Abstract
Background
High-dose methotrexate (HDMTX)-induced acute kidney injury is a rare but life-threatening complication. The methotrexate rescue agent glucarpidase rapidly hydrolyzes methotrexate to inactive metabolites. We retrospectively reviewed glucarpidase use in pediatric cancer patients at our institution and evaluated whether subsequent resumption of HDMTX was tolerated.
Methods
Clinical data and outcomes of all patients who received glucarpidase after HDMTX administration were reviewed.
Results
Of 1,141 patients treated with 4,909 courses of HDMTX, 20 patients (1.8% of patients, 0.4% of courses) received 22 doses of glucarpidase. The median glucarpidase dosage was 51.6 units/kg (range, 13 – 65.6 units/kg). At the time of administration, the median plasma methotrexate concentration was 29.1 µM (range, 1.3 – 590.6 µM). Thirteen of the 20 patients received a total of 39 courses of HDMTX therapy after glucarpidase. The median time to complete methotrexate excretion was 355 hours (range, 244 – 763 hours) for the HDMTX course during which glucarpidase was administered, 90 hours (range, 66 – 268 hours) for the next HDMTX course, and 72 hours (range, 42 – 116 hours) for subsequent courses. The median peak serum creatinine during these HDMTX courses was 2.2 mg/dL (range, 0.8 – 9.6 mg/dL), 0.8 mg/dL (range, 0.4 – 1.6 mg/dL), and 0.6 mg/dL (range, 0.4 – 0.9 mg/dL), respectively. One patient experienced nephrotoxicity upon rechallenge with HDMTX. Renal function eventually returned to baseline in all patients and no patient died as a result of methotrexate toxicity.
Conclusion
It is possible to safely resume HDMTX therapy after glucarpidase treatment for HDMTX-induced acute kidney injury.
Keywords: Carboxypeptidases, gamma-Glutamyl hydrolase, methotrexate/administration and dosage, methotrexate/adverse effects, compassionate use trials, renal insufficiency/chemically induced, pediatric
Background
Methotrexate is one of the most widely used and studied chemotherapeutic agents. It has clinical activity against a number of malignancies, including acute lymphoblastic leukemia (ALL), lymphoma, osteosarcoma, brain cancers, and head and neck cancers.1 High-dose methotrexate (HDMTX), defined as a dose ≥1 g/m2, has been associated with severe toxicities in as many as 10% of patients, with a mortality rate as high as 6% in early studies.2,3 With the institution of standardized supportive care measures, including aggressive hydration, urine alkalinization, and leucovorin rescue, HDMTX now carries a much lower risk of severe toxicity. However, significant morbidity and mortality continue to be associated with HDMTX-induced renal dysfunction.4–24
HDMTX-induced acute kidney injury and the resulting delay in methotrexate elimination can lead to prolonged elevation of systemic methotrexate concentrations and serious toxicity, including myelosuppression, mucositis, and dermatitis.2 Several therapeutic interventions have been investigated for patients who experience HDMTX-induced acute kidney injury. Pharmacokinetically-guided leucovorin rescue is routinely used after HDMTX to counteract the effects of methotrexate on normal cells.2,25–28 While it has been reported that 13 patients experiencing extremely high methotrexate levels were successfully treated with high-dose leucovorin alone, with limited morbidity and with recovery from toxicity29, another group reported that of 3,887 patients receiving HDMTX, 1.8% developed acute kidney injury, with a resulting mortality rate of 4.4%.7 Extracorporeal methods of methotrexate removal (peritoneal dialysis, hemodialysis, hemoperfusion, and plasmapheresis) have variable efficacy and are associated with numerous comorbidities; further, methotrexate concentrations often rebound to toxic levels after extracorporeal therapy is stopped.2,7
Use of the bacterial carboxypeptidase G class of enzymes has demonstrated effectiveness in the treatment of HDMTX-induced acute kidney injury.3–24 A recombinant form of bacterial carboxypeptidase, glucarpidase (carboxypeptidase-G2, CPDG2) has been developed to treat patients with HDMTX-induced acute kidney injury. Glucarpidase hydrolytically cleaves the carboxyl-terminal glutamate residue from extracellular folates and folate analogues, forming the non-cytotoxic metabolites 4-[[2,4-diamino-6-(pteridinyl)methyl]-methylamino]-benzoic acid (DAMPA) and glutamic acid.3 Glucarpidase is reported to rapidly reduce systemic methotrexate concentrations by more than 95% within one hour of administration, and unlike extracorporeal methods, it causes little rebound in systemic methotrexate concentrations.5,8 Aside from allergic-type reactions, which occur in approximately 4% of patients, glucarpidase has little treatment-related toxicity.3 This agent is available under an investigational new drug (IND) treatment protocol for which safety information as well as blood samples to determine antibody formation are requested by the sponsor.
Despite the growing body of literature supporting the use of glucarpidase as a rescue agent for HDMTX-induced acute kidney injury, few reports offer recommendations about the safety of resuming HDMTX therapy after administration of this agent.2–7 Many cancer treatment protocols require multiple courses of HDMTX, and prognosis may be adversely affected when they are delayed or omitted. We therefore reviewed the use of glucarpidase in a cohort of pediatric patients receiving HDMTX therapy and assessed the safety and tolerability of resuming HDMTX after glucarpidase treatment.
Methods
Patients
After approval by the St. Jude Children’s Research Hospital (St. Jude) Institutional Review Board, we reviewed the records of pediatric oncology patients receiving HDMTX (defined as ≥1 g/m2) who were treated with intravenous glucarpidase for HDMTX-induced acute kidney injury between October 1, 1998 and December 31, 2010. All data were obtained from the medical records of the identified patients. These data included demographic information, diagnosis, methotrexate dosage, methotrexate plasma concentrations, glucarpidase dose, indicators of HDMTX toxicity, and serum creatinine values.
