Abstract
OBJECTIVE:
Nonalcoholic fatty liver disease (NAFLD) and polycystic ovary syndrome (PCOS) both are known to be associated with insulin resistance and metabolic syndrome (MS). The aim of the study was to determine the presence of NAFLD and associated factors of hepatic steatosis in women with PCOS.
MATERIALS AND METHODS:
A cross-sectional hospital based study of 54 women with PCOS and 55 healthy controls who were age and weight matched were included. Anthropometric parameters, biochemical and hormonal investigations were done in all the patients. Insulin resistance was calculated by Homeostasis model assessment (HOMA). Abdominal ultrasonography and biochemical tests were used to determine the presence of hepatic steatosis after excluding other causes liver disease.
RESULTS:
Women with PCOS had a higher prevalence of hepatic steatosis (67% vs 25%, P = 0.001) MS (35% vs. 7%, P < 0.01) and elevated transaminases (31% vs. 7%, P = 0.03) than controls. All patients with PCOS and controls with MS had presence of hepatic steatosis. Age, BMI, waist-hip ratio, HOMA-IR, HDL and PCOS diagnosis were the factors associated with presence of hepatic steatosis.
CONCLUSION:
NAFLD is commonly present in women with PCOS in combination with other metabolic derangements. Evaluation for liver disease should be considered at an earlier age in women with PCOS, particularly those who have an evidence of MS.
KEY WORDS: Insulin resistance, metabolic syndrome, non-alcoholic fatty liver disease, polycystic ovary syndrome
INTRODUCTION
Polycystic ovary syndrome (PCOS) is the commonest endocrinopathy of premenopausal women affecting nearly 10% of the population.[1,2] It is characterized by chronic anovulation, hyperandrogenism and/or polycystic ovaries. This disease is associated with significant morbidity in terms of both reproductive and metabolic abnormalities. Women with PCOS commonly display a clustering of metabolic derangements that contribute to increased cardiovascular risk associated with this disease. Insulin resistance possibly plays central role in the pathogenesis of PCOS. Insulin resistance has been demonstrated in both obese and non-obese women with PCOS.[3] Nonalcoholic fatty liver disease (NAFLD) often called as hepatic manifestation of metabolic syndrome (MS) is characterized by the accumulation of fat in the liver in the absence of excessive alcohol consumption is the most common cause of liver disease in western countries. NAFLD encompasses a spectrum of histological changes extending from benign simple steatosis (nonalcoholic fatty liver, NAFL) to steatohepatitis (NASH), progressive fibrosis and, ultimately, cirrhosis.[4,5] Although natural history of NAFLD still remains unclear and hepatic steatosis is a benign condition in majority of the patients, its recognition is relevant since it has potential to evolve into end-stage liver disease and its association with cardiovascular risk factors.[6,7] NAFLD has been associated with insulin resistance[8,9,10] and obesity, particularly central.[11] It is therefore understandable that an association may exist between NAFLD and PCOS given that insulin resistance is the common feature and both disorders can be linked with MS. Brown et al.[12] were the first to report the evidence for association of NAFLD and PCOS when they documented NASH on liver biopsy in a young patient with PCOS. Although, existing data of NAFLD in women with PCOS is very scarce. Since then many workers have shown an increased prevalence of NAFLD in women with PCOS based on abnormal aminotransferase levels and/or abnormal ultrasonographic evidence of hepatic steatosis.[13,14,15,16,17,18,19,20]
A high prevalence of PCOS has been reported in Indian women[21] with higher fasting insulin levels[22,23] and greater insulin resistance compared with Caucasians.[24] In view of increasing prevalence of MS in general and expectedly in young women with PCOS we planned to undertake this study with the aim to identify which factors are associated with presence of NAFLD in this subset of patients. The patients with PCOS were evaluated with abdominal ultrasonography and biochemical testing in order to assess the presence of NAFLD and its association with various metabolic and hormonal factors was studied.
