Abstract
Purpose of review
The thalidomide tragedy of the early 1960s resulted in a great number of studies and reports involving many specialties of medicine. Because of the estimated large number of affected children (5000+) worldwide exposed to this potent teratogen, and the many informative cases in which the exposure time was known, a teratogenic timetable was constructed relating affected structures to the time of exposure. This demonstrated that thalidomide had a teratogenic effect between approximately 20 to 36 days after fertilization.
Recent findings
We found that Duane syndrome and its variants were prominent in individuals who were exposed to thalidomide early in the sensitive period (days 20 to 26±). Other anomalies associated with this early effect were aberrant tearing, facial nerve palsy, ear malformations, and autism. Structural eye malformations were less frequent in this early phase, appearing slightly later in the sensitive period.
Summary
This study summarizes the ophthalmologic findings from a number of studies and compares them with respect to the implications of time of exposure. Because the timing of anomalies such as external ear and limb malformations are well established in the thalidomide literature, correlation with associated eye anomalies gives insight into the approximate timing of the causative teratogen exposure.
Keywords: aberrant tearing, Duane syndrome, facial nerve palsy, thalidomide embryopathy
Introduction
Thalidomide is an extremely potent teratogenic drug capable of causing severe systemic malformations to an exposed fetus during the sensitive period. Thalidomide [α-(N-phthalimido)-glutarimide] was synthesized in 1954 by Chemie Grünenthal under the brand name of Contergan and later licensed in more than 40 countries worldwide [1]. One exception to the general availability of thalidomide occurred in the USA. The United States Food and Drug Administration (FDA) had not approved the drug for unrestricted use because of concerns raised by Francis Kelsey [2], an FDA physician. The ensuing delay was long enough to have the teratogenic effects recognized worldwide. Thus the number of cases of thalidomide embryopathy in the USA was very small. Manufactured under several trade names, thalidomide was a popular treatment for anxiety, insomnia, gastritis, and hyperemesis and advertised as safe and harmless for pregnant women even in high doses. Its teratogenicity in humans and higher mammals was not anticipated [1,3]. The severe teratogenic effects of thalidomide led to passage of new regulatory legislation for pharmaceuticals [4].
In the late 1950s and early 1960s, Lenz [5] noted that in Germany there was an alarming increase in the number of infants born with hypoplastic and aplastic malformations of the extremities. This observation was also noted in other countries [6,7]. In addition to limb anomalies, many other structural and functional anomalies were reported [1,8–11]. Ocular motility dysfunction and structural eye malformations, along with craniofacial anomalies, were prominent [12–20]. Within a few months of the initially reported cases, the drug was withdrawn from commercial sale in most European countries, but not before approximately 5000 cases of malformed live births occurred worldwide. As the survival rate of thalidomide-exposed embryos is estimated to be between 40 and 50%; more than 10 000 pregnancies may have been affected [1].
The literature suggested that exposure to thalidomide caused malformations primarily between 20 and 36 days after fertilization (34–50 days after the last menstrual cycle) [1,5,10]. Thalidomide differs from some teratogens in that the clinical dosage seems not to be as significant as the time of intake of the drug. It is quite rapidly hydrolyzed and because of this short action time, extreme potency, and the large number of women who took thalidomide, informative histories could be ascertained [20]. Many women knew the exact date of intake and the number of pills they took, and a correlation between time of drug intake and resultant malformations could be made [8,9,12,15,21]. From this information, summary timetables of the causation of various malformations were developed (Fig. 1) [10,21].
Figure 1. Summary timetable of thalidomide embryopathy based on observations in the literature.
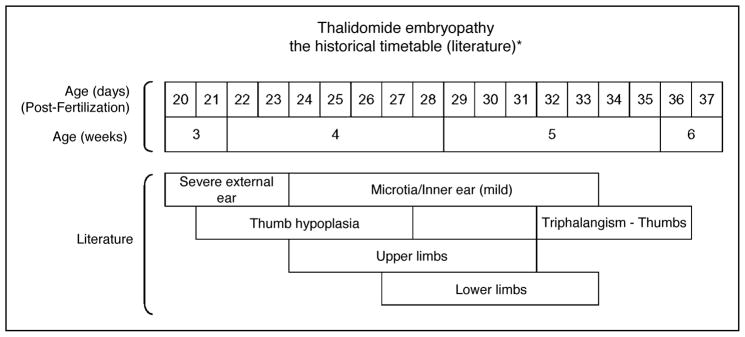
The sensitive period is 20–36 days after fertilization. If calculated from the last menstrual period, it would be approximately days 34–50 [5,10,21].
