Abstract
Endoplasmic reticulum (ER) stress is becoming recognized as an important contributing factor in various diseases, including diabetes mellitus. Prolonged ER stress can cause β-cell apoptosis; however, the underlying mechanism(s) that contribute to this process are not well understood. Early reports suggested that arachidonic acid metabolites and a Ca2+-independent phospholipase A2 (iPLA2) activity play a role in β-cell apoptosis. The PLA2 family of enzymes catalyse the hydrolysis of the sn-2 substituent (i.e. arachidonic acid) of membrane phospholipids. In light of our findings that the pancreatic islet β-cells are enriched in arachidonate-containing phospholipids and express the group VIA iPLA2β, we considered the possibility that iPLA2β participates in ER stress-induced β-cell apoptosis. Our work revealed a novel mechanism, involving ceramide generation and triggering of mitochondrial abnormalities, by which iPLA2β participates in the β-cell apoptosis process. Here, we review our evidence linking ER stress, β-cell apoptosis and iPLA2β. Continued studies in this area will increase our understanding of the contribution of iPLA2β to the evolution of diabetes mellitus and will further our knowledge of factors that influence β-cell health in diabetes mellitus and identify potential targets for future therapeutic interventions to prevent β-cell death.
Keywords: apoptosis, β-cell, ceramides, iPLA2β, mitochondria
Introduction
Diabetes mellitus is the most prevalent human metabolic disease, and it results from loss and/or dysfunction of β-cells in pancreatic islets. Type 2 diabetes mellitus (T2DM) results from a progressive decline of β-cell function and chronic insulin resistance [1]. Autopsy studies indicate that the β-cell mass in obese T2DM patients is smaller than that in obese non-diabetic subjects [2–4] and that the loss in β-cell function in non-obese T2DM is associated with decreases in β-cell mass [5,6]. β-Cell mass is regulated by a balance between β-cell growth, resulting from β-cell replication and neogenesis, and β-cell death resulting from apoptosis [7–9]. Findings in both rodent models of T2DM [10] and human T2DM [6] and other observations [11–13] suggest that the decrease in β-cell mass in T2DM is not because of reduced β-cell proliferation or neogenesis but may be a consequence of increased β-cell apoptosis. Emerging evidence suggests that cytokine-mediated β-cell apoptosis is also a prominent contributor to β-cell death during the development of autoimmune type 1 diabetes mellitus (T1DM) [14–17]. It is therefore important to understand the mechanisms underlying β-cell apoptosis if this process is to be prevented or delayed.
Apoptosis can be mediated via an extrinsic (death receptor) or intrinsic (mitochondrial) pathway [18,19]. Recently, apoptosis as a result of prolonged endoplasmic reticulum (ER) stress has gained recognition [18,20–22] and this process has been implicated as a causative factor in Alzheimer's and Parkinson's diseases and cancer [23]. Several studies suggest that ER stress can also cause β-cell apoptosis, a consequence of which is diabetes mellitus. For instance, β-cell death in the Akita [24,25] and NOD.k iHEL non-immune [26] diabetic mouse models is reported to be due to ER stress and mutations in genes encoding the ER-stress transducer pancreatic ER kinase (PERK) [27], and the ER-resident proteins involved in the degradation of malfolded ER proteins have been linked to diminished β-cell health clinically [28,29]. Additionally, ER stress is thought to play a role in the autoimmune destruction of β-cells during the development of T1DM [17,30–34].
The ER, in addition to serving as a cellular Ca2+ store, is the site where secretory proteins are synthesized, assembled, folded and posttranslationally modified. Interruption of any of these functions can lead to production of malfolded proteins and their accumulation in the ER. When an imbalance between the load of client proteins on the ER and the ER's ability to process the load occurs, it results in ER stress [35,36]. Prolonged ER stress promotes induction of stress factors and activation of caspase-12, localizedintheER [19,21,37], andcansubsequently lead to downstream activation of caspase-3, a protease that is central to the execution of apoptosis [38]. Being a site for Ca2+ storage, the ER responds to various stimuli to release Ca2+ and is therefore extremely sensitive to changes in cellular homeostasis. Thapsigargin, which depletes ER Ca2+ stores by inhibiting sarco-endoplasmic reticulum Ca2+-ATPase (SERCA) Ca2+ pumps, causes ER stress in pancreatic islets and promotes hydrolysis of arachidonic acid. We found that the accumulation in arachidonic acid is suppressed by a bromoenol lactone (BEL) suicide substrate inhibitor of the group VI Ca2+-independent phospholipase A2 (iPLA2) [39]. These observations raised the possibility that iPLA2β is activated during ER stress in β-cells.
