Abstract
Background
Vitamin D deficiency is highly prevalent and associated with dyslipidemia and cardiovascular disease. The impact is unknown of correcting vitamin D deficiency on blood lipids, strong cardiovascular disease prognostic factors.
Methods and Results
To determine relationships between 25-hydroxyvitamin D levels and lipids, we analyzed 4.06 million de-identified patient laboratory test results from September 2009 through February 2011. We performed a cross-sectional study of this population to determine associations between 25-hydroxyvitamin D levels and lipids across clinically defined strata. We also conducted a retrospective cohort study of vitamin D deficient patients to investigate how changes in 25-hydroxyvitamin D levels relate to changes in lipid levels. After exclusions, 108,711 patients with serial testing were selected for cross-sectional analysis. Compared to vitamin D deficient patients (<20 ng/ml), those with optimal levels ≥30 ng/ml) had lower mean total cholesterol (−1.9 mg/dl [95% CI (−1.2, −2.7 mg/dl)]; p <.0001), lower LDL cholesterol (−5.2 mg/dl [95% CI (−4.5, −5.8 mg/dl)]; p <.0001), higher HDL cholesterol (4.8 mg/dl [95% CI (4.5, 5.0 mg/dl)]; p <.0001), and lower triglycerides (−7.5 mg/dl [95% CI (−6.2, −8.7 mg/dl)]; p <.0001). For the retrospective cohort analysis, raising vitamin D levels from <20 ng/ml to ≥30 ng/ml (n = 6,260), compared to those remaining <20 ng/ml (n = 2,332), was associated with a mean increase in total cholesterol (0.77 mg/dl [95% CI (0.18, 1.36 mg/dl)]; p = .01) and HDL cholesterol (0.42 mg/dl [95% CI (0.08, 0.76 mg/dl)]; p = 0.02), but non-significant changes in LDL cholesterol (0.32 mg/dl [95% CI (−0.01, 0.66 mg/dl)]; p = .06) and triglycerides (0.04 mg/dl [95% CI (−2.16, 2.23 mg/dl)]; p = .97)
Conclusions
While vitamin D deficiency is associated with an unfavorable lipid profile in cross-sectional analyses, correcting for a deficiency might not translate into clinically meaningful changes in lipid concentrations, although data from intervention trials is required to confirm these findings.
Keywords: cholesterol, cholesterol reduction, lipids, vitamin D
Introduction
Vitamin D is a steroid hormone that is present in some foods, but is synthesized mainly in response to ultraviolet light exposure. After ingestion or endogenous synthesis, vitamin D is hydroxylated by the liver to form 25-hydroxyvitamin D (25(OH)D), the predominant form of vitamin D in circulation.1 Two forms are important in humans: ergocalciferol (vitamin D2) and cholecalciferol (vitamin D3). Vitamin D2 is synthesized by plants whereas vitamin D3 is synthesized in the skin upon exposure to specific ultraviolet B (UVB) rays. Foods may be fortified with and supplements may include either vitamin D2 or D3.
Epidemiologic studies suggest an inverse association between circulating levels of 25(OH)D and cardiovascular risk biomarkers, including an atherogenic lipid profile.2, 3 Vitamin D deficiency is highly prevalent and can be effectively treated through oral repletion. However, a role for supplementation in modifying cardiovascular risk has not been well defined, and, it is unclear whether vitamin D status is causally related to disease or merely a marker of health.4 This is relevant for practitioners as well as the general population, because of the increasing consumption of pharmacologic doses of vitamin D sold over-the-counter.
Cross-sectional studies are unable to assess the longitudinal effects of changes in 25(OH)D levels on standard cardiovascular risk biomarkers. Although, randomized clinical trials of vitamin D supplementation would provide a higher level of evidence, studies to date have shown conflicting results.5–14 These studies were limited by relatively small sample sizes, confounding effects of vitamin D with additional calcium supplementation, and study designs that did not specifically target vitamin D deficiency, or did not use a sufficient dose of vitamin D to achieve a consensus “optimal” level of ≥30 ng/ml.
In the absence of definitive evidence from randomized, controlled trials (RCT), data mining is becoming an increasingly valuable tool for rapidly and cost-effectively generating and testing hypotheses. Quest Diagnostics has the largest private database of patient laboratory test data. We analyzed de-identified results from this database to compare cross-sectional and longitudinal approaches to studying the relationship between 25(OH)D levels and blood lipids. In the cross-sectional approach, we studied the association between 25(OH)D levels and the lipid panel in a large population derived from medical practices broadly across the United States. For the longitudinal approach, we identified a cohort from the same population to determine how changes in 25(OH)D levels are related to changes in lipid levels.
Given the absence of clear evidence from RCT, we believe our longitudinal cohort analysis introduces a novel approach to exploring these important biomarker relationships. We studied a very large national sample relatively quickly and inexpensively, whereas an analogous prospective, randomized, controlled trial would take years to complete and possibly be prohibitively expensive. Because vitamin D deficiency and dyslipidemia are so prevalent, it is important for clinicians to have better evidence on which to base treatment decisions in a timely manner. We believe that our longitudinal analysis fills this gap between cross-sectional reports and a resource-intensive clinical trial, the results of which would not be available for many years.
Methods
Patients
Quest Diagnostics has more than 145 million annual patient encounters across the United States. Test results are stored in a private, clinical database. 4.06 million patient records included simultaneous 25(OH)D and lipid panel tests between September 2009 and February 2011, and were selected and de-identified for analysis.
