest is the mean level of serum prolactin of animals administered with 1.0 mg/kg B.W. that showed a significant increase. This might be the ability of nicotine to reduce the turnover and release of dopamine through occupying mu or kappa receptors of endorphins or through cholinergic receptors for nicotine[19,20] presented on dopaminergic cells. Local reduction of dopamine could lead to over-production of prolactin by lactotroph cells.[21]
Hyperprolactinemia in men typically leads to hypogonadism resulting in decreased libido, erectile dysfunction, and abnormal semen quality.[22] Hyperprolactinemia impairs male gonadal functions by acting at various levels, possibly by decreasing GnRH pulse generator activity and/or by decreasing LH and FSH secretion, and might be inhibiting testosterone secretion at the level of Leydig cells as well.[23] Prolactin receptors have been demonstrated in the spermatogonia, seminiferous epithelium, and spermatocytes, suggesting that prolactin might have a role to play in normal spermatogenesis.[24] Hence, abnormalities in sperm quality and decreased libido observed in the previous study can also be attributed to the increased in serum prolactin since hyperprolactinemia is attributed to impaired germ cell function due to low FSH and decreased intra-testicular testosterone.[25]
The increased serum prolactin level observed in this group is consistent with other earlier studies[26] but contrary to that of Anderson group.[19] However, some scientists have reported a reduction of prolactin in rats due to the short term effects of cigarette smoke on prolactin[27] or no effects at all.[28] Jurasovicx et al.,[29] found that smoking was significantly associated with a decrease in serum prolactin.
There was an appreciable decrease in the serum prolactin level of rats in the recovery group showing that the effect of nicotine on serum prolactin can be ameliorated by nicotine cessation. This also agrees with earlier findings that observed that there was no difference in hormone concentrations between ex-smokers and non-smokers[30] suggesting that quitting smoking helps to reduce the levels of this hormone.
CONCLUSION
In summary, our results are compatible with the hypothesis that nicotine through tobacco consumption may act as an endocrine disruptor on male hormone profile, specifically on LH, FSH, testosterone, and prolactin levels. It is plausible that the decreased testosterone level is associated with testicular dysfunction rather than a pituitary disorder. However, the effects of nicotine on reproductive hormones were dose dependent and the observed effects were ameliorated with withdrawal.
Footnotes
Source of Support: The authors acknowledge the tertiary education trust fund (TET fund) of Nigeria for providing fund for this research work
Conflict of Interest: None declared
REFERENCES
- 1.Centers of Disease control and prevention. Annual smoking attributable mortality, years of potential life lost, and economic costs–United states, 1995-1999. MMWR Morb Mortal Wkly Rep. 2002;51:300–3. [PubMed] [Google Scholar]
- 2.Langgassner J. Rauchgewohnheiten der österreichischen Bevölkerung. Statistische Nachrichten. 1996;5:319–26. [Google Scholar]
- 3.Benowitz NL, Jacob P, Herrera B. Nicotine intake and dose response when smoking reduced nicotine content cigarettes. Clin Pharmacol Ther. 2006;80:703–14. doi: 10.1016/j.clpt.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 4.Henningfield JE, Goldberg SR. Pathophysiology of tobacco dependence. In: Bloom FE, Kupfer DJ, editors. Psychopharmacology: The fourth Generation of Progress. New York: Raven Press; 1983. pp. 1715–29. [Google Scholar]
- 5.Oyeyipo IP, Raji Y, Emikpe BO, Bolarinwa AF. Effects of nicotine on sperm characteristics and fertility profile in adult male rats: A possible role of cessation. J Reprod Infertil. 2011;12:201–7. [PMC free article] [PubMed] [Google Scholar]
- 6.Yesilli C, Mungan G, Seckiner I, Akduman B, Aclukgozs, Attan K. Effect of varicoceletomy in sperm creatin kinese, HSPA 2 Chiperone protein (Creatine kinase – M type) LDH, LDH-X and lipid peroxidation product levels in infertile men with variocele. Urology. 2005;66:610–5. doi: 10.1016/j.urology.2005.03.078. [DOI] [PubMed] [Google Scholar]
- 7.Gawoski CL, Carmines EL, Faqi AS, Rajerdaran N. In uterus and lacftation exposure of rats to IR4F reference cigarette mainstream smoke: Effect on prenatal and postnatal development. Toxicol Sci. 2004;79:157–69. doi: 10.1093/toxsci/kfh083. [DOI] [PubMed] [Google Scholar]
- 8.Oyeyipo IP, Raji Y, Emikpe BO, Bolarinwa AF. Effects of oral administration of nicotine on organ weight, serum testosterone level and testicular pathology in adult male rats. Niger J Physiol Sci. 2010;25:81–6. [PubMed] [Google Scholar]