MATERIALS AND METHODS
Fifty-four premenopausal women with PCOS diagnosed by Rotterdam criteria (Rotterdam ESHRE/ASRM-sponsored PCOS consensus workshop group, 2003)[25] from endocrine clinic of a teaching hospital between January 2008 to December 2010 were enrolled for a descriptive cross-sectional study. Written informed consent was obtained from all the participants. We included aged 55 years and weight-matched normal healthy women as controls with normal menstrual cycles with no evidence of hyperandrogenism and with normal ovarian morphology on pelvic ultrasonography. Ethical approval was obtained from Institutional Ethics committee.
Inclusion and exclusion criteria
Women with any two of the following were included: oligomenorrhea/oligo-ovulation, clinical or biochemical hyperandrogenism and polycystic ovaries on ultrasound. Oligomenorrhea was defined as absence of menstruation ≥35 days, amenorrhea-no menstruation for >6 months. Clinical hyperandrogenism-modified Ferriman-Gallway score ≥8, with or without acne and/or androgenic alopecia. Women excluded from the study were those with inherited disorders of insulin resistance, type 2 diabetes mellitus, hypertension, Cushing's syndrome, hyperprolactinemia, untreated hypothyroidism, congenital adrenal hyperplasia, with an androgen-secreting adrenal/ovarian tumor and those taking corticosteroids, antiepileptic or antipsychotic drugs, insulin sensitizers, hormonal contraceptives, antituberculosis drugs in past three months, currently pregnant or in the first postpartum year. The patients with alcohol consumption >20 gm/day, history of chronic viral hepatitis, hemochomatosis, autoimmune hepatitis, drug-toxin-induced liver injury or chronic liver disease were also excluded. All the patients and controls underwent a clinical examination, biochemical evaluation and abdominal and pelvic ultrasonographic assessment. The clinical examination was done during which anthropometric measurements (weight, height, hip and waist circumference) and blood pressure were recorded. Biochemical evaluation comprised of routine hematological profile, urea, creatinine, fasting glucose and insulin, aspartate aminotransferase (AST) and alanine aminotransferases (ALT), γ-glutamyltransaminase (γ-GT), total and direct bilirubin, alkaline phosphatase (ALP), hepatitis B surface antigen, anti hepatitis C antibodies, antinuclear antibodies, serum ceruloplasmin, ferritin and lipid profile. The serum levels of prolactin, thyroxin, total testosterone, dehydroepiandrosterone sulfate, follicle stimulating hormone, luteinizing hormone, and 17-hydroxy progesterone were measured in all cases and controls. Homeostasis model assessment (HOMA) method for insulin resistance was calculated by the formula: Fasting serum insulin (micro units/ml) × fasting serum glucose (mill moles per liter)/22.5.
MS was diagnosed in patients with PCOS and controls based on the presence of three or more of the following: (i) waist circumference ≥88 cm (ii) serum triglyceride ≥150 mg/dl (iii) HDL cholesterol <50 mg/dl (iv) serum fasting glucose >100 mg/dl (v) blood pressure ≥130/85 mHg according to NCEP/ATP III, 2001criteria.[26]
Ultrasonography
Ultrasonography was performed by the same radiologist who was blinded for subject's medical history and diagnosis. A high resolution B-mode scanner of General Electric Logic 5 with a 3.5 Hz convex-array probe was used. Heapatic steatosis was defined as diffuse increase in fine echoes in liver parenchyma with impaired visualization of intrahepatic vessels and the diaphragm.[27]
Statistical analysis
The Statistical Package for the Social Sciences (SPSS version 13.0, Inc., Chicago, IL, USA) was used for all statistical analysis. The values of continuous variables were presented as mean ± SD and of categorical variables as absolute number and percentages. Differences in variables between groups (patients with PCOS and controls) and subgroups were tested with Mann-Whitney “U” test and χ2 test as appropriate. Univariate regression analysis was done to evaluate the effect of age, BMI, waist circumference, HOMA-IR, lipid and hormonal parameters along with PCOS diagnosis in all the subjects on the presence of hepatic steatosis. Multivariate analysis was performed for each of the independent variables that were significantly related to hepatic steatosis by univariate regression analysis. A P < 0.05 was considered statistically significant.