Cross-Sectional Study Population
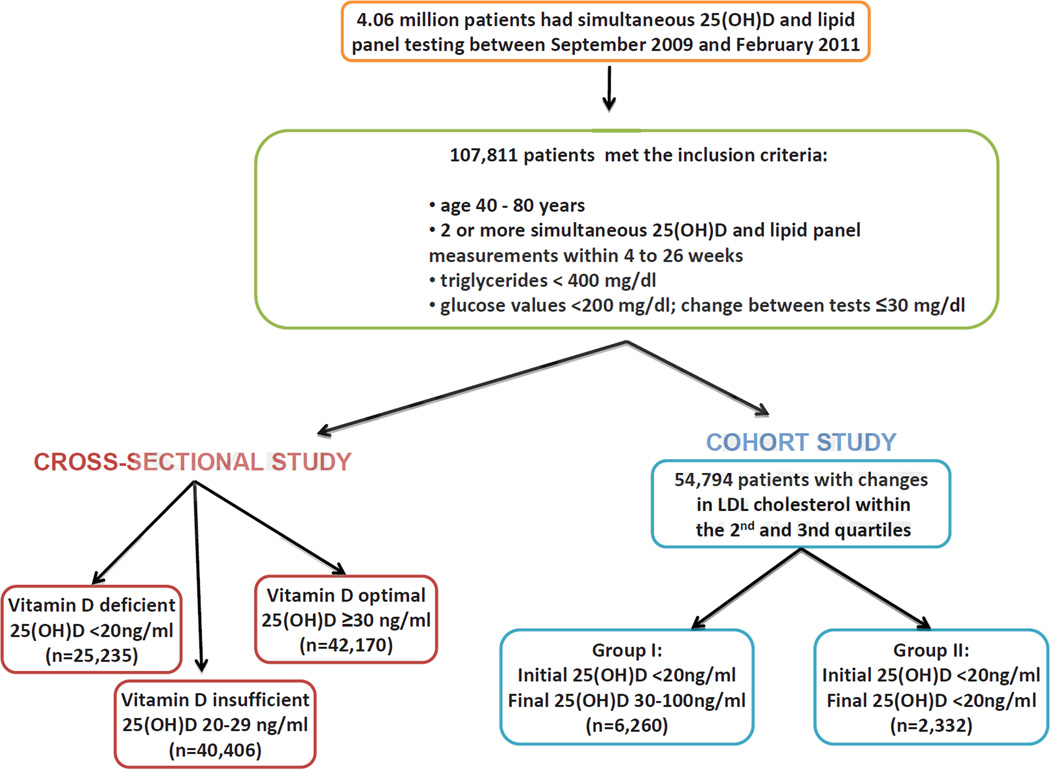
Of these patients, 107,811 records met the following inclusion criteria: ages 40 to 80 years; had two or more simultaneous 25(OH)D, lipid panels, and glucose tests within 4 and 26 weeks apart, inclusive; triglycerides <400 mg/dl and glucose <200 mg/dl for both tests; and the absolute difference between the first and second glucose values ≤30 mg/dl (Figure 1). The glucose restrictions were intended to exclude patients who fasted inconsistently, or may not have fasted, or who had poorly-controlled diabetes. We stratified 25(OH)D results into consensus clinical strata: deficient <20 ng/ml, n = 25,235), insufficient (20 – 29 ng/ml, n = 40,406), and optimal (≥30 ng/ml, n = 42,170) 25(OH)D.
Figure 1.
Description of Patient Populations for Cross-Sectional and Cohort Studies. To convert values of 25-hydroxyvitamin D to nmol/l multiply by 2.496.
Longitudinal Study Population
From these 107,811 patients, we determined the distribution of LDL cholesterol level change between the initial and final visits (Figure 2). To exclude patients who may have had changes in lipid-lowering therapy, the analysis was limited to patients in the second and third quartile of the LDL cholesterol change distribution. This excluded patients whose change in LDL cholesterol measurements were ≤15 or >10 mg/dl. 54,794 patients met this criterion, and from these, two sub-groups were selected: Group I (the “repletion” group) included 6,260 patients whose initial 25(OH)D concentration was <20 ng/ml and whose immediate subsequent 25(OH)D concentration was ≥30 and ≤100 ng/ml. Group II (the “control” group) included 2,332 patients whose initial and immediate subsequent 25(OH)D concentrations were both <20 ng/ml.
Figure 2.
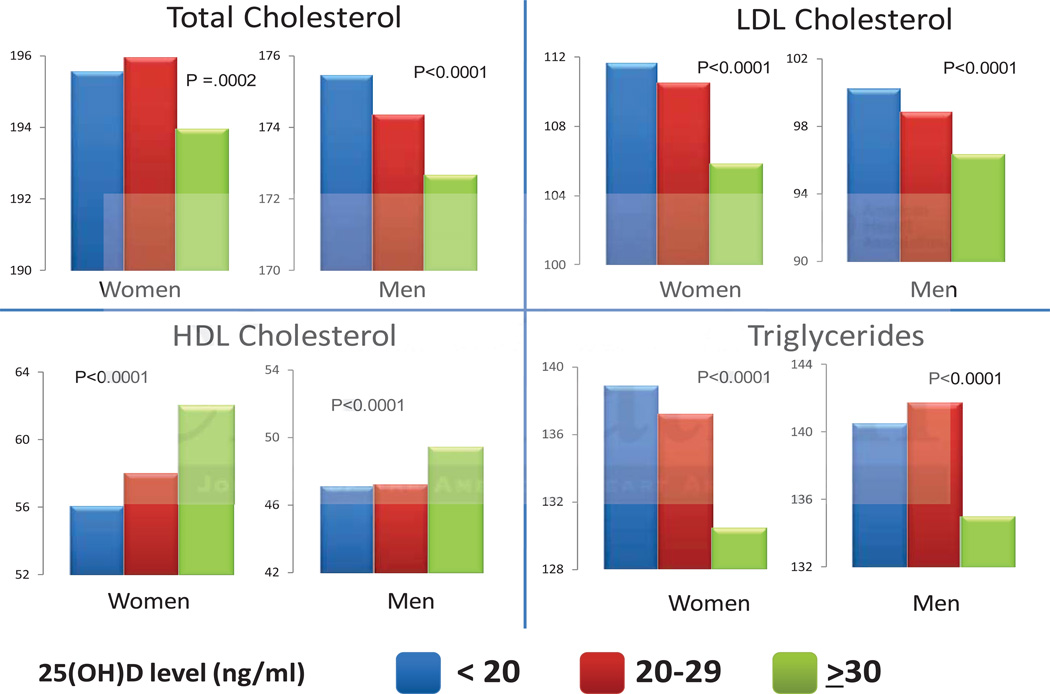
Associations Between 25(OH)D Levels and Lipid Parameters. Patients within each stratum of 25(OH)D level were matched for age and month of testing. P-values indicate the comparisons of 25(OH)D of <20 and ≥30 ng/ml groups. To convert values of total cholesterol, LDL cholesterol, and HDL cholesterol to mmol/l multiply by 0.02586. To convert values of triglycerides to mmol/l multiply by 0,1129.
