Abstract
AIMS:
A variety of stress factors are known to inhibit male reproductive functions. So this study was conducted in order to investigate the effects of honey and vitamin E on the germinative and somatic cells of testes of rats exposed to noise stress.
MATERIALS AND METHODS:
Mature male wistar rats (n = 24) were randomly grouped as follows: Group 1 (honey + noise stress), 2 (vitamin E + noise stress), 3 (noise stress,) and 4 as the control group. In groups 1, 2, and 3, rats were exposed to noise stress. In groups 1 and 2, rats also were given honey and vitamin E, respectively, orally for 50 days. After that, the germinative and somatic cells of testes parenchyma were isolated by digesting the whole testes by a standard method. Next, viability, apoptosis, and necrosis of the cells were evaluated by TUNEL kit and flow cytometry.
RESULTS:
The rates of apoptosis and necrosis of the testicular cells were increased (P = 0.003 and P = 0.001, respectively), but viability of these cells decreased in testes of rats exposed to noise stress (P = 0.003). However, administration of honey and vitamin E were significantly helpful in keeping the cells of testis parenchyma alive, which suffers from noise pollution (P < 0.05 and P < 0.05, respectively).
CONCLUSIONS:
Noise stress has negative influences on the cells of testicular tissue by increasing apoptotic and necrotic cells. However, the associated enhancement in healthy cells suggests that honey and vitamin E have positive influences on the testis parenchyma.
KEY WORDS: Apoptosis, germ cells, honey, noise stress, vitamin E
INTRODUCTION
A variety of stress factors (i.e., exposure to microorganisms, hyperthermia, heavy metals, prolonged immobilization, swimming stress, and noise) are known to inhibit male reproductive functions.[1,2,3,4,5] Noise is one of the major problem today that enhances the apoptosis of the testicular germ cells.[6] This environmental stress may cause increase in the number of free radicals (i.e., hydrogen peroxide [H2O2] and reactive oxygen species [ROS]) that can cause apoptosis in the spermatogenic cell lineage to decrease the rate of normal sperms.[7,8,9,10,11,12]
A variety of drugs have been designed to overcome the infertility problem of men. Many of these may lack specificity, have low efficiency, or have potentially severe side effects on the treated men. It is generally believed that the antioxidants prevent infertility in men via scavenging of free radicals.[13,14]
Honey is full of enzymatic and non-enzymatic antioxidants such as catalase, ascorbic acid, flavonoids, and alkaloids.[15,16,17] It acts against oxidative stress and prevents oxygen contact with non-saturated fatty acids and also lipoprotein oxidation.[18] In addition, honey is rich in fructose, glucose, minerals, magnesium, potassium, calcium, sodium chloride, sulfur, ferrous, phosphate, and vitamins C, B6, B5, B3, B2, and B1.[19]
The efficacy of honey on the fertility of males of different species was also tested in some studies. Generally, it was suggested that honey can affect the spermatogenesis process and sperm fertility in males.[20,21]
Vitamin E is another antioxidant that presents in cell membranes, it neutralizes H2O2 and prevents cell membrane damage.[22] Indeed, α-tocopherol is the most biologically active form of vitamin E and is the most abundant form in the body. Although the absorption processes of all forms of vitamin E are same, the alpha form is found more in the organs and blood.[23] Vitamin E has positive effects on the pathogenesis of testis and sperm fertility. Food supplemental prescriptions that contain vitamin E may contribute to the functional improvement of spermatozoa.[24,25] Also, vitamin E decreases lipid peroxidation produced in oxidative stress and enhances the motility and fertilization power of sperms.[26] To our knowledge, there has been no research regarding the effects of both honey and vitamin E on the testes of rat exposed to noise stress. Therefore, this study suggests that treatment by antioxidants like honey and vitamin E may compensate the oxidative damages as a result of noise stress on the germinative and somatic cells of testis parenchyma.
MATERIALS AND METHODS
Honey and vitamin E solutions preparation
Honey was obtained from a beekeeping center in Uremia, Iran and prepared as a 5% solution. Vitamin E was obtained from Iran Daru Co., Iran.