RESULTS
The clinical and laboratory parameters of patients with PCOS and age and weight-matched healthy controls are listed in Table 1. The women with PCOS had significantly higher central adiposity indicated by higher waist-hip ratio, higher levels of total testosterone, fasting insulin, glucose, alanine aminotransferase (ALT), aspartate aminotransferase (AST) and γ-glutamyltransaminase (γ-GT). They also had lower HDL levels than the controls. Overall, 38/54 women with PCOS were found to be insulin resistant which was defined as value > 75th percentile of HOMA-IR of controls, was > 1.6.
Table 1.
Clinical, biochemical and hormonal characteristics of polycystic ovary syndrome patients and controls
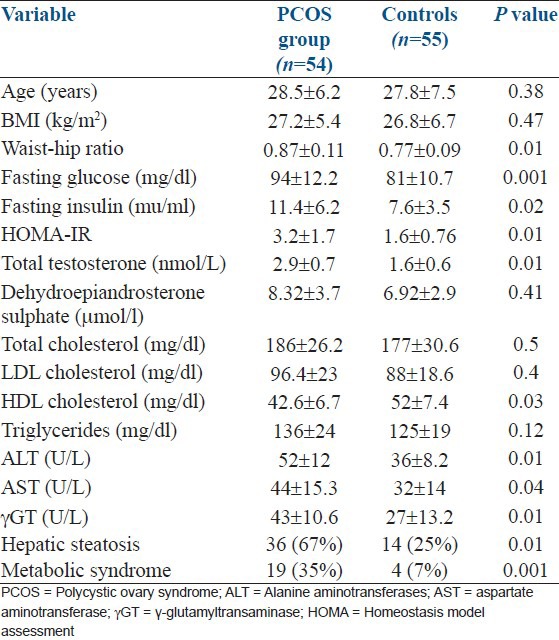
High transaminase levels (>45U/L) were detected in 17/54 women with PCOS and in 4/55 controls. Hepatic steatosis was detected in 36/54 patients with PCOS and in 14/55 controls. Elevated transaminases were present in 20/36 patients and 4/14 controls of all women with hepatic steatosis (n = 50) whereas hepatic steatosis was detected in all women who had elevated transaminases. All patients and controls who had MS had hepatic steatosis as well.
Subgroup analysis
PCOS patients and controls with hepatic steatosis
Patients with PCOS who had hepatic steatosis had a greater waist-hip ratio, higher fasting glucose and insulin and lower HDL levels [Table 2]. They also had high testosterone and HOMA-IR values than controls. Patients with PCOS who had elevated transaminases had higher BMI, HOMA-IR and fasting insulin levels than PCOS patients with normal transaminases.
Table 2.
Clinical, biochemical and hormonal characteristics of polycystic ovary syndrome patients and controls with and without hepatic steatosis
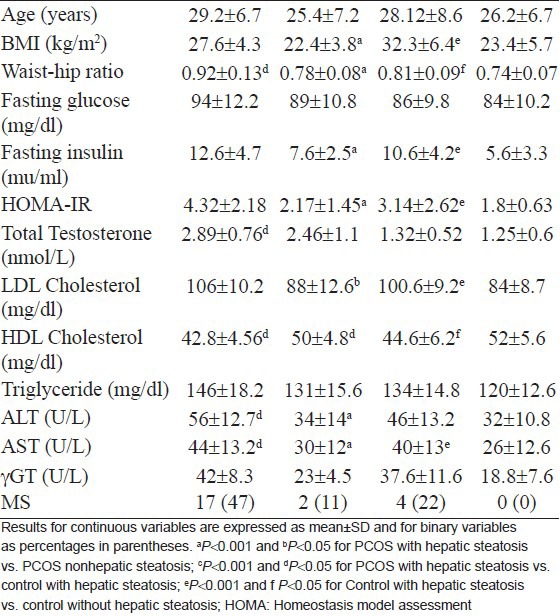
PCOS patients with and without hepatic steatosis
PCOS patients with hepatic steatosis were significantly older, had higher BMI, waist-hip ratio, fasting insulin, HOMA-IR, LDL, ALT and AST than PCOS patients without hepatic steatosis. Patients with PCOS who had elevated transaminases had higher BMI, waist-hip ratio, fasting insulin, HOMA-IR and lower HDL levels than those with normal transaminases.