Laboratory Methods
25(OH)D measurements were performed by liquid chromatography with tandem quadrupole mass spectrometers (LC-MS/MS) (ThermoFisher, San Jose, CA). This method measures the area under the curve (AUC) for vitamin D2, vitamin D3, and total 25(OH)D. The cross-sectional study only reports values based on the AUC for 25(OH)D and if the AUC was less than 4.0 ng/ml a value of 3.9 ng/ml was assigned. In the longitudinal study total 25(OH)vitamin D and 25-hydroxyvitamin D3 were based on the AUC for 25(OH)D and 25-hydroxyvitamin D3, whereas 25-hydroxyvitamin D2 was the AUC for 25(OH)D minus the AUC for 25-hydroxyvitamin D3. In the longitudinal study, 0.3% of patients had total 25(OH)D less than 4.0 ng/ml and these values were arbitrarily set to 3.9 ng/ml; 3% of patients had 25-hydroxyvitamin D3 less than 4 ng/ml and these values were arbitrarily set to zero. Accuracy of testing was monitored by participation in the Vitamin D External Quality Assurance (DEQAS) program.15
Both glucose and calcium measurements were performed on Beckman Coulter AU analyzers using glucose hexokinase and calcium arsenazo methods, respectively (Beckman Coulter, Inc. Brea, CA). Accuracy of testing was monitored by participation in the College of American Pathologists General Chemistry Survey (College of American Pathologists, Northfield, IL). The Friedewald equation was used to calculate LDL cholesterol levels.16
Statistical Analysis
In the cross-sectional study, the Least Squares (LS) Means from GLM regression models were used to detect associations between baseline 25(OH)D and the lipid measurements. Each lipid value was regressed against initial 25(OH)D status and age. Dummy variables were used to control for gender and month of testing. Pairwise comparisons of LS Means between the 25(OH)D groups were evaluated using the Tukey method. Statistical significance was taken as p<0.05.
In the longitudinal study, baseline characteristics for the lipid panel, 25(OH)D, glucose, calcium, age, gender, weeks between testing, and the top 10 ICD-9 codes were calculated for patients in the repletion and control groups. To compare baseline statistics between repletion and control groups, the two-sided, unpaired Student’s t-test was used for quantitative variables; and the two proportion z-test for categorical variables, such as gender and top ICD-9 codes. Inter-group differences in lipid changes between the repletion and control groups were assessed using unpaired two-sample t-tests. Statistical significance was taken as p<0.05.
For additional analysis assessing the impacts of seasonality and the interval between testing on the longitudinal study, see Supplemental Material.
Human Subject Protection
This study was reviewed by The Rockefeller University Institutional Review Board and Western Institutional Review Board and considered “an exempt activity requiring no further IRB review.”
Results
To determine the association between 25(OH)D levels and components of the lipid panel, we performed a cross-sectional analysis in a sample population stratified by consensus clinical 25(OH)D levels: those with deficient (<20 ng/ml), insufficient (20–29 ng/ml) and optimal (≥30 ng/ml) 25(OH)D levels (Figure 1). Mean 25(OH)D levels for these groups were 14.2, 24.8, and 40.0 ng/ml, respectively. Compared to the group with deficient levels, the group with optimal 25(OH)D levels had a statistically significant, healthier lipid panel: lower total and LDL cholesterol, higher HDL cholesterol and lower triglycerides (Table 1). The intermediate group with insufficient 25(OH)D levels showed a directionally consistent, intermediate association with a favorable lipid panel as compared to the group with deficient 25(OH)D levels. These associations were similar for both men and women.
Table 1.
Cross-sectional associations between 25(OH)D and lipids. Lipid parameters are mean values (mg/dl)
| 25(OH)D strata (ng/ml) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lipid Parameter |
<20 | 20–29 | ≥30 | <20 vs. ≥30 | 95% CI) | P value | <20 vs 20–29 | 95% CI) | P value | 20–29 vs ≥30 | 95% CI) | P value | |
| Women (# of patients) | (16,812) | (25,694) | (30,011) | ||||||||||
| Total | |||||||||||||
| Cholesterol | 195.6 | 196.0 | 194.0 | 1.58 | (0.65, 2.52) | .0002 | −0.38 | (−1.34, 0.58) | 0.62 | 1.96 | (1.14, 2.78) | <.0001 | |
| LDL | |||||||||||||
| Cholesterol | 111.7 | 110.6 | 106.0 | 5.73 | (4.91, 6.56) | <.0001 | 1.11 | (0.27, 1.96) | <.01 | 4.62 | (3.89, 5.34) | <.0001 | |
| HDL | |||||||||||||
| Cholesterol | 56.2 | 58.1 | 62.1 | −5.88 | (−6.25, −5.52) | <.0001 | −1.86 | (−2.24, −1.49) | <.0001 | −4.02 | (−4.34, −3.70) | <.0001 | |
| Triglycerides | 139.0 | 137.3 | 130.5 | 8.48 | (7.01, 9.95) | <.0001 | 1.37 | (0.16, 3.17) | 0.03 | 6.81 | (5.52, 8.10) | <.0001 | |
| Men (# of patients) | (8423) | (14712) | (12159) | ||||||||||
| Total | |||||||||||||
| Cholesterol | 175.5 | 174.4 | 172.7 | 2.80 | (1.47, 4.12) | <.0001 | 1.09 | (−0.18, 2.37) | 0.11 | 1.71 | (0.57, 2.84) | <.002 | |
| LDL | |||||||||||||
| Cholesterol | 100.3 | 98.9 | 96.4 | 3.87 | (2.71, 5.02) | <.0001 | 1.39 | (0.28, 2.50) | <.01 | 2.47 | (1.49, 3.46) | <.0001 | |
| HDL | |||||||||||||
| Cholesterol | 47.2 | 47.3 | 49.5 | −2.22 | (−2.67, −1.78) | <.0001 | −0.10 | (−0.53, 0.32) | 0.84 | −2.12 | (−2.50, −1.74) | <.0001 | |
| Triglycerides | 140.6 | 141.8 | 135.1 | 5.54 | (3.23, 7.86) | <.0001 | −1.20 | (−3.42, 1.01) | 0.41 | 6.75 | (4.68, 8.72) | <.0001 | |
To explore this further we performed a cohort analysis to determine if an increase in 25(OH)D, from a deficient to an optimal level, was associated with an improvement in the lipid panel. 107,811 patients satisfied the inclusion criteria (Figure 1). To minimize the confounding effect of possible changes in lipid lowering therapy, we further excluded individuals whose change in LDL cholesterol level was in the first or fourth quartiles of change in LDL cholesterol. From the remaining patients (n = 54,794), we used changes in 25(OH)D levels to identify repletion and control groups, Group I (n = 6,260) and Group II (n = 2,332), respectively.
The average age of patients in Group I was 60.6 (±10.6) years, compared to 58.9 (±10.9) years for patients in Group II (Table 2). Both groups had a similar proportion of women (approximately two-thirds). Baseline 25(OH)D levels for Group I were 14.3 ±3.8 ng/ml, compared to 13.2 ±3.9 ng/ml for Group II. Baseline lipid, glucose, and calcium levels were clinically similar between groups, though the control group had statistically higher LDL cholesterol, lower triglycerides, and lower calcium levels.