Animal preparations
Mature wistar male rats (n = 24, 200 ± 15 g) were obtained from the Laboratory Animal Reproduction and Breeding Center, Ahvaz, Iran. Rats were separated into 4 groups (n = 6), randomly. The cages of groups 1-3 were placed in a woody and acoustic room (dimensions of 3 × 4 × 3 m) and then, routinely, exposed to 90-130 db voice with 300-350 Hz frequency of noise emitted by a white noise generator from 7:00 PM to 7:00 AM for 50 days.[27] It is worth mentioning that the timer was set in a way that the sound-generating (speech) device was turned on for 1 h and turned off for 15-60 min and then turned on again.
Group 1 was exposed to voice along with feeding of 5% honey solution by gavage method.[28] Group 2 was exposed to voice waves along with receiving of vitamin E (75 mg/ml) by gavage method[23] and group 3 was exposed to the voice without any complementary material. Group 4 (control) was kept in natural condition without any stress.
Digestion rats testes procedure
After 50 days that equals with the duration of one spermatogenesis cycle of rat, testes of rats of all groups were removed and then testicular cells of them were cultured in Dulbecco's Modified Eagle Medium (DMEM) medium with 10% fetal bovine serum (FBS) and penicillin/streptomycin. For this purpose, briefly after separating the capsule under stereo microscope, seminiferous tubules were digested in two steps. First, seminiferous tubules were placed in tubes contains 1 mg/ml collagenase type IV and 200-700 μg/ml DNaseI for 15 min in 37°C incubator with gently pipetting action. Next, the tubes were centrifuged (100×g for 5 min), the supernatant was discarded, and the cells were resuspend in 1 ml trypsin-ethylenediamminetetraacetate (EDTA; Sigma) and 200 μg/ml DNaseI for 5 min in 37°C incubator. The activity of trypsin was stopped by adding 10% FBS to cell suspension. The cells were washed in PBS solution and incubated in fixing buffer (1% paraformaldehyde solution). Next, the cells were centrifuged (300×g for 5 min), washed twice in PBS, resuspended in solution containing DNA-labeling, and then incubated (37°C) for 1 hour. Subsequently, the supernatant was discarded and the cells pre-coated with an antibody-anti Brdu solution (at room temperature for 5 min). The cells were then incubated in propidium iodide/RNse staining buffer according to the manufacturer's instructions. Finally, after removing of propidium iodide/RNse staining, the cells were washed again with PBS twice, and assayed for apoptosis by flow cytometry and TANELL kit (Invitrogen, CA, USA) according to the manufacturer's directions.
Statistical analysis
All data are presented as mean ± standard deviation (SD). The significance of differences among different groups was assessed by one-way analysis of variance (ANOVA). The acceptance level of significance was P < 0.05. Data was evaluated by SPSS for windows (SPSS Inc., Chicago, Illinois, USA) version 12.0.1 with 95% confidence interval level.
RESULTS
In the control group, the mean percentage of apoptotic, necrotic, and live cells were 0.9 ± 0.1, 0.24 ± 0.01, and 0.98.41 ± 0.06, respectively. The level percentage of apoptotic and necrotic cells in the noise group (89.31 ± 0.5 and 9.88 ± 0.3, respectively) was increased as compared to the control group (P = 0.003 and P = 0.001, respectively). Also, the mean percentage of viability of cells was decreased when compared with that in the control group (P = 0.003). However, in the case of group pre-treated with honey, the mean percentage of apoptotic and necrotic cells (1.89 ± 0.7 and 0.26 ± 0.01, respectively) decreased when compared with that in the noise group (P = 0.002 and P = 0.002, respectively). The level of apoptotic and necrotic cells in groups pre-treated with vitamin E (3.42 ± 0.6 and 0.43 ± 0.08, respectively) was decreased when compared with that in the noise group (P = 0.01 and P = 0.01, respectively). It is noteworthy that honey increased the live cells percentage more than did vitamin E (97.1% ± 0.03 vs. 95.56 ± 0.07, respectively, P < 0.05), but the necrotic cells level was similar between both honey and vitamin E treated groups (P < 0.05) [Figure 1].
Figure 1.
