Abstract
Rationale
Cannabinoid CB1 antagonists/inverse agonists suppress food-motivated behaviors and are being evaluated as potential appetite suppressants. It has been suggested that the effects of CB1 antagonism on food motivation could be related to actions on mesolimbic dopamine (DA). If this were true, then the effects of interference with cannabinoid CB1 transmission should closely resemble the effects of interference with DA transmission.
Objective
To directly compare the effects of DA antagonists with those of CB1 antagonists/inverse agonists, the present studies employed a concurrent lever-pressing/chow-intake procedure. With this task, interference with DA transmission shifts choice behavior such that lever pressing for a preferred food is decreased but chow intake is increased.
Results
Rats treated with IP injections of the DA D1 antagonist SCH39166 (ecopipam; 0.05–0.2 mg/kg) or the D2 antagonist eticlopride (0.025–0.1 mg/kg) showed substantial decreases in lever pressing and concomitant increases in chow consumption. In contrast, IP administration of the CB1 neutral antagonist AM4113 (4.0–16.0 mg/kg) or the CB1 antagonist/inverse agonist AM251 (2.0–8.0 mg/kg) decreased operant responding for pellets, but there was no corresponding increase in chow intake.
Conclusions
These effects of CB1 antagonists/inverse agonists were similar to those produced by the appetite suppressant fenfluramine and by prefeeding. In contrast, low doses of DA antagonists leave primary food motivation intact, but shift behaviors toward food reinforcers that can be obtained with lower response costs. These results suggest that the effects of interference with CB1 transmission are readily distinguishable from those of reduced DA transmission.
Keywords: Operant, Instrumental, Behavior, Reinforcement, Motivation, Dopamine, Behavioral economics, Reward
Introduction
Several lines of evidence indicate that cannabinoid systems influence feeding and food-motivated behaviors. CB1 agonists have been shown to elevate levels of food intake (Jamshidi and Taylor 2001; Kirkham et al. 2002; Williams and Kirkham 1999). Moreover, food intake is impaired by CB1 receptor inverse agonists such as rimonabant (SR141716A), AM251, and AM1387 (Arnone et al. 1997; Colombo et al. 1998; Simiand et al. 1998; Williams and Kirkham 1999; McLaughlin et al. 2003, 2005, 2006; Pi-Sunyer et al. 2006; Salamone et al. 2007a), as well as by neutral CB1 antagonists including AM4113 and O-2050 (Gardner and Mallet 2006; Salamone et al. 2007a; Sink et al. 2007). Drugs that interfere with CB1 receptor transmission also have been shown to impair food-reinforced behavior (Freedland et al. 2000; McLaughlin et al. 2003, 2006; Ward and Dykstra 2005; Salamone et al. 2007a; Sink et al. 2007). Although studies have demonstrated that the suppression of food intake produced by drugs that interfere with CB1 transmission is not related to motor impairments that directly impair ingestion or food handling (McLaughlin et al. 2005), the precise mechanisms mediating the effects of CB1 antagonists/inverse agonists on food-related behaviors remain uncertain. Various brain systems have been implicated in the food-related effects of cannabinoid drugs, including the hypothalamus, nucleus accumbens shell, and the parabrachial nucleus (Jamshidi and Taylor 2001; Kirkham et al. 2002; Di Marzo et al. 2001; Di Patrizio and Simansky 2006; Soria-Gomez et al. 2007). Another suggestion has been that drugs that act on cannabinoid receptors could be modulating dopamine (DA) transmission, particularly in the nucleus accumbens (Cohen et al. 2002; Wenger et al. 2003; Gardner 2005). CB1 receptor stimulation can increase extracellular DA in nucleus accumbens (Gardner and Lowinson 1991; Tanda et al. 1997; Gardner 2005), and this effect can be blocked by CB1 receptor antagonism (Tanda et al. 1997). Recent evidence indicates that the CB1 receptor antagonist/inverse agonist rimonabant can reduce the increase in extracellular DA in nucleus accumbens that accompanies consumption of a palatable food (Melis et al. 2007), and it has been suggested that interference with CB1 receptor transmission could reduce feeding by reducing mesolimbic DA activity (Melis et al. 2007).
Nevertheless, there appear to be some difficulties with the idea that drugs that interfere with CB1 transmission act on food-motivated behaviors by decreasing activity of DA systems that are hypothesized to mediate primary food motivation. There is considerable evidence demonstrating that the mesolimbic DA system does not mediate primary food “reward,” motivation, or appetite (Salamone et al. 1997, 2003, 2005, 2007b; Salamone and Correa 2002; Kelley et al. 2005). Several studies have shown that nucleus accumbens DA depletion or antagonism does not suppress food intake (Ungerstedt 1971; Koob et al. 1978; Salamone et al. 1993; Baldo et al. 2002); thus, it does not seem highly likely that CB1 antagonists decrease food intake because they reduce accumbens DA release. In addition, there are very few studies that have offered direct comparisons between the behavioral effects of DA and CB1 antagonists. It is reasonable to suggest that, if CB1 antagonists or inverse agonists are exerting their effects on food motivation by reducing DA transmission, then the effects of these CB1-related drugs should closely resemble the effects of DA antagonists. The present studies were undertaken to assess this possibility. These experiments employed a concurrent lever-pressing/chow-feeding procedure that has been used to assess the effects of DA manipulations (Salamone et al. 1991, 1997, 2002, 2003, 2007b; Cousins et al. 1993; Cousins and Salamone 1994; Nowend et al. 2001; Farrar et al. 2007). With this procedure, rats can press a lever to receive a preferred food (Bioserv pellets) or, alternatively, they may approach and consume less-preferred lab chow that is concurrently available in the operant chamber (Salamone et al. 1991, 1996, 1997; Cousins et al. 1993, 1994; Cousins and Salamone 1994; Nowend et al. 2001; Farrar et al. 2007). Under baseline conditions, rats pressing on a fixed-ratio 5 (FR5) schedule typically obtain most of their food by pressing the lever, consuming very little of the laboratory chow. In rats performing this task, prefeeding to reduce food motivation was shown to suppress both lever pressing and chow intake (Salamone et al. 1991). In contrast, low-to-moderate doses of DA antagonists, injected systemically or directly into the accumbens, produced a very different pattern of effects. Systemic injections of DA antagonists with varying selectivity profiles, including cis-flupenthixol, haloperidol, raclopride, SCH23390, and SKF83566, all decreased lever pressing for food but substantially increased intake of the concurrently available chow (Salamone et al. 1991, 1996, 2002; Cousins et al. 1994; Koch et al. 2000). The low dose of haloperidol that produced this shift in behavior (0.1 mg/kg) did not alter intake of the preferred or nonpreferred foods, nor did DA antagonism change food preferences in free-feeding choice tests (Salamone et al. 1991). The shift from lever pressing to chow intake that is produced by systemic DA antagonists was induced by accumbens DA depletions and intra-accumbens injections of DA antagonists into core or shell regions (Salamone et al. 1991; Cousins et al. 1993; Cousins and Salamone 1994; Sokolowski and Salamone 1998; Koch et al. 2000; Nowend et al. 2001).
