Abstract
Our work characterizes the effects of opiate (morphine) dependence on auditory brainstem and visual evoked responses in a rhesus macaque model of neuro-AIDS utilizing a chronic continuous drug delivery paradigm. The goal of this study was to clarify whether morphine is protective, or if it exacerbates simian immunodeficiency virus (SIV) related systemic and neurological disease. Our model employs a macrophage tropic CD4/CCR5 co-receptor virus, SIVmac239 (R71/E17), which crosses the blood brain barrier shortly after inoculation and closely mimics the natural disease course of human immunodeficiency virus (HIV) infection. The cohort was divided into 3 groups: morphine only, SIV only, and SIV + morphine. Evoked potential (EP) abnormalities in sub-clinically infected macaques were evident as early as eight weeks post-inoculation. Prolongations in EP latencies were observed in SIV-infected macaques across all modalities. Animals with the highest CSF viral loads and clinical disease showed more abnormalities than those with sub-clinical disease, confirming our previous work (Raymond et al, 1998, 1999, 2000). Although some differences were observed in auditory and visual evoked potentials in morphine treated compared to untreated SIV-infected animals, the effects were relatively small and not consistent across evoked potential type. However, morphine treated animals with subclinical disease had a clear tendency toward higher virus loads in peripheral and CNS tissues (Marcario et al., 2008) suggesting that if had been possible to follow all animals to end-stage disease, a clearer pattern of evoked potential abnormality might have emerged.
Keywords: SIV, monkey, morphine, evoked potentials, opiates, neuro-AIDS
Introduction
It has been established that HIV can infect the central nervous system and lead to HIV-1-associated motor/cognitive disorder and AIDS dementia complex, but the causes of these deficits are poorly understood (Bacellar et al, 1994; Ciardi et al, 1990; Diesing et al, 2002; Everall, et al, 1993; Glass et al, 1993; Kanzer, 1990; Lipton and Gendelman 1995; McArthur et al, 1993, McArthur et al, 1999; Petito et al, 1986; Price et al, 1988). Combined HIV infection and opiate dependence is a common problem, with estimates that drug abuse may be the second leading method of HIV transmission in the United States (Gayle, 2000). Thus, the interaction of the two leads to questions concerning the effects of drugs of abuse on disease severity and rate of progression (Ansari, 2004; Donahoe, 2004; Kapadia et al, 2005). Morphine has been found to influence both innate and adaptive immunity (Roy and Loh, 1996; Sharp et al, 1998) via opioid receptors on immune cells (McCarthy et al, 2001). Electrophysiological studies investigating the influence of morphine on HIV-related neuropathology are limited; however, a feline model utilizing both multiple acute and escalating dose (followed by withdrawal) drug delivery paradigms suggests a protective role of morphine on feline immunodeficiency virus (FIV)-related auditory brainstem response (ABR) abnormalities (Barr et al, 2000; Barr et al, 2003). In-vitro and in-vivo studies of the effects of opiates on AIDS severity and rate of progression have been disparate (Chuang et al, 1993; Donahoe et al, 1993; Donahoe, 2004; Kumar et al, 2006; Marcario et al, 2008; Suzuki et al, 2002). The results of various epidemiological studies vary with some showing that opiates may have a protective role (Spijkerman et al, 1995; Spijkerman et al, 1996a), others showing a deleterious role (Bouwman et al, 1998; Hutchinson et al, 1997; Krol et al, 1999), and still others finding no effect (Pezzotti et al, 1999; Prins and Veugelers, 1997; Thorpe et al, 2004) on HIV-related pathology and/or mortality rates. Unfortunately, human studies are often confounded by differences in drug abuse pattern and duration, polydrug use, as well as widespread implementation of antiviral therapies (Everall, 2004; Kapadia et al, 2005). However, a recent study by Thorpe et al (2004), based on a large population of HIV-infected women who were either “hard-drug” users or not, is significant in that it was possible to achieve better control of multiple variables. The authors concluded that “hard-drug” use had no effect on CD4 cell percentage or HIV RNA level in support of most prior epidemiological evidence. Recently, we also reported that morphine had no significant effect on viremia, CSF viral titers or survival in SIV-infected macaques over the time course of the study (Marcario et al, 2008). However, morphine was associated with a tendency for greater build-up of virus in the brains of infected animals.
Simian immunodeficiency viruses (SIV) provide excellent models for studying HIV-related neuropathology over a compressed disease time course. The bone marrow passaged macrophage tropic CD4/CCR5 coreceptor virus, SIVmac239 (R71/E17), used in this study is biologically similar to the HIV phenotype (R5 HIV) which commonly infects patients since both utilize the CCR5 receptor (R5 virus) for entry into macrophages (Alkhatib et al, 1996; Chen et al, 1997; Deng et al, 1996; Dragic et al, 1996; Kirchhoff et al, 1997; Marcario et al, 2008; Marx and Chen, 1998). The SIVmac239 (R71/E17) virus has been shown to cross the blood brain barrier shortly after inoculation (Sharma et al, 1992; Narayan et al, 1997) and productively replicates primarily in memory CD4+ T cells in the gut-associated lymphoid tissue (GALT) and spleen (Veazey et al, 1998; Veazey et al, 2000a, b). Our neuropathological and stereological studies, utilizing a cohort of SIVmacR71/17E inoculated Indian origin rhesus macaques have demonstrated many of the features of HIV-1 encephalopathy (Raghavan, et al, 1999; Marcario et al, 2004) as well as a significant loss of neurons in the lateral geniculate nucleus (Berman et al, 1998), the globus pallidus, and the substantia nigra (Marcario et al, 2004). Thus, our model not only closely mimics the natural disease course of HIV infection but also demonstrates many of the hallmarks associated with HIV-related neurological disease.
SIV-infected animals exhibit altered functional integrity of motor and sensory pathways as well as impaired behavioral performance with the progression of disease (Gold et al, 1998; Horn et al, 1998; Marcario et al, 1999a, b; Murray et al, 1992; Prospero-Garcia et al, 1996; Raymond et al, 1998; Raymond et al, 1999; Raymond et al, 2000; Weed et al, 2004). In previous studies using a cohort of SIVmacR71/17E inoculated rhesus macaques, we demonstrated that ABR and VEP peak latency shifts generally correlate with underlying neuropathology at the level of the auditory brainstem nuclei and visual pathway structures (Raymond et al, 1998; Raymond et al, 2000). ABR studies, utilizing SIV-infected monkeys and HIV-positive patients, suggest greater dysfunction in rostral regions of the auditory brainstem pathway with disease progression (Castello et al, 1998; Hausler et al, 1991; Horn et al, 1998; Pagano et al, 1992; Prospero-Garcia et al, 1996; Raymond et al, 1998). VEP abnormalities have been found to be related to retinopathy, optic nerve axonal pathology, and retrochiasmic pathway and primary visual cortex dysfunction (Mahadevan et al, 2006). ABR and VEP abnormalities in HIV-positive patients are well documented and provide a valuable tool in the assessment of peripheral and central nervous system (CNS) dysfunction in both symptomatic and asymptomatic seropositive patients (Castello et al, 1998; Farnarier et al, 1990; Iragui et al, 1996; Koralnik et al, 1990; Mahadevan et al, 2006; Malessa et al, 1995; Mwanza et al, 2004; Pierelli et al, 1993). Hence, electrophysiological monitoring provides an excellent indicator of early and late central and peripheral functional abnormalities and represents an effective method for assessing the influence of drugs of abuse on physiological integrity.
The goal of the present study was to characterize the electrophysiological consequences of SIV infection combined with opiate (morphine) dependence in a nonhuman primate model of neuro-AIDS.
Material and Methods
Subjects
Our cohort consisted of sixteen, 6- to 7-year-old, male Indian origin rhesus macaques (Macaca mulatta) which were SIV and herpes B virus negative. Animals were individually caged and housed in an AAALAC-accredited facility which was maintained at 25.0°C. Random assignment was employed to divide the cohort into three groups: morphine only (Group M, n = 5), SIV only (Group V, n = 5), and SIV + morphine (Group VM, n = 6). ABRs and VEPs were recorded during the control (35 weeks), morphine dependency (26 weeks) and post-inoculation period (33 weeks). Based on the type of disease progression animals were classified as clinical (animals that had to be euthanized before the end of the study due to the presence of serious clinical symptoms, n = 4) or sub-clinical (animals that were terminated at the end of the study and lacked serious clinical symptoms, n = 7). Table 1 summarizes the experimental groups (M, V, VM), periods (control, morphine dependency, post-inoculation) and procedures. All experimental procedures were in compliance with those outlined in the Guide for the Care and Use of Laboratory Animals.
Table 1.
Experimental groups, periods and procedures
| Group | Control period | Morphine dependency period (2.5mg/kg i.m. every 6h, daily) | Postinoculation period |
|---|---|---|---|
| VM (n=6) | Control data | Morphine only | SIV + morphine |
| V (n = 5) | Control data | Sham saline injections | SIV only |
| M (n = 5) | Control data | Morphine only | Morphine only |
| Time (weeks) | 35 | 26 | 33 |
| # EP recording sessions | 3 | 2 | 8 |
| # Virological samples | 0 | 0 | 14 |
VM SIV + morphine, V SIV only, M morphine only, EP evoked potential
Drug delivery paradigm
This study utilized a continuous, chronic (59 weeks) model of morphine dependence. Initially, animals were acclimated to the intramuscular (i.m.) hindlimb injection protocol by first giving sterile saline injections. All animals were behaviorally trained and tested in another part of the protocol. To minimize interference with behavioral performance, the morphine dosage was gradually increased from 1 mg/kg/injection in week 1 to 2.5 mg/kg/injection in week 17. Following the gradual ramp-up, the 2.5 mg/kg/injection dose was maintained for the duration of the experiment. Sham saline injections of equivalent volume were given to Group V subjects. All animals were injected every 6 hours (6:00 AM, 12:00 PM, 6:00 PM and 12 AM), 7 days a week. The total final daily dose was 10 mg/kg. To minimize tissue damage and reduce the potential for infection, injections were alternated between the two legs and hair at the site of injection was regularly shaved.