Glucarpidase administration
Glucarpidase was provided through various IND protocols and per-patient emergency use over the 12 year study period. After reconstitution with normal saline, glucarpidase (protocol dosage, 50 units/kg) was administered intravenously over 5 minutes. The criteria for administration of glucarpidase varied among protocols. All patients who received glucarpidase had an increased serum creatinine concentration of ≥1.5 times the baseline value. The plasma methotrexate concentration was >50 µM for all osteosarcoma patients at 24 hours from the start of the methotrexate infusion. The plasma methotrexate concentration was >10 µM for all other patients with the exception of one, who had received glucarpidase prior to the 42 hour methotrexate level. In the event that a full glucarpidase dose was not available at the time of enrollment, the total available dose was administered promptly, and the remainder was administered upon procurement to equal a total dose of 50 units/kg. Supportive measures (hyper-hydration, urine alkalinization with intravenous sodium bicarbonate, and leucovorin rescue) were continued on the basis of plasma methotrexate concentrations and renal function indicators. Written informed consent was obtained from the parent or guardian and consent or assent from the patient as appropriate prior to glucarpidase administration.
Methotrexate Toxicity
Methotrexate toxicity data for patients enrolled on primary cancer treatment protocols were recovered from previously compiled protocol databases and were graded by using version 2.0, 3.0 or 4.0 of the National Cancer Institute (NCI) Common Toxicity Criteria, according the current version at the time of treatment. For patients not enrolled on cancer treatment protocol, toxicity data were obtained by chart review and were graded by using the NCI Common Toxicity Criteria version 4.0.
Serum creatinine and urine output were monitored at least daily until complete methotrexate excretion could be confirmed or until acute kidney injury resolved. Acute kidney injury was defined as a rise in serum creatinine to >1.5 times the patient’s baseline serum creatinine value. Recovery of renal function was defined as the return of serum creatinine to ≤1.5 times the baseline serum creatinine value.
Methotrexate assays and pharmacokinetics
Blood samples were collected in EDTA-containing tubes. The plasma was separated and plasma methotrexate concentrations were measured locally within 1 hour of collection by using a commercially available fluorescence polarization immunoassay (FPIA) on the TDx® analyzer (Abbott Laboratories, Abbott Park, IL, USA). In a subset of patients, remaining plasma was stored at −80°C for subsequent analysis by high performance liquid chromatography (HPLC) using a modification of the sample extraction procedure of Steinborner and Henion.30 Briefly, 500 µl of patient plasma was mixed with 750 µl of acetonitrile; 10 µl of aminopterin and N-10-trifluoroacetyl pteroic acid (Irvine Chemistry Laboratory, Anaheim, CA) was added to the tube to serve as internal standard. The acetonitrile-plasma mixture was agitated on a vortex mixer for 25–30 seconds and then centrifuged. The liquid phase was mixed with 750 µl of chloroform, and the aqueous phase was then transferred to a separate tube and dried under nitrogen at room temperature. The dried samples were reconstituted in 100 µl of mobile phase (3% acetonitrile in 10 mM sodium acetate pH 5.0) and filtered through a 0.2 µm membrane prior to injection onto the liquid chromatography system.
Plasma methotrexate or DAMPA concentrations were measured on a Waters (Milford, MA) Acquity H-Class ultra performance liquid chromatography system. Reconstituted patient samples, controls, or calibrators (20 µl) were injected into the system. The analytical column was a Waters Acquity BEH C18 (2.1mm × 100mm × 1.7µm). The mobile phase gradient ran linearly from 3% to 16 % A over 14 minutes (A= acetonitrile and B= 10 mM sodium acetate pH 5.0). The total run time was 24 minutes and the flow rate was 0.3 ml/minute. The column eluate was monitored for absorbance at 313 nm. The retention time was 9.0 min for MTX, 13.1 min for DAMPA, and 6.4 and 8.0 minutes for aminotpterin and N-10-(trifluoroacetyl)pteroic acid. The dynamic range of the assay was 0.01–10 µM.
The time to complete methotrexate excretion was defined as the time elapsed between the start of the methotrexate infusion to the time at which the plasma methotrexate concentration fell below 0.1 µM, or to the time at which the last methotrexate concentration was obtained for a given HDMTX course in cases in which the last documented measurement was not <0.1 µM.
Methotrexate dosing
The dose and infusion duration of methotrexate for each patient was determined by the cancer diagnosis and therapeutic protocol. Osteosarcoma patients were treated with 12 g/m2 methotrexate infused over 4 hours. Patients with non-lymphoblastic lymphoma were treated with 8 g/m2 methotrexate infused over 4 hours. Each patient’s physician had the discretion to deviate from these recommended regimens in the event of methotrexate toxicity. For patients with ALL and lymphoblastic lymphoma treated per the Total XV31 and Total XVI studies, each dose of HDMTX was targeted to a steady state plasma concentration of 65 µM based on the clearance of the previous course. The adjusted HDMTX dosage calculated by this targeting method is referred to as the “full recommended dosage” in this report.
Data analysis
Descriptive statistics are used to characterize the study group. Because of the non-normal distribution of data, median values and ranges are reported.
Results
Clinical course of glucarpidase treatment
Twenty of 1,141 (1.8%) patients who were treated with HDMTX at St. Jude received glucarpidase between October 1, 1998 and December 31, 2010. These patients had primary diagnoses of acute lymphoblastic leukemia (n=10), osteosarcoma (n=6), B-cell lymphoblastic lymphoma (n=2), relapsed Hodgkin lymphoma (n=1), and anaplastic large-cell lymphoma (n=1). During the study period 4,909 courses of HDMTX were administered; thus 0.4% of HDMTX courses required glucarpidase (Table 1). The HDMTX course during which glucarpidase was administered ranged from the first through the eighth course of HDMTX (median, third course).
Table 1.