The present experiments were designed to study the effects of systemic injections of CB1 antagonists and inverse agonists on performance of the concurrent choice task, and to compare the actions of these drugs against those of highly selective D1- and D2-family DA antagonists. AM251 is a CB1 antagonist/inverse agonist that has been shown to suppress food intake and food-reinforced operant responding (McLaughlin et al. 2003, 2005). AM4113 is a novel CB1 receptor neutral antagonist. It was recently reported that this drug effectively reduces feeding and food-motivated behaviors similarly to AM251, but unlike AM251, AM4113 did not induce behaviors associated with nausea and malaise (Sink et al. 2007). Neither of these compounds has been evaluated for their effects on the concurrent lever-pressing/chow-intake choice procedure. For comparison purposes, two DA antagonists that have not previously been assessed for their effects in the concurrent choice task were studied as well. Previous work has shown that D1-family antagonists SCH23390 (Cousins et al. 1994; Nowend et al. 2001) and SKF83566 (Salamone et al. 2002) decrease lever pressing and increase chow consumption on the concurrent choice task. To broaden the pharmacological knowledge in this area, and provide direct comparisons with the effects of AM251 and AM4113, the D1-family antagonist SCH39166 (ecopipam) was studied in the present work. SCH39166 binds to D1 receptors with high affinity, but unlike SCH23390, SCH39166 binds to 5HT2A and 5HT2C receptors with a very low affinity (Alburges et al. 1992). Previous studies employing the concurrent choice task have examined the effects of haloperidol and raclopride (Salamone et al. 1991, 2002), and thus, for the present work, the potent and selective D2-family antagonist eticlopride was investigated. Because interference with CB1 transmission is thought to decrease appetite or produce food aversions (McLaughlin et al. 2005; Sink et al. 2007), it was hypothesized that the CB1 antagonist and inverse agonist drugs would both suppress lever pressing but would fail to increase chow intake. In contrast, it was expected that both DA antagonists would produce a reallocation of behavior resulting in reduced lever pressing and increased chow intake.
Materials and methods
Animals
Adult male Sprague-Dawley rats (Harlan Sprague-Dawley, Indianapolis, IN, USA) weighing at least 300 g at the beginning of the experiments were housed in a colony maintained at 23°C, with a 12-h light/dark cycle (lights on 07:00). Rats (total N=34) were food-deprived to 85% of their free-feeding body weight for initial operant training and were allowed modest weight growth (i.e., an additional 5–10%) during the experiment. Water was available ad libitum in the home cages. Animal protocols were approved by the University of Connecticut Institutional Animal Care and Use Committee, and the studies were conducted according to NIH guidelines for animal care and use.
Behavioral procedures
Behavioral sessions were conducted in operant conditioning chambers (28×23×23 cm; Med Associates, St. Albans, VT, USA). Animals were initially trained to lever-press for 4 days (30-min sessions; 45-mg pellets, Bioserve, French-town, NJ, USA, were used for all operant behavior tests) and then were changed to an FR5 schedule (30-min sessions, 5 days/week) and trained for several weeks. Rats were then trained on the concurrent FR5/chow-feeding procedure following the initial FR5 training. For this procedure, weighed amounts of lab chow (LC, Prolab 3000 LabDiet, Purina Mills, St. Louis, MO, USA; typically 15–20 g, three large pieces) were concurrently available on the floor of the chamber during the FR5 sessions. At the end of the session, rats were immediately removed from the chamber. Food intake was determined by weighing the remaining food, including spillage. Rats were trained until achievement of stable baseline levels of chow intake and lever pressing (i.e., consistent responding over 1,200 lever presses per 30 min) before any drug testing began.
Pharmacological agents
AM251 and AM4113 (synthesized in the Makriyannis Laboratory) were dissolved in a vehicle of dimethylsulfoxide (DMSO; Fisher, Waltham, MA, USA), Tween-80 (Fisher), and 0.9% saline in a 1:1:8 ratio. This mixture also served as the vehicle control treatment in experiments 1 and 2. Doses and pretreatment times for AM251 and AM4113 were chosen based upon pilot studies and previously published research (McLaughlin et al. 2003; Sink et al. 2007). They were selected to produce a suppression of lever pressing that was comparable to that produced by the DA antagonists. SCH39166 (ecopipam; (6aS-trans)-11-Chloro-6,6a,7,8,9, 13b-hexahydro-7-methyl-5H-benzo[d] aphtha[2,1-b]azepin-12-ol hydrobromide), obtained from Tocris Bioscience (Ellisville, MO, USA), was dissolved in a 0.3% tartaric acid solution (pH=4.0), which was also used as the vehicle control condition for experiment 3. Eticlopride (S(−)-3-Chloro-5-ethyl-N-[(1-ethyl-2-pyrrolidinyl)methyl]-6-hydroxy-2-methoxybenzamide hydrochloride) was obtained from Sigma Chemical (St. Louis, MO, USA) and was dissolved in a 0.9% saline solution, which was also used as the vehicle control condition for experiment 4. SCH39166 and eticlopride doses and pretreatment times were selected based upon pilot studies. For all experiments, drug or vehicle were administered IP (see descriptions of individual experiments for drug administration schedule).
Experimental procedures
The rats were thoroughly trained on the concurrent FR5/chow-feeding procedure (see above) before drug testing began, and different groups of rats were used for each experiment. All experiments used a within-groups design, with all rats receiving all IP drug treatments in their particular experiment in a randomly varied order (one treatment per week; no treatment sequences were repeated across different animals in the same experiment). Baseline training (i.e., nondrug) sessions were conducted four additional days per week. The following treatments and testing times were used for each experiment:
Experiment 1. AM251: 1.0 ml/kg vehicle, 2.0, 4.0, and 8.0 mg/kg AM251 IP (30 min before testing; n=9)
Experiment 2. AM4113: 1.0 ml/kg vehicle, 4.0, 8.0, and 16.0 mg/kg AM251 IP (30 min before testing; n=7)
Experiment 3. SCH39166: 1.0 ml/kg vehicle, 0.05, 0.1, and 0.2 mg/kg SCH39166 IP (20 min before testing; n=8)
Experiment 4. eticlopride: 1.0 ml/kg vehicle, 0.025, 0.05, and 0.1 mg/kg eticlopride IP (30 min before testing; n=10)
Statistical analyses
Total number of lever presses and gram quantity of chow intake in the concurrent choice experiments were analyzed with repeated measures analysis of variance (ANOVA). When the overall ANOVA was significant, nonorthogonal planned comparisons using the overall error term were used to compare each treatment with the vehicle control. The alpha level for each comparison was kept at 0.05 because the number of comparisons was restricted to the number of treatments minus one (Keppel 1982). In addition, correlational analyses were used to measure the relation between lever pressing and chow consumption for each drug study, with the data collapsed across injection treatments within each study.