Controls with and without hepatic steatosis
The controls with hepatic steatosis were older, had higher BMI, waist-hip ratio, HOMA-IR, fasting insulin and glucose, triglycerides and LDL cholesterol. They also had high aminotransaminase and γ-GT levels and nonsignificantly lower HDL levels.
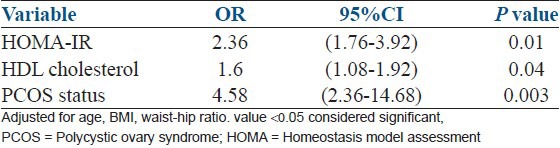
In univariate regression analysis, age, BMI, waist-hip ratio, HOMA-IR, HDL and PCOS diagnosis were the factors significantly related to the presence of hepatic steatosis. Multivariate regression model showed only HOMA-IR, HDL and PCOS diagnosis remained significantly related to the hepatic steatosis after adjustment for age, BMI and waist-hip ratio [Table 3].
Table 3.
Multivariate logistic regression analysis for hepatic steatosis as dependent variable (n=109)
DISCUSSION
NAFLD in recent years has shown a tendency to shift up in the list of causes of hepatomegaly. It is emerging as the most common chronic liver disease in western countries. The prevalence of fatty liver in general population of India has been shown to be similar to the estimates reported from the western countries. PCOS is the most common endocrinopathy affecting women of reproductive age. The consequences of the PCOS extend beyond the reproductive axis; perpetual sequence of hormonal and metabolic aberrations in PCOS patients may commence early and extend throughout life. Women with this disorder are at substantial risk for developing metabolic abnormalities such as glucose intolerance, dyslipidemia and MS.[28,29] The prevalence of MS in women with PCOS appears to be significantly higher than the one estimated in their age-matched counterparts from the general population. The PCOS-MS interrelationship is not restricted to Caucasian women with PCOS. A high MS prevalence has been documented in Brazilian, Chinese, Korean, Indian and in multiracial PCOS populations.[30,31,32,33,34] Increasing evidence suggests that PCOS is also associated with certain nontraditional markers of cardiovascular risk, such as low grade inflammation, oxidative stress, endothelial dysfunction and arterial stiffness. More recently, a link between PCOS and NAFLD has been demonstrated.[12,13,14,15,16,17,18,19,20,35,36,37,38,39,40] The prevalence of NAFLD in women with PCOS may be as high as 40-55%,[14,15] whereas a recent study found that the majority of women with NAFLD had evidence of PCOS.[17] Like PCOS, NAFLD has a strong association with insulin resistance, the MS, and obesity. NAFLD is also a powerful independent predictor of cardiovascular disease. There is a strong association between NAFLD and PCOS consistent with the central role of insulin resistance in the pathogenesis of both conditions. Presence of PCOS with NAFLD has been diagnosed by aspartate AST elevation and/or ultrasound in most of the studies. Conversely, 10 of 14 (71%) female patients in childbearing years with histologically diagnosed NAFLD also had revealed PCOS,[17] indicating a close relationship between these two entities.
In the present study increased prevalence of hepatic steatosic, elevated liver transaminase and MS was observed in women with PCOS than their age and weight-matched healthy counterparts. This shows that NAFLD is relatively common in these patients. The factors that might have contributed to the increased prevalence are increased central adiposity, insulin resistance, dyslipidemia and higher levels of testosterone. In our study, subgroup analysis between PCOS patients with and without hepatic steatosis as well as controls with and without hepatic steatosis showed that age, obesity waist-hip ratio, insulin resistance and dyslipidemia were the factors associated with the presence of hepatic steatosis, in concordance with the findings of other workers. Significance of increased androgen bioavailability in the pathogenesis of NAFLD in women with PCOS has been suggested by some workers.[38,39] It should be addressed in future studies, since in our study causality of the association could not be demonstrated. Gutierrez-Grobe et al.[40] carried out a cross-sectional study, the aim was to study prevalence of NAFLD in premenopausal, postmenopausal and PCOS women. The results suggested that NAFLD is more prevalent in postmenopausal and women with PCOS than those premenopausal ones. The estrogens may have a protective effect of against NAFLD in women.