Characteristics of 20 patients at the time they received glucarpidase for delayed methotrexate clearance out of 1,141 patients who received HDMTX.
| All Patients | Osteosarcoma | ALL | Other | |
|---|---|---|---|---|
| Percent (No.) of patients who received glucarpidase | 1.8% (20 of 1,141) | 8% (6 of 75) | 1.3% (10 of 741) | 1.2% (4 of 325) |
| Age (years) | ||||
| Median | 12.1 | 11.7 | 6.5 | 19.2 |
| Range | 4.1 – 20.4 | 10.7 – 20.4 | 4.6 – 18.7 | 4.1 – 20.2 |
| Gender | ||||
| Female | 6 | 3 | 2 | 1 |
| Male | 14 | 3 | 8 | 3 |
| MTX dosing | ||||
| Dose (g/m2) | ||||
| Median | 5.696 | 12 | 5 | 6.109 |
| Range | 3.338 – 12 | 12 | 3.352 – 8 | 3.338 – 8 |
| Infusion duration (hrs) | ||||
| Median | 18 | 4 | 24 | 11 |
| Range | 4 – 24 | 4 – 6 | 4 – 24 | 4 – 20 |
| Course where toxicity occurred | ||||
| Median | 3 | 5 | 2 | 1 |
| Range | 1 – 8 | 1 – 8 | 1 – 4 | 1 – 2 |
| Glucarpidase | ||||
| Number of doses | 24 | 8 | 10 | 6 |
| 1st Dose (units/kg) | ||||
| Median | 51.6 | 59 | 50.8 | 39.3 |
| Range | 13 – 65.6 | 15 – 65.6 | 13 – 63 | 21 – 65 |
| Time to 1st glucarpidase dose (hrs) | ||||
| Median | 45.9 | 30 | 47.8 | 45.9 |
| Range | 26.3 – 95 | 28 – 46.5 | 26.3 – 95 | 28.8 – 48 |
| MTX decrease measured by TDx (%) a | ||||
| Median | 80 | 82.4 | 78.4 | 85.9 |
| Range | 61.9 – 95.9 | 71.1 – 93.2 | 61.9 – 94.6 | 70.5 – 95.9 |
| MTX decrease measured by HPLC (%) b | ||||
| Median | 99.6 | 99.5 | 99.7 | - |
| Range | 99.2 – 99.9 | 99.2 – 99.6 | 99.6 – 99.9 | - |
| Plasma MTX concentrations by TDx | ||||
| 20 – 24 hrs post-MTX (M) | ||||
| Median | 138.0 | 353.1 | 114.42 | 99.2 |
| Range | 29.2 – 462.9 | 158.8 – 462.9 | 65.7 – 222.1 | 29.2 – 258 |
| Prior to glucarpidase (M) | ||||
| Median | 29.1 | 267.3 | 18.1 | 54.6 |
| Range | 1.3 – 590.6 | 32.2 – 590.6 | 1.3 – 222.1 | 16.5 – 239.8 |
| Time to complete MTX excretion (hrs) | ||||
| Median | 355 | 407 | 344 | 415 |
| Range | 244 – 763 | 295 – 763.2 | 245 – 497 | 259.2 – 540 |
| Serum Creatinine (mg/dL) | ||||
| Prior to glucarpidase | ||||
| Median | 1.9 | 2.3 | 1.8 | 1.5 |
| Range | 0.6 – 3.5 | 1.6 – 2.8 | 0.6 – 2.8 | 1.2 – 3.5 |
| % increase from baseline | ||||
| Median | 258.3 | 325.0 | 258.3 | 196.7 |
| Range | 33.3 – 566.7 | 155.6 – 500.0 | 133.3 – 566.7 | 33.3 – 400.0 |
| Max | ||||
| Median | 2.2 | 2.7 | 1.8 | 4.9 |
| Range | 0.8 – 9.6 | 2.1 – 3.7 | 0.8 – 2.3 | 1.3 – 9.6 |
| Recovery of Renal Function (days) c | ||||
| Median | 21 | 26 | 24 | 20 |
| Range | 7 – 56 | 17 – 34 | 8 – 56 | 7 – 42 |
HDMTX, high-dose methotrexate; MTX, methotrexate; TDx, fluorescence polymerization immunoassay; HPLC, high performance liquid chromatography; g, grams; m2, meters2, hrs, hours; ALL, acute lymphoblastic leukemia.
The percent decrease is the decrease from the MTX level prior to glucarpidase to the first level obtained after glucarpidase administration.
The percent decrease is the decrease from the MTX level prior to glucarpidase to the first level obtained after glucarpidase administration.
Recovery of renal function was defined as the return of serum creatinine to ≤1.5 times the pretreatment (baseline) level.
Four patients received two glucarpidase doses; however, only two of those patients were given the full recommended dosage of 50 units/kg twice. Because there was a limited amount of glucarpidase immediately available, two patients received less than 50 units/kg and were given a “make up” dose to complete the full recommended dosage within 48 hours of the first dose. The median initial glucarpidase dosage for all patients was 51.6 units/kg (range, 13.0 – 65.6 units/kg). The median time from the start of the HDMTX infusion to administration of the first glucarpidase dose was 45.9 hours (range, 26.3 – 95 hours).