Viral inoculation
Animals in Group VM (SIV + Morphine) and Group V (SIV Only) were inoculated via femoral bone marrow injection with neurovirulent SIVmac(R71/17E) provided by Dr. O. Narayan. Each animal in Group VM and Group V received 0.5 ml of each viral homogenate (R71 and 17E) for a total volume of 1 ml that contained approximately 1000 TCID50. Animals in Group M were sham inoculated via femoral bone marrow saline injection.
Electrophysiological testing procedures
Following an initial injection of atropine (0.04 mg/kg i.m.) and ketamine (10 mg/kg i.m.), anesthesia was maintained with subsequent doses of ketamine at 20 – 30 min intervals. We have confirmed what others have reported, that the latency of EPs does not vary with the depth of ketamine anesthesia in the dose range we used (Ghaly et al, 1990; Ghaly et al, 2001). Electroencephalograms and EPs were recorded via subdermal platinum needle electrodes (Grass™ model F-E2; West Warwick, RI). The right shoulder served as the noncephalic ground. Animals were placed lying prone inside a Faraday cage. Body temperature was measured every 30 minutes with an infrared tympanic thermometer. Temperature was maintained with a heating pad throughout testing. A custom Windows™ compatible software program (Neural Averager, Larry Shupe, Seattle, WA) written for Cambridge Electronic Design (CED 1401; Cambridge, England) hardware was used for data acquisition and averaging. All averaged records were saved for later off-line analysis. During each EP recording session, all EPs were repeated twice to confirm reproducibility.
Auditory brainstem responses
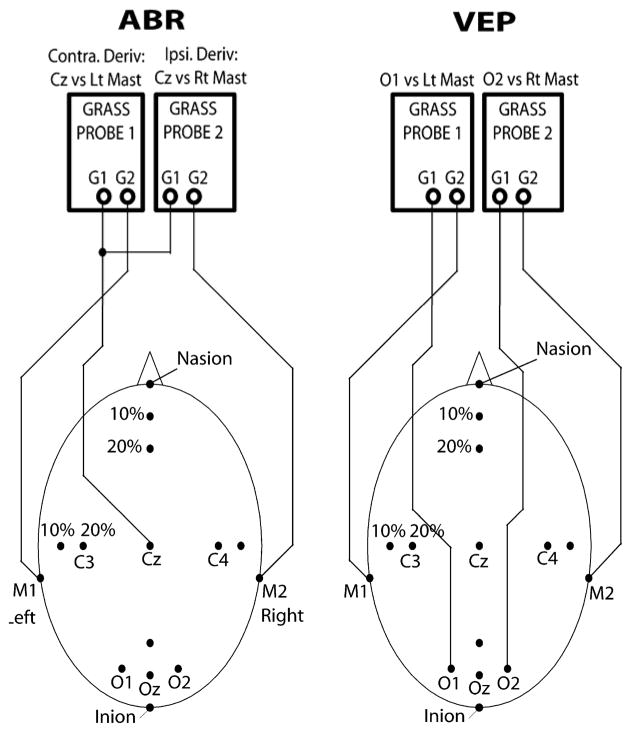
ABRs were recorded using Grass™ AC amplifiers (Model P511 and HIP5 high impedance preamplifiers; West Warwick, RI) with low and high filter settings of 100 Hz and 3 KHz respectively and a gain of 200K. A Grass™ auditory click stimulator (Model S10CTCMA; West Warwick, RI) was used to generate condensation polarity clicks (100-μs square wave pulses) at a frequency of 11.3 Hz. Clicks were presented monaurally (right ear only) via insert earphones (E-A-RTone™ tubephone Model 3A; Cabot Safety Corporation, Indianapolis, IN) at 80 dB sound pressure level. A total of 2000 stimulus repetitions were averaged and recorded utilizing the Neural Averager software. The vertex (Cz) served as the active (+) recording electrode site. Reference electrodes (−) were placed at the right mastoid (M2) and left mastoid (M1). A schematic of the ABR electrode montage is provided in Figure 1.
Figure 1.
Auditory brainstem response (ABR) and visual evoked potential (VEP) electrode montage. ABRs were recorded with the active (+) recording electrode at the vertex (Cz). VEPs were recorded with the active (+) electrodes placed at O1 and O2 overlying the left and right occipital cortices, respectively. For both ABRs and VEPs, reference electrodes (−) were placed at the right mastoid (M2) and left mastoid (M1).
Visual evoked potentials
VEPs were recorded using Grass™ AC amplifiers (Model P511 and HIP5 high impedance preamplifiers) with low and high filter settings of 1 Hz and 100 Hz respectively and a gain of 50K. A Grass™ photic stimulator (Model PS33; West Warwick, RI) with an illumination intensity setting of 16 was placed 45 cm from the animal’s head. The animal’s eyelids were retracted to achieve full exposure to the pupils. The stimulator generated binocular flash stimuli at a frequency of 2 Hz. A total of 200 stimulus repetitions were averaged and measured utilizing Windows based Neural Averager software (Larry Shupe, Seattle). VEPs were recorded with the active (+) electrodes placed at O1 and O2 overlying the left and right occipital cortices, respectively. Throughout the text, (O1) and (O2) are used to indicate the source of the signal. Placement was approximately 12 mm rostral to the inion and 12 mm lateral to the midline. Reference electrodes (−) were placed at the right mastoid (M2) and left mastoid (M1). A schematic of the VEP electrode montage is provided in Figure 1.
Statistical analysis
ABR and VEP parameters were assessed longitudinally for differences related to group (VM, V and M) and disease progression type (C = clinical, S = sub-clinical). In order to assess the equivalence of the three groups (VM, V and M) prior to initiation of the morphine protocol, we compared the groups based on the final observation taken during the control period using an omnibus ANOVA F-test for each dependent variable. The control period was used as a covariate in order to correct for differences between the groups during the control period. Relevant p-values were calculated using SAS. A type I error rate of 5% was used to determine statistical significance. Table 1 summarizes the experimental groups and periods.
To assess the effect of morphine on SIV-related evoked potential peak latency shifts, we estimated the time slope (i.e., average change in dependent measure per week) for all three groups (V, M, and VM) during the post-inoculation period. This was accomplished using a mixed model for each dependent variable that included time (in weeks), group, and time-by-group interaction as predictors, with the last observation from the morphine dependency period included as a covariate. The model was fit with a first-order autoregressive structure to account for dependencies among the repeated (within-subject) observations. Using the slope estimates generated by this model, we used contrasts to conduct pairwise comparisons between the M and VM groups, V and VM groups, and V and M groups.
To compare disease progression type (C = clinical, S = sub-clinical) means during the post-inoculation period, mixed models including disease progression type (C, S), time (in weeks), and time-by-disease progression type interactions with first-order autoregressive covariance structure were fitted to the ABR and VEP data. The last observation from the morphine dependency period was included as a covariate.
For each dependent variable, we also computed least squares mean estimates of the final and maximum observations for the three groups (V, M, and VM) during the post-inoculation period using a general linear model with group as a predictor. The last observation from the morphine dependency period was included as a covariate. We then used contrasts to conduct pairwise comparisons (VM vs. V, VM vs. M, V vs. M) of these mean estimates. Similarly, for each dependent variable, we also computed least squares mean estimates of the final and maximum observations for the disease progression type (C, S) during the post-inoculation period using a general linear model with disease progression type as a predictor. The last observation from the morphine dependency period was included as a covariate.
In addition to group analysis, we conducted single subject analysis using each monkey as its own control. Deviations in measured latencies that exceeded two standard deviations of the mean during the control period were considered significant (Raymond et al, 1998, 1999, 2000). To obtain a better estimate of the variability, the standard deviation was calculated from the data of all monkeys in a particular group but comparisons were with each monkey’s own control period mean. For the single subject analysis, imputations were performed on absent values. Absent values resulted from instances when EPs were absent (undetectable) due to disease severity. For the imputed analyses, the maximum value for a specific dependent variable across all animals was substituted for absent values.
Results
Disease Profile and onset of conduction delays in relation to disease progression
Based on disease profile, macaques were classified as either clinical (animals that had to be euthanized before the end of the study due to disabling clinical symptoms) or sub-clinical (animals that lacked disabling clinical symptoms and were terminated at the end of the study). Table 2 summarizes the time course of the onset of EP abnormalities in individual monkeys, defined as latencies exceeding two standard deviations of the control period mean. Four of eleven inoculated monkeys (98D264, 98C065, 98C001, 98C089) developed clinical disease (two macaques in each of the VM and V groups, Table 2). Clinical signs were evident as early as 13 weeks post-inoculation. Seven of eleven inoculated monkeys exhibited sub-clinical disease and were euthanized at a predetermined date (30 and 31 weeks post-inoculation). There were no statistically significant differences in general survival rate, viremia or CSF viral load between the VM and V groups over the time course of the study (Table 2; Marcario et al, 2008).
Table 2.