Swedish thalidomide study (1987–1989)
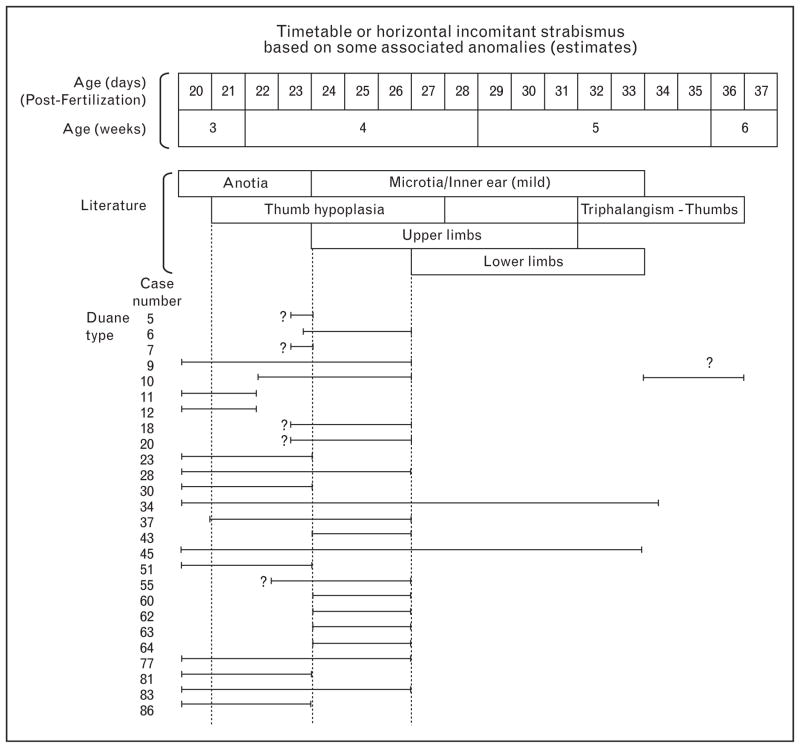
During the 1960s there had been litigation against the drug company in Sweden and about 100 children were identified as meeting the criteria for thalidomide embryopathy. From 1987 to 1989, a total of 86 of these patients were examined [22–24]. The patients were 27–30 years old, although the diagnosis of thalidomide embryopathy had been made when they were children. The purpose of this study was to describe the ophthalmologic findings and then compare them with the associated limb and ear anomalies in which the teratogenic susceptibility timetables were well documented. Table 1 summarizes the ophthalmologic findings and Table 2 summarizes the limb and systemic anomalies. There was a surprisingly large number of patients with incomitant strabismus, usually of the Duane type [22–25], but variations, such as abduction deficits without palpebral fissure change, were also noted [22,25]. Each case was then analyzed as to their associated limb or ear anomalies. An example of this analysis with Duane type ocular motility abnormality can be seen in Fig. 2. It is convincing that exposure was early in the thalidomide sensitive period, from days 24 to 26 after fertilization, although it was difficult to refine this timeline further since there were no distinguishing characteristics of associated anomalies between days 24 and 26. There were some patients who had exposure throughout the sensitive period, but most fall into the early group with associated anomalies limited to ear, thumb, and upper limb. It is unknown whether there were more mothers who took thalidomide for a longer period, but one might speculate that many of those with more severe systemic anomalies in the later period had spontaneous abortions.
Table 1.
Ocular anomalies documented in the Swedish Thalidomide Study (1987–1989)a
| Anomaly | No. of (%) affecteda |
|---|---|
| Strabismus – incomitant | 37 (44%) |
| Typical Duane syndrome with pronounced limitation abduction and fissure change on adduction [26] | |
| Marked limitation abduction and adduction [7] | |
| Abduction deficit only [4] | |
| Strabismus – comitant (all esotropia) | 6 (7%) |
| Aberrant lacrimation | 17 (20%) |
| Coloboma (uveal or optic disc) | 3 |
| Microphthalmos | 2 |
| Glaucoma | 1 |
| Lipodermoid of conjunctiva | 1 |
| Hypertelorism | 1 |
| Retinal myelinated nerve fibers | 2 |
| Ptosis | 2 |
Reproduced with permission [24].
N =86, except strabismus N = 84.
Table 2.
Limb and systemic anomalies in Swedish Thalidomide Study (1987–1989) (N=86)
| Limb and systemic anomalies in Swedish Thalidomide Study (1987–1989) (N = 86) | ||
|---|---|---|
| Anomalies/site of anomaly | No. (%) affected | |
| Thumbs | 70 (81%) | |
| Upper limb (excluding thumb) | 59 (69%) | |
| Lower limb | 21 (24%) | |
| Ears/hearing | 33 (38%) | |
| Facial nerve palsy | 17 (20%) | |
| Kidney | 12 (14%) |
|
| Cardiovascular | 7 (8%) | |
| Chest/lung | 4 (5%) | |
| Genitalia | 3 (3%) | |
| Anal atresia | 4 (5%) | |
| Choanal atresia | 2 (2%) | |
| Dental anomalies | 4 (5%) | |
| Mental retardation (moderate to severe) | 5 (6%) | |
| Severe autism ** | 4 (5%) | |
Reproduced with permission [24].