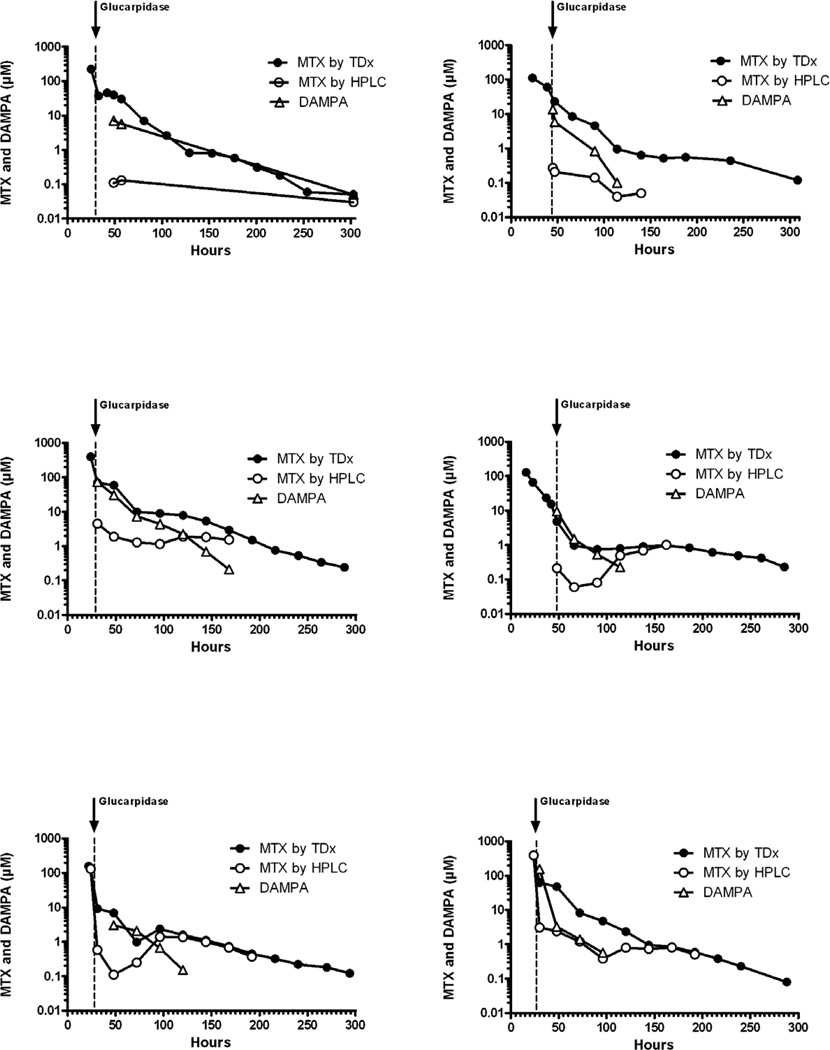
The median plasma methotrexate concentration was 138.0 µM (range, 29.2 – 462.9 µM) at 20 – 24 hours after the start of the methotrexate infusion and was 29.1 µM (range, 1.3 – 590.6 µM) prior to glucarpidase administration as measured by TDx. Glucarpidase administration resulted in a rapid decrease in methotrexate plasma concentrations. The median percent decrease in methotrexate after glucarpidase administration was 80% (range, 61.9 – 95.9%) as measured by TDx by comparing the last plasma methotrexate concentration before glucarpidase and the first concentration obtained after glucarpidase administration. For the six patients whose methotrexate concentrations were measured by HPLC, plasma methotrexate concentrations were reduced by a median of 99.6% (range, 99.2% – 99.9%) at the time of the first plasma methotrexate level following glucarpidase administration. DAMPA was also detectable by HPLC almost immediately following glucarpidase administration (Figure 1). The median time to complete methotrexate excretion was 355 hours (range, 244 – 763 hours).
Figure 1. Plasma levels of methotrexate and DAMPA after glucarpidase.
Plasma methotrexate and DAMPA concentration vs. time after glucarpidase treatment. Methotrexate (MTX) was measured by fluorescence polymerization immunoassay (●) and HPLC (○), and DAMPA was measured by HPLC (△), in the six patients who had assays by both methods. The arrow indicates the time of glucarpidase administration.
The median serum creatinine value prior to glucarpidase administration was 1.9 mg/dL (range, 0.6 – 3.5 mg/dL), and the median peak serum creatinine was 2.2 mg/dL (range, 0.8 – 9.6 mg/dL). Recovery of renal function, defined as the return of serum creatinine to ≤1.5 times the pretreatment (baseline) level, occurred at a median of 21 days (range, 7 – 56 days).
During the HDMTX course in which glucarpidase was given, seven patients experienced a grade 3 or 4 serum creatinine increase (>3 times the upper limit of normal) (Table2). All patients had at least a doubling of serum creatinine from baseline. One patient developed oliguria concomitantly with acute kidney injury, while all others maintained urine output >1 ml/kg/hr. One patient received hemodialysis for electrolyte abnormalities. Two patients developed grade 4 neutropenia (absolute neutrophil count <500/mm3) and one developed grade 3 neutropenia (absolute neutrophil count 500 – 1,000/mm3). Four patients had a grade 3 infection requiring intravenous antibiotics. One patient experienced grade 3 mucositis. There were no treatment-related deaths.
Table 2.