Results
Experiments 1 and 2: effects of AM251 and AM4113 on the concurrent FR5/chow-feeding procedure
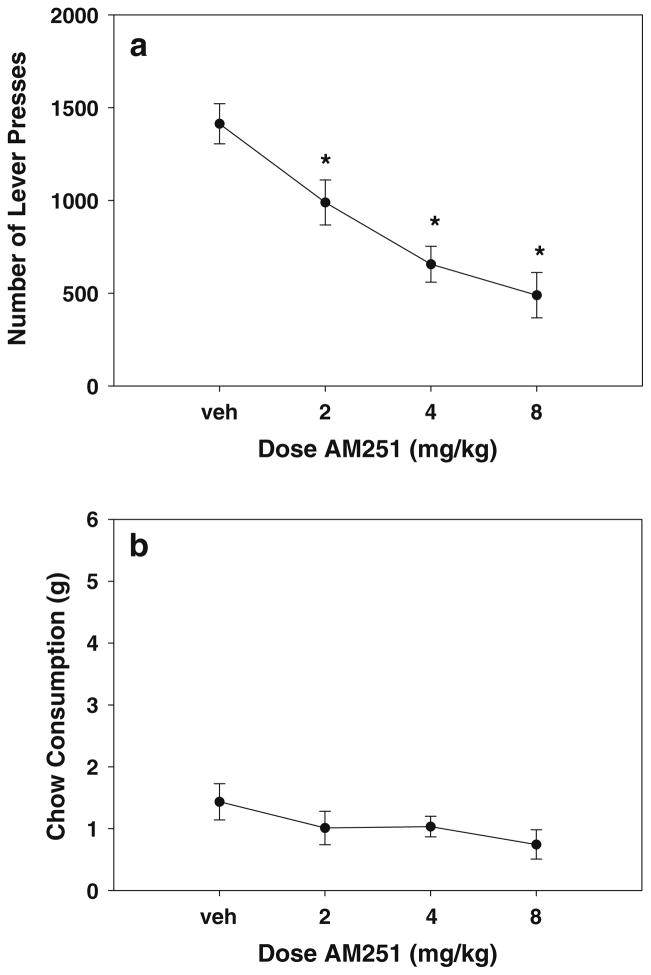
The results of experiment 1 are shown in Fig. 1. There was an overall significant effect of AM251 treatment on lever-pressing [Fig. 1a; F(3,24)=14.1, p<0.001]. Planned comparisons showed that AM251 produced a significant reduction in lever pressing compared to vehicle control for all doses (p<0.05). There was no overall significant effect on chow consumption [Fig. 1b; F(3,24)=1.118, p= 0.361], although linear regression analysis suggested that there was a tendency for levels of chow intake to decrease as a function of dose [F(1,34)=3.48, p=0.07]. In addition, six of nine rats showed lower levels of chow intake at the high dose of AM251 compared to vehicle. The results of experiment 2 (AM4113) are shown in Fig. 2. There was an overall significant effect of drug treatment on lever-pressing [Fig. 2a; F(3,18)=21.488, p<0.001]. Planned comparisons showed that AM4113 produced a significant reduction in lever pressing compared to vehicle control for all doses (p< 0.05). Neither the overall ANOVA nor regression analyses demonstrated dose-related changes in chow consumption (Fig. 2b). Nevertheless, five of seven rats showed lower levels of chow intake after treatment with the high dose of AM4113 compared to vehicle.
Fig. 1.
Effects of the cannabinoid CB1 receptor antagonist/inverse agonist AM251. a Mean (±SEM) number of lever presses after treatment with vehicle and various doses of AM251. b Mean (±SEM) intake of lab chow (in grams) after treatment with vehicle and various doses of AM251. Asterisks p<0.05, different from vehicle, planned comparison
Fig. 2.
Effects of the cannabinoid CB1 receptor neutral antagonist AM4113. a Mean (±SEM) number of lever presses after treatment with vehicle and various doses of AM4113. b Mean (±SEM) intake of lab chow (in grams) after treatment with vehicle and various doses of AM4113. Asterisks p<0.05, different from vehicle, planned comparison
Experiments 3 and 4: effects of SCH39166 and eticlopride on the concurrent FR5/chow-feeding procedure
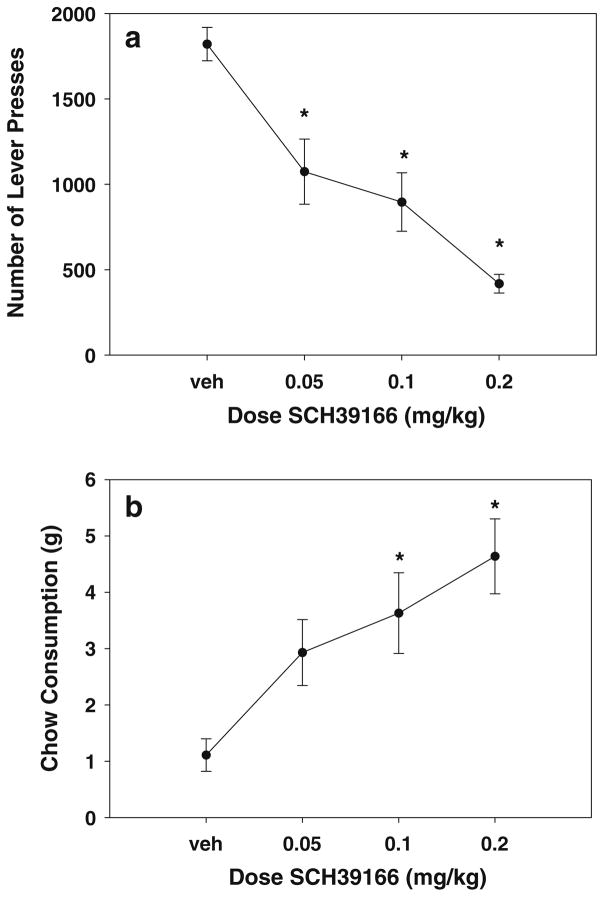
The results of experiment 3 are summarized in Fig. 3. SCH39166 produced a significant decrease in lever pressing [F(3,21)=19.943, p<0.001; Fig. 3a] and, at the same time, significantly increased chow consumption [F(3,21)=6.821, p=0.002; Fig. 3b]. Planned comparisons showed that SCH39166 significantly reduced lever pressing at all doses compared to vehicle and increased chow consumption compared to vehicle control at doses of 0.1 and 0.2 mg/kg (p<0.05). Figure 4 shows the results of experiment 4. Eticlopride produced a significant decrease in lever pressing [F(3,27)=22.57, p<0.001; Fig. 4a] and also significantly increased chow intake [F(3,27)=14.780, p<0.001; Fig. 4b]. Planned comparisons showed that eticlopride produced significant reductions in lever pressing and increases in chow consumption at both the 0.05 and 0.1 mg/kg doses compared to vehicle (p<0.05).