Unable to comment on possible milder cases of autism as protocol did not include a formal psychiatric evaluation. However, for these four patients, there was a later formal evaluation by psychiatrists.
Figure 2. Timetable of Duane syndrome based on associated ear and limb anomalies of each patient compared with the thalidomide timetable established from studies conducted in Sweden.
For example, patient 9, who had moderate ear anomalies, upper limb but no lower limb malformations suggests that thalidomide was taken in the early to middle phase of the sensitive period. Some of the beginning and end points are estimates (e.g., the difficulty in separating anotia from microtia and how to plot the few patients with very mild or questionable limb anomalies). Other problem patients include patients 5 and 7, who only had a few malformations. Even with the less clear examples, there was no question that Duane syndrome was associated with an early thalidomide effect within the 20–36 day overall sensitive period. Data for other types of incomitant strabismus, facial nerve palsy, and aberrant lacrimation were also consistent with an early thalidomide effect [22].
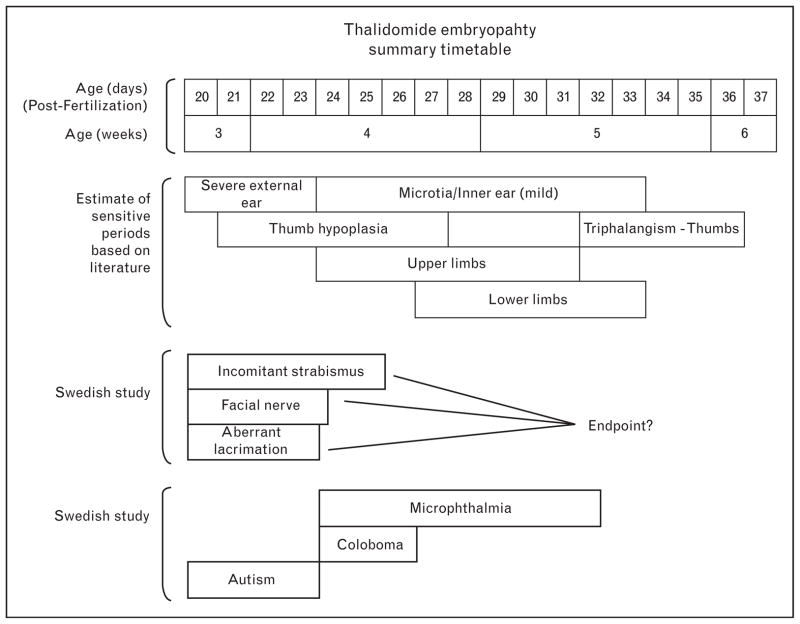
The other frequent ocular-related abnormalities included aberrant tearing in 17 (20%) and coloboma and/or microphthalmia in five. Facial nerve palsy was noted in 17 patients (20%) and, like aberrant tearing, was associated with early exposure, while the structural anomalies of coloboma and microphthalmia seemed to occur slightly later. A surprising observation not previously noted in the literature was that at least four individuals in the early-effect group had severe autism [26]. This was of considerable interest to researchers in autism as it suggests that an early insult in embryogenesis could lead to a functional disturbance involving higher brain centers [26,27]. A summary of these associations has been shown in Fig. 3.
Figure 3. Summary estimates of sensitive periods for ophthalmic malformations based on associated anomalies manifested by most patients (see .
Figure 2 for Duane syndrome, the most frequent incomitant strabismus)
All clinical forms of incomitant strabismus had similar associated anomalies. These blocks of sensitive periods for the development of eye anomalies and autism are estimations and may be shorter or slightly longer than indicated. The thalidomide timetable for the ear, thumb, and limb anomalies was derived from the literature [8,10,21,22].
Congenital aberrant tearing
Congenital aberrant (anomalous) tearing includes tearing when eating, absence of emotional (psychic) lacrimation, or an unusual late onset of tearing. Inappropriate tearing associated with eating or sucking is often referred to as paradoxical gustolacrimal tearing or ‘crocodile tears’. Bogorad [28] suggested the term ‘crocodile tears’ came from the myth that crocodiles cried when eating their prey.
Although the number of patients with Duane syndrome who also have anomalous tearing are few, the association of anomalous tearing with Duane syndrome is exceedingly strong. There are many case reports and small series that support this observation [29–31]. Although reported in connection with other teratogens and syndromes, these two examples of anomalous innervation are usually isolated findings [31]. A congenital lack of emotional tearing, with a normal cornea, suggests adequate basal tearing. Lack of emotional tearing is also often associated with crocodile tears and Duane syndrome.
A book summarizing the Japanese experience of a large number of individuals with thalidomide embryopathy was edited by Kida [10] in 1987. Thalidomide was on the market in Japan for 5 years (1958–1963) and was sold without a prescription. Arimoto [19] conducted detailed ocular examinations and reported Duane syndrome in 31 (23%), crocodile tears syndrome in 24 (18%), and facial nerve palsy in 38 patients (28%). Arimoto [19] also postulated that the susceptible period was approximately 20–24 days after fertilization for these malformations.