Subject disease profile and time course
| Group | Animal | Disease type | Survival time (weeks pi, pm) | Onset ABR abnormality (weeks pi, pm) | Onset VEP abnormality (weeks pi, pm) | Onset clinical symptoms (weeks pi, pm) | Terminal virus load plasma (copies/mL) | Terminal virus load CSF (copies/mL) | Symptoms |
|---|---|---|---|---|---|---|---|---|---|
| VM (n=6) | 98C053 | S | 31, 57 | – | – | – | 9, 800 | 17, 900 | – |
| 98D264 | C | 16, 42 | 12, 38 | 8, 34 | 13, 39 | 43,151,780 | 25,385,640 | Severe tremor and ataxia | |
| 98C065 | C | 15, 41 | 12, 38 | 15, 41 | 13, 38 | 49,258,480 | 25,138,180 | Severe tremor and ataxia | |
| 98D276 | S | 31, 57 | – | – | – | 6,444,920 | 112,920 | – | |
| 98C095 | S | 30, 56 | 21, 47 | 30, 56 | 24, 50 | 73,972,400 | 82,550 | Mild weight and appetite loss | |
| 98C088 | S | 31, 57 | 8, 34 | – | – | 37,747,380 | 98,540 | – | |
| V (n=5) | 98D388 | S | 31, NA | 8, NA | 8, NA | – | 11,240 | 7,080 | – |
| 98C001 | C | 29, NA | 12, NA | 16, NA | 54,186,180 | 138,928,140 | Untreatable diarrhea and severe weight loss | ||
| 98C089 | C | 13, NA | 12, NA | 4, NA | 13, NA | 30,618,660 | 175,497,620 | Acute seizures. paralysis | |
| 98D321 | S | 30, NA | 12, NA | 12, NA | 25, NA | 17,828,120 | 22,200 | Mild tremor | |
| 98C025 | S | 31, NA | – | – | – | 27,318,940 | 272,820 | – | |
| M (n=5) | 98C067 | NA | NA, 59 | – | – | NA, 44 | NA | NA | Mild tremor |
| 98C070 | NA | NA, 34 | – | – | NA, 24 | NA | NA | Leg infection at injection site | |
| 98C097 | NA | NA, 59 | – | – | NA, 59 | NA | NA | Mild tremor | |
| 98C110 | NA | NA, 34 | – | – | NA, 18 | NA | NA | Leg infection at injection site | |
| 98C042 | NA | NA, 59 | – | – | – | NA | NA | – |
ABR latency changes
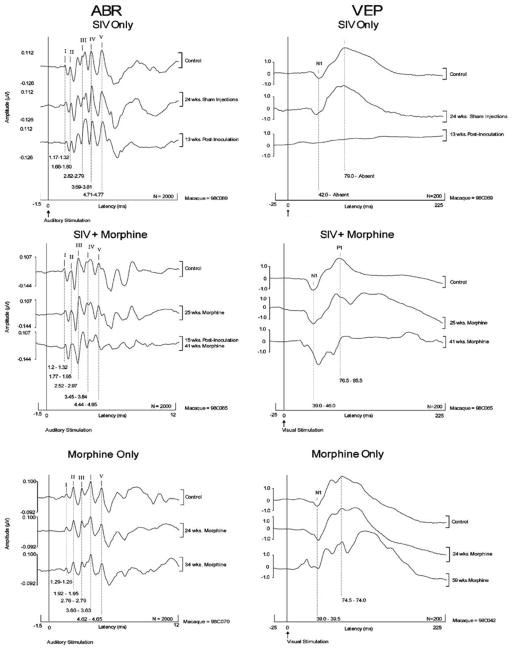
The present study demonstrates that significant post-inoculation increases occurred in ABR parameters confirming our earlier work (Raymond et al, 1998). Figure 2 provides representative examples of serial ABRs recorded at similar time points during the control and morphine dependency periods. The final recording was just prior to necropsy. As observed in human ABR recordings, within monkey ABR waveform morphology and peak latencies are highly stable and reproducible over time, although there is significant across animal variability. As shown in the examples of Figure 2, significant peak latency and interpeak interval (IPI) shifts did not occur during the morphine dependency period. Of particular interest are the post-inoculation latency shifts in ABR peaks II and IV in macaque 98C089 (group V) and peaks III, IV, and V in macaque 98C065 (group VM). Both of these animals had clinical disease. Prolongations in peak latencies have been related to delays in conduction velocity associated with demyelination.
Figure 2.
Representative examples of serial ABRs and VEPs recorded at similar time points during the control and morphine dependency periods. The final recording was just prior to necropsy. Note the post-inoculation ABR latency increases of peaks III-V in macaque 98C065 (group VM, animal with clinical disease) and peaks II and IV in macaque 98C089 (group V, animal with clinical disease). Note also the complete loss of the VEP associated with end-stage disease in macaque 98C089 (group V, animal with clinical disease) and the significant end-stage increase in the VEP N1 peak in macaque 98C065 (group VM, animal with clinical disease).
The results of the current study confirm that ABR testing can be a valuable tool in assessing the influence of drugs of abuse on physiological integrity. Table 2 shows that ABR abnormalities occurred prior to the onset of clinical symptoms in three animals with clinical disease [98D264 (group VM), 98C065 (group VM), 98C089 (group V)] and two animals with sub-clinical disease [98C095 (group VM), 98D321 (group V)]. A total of four animals with subclinical disease exhibited ABR abnormalities, further emphasizing the value of evoked potential studies for the early detection of functional abnormalities. Increases in peak latency in the subclinical animals typically corresponded with peaks in CSF and/or plasma viral loads. Our findings indicate that animals with clinical disease showed ABR abnormalities as early as 12 weeks post-inoculation. Although the onset of ABR abnormalities varied between animals, at end-stage, most of the animals with clinical disease exhibited increases greater than two standard deviations above the control mean in at least one ABR parameter (Table 2). It is worth noting that three macaques with sub-clinical infections did not show statistically significant increases in ABR latencies throughout the course of the study.
Table 3 provides a within period (morphine dependency, post-inoculation) single subject analysis of the number of monkeys which exhibited latencies or IPIs greater than 2 SDs above the control period mean. No ABR abnormalities occurred during the morphine dependency period in macaques in either the VM, V or M group (Table 3). Table 3 also shows that the majority of increases in post-inoculation peak latencies occurred in the later peaks (i.e., peaks III-V). Additional significant findings were the changes in interpeak intervals: 1) I-III (3 VM and 1 V macaque), 2) III-V (2 VM macaques), and 3) I-V (2 VM macaques). IPI increases occurred more frequently in animals in the VM group. The presence of peak III-V latency shifts and III-V IPI shifts suggest greater dysfunction in rostral (pons and midbrain) regions of the auditory brainstem pathway with disease progression. Although our descriptive statistics (Table 3) suggest greater abnormalities in the VM macaques compared to V macaques, our group analyses revealed no statistically significant group differences between: VM & V, VM & M, and V & M. However, statistically significant group differences did occur between the clinical and sub-clinical macaques. Specifically, animals with clinical disease showed greater ABR abnormalities in peak III (p = 0.0110) than animals with sub-clinical disease confirming our previous findings (Raymond et al, 1998).
Table 3.
Single subject statistical analysis of ABR and VEP parameters
| Modality | Parameter | Number of monkeys with EP latencies exceeding 2 SDs of that monkey’s control period mean
|
|||||
|---|---|---|---|---|---|---|---|
| Group VM period
|
Group V period
|
Group M period
|
|||||
| Morphine | Postinoc | Morphine | Postinoc | Morphine | Postmorph | ||
| ABR latency (ms) | Peak I | – | – | – | – | – | – |
| Peak II | – | – | – | 3 | – | – | |
| Peak III | – | 4 | – | 1 | – | – | |
| Peak IV | – | 3 | – | 2 | – | – | |
| Peak V | – | 3 | – | – | – | – | |
| I–III IPI | – | 3 | – | 1 | – | – | |
| III–V IPI | – | 2 | – | – | – | – | |
| I–V IPI | – | 2 | – | – | – | – | |
| VEP latency (ms) | N1 (O1) | – | 1 | – | 1 | – | – |
| P1 (O1) | – | 3 | – | 4 | – | – | |
| N1 (O2) | – | 1 | – | 2 | – | – | |
| P1 (O2) | – | 2 | – | 4 | – | – | |
VEP latency changes
As shown in Table 2, VEP abnormalities occurred 4–9 weeks earlier than the onset of clinical symptoms in three out of four animals with clinical disease [98D264 (group VM), 98C001 (group V), 98C089 (group V)]. In fact, one animal with clinical disease (98C089) showed VEP abnormalities as early as 4 weeks post-inoculation. While the onset of VEP abnormalities varied between animals, at end-stage, all of the animals with clinical disease exhibited increases greater than 2 SDs above the control mean in at least one VEP parameter (Table 2).
Our flash-evoked VEPs were less reproducible than the ABRs. Nevertheless, it was clear that some end-stage VEP deficits were more severe than ABR abnormalities. This is illustrated in Figure 2 which shows the complete loss of the VEP associated with end-stage disease (98C089, group V, animal with clinical disease). This observation is consistent with our previous study in which two rapid progressors exhibited undetectable VEP peaks at end-stage disease (Raymond et al, 2000). Note also the significant end-stage increase in the N1 peak in macaque 98C065 (group VM, animal with clinical disease). No significant change in VEP parameters was evident in the morphine only animal illustrated in Figure 2 (macaque 98C042).
Table 3 provides single subject, within period (morphine dependency, post-inoculation) analysis of the number of animals which exhibited latency increases greater than 2 SDs above the control period mean. None of the animals in any group exhibited latencies greater than 2 SDs during the morphine dependency period (pre-inoculation). During the post-inoculation period, 11 VEP abnormalities were found in the V group compared to 7 in the VM group. No abnormalities were found in the M group of animals during the post-inoculation period.
Our group analysis revealed significant findings (Table 4). First, VM macaques showed greater abnormalities in P1 (O1) (p = 0.0383) compared to V group animals. Our group analyses also showed significant group abnormalities in VM macaques compared to M macaques for the following parameters: P1 (O1), P1 (O2) and N1 (O2). No statistically significant group differences were obtained between the clinical and sub-clinical macaques for any of the VEP parameters.
Table 4.
Group statistical analysis of VEP parameters
| Modality3 | Parameter | Group differences (pairwise differences p values)
|
Directionality of group differences | |||
|---|---|---|---|---|---|---|
| VM and V | VM and M | V and M | C and S | |||
| VEP | N1 (O1) | – | – | – | – | – |
| P1 (O1) | 0.0383 | 0.0068 | – | – | VM > V, VM > M | |
| N1 (O2) | – | 0.0164 | – | – | VM> M | |
| P1 (O2) | – | 0.0010 | – | – | VM> M | |
Discussion
We assessed the influence of well maintained, chronic (59 weeks) morphine dependence (2.5 mg/kg i.m., every 6 hours, 7 days a week) on ABRs and VEPs in a cohort of sixteen Indian origin rhesus macaques which were divided into three groups: 1) Morphine only (M), 2) SIV only (V), and 3) SIV + Morphine (VM). Prolongations in ABR and VEP latencies were observed in SIV-infected animals with and without morphine dependence confirming our previous work (Raymond et al 1998, 1999, 2000). Although more ABR abnormalities were found in group VM than group V (17 vs 7, Table 3), it is important to note that a similar fraction of monkeys in each group had abnormalities (4/6 for VM vs 3/5 for V, Table 2) and, hence, it is difficult to conclude that morphine significantly increased incidence compared to virus alone. The data on VEPs showed more abnormalities in the V group than the VM group (11 vs 7, Table 3) and also a greater fraction of monkeys in the V group had abnormalities than in the VM group (4/5 for V vs 3/6 for VM, Table 2), although the numbers are small. Also, the animal that showed complete loss of the VEPs was in the V group (Figure 2). These results might actually be used to argue in favor of a protective effect of morphine but a conservative interpretation would be that morphine clearly did not increase the number of SIV-infected animals with VEP abnormalities of the time period of this study.