Table 2.
Baseline Characteristics for the Cohort Study. Values are shown as mean (+/− SD).
| Group I | Group II | P-value | |
|---|---|---|---|
| Number | 6,260 | 2,332 | |
| Age (years) | 60.6 (+/−10.6) | 58.9 (+/−10.9) | <.0001 |
| Gender (% women) | 66.0 | 66.7 | 0.40 |
| Time between measurements (weeks) | 17.6 (+/−5.3) | 17.3 (+/−5.7) | 0.01 |
| Initial 25(OH)D (ng/ml) | 14.3 (+/−3.8) | 13.2 (+/−3.9) | <.0001 |
| Total Cholesterol (mg/dl) | 178.9 (+/− 37.9) | 180.2 (+/− 39.5) | 0.18 |
| LDL Cholesterol (mg/dl) | 98.8 (+/− 32.5) | 101.4 (+/− 33.4) | 0.001 |
| HDL Cholesterol (mg/dl) | 53.5 (+/− 15.6) | 53.5 (+/− 15.9) | 0.53 |
| Triglycerides (mg/dl) | 133.5 (+/− 63) | 128.0 (+/− 63) | 0.0001 |
| Glucose (mg/dl) | 101.8 (+/− 20.2) | 101.0 (+/− 21.1) | 0.13 |
| Calcium (mg/dl) | 9.41 (+/−0.40) | 9.35 (+/− 0.45) | <.0001 |
| Creatinine (mg/dl) | 0.92 (+/− 0.34) | 0.92 (+/− 0.43) | 0.74 |
Table 3 shows the frequency of the most common ICD-9 codes listed on the initial laboratory requisitions. Patients in Group I were more likely to receive ICD-9 codes indicative of dyslipidemia and hypertension compared to controls. However, there was no statistical difference between groups in the designation of vitamin D deficiency or the common co-morbidities of diabetes and hypothyroidism.
Table 3.
Most Common ICD-9 Diagnosis Codes
| Rank | ICD-9 Code |
Description | % of Patients with the ICD-9 Code | P-value | |
|---|---|---|---|---|---|
| Group I (Repletion) | Group II (Control) | ||||
| 1 | 272 | Disorders of lipoid metabolism | 56 | 49.1 | <0.0001 |
| 2 | 401 | Essential hypertension | 37.9 | 33.5 | <0.0001 |
| 3 | 250 | Diabetes mellitus | 25.8 | 25.9 | 0.48 |
| 4 | 780 | General symptoms | 12.6 | 11.3 | 0.04 |
| 5 | V70 | General medical examination | 11.6 | 10.7 | 0.11 |
| 6 | 244 | Acquired hypothyroidism | 11.4 | 10.1 | 0.04 |
| 7 | 268 | Vitamin D deficiency | 9.7 | 9.5 | 0.42 |
| 8 | V58 | Other and unspecified aftercare | 6.8 | 5.9 | 0.05 |
| 9 | 790 | Nonspecific findings on examination of blood | 5.9 | 6 | 0.48 |
| 10 | 733 | Other disorders of bone and cartilage | 5 | 4.3 | 0.07 |
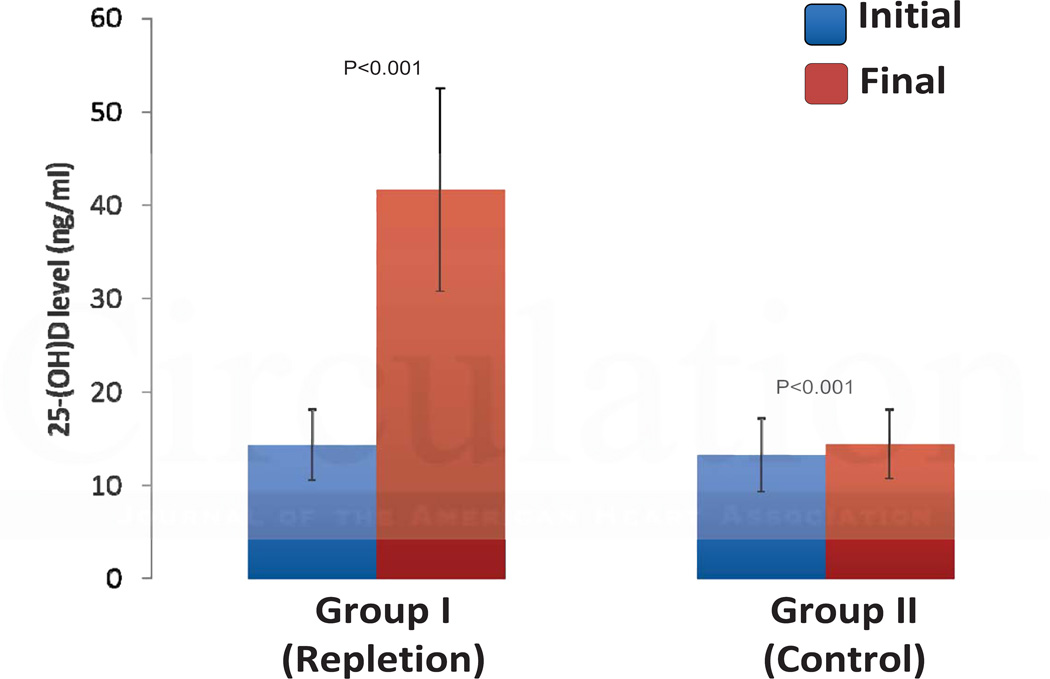
For Group I, the repletion group, 25(OH)D levels increased an average of 27.3 ng/ml, from a mean value of 14.3 ±3.8 ng/ml to 41.6 ±10.9 ng/ml (Figure 3). In contrast, initial and final mean 25(OH)D levels remained deficient for Group II, the control group, (13.2 ±3.9 vs. 14.1 ±3.7 ng/ml), an increase of only 0.9 ng/ml. Since vitamin D2 is not endogenously produced, we analyzed 25-hydroxyvitamin D2 and D3 levels at initial and final visits for both Groups I and II to provide evidence that the increase in Group I was due to supplementation. In the repletion group, 25-hydroxyvitamin D2 levels were detectable (>4 ng/ml) in only 4.6% of patients at baseline, but in 69.2% at the final measurement. In the control group, 25-hydroxyvitamin D2 levels were detectable in 5.3% of patients at baseline and in 12.1% at the final measurement. Correspondingly, in the repletion group, 25-hydroxyvitamin D2 increased 22.2 ng/ml of a total 25(OH)D increase of 27.3 ng/ml, compared to the control group where 25-hydroxyvitamin D2 levels increased 0.6 ng/ml of a total 25(OH)D increase of 0.9 ng/ml. Thus 81% of the increase in 25-hydroxyvitamin D levels in the repletion group was due to 25-hydroxyvitamin D2, proving a major role for exogenous supplementation. Since vitamin D3 is made endogenously but can also come from supplementation, this is a minimum figure.