The PLA2s are a diverse group of enzymes that catalyse the hydrolysis of the sn-2 substituent from glycerophospholipid substrates to yield a free fatty acid and a 2-lysophospholipid [40]. In pancreatic islet β-cell organelles, similar to brain tissue [41], arachidonic acid is a major sn-2 substituent of membrane phospholipids [42,43]. Arachidonic acid and its oxygenated metabolites are potent bioactive mediators that can regulate physiological and pathophysiological processes. At present, the recognized PLA2s are classified into 15 groups based on their Ca2+ requirement for activation and sequence homology [44,45]. Among the iPLA2s, the β-isoform of iPLA2 (iPLA2β) does not require Ca2+ for activity [45]. The iPLA2β enzyme is activated by ATP, inhibited by a BEL suicide substrate [46], predominantly cytosolic under basal conditions, and has unique features that enhance the possibility that it can participate in multiple biological processes [45,47]. These include ankyrin repeats, acyl-CoA esterase activity, caspase-3 cleavage consensus sequence, bipartite nuclear localization sequence and calmodulin-binding domain. In addition, the iPLA2β gene promoter contains a sterol regulatory element (SRE), and transcription of the iPLA2β gene is regulated in a sterol-dependent manner [48].
The iPLA2β has been proposed to be involved in phospholipid remodelling, maintenance of phosphatidylcholine mass, signal transduction, cell proliferation and other biological processes [45]. Furthermore, a number of studies have linked iPLA2β with apoptosis in non-β-cells. Our studies revealed that in pancreatic islets iPLA2β is expressed predominantly in the β-cells and that it, as expected, is activated by ATP and inhibited by BEL [46,49–51]. In view of subsequent demonstrations that inhibition of SERCA induces apoptosis through a Ca2+-independent mechanism [52] and that iPLA2β over-expression induces U937 cell apoptosis [53], we considered the possibility that iPLA2β participates in ER stress-induced β-cell apoptosis. Here, we review our observations over the past 5 years that provide evidence for the involvement of iPLA2β in ER stress-induced β-cell apoptosis via a previously unrecognized mechanism of ceramide generation in the β-cell.
iPLA2β Role in β-Cells Undergoing ER Stress
Link Between ER Stress-induced β-Cell Apoptosis and iPLA2β
A potential involvement of iPLA2β in β-cell apoptosis was suggested by studies from Polonsky [52] and Kudo [53]. The first study showed that thapsigargin and other SERCA inhibitors induce MIN-6 cell apoptosis by a mechanism that did not require an increase in [Ca2+]i but one that involved generation of arachidonic acid metabolites. This implied involvement of a PLA2 that manifested activity in the absence of Ca2+. In the latter study, overexpression of iPLA2β, but not cPLA2, increased the incidence of human monocytic U397 cells exposed to TNFα/cycloheximide. Interestingly, cells undergoing apoptosis expressed both the expected full-length iPLA2β and an additional immunoreactive band of smaller size. This band was deduced to be a truncated iPLA2β generated by caspase-3-mediated cleavage of the full-length iPLA2β. Surprisingly, the truncated iPLA2β was found to manifest greater activity and overexpression of this protein induced more pronounced apoptosis than the full-length iPLA2β. These findings suggested that activation of iPLA2β is part of the mechanism that contributes to apoptotic cell death. These reports caused us to consider whether iPLA2β participates in ER stress-induced β-cell apoptosis. To address this possibility, we characterized the effects of inducing ER stress in rat INS-1 cells [54,55], which express an iPLA2β activity [51].
Exposure of INS-1 cells to thapsigargin promoted time-dependent increases in ER stress and apoptotic factors (Grp78/Bip, pPERK, p-eIF2a, CHOP and activated caspase-12). Prolonged ER stress led to the activation of caspase-3, cleavage of PARP and INS-1 cell apoptosis. Inhibition of iPLA2β, however, suppressed, whereas overexpression of iPLA2β amplified the ER stress-induced INS-1 cell apoptosis. These observations are consistent with the participation of iPLA2β in ER stress-induced β-cell apoptosis.
To eliminate the possibility that the above-described observations in INS-1 cells were as a result of non-specific effects of chemical inhibitors or a consequence of iPLA2β overexpression, we examined whether iPLA2β plays a role in the spontaneous development of ER stress. For these studies, we used a β-cell line (Ins2+/AK) established from Akita mice [56] which harbours a spontaneous mutation of the insulin 2 gene (Ins2) (C96Y). This mutation causes misfolding of insulin in the ER leads to the development of hyperglycaemia/diabetes as a consequence of ER stress-induced β-cell apoptosis [57,58]. Akita β-cells express higher pPERK and activated caspase-3 and undergo a higher incidence of apoptosis under basal conditions, in comparison with wild-type β-cells [59]. As expected, ER stress accelerated and/or amplified these outcomes in the Akita β-cells, relative to wild-type β-cells. Consistent with our observations in INS-1 cells, iPLA2β expression was greater in the Akita β-cells than in the wild-type β-cells. Importantly, both basal and ER stress-associated apoptosis were suppressed in Akita cells treated with iPLA2β-targeted siRNAs.
These findings confirm our hypothesis that iPLA2β expression/activation is a critical step in ER stress-mediated β-cell apoptosis. The physiological relevance of these observations in cell lines is underscored by our most recent work (manuscripts in preparation) in islets from iPLA2β-null, islet β-cell-specific iPLA2β transgenic and NOD mice as well as in native human pancreatic islets. Furthermore, these observations are the first to identify regulation of iPLA2β expression during the progression of a biological process.
