Abstract
In this study we transplanted bone marrow mononuclear cells (BM-MNCs) or microglia into rats that had undergone permanent cerebral ischemia and observed the distribution or morphology of transplanted cells in vivo. In addition, we also compared the effects of BM-MNCs and microglia on infarct volume, brain water content, and functional outcome after permanent cerebral ischemia. BM-MNCs and microglia were obtained from femur and brain, respectively, of newborn rats. Adult rats were injected with vehicle or 3 million BM-MNCs or microglia via the tail vein 24 h after permanent middle cerebral artery occlusion (pMCAO). The distribution or morphologic characteristics of transplanted BM-MNCs (BrdU/double-stained CD34 and CD45) and microglia (BrdU/double-stained Iba-1) were detected with immunofluorescent staining at 3 or 7 and 14 days after pMCAO. Functional deficits were assessed by the modified neurologic severity score at 1, 3, 7 and 14 days after pMCAO. Brain water content was assessed with dry–wet weight method at 3 days, and infarct volume was determined with 2,3,5-triphenyltetrazolium chloride (TTC) staining at 14 days. More BrdU/double-stained CD45- and Iba-1-positive cells were observed than BrdU/double-stained CD34-positive cells around the infarcted area. Some infused microglia show the morphology of innate microglia at 7 days and the increased numbers can be detected at 14 days after pMCAO. BM-MNC-treated rats showed a significant reduction in infarct volume and brain water content compared to vehicle- and microglia-treated rats. In addition, BM-MNC treatment reduced neurologic deficit scores compared to those in the other groups. The results provide evidence that infusion of BM-MNC, but not microglia, is neuroprotective after permanent cerebral ischemia.
Keywords: Bone marrow mononuclear cells, Cerebral ischemia, Microglia, Therapeutic effect
1. Introduction
Currently, use of tissue plasminogen activator is the only strategy available for protecting the brain after ischemic stroke. However, recent research has shown cell therapy to be a promising new avenue for the treatment of stroke [1,2]. Transplanting different cell types, such as neural stem cells, progenitor cells, cord blood cells, and bone marrow stromal cells (BMSCs) could minimize neural injury and potentially restore function of damaged tissue [3,4].
One source of cells shown to improve functional outcome in animal models of ischemic stroke is bone marrow. Although studies have demonstrated the high therapeutic value of BMSCs for ischemic stroke in animals [5–8], BMSCs are generally considered more difficult to culture and purify than bone marrow mononuclear cells (BM-MNCs). BM-MNCs comprise mesenchymal and hematopoietic stem cells. Clinical research into their use in heart disease therapy has drawn attention to their potential therapeutic value [9]. Indeed, BM-MNCs have been shown to differentiate into microglia-like cells and may provide a therapeutic effect for Alzheimer’s disease in animal models [10,11]. Several laboratories have reported that BM-MNCs migrate to the boundaries of infarcts and promote stroke recovery after transient cerebral ischemia in rats [12–14]. Furthermore, in multiple small clinical trials, intravascular injection of BM-MNCs appears to be safe and feasible in stroke patients [15,16].
Microglia are resident monocyte-lineaged cells in the brain. They can be activated with the characteristic of monocytes and macrophages after stroke. However, published results are conflicting in regard to the role of microglia in the pathophysiology of cerebral infarction. In a recent report, Narantuya et al. [17] found that human microglia transplantation in a rat transient cerebral ischemia model provides neuroprotection and improves functional recovery. However most investigators have concluded that microglia are detrimental when activated in the acute phase of stroke [18,19].
To the best of our knowledge, no study has examined the therapeutic effects of BM-MNCs and primary microglia on stroke outcomes in the permanent cerebral ischemia model. The aims of our study were to observe the morphologic characteristics of transplanted microglia and the distribution of exogenous BM-MNCs and microglia in vivo and evaluate whether transplantation of each cell type affects infarct volume, brain water content, and functional recovery after permanent cerebral ischemia in rats.