All women with PCOS and controls who had MS were also detected to have hepatic steatosis. This finding is in agreement with data that confers NAFLD to be considered as a feature of MS, a cluster of cardiovascular risk factors known to predict long-term cardiovascular events, and may be seen as an additional marker of cardiometabolic risk.
Strong epidemiological data now exist linking NAFLD and long-term risk of adverse cardiovascular outcomes.[41]
In a systematic review to assess the literature for associations between PCOS and NAFLD, Baranova et al.[42] observed that insulin resistance, a hallmark of MS is observed in 50-80% of women with PCOS and patients with NAFLD. In most studies, the prevalence of both PCOS and NAFLD rises proportionally to the degree of insulin resistance. NAFLD is considered as the hepatic manifestation of MS since it worsens insulin resistance, predicts emergence of metabolic complications and increases the risk of cardiovascular events. Similarly, it seems appropriate to consider PCOS as the ovarian manifestation of MS. Both these conditions can coexist and may respond to similar therapeutic strategies. Being a cross-sectional study we could not establish the cause-effect relationship between the two diseases. In the Medline search we could not find any similar study that has looked into prevalence of NAFLD in women with PCOS in our country. Possibly we are the first one to address this issue in our population. NAFLD emerged from an anecdotal disease to the most common cause of incident chronic liver disease in the western world. Natural history studies have shown its potential to cause serious liver damage in form of cirrhosis and hepatocellular carcinoma and ultimately increased liver related morbidity and mortality. The recognition of insulin resistance as an underlying pathogenesis of steatosis contributed to the identification of cause which is amenable to treatment. Therefore, screening for liver injury in patients with metabolic risk factors should find a place in our clinical practice. The priorities of future research should be optimization of noninvasive screening techniques, identification of patients who are at increased risk for progression of liver injury and to find out therapeutic strategies.
Limitations of our study were its small sample size and most of the patients with PCOS were obese so we could not analyze lean PCOS patients as a separate subgroup. Further, larger studies evaluating NAFLD in phenotypically different PCOS are needed for the most appropriate screening method and effective interventions. Another limitation of the study was use of ultrasonography and not the liver biopsy which is thought to be the gold standard for diagnosis of NAFLD. Now, ultrasonography is the most widely used imaging method noninvasive, less time taking feasible and cost effective for detecting fatty liver detection with 91% sensitivity and 93% specificity in presence of > 30% hepatic steatosis.[43] However, for grading of severity of hepatic steatosis and detection of inflammation and fibrosis liver biopsy is required. Although several of biomarkers are, in general, useful for the diagnostic evaluation of a patient with suspected NAFLD, they lack the specificity and sensitivity to distinguish fatty liver from NASH and to determine the presence and stage of fibrosis.[44]
NAFLD and PCOS both independently have been incriminated with MS and increased risk for future cardiovascular events. So, early detection of NAFLD in PCOS is important because early intervention will decrease or eliminate the possibility of disease progression.