Link Between ER Stress-induced Ceramide Generation and iPLA2β
Ceramides are lipid messengers that can suppress cell growth and induce apoptosis [60] and they can be generated via multiple mechanisms (de novo synthesis, sphingomyelin hydrolysis, inhibition of ceramide degradation or salvage pathway). While examining lipid profiles that might be associated with ER stress, we unexpectedly found a temporal increase in ceramides in the INS-1 cells [61]. Intriguingly, examination of the activated pathway revealed that ER stress promotes ceramide generation by inducing neutral sphingomyelinase (NSMase) [55] and that inactivation of iPLA2β by BEL not only suppresses ceramide accumulation but also inhibits NSMase induction. These findings suggested that the ceramide generation during ER stress in β-cells occurs by an iPLA2β-dependent induction of NSMase. Consistent with these findings, overexpression of iPLA2β amplified NSMase induction, sphingomyelin hydrolysis and ceramide generation, whereas inhibition or knockdown of NSMase suppressed sphingomyelin hydrolysis, ceramide generation, DNA laddering and TUNEL positivity in INS-1 cells. Taken together with the evidences of increased basal expression of NSMase and lower total pool of sphingomyelin pools in the Akita, relative to wild-type, β-cells and inhibition of ER stress-associated NSMase induction in both cells by BEL, our findings reveal that the iPLA2β/ceramide axis plays an important role during ER stress-induced β-cell apoptosis.
Link Between ER Stress, Mitochondrial Apoptotic Pathway and iPLA2β
Ceramide accumulation has been linked to apoptosis through the intrinsic (mitochondrial) pathway. Although the ER- and mitochondria-associated apoptotic pathways can be activated independently, it has been suggested that induction of ER stress may lead to the triggering of intrinsic apoptotic processes [62,63]. Our findings suggest that such a scenario evolves in β-cells undergoing ER stress as cytochrome c and Smac accumulate in the cytosol of thapsigargin-treated INS-1 cells and this response is magnified in cells that overexpress iPLA2β [55]. These findings suggested the unexpected involvement of iPLA2β in the mitochondrial triggering process following induction of ER stress. Because the release of cytochrome c is associated with alterations in the mitochondrial membrane integrity, assessment of mitochondrial membrane potential (Δψ) and permeability transition pore (PTP) revealed a loss in Δψ and activation (opening) of the PTP in ER-stressed cells. These outcomes were amplified in iPLA2β-overexpressing cells and suppressed by the inactivation of iPLA2β by BEL. Basal Δψ was also compromised in the Akita β-cells, in comparison with the wild-type β-cells. Furthermore, inhibition or knockdown of NSMase suppressed the loss in Δψ and activation of PTP. These findings therefore indicate that the iPLA2β –ceramide axis plays a critical role in activating the mitochondrial apoptotic pathway in insulin-secreting cells during ER stress.
Regulation of iPLA2β During ER Stress
To date, our findings suggest that there are at least three mechanisms that can potentially regulate iPLA2β activity in β-cells during ER stress: translocation of iPLA2β to subcellular organelles, proteolytic processing of iPLA2β to a truncated, more highly active form and increases in iPLA2β expression. Like cPLA2, iPLA2β translocates from cytosol to various subcellular organelles on exposure to certain stimuli [64]. Earlier, we observed that ER stress promotes the accumulation of iPLA2β in the perinuclear region [61]. Subsequent studies revealed that following induction of ER stress, iPLA2β accumulates first in the ER [54] and then in the mitochondria [55]. In parallel, enzymatic activity assays revealed increases in nuclear-, ER- and mitochondria-associated iPLA2β catalytic activity. The increase in mitochondria-associated iPLA2β is accompanied by a concurrent decrease in ER iPLA2β, suggesting that iPLA2β translocates from the ER to the mitochondria during the evolution of ER stress [65]. Consistent with such iPLA2β mobilization, sphingomyelin hydrolysis and ceramide accumulation were increased in the ER and mitochondria fractions of INS-1 cells undergoing ER stress and both outcomes were twofold greater in iPLA2β-overexpressing INS-1 cells [55]. This is an intriguing scenario because pancreatic islet β-cell organelles are enriched in arachidonic acid-containing membrane phospholipids [42,43] and arachidonic acid and arachidonoyl-CoA can activate NSMase [66]. Thus, in addition to inducing NSMase expression, products derived from iPLA2β activation can stimulate NSMase activity. Our findings therefore suggest that ER stress promotes association of iPLA2β with subcellular organelles (nucleus, ER and mitochondria) where it generates bioactive lipids that are recognized contributors to the apoptotic process.
Curiously, the iPLA2β that accumulates in the nucleus is predominantly a truncatedprotein [61]. ThistruncatediPLA2β isoform is analogous to the one previously reported to be a product of caspase-3-catalysed cleavage at the caspase-3 consensus site of iPLA2β in the N-terminal region [53]. Caspase-3 inhibition prevents generation of the truncated iPLA2β product. Mobilization of the truncated iPLA2β, described as being more active than the full-length iPLA2β [53] in the nucleus, supports the possibility that the shorter iPLA2β itself or products derived from iPLA2β-mediated hydrolysis of nuclear membrane phospholipids could regulate transcription of genes, such as the one encoding NSMase.