2. Materials and methods
2.1. Animals
All studies were performed in accordance with the NIH and institutional guidelines for animal research under a protocol approved by the Institutional Animal Care and Use Committee at Zhengzhou University, Zhengzhou, China. Adult, male Sprague-Dawley rats (260–300 g, 3 months old) were purchased from the Animal Experimental Center of Zhengzhou University. They were housed under standard conditions with a 12-h light/dark cycle and given free access to food and water throughout the study. All procedures were designed to minimize the number of animals used and their suffering.
2.2. Preparation of BM-MNCs and microglia
BM-MNCs were collected from femurs of 1- or 2-day-old Sprague-Dawley rats (n = 48) and were purified by density gradient centrifugation as previously described [20]. Flow cytometry analysis (FACSCalibur, BD Biosciences, San Jose, CA) with monoclonal anti-CD34 (a marker of hematopoietic stem/progenitor cells, Santa Cruz Biotechnology, Santa Cruz, CA) and anti-CD45 (a marker of monocytes and a negative marker of mesenchymal stem cells, BD Biosciences) antibodies showed that 11.3 ± 1.28% of the collected BM-MNCs were positive for CD34 and 86.1 ± 2.12% were positive for CD45.
Rats used for the collection of BM-MNCs were also used for the culture of microglia. Microglia were isolated from mixed glial culture with a slightly modified version of a previously described method [21]. Briefly, the whole brains of rats were stripped of meninges, mechanically dissociated, and filtered through 70-μm nylon mesh to recover dissociated cells. To harvest enough cells, the dissociated cells were centrifugated and resuspended in Dulbecco’s Modified Eagle’s Medium (DMEM-F12; HyClone, Logan, UT) supplemented with 10% fetal bovine serum (FBS; HyClone) and antibiotics. This preparation of mixed microglia was seeded onto culture dishes coated with poly-L-lysine (Corning, Cambridge, MA) and incubated for 7 days at 37 °C. We then used a previously described method to obtain an enriched culture of microglial cells [22,23]. The mixed glia culture was shaken, and the primary selected microglia were cultured in DMEM that contained 10% FBS (2 × 105 cells/mL). Immunocytochemical analysis was carried out as follows to identify the purity of microglia. Microglia were fixed with 4% paraformaldehyde and incubated with rabbit anti-CD11b/c antibody (Santa Cruz Biotechnology, Santa Cruz, CA) in phosphate-buffered saline (PBS) containing 5% goat serum and 3% Triton X-100. Then the cells were rinsed with PBS for three times and incubated with goat anti-rabbit IgG (Santa Cruz Biotechnology, Santa Cruz, CA). Stained cells were viewed and analyzed under a bright-field microscope. Immunocytochemical analysis indicated that the purity of the cultured microglia was more than 95%.
We also prepared a portion of BM-MNCs and microglia to test their migration capabilities. We labeled the cells with bromodeoxyuridine (BrdU) by incubating them in cell culture medium containing 12 μg/mL BrdU (Sigma, St. Louis, MO, USA) for 24 h. Incorporation of BrdU into the cells was confirmed by immunocytochemistry. The experimental procedure was the same as that used for detection of CD11b/c above except that rabbit anti-BrdU antibody (Santa Cruz Biotechnology, Santa Cruz, CA) was used.
2.3. Permanent middle cerebral artery occlusion (pMCAO)
Adult, male rats were anesthetized by an intraperitoneal injection of 10% chloral hydrate (400 mg/kg). pMCAO was induced by occluding the left middle cerebral artery (MCA) with a nylon monofilament as described previously [24,25]. Body temperature of the rats was monitored throughout surgery by a rectal probe and maintained at 37 ± 0.5 °C with a heating pad. After surgery, the wound was sutured and rats were returned to their cages, which were maintained at 29 °C. Sham-operated rats (n = 16) were subjected to the same surgical procedure, except that the MCA was not occluded. Successful MCAO was defined as more than 80% decrease in cerebral blood flow and was confirmed by Laser-Doppler flowmetry. Rats were excluded from the study if the cerebral blood flow did not decrease by more than 80% and the modified neurological severity score (mNSS) less than 12 or more than 16 at day 1 after pMCAO. The mortality was recorded during the experimental and recovery period.