Figure 3.
Changes in 25(OH)D Levels in the Cohort Study. Graph display the mean 25(OH)D riglycerides and standard deviation. To convert values of 25-hydroxyvitamin D to nmol/l multiply by 2.496.
At the final visit, total cholesterol decreased 2.04 mg/dl in the repletion group compared to a 2.81 mg/dl decrease in the control group, for a relative increase of 0.77 mg/dl (p = .01) in the repletion group (Table 4). An increase in 25(OH)D levels was also associated with an increase in HDL cholesterol 0.42 mg/dl (p = .02). No statistically significant inter-group differences were observed for changes in LDL cholesterol or triglycerides.
Table 4.
Changes in Lipid Parameters for Repletion and Control Groups
| Lipid Parameter (mg/dL) | Group I (Repletion) | Group II (Control) | Inter-group Difference | 95% CI |
|---|---|---|---|---|
| Total Cholesterol | −2.04 | −2.81 | 0.77 | (0.18, 1.36) |
| LDL Cholesterol | −2.00 | −2.32 | 0.32 | (−0.01, 0.66) |
| HDL Cholesterol | 0.23 | −0.19 | 0.42 | (0.08, 0.76) |
| Triglycerides | −1.48 | −1.51 | 0.04 | (−2.16, 2.23) |
Discussion
We analyzed a large national clinical laboratory database to determine relationships between 25(OH)D levels and components of the lipid panel. In the cross-sectional analysis, the “optimal” group relative to the “deficient” 25(OH)D group displayed lower total cholesterol (−1.9 mg/dl), lower LDL cholesterol (−5.2 mg/dl), higher HDL cholesterol (4.8 mg/dl), and lower triglycerides (−7.5 mg/dl). The statistically significant cross-sectional association between 25(OH)D levels and components of the lipid panel is consistent with other cross-sectional studies and suggests a possible causal relationship. However, association studies cannot be used to infer causality. Indeed, in contrast to cross-sectional data, the longitudinal analysis showed that increasing 25(OH)D levels from the deficient to optimal range (“repletion” group), compared to remaining in the deficient range (“control” group), was associated with small and clinically minimal effects on total cholesterol 0.8 mg/dl increase) and HDL cholesterol (0.4 mg/dl increase), and no significant changes in LDL cholesterol or triglycerides levels. These longitudinal data contrast with the purported benefits of vitamin D repletion on the lipid profile inferred from cross-sectional studies.17–20
These novel findings and approach provide a different type of evidence for clinical practice guidelines than existing association studies. Clinicians are still awaiting the results of large, randomized, placebo-controlled outcome trials of vitamin D supplementation.21 In the absence of clinical trials, this novel, inexpensive approach fills a gap for quickly examining the effect of vitamin D repletion on the lipid panel, a major predictive biomarker of cardiovascular risk. Moreover, and particularly important for patient-oriented research, the data were obtained from patient encounters, in a settings reflective of true clinical practice across the United States.
Our cross-sectional analysis is concordant with other vitamin D/lipid association studies showing that higher 25(OH)D levels are associated with a healthier lipid profile.17–20 This validates the use of the Quest Diagnostics database because it replicated the known associations between 25(OH)D and the lipid profile found in other cross-sectional studies. For example, the largest published association study (n = 15,088), based on NHANES III, found that mean 25(OH)D levels were lower in subjects with hypertriglyceridemia and hypercholesterolemia.17 The same study also compared the age-, sex-, and race-adjusted prevalence rates of cardiovascular risk factors between the highest (≥92.4 nmol/l [≥37ng/ml]) and lowest (≤52.4 nmol/l [<21 ng/ml]) quartiles of 25(OH)D levels and found that hypertriglyceridemia was more prevalent in the lowest quartile of 25(OH)D levels. Using a similar approach of stratifying by 25(OH)D levels, we were able to supplement the findings of the NHANES III analysis by also identifying trends in LDL and HDL cholesterol levels.
Given the large size of our database, we were able to control for patient age, gender, and month of testing. We had enough patient records to match patients into clinically relevant strata of 25(OH)D levels: <20, 20–29, and 30–100 ng/ml. Therefore, we were able to achieve higher statistical power to identify clinically significant relationships, which show a step-wise association of higher 25(OH)D levels with better lipid panel results. Furthermore, compared to NHANES III, our data contained a greater proportion of patients with hypertension (32.5% vs. 28.4%) and diabetes (29.9% vs. 8.4%), which is more reflective of the patient population at increased cardiovascular risk and those seeking medical care.17
The difference in lipid levels between the optimal and deficient vitamin D groups in our cross-sectional study suggests a possible 12% reduction in the imputed relative risk of cardiovascular disease!22, 23 Association studies, however, do not prove cause and effect.
RCT have been published to investigate a causal relationship between vitamin D supplementation and changes in the lipid panel.5–9, 11 The largest was the Women’s Health Initiative (WHI). A subgroup analysis of 1,191 women found no effect of vitamin D supplementation on lipids over a 5-year period.7 However, the WHI highlights many pitfalls common to prior studies, preventing a definitive conclusion of the effect of vitamin D repletion on lipids.24 First, many study participants were not vitamin D deficient at baseline. Furthermore, the dose of vitamin D (400 IU per day) was likely too small to meaningfully separate treatment and placebo groups, especially when subjects in both arms were allowed to take non-study vitamin D supplements. A third limitation was the co-administration of calcium supplements, which may have confounded the effect of vitamin D. There was also relatively poor compliance: only 60% of participants took >80% of the study drug. Other trials suffered from small sample sizes that lacked sufficient statistical power.5–14, 25 Thus, there are no intervention studies that clearly address the effect of vitamin D repletion on lipid levels.
A new generation of vitamin D supplementation trials may provide more definitive evidence of vitamin D supplementation on heart disease outcomes.21 These well-powered trials use a higher dose of vitamin D than the WHI and will test the effect of vitamin D without calcium. However, they do not specifically target vitamin D deficiency and the results will be unavailable for several years.