ER stress induces iPLA2β expression in both wild-type and Akita β-cell [59], suggesting that a pathway that promotes iPLA2β expression is activated during ER stress in the β-cells. One report identified the presence of a SRE in the iPLA2β gene [48]. Because SRE-binding proteins (SREBPs) are known to be induced and processed to the mature (active) forms (mSREBPs) during stress and also in response to glucolipotoxicity and thapsigargin in β-cells [67], we considered the possibility that the increase in iPLA2β expression observed in our studies was induced by elevations in mSREBPs. Indeed, expression of mSREBP-1 was higher in the Akita than in the wild-type β-cells and increased in response to ER stress. A dominant-negative SREBP-1 [68] suppressed both ER stress-induced processing of SREBP-1 and expression of iPLA2β [59], consistent with an SREBP-mediated regulation of iPLA2β expression in β-cells undergoing ER stress.
Summary and Conclusions
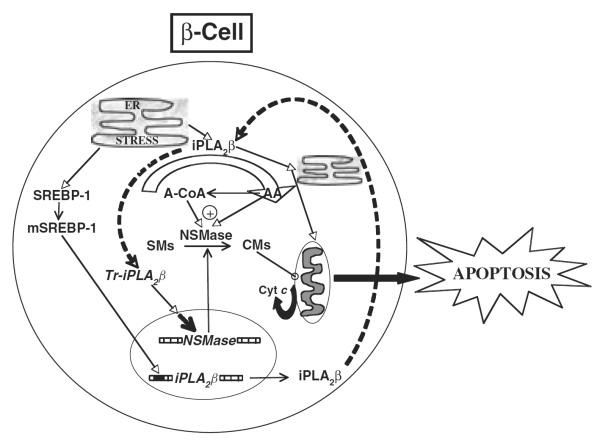
β-Cell apoptosis as a consequence of prolonged ER stress is becoming recognized as a contributing factor in the development and progression of diabetes mellitus. Our observations reveal a role for iPLA2β-mediated ceramide generation via NSMase during ER stress-induced β-cell apoptosis, as summarized in figure 1. Evidence gathered in cell lines, native pancreatic islets and diabetic animal models indicates participation of the iPLA2β in this process. To date, we have determined that ER stress promotes activation of iPLA2β, accumulation of iPLA2β in subcellular organelles that are integral to the apoptotic process, proteolytic cleavage of iPLA2β to a more active product, ceramide generation by an iPLA2β-dependent mechanism and mitochondrial abnormalities by an iPLA2β/ceramide-dependent mechanism, and that ER stress-induced β-cell apoptosis is amplified by iPLA2β overexpression and suppressed by the inactivation of iPLA2β. Also recognized was a regulation of iPLA2β expression during a physiological response by mSREBP-1. Furthermore, β-cells from a spontaneous model of ER stress express higher iPLA2β and NSMase and exhibit a profile similar to that evidenced in INS-1 cells exposed to SERCA inhibitors, strengthening a link between ER stress, β-cell apoptosis and iPLA2β. Finally, evidence of higher iPLA2β and NSMase expression in native pancreatic islets from the Akita and NOD (a model of autoimmune T1DM) mice supports the possibility that activation of these enzymes contributes to β-cell apoptosis and the eventual onset and development of diabetes mellitus in these animals.
Figure 1.
Proposed role of the cytosolic group VIA Ca2+-independent phospholipase A2 (iPLA2β)–ceramide axis in endoplasmic reticulum (ER) stress-induced β-cell apoptosis. ER stress in the β-cell promotes the expression/activity of iPLA2β, leading to the induction of NSMase and increased hydrolysis of sphingomyelins from β-subcellular organelles and accumulation of ceramides in the cytosol. These lipid second messengers activate the mitochondrial apoptotic processes and cause loss in ΔΨ, activation of permeability transition pore and release of cytochrome c into the cytosol. The latter interacts with other factors to activate caspases leading to apoptosis of the β-cell. Consistent with a role for iPLA2β-mediated ceramide generation via NSMase in this process, inactivation of iPLA2β or chemical inhibition or knockdown of NSMase suppresses NSMase expression and ceramide generation, cytochrome c release and apoptosis [54]. The broken arrows linking iPLA2β and truncated iPLA2β (Tr-iPLA2β) indicate a series of events leading to the generation of a caspase-3-processed highly active iPLA2β.
Ongoing work in our laboratory is focused on identifying specific targets of iPLA2β and products of its activation that play a role in the cellular events that lead to β-cell apoptosis. Among the emerging paradigms in these studies is the concept that β-cell apoptosis can be, in part, attributed to iPLA2β-dependent modification of the unfolded protein response or triggering alternative splicing of the pre-mRNAs encoding pro-apoptotic factors. These and other studies will further our understanding of the role of iPLA2β in β-cell apoptosis and enable us to more precisely define its contribution to the onset and progression of diabetes. The findings will add to our knowledge of factors that influence β-cell health in diabetes mellitus and identify potential targets for future therapeutic interventions to prevent β-cell death.
Acknowledgements
The authors would like to thank the expert technical assistance of Dr Mary Wohltmann and Mr Alan Bohrer for contributing to the work reviewed here. We would also like to thank Dr John Turk (Washington University School of Medicine, St Louis, MO) for providing the iPLA2β-null and iPLA2β-Tg mice and the ICR Basic Science Islet Distribution Program and Washington University/Juvenile Diabetes Research Foundation (Award no. 31–2008-382 to Dr Thalachallour Mohanakumar) for providing (to approved user SR) the human islets. The work was supported, in whole or in part, by grants from the National Science Foundation (MCB 0544068), National Institutes of Health (R01-DK69455, R37-DK34388, P41-RR00954, P60-DK20579, P30-DK56341) and the American Diabetes Association.