2.4. Injection of cells
Eighty rats that underwent pMCAO were randomly divided into four groups as follows: no treatment (16 rats), vehicle treatment (16 rats), treatment with microglia (24 rats), treatment with BM-MNCs (24 rats). Cell-treated rats were administered 3 million primary microglia or BM-MNCs by tail vein infusion at 24 h after MCA occlusion. Vehicle-treated rats were injected with an equal volume of cell culture medium. Blood pH, arterial blood gases (PaO2, PaCO2), and body temperature were measured before the pMCAO procedure, 30 min after the procedure, and 30 min after cell injection.
2.5. Assessment of migration capabilities of microglia and BM-MNCs
On day 3 after pMCAO, four rats each from microglia- and BM-MNC-treated groups were anesthetized with pentobarbital sodium solution, and brains were cut into 30-μm sections for detection of BrdU-labeled cells. Briefly, sections were blocked for 2 h with 1% bovine serum albumin (BSA) in PBS-Tween-20 (PBS-T) and then incubated overnight at 4 °C with rabbit anti-BrdU (1:200; Santa Cruz Biotechnology, Santa Cruz, CA) in PBS-T containing 1% BSA. The sections were then rinsed three times in PBS-T (10 min each) and finally incubated for 2 h at room temperature with CFL555-0 conjugated secondary antibody (1:200; Santa Cruz Biotechnology, Santa Cruz, CA). The stained cells around the infarcted area were observed under a fluorescence microscope (Olympus CKX41, Olympus, Japan). Images of the areas were captured with a digital camera (Olympus, Japan) by an investigator blinded to experimental group. In addition, double staining of BrdU (1:200; Santa Cruz Biotechnology, Santa Cruz, CA)/CD34 (1:200; Santa Cruz Biotechnology, Santa Cruz, CA) and CD45 (1:200; Santa Cruz Biotechnology, Santa Cruz, CA) was also conducted at 3 days after occlusion (n = 4). Double staining of BrdU (1:200; Santa Cruz) and ionized calcium-binding adaptor molecule 1 (Iba-1) (1:500; Wako Pure Chemical Industries, Osaka, Japan) was carried out at 3, 7 and 14 days after occlusion (n = 4). The sections were incubated overnight at 4 °C with the above antibodies. After rinsing in PBS-T for 10 min × 3, the sections were incubated for 2 h at room temperature with CFL555-or CFL488-conjugated secondary antibody (1:200; Santa Cruz Biotechnology, Santa Cruz, CA). The sections were then rinsed at 4 °C in PBS-T and PBS for 10 min × 3, and coverslipped with VECTASHIELD (Santa Cruz Biotechnology, Santa Cruz, CA). Double stained cells were observed under a fluorescent microscope (Olympus CKX41, Olympus, Japan).
2.6. Analysis of brain water content
The rats (n = 6) were anesthetized and decapitated on day 3 after pMCAO as previously described [26] for determination of water content. Briefly, cerebral tissue was divided with a blade into two hemispheres from the anatomic midline. The left hemisphere of these rats was immediately weighed with an electronic analytical balance to obtain the wet weight. Then brain samples were dried at 100 °C in an electric blast drying oven for 24 h to obtain the dry weight. Brain water content was calculated as: (wet weight − dry weight)/wet weight × 100% [27].
