Abstract
OBJECTIVES:
The objective was to highlight the frequency, clinical profile, and predisposing factors of ectopic pregnancy (EP) in a general hospital.
MATERIALS AND METHODS:
This descriptive study was conducted at the Obstetrics and Gynaecology department of Hera General hospital, Makkah, Saudi Arabia, from July 1, 2009 to December 29, 2010. Data were collected on chief medical complaints, sociodemographic characteristics, past obstetrics and gynecological history, management done, and outcome of management. Data were analyzed using Microsoft Office Excel (version 2007).
RESULTS:
Out of total 7564 pregnancies, 44 (0.58%) patients were diagnosed as EP. Out of 44, 22 (50%) patients presented within 24 h of onset of symptoms. Mean age was 28 ± 7 years. Multigravida were predominant in 25 (57%), and 21 (48%) had gestational age of 6-8 weeks at the time of presentation; the common presenting features were amenorrhea (41, 93.2%), abdominal pain (39, 88.6%), and tenderness (38, 86%). Previous pelvic surgery (13, 29.5%), infertility treatment (11, 25%), and pelvic inflammatory disease (10, 22.7%) were the common predisposing factors. Twenty-five (57%) presented with ruptured EP and were operated within 24 h, and the remaining were kept under observation till further diagnosis. After confirming the diagnosis, 12/19 underwent laparoscopy, whereas 7/19 received medical treatment. Surgery confirmed fallopian tube pregnancies in 35 (94.5%). No mortality was observed.
CONCLUSION:
Previous pelvic surgeries were the major etiological factor for EP. Other factors were infertility treatment and pelvic inflammatory disease. The most common site of EP was fallopian tubes.
KEY WORDS: Ectopic pregnancy, pelvic inflammatory disease, predisposing factors, tubal pregnancy
INTRODUCTION
Ectopic pregnancy (EP) is a pregnancy in which fertilized ovum implants other than the endometrial lining of the uterus. Incidence of EP ranges between 0.25% to 2% of all pregnancies.[1] In 95% of EPs, fertilized ovum implants in the tubes, but rarely in other organs like abdomen, ovaries, cervix, spleen, omentum, cessarian section scar, and intramural. The death rate is about 1 per 2000 EPs and 15% of all maternal deaths.[2,3,4,5] Incidence of EP is nearly double in Black women who also have a four times greater risk of EP-related mortality than White women.[6] The risk factors for EP include pelvic inflammatory disease, previous tubal surgery, previous tubal pregnancy, progestin contraceptive, assisted reproduction, ovulation induction, induced abortion, salpingitis isthmica nodosa, smoking, and diethyl stilbestrol exposure.[7,8] Most of the tubal pregnancies become symptomatic within 12 weeks, but a small number of them progress beyond this gestation and are diagnosed late.[9]
Proper management of EP needs early diagnosis, resuscitation, timely treatment, and follow-up. Early diagnosis of EP is a difficult task but can be possible with the help of quantitative β-human chorionic gonadotropin (β-hCG) level, transvaginal ultrasonography, and laparoscopy.[10,11]
Knowledge of the risk factors for EP helps in rapid diagnosis and could reduce the need for surgery and suggest actions to improve prognosis. However, several studies assessing risk factors for EP have been published from developed countries.[7] Saudi women have different characteristics, like cultural, religious, sociodemographic, sexual behavior, beliefs, and contraception practices, than those of developed countries and may have different risk factors for EP.
The purpose of this study was to highlight the frequency and predisposing factors of EP, and to elucidate the outcome after different treatment modalities in a general hospital.
MATERIALS AND METHODS
This retrospective descriptive study was conducted at Hera General Hospital, Makkah, Saudi Arabia, over a duration of 18 months from July 1, 2009 to December 31, 2010.
The study subjects included all females in their reproductive age who were admitted at the Obstetrics and Gynaecology department with suspicion of EP. Data were retrieved from the medical record office by exploring the patients’ files in detail. Patients’ files were looked for age; gravidity; gestational age; clinical presenting features; and EP risk factors, like history of previous abortion, infertility treatment, current use of intrauterine contraceptive device (IUCD), history of previous tubal surgery and pelvic surgery, previous ectopic and/or pelvic inflammatory disease. The duration of hospital stay was also recorded. Peak level of β-hCG, transvaginal ultrasonographic findings, mode of treatment, and type of surgical procedure were also retrieved. A woman experiencing several EPs during the study period generated multiple case entries, one for each EP episode. In this study, seven women experienced two, and two women experienced three EPs. The duration from symptoms’ onset to admission and admission to definite treatment was also recorded.