Footnotes
Conflict of Interests
The authors do not declare any conflict of interest relevant to this manuscript.
References
- 1.DeFronzo RA. Lilly lecture 1987. The triumvirate: beta-cell, muscle, liver. A collusion responsible for NIDDM. Diabetes. 1988;37:667–687. doi: 10.2337/diab.37.6.667. [DOI] [PubMed] [Google Scholar]
- 2.Clark A, Wells CA, Buley ID, et al. Islet amyloid, increased A-cells, reduced B-cells and exocrine fibrosis: quantitative changes in the pancreas in type 2 diabetes. Diabetes Res. 1988;9:151–159. [PubMed] [Google Scholar]
- 3.Kloppel G, Lohr M, Habich K, et al. Islet pathology and the pathogenesis of type 1 and type 2 diabetes mellitus revisited. Surv Synth Pathol Res. 1985;4:110–125. doi: 10.1159/000156969. [DOI] [PubMed] [Google Scholar]
- 4.Stefan Y, Orci L, Malaisse-Lagae F, et al. Quantitation of endocrine cell content in the pancreas of nondiabetic and diabetic humans. Diabetes. 1982;31:694–700. doi: 10.2337/diab.31.8.694. [DOI] [PubMed] [Google Scholar]
- 5.Butler AE, Janson J, Bonner-Weir S, et al. β-Cell deficit and increased β-cell apoptosis in humans with type 2 diabetes. Diabetes. 2003;52:102–110. doi: 10.2337/diabetes.52.1.102. [DOI] [PubMed] [Google Scholar]
- 6.Yoon KH, Ko SH, Cho JH, et al. Selective β-cell loss and α-cell expansion in patients with type 2 diabetes mellitus in Korea. J Clin Endocrinol Metab. 2003;88:2300–2308. doi: 10.1210/jc.2002-020735. [DOI] [PubMed] [Google Scholar]
- 7.Bernard C, Berthault M-F, Saulnier C, et al. Neogenesis vs. apoptosis as main components of pancreatic β-cell mass changes in glucose-infused normal and mildly diabetic adult rats. FASEB J. 1999;13:1195–1205. doi: 10.1096/fasebj.13.10.1195. [DOI] [PubMed] [Google Scholar]
- 8.Pick A, Clark J, Kubstrup C, et al. Role of apoptosis in failure of beta-cell mass compensation for insulin resistance and beta-cell defects in the male Zucker diabetic fatty rat. Diabetes. 1998;47:358–364. doi: 10.2337/diabetes.47.3.358. [DOI] [PubMed] [Google Scholar]
- 9.Vinik A, Rafaeloff R, Pittenger G, et al. Induction of pancreatic islet neogenesis. Horm Metab Res. 1997;29:278–293. doi: 10.1055/s-2007-979037. [DOI] [PubMed] [Google Scholar]
- 10.Butler AE, Janson J, Soeller WC, et al. Increased β-cell apoptosis prevents adaptive increase in β-cell mass in mouse model of type 2 diabetes: evidence for role of islet amyloid formation rather than direct action of amyloid. Diabetes. 2003;52:2304–2314. doi: 10.2337/diabetes.52.9.2304. [DOI] [PubMed] [Google Scholar]
- 11.Cerasi E, Kaiser N, Leibowitz G. Type 2 diabetes and beta cell apoptosis. Diabetes Metab. 2000;26:13–16. [PubMed] [Google Scholar]
- 12.Chandra J, Zhivotovsky B, Zaitsev S, et al. Role of apoptosis in pancreatic beta-cell death in diabetes. Diabetes. 2001;50:S44–S47. doi: 10.2337/diabetes.50.2007.s44. [DOI] [PubMed] [Google Scholar]
- 13.Sesti G. Apoptosis in the beta cells: cause or consequence of insulin secretion defect in diabetes? Ann Med. 2002;34:444–450. doi: 10.1080/078538902321012397. [DOI] [PubMed] [Google Scholar]
- 14.Araki E, Oyadomari S, Mori M. Impact of endoplasmic reticulum stress pathway on pancreatic β-cells and diabetes mellitus. Exp Biol Med. 2003;228:1213–1217. doi: 10.1177/153537020322801018. [DOI] [PubMed] [Google Scholar]
- 15.Lee M-S, Chang I, Kim S. Death effectors of β-cell apoptosis in type 1 diabetes. Mol Genet Metab. 2004;83:82–92. doi: 10.1016/j.ymgme.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 16.Mathis D, Vence L, Benoist C. Beta-cell death during progression to diabetes. Nature. 2001;414:792–798. doi: 10.1038/414792a. [DOI] [PubMed] [Google Scholar]
- 17.Oyadomari S, Takeda K, Takiguchi M, et al. Nitric oxide-induced apoptosis in pancreatic beta cells is mediated by the endoplasmic reticulum stress pathway. Proc Natl Acad Sci U S A. 2001;98:10845–10850. doi: 10.1073/pnas.191207498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Diaz-Horta O, Kamagate A, Herchuelz A, et al. Na/Ca exchanger overexpression induces endoplasmic reticulum-related apoptosis and caspase-12 activation in insulin-releasing BRIN-BD11 cells. Diabetes. 2002;51:1815–1824. doi: 10.2337/diabetes.51.6.1815. [DOI] [PubMed] [Google Scholar]
- 19.Mehmet H. Caspases find a new place to hide. Nature. 2000;403:29–30. doi: 10.1038/47377. [DOI] [PubMed] [Google Scholar]