It is noteworthy that the tearing findings in the Japanese study are similar to those of the Swedish thalidomide study and aberrant tearing has also been reported with thalidomide embryopathy in a number of other studies [17,32–35]. Table 3 summarizes these studies. Other reports on thalidomide investigations often do not even mention tearing symptoms or findings. One wonders whether the appropriate questions were asked. This lack of investigation of these symptoms may also exist with reports of the nonsyndromic Duane patients.
Table 3.
Thalidomide embryopathy associated with congenital aberrant tearing in some or all cases
| Author | Series type | No. of cases with aberrant tearing | Age/sex | Motility | Crocodile tears | Emotional tearing | Seventh nerve palsy | Comments |
|---|---|---|---|---|---|---|---|---|
| Arimoto [19] | 138 cases of thalidomide | 23 | NI | 23 DS | 23 cases | NI | 20 | |
| Maruo et al. [32] | 266 cases of DS including 23 cases of thalidomide | 27 | NI/121 M; 145 F | 23 bilateral DS; 4 unilateral DS | 27 cases | NI | NI | 18 of 23 cases with history of thalidomide had aberrant tearing |
| Miller [31] | 86 cases of thalidomide | 17 | NI/12 M; 15 F | 16 bilateral DS; 1 abduction ↓ | 15 cases | Absent 10 | 12 | Hearing loss 7; autism 2 |
| Trieschmann [33] | All 3 cases of thalidomide | 3 | 1) 10 mo 2) 9 y/F 3) 11 y/F | 1) DS OU 2) Abduction ↓ OU 3) NI | 1) OU 2) OU 3) OD worse than OS | NI | 1) R/L 2) R/L 3) R | Schirmer test scores (mm) 8 OD/5 OS→ eating 20/16 deaf; tearing ↑ with eating apple |
| Uemura and Tamura [34] | 4 cases of thalidomide HX | 10 | 3–6 y/3 F 7 M | Bilateral 9; 1 OD | 7 cases OU; 3 cases OS | NI | 6 | 4 thalidomide history; 3 CNS malformations; 1 deaf; Schirmer after eating on 2 cases, both ↑ |
| Takemori et al. [35] | Thalidomide series of 18 cases with ear anomalies | 7 | NI/12 M; 6 F | 11 abduction ↓OU; 1 abduction ↓unilateral | 7 cases | NI | 3 OU; 3 unilateral | 11 external ears; absence of stapes; inner ear 15; vestibular hypoplasia 15; 4 limb anomalies; Schirmer 11/8 mm → 34/28 mm after eating sour apple |
CNS, central nervous system; DS, Duane syndrome; NI, no information; OD, right eye; OS, left eye; OU, both eyes; ↑, increased; ↓, decreased. Reproduced with permission from [31].
Zhang [29], in an article from China in 1997, summarized 201 cases of Duane retraction syndrome, in which crocodile tears were noted in 26 cases (13%). In 2002, he reported 25 cases of gustatory tearing associated with Duane syndrome [30].
Lack of emotional (psychic) tearing is another unusual form of anomalous tearing. This information will rarely be volunteered by the patient unless specific questions are asked. In our thalidomide series, lack of psychic tearing was always associated with Duane syndrome and usually, but not always, with tearing when eating.
A recent second Swedish thalidomide study by the authors was a multidisciplinary design [31]. Because many members of the original cohort of Swedish patients with thalidomide embryopathy declined to participate in the new comprehensive study, our results are only for a subset of the Swedish patients plus a few new cases, and thus there may be some bias in the results. One goal was to add more information on tearing symptoms in these thalidomide survivors (44–46 years old) by adding a baseline Schirmer I test and repeating the test while the patient is chewing. Although there may be reservations about the accuracy and reproducibility of the Schirmer tests, certain findings were very interesting. Eleven individuals complained of anomalous tearing. Nine of 11 individuals had Duane syndrome and seven of these showed increased tearing greater than 10 mm in the Schirmer test after chewing. All individuals with lack of emotional tears in this group (seven) had Duane syndrome. Eight with Duane syndrome and a very low Schirmer score but no history of dry eyes. Seven of these showed a 10 mm or greater increased Schirmer score on eating.