Our group statistical analysis failed to reveal many significant differences. Nevertheless, one noteworthy significant difference was observed. Peak P1 (O1) of the visual evoked potential in the VM group showed a greater increase in latency than the V group. Differences were found between the VM and M groups but, surprisingly, none were found between the V and M groups. A major limitation impeding our group analysis was the relatively small number of monkeys in each group in relation to effect magnitude.
Macaques in both the VM and V groups exhibited greater dysfunction in later peaks of the ABR suggesting dysfunction at the level of the superior olivary nucleus (hypothesized generator of peak III and possibly peak IV), lateral lemniscus tracts and nuclei (hypothesized generator of peak IV and possibly peak V), and/or inferior colliculus (hypothesized generator of peak V) (Buchwald and Huang, 1975; Curio and Oppel, 1988; Hashimoto 1982; Hashimoto et al, 1981; Legatt et al, 1986; Moller and Burgess, 1986; Moller and Jannetta, 1982a, b; Moller et al, 1981a, b; Moller et al, 1994). VEP abnormalities, in some cases including complete loss of the VEP (macaque 98C089), could result from pathophysiology anywhere along the visual pathway. The occipital cortex is the most likely generator for the VEP (Kraut et al, 1985), but loss or slowing of the flash-evoked visual volley at any point along the pathway would affect the potential generated at the cortex.
In agreement with our previous findings (Raymond et al, 1998, 1999, 2000), the time course of the development of overt clinical disease (i.e., clinical vs sub-clinical animals) was an excellent indicator of severity of neural pathology and CSF viral load. Animals which developed clinical disease (4/11 inoculated animals; two animals in each of the VM and V groups) consistently exhibited: 1) more severe sensory electrophysiological abnormalities, and 2) elevated CSF viral RNA copies. In previous work on this same cohort of monkeys, we showed that clinical disease was also associated with higher viral concentrations in the basal ganglia, occipital cortex, parietal cortex, deep white matter, and brainstem (Marcario et al, 2008).
Disease Profile
Four of eleven inoculated monkeys (98D264, 98C065, 98C001, 98C089) developed clinical disease (two macaques in each of the VM and V groups, Table 2). It should be noted that some animals in the morphine only group also developed clinical signs. Mild tremor was present in two animals (macaques 98C067 and 98C097, Table 2). Tremor has been associated with opioid withdrawal in both humans and nonhuman primates (Seevers, 1936). Given the fact that our animals were injected with morphine every 6 hours and did not exhibit signs of withdrawal (i.e., dysphoria, gastrointestinal problems), it is unlikely that the observed tremor was related to fluctuations in plasma opioid levels. Lalley et al (1975) observed that intracaudate injection of morphine in unanesthetized cats produced resting tremor thought to be associated with reduced dopamine function and it is possible that the mild tremor we observed in some monkeys may have had a similar origin.
Necrotizing myositis/dermatitis was present at the site of morphine injection in 2/5 animals (macaques 98C070 and 98C110) in the morphine only group. It is noteworthy that cutaneous complications associated with injection drug use are well documented and include cutaneous infections (Beaufoy 1993; Binswanger et al, 2000; Spijkerman et al, 1996b), necrotizing fasciitis (Callahan et al, 1998; Chen et al, 2001) and necrotizing ulcers (Bruckner et al, 1982; Scott et al, 1997). The possibility of local immune suppression at the site of morphine injections can be raised although it would seem that the VM group monkeys would also be affected. However, the numbers of monkeys in our groups might be too small to draw conclusions. A synergistic effect between cocaine and cutaneous streptococcal infection has been suggested by Hoeger et al (1996). However, the direct cytotoxic effects of opiates must also be considered (Bruckner et al, 1982; Scott et al, 1997).
Virological, immunological and neuropathological correlations
Clinical disease was associated with high CSF viral RNA copies and more severe EP abnormalities. For example, once viral loads exceeded 107 to 108 copies of viral RNA/ml CSF, all macaques with clinical disease showed prolongations in at least one VEP parameter and three out of four macaques with clinical disease (macaques 98D264, 98C065, 98C089) had increases in at least one ABR parameter. Although CSF viral loads were a good indicator of EP abnormalities it should be noted that in macaque 98C095 (group VM, sub-clinical), increases in ABR peaks III-V and I-III IPIs, III-V IPIs, and I-V IPIs as well as VEP P1 (O1) and P1 (O2) corresponded with plasma viral loads exceeding 107 copies of viral RNA/ml plasma while CSF viral loads remained relatively low.
Viral RNA was recovered from plasma and CSF samples of all inoculated macaques (n = 11) indicating productive infection. There was no statistically significant difference in CSF (Wilcoxon-Mann-Whitney test, p = 0.095 to 0.792) and plasma (Wilcoxon-Mann-Whitney test, p = 0.43 to 0.99) viral RNA copies between the V and VM groups at the examined time points throughout the duration of the study (Marcario et al, 2008). However, there was a statistically significant difference in CSF viral RNA copies between macaques with clinical disease versus those with sub-clinical infections (Wilcoxon-Mann-Whitney test, p = 0.006, clinical > sub-clinical) for the average of the final two measurement weeks prior to necropsy. Plasma viral RNA copies were significantly higher in macaques with clinical disease compared to those with sub-clinical infections (Wilcoxon-Mann-Whitney test, p = 0.006 to 0.042) at all examined time points except the average of 3–4 weeks p.i. (p = 0.073) and the average of the final two measurement weeks prior to necropsy (Marcario et al, 2008).
Following inoculation, peripheral blood mononuclear cells from macaques in the V and VM groups were examined for development of cell mediated immunity responses to the virus. We found a morphine-associated loss of ELISPOT responses (Marcario et al, 2008). Specifically, all animals in the V group developed ELISPOT responses; however, none of the animals in the VM group developed any ELISPOT responses during any of the sampling periods. Although there was no statistically significant difference in CSF and plasma viral RNA copies between the V and VM groups, the results suggest that morphine could have a gradual influence on SIV-related pathogenesis. In addition, although there were no statistically significant differences in SIV gag levels between the V and VM groups for the tested peripheral tissues or brain regions, higher tissue viral concentrations were found in sub-clinical VM animals compared to sub-clinical V animals, as 15 of 16 tissue samples in the VM group compared to 4 of 12 tissue samples in the V group exceeded 106 copies of viral RNA. In addition, sub-clinical animals in the VM group exhibited a trend toward higher viral titers in the brain (14 of 20 brain regions had viral titers exceeding 104 copies of viral RNA) than subclinical animals in the V group (4 of 15 brain regions had viral titers exceeding 104 copies of viral RNA). These data, coupled with the morphine associated suppression of cell mediated immune responses (i.e., loss of ELISPOT responses), suggest that, with time, macaques in the VM group with sub-clinical infections might have eventually progressed more rapidly to clinical AIDS than animals in the V group animals.
The macaques with clinical disease (4/11 inoculated animals) exhibited the most severe neuropathological and EP abnormalities. Lentivirus-induced neuropathology included multinucleate giant cell reactions, perivascular mononuclear infiltrates, meningoencephalitis, microglial nodule formation, and radiculoneuritis. Macaques with sub-clinical disease (7/11 inoculated animals) did not exhibit the hallmarks of AIDS-related neuropathology. However, it should be noted that although not a classical lentiviral encephalitis, macaque 98D388 (group V, subclinical) showed a minimal, single site perivascular cuff. In addition, macaque 98C088 (group VM, subclinical) exhibited mild meningitis, specifically the meninges contained a focal site of lymphoid inflammation. Although there were no obvious pathological findings in most of the macaques in the M group, there was a focal site glial nodule composed of macrophages/microglia within the basal ganglia, proximate to the internal capsule in macaque 98C097 (group M).
In a previous study utilizing a cohort of SIVmacR71/17E inoculated macaques, we found inflammatory pathology in animals with clinical disease in the optic nerves and tracts, lateral geniculate bodies, superior colliculi, and calcarine cortices (Raymond et al, 2000). Additional pathology included areas of spongiform vacuolation, neuronal loss, and a reactive gliosis in cortical layers III and IV of the visual cortex (Raymond et al, 2000). We also showed mild to severe degrees of lentivirus-induced auditory pathway pathology found in the cochlear nucleus, lateral lemniscus, olivary complex, inferior colliculus and medial geniculate body (Raymond et al, 1998). Although exceptions exist, our previous work suggests that the degree of lentivirus-induced neuropathology generally correlates with the severity of EP abnormalities (Raymond et al, 1998). Similar pathology can be assumed to have contributed to the increases in ABR peaks III-V and I-III IPIs, III-V IPIs, I-V IPIs, as well as, VEP N1 and P1 latencies observed in the present study.