Data were analyzed using Microsoft Office Excel (version 2007). Numerical data were subjected to descriptive analysis, with mean ± standard deviation (SD). Categorical data were analyzed as frequency and percentage.
Ethical issues
The Institutional Review Board of Hera General Hospital, Makkah, Saudi Arabia, granted us permission to conduct this study.
RESULTS
During the study period, a total of 44 patients were diagnosed as EP. Incidence was 0.58% out of 7654 pregnant women admitted at the Department of Obstetrics and Gynaecology (5.8/1000 pregnancies).
The mean age of the women was 27.9 ± 7 years and only 2 (4.5%) were more than 40 years old. Twenty-five (56.8%) were multigravida whereas 21 (47.7%) presented at 7-8 weeks of gestation. The range of gestational age was 3-9 weeks at presentation. Only 24 (54.5%) patients presented within 24 h, 6 patients within 1-2 days, and 14 patients presented after 2 days of onset of symptoms of EP. The most common presenting feature was amenorrhea (41, 93.2%), followed by abdominal pain (39, 88.6%) and abdominal tenderness (38, 86%). Nineteen patients had β-hCG level below 1000 mIU/ml. Transvaginal ultrasound was performed in 38 patients, whereas six patients underwent abdominal ultrasound. Fluid in Pouch of Douglas was found in 21 (55.3%) cases and ectopic mass in 13 (34.2%) cases [Tables 1 and 2].
Table 1.
Demography and obstetrical history
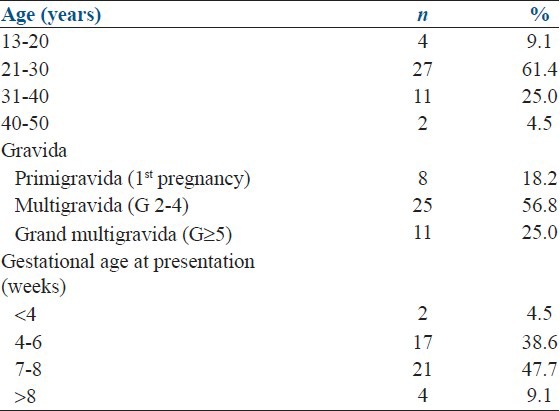
Table 2.
Clinical presentation of ectopic pregnancy
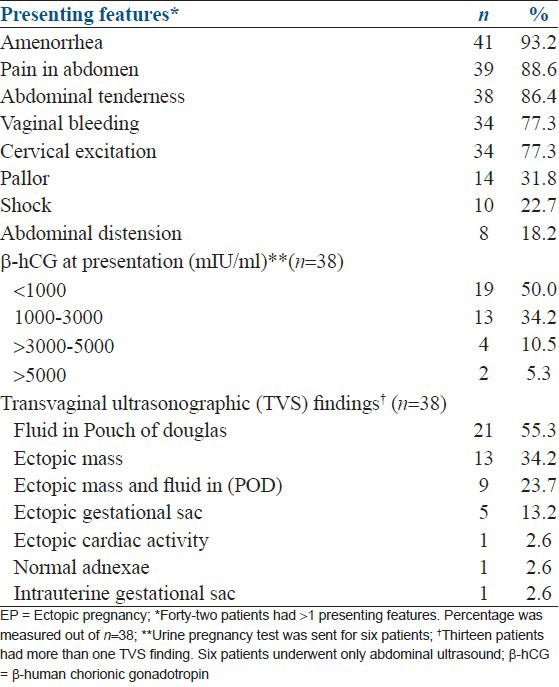
Sixteen (36.3%) females presented with hemoglobin levels of 8-10 gm%, followed by 13 (29.5%) with ≥ 10-12 gm%, 9 (20.5%) with ≥ 12-14 gm%, and 6 (13.6%) with ≤ 8 gm%. Twenty-three (52.3%) patients did not receive any blood transfusion during their hospital stay, but 11 patients received one, 5 received two, 3 received three, and 2 patients received ≥ 4 units of blood transfusion.
Out of 44 patients, 25 underwent emergency laparotomy whereas the remaining 19 patients were admitted for observation and further diagnosis. When diagnosis was proved, 12 underwent laparoscopy whereas seven received medical treatment by methotrexate (MTX) and it was successful in all cases.
On the other hand, salpingectomy was performed in 33 (89.1%) cases followed by salpingostomy in 2 (5.4%), whereas one case underwent salpingotomy and resection of ectopic mass was performed for the remaining one patient.
Pre-operative findings for 37 cases revealed that 35 (94.6%) females had EP in the fallopian tube, whereas one case had within the ovary and one had heterotopic pregnancy, i.e., combined intra- and extra-uterine. Average hospital stay after surgery was 4 days in laparotomy and 2 days in laparoscopy. However, only one patient had a post-laparotomy wound infection.