2.7. Determination of infarct volume
Rats used for the determination of infarct volume were anesthetized with pentobarbital sodium solution and killed on day 14 after pMCAO (n = 6). Brains of these rats were removed and cut into five 2-mm-thick coronal sections on a Vibratome (Vibratome, St. Louis, MO). The brain slices were quickly immersed in 1% 2,3,5-triphenyltetrazolium chloride (TTC) (Sigma, St. Louis, MO, USA) in 0.1 M PBS (pH 7.4) for 20 min at room temperature and then stored in phosphate-buffered 4% paraformaldehyde overnight before analysis. The area of damaged parenchyma (unstained tissue) was measured on the posterior surface of each slice using Sigma scan Pro 5.0 (SPSS Science). Each infarct area was then multiplied by the ratio of the surface of the infarcted (ipsilateral) hemisphere to the intact (contralateral) hemisphere at the same level to correct for brain swelling. The total volume of damaged tissue (in cubic millimeters) was then calculated by linear integration of the corrected lesion areas [26].
2.8. Neurologic assessment
The modified neurological severity score (mNSS) was used by an investigator blinded to treatment group to test the neurological scores of rats on days 1, 3, 7 and 14 after pMCAO or sham surgery (n = 10) [17]. The mNSS comprises motor, sensory, balance, and reflex tests. Neurologic function was graded on a scale of 0–22 (no deficit = 0, maximal deficit = 22) [28].
2.9. Statistical analysis
Results are expressed as mean ± SEM. Repeated measures ANOVA followed by Bonferroni post hoc test was used to determine changes in neurologic scores after pMCAO. One-way ANOVA followed by LSD test was used to determine changes in brain water content and infarct volume after pMCAO. P < 0.05 was considered statistically significant.
3. Results
3.1. Mortality, physiologic parameters and migration of microglia and BM-MNCs
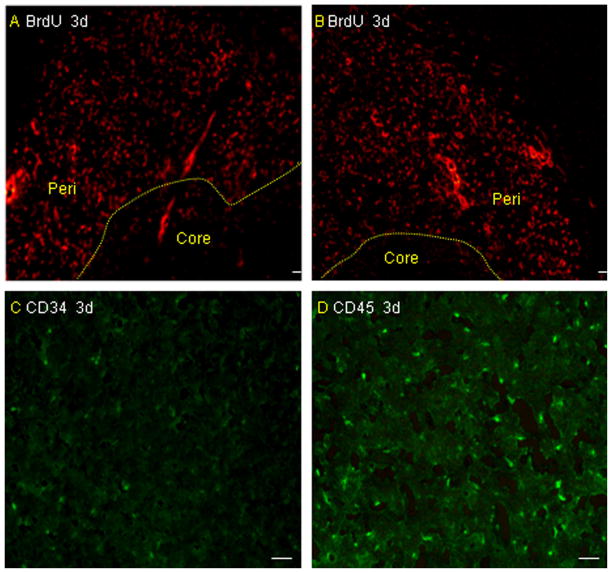
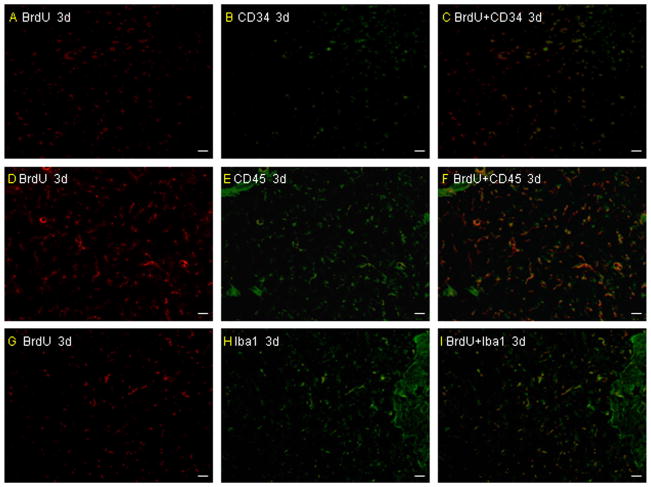
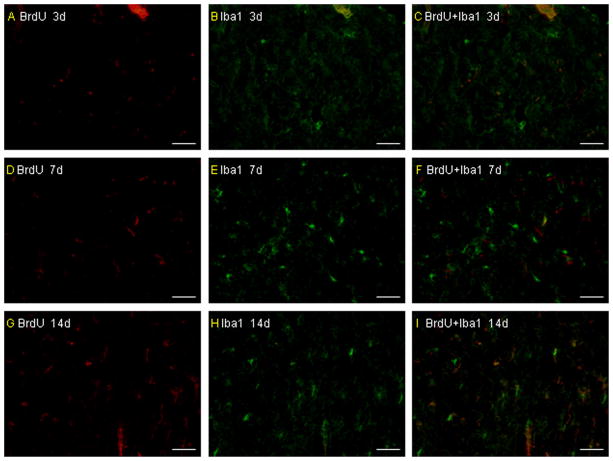