Comparison to previous studies of evoked potentials
The influence of chronic (59 weeks) morphine dependence on SIV-related auditory neuropathology has to our knowledge never been addressed in primates prior to this study. Studies utilizing a feline model which employed both multiple acute and escalating dose (followed by withdrawal) drug delivery paradigms suggest a protective role of morphine on feline immunodeficiency virus (FIV)-related ABR abnormalities (Barr et al, 2000; Barr et al, 2003). Specifically, Barr et al (2003), using an escalating dose of morphine (1.0 – 2.0 mg/kg/day) followed by withdrawal, found that although significant post-inoculation ABR Peak 4 latency increases occurred in FIV-infected animals without morphine (V group), there was no significant difference in Peak 4 latencies between control animals (saline-treated, uninfected animals) and morphine treated, FIV-infected animals (VM group). Similarly, in a cat study of multiple acute morphine exposures (1 injection of 2.0 mg/kg/day for 2 consecutive days/week), Barr et al (2000) found that significant post-inoculation increases in ABR peak 4 did not occur in morphine treated, FIV-infected animals (VM group) in contrast to FIV-only animals (V group) which developed significant peak 4 increases after 16 weeks post-inoculation.
The above findings are in accord with previous studies utilizing a simian model of chronic (i.e., low-stress) morphine dependence which reported a protective role (lower viral loads, slower disease progression, although no neurophysiological studies) of morphine on SIV-related disease progression (Donahoe et al, 1993; Donahoe, 2004). The nature of the observed neuroprotective influence of morphine could be related to a reduction in elevated excitotoxic extracellular glutamate levels associated with lentivirus (Barr et al, 2000; Lipton, 1998; Manzoni and Williams, 1999; Sepulveda et al, 1998; Thorlin et al, 1998). Although the exact immunoprotective mechanisms of morphine are unknown, the above mentioned studies suggest that morphine dosage as well as the drug delivery paradigm, for example, length of exposure, may influence disease outcome (Barr et al, 2000; Donahoe, 2004; Kapadia et al, 2005).
Possible role of viral strain and morphine drug regimen in simian studies
The SIV model provides an excellent paradigm for studying HIV-related pathogenesis. Previous studies have reported enhanced SIV and/or SHIV replication in morphine-treated macaques (Chuang, 1993; Kumar et al, 2006). Specifically, Kumar et al (2006) inoculated macaques with a combination of SHIVKU, SHIV89.6P and SIV/17E-Fr and reported that morphine treated and virus infected animals exhibited: 1) higher CSF and plasma viral loads, 2) more rapid blood brain barrier viral migration with SHIVKU, and 3) more rapid disease onset. Simian studies suggesting a protective role of morphine inoculated animals with SIVsmm9 and used relatively high doses of morphine (Donahoe et al, 1993; Donahoe, 2004). Incongruent findings regarding morphine’s possible effects may be due in part to differences in viral strain resulting in different CD4 profiles and severity of neurovirulence. Specifically, SIVsmm9 may not be as virulent as viruses used in other studies (Fultz et al, 1986; Suzuki et al, 2002). Morphine dose may also be a factor, although the dosages used in all the monkey studies, including this one, are sufficient to produce clear dependence.
Comparison with epidemiological studies
Epidemiological studies of opiate dependence on HIV disease progression in humans have yielded inconsistent results. Several reports suggest higher rates of HIV encephalitis and microglial activation in heroin addicts (Bell et al, 1998; Davies et al, 1997; Tomlinson et al, 1999) as well as an increased incidence of opportunistic infections in “hard-drug” users (Thorpe et al, 2004). In an effort to dissect the role of HIV infection and drugs of abuse on neuropathology, Bell et al. (2002) compared microglial activation and other parameters in drug users with HIV encephalitis (HIVE), HIV-negative drug users and controls without HIV or drug use. Most noteworthy was the fact that not only did the HIVE- drug using group show increased microglial activation compared to controls, but so did the HIV-negative drug users. An interaction between these separate effects on microglial activation might provide a mechanism for potentiation of disease in drug users. A later study (Arango et al., 2004) comparing HIVE drug users with HIVE non–drug users revealed a trend in the thalamus toward greater microglial activation in the drug users, although this difference did not achieve statistical significance. Multi-drug use characterized the subjects in these studies though most were opiate users.
Both lower or higher mortality rates associated with combined HIV infection and drug use have been reported (Alcabes and Friedland, 1995; Selwyn et al, 1992). However, the majority of epidemiological studies report that opiate use has neither a protective nor a deleterious role in HIV-related disease (Pezzotti et al, 1999; Prins and Veugelers, 1997; Thorpe et al, 2004). The study by Thorpe et al (2004) is particularly significant in that it was based on a large population of HIV-infected women some of whom were hard drug users (including heroin/opiates) that could be compared with a group of non-drug users. Not only were the drug and non-drug groups more comparable than is often the case in epidemiological studies of men, but it was also possible in this study to confirm self reports of drug use with blood samples. The authors conclude that “In this large cohort of HIV-infected women, we found no effect of hard drugs on virological or immunological markers of HIV disease progression, nor did hard-drug using women progress more rapidly to death than nonusers. However, a greater number of hard-drug–using women did develop class C events (AIDS defining, e.g., pneumonia) than non-users, both before enrollment and during the study period.” Overall, our results on ABR and visual evoked potentials provide evidence consistent with these conclusions. However, an important caveat must be raised. Morphine treatment was associated with a tendency for greater build-up of virus in the brains of infected animals suggesting that if we were able to follow these animals for a longer period of time, a clearer picture of morphine related evoked potential abnormalities might have emerged (Marcario et al, 2008).
Conclusions
Increases in EP latencies were observed in SIV-infected macaques across all modalities. Animals with the highest CSF viral loads and clinical disease showed more abnormalities than those with sub-clinical disease, confirming our previous work (Raymond et al, 1998, 1999, 2000). Our evoked potential analysis of morphine’s effects on SIV disease severity and progression yielded mixed results. More ABR abnormalities were observed in the VM compared to the V group (17 vs 7). In contrast, the fraction of animals with ABR abnormalities was similar for both the VM and V groups (4/6 for VM vs 3/5 for V) and the number of animals with VEP abnormalities was either not different or slightly higher for the V group compared to the VM group (11 vs 7). The fraction of animals with VEP abnormalities was also similar given the small sample size (4/5 for V vs 3/6 for VM). Overall, while some differences were observed, they were relatively small and not consistent across evoked potential type. However, we must qualify this by stating that the morphine treated animals did show complete inhibition of ELISPOT responses against the virus and VM animals with subclinical disease also had a tendency toward higher viral titers in peripheral and CNS tissues than untreated animals (Marcario et al., 2008). It is possible that if all animals had been followed to end-stage disease a clearer pattern of greater evoked potential dysfunction in the VM group compared to the V group might have emerged.
Acknowledgments
The authors thank Sarah Karina, Glaukia Cavalcanti and Kip Fogle for their assistance with behavioral training and morphine/saline injections, as well as Heather Hudson and Darcy Griffin for assistance with morphine/saline injections. We also thank Ian Edwards and James Rengel for their expert technical contributions and Drs. Zhuang Li and David Pinson for assistance with necropsies and pathological analyses. This work was supported by NIH grants DA12827, HD02528, and COBRE P20RR16443.
Footnotes
Meeting presentations: Psychoneuroimmunology Research Society, May 2007; USA-Caribbean Conference: HIV/AIDS and Drug Abuse, Dec 2006; Society on NeuroImmune Pharmacology, April 2006 and April 2005; Association for Research in Otolaryngology 29th MidWinter Meeting, Feb. 2006; Society for Neuroscience, Oct. 2004.