These findings lead to several considerations beyond the thalidomide population. Do our routine adult patients with Duane syndrome have a greater prevalence of ‘dry eyes’ with or without symptoms? Do some have increased tearing during eating that gives an overall total adequate tearing? Jacklin [36] mentioned a patient with unilateral dry eye and crocodile tears who carried an apple to eat to relieve his low tearing symptoms. Is there a connection between these two forms of aberrant innervation? Cells destined to form the sixth and seventh nerve nucleus and the superior lacrimal nucleus in the brain stem, are felt to be in close proximity in early embryogenesis. Ramsay and Taylor [37] proposed that there was nuclear damage or dysgenesis in the vicinity of the abducens nucleus, with the lacrimal finding being the result of innervation of the lacrimal gland by fibers subserving salivation. The observations in thalidomide embryopathy of an overwhelming association between facial nerve palsy, Duane syndrome, and aberrant tearing strongly support a theory of a common nuclear location during embryogenesis. There may also be some damage in the lacrimal nucleus to cells that are destined to be connected with higher centers that would normally be responsible for emotional tearing.
Möbius sequence
There is a resemblance between some individuals with anomalies connected with early exposure to thalidomide and those with Möbius sequence [38,39]. Möbius sequence is classically described as a condition characterized by involvement of the sixth and seventh cranial nerves frequently associated with limb and facial anomalies. This constellation of cranial nerve anomalies is similar to some individuals with thalidomide embryopathy due to early exposure in the sensitive period. In 1995, a multidiscipline, prospective study was done in Sweden involving 25 patients exposed to thalidomide who had Möbius sequence [40,41]. One of the motivating factors for this study was to investigate functional issues such as autism and tearing abnormalities, which had been observed in the thalidomide early exposure group. There had been few articles in the older literature that reported autism in individuals with Möbius sequence [42]. In this 1995 study, there were 21 patients old enough to have a psychiatric examination and eight met the diagnostic findings of autism disorder [43]. Six patients in the study were noted to have some tearing abnormality. These comparisons became more interesting with the reports from South America, especially Brazil, that children were born with findings compatible with the diagnosis of Möbius sequence whose mothers had unsuccessfully attempted abortion with a drug, misoprostol [44]. In a study in Recife, Brazil, of 28 patients with Möbius sequence, there was a history of misoprostol exposure early in pregnancy in 17 [39]. Autism was present in seven patients and was represented in both groups. Abnormal tearing was also reported in some [31,39,45].
What has been learned?
The potent teratogenicity appropriately discouraged other uses of thalidomide, although the drug has subsequently been tried for many diseases and medical problems. At present the FDA has approved its use for erythema nodosum leprosum (ENL) and multiple myeloma. Other uses are off label or on an experimental protocol [46–48]. The effectiveness of thalidomide in any particular condition will depend on its mechanism of action and at this time these mechanisms are only understood in a few situations [24,46–49]. It has been shown to have some sedative, anti-inflammatory, immunomodulatory, and antiangiogenic activity [24,40,46]. Nonetheless, it should not be used during pregnancy or in women of child bearing age who are fertile and not using contraception.
Thalidomide is a potent teratogen capable of causing severe ocular and systemic malformations when the fetus is exposed during the early 4th week of pregnancy (days 20–26±).
The most frequent ocular manifestation is incomitant strabismus, mostly of the Duane type syndrome type, but also including isolated abduction deficit with no palpebral fissure changes (exposure days 20–24±). Associated nonocular anomalies include external ear malformations, hearing deficits, facial nerve palsy, and thumb anomalies. As shown in Fig. 4, more severe upper limb, lower limb, and inner ear malformations occurred later in the sensitive period. Systemic anomalies involving the heart, kidney, and gastrointestinal system were not easy to associate with a particular exposure time but seemed to involve the mid to late periods.
There were no cases of comitant strabismus associated with early exposure, but in the cases observed (7) all had limb anomalies, suggesting a later exposure. The seven cases of concomitant strabismus noted in the thalidomide study had associated anomalies characteristic of later exposure to thalidomide (Fig. 5).
The association of Duane syndrome and facial nerve palsy is similar to characteristic findings in Möbius sequence, which can at times be caused by a teratogen, although the exposure time by history seems later.
Aberrant tearing, either inappropriate lacrimation with eating or lack of emotional tearing, is rare but frequently noted in individuals with embryopathy from early exposure to thalidomide. Most cases are associated with Duane syndrome.
Structural anomalies of the eye such as microphthalmos and/or uveal colobomas occur infrequently and suggest a slightly later, but abutting, exposure time. This later period is thought to be the thalidomide exposure time for many systemic malformations that might result in a spontaneous abortion. When eye malformations occur with serious systemic malformations, the fetus is more likely to be spontaneously aborted and the eye anomalies may never be noted.
Figure 4. Malformations of shoulders, upper limbs and hands in thalidomide embryopathy.