Fig. 3.
Effects of the dopamine D1 receptor antagonist SCH39166. a Mean (±SEM) number of lever presses after treatment with vehicle and various doses of SCH39166. b Mean (±SEM) intake of lab chow (in grams) after treatment with vehicle and various doses of SCH39166. Asterisks p<0.05, different from vehicle, planned comparison
Fig. 4.
Effects of the dopamine D2 receptor antagonist eticlopride. a Mean (±SEM) number of lever presses after treatment with vehicle and various doses of eticlopride. b Mean (±SEM) intake of lab chow (in grams) after treatment with vehicle and various doses of eticlopride. Asterisks p<0.05, different from vehicle, planned comparison
Correlational analyses across experiments 1–5
One of the statistical markers that has been used in previous experiments to characterize the shift from lever pressing to chow intake induced by DA antagonism has been the presence or absence of an inverse correlation between these two variables. For these analyses, data within each experiment were collapsed across all treatment conditions. In the first two experiments, data from rats treated with either AM251 or AM4113 failed to show a significant relation between lever pressing and chow intake (AM251: r=−0.057, NS; AM4113: r=0.070, NS). In contrast, a significant and robust inverse correlation was observed with the DA antagonists (SCH39166: r=−0.798, df=30, p< 0.001; eticlopride: r=−0.649, df=38, p<0.001).
Discussion
In the studies described above, a concurrent lever-pressing/feeding task was used to compare the effects of drugs that interfere with cannabinoid CB1 transmission with those of drugs that act as DA antagonists. These experiments were undertaken to determine if CB1 antagonists/inverse agonists could produce effects on food-motivated behavior that closely resemble those produced by DA antagonists. The DA antagonists SCH39166 and eticlopride both showed similar effects, i.e., there was a shift from lever pressing to chow intake, such that drug-induced decreases in lever pressing were accompanied by increases in chow intake. In contrast, the cannabinoid antagonist/inverse agonist AM251 and the CB1 neutral antagonist AM4113 failed to increase chow intake at doses that suppressed food-reinforced lever pressing. Although lever pressing and chow intake showed a robust inverse correlation in rats treated with DA antagonists, these variables were unrelated in rats treated with the drugs that impaired cannabinoid CB1 transmission. Overall, these data indicate that CB1 antagonists/inverse agonists and DA antagonists produce different types of behavioral effects, which seems to argue against the idea that interference with CB1 transmission suppresses food-motivated behavior because of actions mediated by reductions in DA transmission.
Experiments 1 and 2 demonstrated that the CB1 antagonist/inverse agonist AM251 and the neutral CB1 antagonist AM4113 both decreased food-reinforced lever pressing. These data are consistent with previous studies showing that interference with cannabinoid CB1 transmission can suppress food-reinforced instrumental behavior (Freedland et al. 2000; McLaughlin et al. 2003, 2006; Ward and Dykstra 2005; Salamone et al. 2007a; Sink et al. 2007). However, these earlier studies were conducted using conventional operant schedules. In the present work, rats were tested on a concurrent choice procedure, in which a less-preferred food (lab chow) was also available in the chamber. The dose ranges of AM251 and AM4113 that were tested produced a wide variation in operant response suppression (i.e., approximately 30–87.5% reductions), matching the effects produced by DA antagonists. Nevertheless, the decreases in lever pressing produced by AM251 and AM4113 were not accompanied by any increases in chow intake. In fact, there was a slight tendency for AM251 and AM4113 to decrease chow intake as dose increased, although a floor effect due to low baseline levels of chow intake probably prevented a significant effect from being observed in the present study. Previous papers that assessed chow intake separately showed that the doses of AM251 and AM4113 used in the present study also suppressed chow intake (McLaughlin et al. 2003; Sink et al. 2007). The present results with AM251 and AM4113 are similar to those reported previously for other manipulations that are thought to affect aspects of appetite. In earlier work employing the concurrent choice task, prefeeding to reduce food motivation and administration of appetite suppressants such as amphetamine and fenfluramine all failed to increase chow intake under conditions that suppressed food-reinforced lever pressing (Salamone et al. 1991, 2002; Cousins et al. 1994). Thus, although the effects of cannabinoid CB1 receptor antagonists/inverse agonists on performance of the concurrent choice task differed markedly from those produced by DA antagonists (see detailed discussion below), they were similar to those produced by other manipulations that are known to suppress appetite. Taken together, these results suggest that interference with CB1 transmission suppresses food-reinforced behavior because of broad effects on directional aspects of primary food motivation (i.e., processes directing behavior towards consumption of food). The precise mechanisms underlying these effects are unknown. Several papers have concluded that CB1 inverse agonists may act to suppress appetite (Arnone et al. 1997; Colombo et al. 1998; Simiand et al. 1998; McLaughlin et al. 2003, 2005, 2006; see review by Salamone et al. 2007a). It has been suggested that competition from other responses (e.g., grooming, scratching) could contribute to the decreases in feeding produced by rimonabant (Tallett et al. 2007), and it has also been reported that CB1 inverse agonists can produce food aversions (McLaughlin et al. 2005; Salamone et al. 2007a; but see also Thornton-Jones et al. 2005). A recent study has demonstrated that the neutral antagonist, AM4113, suppressed food intake at doses that did not induce conditioned gaping, which is a sign of nausea in rats (Sink et al. 2007). Yet, regardless of the particular mechanism involved, it does appear that interference with CB1 transmission suppresses food-motivated instrumental behavior because of direct actions on food intake regulation mechanisms.
The results seen after administration of DA antagonists differed markedly from those produced by AM251 and AM4113. Consistent with a large body of data, the D1-family antagonist SCH31966 and the D2-family antagonist eticlopride both produced a shift in behavior on the choice task, i.e., drug-induced decreases in lever pressing were accompanied by increases in chow intake. Previous work has shown that systemic injections of DA antagonists with varying selectivity profiles, including nonselective and D1-and D2-family selective drugs, all decreased lever pressing for food but substantially increased consumption of the concurrently available chow (Salamone et al. 1991, 1996, 2002; Cousins et al. 1994; Koch et al. 2000). Although D1-and D2-family antagonists have been reported to have different effects on several behaviors, including meal patterning (Clifton et al. 1991; Clifton 1995), they produce similar effects on response allocation as measured by the concurrent lever-pressing/feeding task. In the present studies, as well as in previous work (Cousins et al. 1993; Sokolowski and Salamone 1998; Salamone et al. 2002), there was a robust inverse correlation between lever pressing and chow intake in rats treated with DA D1- and D2-family antagonists. This correlation was not seen in animals treated with AM251 or AM4113 (experiments 1–2), nor was it previously observed in rats treated with the appetite suppressant fenfluramine (Salamone et al. 2002).