- 20.Bitko V, Barik S. An endoplasmic reticulum-specific stress-activated caspase (caspase-12) is implicated in the apoptosis of A549 epithelial cells by respiratory syncytial virus. J Cell Biochem. 2001;80:441–454. doi: 10.1002/1097-4644(20010301)80:3<441::aid-jcb170>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 21.Nakagawa T, Zhu H, Morishima N, et al. Caspase-12 mediates endoplasmicreticulum-specific apoptosis and cytotoxicity by amyloid-beta. Nature. 2000;403:98–103. doi: 10.1038/47513. [DOI] [PubMed] [Google Scholar]
- 22.Rao RV, Castro-Obregon S, Frankowski H, et al. Coupling endoplasmic reticulum stress to the cell death program. An Apaf-1-independent intrinsic pathway. J Biol Chem. 2002;277:21836–21842. doi: 10.1074/jbc.M202726200. [DOI] [PubMed] [Google Scholar]
- 23.Hosoi T, Ozawa K. Endoplasmic reticulum stress in disease: mechanisms and therapeutic opportunities. Clin Sci. 2009;118:19–29. doi: 10.1042/CS20080680. [DOI] [PubMed] [Google Scholar]
- 24.Oyadomari S, Araki E, Mori M. Endoplasmic reticulum stress-mediated apoptosis in pancreatic beta-cells. Apoptosis. 2002;7:335–345. doi: 10.1023/a:1016175429877. [DOI] [PubMed] [Google Scholar]
- 25.Oyadomari S, Koizumi A, Takeda K, et al. Targeted disruption of the Chop gene delays endoplasmic reticulum stress-mediated diabetes. J Clin Invest. 2002;109:525–532. doi: 10.1172/JCI14550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Socha L, Silva D, Lesage S, et al. The role of endoplasmic reticulum stress in nonimmune diabetes: NOD.k iHEL, a novel model of β-cell death. Ann N Y Acad Sci. 2003;1005:178–183. doi: 10.1196/annals.1288.022. [DOI] [PubMed] [Google Scholar]
- 27.Harding HP, Zeng H, Zhang Y, et al. Diabetes mellitus and exocrine pancreatic dysfunction in perk−/− mice reveals a role for translational control in secretory cell survival. Mol Cell. 2001;7:1153–1163. doi: 10.1016/s1097-2765(01)00264-7. [DOI] [PubMed] [Google Scholar]
- 28.Delepine M, Nicolino M, Barrett T, et al. EIF2AK3, encoding translation initiation factor 2-alpha kinase 3, is mutated in patients with Wolcott-Rallison syndrome. Nat Genet. 2000;25:406–409. doi: 10.1038/78085. [DOI] [PubMed] [Google Scholar]
- 29.Takeda K, Inoue H, Tanizawa Y, et al. WFS1 (Wolfram syndrome 1) gene product: predominant subcellular localization to endoplasmic reticulum in cultured cells and neuronal expression in rat brain. Hum Mol Genet. 2001;10:477–484. doi: 10.1093/hmg/10.5.477. [DOI] [PubMed] [Google Scholar]
- 30.Araki E, Oyadomari S, Mori M. Endoplasmic reticulum stress and diabetes mellitus. Intern Med. 2003;42:7–14. doi: 10.2169/internalmedicine.42.7. [DOI] [PubMed] [Google Scholar]
- 31.Cardozo AK, Ortis F, Storling J, et al. Cytokines downregulate the sarcoendoplasmic reticulum pump Ca2+ ATPase 2b and deplete endoplasmic reticulum Ca2+, leading to induction of endoplasmic reticulum stress in pancreatic β-cells. Diabetes. 2005;54:452–461. doi: 10.2337/diabetes.54.2.452. [DOI] [PubMed] [Google Scholar]
- 32.Fonseca SG, Fukuma M, Lipson KL, et al. WFS1 is a novel component of the unfolded protein response and maintains homeostasis of the endoplasmic reticulum in pancreatic β-cells. J Biol Chem. 2005;280:39609–39615. doi: 10.1074/jbc.M507426200. [DOI] [PubMed] [Google Scholar]
- 33.Ueda K, Kawano J, Takeda K, et al. Endoplasmic reticulum stress induces WFS1 gene expression in pancreatic β-cells via transcriptional activation. Eur J Endocrinol. 2005;153:167–176. doi: 10.1530/eje.1.01945. [DOI] [PubMed] [Google Scholar]
- 34.Yamada T, Ishihara H, Tamura A, et al. WFS1-deficiency increases endoplasmic reticulum stress, impairs cell cycle progression and triggers the apoptotic pathway specifically in pancreatic β-cells. Hum Mol Genet. 2006;15:1600–1609. doi: 10.1093/hmg/ddl081. [DOI] [PubMed] [Google Scholar]
- 35.Aridor M, Balch WE. Integration of endoplasmic reticulum signaling in health and disease. Nat Med. 1999;5:745–751. doi: 10.1038/10466. [DOI] [PubMed] [Google Scholar]
- 36.Ron D. Translational control in the endoplasmic reticulum stress response. J Clin Invest. 2002;110:1383–1388. doi: 10.1172/JCI16784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harding HP, Ron D. Endoplasmic reticulum stress and the development of diabetes: a review. Diabetes. 2002;51:S455–S461. doi: 10.2337/diabetes.51.2007.s455. [DOI] [PubMed] [Google Scholar]