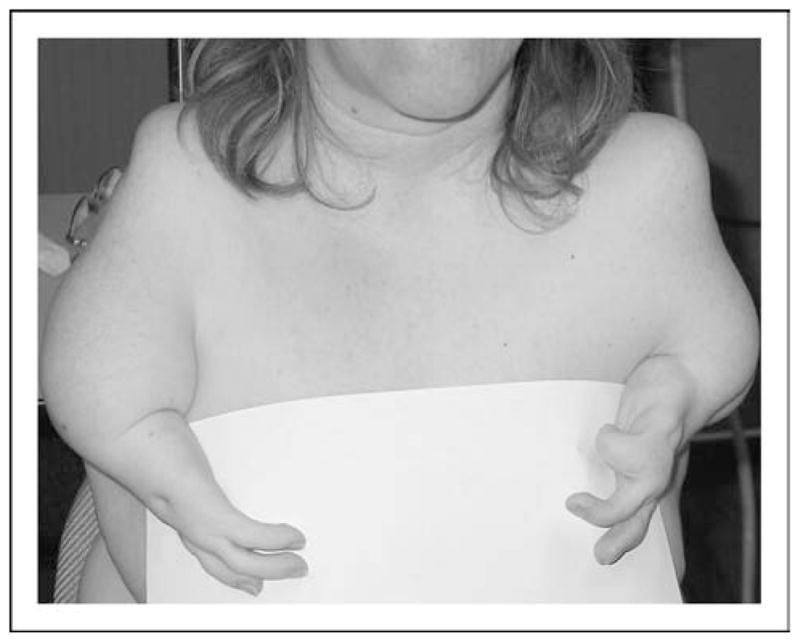
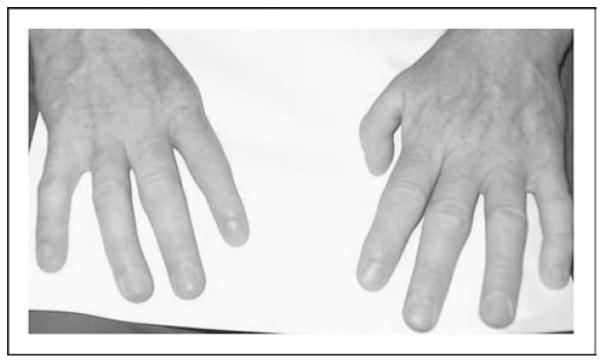
Figure 5. Hand malformations in thalidomide embryopathy, showing aplasia of the right and hypoplasia of the left thumb.
Conclusion
There have been many inspiring examples of the individuals with thalidomide embryopathy coping and overcoming their major handicaps. Many of these survivors agreed to the multiple studies done on thalidomide embryopathy. We owe much to these patients. Although the drug was only on the market in most countries for a few years in the early 1960s, these studies have given information on the time and location of the developmental insult that results in specific malformations and functional defects. There is overwhelming evidence that a disturbance in development at a very early time (days 20–26±), probably in the area of the sixth nerve nucleus, can result in Duane syndrome in association with aberrant tearing and facial nerve palsy. Möbius sequence and colobomatous microphthalmia appear to be manifestations of slightly later exposure.
Key points.
The thalidomide tragedy resulted in an increased awareness of possible teratogenic causes of ocular malformations.
The well established timetable of the period of sensitivity of this drug for some limb and ear anomalies allowed comparison with the associated ocular malformation and therefore speculation as to the sensitive period for these ophthalmologic malformations.
The high frequency of Duane syndrome noted in affected individuals was associated with early exposure to thalidomide (days 20 to ±24).
Acknowledgments
This study was supported in part by grant U19HD/DC35466 from Collaborative Programs of Excellence in Autism, National Institute of Child Health and Human Development, Rockville, Maryland; core grant EY 1792 from the National Eye Institute, Bethesda, Maryland; an unrestricted research grant from Research to Prevent Blindness Inc., New York, New York; and the Lions of Illinois Foundation, Maywood, Illinois (Dr Miller). This study was also supported in part by the Gothenburg Medical Society, Gothenburg, Sweden (Dr Strömland).
Footnotes
Conflicts of interest
There are no conflicts of interest.