The shift in behavior from lever pressing to chow intake that is seen after systemic DA antagonism is not seen after local depletions of DA in anterior or ventrolateral neo-striatum (Cousins et al. 1993), but it is observed after local DA depletion or antagonism in the nucleus accumbens (Salamone et al. 1991; Cousins et al. 1993; Sokolowski and Salamone 1998; Koch et al. 2000; Nowend et al. 2001). Thus, rats that have been injected with low doses of DA antagonists, as well as those that have accumbens DA depletions, remain directed towards the acquisition and consumption of food despite their diminished level of lever pressing. These data have been interpreted as indicating that DA is involved in activational aspects of motivation (e.g., increased activity, exertion of effort, overcoming response costs) and response allocation in effort-related choice tasks, but that fundamental aspects of primary food motivation and appetite are left intact after low doses of DA antagonists or interference with accumbens DA transmission (Salamone et al. 1991, 1997, 2002, 2003, 2005, 2006, 2007b; Aberman et al. 1998; Aberman and Salamone 1999; Kelley et al. 2005; Baldo and Kelley 2007). This observation is consistent with several previous studies. In separate food intake tests, low doses of DA antagonists that reduced lever pressing did not suppress chow intake (Rolls et al. 1974; Fibiger et al. 1976; Rusk and Cooper 1994) or intake of Bioserve pellets (Salamone et al. 1991). Similar findings have been reported for water-reinforced responding (Ljungberg 1987, 1988, 1990). Previous studies showed that a 0.1-mg/kg dose of haloperidol did not suppress chow intake or reduce time spent feeding on chow (Salamone et al. 1990), although this dose has been shown consistently to suppress lever pressing and increase chow intake in the concurrent choice task (Salamone 1986; Salamone et al. 1991, 1996; Farrar et al. 2007). Clifton et al. (1991) reported that low doses of the D1 antagonist SCH23390 had little effect on food intake and that low doses of the D2 antagonists raclopride and YM-09151-2 actually increased meal duration and size. A number of studies have demonstrated that fundamental aspects of food motivation, including perception and discrimination of food reward magnitude, and appetitive taste reactivity to food, are left intact after DA antagonists or accumbens DA depletions (Martin-Iverson et al. 1987; Salamone et al. 1990, 1994; Treit and Berridge 1990; Cousins et al. 1996; Aparicio 1998; Berridge and Robinson 1998; Berridge 2000; for reviews, see Salamone et al. 1997, 1999, 2003, 2005, 2006, 2007b). Injections of D1- or D2-family antagonists into either the core or shell subregions of nucleus accumbens, in doses that suppressed locomotion, failed to suppress food intake (Baldo et al. 2002). Taking all these findings together with the present data, it would be extremely difficult to argue that low doses of DA antagonists, or accumbens DA depletions, suppress food-reinforced lever pressing because of a reduction in appetite or a general loss of food motivation (Salamone et al. 1991, 1997, 2002, 2007b; Ikemoto and Panksepp 1996, 1999; Kelley et al. 2005). Nevertheless, as discussed above, this does appear to be a reasonable explanation for the effects of CB1 antagonists and inverse agonists on food-reinforced behavior.
Other studies have reported that interference with cannabinoid CB1 receptor function produces behavioral effects that are different from those produced by DA antagonism or DA depletion. For example, food consumption can be suppressed by high doses of DA antagonists (e.g., 0.2 mg/kg or greater) and whole neostriatal DA depletions, and a major parameter of food intake that is affected by these conditions is the rate of feeding (i.e., grams intake per minute spent eating; Blundell 1987; Salamone et al. 1990, 1993). Although nucleus accumbens DA depletions have little effect on food intake (Koob et al. 1978; Salamone et al. 1993), local depletions of DA in the ventrolateral neostriatum impaired food intake primarily by reducing feeding rate and disrupting forepaw used during food handling, with little effect on time spent feeding (Salamone et al. 1993). In contrast, doses of AM251 that impaired food intake had substantial effects on time spent feeding but did not significantly affect feeding rate or food handling (McLaughlin et al. 2005). Taken together, detailed analyses conducted across different types of behavioral tests (i.e., response allocation in effort-related choice, food intake) seem to suggest that, although interference with DA and CB1 receptor function can affect aspects of food-related behavior, the specific patterns of effects produced by each class of drugs (i.e., DA antagonists and CB1 antagonists/inverse agonists) are quite distinct from each other. Thus, despite results indicating that modulation of CB1 receptors can alter aspects of DA transmission (Gardner 2005; Melis et al. 2007), it does not appear that CB1 antagonists or inverse agonists are exerting their effects on food-motivated behavior in a manner that is directly mediated via reduced DA transmission in nucleus accumbens.
Acknowledgments
This work was supported by a grant to J.S. and A.M. from the National Institute of Drug Abuse.