Methotrexate-related toxicities during the glucarpidase course
| Common Toxicity Criteria Grade (% of patients) | ||
|---|---|---|
| Toxicity | 3 | 4 |
| Serum creatinine elevation | 6 (30%) | 1 (5%) |
| Oral mucositis | 1 (5%) | - |
| Vomiting | 2 (10%) | - |
| Diarrhea | - | - |
| Myelosuppression | 1 (5%) | 2 (10%) |
| Infection | 4 (20%) | - |
Rechallenge with high-dose methotrexate
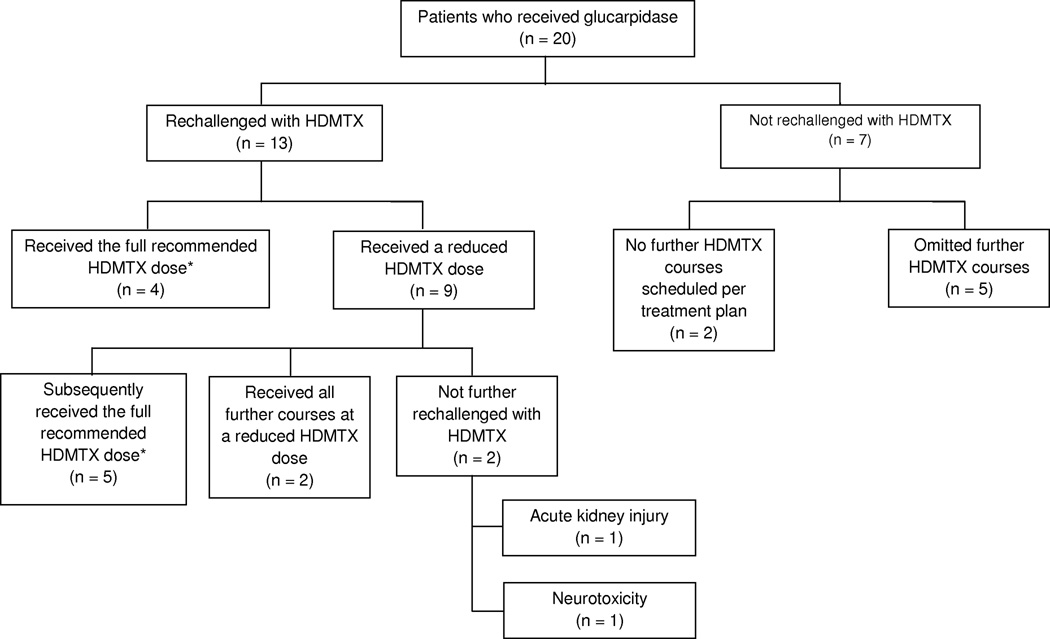
Thirteen patients were rechallenged with HDMTX after receiving glucarpidase (Table 3). Two of the twenty patients were not scheduled to receive further HDMTX as dictated by their treatment plan and five patients had all future HDMTX courses omitted at the discretion of the treating physician. Nine of the patients who were rechallenged received a reduced HDMTX dosage (dose reductions ranged from 50% to 75% of the recommended HDMTX dosage) and four patients received the full recommended dosage of HDMTX for the course immediately after glucarpidase (Figure 2). Three of the four patients who received the full recommended dosage of HDMTX were treated for acute lymphoblastic leukemia or lymphoblastic lymphoma and therefore had their dosage targeted based on the clearance of their previous course. Although those three patients received the full recommended dosage calculated in this way, the actual individualized HDMTX dosage received was 33%, 68%, and 75% of the dosage administered during the glucarpidase course because the calculation of each recommended dose depends heavily on the methotrexate clearance during previous courses. One osteosarcoma patient received the full recommended 12 g/m2 HDMTX dosage for the next course following glucarpidase. All patients had serum creatinine values return to the normal range for their age before being rechallenged with HDMTX. The median time to HDMTX rechallenge, defined as the time elapsed between the start of the HDMTX course in which glucarpidase was administered and the start of the next HDMTX infusion, was 28 days (range, 18 – 70 days). All patients who were rechallenged were admitted the evening prior to the next HDMTX course for prehydration and had the rate of intravenous hydration and subsequent leucovorin dosing adjusted by a clinical pharmacist.
Table 3.
Characteristics of patients rechallenged with HDMTX following glucarpidase administration.
| HDMTX course immediately following glucarpidase | All other HDMTX courses for the same patients | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of patients rechallenged |
Time to MTX rechallenge (days) a [median (range)] |
% of full HDMTX dosage administered at rechallenge [median (range)] |
HDMTX Dosage (g/m2) [median (range)] |
Max Scr (mg/dL) [median (range)] |
SCr % increase from baseline [median (range)] |
Time to complete MTX excretion (hrs) [median (range)] |
Total number of courses |
HDMTX Dosage (g/m2) [median (range)] |
Max Scr (mg/dL) [median (range)] |
SCr % increase from baseline [median (range)] |
Time to complete MTX excretion (hrs) [median (range)] |
|
| All Patients (n = 20) |
13 | 28 (18 – 70) |
67 (50 – 100) |
2.486 (1.6 – 12) |
0.8 (0.4 – 1.6) |
25 (0 – 128.6) |
90 (66 – 268) |
26 | 8.5 (2 – 12) |
0.6 (0.4 – 0.9) |
22.5 (0 – 50) |
72 (42 – 116) |
| Osteosarcoma (n = 6) |
4 | 43 (20 – 70) |
67 (50 – 100) |
8 (6 – 12) |
0.8 (0.5 – 1.6) |
42.5 (14.3 – 128.6) |
96 (72 – 268) |
13 | 12 (12 – 12) |
0.6 (0.5 – 0.9) |
25 (0 – 50) |
72 (72 – 116) |
| ALL (n = 10) |
7 | 27 (18 – 32) |
67 (50 – 100) |
2.02 (1.6 – 2.714) |
0.8 (0.5 – 0.9) |
25 (0 – 100) |
90 (66 – 114) |
10 | 3.153 (2 – 5) |
0.6 (0.4 – 0.9) |
16.3 (0 – 33.3) |
66 (42 – 92) |
| Other (n = 4) |
2 | 26 (23 – 29) |
80 (60 – 100) |
2.243 (2 – 2.486) |
0.8 (0.4 – 1.1) |
28.6 (0 – 57.1) |
78 (66 – 90) |
3 | 3.1 (3 – 4.5) |
0.4 (0.4 – 0.8) |
33.3 (14.3 – 33.3) |
42 (42 – 66) |
HDMTX, high-dose methotrexate; g, grams; SCr, serum creatinine; MTX, methotrexate; mg, milligram; dL, deciliter; hrs, hours; ALL, acute lymphoblastic leukemia
The time to methotrexate rechallenge was the time elapsed between the start of the HDMTX course in which glucarpidase was administered and the start of the next HDMTX infusion.
Figure 2. High-dose methotrexate rechallenge.
Disposition of 20 patients who received glucarpidase, whether they were rechallenged with high-dose methotrexate (HDMTX) following glucarpidase use, whether they received the full recommended HDMTX dose or a reduced dose for the course immediately after glucarpidase, and whether the full recommended dose was ever given.
* HDMTX doses for ALL and lymphoblastic lymphoma patients were targeted to a steady state concentration of 65 µM based on the clearance of the previous HDMTX course. Full dose was defined as the administration of the recommended targeted dose.