References
- Alcabes P, Friedland G. Injection drug use and human immunodeficiency virus infection. Clin Infect Dis. 1995;20:1467–1479. doi: 10.1093/clinids/20.6.1467. Review. [DOI] [PubMed] [Google Scholar]
- Alkhatib G, Combadiere C, Broder CC, Feng Y, Kennedy PE, Murphy PM, Berger EA. CC CKR5: a RANTES, MIP-1alpha, MIP-1beta receptor as a fusion cofactor for macrophage-tropic HIV-1. Science. 1996;272:1955–1958. doi: 10.1126/science.272.5270.1955. [DOI] [PubMed] [Google Scholar]
- Arrango JC, Simmonds P, Brettle RP, Bell JE. Does drug abuse influence the microglial response in AIDS and HIV encephalitis? AIDS. 2004;18 (suppl 1):S69–S74. [PubMed] [Google Scholar]
- Ansari AA. Drugs of abuse and HIV–a perspective. J Neuroimmunol. 2004;147:9–12. doi: 10.1016/j.jneuroim.2003.10.006. [DOI] [PubMed] [Google Scholar]
- Bacellar H, Munoz A, Miller EN, Cohen BA, Besley D, Selnes OA, Becker JT, McArthur JC. Temporal trends in the incidence of HIV-1–related neurologic diseases: multicenter AIDS cohort study, 1985–1992. Neurology. 1994;44:1892–1900. doi: 10.1212/wnl.44.10.1892. [DOI] [PubMed] [Google Scholar]
- Barr MC, Billaud JN, Selway DR, Huitron-Resendiz S, Osborn KG, Henriksen SJ, Phillips TR. Effects of multiple acute morphine exposures on feline immunodeficiency virus disease progression. J Infect Dis. 2000;182:725–732. doi: 10.1086/315789. [DOI] [PubMed] [Google Scholar]
- Barr MC, Huitron-Resendiz S, Sanchez-Alavez M, Henriksen SJ, Phillips TR. Escalating morphine exposures followed by withdrawal in feline immunodeficiency virus-infected cats: a model for HIV infection in chronic opiate abusers. Drug Alcohol Depend. 2003;72:141–149. doi: 10.1016/s0376-8716(03)00195-9. [DOI] [PubMed] [Google Scholar]
- Beaufoy A. Infections in intravenous drug users: a two-year review. Can J Infect Control. 1993;8:7–9. [PubMed] [Google Scholar]
- Bell JE, Brettle RP, Chiswick A, Simmonds P. HIV encephalitis, proviral load and dementia in drug users and homosexuals and AIDS. Effect of neocortical involvement. Brain. 1998;121:2043–2052. doi: 10.1093/brain/121.11.2043. [DOI] [PubMed] [Google Scholar]
- Bell JE, Arango JC, Robertson R, Brettle RP, Leen C, Simmonds P. HIV and drug misuse in the Edinburgh cohort. J Acquir Immune Defic Syndr. 2002;31:S35–S42. doi: 10.1097/00126334-200210012-00003. [DOI] [PubMed] [Google Scholar]
- Berman NE, Raymond LA, Warren KA, Raghavan R, Joag SV, Narayan O, Cheney PD. Fractionator analysis shows loss of neurons in the lateral geniculate nucleus of macaques infected with neurovirulent simian immunodeficiency virus. Neuropathol Appl Neurobiol. 1998;24:44–52. doi: 10.1046/j.1365-2990.1998.00095.x. [DOI] [PubMed] [Google Scholar]
- Binswanger IA, Kral AH, Bluthenthal RN, Rybold DJ, Edlin BR. High prevalence of abscesses and cellulitis among community-recruited injection drug users in San Francisco. Clin Infect Dis. 2000;30:579–581. doi: 10.1086/313703. [DOI] [PubMed] [Google Scholar]
- Bouwman FH, Skolasky RL, Hes D, Selnes OA, Glass JD, Nance-Sproson TE, Royal W, Dal Pan GJ, McArthur JC. Variable progression of HIV-associated dementia. Neurology. 1998;50:1814–1820. doi: 10.1212/wnl.50.6.1814. [DOI] [PubMed] [Google Scholar]
- Bruckner JV, Jiang WD, Ho BT, Levy BM. Histopathological evaluation of cocaine-induced skin lesions in the rat. J Cutan Pathol. 1982;9:83–95. doi: 10.1111/j.1600-0560.1982.tb01045.x. [DOI] [PubMed] [Google Scholar]
- Buchwald JS, Huang C. Far-field acoustic response: origins in the cat. Science. 1975;189:382–384. doi: 10.1126/science.1145206. [DOI] [PubMed] [Google Scholar]
- Callahan TE, Schecter WP, Horn JK. Necrotizing soft tissue infection masquerading as cutaneous abcess following illicit drug injection. Arch Surg. 1998;133:812–817. doi: 10.1001/archsurg.133.8.812. [DOI] [PubMed] [Google Scholar]
- Castello E, Baroni N, Pallestrini E. Neurotological auditory brain stem response findings in human immunodeficiency virus-positive patients without neurologic manifestations. Ann Otol Rhinol Laryngol. 1998;107:1054–1060. doi: 10.1177/000348949810701210. [DOI] [PubMed] [Google Scholar]
- Chen JL, Fullerton KE, Flynn NM. Necrotizing fasciitis associated with injection drug use. Clin Infect Dis. 2001;33:6–15. doi: 10.1086/320874. [DOI] [PubMed] [Google Scholar]
- Chen Z, Zhou P, Ho DD, Landau NR, Marx PA. Genetically divergent strains of simian immunodeficiency virus use CCR5 as a coreceptor for entry. J Virol. 1997;71:2705–2714. doi: 10.1128/jvi.71.4.2705-2714.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang LF, Killam KF, Jr, Chuang RY. Increased replication of simian immunodeficiency virus in CEM x174 cells by morphine sulfate. Biochem Biophys Res Commun. 1993;195:1165–1173. doi: 10.1006/bbrc.1993.2167. [DOI] [PubMed] [Google Scholar]
- Ciardi A, Sinclair E, Scaravilli F, Harcourt-Webster NJ, Lucas S. The involvement of the cerebral cortex in human immunodeficiency virus encephalopathy: a morphological and immunohistochemical study. Acta Neuropathol. 1990;81:51–59. doi: 10.1007/BF00662637. [DOI] [PubMed] [Google Scholar]
- Curio G, Oppel F. Intraparenchymatous ponto-mesencephalic field distribution of brain-stem auditory evoked potentials in man. Electroencephalogr Clin Neurophysiol. 1988;69:259–265. doi: 10.1016/0013-4694(88)90134-4. [DOI] [PubMed] [Google Scholar]
- Davies J, Everall IP, Weich S, McLaughlin J, Scaravilli F, Lantos PL. HIV-associated brain pathology in the United Kingdom: an epidemiological study. AIDS. 1997;11:1145–1150. doi: 10.1097/00002030-199709000-00010. [DOI] [PubMed] [Google Scholar]
- Deng H, Liu R, Ellmeier W, Choe S, Unutmaz D, Burkhart M, Di Marzio P, Marmon S, Sutton RE, Hill CM, Davis CB, Peiper SC, Schall TJ, Littman DR, Landau NR. Identification of a major co-receptor for primary isolates of HIV-1. Nature. 1996;381:661–666. doi: 10.1038/381661a0. [DOI] [PubMed] [Google Scholar]
- Diesing TS, Swindells S, Gelbard H, Gendelman HE. HIV-1-associated dementia: a basic science and clinical perspective. AIDS Read. 2002;12:358–368. Review. [PubMed] [Google Scholar]
- Donahoe RM. Multiple ways that drug abuse might influence AIDS progression: clues from a monkey model. J Neuroimmunol. 2004;147:28–32. doi: 10.1016/j.jneuroim.2003.10.011. [DOI] [PubMed] [Google Scholar]
- Donahoe RM, Byrd LD, McClure HM, Fultz P, Brantley M, Marsteller F, Ansari AA, Wenzel D, Aceto M. Consequences of opiate-dependency in a monkey model of AIDS. Adv Exp Med Biol. 1993;335:21–28. doi: 10.1007/978-1-4615-2980-4_4. Review. [DOI] [PubMed] [Google Scholar]
- Dragic T, Litwin V, Allaway GP, Martin SR, Huang Y, Nagashima KA, Cayanan C, Maddon PJ, Koup RA, Moore JP, Paxton WA. HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5. Nature. 1996;381:667–673. doi: 10.1038/381667a0. [DOI] [PubMed] [Google Scholar]
- Everall IP. Interaction between HIV and intravenous heroin abuse? J Neuroimmunol. 2004;147:13–15. doi: 10.1016/j.jneuroim.2003.10.008. [DOI] [PubMed] [Google Scholar]
- Everall I, Luthert P, Lantos P. A review of neuronal damage in human immunodeficiency virus infection: its assessment, possible mechanism and relationship to dementia. J Neuropathol Exp Neurol. 1993;52:561–566. doi: 10.1097/00005072-199311000-00002. [DOI] [PubMed] [Google Scholar]
- Farnarier G, Somma-Mauvais H. Multimodal evoked potentials in HIV infected patients. Electroencephalogr Clin Neurophysiol Suppl. 1990;41:355–369. doi: 10.1016/b978-0-444-81352-7.50043-1. [DOI] [PubMed] [Google Scholar]
- Fultz PN, McClure HM, Anderson DC, Swenson RB, Anand R, Srinivasan A. Isolation of a T-lymphotropic retrovirus from naturally infected sooty mangabey monkeys (Cercocebus atys) Proc Natl Acad Sci USA. 1986;83:5286–5290. doi: 10.1073/pnas.83.14.5286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gayle H. An overview of the global HIV/AIDS epidemic, with a focus on the United States. AIDS. 2000;14(Suppl 2):S8–17. [PubMed] [Google Scholar]
- Ghaly RF, Ham JH, Lee JJ. High-dose ketamine hydrochloride maintains somatosensory and magnetic motor evoked potentials in primates. Neurol Res. 2001;23:881–886. doi: 10.1179/016164101101199342. [DOI] [PubMed] [Google Scholar]
- Ghaly RF, Stone JL, Aldrete JA, Levy WJ. Effects of incremental ketamine hydrochloride doses on motor evoked potentials (MEPs) following transcranial magnetic stimulation: a primate study. J Neurosurg Anesthesiol. 1990;2:79–85. doi: 10.1097/00008506-199006000-00004. [DOI] [PubMed] [Google Scholar]
- Glass JD, Wesselingh SL, Selnes OA, McArthur JC. Clinical-neuropathologic correlation in HIV-associated dementia. Neurology. 1993;43:2230–2237. doi: 10.1212/wnl.43.11.2230. [DOI] [PubMed] [Google Scholar]
- Gold LH, Fox HS, Henriksen SJ, Buchmeier MJ, Weed MR, Taffe MA, Huitron-Resendiz S, Horn TF, Bloom FE. Longitudinal analysis of behavioral, neurophysiological, viral and immunological effects of SIV infection in rhesus monkeys. J Med Primatol. 1998;27:104–112. doi: 10.1111/j.1600-0684.1998.tb00234.x. [DOI] [PubMed] [Google Scholar]