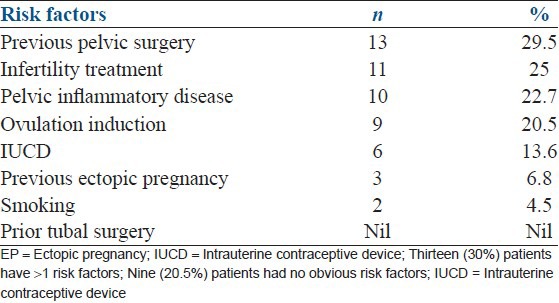
Eighty percent (n = 35) had some risk factors, whereas previous pelvic surgery was most frequently seen (13, 29.5%) followed by history of pelvic inflammatory disease (10, 22.7%) [Table 3].
Table 3.
Predisposing risk factors for the ectopic pregnancy
DISCUSSION
In our study, the rate of EP was 0.58% (5.8/1000 pregnancies). The rate of EP was 1.9% as reported by Lozeau and Potter[12] in USA, and 1.04% and 1% by Bangash and Ahmad,[13] and Waseem,[14] respectively, in Pakistan. Incidence in our study is lower than the reported incidence because of study settings. Our study was conducted in a secondary healthcare center, which is why we were not receiving referral cases from the whole of the Makkah region, and incidence represented the incidence in Hera General Hospital rather than the general population of the region.
Our study is comparable to a number of local as well as international studies.[15,16,17,18] MTX treatment of EP was safe and effective for selected patients with unruptured tubal EP, with no major side effects as found by Dhar et al.[19]
A paper presented in a conference proceeding highlighted management of advanced EP, i.e., in Bahrain, managements of EP were as follows: laparotomy and salpingectomy (84.2% and 5.3%), MTX alone (5.3%) and MTX followed by laparotomy and salpingectomy (10.5%), whereas in Qatar it was laparoscopy and salpingectomy (77.6%), MTX alone (19.4%), and MTX followed by laparoscopy and salpingectomy (3%). Laparoscopy in Qatar and laparotomy in Bahrain were the treatment of choice to treat advance ectopic cases.[20] Lozeau and Potter[12] reported a success rate of 88% in a single-dose versus 93% in a multiple-dose regimen of MTX. Transvaginal ultrasound showed a high predictive value of EP.[12,14,21] Salpingostomy was concluded as a better option than salpingectomy regarding the future fertility outcome in one study.[22] Mortality rate was low in the study of Bangash and Ahmad.[13]
We tried in our study to increase the clinical knowledge and the degree of suspicion of the physicians to diagnose the EP as early as possible. This will avoid delay in presentation and diagnosis, which is vital to avoid morbidity and mortality in patients with EP.
RECOMMENDATIONS
After a review of the literature and our study results, we are in a position to recommend the following steps at three levels i.e., public, primary healthcare, and specialist center. Aim should be early diagnosis and prompt treatment of EP without unnecessary delay in presentation, diagnosis, and treatment.
We should launch a public awareness program about the risk factors to educate all females through electronic media like TV, radio, or newspapers. All these patients should register themselves at a specialist hospital for care of their pregnancy where specialist gynecologists and facilities for diagnosis and treatment of EP are available.
General practitioners working in primary healthcare centers should be educated to have a high index of suspicion for EP.
At specialist-level hospitals, all females (at their child-bearing age) presenting with hemodynamic instability or pain in the lower abdomen should be admitted and immediate investigations like pregnancy test, β-hCG, and ultrasound (both trans-abdominal and trans-vaginal) should be ensured even if there is no history of amenorrhea. Early diagnosis and prompt treatment are the ultimate goal.
CONCLUSION
This study found that previous pelvic surgery, infertility treatment, and pelvic inflammatory disease were the major predisposing factors for EP. The most common site was the fallopian tube and the success rate of MTX was found to be 100%. To avoid emergency laparotomy that has its own post-operative complications, timely diagnosis of EP should be encouraged.
ACKNOWLEDGEMENTS
We are thankful to Mr. Turky Albishri, Senior Researcher, King Abdullah Medical City, for his technical support.