- 9.Seyler LE, Pomerleau OF, Fertig JB, Hunt D, Parker K. Pituitary hormone response to cigarette smoking. Pharmacol Biochem Behav. 1986;24:159–62. doi: 10.1016/0091-3057(86)90062-6. [DOI] [PubMed] [Google Scholar]
- 10.Vine MF. Smoking and male reproduction: A review. Int J Androl. 1996;19:323–37. doi: 10.1111/j.1365-2605.1996.tb00523.x. [DOI] [PubMed] [Google Scholar]
- 11.Kapoor D, Jones TH. Smoking and hormones in health and endocrine disorders. Eur J Endocrinol. 2005;152:491–9. doi: 10.1530/eje.1.01867. [DOI] [PubMed] [Google Scholar]
- 12.Blanco-Muñoz J, Lacasaña M, Aguilar-Garduño C. Effect of current tobacco on the male reproductive hormone profile. Sci Total Environ. 2012;426:100–5. doi: 10.1016/j.scitotenv.2012.03.071. [DOI] [PubMed] [Google Scholar]
- 13.Ojeda SR, Urbanski HF. Puberty in rat. In: Knobil E, Neil JE, editors. The Physiology of Reproduction. 2nd ed. New York II: Raven Press; 1994. pp. 363–409. [Google Scholar]
- 14.Huthaniemi IT, Toppari J. Endocrine, paracrine, and autocrine regulation of testicular steroidogenesis. In: Mukhopadhya AK, Raizada MK, editors. Tissue Renin Angiotensin System. New York: Plenum Press; 1995. pp. 33–53. [DOI] [PubMed] [Google Scholar]
- 15.Yamamoto Y, Isoyama E, Sofikitis N. Effects of smoking on testicular function and fertilizing potential in rats. J Urol. 1998;26:45–8. doi: 10.1007/s002400050022. [DOI] [PubMed] [Google Scholar]
- 16.Trummer H, Habermann H, Haas J, Pummer K. The impact of cigarette smoking on human semen parameters and hormones. Hum Reprod. 2002;17:1554–9. doi: 10.1093/humrep/17.6.1554. [DOI] [PubMed] [Google Scholar]
- 17.Ramlau-Hansen CH, Thulstrup AM, Aggerholm AS, Jensen MS, Toft G, Bonde JP. Is smoking a risk factor for decreased semen quality? A cross-sectional analysis. Hum Reprod. 2007;22:188–96. doi: 10.1093/humrep/del364. [DOI] [PubMed] [Google Scholar]
- 18.Patterson TR, Stringham JD, Meikle AW. Nicotine and cotinine inhibit steroidogenesis in mouse Leydig cells. Life Sci. 1990;46:265–72. doi: 10.1016/0024-3205(90)90032-m. [DOI] [PubMed] [Google Scholar]
- 19.Andersen AN, Semczuk M, Tabor A. Prolactin and pituitary gonadal function in cigarette smoking infertile patients. Andrologia. 1984;16:391–6. doi: 10.1111/j.1439-0272.1984.tb00381.x. [DOI] [PubMed] [Google Scholar]
- 20.Shieh KR, Pan JT. Nicotinic control of tuberoinfundibular dopaminergic neuron activity and prolactin secretion: Diurnal rhythm and involvement of endogenous opioidergic system. Brain Res. 1997;756:266–72. doi: 10.1016/s0006-8993(97)00157-1. [DOI] [PubMed] [Google Scholar]
- 21.Shi J, Hui L, Xu Y, Wang F, Huang W, Hu G. Sequence variations in the μ-opioid receptor gene (OPRM1) associated with human addiction to heroin. Hum Mutat. 2002;19:459–60. doi: 10.1002/humu.9026. [DOI] [PubMed] [Google Scholar]
- 22.Corsello SM, Ubertini G, Altomare M, Lovicu RM, Migneco MG, Rota CA, et al. Giant prolactinomas in men: Efficacy of cabergoline treatment. Clin Endocrinol (Oxf) 2003;58:662–70. doi: 10.1046/j.1365-2265.2003.01770.x. [DOI] [PubMed] [Google Scholar]
- 23.Horseman ND, Gregerson KA. Prolactin. In: DeGroot LJ, Jameson JL, editors. Endocrinology. Philadelphia: Elsevier Saunders; 2006. pp. 309–21. [Google Scholar]
- 24.Hondo E, Kurohmaru M, Sakai S, Ogawa K, Hayashi Y. Prolactin receptor expression in rat spermatogenic cells. Biol Reprod. 1995;52:1284–90. doi: 10.1095/biolreprod52.6.1284. [DOI] [PubMed] [Google Scholar]
- 25.Molitch ME. Prolactin in human reproduction. In: Strauss JF III, Barbieri RL, editors. Yen and Jaffe's Reproductive Endocrinology: Physiology, Pathophysiology, and Clinical Management. 6th ed. Philadelphia, PA: Elsevier Inc; 2009. pp. 57–78. [Google Scholar]
- 26.Nordberg A, Wahlstrom G, Arnelo U, Larsson C. Effect of long term nicotine treatment on [3H] nicotine binding site in the rat brain. Drug Alcohol Depend. 1985;16:9–17. doi: 10.1016/0376-8716(85)90077-8. [DOI] [PubMed] [Google Scholar]
- 27.Berta L, Fortunati N, Gennari P, Appendino M, Casella A, Frairia R. Influence of cigarette smoking on pituitary and sex hormone balance in healthy premenopausal women. Fertil Steril. 1991;56:788–9. doi: 10.1016/s0015-0282(16)54620-2. [DOI] [PubMed] [Google Scholar]
- 28.Law MR, Cheng R, Hackshaw AK, Allaway S, Hale AK. Cigarette smoking, sex hormones and bone density in women. Eur J Epidemiol. 1997;13:553–8. doi: 10.1023/a:1007389712487. [DOI] [PubMed] [Google Scholar]
- 29.Jurasovic’ J, Cvitkovic’ P, Pizent A, Colak B, Telisman S. Semen quality and reproductive endocrine function with regard to blood cadmium in Croatian male subjects. Biometal. 2004;17:735–43. doi: 10.1007/s10534-004-1689-7. [DOI] [PubMed] [Google Scholar]
- 30.Gill-Sharma MK. Prolactin and male fertility: the long and short feedback regulation. Int J Endocrinol. 2009;687259:1–13. doi: 10.1155/2009/687259. [DOI] [PMC free article] [PubMed] [Google Scholar]