CONCLUSIONS
Our study shows that NAFLD is common in patients with PCOS in combination with other metabolic abnormalities. Since we have greater propensity for being insulin resistant, it is particularly important that efforts should be made for early detection of NAFLD in these subsets of patients.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Ehrmann DA. Polycystic ovary syndrome. N Engl J Med. 2005;352:1223–36. doi: 10.1056/NEJMra041536. [DOI] [PubMed] [Google Scholar]
- 2.Azziz R, Woods KS, Reyna R, Key TJ, Knochenhauer ES, Yildiz BO. The prevalence and features of the polycystic ovary syndrome in an unselected population. J Clin Endocrinol Metab. 2004;89:2745–9. doi: 10.1210/jc.2003-032046. [DOI] [PubMed] [Google Scholar]
- 3.Dunaif A, Segal KR, Futterweit W, Dobrjansky A. Profound Peripheral insulin resistance, Independent of obesity, in polycystic ovary syndrome. Diabetes. 1989;38:1165–74. doi: 10.2337/diab.38.9.1165. [DOI] [PubMed] [Google Scholar]
- 4.Farrell GC, Larter CZ. Nonalcoholic fatty liver disease: From steatosis to cirrhosis. Hepatology. 2006;43:S99–112. doi: 10.1002/hep.20973. [DOI] [PubMed] [Google Scholar]
- 5.Clark JM, Diehl AM. Nonalcoholic fatty liver disease: An underrecognized cause of cryptogenic cirrhosis. JAMA. 2003;289:3000–4. doi: 10.1001/jama.289.22.3000. [DOI] [PubMed] [Google Scholar]
- 6.Targher G. Non-alcoholic fatty liver disease, the metabolic syndrome and the risk of cardiovascular disease: The plot thickens. Diabet Med. 2007;24:1–6. doi: 10.1111/j.1464-5491.2007.02025.x. [DOI] [PubMed] [Google Scholar]
- 7.Bellentani S, Bedogni G, Tiribelli C. Liver and heart: A new link? J Hepatol. 2008;49:300–2. doi: 10.1016/j.jhep.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 8.Agarwal N, Sharma BC. Insulin resistance and clinical aspects of non-alcoholic steatohepatitis (NASH) Hepatol Res. 2005;33:92–6. doi: 10.1016/j.hepres.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 9.Ahima RS. Insulin resistance: Cause or consequence of nonalcoholic steatohepatitis? Gastroenterology. 2007;132:444–6. doi: 10.1053/j.gastro.2006.11.048. [DOI] [PubMed] [Google Scholar]
- 10.Bloomgarden ZT. Nonalcoholic fatty liver disease and insulin resistance in youth. Diabetes Care. 2007;30:1663–9. doi: 10.2337/dc07-zb06. [DOI] [PubMed] [Google Scholar]
- 11.Angelico F, Del BM, Conti R, Francioso S, Feole K, Fiorello S, et al. Insulin resistance, the metabolic syndrome, and nonalcoholic fatty liver disease. J Clin Endocrinol Metab. 2005;90:1578–82. doi: 10.1210/jc.2004-1024. [DOI] [PubMed] [Google Scholar]
- 12.Brown AJ, Tendler DA, McMurray RG, Setji TL. Polycystic ovary syndrome and severe nonalcoholic steatohepatitis: Beneficial effect of modest weight loss and exercise on liver biopsy findings. Endocr Pract. 2005;11:319–24. doi: 10.4158/EP.11.5.319. [DOI] [PubMed] [Google Scholar]
- 13.Setji TL, Holland ND, Sanders LL, Pereira KC, Diehl AM, Brown AJ. Nonalcoholic steatohepatitis and nonalcoholic fatty liver disease in young women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2006;91:1741–7. doi: 10.1210/jc.2005-2774. [DOI] [PubMed] [Google Scholar]
- 14.Cerda C, Perez-Ayuso RM, Riquelme A, Soza A, Villaseca P, Sir-Petermann T, et al. Nonalcoholic fatty liver disease in women with polycystic ovary syndrome. J Hepatol. 2007;47:412–7. doi: 10.1016/j.jhep.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 15.Gambarin-Gelwan M, Kinkhabwala SV, Schiano TD, Bodian C, Yeh HC, Futterweit W. Prevalence of nonalcoholic fatty liver disease in women with polycystic ovary syndrome. Clin Gastroenterol Hepatol. 2007;5:496–501. doi: 10.1016/j.cgh.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 16.Preiss D, Sattar N, Harborne L, Norman J, Fleming R. The effects of 8 months of metformin on circulating GGT and ALT levels in obese women with polycystic ovarian syndrome. Int J Clin Pract. 2008;62:1337–43. doi: 10.1111/j.1742-1241.2008.01825.x. [DOI] [PubMed] [Google Scholar]