Contributor Information
K. S. Sink, Department of Psychology, University of Connecticut, Storrs, CT 06269-1020, USA
V. K. Vemuri, Center for Drug Discovery, Northeastern University, 360 Huntington Avenue, Boston, MA 02115, USA
T. Olszewska, Center for Drug Discovery, Northeastern University, 360 Huntington Avenue, Boston, MA 02115, USA
A. Makriyannis, Center for Drug Discovery, Northeastern University, 360 Huntington Avenue, Boston, MA 02115, USA
J. D. Salamone, Email: john.salamone@uconn.edu, Department of Psychology, University of Connecticut, Storrs, CT 06269-1020, USA. Division of Behavioral Neuroscience, Department of Psychology, University of Connecticut, Storrs, CT 06269-1020, USA
References
- Aberman JE, Salamone JD. Nucleus accumbens dopamine depletions make rats more sensitive to high ratio requirements do not affect primary food reinforcement. Neuroscience. 1999;92:545–552. doi: 10.1016/s0306-4522(99)00004-4. [DOI] [PubMed] [Google Scholar]
- Aberman JE, Ward SJ, Salamone JD. Effects of dopamine antagonists and accumbens dopamine depletions on time-constrained progressive ratio performance. Pharmacol Biochem Behav. 1998;61:341–348. doi: 10.1016/s0091-3057(98)00112-9. [DOI] [PubMed] [Google Scholar]
- Alburges ME, Hunt ME, McQuade RD, Wamsley JK. D1-receptor antagonists: comparison of [3H]SCH39166 to [3H] SCH23390. J Chem Neuroanat. 1992;5:357–366. doi: 10.1016/0891-0618(92)90051-q. [DOI] [PubMed] [Google Scholar]
- Aparicio C. Assessing haloperidol in rats with the barrier choice paradigm. Suma Psicol. 1998;5:1–20. (article in Spanish) [Google Scholar]
- Arnone M, Maruani J, Chaperon F, Thiébot M-H, Poncelet M, Soubrié P, Le Fur G. Selective inhibition of sucrose and ethanol intake by SR 141716, an antagonist of central cannabinoid (CB1) receptors. Psychopharmacology. 1997;132:104–106. doi: 10.1007/s002130050326. [DOI] [PubMed] [Google Scholar]
- Baldo BA, Kelley AE. Discrete neurochemical coding of distinguishable motivational processes: insights from nucleus accumbens control of feeding. Psychopharmacology (Berl) 2007;191:439–459. doi: 10.1007/s00213-007-0741-z. [DOI] [PubMed] [Google Scholar]
- Baldo BA, Sadeghian K, Basso AM, Kelley AE. Effects of selective dopamine D1 or D2 receptor blockade within nucleus accumbens subregions on ingestive behavior and associated motor activity. Behav Brain Res. 2002;137:165–177. doi: 10.1016/s0166-4328(02)00293-0. [DOI] [PubMed] [Google Scholar]
- Berridge KC. Measuring hedonic impact in animals and infants: microstructure of affective taste reactivity patterns. Neurosci Biobehav Rev. 2000;24:173–198. doi: 10.1016/s0149-7634(99)00072-x. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res Rev. 1998;28:309–369. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- Blundell JE. Structure, process and mechanism: case studies in the psychopharmacology of feeding. In: Iversen LL, Iversen SD, Snyder SH, editors. Handbook of psychopharmacology. Vol. 19. Plenum; New York: 1987. pp. 123–182. [Google Scholar]
- Clifton PG. Effects of SCH39166 and domperidone on the meal patterning of male rats. Pharmacol Biochem Behav. 1995;52:265–270. doi: 10.1016/0091-3057(95)00094-d. [DOI] [PubMed] [Google Scholar]
- Clifton PG, Rusk IN, Cooper SJ. Effects of dopamine D1 and dopamine D2 antagonists on the free feeding and drinking patterns of rats. Behav Neurosci. 1991;105:272–281. doi: 10.1037//0735-7044.105.2.272. [DOI] [PubMed] [Google Scholar]
- Cohen C, Perrault G, Voltz C, Steinberg R, Soubrie P. SR141716, a central cannabinoid (CB1) receptor antagonist, blocks the motivational and dopamine-releasing effects of nicotine in rats. Behav Pharmacol. 2002;13:451–463. doi: 10.1097/00008877-200209000-00018. [DOI] [PubMed] [Google Scholar]
- Colombo G, Agabio R, Diaz G, Lobina C, Reali R, Gessa GL. Appetite suppression and weight loss after the cannabinoid antagonist SR 141716. Life Sci. 1998;63:PL113–PL117. doi: 10.1016/s0024-3205(98)00322-1. [DOI] [PubMed] [Google Scholar]
- Cousins MS, Salamone JD. Nucleus accumbens dopamine depletions in rats affect relative response allocation in a novel cost/benefit procedure. Pharmacol Biochem Behav. 1994;49:85–91. doi: 10.1016/0091-3057(94)90460-x. [DOI] [PubMed] [Google Scholar]
- Cousins MS, Sokolowski JD, Salamone JD. Different effects of nucleus accumbens and ventrolateral striatal dopamine depletions on instrumental response selection in the rat. Pharmacol Biochem Behav. 1993;46:943–951. doi: 10.1016/0091-3057(93)90226-j. [DOI] [PubMed] [Google Scholar]
- Cousins MS, Wei W, Salamone JD. Pharmacological characterization of performance on a concurrent lever pressing/feeding choice procedure: effects of dopamine antagonist, cholinomimetic, sedative and stimulant drugs. Psychopharmacology. 1994;116:529–537. doi: 10.1007/BF02247489. [DOI] [PubMed] [Google Scholar]
- Cousins MS, Atherton A, Turner L, Salamone JD. Nucleus accumbens dopamine depletions alter relative response allocation in a T-maze cost/benefit task. Behav Brain Res. 1996;74:189–197. doi: 10.1016/0166-4328(95)00151-4. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, Goparaju S, Wang L, Liu J, Batkai S, Jarai Z. Leptin-regulated endocannabinoids are involved in maintaining food intake. Nature. 2001;410:822–825. doi: 10.1038/35071088. [DOI] [PubMed] [Google Scholar]
- Di Patrizio NV, Simansky KJ. Program No. 456.2. Neuroscience Meeting Planner. Society for Neuroscience; Atlanta: 2006. Differential roles for cannabinoid and mu-opioid signaling systems of the parabrachial nucleus in modulating intake of standard chow and high-fat/high-sucrose diet. (online) [Google Scholar]
- Farrar AM, Pereira M, Velasco F, Hockemeyer J, Muller CE, Salamone JD. Adenosine A(2A) receptor antagonism reverses the effects of dopamine receptor antagonism on instrumental output and effort-related choice in the rat: implications for studies of psychomotor slowing. Psychopharmacology. 2007;191:579–586. doi: 10.1007/s00213-006-0554-5. [DOI] [PubMed] [Google Scholar]
- Fibiger HC, Carter DA, Phillips AG. Decreased intracranial self-stimulation after neuroleptics or 6-hydroxydopamine: evidence for mediation by motor deficits rather than by reduced reward. Psychopharmacology. 1976;47:21–27. doi: 10.1007/BF00428696. [DOI] [PubMed] [Google Scholar]
- Freedland CS, Poston JS, Porrino LJ. Effects of SR141716A, a central cannabinoid receptor antagonist, on food-maintained responding. Pharmacol Biochem Behav. 2000;67:265–270. doi: 10.1016/s0091-3057(00)00359-2. [DOI] [PubMed] [Google Scholar]
- Gardner EL. Endocannabinoid signaling system and brain reward: emphasis on dopamine. Pharmacol Biochem Behav. 2005;81:263–284. doi: 10.1016/j.pbb.2005.01.032. [DOI] [PubMed] [Google Scholar]