- 38.Cohen GM. Caspases: the executioners of apoptosis. Biochem J. 1997;326:1–16. doi: 10.1042/bj3260001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nowatzke W, Ramanadham S, Ma Z, et al. Mass spectrometric evidence that agents that cause loss of Ca2+ from intracellular compartments induce hydrolysis of arachidonic acid from pancreatic islet membrane phospholipids by a mechanism that does not require a rise in cytosolic Ca2+ concentration. Endocrinology. 1998;139:4073–4085. doi: 10.1210/endo.139.10.6225. [DOI] [PubMed] [Google Scholar]
- 40.Gijon MA, Leslie CC. Phospholipases A2. Semin Cell Dev Biol. 1997;8:297–303. doi: 10.1006/scdb.1997.0151. [DOI] [PubMed] [Google Scholar]
- 41.Freysz L, Bieth R, Judes C, et al. Quantitative distribution of phospholipids in neurons and glial cells isolated from rat cerebral cortex. J Neurochem. 1968;15:307–313. doi: 10.1111/j.1471-4159.1968.tb11615.x. [DOI] [PubMed] [Google Scholar]
- 42.Ramanadham S, Bohrer A, Gross RW, et al. Mass spectrometric characterization of arachidonate-containing plasmalogens in human pancreatic islets and in rat islet beta-cells and subcellular membranes. Biochemistry. 1993;32:13499–13509. doi: 10.1021/bi00212a015. [DOI] [PubMed] [Google Scholar]
- 43.Ramanadham S, Bohrer A, Mueller M, et al. Mass spectrometric identification and quantitation of arachidonate-containing phospholipids in pancreatic islets: prominence of plasmenylethanolamine molecular species. Biochemistry. 1993;32:5339–5351. doi: 10.1021/bi00071a009. [DOI] [PubMed] [Google Scholar]
- 44.Schaloske RH, Dennis EA. The phospholipase A2 superfamily and its group numbering system. Biochim Biophys Acta Mol Cell Biol Lipids. 2006;1761:1246–1259. doi: 10.1016/j.bbalip.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 45.Lei X, Barbour SE, Ramanadham S. Group VIA Ca2+-independent phospholipase A2 (iPLA2β) and its role in β-cell programmed cell death. Biochimie. 2010;92:627–637. doi: 10.1016/j.biochi.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ma Z, Ramanadham S, Kempe K, et al. Pancreatic islets express a Ca2+-independent phospholipase A2 enzyme that contains a repeated structural motif homologous to the integral membrane protein binding domain of ankyrin. J Biol Chem. 1997;272:11118–11127. [PubMed] [Google Scholar]
- 47.Turk J, Ramanadham S. The expression and function of a group VIA calcium-independent phospholipase A2 (iPLA2β) in beta-cells. Can J Physiol Pharmacol. 2004;82:824–832. doi: 10.1139/y04-064. [DOI] [PubMed] [Google Scholar]
- 48.Seashols SJ, del Castillo Olivares A, Gil G, et al. Regulation of group VIA phospholipase A2 expression by sterol availability. Biochim Biophys Acta Mol Cell Biol Lipids. 2004;1684:29–37. doi: 10.1016/j.bbalip.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 49.Gross RW, Ramanadham S, Kruszka KK, et al. Rat and human pancreatic islet cells contain a calcium ion independent phospholipase A2 activity selective for hydrolysis of arachidonate which is stimulated by adenosine triphosphate and is specifically localized to islet beta-cells. Biochemistry. 1993;32:327–336. doi: 10.1021/bi00052a041. [DOI] [PubMed] [Google Scholar]
- 50.Ramanadham S, Gross RW, Han X, et al. Inhibition of arachidonate release by secretagogue-stimulated pancreatic islets suppresses both insulin secretion and the rise in beta-cell cytosolic calcium ion concentration. Biochemistry. 1993;32:337–346. doi: 10.1021/bi00052a042. [DOI] [PubMed] [Google Scholar]
- 51.Ramanadham S, Hsu F-F, Bohrer A, et al. Studies of the role of group vi phospholipase A2 in fatty acid incorporation, phospholipid remodeling, lysophosphatidylcholine generation, and secretagogue-induced arachidonic acid release in pancreatic islets and insulinoma cells. J Biol Chem. 1999;274:13915–13927. doi: 10.1074/jbc.274.20.13915. [DOI] [PubMed] [Google Scholar]
- 52.Zhou Y-P, Teng D, Dralyuk F, et al. Apoptosis in insulin-secreting cells. Evidence for the role of intracellular Ca2+ stores and arachidonic acid metabolism. J Clin Invest. 1998;101:1623–1632. doi: 10.1172/JCI1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Atsumi G, Murakami M, Kojima K, et al. Distinct roles of two intracellular phospholipase A2s in fatty acid release in the cell death pathway. Proteolytic fragment of type IVA cytosolic phospholipase A2 alpha inhibits stimulus-induced arachidonate release, whereas that of type VI Ca2+-independent phospholipase A2 augments spontaneous fatty acid release. J Biol Chem. 2000;275:18248–18258. doi: 10.1074/jbc.M000271200. [DOI] [PubMed] [Google Scholar]