- 17.Brzozowska MM, Ostapowicz G, Weltman MD. An association between non-alcoholic fatty liver disease and polycystic ovarian syndrome. J Gastroenterol Hepatol. 2009;24:243–24. doi: 10.1111/j.1440-1746.2008.05740.x. [DOI] [PubMed] [Google Scholar]
- 18.Targher G, Solagna E, Tosi F, Castello R, Spiazzi G, Zoppini G, et al. Abnormal serum alanine aminotransferase levels are associated with impaired insulin sensitivity in young women with polycystic ovary syndrome. J Endocrinol Invest. 2009;32:695–700. doi: 10.1007/BF03345743. [DOI] [PubMed] [Google Scholar]
- 19.Schwimmer JB, Khorram O, Chiu V, Schwimmer WB. Abnormal aminotransferase activity in women with polycystic ovary syndrome. Fertil Steril. 2005;83:494–7. doi: 10.1016/j.fertnstert.2004.08.020. [DOI] [PubMed] [Google Scholar]
- 20.Markou A, Androulakis II, Mourmouris C, Tsikkini A, Samara C, Sougioultzis S, et al. Hepatic steatosis in young lean insulin resistant women with polycystic ovary syndrome. Fertil Steril. 2010;93:1220–6. doi: 10.1016/j.fertnstert.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 21.Rodin DA, Bano G, Bland JM, Taylor K, Nussey SS. Polycystic ovaries and associated metabolic abnormalities in Indian subcontinent Asian women. Clin Endocrinol (Oxf) 1998;49:91–9. doi: 10.1046/j.1365-2265.1998.00492.x. [DOI] [PubMed] [Google Scholar]
- 22.Norman RJ, Mahabeer S, Master S. Ethnic differences in insulin and glucose response to glucose between white and Indian women with polycystic syndrome. Fertil Steril. 1995;63:58–62. doi: 10.1016/s0015-0282(16)57297-5. [DOI] [PubMed] [Google Scholar]
- 23.Wijeyaratne CN, Balen AH, Barth JH, Belchetz PE. Clinical manifestations and insulin resistance (IR) in polycystic ovary syndrome (PCOS) among South Asians and Caucasians: Is there a difference? Clin Endocrinol (Oxf) 2002;57:343–50. doi: 10.1046/j.1365-2265.2002.01603.x. [DOI] [PubMed] [Google Scholar]
- 24.Kulshreshtha B, Ganie MA, Praveen EP, Gupta N, Lal Khurana M, Seith A, et al. Insulin response to oral glucose in healthy, lean young women and patients with polycystic ovary syndrome. Gynecol Endocrinol. 2008;24:637–43. doi: 10.1080/09513590802342858. [DOI] [PubMed] [Google Scholar]
- 25.The Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group, authors. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81:19–25. doi: 10.1016/j.fertnstert.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 26.Executive summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) J Am Med Assoc. 2001;285:2486–97. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 27.Saadeh S, Younossi ZM, Remer EM, Gramlich T, Ong JP, Hurley M, et al. The utility of radiological imaging in non alcoholic hepatic disease. Gasteroenterology. 2002;123:745–50. doi: 10.1053/gast.2002.35354. [DOI] [PubMed] [Google Scholar]
- 28.Moran LJ, Misso ML, Wild RA, Norman RJ. Impaired glucose tolerance, type 2 diabetes and metabolic syndrome in polycystic ovary syndrome: A systematic review and meta-analysis. Hum Reprod Update. 2010;16:347–63. doi: 10.1093/humupd/dmq001. [DOI] [PubMed] [Google Scholar]
- 29.Cussons AJ, Stuckey BG, Watts GF. Cardiovascular disease in the polycystic ovary syndrome: New insights and perspectives. Atherosclerosis. 2006;185:227–39. doi: 10.1016/j.atherosclerosis.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 30.Soares EM, Azevedo GD, Gadelha RG, Lemos TM, Maranhão TM. Prevalence of the metabolic syndrome and its components in Brazilian women with polycystic ovary syndrome. Fertil Steril. 2008;89:649–55. doi: 10.1016/j.fertnstert.2007.03.081. [DOI] [PubMed] [Google Scholar]