- Gardner EL, Lowinson JH. Marijuana’s interaction with brain reward systems: update 1991. Pharmacol Biochem Behav. 1991;40:571–580. doi: 10.1016/0091-3057(91)90365-9. [DOI] [PubMed] [Google Scholar]
- Gardner A, Mallet PE. Suppression of feeding, drinking, and locomotion by a putative cannabinoid receptor ‘silent antagonist’. Eur J Pharmacol. 2006;530:103–106. doi: 10.1016/j.ejphar.2005.11.032. [DOI] [PubMed] [Google Scholar]
- Ikemoto S, Panksepp J. Dissociations between appetitive and consummatory responses by phamacological manipulations of reward-relevant brain regions. Behav Neurosci. 1996;110:331–345. doi: 10.1037//0735-7044.110.2.331. [DOI] [PubMed] [Google Scholar]
- Ikemoto S, Panksepp J. The role of nucleus accumbens dopamine in motivated behavior: a unifying interpretation with special reference to reward-seeking. Brain Res Rev. 1999;31:6–41. doi: 10.1016/s0165-0173(99)00023-5. [DOI] [PubMed] [Google Scholar]
- Jamshidi N, Taylor DA. Anandamide administration into the ventromedial hypothalamus stimulates appetite in rats. Br J Pharmacol. 2001;134(6):1151–1154. doi: 10.1038/sj.bjp.0704379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley AE, Baldo BA, Pratt WE, Will MJ. Corticostriatal–hypothalamic circuitry and food motivation: integration of energy, action and reward. Physiol Behav. 2005;86:773–795. doi: 10.1016/j.physbeh.2005.08.066. [DOI] [PubMed] [Google Scholar]
- Keppel G. Design and analysis: a researchers handbook. Prentice-Hall: Englewood Cliffs; 1982. [Google Scholar]
- Kirkham TC, Williams CM, Fezza F, Di Marzo V. Endocanna-binoid levels in rat limbic forebrain and hypothalamus in relation to fasting, feeding and satiation: stimulation of eating by 2-arachidonoyl glycerol. Br J Pharmacol. 2002;136:550–557. doi: 10.1038/sj.bjp.0704767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch M, Schmid A, Scnhnitzler HU. Role of nucleus accumbens dopamine D1 and D2 receptors in instrumental and Pavlovian paradigms of conditioned reward. Psychopharmacology. 2000;152:67–73. doi: 10.1007/s002130000505. [DOI] [PubMed] [Google Scholar]
- Koob GF, Riley SJ, Smith SC, Robbins TW. Effects of 6-hydroxydopamine lesions of the nucleus accumbens septi and olfactory tubercle on feeding, locomotor activity, and amphetamine anorexia in the rat. J Comp Physiol Psychol. 1978;92:917–927. doi: 10.1037/h0077542. [DOI] [PubMed] [Google Scholar]
- Ljungberg T. Blockade by neuroleptics of water intake and operant responding in the rat: anhedonia, motor deficit or both? Pharmacol Biochem Behav. 1987;27:341–350. doi: 10.1016/0091-3057(87)90578-8. [DOI] [PubMed] [Google Scholar]
- Ljungberg T. Scopolamine reverses haloperidol-attenuated lever pressing for water but not haloperidol-attenuated water intake in the rat. Pharmacol Biochem Behav. 1988;29:205–211. doi: 10.1016/0091-3057(88)90298-5. [DOI] [PubMed] [Google Scholar]
- Ljungberg T. Differential attenuation of water intake and water-rewarded operant responding by repeated administration of haloperidol and SCH 23390 in the rat. Pharmacol Biochem Behav. 1990;35:111–115. doi: 10.1016/0091-3057(90)90213-2. [DOI] [PubMed] [Google Scholar]
- Martin-Iverson MT, Wilke D, Fibiger HC. Effect of haloperidol and d-amphetamine on perceived quantitiy of food and tones. Psychopharmacology. 1987;93:374–381. doi: 10.1007/BF00187260. [DOI] [PubMed] [Google Scholar]
- McLaughlin PJ, Winston K, Swezey L, Wisniecki A, Aberman J, Tardif DJ, Betz AJ, Ishiwari K, Makriyannis A, Salamone JD. The cannabinoid CB1 antagonists SR 141716A and AM 251 suppress food intake and food-reinforced behavior in a variety of tasks in rats. Behav Pharmacol. 2003;14:583–588. doi: 10.1097/00008877-200312000-00002. [DOI] [PubMed] [Google Scholar]
- McLaughlin PJ, Winston KM, Limebeer CL, Parker LA, Makriyannis A, Salamone JD. The cannabinoid CB1 antagonist AM251 produces food avoidance and behaviors associated with nausea but does not impair feeding efficiency in rats. Psychopharmacology. 2005;180:286–293. doi: 10.1007/s00213-005-2171-0. [DOI] [PubMed] [Google Scholar]
- McLaughlin PJ, Qian L, Wood JT, Wisniecki A, Winston KM, Swezey LA, Makriyannis A, Salamone JD. Suppression of food intake and food-reinforced behavior produced by the novel CB1 receptor antagonist/inverse agonist AM1387. Pharmacol Biochem Behav. 2006;83:396–402. doi: 10.1016/j.pbb.2006.02.022. [DOI] [PubMed] [Google Scholar]
- Melis T, Succu S, Sanna F, Boi A, Argiolas A, Melis MR. The cannabinoid antagonist SR 141716A (Rimonabant) reduces the increase of extra-cellular dopamine release in the rat nucleus accumbens induced by a novel high palatable food. Neurosci Lett. 2007;419:231–235. doi: 10.1016/j.neulet.2007.04.012. [DOI] [PubMed] [Google Scholar]
- Nowend KL, Arizzi M, Carlson BB, Salamone JD. D1 or D2 antagonism in nucleus accumbens core or dorsomedial shell suppresses lever pressing for food but leads to compensatory increases in chow consumption. Pharmacol Biochem Behav. 2001;69:373–382. doi: 10.1016/s0091-3057(01)00524-x. [DOI] [PubMed] [Google Scholar]
- Pi-Sunyer FX, Aronne LJ, Heshmati HM, Devin J, Rosenstock J RIO-North America Study Group. Effect of rimonabant, a cannabinoid-1 receptor blocker, on weight and cardiometabolic risk factors in overweight or obese patients: RIO-North America: a randomized controlled trial. JAMA. 2006;295:761–775. doi: 10.1001/jama.295.7.761. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Rolls BJ, Kelly PH, Shaw SG, Wood RJ, Dale R. The relative attenuation of self-stimulation, eating and drinking produced by dopamine-receptor blockade. Psychopharmacologia. 1974;38:219–230. doi: 10.1007/BF00421374. [DOI] [PubMed] [Google Scholar]
- Rusk IN, Cooper SJ. Parametric studies of selective D1 and D2 antagonists: effects on appetitive and feeding behavior. Behav Pharmacol. 1994;5:615–622. doi: 10.1097/00008877-199410000-00007. [DOI] [PubMed] [Google Scholar]