After rechallenge with one course of HDMTX, the median peak serum creatinine (0.8 mg/dL; range, 0.4 – 1.6 mg/dL) and the median percent creatinine increase over baseline (25%; range, 0 – 128.6%) were lower than those observed after the previous course. The median time to complete methotrexate excretion was 90 hours (range, 66 – 268 hours). The thirteen patients who were rechallenged with HDMTX received a total of 39 HDMTX courses after glucarpidase (1 – 6 courses per patient) (Table 3). These methotrexate dosages ranged from 50% to 100% of the recommended dosage. For all other additional HDMTX courses following the initial rechallenge, the median peak serum creatinine was 0.6 mg/dL (range, 0.4 – 0.9 mg/dL), or 22.5% over baseline values (range, 0 – 50%). All but two patients who were rechallenged either completed all recommended courses of HDMTX or were scheduled to do so at the time of analysis. One patient experienced renal dysfunction and delayed methotrexate elimination upon rechallenge with HDMTX and another experienced neurotoxicity that was unrelated to renal dysfunction. None of the patients required further glucarpidase with subsequent HDMTX courses.
Discussion
This study describes the incidence of glucarpidase use in a pediatric oncology institution and the feasibility of resuming HDMTX therapy in patients who have received glucarpidase for HDMTX-induced acute kidney injury. HDMTX-induced acute kidney injury can cause prolonged elevation of systemic methotrexate concentrations and potentially cause serious toxicity.1 The cornerstones of prevention and control of HDMTX-induced acute kidney injury are aggressive hydration and alkalinization. When these methods fail to prevent acute kidney injury from occurring, glucarpidase provides a method of reducing plasma methotrexate concentrations quickly and safely. The incidence observed in our population (1.8%) is similar to that previously reported in the osteosarcoma population.7 Widemann et al.20 reported that delaying the administration of glucarpidase more than 96 hours from the start of the HDMTX infusion may increase the risk of grade 4 and 5 toxicity. All of our twenty patients received glucarpidase within 96 hours (median, 45.9 hours; range, 26.3 – 95.0 hours) after the start of the methotrexate infusion. Renal function eventually recovered to baseline in all patients, and no patient died as a result of methotrexate toxicity.
Measurement of plasma methotrexate by TDx is known to overestimate the methotrexate concentration due to the cross reactivity of methotrexate with its metabolite DAMPA.5,8 For research purposes, methotrexate was assayed in a subset of our 20 patients by HPLC, and these results indicated that plasma methotrexate was reduced by more than 99% by glucarpidase (Figure 1). However, in the absence of an HPLC assay, commercial methods can be used to guide the duration of leucovorin rescue because DAMPA metabolite levels become insignificant as the plasma methotrexate concentration approaches 0.1 µM (Figure 1).
Previous experience to guide the resumption of HDMTX following glucarpidase administration for HDMTX-induced acute kidney injury is limited to case reports and one case series.6 In the largest group to date, thirteen of our 20 patients who received glucarpidase were rechallenged with HDMTX at dosages ranging from 50% to 100% of full dosage. In leukemia patients, dosages ranged from 50% to 100% of the recommended HDMTX dosage. Three out of five leukemia patients who tolerated a reduced dose were subsequently given the full recommended HDMTX dose. In osteosarcoma patients, rechallenge doses again ranged from 50% to 100% of the recommended full dosage. Two of these patients who were rechallenged with a reduced HDMTX dose tolerated the full dose during the next course, while one patient again experienced delayed elimination and was not rechallenged further. Two rechallenged patients had B-lineage lymphoblastic lymphoma and were treated per the St. Jude Total XV acute lymphoblastic leukemia protocol, in which the HDMTX dosage was individualized on the basis of systemic clearance. One of these patients resumed the full dose of HDTMX immediately after glucarpidase, while the other resumed the full dose after a single reduced-dose cycle.
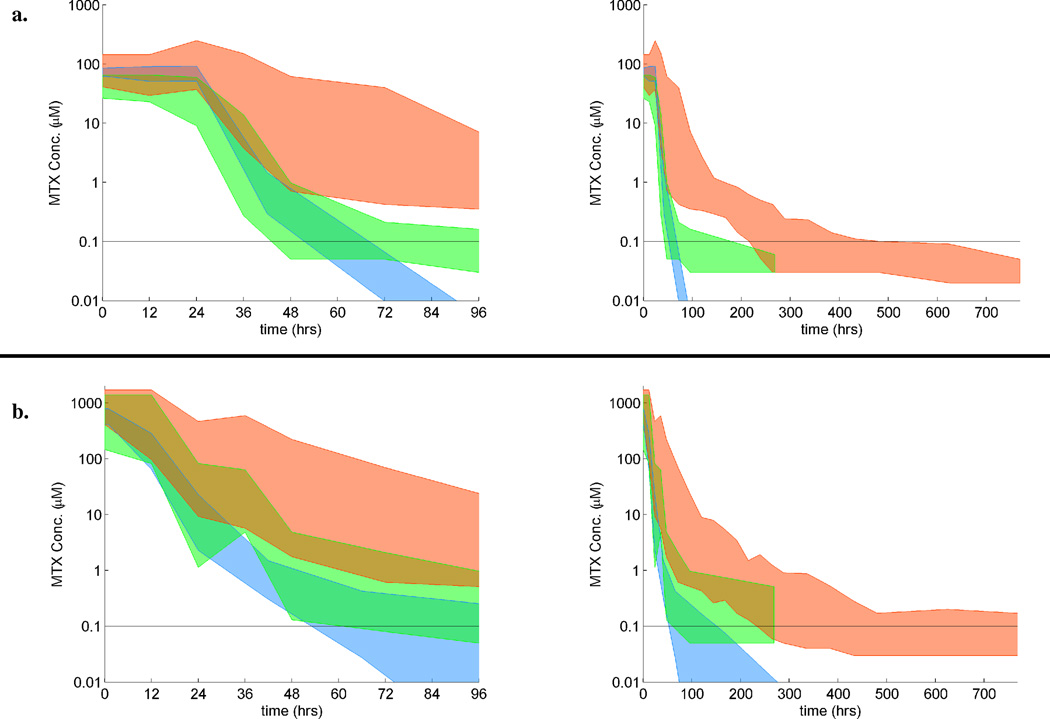
Eleven of 13 patients who were rechallenged with HDMTX tolerated the therapy well (Table 3). The clearance profile of methotrexate for the course immediately after glucarpidase administration more closely resembled that of the expected clearance of methotrexate (Figure 3). The median time to complete methotrexate excretion fell from 355 hours during the course requiring glucarpidase to 90 hours for the course immediately after glucarpidase and then to 72 hours for all subsequent courses. In historical controls, the complete methotrexate elimination time following HDMTX was 66 – 72 hours. These results show that methotrexate elimination recovers to baseline as renal function recovers.