- 31.Cheung LP, Ma RC, Lam PM, Lok IH, Haines CJ, So WY, et al. Cardiovascular risks and metabolic syndrome in Hong Kong Chinese women with polycystic ovary syndrome. Hum Reprod. 2008;23:1431–8. doi: 10.1093/humrep/den090. [DOI] [PubMed] [Google Scholar]
- 32.Park HR, Choi Y, Lee HJ, Oh JY, Hong YS, Sung YA. The metabolic syndrome in young Korean women with polycystic ovary syndrome. Diabetes Res Clin Pract. 2007;77:S243–6. doi: 10.1016/j.diabres.2007.01.065. [DOI] [PubMed] [Google Scholar]
- 33.Bhattacharya SM. Prevalence of metabolic syndrome in women with polycystic ovary syndrome, using two proposed definitions. Gynaecol Endocrinol. 2010;26:516–20. doi: 10.3109/09513590903367010. [DOI] [PubMed] [Google Scholar]
- 34.Glueck CJ, Papanna R, Wang P, Goldenberg N, Sieve-Smith L. Incidence and treatment of metabolic syndrome in newly referred women with confirmed polycystic ovarian syndrome. Metabolism. 2003;52:908–15. doi: 10.1016/s0026-0495(03)00104-5. [DOI] [PubMed] [Google Scholar]
- 35.Chitturi S, Abeygunasekera S, Farrell GC, Holmes-Walker J, Hui JM, Fung C, et al. NASH and insulin resistance: Insulin hyper secretion and specific association with the insulin resistance syndrome. Hepatology. 2002;35:373–9. doi: 10.1053/jhep.2002.30692. [DOI] [PubMed] [Google Scholar]
- 36.Zheng RH, Ding CF. Prevalence of nonalcoholic fatty liver disease in patients with polycystic ovary syndrome: A case-control study. Zhonghua Fu Chan Ke Za Zhi. 2008;43:98–101. [PubMed] [Google Scholar]
- 37.Economou F, Xyrafis X, Livadas S, Androulakis II, Argyrakopoulou G, Christakou CD, et al. In overweight/obese but not in normal-weight women, polycystic ovary syndrome is associated with elevated liver enzymes compared to controls. Hormones (Athens) 2009;8:199–206. doi: 10.14310/horm.2002.1236. [DOI] [PubMed] [Google Scholar]
- 38.Kauffman RP, Baker TE, Baker V, Kauffman MM, Castracane VD. Endocrine factors associated with non-alcoholic fatty liver disease in women with polycystic ovary syndrome: Do androgens play a role? Gynecol Endocrinol. 2010;26:39–46. doi: 10.3109/09513590903184084. [DOI] [PubMed] [Google Scholar]
- 39.Vassilatou E, Lafoyianni S, Vryonidou A, Ioannidis D, Kosma L, Katsoulis K, et al. Increased androgen bioavailability is associated with non-alcoholic fatty liver disease in women with polycystic ovary syndrome. Hum Reprod. 2010;25:212–20. doi: 10.1093/humrep/dep380. [DOI] [PubMed] [Google Scholar]
- 40.Gutierrez-Grobe Y, Ponciano-Rodríguez G, Ramos MH, Uribe M, Méndez-Sánchez N. Prevalence of non alcoholic fatty liver disease in premenopausal, postmenopausal and polycystic ovary syndrome women. The role of estrogens. Ann Hepatol. 2010;9:402–9. [PubMed] [Google Scholar]
- 41.Targher G, Marra F, Marchesini G. Increased risk of cardiovascular disease in non-alcoholic fatty liver disease: Causal effect or epiphenomenon? Diabetologia. 2008;51:1947–53. doi: 10.1007/s00125-008-1135-4. [DOI] [PubMed] [Google Scholar]
- 42.Baranova A, Tran TP, Birerdinc A, Younossi ZM. Systematic review: Association of polycystic ovary syndrome with metabolic syndrome and non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2011;33:801–14. doi: 10.1111/j.1365-2036.2011.04579.x. [DOI] [PubMed] [Google Scholar]
- 43.Palmentieri B, de Sio I, La Mura V, Masarone M, Vecchione R, Bruno S, et al. The role of bright liver echo pattern on ultrasound B-mode examination in the diagnosis of liver steatosis. Dig Liver Dis. 2006;38:485–9. doi: 10.1016/j.dld.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 44.Wieckowska A, Feldstein AE. Diagnosis of nonalcoholic fatty liver disease: Invasive versus noninvasive. Semin Liver Dis. 2008;28:386–95. doi: 10.1055/s-0028-1091983. [DOI] [PubMed] [Google Scholar]