- Salamone JD. Different effects of haloperidol and extinction on instrumental behaviors. Psychopharmacology. 1986;88:18–23. doi: 10.1007/BF00310507. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Correa M. Motivational views of reinforcement: implications for understanding the behavioral functions of nucleus accumbens dopamine. Behav Brain Res. 2002;137:3–25. doi: 10.1016/s0166-4328(02)00282-6. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Zigmond MJ, Stricker EM. Characterization of the impaired feeding behavior in rats given haloperidol or dopamine-depleting brain lesions. Neuroscience. 1990;39:17–24. doi: 10.1016/0306-4522(90)90218-s. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Steinpreis RE, McCullough LD, Smith P, Grebel D, Mahan K. Haloperidol and nucleus accumbens dopamine depletion suppress lever pressing for food but increase free food consumption in a novel food-choice procedure. Psychopharmacology. 1991;104:515–521. doi: 10.1007/BF02245659. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Mahan K, Rogers S. Ventrolateral striatal dopamine depletions impair feeding and food handling in rats. Pharmacol Biochem Behav. 1993;44:605–610. doi: 10.1016/0091-3057(93)90174-r. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Cousins MS, Bucher S. Anhedonia or anergia? Effects of haloperidol and nucleus accumbens dopamine depletion on instrumental response selection in a T-maze cost/benefit procedure. Behav Brain Res. 1994;65:221–229. doi: 10.1016/0166-4328(94)90108-2. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Cousins MS, Maio C, Champion M, Turski T, Kovach J. Different behavioral effects of haloperidol, clozapine and thioridazine in an instrumental lever pressing/feeding procedure. Psychopharmacology. 1996;125:105–112. doi: 10.1007/BF02249408. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Cousins MS, Snyder BJ. Behavioral functions of nucleus accumbens dopamine: empirical and conceptual problems with the anhedonia hypothesis. Neurosci Biobehav Rev. 1997;21:341–359. doi: 10.1016/s0149-7634(96)00017-6. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Aberman JE, Sokolowski JD, Cousins MS. Nucleus accumbens dopamine and rate of responding: neuro-chemical and behavioral studies. Psychobiology. 1999;27:236–247. [Google Scholar]
- Salamone JD, Arizzi M, Sandoval MD, Cervone KM, Aberman JE. Dopamine antagonsts alter response allocation but do not suppress appetite for food in rats: contrast between the effects of SKF 83566, raclopride and fenfluramine on a concurrent choice task. Psychopharmacology. 2002;160:371–380. doi: 10.1007/s00213-001-0994-x. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Correa M, Mingote S, Weber SM. Nucleus accumbens dopamine and the regulation of effort in food-seeking behavior: implications for studies of natural motivation, psychiatry, and drug abuse. J Pharmacol Exp Ther. 2003;305:1–8. doi: 10.1124/jpet.102.035063. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Correa M, Mingote SM, Weber SM. Beyond the reward hypothesis: alternative functions of nucleus accumbens dopamine. Curr Opin Pharmacol. 2005;5:34–41. doi: 10.1016/j.coph.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Correa M, Mingote SM, Weber SM, Farrar AM. Nucleus accumbens dopamine and the forebrain circuitry involved in behavioral activation and effort-related decision making: implications of understanding anergia and psychomotor slowing and depression. Curr Psychiatr Rev. 2006;2:267–280. [Google Scholar]
- Salamone JD, McLaughlin PJ, Sink K, Makriyannis A, Parker LA. Cannabinoid CB(1) receptor inverse agonists and neutral antagonists: effects on food intake, food-reinforced behavior and food aversions. Physiol Behav. 2007a;91:383–388. doi: 10.1016/j.physbeh.2007.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamone JD, Correa M, Farrar A, Mingote SM. Effort-related functions of nucleus accumbens dopamine and associated forebrain circuits. Psychopharmacology. 2007b;191:461–482. doi: 10.1007/s00213-006-0668-9. [DOI] [PubMed] [Google Scholar]
- Simiand J, Keane M, Keane PE, Soubrie P. SR141716, a CB1 cannabinoid receptor antagonist, selectively reduces sweet food intake in marmoset. Behav Pharmacol. 1998;9:179–181. [PubMed] [Google Scholar]
- Sink KS, McLaughlin PJ, Wood JA, Brown C, Fan P, Vemuri VK, Pang Y, Olzewska T, Thakur GA, Makriyannis A, Parker LA, Salamone JD. The novel cannabinoid CB(1) receptor neutral antagonist AM4113 suppresses food intake and food-reinforced behavior but does not induce signs of nausea in rats. Neuropsychopharmacology. 2007 doi: 10.1038/sj.npp.1301476. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolowski JD, Salamone JD. The role of nucleus accumbens dopamine in lever pressing and response allocation: effects of 6-OHDA injected into core and dorsomedial shell. Pharmacol Biochem Behav. 1998;59:557–566. doi: 10.1016/s0091-3057(97)00544-3. [DOI] [PubMed] [Google Scholar]
- Soria-Gomez E, Matias I, Rueda-Orozco PE, Cisneros M, Petrosino S, Navarro L, Di Marzo V, Prospero-Garcia O. Pharmacological enhancement of the endocannabinoid system in the nucleusaccumbens shell stimulates food intake and increases c-Fos expression in the hypothalamus. Br J Pharmacol. 2007;151(7):1109–1116. doi: 10.1038/sj.bjp.0707313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallett AJ, Blundell JE, Rodgers RJ. Grooming, scratching and feeding: role of response competition in acute anorectic response to rimonabant in male rats. Psychopharmacology. 2007;195(1):27–39. doi: 10.1007/s00213-007-0880-2. [DOI] [PubMed] [Google Scholar]
- Tanda G, Pontieri FE, Di Chiara G. Cannabinoid and heroin activation of mesolimbic dopamine transmission by a common μ1 opioid receptor mechanism. Science. 1997;276:2048–2050. doi: 10.1126/science.276.5321.2048. [DOI] [PubMed] [Google Scholar]
- Thornton-Jones ZD, Vickers SP, Clifton PG. The cannabinoid CB1 receptor antagonist SR141716A reduces appetitive and consummatory responses for food. Psychopharmacology. 2005;179:452–460. doi: 10.1007/s00213-004-2047-8. [DOI] [PubMed] [Google Scholar]
- Treit D, Berridge KC. A comparison of benzodiazepine, serotonin, and dopamine agents in taste-reactivity paradigm. Pharmacol Biochem Behav. 1990;37:451–456. doi: 10.1016/0091-3057(90)90011-6. [DOI] [PubMed] [Google Scholar]
- Ungerstedt U. Adipsia and aphagia after 6-hydroxydopamine induced degeneration of the nigrostriatal dopamine system. Acta Physiol Scand Suppl. 1971;367:95–122. doi: 10.1111/j.1365-201x.1971.tb11001.x. [DOI] [PubMed] [Google Scholar]
- Ward SJ, Dykstra LA. The role of CB1 receptors in sweet versus fat reinforcement: effect of CB1 receptor deletion, CB1 receptor antagonism (SR141716A) and CB1 receptor agonism (CP-55940) Behav Pharmacol. 2005;16:381–388. doi: 10.1097/00008877-200509000-00010. [DOI] [PubMed] [Google Scholar]
- Wenger T, Moldrich G, Furst S. Neuromorphological background of cannabis addiction. Brain Res Bull. 2003;61:125–128. doi: 10.1016/s0361-9230(03)00081-9. [DOI] [PubMed] [Google Scholar]
- Williams CM, Kirkham TC. Anandamide induces overeating: mediation by central cannabinoid (CB1) receptors. Psychopharmacology. 1999;143:315–317. doi: 10.1007/s002130050953. [DOI] [PubMed] [Google Scholar]