Figure 3. Range of MTX plasma concentrations for patients during the HDMTX course in which glucarpidase was given, the first HDMTX course after glucarpidase, and the expected range for dose.
Methotrexate (MTX) plasma concentrations (µM) vs. time during the HDMTX courses during which glucarpidase was given (red), the next HDMTX course (green), and the expected range (blue). Colored regions represent the range of methotrexate concentrations for (a) patients who received a 24-hour infusion of 5 g/m2 of MTX or a dose targeted to a MTX steady-state concentration of 65 µM and (b) patients who received a 4-hour infusion of 8 – 12 g/m2 of MTX. MTX doses may have been reduced for the first course after glucarpidase. Each set of two panels shows data for a 96-hour and a 700-hour time period, respectively. The expected ranges for dose were obtained from the pharmacokinetic data for previous cohorts of St. Jude patients with acute lymphoblastic leukemia and osteosarcoma
In conclusion, the use of glucarpidase is a strategy to effectively rescue patients with HDMTX-induced acute kidney injury and is associated with complete renal recovery. The resumption of HDMTX after glucarpidase administration was well tolerated, although close monitoring of both renal function and plasma methotrexate levels was necessary.
Acknowledgments
Funding Support: American Lebanese Syrian Associated Charities (ALSAC), Cancer Center Support grant CA21765
Footnotes
Financial Disclosures: The authors declare no financial conflicts of interest.
Glucarpidase is an infrequently used but effective intervention for high-dose methotrexate-induced acute kidney injury. Resumption of high-dose methotrexate after glucarpidase administration is feasible.
References
- 1.Treon SP, Chabner BA. Concepts in use of high-dose methotrexate therapy. Clin Chem. 1996;42:1322–1329. [PubMed] [Google Scholar]
- 2.Widemann BC, Adamson PC. Understanding and managing methotrexate nephrotoxicity. Oncologist. 2006;11:694–703. doi: 10.1634/theoncologist.11-6-694. [DOI] [PubMed] [Google Scholar]
- 3.Patterson DM, Lee SM. Glucarpidase following high-dose methotrexate: update on development. Expert Opin Biol Ther. 2010;10:105–111. doi: 10.1517/14712590903468677. [DOI] [PubMed] [Google Scholar]
- 4.Park ES, Han KH, Choi HS, Shin HY, Ahn HS. Carboxypeptidase-G2 rescue in a patient with high dose methotrexate-induced nephrotoxicity. Cancer Res Treat. 2005;37:133–135. doi: 10.4143/crt.2005.37.2.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schwartz S, Borner K, Muller K, et al. Glucarpidase (carboxypeptidase g2) intervention in adult and elderly cancer patients with renal dysfunction and delayed methotrexate elimination after high-dose methotrexate therapy. Oncologist. 2007;12:1299–1308. doi: 10.1634/theoncologist.12-11-1299. [DOI] [PubMed] [Google Scholar]
- 6.Snyder RL. Resumption of high-dose methotrexate after methotrexate-induced nephrotoxicity and carboxypeptidase G2 use. Am J Health Syst Pharm. 2007;64:1163–1169. doi: 10.2146/ajhp060187. [DOI] [PubMed] [Google Scholar]
- 7.Widemann BC, Balis FM, Kempf-Bielack B, et al. High-dose methotrexate-induced nephrotoxicity in patients with osteosarcoma. Cancer. 2004;100:2222–2232. doi: 10.1002/cncr.20255. [DOI] [PubMed] [Google Scholar]
- 8.Buchen S, Ngampolo D, Melton RG, et al. Carboxypeptidase G2 rescue in patients with methotrexate intoxication and renal failure. Br J Cancer. 2005;92:480–487. doi: 10.1038/sj.bjc.6602337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DeAngelis LM, Tong WP, Lin S, Fleisher M, Bertino JR. Carboxypeptidase G2 rescue after high-dose methotrexate. J Clin Oncol. 1996;14:2145–2149. doi: 10.1200/JCO.1996.14.7.2145. [DOI] [PubMed] [Google Scholar]
- 10.Esteve MA, Devictor-Pierre B, Galy G, et al. Severe acute toxicity associated with high-dose methotrexate (MTX) therapy: use of therapeutic drug monitoring and test-dose to guide carboxypeptidase G2 rescue and MTX continuation. Eur J Clin Pharmacol. 2007;63:39–42. doi: 10.1007/s00228-006-0212-1. [DOI] [PubMed] [Google Scholar]
- 11.Hum M, Kamen BA. Successful carboxypeptidase G2 rescue in delayed MTX-elimination due to renal failure. Pediatr Hematol Oncol. 1995;12:521–524. doi: 10.3109/08880019509030765. [DOI] [PubMed] [Google Scholar]
- 12.Krause AS, Weihrauch MR, Bode U, et al. Carboxypeptidase-G2 rescue in cancer patients with delayed methotrexate elimination after high-dose methotrexate therapy. Leuk Lymphoma. 2002;43:2139–2143. doi: 10.1080/1042819021000032953. [DOI] [PubMed] [Google Scholar]
- 13.Mohty M, Peyriere H, Guinet C, Hillaire-Buys D, Blayac JP, Rossi JF. Carboxypeptidase G2 rescue in delayed methotrexate elimination in renal failure. Leuk Lymphoma. 2000;37:441–443. doi: 10.3109/10428190009089446. [DOI] [PubMed] [Google Scholar]