Footnotes
Source of Support: Hera General Hospital, Makkah, KSA
Conflict of Interest: None declared
REFERENCES
- 1.Van Den Eeden SK, Shan J, Bruce C, Glasser M. Ectopic pregnancy rate and treatment utilization. Obstet Gynecol. 2005;105:1052–7. doi: 10.1097/01.AOG.0000158860.26939.2d. [DOI] [PubMed] [Google Scholar]
- 2.Ayinde OA, Aimakhu CO, Adeyanju OA, Omigbodun OA. Abdominal pregnancy at the University College Hospital, Ibadan: A ten year review. Afr J Reprod Health. 2005;9:123–7. [PubMed] [Google Scholar]
- 3.Phupong V, Ultchawadi P. Primary ovarian pregnancy. J Med Assoc Thai. 2005;88:527–9. [PubMed] [Google Scholar]
- 4.Wang CJ, Yuen LT, Chao AS, Lee CL, Yen CF, Soong YK. Caesarean scar pregnancy successfully treated by operative hysteroscopy and suction curettage. BJOG. 2005;112:839–40. doi: 10.1111/j.1471-0528.2005.00532.x. [DOI] [PubMed] [Google Scholar]
- 5.Lee GS, Hur SY, Kown I, Shin JC, Kim SP, Kim SJ. Diagnosis of early intramural pregnancy. J Clin Ultrasound. 2005;33:190–2. doi: 10.1002/jcu.20107. [DOI] [PubMed] [Google Scholar]
- 6.Butts SF, Gibson E, Sammel MD, Shaunik A, Rudick B, Barnhart K. Race, socioeconomic status, and response to methotrexate treatment of ectopic pregnancy in an urban population. Fertil Steril. 2010;94:2789–92. doi: 10.1016/j.fertnstert.2010.06.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shah N, Khan NH. Ectopic pregnancy: Presentation and risk factors. J Coll Physicians Surg Pak. 2005;15:535–8. [PubMed] [Google Scholar]
- 8.Basu A, Candelier C. Ectopic pregnancy with postcoital contraception-a case report. Eur J Contracept Reprod Health Care. 2005;10:6–8. doi: 10.1080/13625180400020895. [DOI] [PubMed] [Google Scholar]
- 9.Tasnim N, Mahmud G. Advance abdominal pregnancy-a diagnostic and management dilemma. J Coll Physicians Surg Pak. 2005;15:493–5. [PubMed] [Google Scholar]
- 10.Farquhar CM. Ectopic pregnancy. Lancet. 2005;366:583–91. doi: 10.1016/S0140-6736(05)67103-6. [DOI] [PubMed] [Google Scholar]
- 11.Sohail S. Hemorrhagic corpus luteum mimicking heterotopic pregnancy. J Coll Physicians Surg Pak. 2005;15:180–1. [PubMed] [Google Scholar]
- 12.Lozeau AM, Potter B. Diagnosis and management of ectopic pregnancy. Am Fam Physcian. 2005;72:1707–14. [PubMed] [Google Scholar]
- 13.Bangash N, Ahmad H. A study of 65 cases of ectopic pregnancy in one year period in military hospital. Pak Armed Forces Med J. 2004;54:205–8. [Google Scholar]
- 14.Waseem T. Proportionate morbidity and risk factors of ectopic pregnancy. Ann King Edward Med Univ. 2004;10:298–300. [Google Scholar]
- 15.Mansoor I. Ectopic pregnancies. Middle East J Emerg Med. 2002;2:13–16. [Google Scholar]
- 16.Aziz S, Al Wafi B, Al Swadi H. Frequency of ectopic pregnancy in a medical centre, Kingdom of Saudi Arabia. J Pak Med Assoc. 2011;61:221–4. [PubMed] [Google Scholar]
- 17.Archibong EI, Sobande AA. Ectopic pregnancy in Abha, Saudi Arabia. A continuing conundrum. Saudi Med J. 2000;21:330–4. [PubMed] [Google Scholar]
- 18.Fageeh WM. Diagnosis and management of ectopic pregnancy in King Abdulaziz University Hospital: A four year experience. JKAU Med Sci. 2008;15:15–25. [Google Scholar]
- 19.Dhar H, Hamdi I, Rathi B. Methotrexate treatment of ectopic pregnancy: Experience at Nizwa Hospital with literature review. Oman Med J. 2011;26:94–8. doi: 10.5001/omj.2011.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mohamed SS, Afifi NN, Al Taher F, Humran YM. Qatar Foundation Annual Research Forum Proceedings, 2011 Nov 20-22. Doha, Qatar: Bloomsbury Qatar Foundation Publishing; 2011. Management of advanced ectopic pregnancy: Comparative study between state of Qatar and Kingdom of Bahrain. BMPS2; p. 116. [Google Scholar]
- 21.Mahmood AM, Aziz A. Tubal ectopic pregnancy; critical evaluation of presentation and management. Prof Med J. 2001;8:208–13. [Google Scholar]
- 22.van Beek JJ, Vollaard ES. Fertility after treatment for ectopic pregnancy: Evaluation of the switch from laparotomy to laparoscopy. Ned Tijdschr Geneeskd. 2005;149:407–12. [PubMed] [Google Scholar]