References
- 1.Lenz W. A short history of thalidomide embryopathy. Teratology. 1988;38:203–215. doi: 10.1002/tera.1420380303. [DOI] [PubMed] [Google Scholar]
- 2.Kelsey FO. Thalidomide update: regulatory aspects. Teratology. 1986;38:217. doi: 10.1002/tera.1420380305. [DOI] [PubMed] [Google Scholar]
- 3.Brent RL, Holmes LB. Clinical and basic science from the thalidomide tragedy: what have we learned about the causes of limb defects? Teratology. 1988;38:241–251. doi: 10.1002/tera.1420380308. [DOI] [PubMed] [Google Scholar]
- 4.Stirling D, Sherman M, Strauss S. Thalidomide: a surprising recovery. J Am Phar Assoc. 1997;NS37:307–313. [PubMed] [Google Scholar]
- 5.Lenz W, Knapp K. Die thalidomide-embryopathie. Dtsch Med Wochenschr. 1962;87:1232–1242. doi: 10.1055/s-0028-1111892. [DOI] [PubMed] [Google Scholar]
- 6.McBride WG. Thalidomide and congenital abnormalities. Lancet. 1961;2:1358. [Google Scholar]
- 7.Smithells RW. Thalidomide and malformations in Liverpool. Lancet. 1962;1:1270–1273. doi: 10.1016/s0140-6736(62)92367-x. [DOI] [PubMed] [Google Scholar]
- 8.Lenz W. Malformations caused by drugs in pregnancy. Am J Dis Child. 1966;112:99–106. doi: 10.1001/archpedi.1966.02090110043001. [DOI] [PubMed] [Google Scholar]
- 9.Pliess G. Thalidomide and congenital abnormalities. Lancet. 1962;2:1128–1129. [Google Scholar]
- 10.Kida M, editor. Thalidomide embryopathy in Japan. Tokyo: Kodansha Ltd; 1987. pp. 3–4.pp. 275 [Google Scholar]
- 11.Smithells RW. Defects and disabilities of thalidomide children. Br Med J. 1973;1:269–272. doi: 10.1136/bmj.1.5848.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miehlke AU, Partsch CJ. Ohrmissbildung, Facialis-und Abducenslähmung als syndrom der Thalidomidschädigung. Arch Ohren-usw Heilk u Z Hals-usw Heilk. 1963;181:154. [Google Scholar]
- 13.Gilkes MT, Strode M. Ocular anomalies in association with developmental limb abnormalities of drug origin. Lancet. 1963;1:1026–1027. doi: 10.1016/s0140-6736(63)92429-2. [DOI] [PubMed] [Google Scholar]
- 14.Otto J. Conterganschäden mit Augenbeteiligung. Bericht Deutsch Ophthal Ges. 1964;65:220–221. [PubMed] [Google Scholar]
- 15.Papst W. Thalidomid und kongenitale anomalien der Augen. Bericht Deutsch Ophthal Ges. 1964;65:209–215. [PubMed] [Google Scholar]
- 16.Cullen JF. Ocular defects in thalidomide babies. Br J Ophthalmol. 1964;48:151–153. doi: 10.1136/bjo.48.3.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zetterström B. Ocular malformation caused by thalidomide. Acta Ophthalmol. 1966;44:391–395. doi: 10.1111/j.1755-3768.1966.tb08048.x. [DOI] [PubMed] [Google Scholar]
- 18.Arimoto H. Ocular findings of thalidomide embryopathy. Jpn J Clin Ophthalmology. 1979;33:501–507. [Google Scholar]
- 19.Arimoto Y. Ophthalmology in thalidomide embryopathy. In: Mida K, editor. Thalidomide embryopathy in Japan. Tokyo: Kadansha; 1987. pp. 143–153. [Google Scholar]
- 20.Tseng S, Pak G, Washenik K, et al. Rediscovering thalidomide: a review of its mechanism of action, side effects, and potential uses. J Am Acad Dermatol. 1996;35:969–979. doi: 10.1016/s0190-9622(96)90122-x. [DOI] [PubMed] [Google Scholar]
- 21.Nowack E. Die sensible Phase bei der Thalidomid-Embryopathie. Human-genetik. 1965;1:516–536. doi: 10.1007/BF00338341. [DOI] [PubMed] [Google Scholar]
- 22.Miller MT. Thalidomide embryopathy: a model for the study of congenital incomitant horizontal strabismus. Trans Am Ophthalmol Soc. 1991;89:623–674. [PMC free article] [PubMed] [Google Scholar]
- 23.Strömland K, Miller MT. Thalidomide embryopathy: revisited 27 years later. Acta Ophthalmol. 1993;71:238–245. doi: 10.1111/j.1755-3768.1993.tb04997.x. [DOI] [PubMed] [Google Scholar]
- 24.Miller MT, Strömland K. Teratogen update: thalidomide: a review, with a focus on ocular findings and new potential uses. Teratology. 1999;60:306–321. doi: 10.1002/(SICI)1096-9926(199911)60:5<306::AID-TERA11>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 25.Miller MT, Strömland K. Ocular motility in thalidomide embryopathy. J Pediatr Ophthalmol Strabismus. 1991;28:47–54. [PubMed] [Google Scholar]
- 26.Strömland K, Nordin V, Miller M, et al. Autism in thalidomide embryopathy: a population study. Dev Med Child Neurol. 1994;36:351–356. doi: 10.1111/j.1469-8749.1994.tb11856.x. [DOI] [PubMed] [Google Scholar]