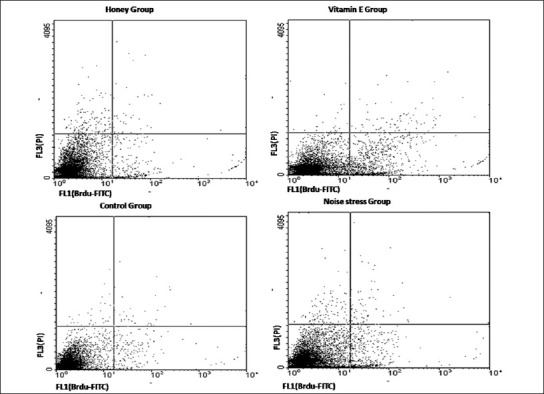
Flowcytometric study. Cells were analyzed for green fluorescence (FITC) and for red fluorescence (PI) by flow cytometry. Percentages of viable, apoptotic and necrotic testicular parenchyma cells were determined by the dot plot of FL1 (in the X axis) to FL3 (in the Y axis). Lower left indicates viability percent and lower right and upper right indicate percentage of apoptosis and necrosis respectively. The comparisons between cell cycle/apoptotic responses of Honey, Vitamin, Noise stress and control groups are showed.
DISCUSSION
In the present study, it was suggested that the noise stress may have negative influences on germinative and somatic cells of testes parenchyma by increasing apoptotic and necrotic cells. Considering the current results, some studies confirmed that, in generally, stress can injure testicular cells.[6] Yazawa et al.,[8] reported the effect of immobilization stress on the apoptotic rate of the germ cells as well.
In normal situation, apoptosis process occurs permanently for maintaining the tissue homeostasis during spermatogenesis.[29] However, high occurrence of apoptosis can cause negative effects in male genital system.[8] Apoptotic activity is dependent on the expression of some apoptosis-related genes and proteins (i.e., caspase, Apaf-1, NF-KB, P53, and death receptors) and also some anti-apoptosis-related genes and proteins (i.e., BCL-2). Indeed, these genes and proteins play a critical role in the apoptosis program.[30,31,32]
Furthermore, some studies have shown that there is a relationship between apoptosis-related proteins and genes and male infertility.[33] In addition, some studies determined whether there is a relationship between steroid hormones or testicular cells and the expression patterns of apoptosis-related proteins and genes.[34]
The results of abovementioned studies showed that reduction or inhibition of gonadotropin as well as steroid hormones secretion can lead to apoptosis of testicular cells.[35,36] Therefore, it seems that every factor that can cause, reduce, or even inhibit gonadotropin and sex hormones secretion, normally can lead to induction of apoptosis in germinative and somatic cells of testes parenchyma.[36]
Therefore, noise stress may disrupt steroid hormones concentration and neuroendocrine gonadal axis in turn is result in increased rate of cellular damage by expressing, as mentioned for testicular tissue, apoptosis-related proteins and genes in testicular cells.
In accordance with the above suggestion, Chandralekha et al.,[37] reported that the testosterone serum level in rats under noise stress (100 dB) was reduced. Also, more structural changes in the testis tissue was observed following the noise stress.[37] In addition, it was reported that apoptosis ratio is negatively correlated with normal morphology and motility of sperm and conversely positively correlated with sperm tail defects.[38,39]
The results in this study also show that the necrosis in cells increased following noise stress. Consequently, it seems that noise stress can directly lead to induce necrosis in cells population and destruct germ cells of the testicular tissue.
Alternatively, this study reported that honey and vitamin E decreased apoptosis and necrosis in cells by noise stress and thereby increased cell viability. In fact, focusing on the above suggestions, the only thing that can be concluded from this finding is that vitamin E and especially honey, being rich in enzymatic and nonenzymatic antioxidants, work by neutralizing the expression patterns of apoptosis-related proteins and genes (may be due to destructive effects of noise stress) or by regulation of anti-apoptotic patterns. Abdul-Ghani et al.,[28] claimed that honey enhances the spermatogenesis process without any disruption in sexual hormone concentrations. However, Asiyah et al.,[40] reported that honey has no more positive influence on the sex hormones concentration in male. In general, each factor that affects the function of interstitial space cells (leydig and myoid cells) as well as epithelium germinal cells (sertoli and germ cells), may consequently lead to suffer a harmful effect on the neuroendocrine gonadal axis and, in turn, on the spermatogenic cell lineage. The above information suggests that honey and vitamin E, because of their effect on both neuroendocrine gonadal axis and testicular cells, may create conditions that modifies or decreases the process of apoptosis and necrosis in the cells. The second assumption is that honey and vitamin E, because of enhanced steroid hormones level to sertoli cells, can have positive effect on the nutrition of germ cells. Mahanem et al.,[41] as well as Syazana[21] reported that if appropriate dose of honey is applied to rats, the spermatogenic cells lineage and sperm rate increases. Naseem et al.,[26] claimed that vitamin E enhances sperm parameters. Therefore, considering the above results and the results of this study, it is suggested that honey and vitamin E can be useful for enhancing the longevity of cells that suffer from deleterious factors such as noise pollution.