- Hashimoto I. Auditory evoked potentials from the human midbrain: slow brain stem responses. Electroencephalogr Clin Neurophysiol. 1982;53:652–657. doi: 10.1016/0013-4694(82)90141-9. [DOI] [PubMed] [Google Scholar]
- Hashimoto I, Ishiyama Y, Yoshimoto T, Nemoto S. Brain-stem auditory-evoked potentials recorded directly from human brain-stem and thalamus. Brain. 1981;104:841–859. doi: 10.1093/brain/104.4.841. [DOI] [PubMed] [Google Scholar]
- Hausler R, Vibert D, Koralnik IJ, Hirschel B. Neuro-otological manifestations in different stages of HIV infection. Acta Otolaryngol Suppl. 1991;481:515–521. doi: 10.3109/00016489109131461. [DOI] [PubMed] [Google Scholar]
- Hoeger PH, Haupt G, Hoelzle E. Acute multifocal skin necrosis: synergism between invasive streptococcal infection and cocaine-induced tissue ischaemia? Acta Derm Venereol. 1996;76:239–241. doi: 10.2340/0001555576239241. [DOI] [PubMed] [Google Scholar]
- Horn TF, Huitron-Resendiz S, Weed MR, Henriksen SJ, Fox HS. Early physiological abnormalities after simian immunodeficiency virus infection. Proc Natl Acad Sci USA. 1998;95:15072–15077. doi: 10.1073/pnas.95.25.15072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson SJ, Brettle RP, Gore SM. Predicting survival in AIDS: refining the model. QJM. 1997;90:685–692. doi: 10.1093/qjmed/90.11.685. [DOI] [PubMed] [Google Scholar]
- Iragui VJ, Kalmijn J, Plummer DJ, Sample PA, Trick GL, Freeman WR. Pattern electroretinograms and visual evoked potentials in HIV infection: evidence of asymptomatic retinal and postretinal impairment in the absence of infectious retinopathy. Neurology. 1996;47:1452–1456. doi: 10.1212/wnl.47.6.1452. [DOI] [PubMed] [Google Scholar]
- Kanzer MD. Neuropatholoy of AIDS. Crit Rev Neurobiol. 1990;5:313–362. [PubMed] [Google Scholar]
- Kapadia F, Vlahov D, Donahoe RM, Friedland G. The role of substance abuse in HIV disease progression: reconciling differences from laboratory and epidemiologic investigations. Clin Infect Dis. 2005;41:1027–1034. doi: 10.1086/433175. [DOI] [PubMed] [Google Scholar]
- Kirchhoff F, Pohlmann S, Hamacher M, Means RE, Kraus T, Uberla K, Di Marzio P. Simian immunodeficiency virus variants with differential T-cell and macrophage tropism use CCR5 and an unidentified cofactor expressed in CEMx174 cells for efficient entry. J Virol. 1997;71:6509–6516. doi: 10.1128/jvi.71.9.6509-6516.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koralnik IJ, Beaumanoir A, Hausler R, Kohler A, Safran AB, Delacoux R, Vibert D, Mayer E, Burkhard P, Nahory A, et al. A controlled study of early neurologic abnormalities in men with asymptomatic human immunodeficiency virus infection. N Engl J Med. 1990;323:864–870. doi: 10.1056/NEJM199009273231303. [DOI] [PubMed] [Google Scholar]
- Krol A, Flynn C, Vlahov D, Miedema F, Coutinho RA, van Ameijden EJ. New evidence to reconcile in vitro and epidemiologic data on the possible role of heroin on CD4+ decline among HIV-infected injecting drug users. Drug Alcohol Depend. 1999;54:145–154. doi: 10.1016/s0376-8716(98)00158-6. [DOI] [PubMed] [Google Scholar]
- Kumar R, Orsoni S, Norman L, Verma AS, Tirado G, Giavedoni LD, Staprans S, Miller GM, Buch SJ, Kumar A. Chronic morphine exposure causes pronounced virus replication in cerebral compartment and accelerated onset of AIDS in SIV/SHIV-infected Indian rhesus macaques. Virology. 2006;354:192–206. doi: 10.1016/j.virol.2006.06.020. [DOI] [PubMed] [Google Scholar]
- Kraut MA, Arezzo JC, Vaughan HG. Intracortical generators of the flash VEP in monkeys. Electroencephalogr Clin Neurophysiol. 1985;62:300–312. doi: 10.1016/0168-5597(85)90007-3. [DOI] [PubMed] [Google Scholar]
- Lalley PM, Rossi GV, Baker WW. Tremor production by intracaudate injections of morphine. Eur J Pharmacol. 1975;32:45–51. doi: 10.1016/0014-2999(75)90321-0. [DOI] [PubMed] [Google Scholar]
- Legatt AD, Arezzo JC, Vaughan HG., Jr Short-latency auditory evoked potentials in the monkey. II. Intracranial generators. Electroencephalogr Clin Neurophysiol. 1986;64:53–73. doi: 10.1016/0013-4694(86)90043-x. [DOI] [PubMed] [Google Scholar]
- Lipton SA. Neuronal injury associated with HIV-1: approaches to treatment. Annu Rev Pharmacol Toxicol. 1998;38:159–177. doi: 10.1146/annurev.pharmtox.38.1.159. Review. [DOI] [PubMed] [Google Scholar]
- Lipton SA, Gendelman HE. Seminars in medicine of the Beth Israel Hospital, Boston. Dementia associated with the acquired immunodeficiency syndrome. N Engl J Med. 1995;332:934–940. doi: 10.1056/NEJM199504063321407. [DOI] [PubMed] [Google Scholar]
- Mahadevan A, Satishchandra P, Prachet KK, Sidappa NB, Ranga U, Santosh V, Yasha TC, Desai A, Ravi V, Shankar SK. Optic nerve axonal pathology is related to abnormal visual evoked responses in AIDS. Acta Neuropathol. 2006;112:461–469. doi: 10.1007/s00401-006-0089-1. [DOI] [PubMed] [Google Scholar]
- Malessa R, Agelink MW, Diener HC. Dysfunction of visual pathways in HIV-1 infection. J Neurol Sci. 1995;130:82–87. doi: 10.1016/0022-510x(95)00002-j. [DOI] [PubMed] [Google Scholar]
- Manzoni OJ, Williams JT. Presynaptic regulation of glutamate release in the ventral tegmental area during morphine withdrawal. J Neurosci. 1999;19:6629–6636. doi: 10.1523/JNEUROSCI.19-15-06629.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcario JK, Manaye KF, SantaCruz KS, Mouton PR, Berman NE, Cheney PD. Severe subcortical degeneration in macaques infected with neurovirulent simian immunodeficiency virus. J Neurovirol. 2004;10:387–399. doi: 10.1080/13550280490521131. [DOI] [PubMed] [Google Scholar]
- Marcario JK, Raymond LA, McKiernan BJ, Foresman LL, Joag SV, Raghavan R, Narayan O, Cheney PD. Motor skill impairment in SIV-infected rhesus macaques with rapidly and slowly progressing disease. J Med Primatol. 1999a;28:105–117. doi: 10.1111/j.1600-0684.1999.tb00258.x. [DOI] [PubMed] [Google Scholar]
- Marcario JK, Raymond LA, McKiernan BJ, Foresman LL, Joag SV, Raghavan R, Narayan O, Hershberger S, Cheney PD. Simple and choice reaction time performance in SIV-infected rhesus macaques. AIDS Res Hum Retroviruses. 1999b;15:571–583. doi: 10.1089/088922299311097. [DOI] [PubMed] [Google Scholar]
- Marcario JK, Riazi M, Adany I, Kenjale H, Fleming K, Marquis J, Nemon O, Mayo MS, Yankee T, Narayan O, Cheney PD. Effect of morphine on the neuropathogenesis of SIVmac infection in indian rhesus macaques. J Neuroimmune Pharmacology. 2008;3:12–25. doi: 10.1007/s11481-007-9085-z. [DOI] [PubMed] [Google Scholar]
- Marx PA, Chen Z. The function of simian chemokine receptors in the replication of SIV. Semin Immunol. 1998;10:215–223. doi: 10.1006/smim.1998.0135. [DOI] [PubMed] [Google Scholar]
- McArthur JC, Hoover DR, Bacellar H, Miller EN, Cohen BA, Becker JT, Graham NM, McArthur JH, Selnes OA, Jacobson LP, et al. Dementia in AIDS patients: incidence and risk factors. Multicenter AIDS cohort study. Neurology. 1993;43:2245–2252. doi: 10.1212/wnl.43.11.2245. [DOI] [PubMed] [Google Scholar]
- McArthur JC, Sacktor N, Selnes O. Human immunodeficiency virus-associated dementia. Semin Neurol. 1999;19:129–150. doi: 10.1055/s-2008-1040831. Review. [DOI] [PubMed] [Google Scholar]
- McCarthy L, Wetzel M, Sliker JK, Eisenstein TK, Rogers TJ. Opioids, opioid receptors, and the immune response. Drug Alcohol Depend. 2001;62:111–123. doi: 10.1016/s0376-8716(00)00181-2. [DOI] [PubMed] [Google Scholar]
- Moller AR, Burgess J. Neural generators of the brain-stem auditory evoked potentials (BAEPs) in the rhesus monkey. Electroencephalogr Clin Neurophysiol. 1986;65:361–372. doi: 10.1016/0168-5597(86)90015-8. [DOI] [PubMed] [Google Scholar]
- Moller AR, Jannetta PJ. Evoked potentials from the inferior colliculus in man. Electroencephalogr Clin Neurophysiol. 1982a;53:612–620. doi: 10.1016/0013-4694(82)90137-7. [DOI] [PubMed] [Google Scholar]
- Moller AR, Jannetta PJ. Auditory evoked potentials recorded intracranially from the brain stem in man. Exp Neurol. 1982b;78:144–157. doi: 10.1016/0014-4886(82)90196-0. [DOI] [PubMed] [Google Scholar]
- Moller AR, Jannetta P, Bennett M, Moller MB. Intracranially recorded responses from the human auditory nerve: new insights into the origin of brain stem evoked potentials (BSEPs) Electroencephalogr Clin Neurophysiol. 1981a;52:18–27. doi: 10.1016/0013-4694(81)90184-x. [DOI] [PubMed] [Google Scholar]
- Moller AR, Jannetta PJ, Moller MB. Neural generators of brainstem evoked potentials. Results from human intracranial recordings. Ann Otol Rhinol Laryngol. 1981b;90:591–596. doi: 10.1177/000348948109000616. [DOI] [PubMed] [Google Scholar]
- Moller AR, Jannetta PJ, Jho HD. Click-evoked responses from the cochlear nucleus: a study in human. Electroencephalogr Clin Neurophysiol. 1994;92:215–224. doi: 10.1016/0168-5597(94)90065-5. [DOI] [PubMed] [Google Scholar]
- Murray EA, Rausch DM, Lendvay J, Sharer LR, Eiden LE. Cognitive and motor impairments associated with SIV infection in rhesus monkeys. Science. 1992;255:1246–1249. doi: 10.1126/science.1546323. [DOI] [PubMed] [Google Scholar]