In the absence of rigorous RCT, we employed the Quest Diagnostics database to conduct a retrospective cohort analysis. This method has several advantages over association studies. Foremost, it presents a different type of evidence by analyzing changes in 25(OH)D over time to directly test the effect of raising 25(OH)D from deficient to optimal levels. The database was large enough to identify 8,592 individuals who met the specified inclusion criteria. The longitudinal patient cohorts were derived from the cross-sectional patient pool. As a surrogate for active intervention, we identified large changes in 25(OH)D levels in serial measurements over a relatively short time period (between 4 and 26 weeks, inclusive). While season and diet can influence 25(OH)D levels, the near tripling of mean 25(OH)D levels are best explained by pharmacologic intervention. This is supported by the large rise in 25-hydroxyvitamin D2 levels, which is exclusively derived from exogenous sources. To better approximate a controlled trial where changes in lipid-lowering therapy would be precluded, we excluded individuals in the first and fourth quartiles of changes in LDL cholesterol level. Our cohort analysis showed that the magnitude and direction of the changes in serial lipid values were remarkably inconsistent with the association data. Therefore, vitamin D repletion may fail to mimic the change in the lipid panel expected from association studies. To our knowledge, this is the first time this discordance has been shown.
It is unclear why vitamin D supplementation appears not to improve the lipid profile. The simplest explanation may be that vitamin D has no effect on lipid metabolism. Vitamin D status may be a surrogate marker of health without a causal role. For example, obesity in particular has been independently associated with low 25(OH)D levels and dyslipidemia.26, 27 Indeed, weight loss raises 25(OH)D levels and improves the lipid profile28, 29. Because vitamin D is fat-soluble, adipose tissue may serve as site of sequestration of vitamin D, effectively trapping it and lowering circulating levels of 25(OH)D. This phenomenon may explain the associations between 25(OH)D levels, as well as the lipid profile and the failure of 25(OH)D repletion to improve the lipid profile. Another possibility is that the lipid profile influences vitamin D levels and not the converse; dyslipidemia itself may lower vitamin D levels. Alternatively, reports have demonstrated that rosuvastatin, atorvastatin, simvastatin and lovastatin can lead to a significant rise in 25(OH)D levels over a period of weeks.30–33 Therefore, statin use may contribute to a healthier lipid profile and higher 25(OH)D levels without a direct effect of vitamin D on lipids. Finally, vitamin D absorbed through the gut may have different effects on lipid metabolism from vitamin D synthesized in the skin. Intestinal epithelium possesses 25-hydroxylase and 1-β-hydroxylase activity.34–36 Therefore, oral vitamin D can be locally converted to the active 1,25-dihydroxyvitamin D metabolite and induce autocrine signals within enterocytes. Indeed, oral vitamin D is known to stimulate Fgf15 production by intestinal epithelia, which alters bile acid homeostasis in mice.37 How changes in FGF19 (the human homolog of Fgf15), in response to vitamin D supplementation, may impact cholesterol, the precursor of bile acids, remains to be studied.
There are limitations to our methods. Patient medical records were unavailable, which may have led to inadequate recognition of factors that influence the lipid panel, such as diabetes and dietary history, as well as potential confounders including body mass index, physical activity, sun exposure or medications.. Also, while we were unable to evaluate the impact of ethnicity, there are no ethnicity-specific thresholds for 25(OH)D deficiency or treatment. Where possible, we developed database rules to approximate exclusion criteria that RCT might employ to explore the causal relationship between vitamin D repletion and lipid panel. We attempted to eliminate patients with poorly-controlled diabetes, as well as patients who may not have been adequately and consistently fasting, by imposing criteria related to glucose levels. Similarly, we attempted to limit the confounding effects of statin therapy by restricting the study population to patients who did not have large changes in LDL cholesterol between the two visits.
Our specific method limited the study population to the middle two quartiles of changes in LDL cholesterol, excluding patients on either end of the distribution. We assessed the sensitivity of our results to this exclusion criterion by replicating the analysis for each of the four quartiles (data upon request). For three of the four quartiles (2nd, 3rd, and 4th), we found no meaningful association of vitamin D repletion with changes in the lipid profile. Only in individuals with the largest decreases in LDL cholesterol (quartile 1 - defined as patients who had a decrease of more than 15 mg/dl in LDL cholesterol within a 4 to 26 week period) was there an association of vitamin D repletion with lowering LDL cholesterol and triglycerides. The mean decrease in LDL cholesterol for patients in this quartile was −42 mg/dl, highly suggestive of medical intervention. In addition, the vitamin D attributable change in LDL cholesterol was less than 14% (−5.8 mg/dl / −42.0 mg/dl) of the total observed change. Patients in this first quartile were most likely confounded by statin therapy, where the sharp changes in LDL cholesterol are more likely due primarily to changes in statin therapy rather than vitamin D supplements. Therefore, we believe our sensitivity analysis validates the use of database rules to exclude patients who are most likely impacted by large, first-order confounding effects outside the scope of the scientific inquiry being pursued.
Also, we do not know the clinical rational for why these patients underwent vitamin D and lipid testing, which may represent other confounding factors. We explored the sensitivity of our results to potential confounding, latent effects that might have been present in baseline characteristics. We developed a propensity score model to define repletion and control groups. We built a logistic regression model that tries to explain membership in the repletion group using available baseline characteristics. Statistically significant predictors included age, initial 25(OH)D result stratum, initial LDL cholesterol level, initial triglycerides level, abnormal calcium results (pre-defined reference laboratory reference range) and ICD-9 code evidence of dyslipidemia (ICD-9 = 272). Stratification matching with balancing verification was used to match control to repletion group results. The results (data upon request) are highly consistent with the findings presented in this paper.
While the longitudinal study was designed as an efficient alternative to a resource intensive RCT, it is an observational study. Therefore, while the results from this large data-mining analysis show that an increase from deficient to optimal 25(OH)D status is not associated with an improvement in the lipid profile, a definitive conclusion on the effect of vitamin D supplementation on lipids should await the results of large RCT. However, until such results are available, this study challenges the utility of extrapolating the cross-sectional associations between vitamin D and lipids into a rational for treating patients with vitamin D supplements to improve the lipid profile.
The epidemiologic evidence, based largely on association studies, suggests a role for higher 25(OH)D levels in protecting against cardiovascular disease. However, this level of evidence does not prove a causal relationship, and until compelling evidence is available to inform clinical practice, longitudinal analyses of large, patient laboratory databases are valuable tools for studying unresolved health questions. With the benefit of serial testing in clinical practice, we now present evidence of an uncoupling between vitamin D and lipids for association versus intervention. In contrast to the cross-sectional association between 25(OH)D levels and a healthier lipid profile, raising 25(OH)D levels from “deficient” to “optimal” in a cohort neither improved nor worsened the lipid profile. This suggests that a higher level of 25(OH)D may simply be a passive marker of better cardiovascular health.