- 14.Nowicki TS, Bjornard K, Kudlowitz D, Sandoval C, Jayabose S. Early recognition of renal toxicity of high-dose methotrexate therapy: a case report. J Pediatr Hematol Oncol. 2008;30:950–952. doi: 10.1097/MPH.0b013e318182e73e. [DOI] [PubMed] [Google Scholar]
- 15.O'Marcaigh AS, Johnson CM, Smithson WA, et al. Successful treatment of intrathecal methotrexate overdose by using ventriculolumbar perfusion and intrathecal instillation of carboxypeptidase G2. Mayo Clin Proc. 1996;71:161–165. doi: 10.4065/71.2.161. [DOI] [PubMed] [Google Scholar]
- 16.Peyriere H, Cociglio M, Margueritte G, Vallat C, Blayac JP, Hillaire-Buys D. Optimal management of methotrexate intoxication in a child with osteosarcoma. Ann Pharmacother. 2004;38:422–427. doi: 10.1345/aph.1D237. [DOI] [PubMed] [Google Scholar]
- 17.Sieniawski M, Rimpler M, Herrmann R, Josting A. Successful carboxypeptidase G2 rescue of a high-risk elderly Hodgkin lymphoma patient with methotrexate intoxication and renal failure. Leuk Lymphoma. 2007;48:1641–1643. doi: 10.1080/10428190701447338. [DOI] [PubMed] [Google Scholar]
- 18.Tuffaha HW, Al Omar S. Glucarpidase rescue in a patient with high-dose methotrexate-induced nephrotoxicity. J Oncol Pharm Pract. 2011;17:136–140. doi: 10.1177/1078155209348720. [DOI] [PubMed] [Google Scholar]
- 19.Vilay AM, Mueller BA, Haines H, Alten JA, Askenazi DJ. Treatment of methotrexate intoxication with various modalities of continuous extracorporeal therapy and glucarpidase. Pharmacotherapy. 2010;30:111. doi: 10.1592/phco.30.1.111. [DOI] [PubMed] [Google Scholar]
- 20.Widemann BC, Balis FM, Kim A, et al. Glucarpidase, leucovorin, and thymidine for high-dose methotrexate-induced renal dysfunction: clinical and pharmacologic factors affecting outcome. J Clin Oncol. 2010;28:3979–3986. doi: 10.1200/JCO.2009.25.4540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Widemann BC, Balis FM, Murphy RF, et al. Carboxypeptidase-G2, thymidine, and leucovorin rescue in cancer patients with methotrexate-induced renal dysfunction. J Clin Oncol. 1997;15:2125–2134. doi: 10.1200/JCO.1997.15.5.2125. [DOI] [PubMed] [Google Scholar]
- 22.Widemann BC, Balis FM, Shalabi A, et al. Treatment of accidental intrathecal methotrexate overdose with intrathecal carboxypeptidase G2. J Natl Cancer Inst. 2004;96:1557–1559. doi: 10.1093/jnci/djh270. [DOI] [PubMed] [Google Scholar]
- 23.Widemann BC, Hetherington ML, Murphy RF, Balis FM, Adamson PC. Carboxypeptidase-G2 rescue in a patient with high dose methotrexate-induced nephrotoxicity. Cancer. 1995;76:521–526. doi: 10.1002/1097-0142(19950801)76:3<521::aid-cncr2820760325>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 24.Zoubek A, Zaunschirm HA, Lion T, et al. Successful carboxypeptidase G2 rescue in delayed methotrexate elimination due to renal failure. Pediatr Hematol Oncol. 1995;12:471–477. doi: 10.3109/08880019509009477. [DOI] [PubMed] [Google Scholar]
- 25.Evans WE, Crom WR, Abromowitch M, et al. Clinical pharmacodynamics of high-dose methotrexate in acute lymphocytic leukemia. Identification of a relation between concentration and effect. N Engl J Med. 1986;314:471–477. doi: 10.1056/NEJM198602203140803. [DOI] [PubMed] [Google Scholar]
- 26.Relling MV, Fairclough D, Ayers D, et al. Patient characteristics associated with high-risk methotrexate concentrations and toxicity. J Clin Oncol. 1994;12:1667–1672. doi: 10.1200/JCO.1994.12.8.1667. [DOI] [PubMed] [Google Scholar]
- 27.Stoller RG, Hande KR, Jacobs SA, Rosenberg SA, Chabner BA. Use of plasma pharmacokinetics to predict and prevent methotrexate toxicity. N Engl J Med. 1977;297:630–634. doi: 10.1056/NEJM197709222971203. [DOI] [PubMed] [Google Scholar]
- 28.Pinedo HM, Zaharko DS, Bull JM, Chabner BA. The reversal of methotrexate cytotoxicity to mouse bone marrow cells by leucovorin and nucleosides. Cancer Res. 1976;36:4418–4424. [PubMed] [Google Scholar]
- 29.Flombaum CD, Meyers PA. High-dose leucovorin as sole therapy for methotrexate toxicity. J Clin Oncol. 1999;17:1589–1594. doi: 10.1200/JCO.1999.17.5.1589. [DOI] [PubMed] [Google Scholar]
- 30.Steinborner S, Henion J. Liquid-liquid extraction in the 96-well plate format with SRM LC/MS quantitative determination of methotrexate and its major metabolite in human plasma. Anal Chem. 1999;71:2340–2345. doi: 10.1021/ac981294y. [DOI] [PubMed] [Google Scholar]
- 31.Pui CH, Campana D, Pei D, et al. Treating childhood acute lymphoblastic leukemia without cranial irradiation. N Engl J Med. 2009;360:2730–2741. doi: 10.1056/NEJMoa0900386. [DOI] [PMC free article] [PubMed] [Google Scholar]