CONCLUSION
It seems that the noise stress has negative influences on the fertility of male. The findings of this study may specify a novel natural therapeutic approach based on modulation of the apoptotic process induced by pathogenesis stress, i.e., noise and enhancing spermatogenesis through honey and vitamin E as possible future antioxidant in fertile men.
ACKNOWLEDGMENTS
This study was supported by a research grant (n=PRC-92) from the Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran. The present study is extracted from master's thesis of Mr. Asghar Rajabzadeh.
Footnotes
Source of Support: Research grant (n=PRC-92) from the Ahvaz Jundishapur University of Medical Sciences
Conflict of Interest: None declared
REFERENCES
- 1.Ozawa N, Goda N, Makino N, Yamaguchi T, Yoshimura Y, Suematsu M. Leydig cell-derived heme oxygenase-1 regulates apoptosis of premeiotic germ cells in response to stress. J Clin Invest. 2002;109:457–67. doi: 10.1172/JCI13190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saki G, Rahim R, Vaysi OA. Effect of forced swimming stress on in-vivo fertilization capacity of rat and subsequent offspring quality. J Hum Reprod Sci. 2010;3:32–4. doi: 10.4103/0974-1208.63120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mingoti GZ, Pereita RN, Monteiro CM. Fertility of male adult rats submitted to force swimming stress. Brazil J Med Biol Res. 2003;36:677–81. doi: 10.1590/s0100-879x2003000500016. [DOI] [PubMed] [Google Scholar]
- 4.Saki G, Razie S, Amirpoor S. Pregnancy rate in female mice exposed to forced swimming stress. Asian J Biol Sci. 2011;4:266–71. [Google Scholar]
- 5.Saki G, Rahim F, Alizadeh K. Effect of forced swimming stress on count, motility and fertilization capacity of the sperm in adult rats. J Hum Reprod Sci. 2009;2:72–5. doi: 10.4103/0974-1208.57226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jalali M, Saki G, Sarkaki AR, Karami K, Nasri S. Effect of noise stress on count, progressive and non-progressive sperm motility, body and genital organ weights of adult male rats. J Hum Reprod Sci. 2012;5:48–51. doi: 10.4103/0974-1208.97801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Srivastava RK, Taylor MF, Mann DR. Effect of immobilization stress on plasma luteinizing hormone, testosterone, and corticosterone concentrations and on 3 beta-hydroxysteroid dehydrogenese activity in the testes of adult rats. Proc Soc Exp Biol Med. 1993;204:231–5. doi: 10.3181/00379727-204-43658. [DOI] [PubMed] [Google Scholar]
- 8.Yazawa H, Sasagawa I, Ishigooka M, Nakada The Effect of immobilization stress on testicular germ cell apoptosis in rats. Hum Reprod. 1999;14:1806–10. doi: 10.1093/humrep/14.7.1806. [DOI] [PubMed] [Google Scholar]
- 9.Wang X, Sharma RK, Sikka SC. Oxidative stress is associated with increased apoptosis leading to spermatoozoa DNA damage in patients with male factor infertility. Fertil Steril. 2003;80:531–5. doi: 10.1016/s0015-0282(03)00756-8. [DOI] [PubMed] [Google Scholar]
- 10.Sharma RK, Agarwal A. Role of reactive oxygen species in male infertility. Urology. 1996;48:835–50. doi: 10.1016/s0090-4295(96)00313-5. [DOI] [PubMed] [Google Scholar]
- 11.Agarwal A, Saleh RA, Bedaiwy MA. Role of reactive oxygen species in the pathophysiology of human reproduction. Fertil Steril. 2003;79:829–43. doi: 10.1016/s0015-0282(02)04948-8. [DOI] [PubMed] [Google Scholar]