Supplementary Material
Acknowledgments
Funding Sources: Supported in part by grant # UL1RR024143 from the National Center for Advancing Translational Sciences (NCATS), National Institutes of Health.
Footnotes
Conflict of Interest Disclosures: Xiaohua Huang, Mouneer Odeh, and Harvey Kaufman are employees of and own stock in Quest Diagnostics.
References
- 1.Zehnder D, Bland R, Williams MC, McNinch RW, Howie AJ, Stewart PM, Hewison M. Extrarenal expression of 25-hydroxyvitamin d(3)-1 alpha-hydroxylase. J Clin Endocrinol Metab. 2001;86:888–894. doi: 10.1210/jcem.86.2.7220. [DOI] [PubMed] [Google Scholar]
- 2.Kim DH, Sabour S, Sagar UN, Adams S, Whellan DJ. Prevalence of hypovitaminosis D in cardiovascular diseases (from the National Health and Nutrition Examination Survey 2001 to 2004) Am J Cardiol. 2008;102:1540–1544. doi: 10.1016/j.amjcard.2008.06.067. [DOI] [PubMed] [Google Scholar]
- 3.Forrest KY, Stuhldreher WL. Prevalence and correlates of vitamin D deficiency in US adults. Nutr Res. 2011;31:48–54. doi: 10.1016/j.nutres.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 4.Rosen CJ. Clinical practice. Vitamin D insufficiency. N Engl J Med. 2011;364:248–254. doi: 10.1056/NEJMcp1009570. [DOI] [PubMed] [Google Scholar]
- 5.Heikkinen AM, Tuppurainen MT, Niskanen L, Komulainen M, Penttila I, Saarikoski S. Long-term vitamin D3 supplementation may have adverse effects on serum lipids during postmenopausal hormone replacement therapy. Eur J Endocrinol. 1997;137:495–502. doi: 10.1530/eje.0.1370495. [DOI] [PubMed] [Google Scholar]
- 6.Nagpal J, Pande JN, Bhartia A. A double-blind, randomized, placebo-controlled trial of the short-term effect of vitamin D3 supplementation on insulin sensitivity in apparently healthy, middle-aged, centrally obese men. Diabet Med. 2009;26:19–27. doi: 10.1111/j.1464-5491.2008.02636.x. [DOI] [PubMed] [Google Scholar]
- 7.Rajpathak SN, Xue X, Wassertheil-Smoller S, Van Horn L, Robinson JG, Liu S, Allison M, Martin LW, Ho GY, Rohan TE. Effect of 5 y of calcium plus vitamin D supplementation on change in circulating lipids: results from the Women's Health Initiative. Am J Clin Nutr. 2010;91:894–899. doi: 10.3945/ajcn.2009.28579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scragg R, Khaw KT, Murphy S. Effect of winter oral vitamin D3 supplementation on cardiovascular risk factors in elderly adults. Eur J Clin Nutr. 1995;49:640–646. [PubMed] [Google Scholar]
- 9.Tuppurainen M, Heikkinen AM, Penttila I, Saarikoski S. Does vitamin D3 have negative effects on serum levels of lipids? A follow-up study with a sequential combination of estradiol valerate and cyproterone acetate and/or vitamin D3. Maturitas. 1995;22:55–61. doi: 10.1016/0378-5122(95)00909-5. [DOI] [PubMed] [Google Scholar]
- 10.Witham MD, Nadir MA, Struthers AD. Effect of vitamin D on blood pressure: a systematic review and meta-analysis. J Hypertens. 2009;27:1948–1954. doi: 10.1097/HJH.0b013e32832f075b. [DOI] [PubMed] [Google Scholar]
- 11.Gannage-Yared MH, Azoury M, Mansour I, Baddoura R, Halaby G, Naaman R. Effects of a short-term calcium and vitamin D treatment on serum cytokines, bone markers, insulin and lipid concentrations in healthy post-menopausal women. J Endocrinol Invest. 2003;26:748–753. doi: 10.1007/BF03347358. [DOI] [PubMed] [Google Scholar]
- 12.Maki KC, Rubin MR, Wong LG, McManus JF, Jensen CD, Lawless A. Effects of vitamin D supplementation on 25-hydroxyvitamin D, high-density lipoprotein cholesterol, and other cardiovascular disease risk markers in subjects with elevated waist circumference. Int J Food Sci Nutr. 2011;62:318–327. doi: 10.3109/09637486.2010.536146. [DOI] [PubMed] [Google Scholar]
- 13.Zittermann A, Frisch S, Berthold HK, Gotting C, Kuhn J, Kleesiek K, Stehle P, Koertke H, Koerfer R. Vitamin D supplementation enhances the beneficial effects of weight loss on cardiovascular disease risk markers. Am J Clin Nutr. 2009;89:1321–1327. doi: 10.3945/ajcn.2008.27004. [DOI] [PubMed] [Google Scholar]
- 14.Andersen R, Brot C, Mejborn H, Molgaard C, Skovgaard LT, Trolle E, Ovesen L. Vitamin D supplementation does not affect serum lipids and lipoproteins in Pakistani immigrants. Eur J Clin Nutr. 2009;63:1150–1153. doi: 10.1038/ejcn.2009.18. [DOI] [PubMed] [Google Scholar]
- 15.Carter GD, Carter CR, Gunter E, Jones J, Jones G, Makin HL, Sufi S. Measurement of Vitamin D metabolites: an international perspective on methodology and clinical interpretation. J Steroid Biochem Mol Biol. 2004;89–90:467–471. doi: 10.1016/j.jsbmb.2004.03.055. [DOI] [PubMed] [Google Scholar]
- 16.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 17.Martins D, Wolf M, Pan D, Zadshir A, Tareen N, Thadhani R, Felsenfeld A, Levine B, Mehrotra R, Norris K. Prevalence of cardiovascular risk factors and the serum levels of 25-hydroxyvitamin D in the United States: data from the Third National Health and Nutrition Examination Survey. Arch Intern Med. 2007;167:1159–1165. doi: 10.1001/archinte.167.11.1159. [DOI] [PubMed] [Google Scholar]