- Mwanza JC, Nyamabo LK, Tylleskar T, Plant GT. Neuro-ophthalmological disorders in HIV infected subjects with neurological manifestations. Br J Ophthalmol. 2004;88:1455–1459. doi: 10.1136/bjo.2004.044289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayan O, Stephens EB, Joag SV, Chebloune Y. Animal models of human immunodeficiency virus neurologic disease. In: Berger JR, Levy RM, editors. AIDS and the nervous system. 2. Lippincott, Raven Publishers; Philadelphia: 1997. [Google Scholar]
- Pagano MA, Cahn PE, Garau ML, Mangone CA, Figini HA, Yorio AA, Dellepiane MC, Amores MG, Perez HM, Casiro AD. Brain-stem auditory evoked potentials in human immunodeficiency virus-seropositive patients with and without acquired immunodeficiency syndrome. Arch Neurol. 1992;49:166–169. doi: 10.1001/archneur.1992.00530260068022. [DOI] [PubMed] [Google Scholar]
- Petito CK, Cho ES, Lemann W, Navia BA, Price RW. Neuropathology of acquired immunodeficiency syndrome (AIDS): an autopsy review. J Neuropathol Exp Neurol. 1986;45:635–646. doi: 10.1097/00005072-198611000-00003. [DOI] [PubMed] [Google Scholar]
- Pezzotti P, Galai N, Vlahov D, Rezza G, Lyles CM, Astemborski J. Direct comparison of time to AIDS and infectious disease death between HIV seroconverter injection drug users in Italy and the United States: results from the ALIVE and ISS studies. AIDS link to intravenous experiences. Italian seroconversion study. J Acquir Immune Defic Syndr Hum Retrovirol. 1999;20:275–282. doi: 10.1097/00042560-199903010-00010. [DOI] [PubMed] [Google Scholar]
- Pierelli F, Soldati G, Zambardi P, Garrubba C, Spadaro M, Tilia G, Pauri F, Morocutti C. Electrophysiological study (VEP, BAEP) in HIV-1 seropositive patients with and without AIDS. Acta Neurol Belg. 1993;93:78–87. [PubMed] [Google Scholar]
- Price RW, Brew B, Sidtis J, Rosenblum M, Scheck AC, Cleary P. The brain in AIDS: central nervous system HIV-1 infection and AIDS dementia complex. Science. 1988;239:586–592. doi: 10.1126/science.3277272. [DOI] [PubMed] [Google Scholar]
- Prins M, Veugelers PJ. Comparison of progression and non-progression in injecting drug users and homosexual men with documented dates of HIV-1 seroconversion. European seroconverter study and the tricontinental seroconverter study. AIDS. 1997;11:621–631. doi: 10.1097/00002030-199705000-00010. [DOI] [PubMed] [Google Scholar]
- Prospero-Garcia O, Gold LH, Fox HS, Polis I, Koob GF, Bloom FE, Henriksen SJ. Microglia-passaged simian immunodeficiency virus induces neurophysiological abnormalities in monkeys. Proc Natl Acad Sci USA. 1996;93:14158–14163. doi: 10.1073/pnas.93.24.14158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavan R, Cheney PD, Raymond LA, Joag SV, Stephens EB, Adany I, Pinson DM, Li Z, Marcario JK, Jia F, Wang C, Foresman L, Berman NE, Narayan O. Morphological correlates of neurological dysfunction in macaques infected with neurovirulent simian immunodeficiency virus. Neuropathol Appl Neurobiol. 1999;25:285–294. doi: 10.1046/j.1365-2990.1999.00185.x. [DOI] [PubMed] [Google Scholar]
- Raymond LA, Wallace D, Berman NE, Marcario J, Foresman L, Joag SV, Raghavan R, Narayan O, Cheney PD. Auditory brainstem responses in a rhesus macaque model of neuro-AIDS. J Neurovirol. 1998;4:512–520. doi: 10.3109/13550289809113495. [DOI] [PubMed] [Google Scholar]
- Raymond LA, Wallace D, Marcario JK, Raghavan R, Narayan O, Foresman LL, Berman NE, Cheney PD. Motor evoked potentials in a rhesus macaque model of neuro-AIDS. J Neurovirol. 1999;5:217–231. doi: 10.3109/13550289909015808. [DOI] [PubMed] [Google Scholar]
- Raymond LA, Wallace D, Raghavan R, Marcario JK, Johnson JK, Foresman LL, Joag SV, Narayan O, Berman NE, Cheney PD. Sensory evoked potentials in SIV-infected monkeys with rapidly and slowly progressing disease. AIDS Res Hum Retroviruses. 2000;16:1163–1173. doi: 10.1089/088922200415018. [DOI] [PubMed] [Google Scholar]
- Roy S, Loh HH. Effects of opioids on the immune system. Neurochem Res. 1996;21:1375–1386. doi: 10.1007/BF02532379. [DOI] [PubMed] [Google Scholar]
- Scott DW, Morrell JI, Vernotica EM. Focal necrotizing panniculitis and vascular necrosis in rats given subcutaneous injections of cocaine hydrochloride. J Cutan Pathol. 1997;24:25–29. doi: 10.1111/j.1600-0560.1997.tb00781.x. [DOI] [PubMed] [Google Scholar]
- Seevers MH. Opiate addiction in the monkey I. methods of study. J Pharmacol Exp Ther. 1936;56:147–156. [Google Scholar]
- Selwyn PA, Alcabes P, Hartel D, Buono D, Schoenbaum EE, Klein RS, Davenny K, Friedland GH. Clinical manifestations and predictors of disease progression in drug users with human immunodeficiency virus infection. N Engl J Med. 1992;327:1697–1703. doi: 10.1056/NEJM199212103272401. [DOI] [PubMed] [Google Scholar]
- Sepulveda MJ, Hernandez L, Rada P, Tucci S, Contreras E. Effect of precipitated withdrawal on extracellular glutamate and aspartate in the nucleus accumbens of chronically morphine-treated rates: an in vivo microdialysis study. Pharmacol Biochem Behav. 1998;60:255–262. doi: 10.1016/s0091-3057(97)00550-9. [DOI] [PubMed] [Google Scholar]
- Sharma DP, Zink MC, Anderson M, Adams R, Clements JE, Joag SV, Narayan O. Derivation of neurotropic simian immunodeficiency virus from exclusively lymphocytetropic parental virus: pathogenesis of infection in macaques. J Virol. 1992;66:3550–3556. doi: 10.1128/jvi.66.6.3550-3556.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp BM, Roy S, Bidlack JM. Evidence for opioid receptors on cells involved in host defense and the immune system. J Neuroimmunol. 1998;83:45–56. Review. [PubMed] [Google Scholar]
- Spijkerman IJ, Koot M, Prins M, Keet IP, van den Hoek AJ, Miedema F, Coutinho RA. Lower prevalence and incidence of HIV-1 syncytium-inducing phenotype among injecting drug users compared with homosexual men. AIDS. 1995;9:1085–1092. doi: 10.1097/00002030-199509000-00016. [DOI] [PubMed] [Google Scholar]
- Spijkerman IJ, Langendam MW, Veugelers PJ, van Ameijden EJ, Keet IP, Geskus RB, van den Hoek A, Coutinho RA. Differences in progression to AIDS between injection drug users and homosexual men with documented dates of seroconversion. Epidemiology. 1996a;7:571–577. doi: 10.1097/00001648-199611000-00002. [DOI] [PubMed] [Google Scholar]
- Spijkerman IJ, van Ameijden EJ, Mientjes GH, Coutinho RA, van den Hoek A. Human immunodeficiency virus infection and other risk factors for skin abscesses and endocarditis among injection drug users. J Clin Epidemiol. 1996b;49:1149–1154. doi: 10.1016/0895-4356(96)00180-1. [DOI] [PubMed] [Google Scholar]
- Suzuki S, Chuang AJ, Chuang LF, Doi RH, Chuang RY. Morphine promotes simian acquired immunodeficiency syndrome virus replication in monkey peripheral mononuclear cells: induction of CC chemokine receptor 5 expression for virus entry. J Infec Dis. 2002;185:1826–1829. doi: 10.1086/340816. [DOI] [PubMed] [Google Scholar]
- Thorpe LE, Frederick M, Pitt J, Cheng I, Watts DH, Buschur S, Green K, Zorrilla C, Landesman SH, Hershow RC. Effect of hard-drug use on CD4 cell percentage, HIV RNA level, and progression to AIDS-defining class C events among HIV-infected women. J Acquir Immune Defic Syndr. 2004;37:1423–1430. doi: 10.1097/01.qai.0000127354.78706.5d. [DOI] [PubMed] [Google Scholar]
- Thorlin T, Roginski RS, Choudhury K, Nilsson M, Ronnback L, Hansson E, Eriksson PS. Regulation of the glial glutamate transporter GLT-1 by glutamate and delta-opioid receptor stimulation. FEBS Lett. 1998;425:453–459. doi: 10.1016/s0014-5793(98)00288-9. [DOI] [PubMed] [Google Scholar]
- Tomlinson GS, Simmonds P, Busuttil A, Chiswick A, Bell JE. Upregulation of microglia in drug users with and without pre-symptomatic HIV infection. Neuropathol Appl Neurobiol. 1999;25:369–379. doi: 10.1046/j.1365-2990.1999.00197.x. [DOI] [PubMed] [Google Scholar]
- Veazey RS, DeMaria M, Chalifoux LV, Shvetz DE, Pauley DR, Knight HL, Rosenzweig M, Johnson RP, Desrosiers RC, Lackner AA. Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science. 1998;280:427–431. doi: 10.1126/science.280.5362.427. [DOI] [PubMed] [Google Scholar]
- Veazey RS, Mansfield KG, Tham IC, Carville AC, Shvetz DE, Forand AE, Lackner AA. Dynamics of CCR5 expression by CD4(+) T cells in lymphoid tissues during simian immunodeficiency virus infection. J Virol. 2000a;74:11001–11007. doi: 10.1128/jvi.74.23.11001-11007.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veazey RS, Tham IC, Mansfield KG, DeMaria M, Forand AE, Shvetz DE, Chalifoux LV, Sehgal PK, Lackner AA. Identifying the target cell in primary simian immunodeficiency virus (SIV) infection: highly activated memory CD4(+) T cells are rapidly eliminated in early SIV infection in vivo. J Virol. 2000b;74:57–64. doi: 10.1128/jvi.74.1.57-64.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weed MR, Gold LH, Polis I, Koob GF, Fox HS, Taffe MA. Impaired performance on a rhesus monkey neuropsychological testing battery following simian immunodeficiency virus infection. AIDS Res Hum Retroviruses. 2004;20:77–89. doi: 10.1089/088922204322749521. [DOI] [PubMed] [Google Scholar]