- 12.Lysiak JJ, Zheng S, Woodsen R, Turner TT. Caspase-9-dependent pathway to murine germ cell apoptosis: Medication by oxidative stress, BAX and caspase 2. Cell Tissue Res. 2007;328:411–9. doi: 10.1007/s00441-006-0341-y. [DOI] [PubMed] [Google Scholar]
- 13.Bauche F, Fouchard MH, Jegou B. Antioxidant system in rat testicular cells. FEBS Lett. 1994;349:392–6. doi: 10.1016/0014-5793(94)00709-8. [DOI] [PubMed] [Google Scholar]
- 14.Gu W, Hecht NB. Developmental expression of glutathione peroxidase, catalase, and manganes superoxide dismutase mRNAs during spermatogenesis in the mouse. J Androl. 1996;17:526–62. [PubMed] [Google Scholar]
- 15.Kamaruddin MY. Honey: A healing for mankind throughout the ages. The Fountain. 1993;1:4–6. [Google Scholar]
- 16.Aljady AM, Kamaruddin MY, Jamal AM, Mohd Y. Biochemical study on the efficacy of Malaysian honey in infected wounds: An animal model. Medical Journal of Islamic Academy of Sciences. 2000;13:125–32. [Google Scholar]
- 17.Perez E, Rodriguez-Malaver AJ, Vit P. Antioxidant capacity of venezuelan honey in wistar rat homogenates. J Med Food. 2006;9:510–6. doi: 10.1089/jmf.2006.9.510. [DOI] [PubMed] [Google Scholar]
- 18.Khalili MI, Sulaiman SA, Boukraa L. Antioxidant properties of honey and its role in preventing health disorder. The Open Nutraceuticals Journal. 2010;3:6–16. [Google Scholar]
- 19.Bogdanov S, Jurendic T, Sieber R, Gallmann P. Honey for nutrition and health: A review. J Am Coll Nutr. 2008;27:677–89. doi: 10.1080/07315724.2008.10719745. [DOI] [PubMed] [Google Scholar]
- 20.Estevinho L, Pereira AP, Moreira L, Dias LG, Pereira E. Antioxidant and antimicrobial effects of phenolic compounds extracts of Northeast Portugal honey. Food Chem Toxicol. 2008;46:3774–9. doi: 10.1016/j.fct.2008.09.062. [DOI] [PubMed] [Google Scholar]
- 21.Syazana NS. Effect of Ggelam honey on sperm quality and testis of rat. Sains Malaysiana. 2011;40:1243–6. [Google Scholar]
- 22.Kessopoulou E, Powers HJ, Sharma KK, Pearson MJ, Russell JM, Cooke ID, et al. A double-blind randomized placebo cross-over controlled trial using the antioxidant vitamin E to treat reactive oxygen species associated male infertility. Fertil steril. 1995;64:825–31. doi: 10.1016/s0015-0282(16)57861-3. [DOI] [PubMed] [Google Scholar]
- 23.Shalaby MA, Elzorba HY. Protective Effect of Celery oil, Vitamin E and their combination against testicular toxicity in male rats. Global Veterinaria. 2010;5:122–8. [Google Scholar]
- 24.Erat M, Ciftci M, Gumustekin K, Gul M. Effect of nicotin and vitamin E on glutathione reductase activity in some rat tissues in vivo and in vitro. Eur J Pharmacol. 2007;554:92–7. doi: 10.1016/j.ejphar.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 25.Yousef MI, Abdallah GA, Kamel KI. Effect of ascorbic acid and vitamin E supplementation on semen quality and biochemical parameters of male rabbits. Anim Reprod Sci. 2003;76:99–111. doi: 10.1016/s0378-4320(02)00226-9. [DOI] [PubMed] [Google Scholar]
- 26.Naseem M, Goh YM, Hafandi A, Amal NM, Kufli CN, Rajion MA. Effect of vitamin E and soybean oil supplementation on sperm parameters in male sprague-Dawley rats. Trop Biomed. 2007;24:45–8. [PubMed] [Google Scholar]
- 27.Karami K, Sarkaki AR. The effect of noise on fertility outcomes of white rats. Sci Med J. 2002;33:45–9. [Google Scholar]