- 18.Cheng S, Massaro JM, Fox CS, Larson MG, Keyes MJ, McCabe EL, Robins SJ, O'Donnell CJ, Hoffmann U, Jacques PF, Booth SL, Vasan RS, Wolf M, Wang TJ. Adiposity, cardiometabolic risk, and vitamin D status: the Framingham Heart Study. Diabetes. 2010;59:242–248. doi: 10.2337/db09-1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fraser A, Williams D, Lawlor DA. Associations of serum 25-hydroxyvitamin D, parathyroid hormone and calcium with cardiovascular risk factors: analysis of 3 NHANES cycles (2001–2006) PLoS One. 2010;5:e13882. doi: 10.1371/journal.pone.0013882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jorde R, Figenschau Y, Hutchinson M, Emaus N, Grimnes G. High serum 25-hydroxyvitamin D concentrations are associated with a favorable serum lipid profile. Eur J Clin Nutr. 2010;64:1457–1464. doi: 10.1038/ejcn.2010.176. [DOI] [PubMed] [Google Scholar]
- 21.Manson JE. Vitamin D and the heart: why we need large-scale clinical trials. Cleve Clin J Med. 2010;77:903–910. doi: 10.3949/ccjm.77gr.10004. [DOI] [PubMed] [Google Scholar]
- 22.Gordon DJ, Probstfield JL, Garrison RJ, Neaton JD, Castelli WP, Knoke JD, Jacobs DR, Jr, Bangdiwala S, Tyroler HA. High-density lipoprotein cholesterol and cardiovascular disease. Four prospective American studies. Circulation. 1989;79:8–15. doi: 10.1161/01.cir.79.1.8. [DOI] [PubMed] [Google Scholar]
- 23.Baigent C, Keech A, Kearney PM, Blackwell L, Buck G, Pollicino C, Kirby A, Sourjina T, Peto R, Collins R, Simes R. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005;366:1267–1278. doi: 10.1016/S0140-6736(05)67394-1. [DOI] [PubMed] [Google Scholar]
- 24.Prentice RL, Anderson GL. The women's health initiative: lessons learned. Annu Rev Public Health. 2008;29:131–150. doi: 10.1146/annurev.publhealth.29.020907.090947. [DOI] [PubMed] [Google Scholar]
- 25.Zittermann A, Gummert JF, Borgermann J. The role of vitamin d in dyslipidemia and cardiovascular disease. Curr Pharm Des. 2011;17:933–942. doi: 10.2174/138161211795428786. [DOI] [PubMed] [Google Scholar]
- 26.Wortsman J, Matsuoka LY, Chen TC, Lu Z, Holick MF. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr. 2000;72:690–693. doi: 10.1093/ajcn/72.3.690. [DOI] [PubMed] [Google Scholar]
- 27.Jousilahti P, Tuomilehto J, Vartiainen E, Pekkanen J, Puska P. Body weight, cardiovascular risk factors, and coronary mortality. 15-year follow-up of middle-aged men and women in eastern Finland. Circulation. 1996;93:1372–1379. doi: 10.1161/01.cir.93.7.1372. [DOI] [PubMed] [Google Scholar]
- 28.Rock CL, Emond JA, Flatt SW, Heath DD, Karanja N, Pakiz B, Sherwood NE, Thomson CA. Weight loss is associated with increased serum 25-hydroxyvitamin D in overweight or obese women. Obesity. 2012 doi: 10.1038/oby.2012.57. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Poobalan A, Aucott L, Smith WC, Avenell A, Jung R, Broom J, Grant AM. Effects of weight loss in overweight/obese individuals and long-term lipid outcomes--a systematic review. Obes Rev. 2004;5:43–50. doi: 10.1111/j.1467-789x.2004.00127.x. [DOI] [PubMed] [Google Scholar]
- 30.Perez-Castrillon JL, Vega G, Abad L, Sanz A, Chaves J, Hernandez G, Duenas A. Effects of Atorvastatin on vitamin D levels in patients with acute ischemic heart disease. Am J Cardiol. 2007;99:903–905. doi: 10.1016/j.amjcard.2006.11.036. [DOI] [PubMed] [Google Scholar]
- 31.Ross AC, Manson JE, Abrams SA, Aloia JF, Brannon PM, Clinton SK, Durazo-Arvizu RA, Gallagher JC, Gallo RL, Jones G, Kovacs CS, Mayne ST, Rosen CJ, Shapses SA. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96:53–58. doi: 10.1210/jc.2010-2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ertugrul DT, Yavuz B, Cil H, Ata N, Akin KO, Kucukazman M, Yalcin AA, Dal K, Yavuz BB, Tutal E. STATIN-D Study: Comparison of the Influences of Rosuvastatin and Fluvastatin Treatment on the Levels of 25 Hydroxyvitamin D. Cardiovasc Ther. 2011;29:146–152. doi: 10.1111/j.1755-5922.2010.00141.x. [DOI] [PubMed] [Google Scholar]
- 33.Tarcin O, Yavuz DG, Ozben B, Telli A, Ogunc AV, Yuksel M, Toprak A, Yazici D, Sancak S, Deyneli O, Akalin S. Effect of vitamin D deficiency and replacement on endothelial function in asymptomatic subjects. J Clin Endocrinol Metab. 2009;94:4023–4030. doi: 10.1210/jc.2008-1212. [DOI] [PubMed] [Google Scholar]
- 34.Bises G, Kallay E, Weiland T, Wrba F, Wenzl E, Bonner E, Kriwanek S, Obrist P, Cross HS. 25-hydroxyvitamin D3-1alpha-hydroxylase expression in normal and malignant human colon. J Histochem Cytochem. 2004;52:985–989. doi: 10.1369/jhc.4B6271.2004. [DOI] [PubMed] [Google Scholar]
- 35.Gupta RP, Hollis BW, Patel SB, Patrick KS, Bell NH. CYP3A4 is a human microsomal vitamin D 25-hydroxylase. J Bone Miner Res. 2004;19:680–688. doi: 10.1359/JBMR.0301257. [DOI] [PubMed] [Google Scholar]
- 36.Theodoropoulos C, Demers C, Mirshahi A, Gascon-Barre M. 1,25-Dihydroxyvitamin D(3) downregulates the rat intestinal vitamin D(3)-25-hydroxylase CYP27A. Am J Physiol Endocrinol Metab. 2001;281:E315–E325. doi: 10.1152/ajpendo.2001.281.2.E315. [DOI] [PubMed] [Google Scholar]
- 37.Schmidt DR, Holmstrom SR, Fon Tacer K, Bookout AL, Kliewer SA, Mangelsdorf DJ. Regulation of bile acid synthesis by fat-soluble vitamins A and D. J Biol Chem. 2010;285:14486–14494. doi: 10.1074/jbc.M110.116004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.