- 54.Lei X, Zhang S, Bohrer A, et al. The Group VIA calcium-independent phospholipase A2 participates in ER stress-induced INS-1 insulinoma cell apoptosis by promoting ceramide generation via hydrolysis of sphingomyelins by neutral sphingomyelinase. Biochemistry. 2007;46:10170–10185. doi: 10.1021/bi700017z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lei X, Zhang S, Bohrer A, et al. Calcium-independent phospholipase A2 (iPLA2β)-mediated ceramide generation plays a key role in the cross-talk between the endoplasmic reticulum (ER) and mitochondria during ER stress-induced insulin-secreting cell apoptosis. J Biol Chem. 2008;283:34819–34832. doi: 10.1074/jbc.M807409200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nozaki JI, Kubota H, Yoshida H, et al. The endoplasmic reticulum stress response is stimulated through the continuous activation of transcription factors ATF6 and XBP1 in Ins2+/Akita pancreatic β-cells. Genes Cells. 2004;9:261–270. doi: 10.1111/j.1356-9597.2004.00721.x. [DOI] [PubMed] [Google Scholar]
- 57.Kayo T, Koizumi A. Mapping of murine diabetogenic gene mody on chromosome 7 at D7Mit258 and its involvement in pancreatic islet and beta cell development during the perinatal period. J Clin Invest. 1988;101:2112–2118. doi: 10.1172/JCI1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yoshioka M, Kayo T, Ikeda T, et al. A novel locus, Mody4, distal to D7Mit189 on chromosome 7 determines early-onset NIDDM in nonobese C57BL/6 (Akita) mutant mice. Diabetes. 1997;46:887–894. doi: 10.2337/diab.46.5.887. [DOI] [PubMed] [Google Scholar]
- 59.Lei X, Zhang S, Barbour SE, et al. Spontaneous development of ER stress that can lead to diabetes mellitus is associated with higher calcium-independent phospholipase A2 (iPLA2β) expression: a role for regulation by SREBP-1. J Biol Chem. 2010;285:6693–6705. doi: 10.1074/jbc.M109.084293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Obeid LM, Hannun YA. Ceramide: a stress signal and mediator of growth suppression and apoptosis. J Cell Biochem. 1995;58:191–198. doi: 10.1002/jcb.240580208. [DOI] [PubMed] [Google Scholar]
- 61.Ramanadham S, Hsu FF, Zhang S, et al. Apoptosis of Insulin-secreting cells induced by endoplasmic reticulum stress is amplified by overexpression of group VIA calcium-independent phospholipase A2 (iPLA2β) and suppressed by inhibition of iPLA2β. Biochemistry. 2004;43:918–930. doi: 10.1021/bi035536m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Berridge MJ. The endoplasmic reticulum: a multifunctional signaling organelle. Cell Calcium. 2002;32:235–249. doi: 10.1016/s0143416002001823. [DOI] [PubMed] [Google Scholar]
- 63.Duchen MR. Mitochondria and calcium: from cell signalling to cell death. J Physiol (Lond) 2000;529:57–68. doi: 10.1111/j.1469-7793.2000.00057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ma Z, Zhang S, Turk J, et al. Stimulation of insulin secretion and associated nuclear accumulation of iPLA2β in INS-1 insulinoma cells. Am J Physiol Endocrinol Metab. 2002;282:E820–E833. doi: 10.1152/ajpendo.00165.2001. [DOI] [PubMed] [Google Scholar]
- 65.Song H, Bao S, Lei X, et al. Evidence for proteolytic processing and stimulated organelle redistribution of iPLA2β. Biochim Biophys Acta Mol Cell Biol Lipids. 2010;298:E1097–E1194. doi: 10.1016/j.bbalip.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Robinson BS, Hii CS, Poulos A, et al. Activation of neutral sphingomyelinase in human neutrophils by polyunsaturated fatty acids. Immunology. 1997;91:274–280. doi: 10.1046/j.1365-2567.1997.d01-2227.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang H, Kouri G, Wollheim CB. ER stress and SREBP-1 activation are implicated in β-cell glucolipotoxicity. J Cell Sci. 2005;118:3905–3915. doi: 10.1242/jcs.02513. [DOI] [PubMed] [Google Scholar]
- 68.Kim JB, Spiegelman B. ADD1/SREBP1 promotes adipocyte differentiation and gene expression linked to fatty acid metabolism. Genes Dev. 1996;10:1096–1107. doi: 10.1101/gad.10.9.1096. [DOI] [PubMed] [Google Scholar]