- 27.Miller MT, Strömland K, Ventura L. Autism associated with conditions characterized by developmental errors in early embryogenesis. Int J Dev Neurosci. 2005;23:201–219. doi: 10.1016/j.ijdevneu.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 28.Bogorad F. Das symptom der Krokodilstränen. Zbl Ophthalmologie. 1930;22:534. [Google Scholar]
- 29.Zhang F. Clinical features of 201 cases with Duane’s retraction syndrome. Clin Med J (Engl) 1997;110:789–791. [PubMed] [Google Scholar]
- 30.Zhang F. A clinical analysis of 25 cases with Duane’s retraction syndrome combined with congenital crocodile tears. Zhonghua Wai Ke Za Zhi. 2002;38:217–219. [PubMed] [Google Scholar]
- 31.Miller MT, Strömland K, Ventura L. Congenital aberrant tearing: a relook. Trans Am Ophthalmol Soc. 2008;106:1–17. [PMC free article] [PubMed] [Google Scholar]
- 32.Maruo T, Kubota N, Arimoto H, Kikuchi R. Duane’s syndrome. Jpn J Ophthal-mol. 1979;23:453–468. [Google Scholar]
- 33.Trieschmann W. Krokodilstränen bei Conterganschäden (Crocodile tears in thalidomide embryopathy) Klin Monatsbl Augenheilkd. 1994;162:546–550. [PubMed] [Google Scholar]
- 34.Uemura Y, Tamura H. Congenital gustato-lacrimal syndrome. Jpn J Clin Ophthalmol. 1968;22:489–495. [Google Scholar]
- 35.Takemori S, Tanaka Y, Suzuki JJ. Thalidomide anomalies of the ear. Arch Otolaryngol. 1976;102:425–427. doi: 10.1001/archotol.1976.00780120073010. [DOI] [PubMed] [Google Scholar]
- 36.Jacklin HN. The gusto-lacrimal reflex (syndrome of crocodile tears): report of cases with tear analysis. Am J Ophthalmol. 1966;61:1521–1526. doi: 10.1016/0002-9394(66)90496-x. [DOI] [PubMed] [Google Scholar]
- 37.Ramsay J, Taylor D. Congenital crocodile tears: a key to the etiology of Duane’s syndrome. Br J Ophthalmol. 1980;64:518–522. doi: 10.1136/bjo.64.7.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Elsahy NI. Moebius syndrome associated with the mother taking thalidomide during gestation. Plast Reconstr Surg. 1973;51:93–95. doi: 10.1097/00006534-197301000-00026. [DOI] [PubMed] [Google Scholar]
- 39.Miller MT, Ventura L, Strömland K. Thalidomide and misoprostol: ophthalmologic manifestations and associations both expected and unexpected. Birth Defects Res A Clin Mol Teratol. 2009;85:667–676. doi: 10.1002/bdra.20609. [DOI] [PubMed] [Google Scholar]
- 40.Strömland K, Sjögreen L, Miller MT, et al. Möbius sequence: a Swedish multidiscipline study. Eur J Pediatr Neurol. 2002;6:35–45. doi: 10.1053/ejpn.2001.0540. [DOI] [PubMed] [Google Scholar]
- 41.Miller MT, Strömland K. The Möbius sequence: a relook. J AAPOS. 1999;3:199–208. doi: 10.1016/s1091-8531(99)70003-0. [DOI] [PubMed] [Google Scholar]
- 42.Gillberg C, Steffenburg S. Autistic behaviour in Moebius syndrome. Acta Paediatr. 1989;79:314–326. doi: 10.1111/j.1651-2227.1989.tb11076.x. [DOI] [PubMed] [Google Scholar]
- 43.Johansson M, Wentz E, Fernell E, et al. Autistic spectrum disorders in Möbius sequence: a comprehensive study of 25 individuals. Dev Med Child Neurol. 2001;43:338–345. doi: 10.1017/s0012162201000627. [DOI] [PubMed] [Google Scholar]
- 44.Gonzalez CH, Marques Dias MJ, Kim CA, et al. Congenital abnormalities in Brazilian children associated with misoprostol misuse in first trimester of pregnancy. Lancet. 1998;351:1624–1627. doi: 10.1016/S0140-6736(97)12363-7. [DOI] [PubMed] [Google Scholar]
- 45.Bandim JM, Ventura LO, Miller MT, et al. Autism and Möbius sequence: an exploratory study of children in northwestern Brazil. Arq Neuropsiquiatr. 2003;61:181–185. doi: 10.1590/s0004-282x2003000200004. [DOI] [PubMed] [Google Scholar]
- 46.Chen M, Doherty SD, Hsu S. Innovative uses of thalidomide. Dermatol Clini. 2010;28:577–586. doi: 10.1016/j.det.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 47.Wu JJ, Huang DB, Pang KR, et al. Thalidomide: dermatological indications, mechanisms of action and side-effects. Br J Dermatol. 2005;153:253–254. doi: 10.1111/j.1365-2133.2005.06747.x. [DOI] [PubMed] [Google Scholar]
- 48.Perri AJ, III, Hsu S. A review of thalidomide’s history and current dermatological applications. Dermatology Online J. 2003;9:5. [PubMed] [Google Scholar]
- 49.Ito T, Ando H, Suzuki T, et al. Identification of a primary target of thalidomide teratogenicity. Science. 2010;327:1345–1350. doi: 10.1126/science.1177319. [DOI] [PubMed] [Google Scholar]