- 28.Abdul-Ghani AS, Dabdoub N, Muhammad R, Abdul-Ghani R, Qazzaz M. Effect of Palestinian honey on spermatogenesis in rats. J Med Food. 2008;11:799–802. doi: 10.1089/jmf.2008.0085. [DOI] [PubMed] [Google Scholar]
- 29.Martincic DS, Virant Klun I, Zorn B, Vrtovec HM. Germ cell apoptosis in the human testis. Pflugers Arch. 2001;442:159–60. doi: 10.1007/s004240100007. [DOI] [PubMed] [Google Scholar]
- 30.Aggarwal A, Misro MM, Maheshwari A, Sehgal N, Nandan D. Adverse effects associated with persistent stimulation of Leydig cells with hCG in vitro. Mol Reprod Dev. 2009;76:1076–83. doi: 10.1002/mrd.21074. [DOI] [PubMed] [Google Scholar]
- 31.Aycan Z, Ustunsalih-Inan Y, Cetinkaya E, Vidinlisan S, Ornek A. Evaluation of low-dose hCG treatment for cryptorchidism. Turk J Pediatr. 2006;48:228–31. [PubMed] [Google Scholar]
- 32.Diemer T, Allen JA, Hales KH, Hales DB. Reactive oxygen disrupts mitochondria in MA-10 tumor leydig cells and inhibits steroidogenic acute regulatory (StAR) protein and steroidogenesis. Endocrinology. 2003;144:2882–91. doi: 10.1210/en.2002-0090. [DOI] [PubMed] [Google Scholar]
- 33.Brugnon F, Van Assche E, Verheyen G, Sion B, Boucher D, Pouly JL, et al. Study of two markers of apoptosis and meiotic segregation in ejaculated sperm of chromosomal translocation carrier patients. Hum Reprod. 2006;21:685–93. doi: 10.1093/humrep/dei401. [DOI] [PubMed] [Google Scholar]
- 34.Moustafa MH, Sharma RK, Thornton J, Mascha E, Abdel-Hafez MA, Thomas AJ, Jr, et al. Relationship between ROS production apoptosis and DNA denaturation in spermatozoa from patients examined for infertility. Hum Repord. 2004;19:129–38. doi: 10.1093/humrep/deh024. [DOI] [PubMed] [Google Scholar]
- 35.Troiano L, Fustini MF, Lovato E, Frasoldati A, Malorni W, Capri M, et al. Apoptosis and spermatogenesis. Evidence from an in vivo model of testosterone withdrawal in the adult rat. Biochem Bioph Res Commun. 1994;202:1315–21. doi: 10.1006/bbrc.1994.2074. [DOI] [PubMed] [Google Scholar]
- 36.Mylchreest E, Sar M, Wallace DG, Foster PM. Fetal testosterone insufficiency and abnormal proliferation of leydig cells and gonocytes in rats exposed to di (nbuty1) phthalate. Reprod Toxicol. 2002;16:19–28. doi: 10.1016/s0890-6238(01)00201-5. [DOI] [PubMed] [Google Scholar]
- 37.Chandralekha GS, Jegenathan R, Charan J. Noise exposure effect on testicular histology, morphology and on male steroidogenic hormone. Malaysian J Med Sci. 2007;14:28–35. [PMC free article] [PubMed] [Google Scholar]
- 38.Nabil A, Tamer S, Uwe P. The relationship between human sperm apoptosis and morphology and the sperm deformity index. Hum Repro. 2007;5:1413–9. doi: 10.1093/humrep/dem016. [DOI] [PubMed] [Google Scholar]
- 39.Chen Z, Hauser R, Trbovich AM, Shifren JL, Dorer DJ, Godfrey-Bailey L, et al. The relationship between human semen characteristics and sperm apoptosis. A pilot study. J Androl. 2006;27:112–20. doi: 10.2164/jandrol.05073. [DOI] [PubMed] [Google Scholar]
- 40.Asiyah HA, Syazana NS, Hashida N. Effects of nicotine and Gelam honey on testis parameters and sperm quality of juvenile rats. Scientific Research and Essays. 2011;6:5471–74. [Google Scholar]
- 41.Mahanem M, SitiAmrah S, Yatiban M, Hasnan J. Effect of ‘Tualang Honey’ on spermatogenesis in rats. Malay J Med Sci. 2007;14:126. [Google Scholar]