The mortality throughout the experiment was 0 (0/16) in the sham group, 25.0% (4/16) in the untreated group, 31.2% (5/16) in the vehicle-treated group, 20.8% (5/24) in the microglia-treated group and 29.2% (7/24) in the BMMNC-treated group. There is no significant difference among the last four groups (P > 0.05). Values for blood pH, arterial blood gases, and rectal temperature remained within normal physiologic ranges throughout the experiment. No differences were observed in any of these variables among the sham-operated group, untreated group, vehicle-treated group, microglia-treated group, and BM-MNC-treated group (all P > 0.05; Table 1). Brain sections from the rats that were administered BrdU-, CD34- and CD45-labeled cells revealed that both microglia and BM-MNCs migrated to the boundaries of infarcts on day 3 after pMCAO (Fig. 1A–D). In addition, BrdU/double-stained microglia (Iba-1) and BM-MNCs (CD34 and CD45) were also detected around the infarcted area (Fig. 2A–I). There are more BrdU/double-stained CD45 and Iba-1 cells than BrdU/double-stained CD34 cells around the infracted area. The infused microglia did not show a ramified or an activated/amoeboid morphology at 3 days after pMCAO, but some infused microglia show a ramified or an activated/amoeboid morphology at 7 days and the increased numbers can be detected at 14 days after pMCAO (Fig. 3A–I).
Table 1.
Physiological variables of each group (mean ± S.E.).
| Experimental group | pH | PaO2 (mm Hg) | PaCO2 (mm Hg) | Temperature (°C) |
|---|---|---|---|---|
| Sham-operated | ||||
| Before surgery | 7.35 ± 0.05 | 97.5 ± 3.5 | 36.1 ± 4.5 | 37.13 ± 1.37 |
| 30 min after surgery | 7.34 ± 0.03 | 98.1 ± 4.1 | 35.7 ± 3.9 | 37.16 ± 2.21 |
| 30 min after injection | 7.32 ± 0.05 | 98.0 ± 3.3 | 36.5 ± 3.2 | 36.93 ± 1.18 |
| Untreated | ||||
| Before surgery | 7.31 ± 0.03 | 98.1 ± 2.8 | 35.2 ± 4.9 | 36.83 ± 2.03 |
| 30 min after surgery | 7.36 ± 0.05 | 97.4 ± 4.1 | 36.1 ± 3.3 | 35.77 ± 1.64 |
| 30 min after injection | 7.34 ± 0.03 | 96.7 ± 3.6 | 35.4 ± 3.8 | 37.04 ± 1.38 |
| Vehicle-treated | ||||
| Before surgery | 7.35 ± 0.06 | 96.6 ± 4.6 | 36.1 ± 3.7 | 37.14 ± 2.37 |
| 30 min after surgery | 7.30 ± 0.03 | 95.8 ± 5.4 | 36.3 ± 4.5 | 35.98 ± 1.52 |
| 30 min after injection | 7.31 ± 0.05 | 98.2 ± 3.9 | 35.7 ± 4.3 | 36.99 ± 2.03 |
| Microglia-treated | ||||
| Before surgery | 7.36 ± 0.04 | 96.1 ± 4.2 | 35.7 ± 4.6 | 37.14 ± 1.02 |
| 30 min after surgery | 7.31 ± 0.03 | 97.4 ± 3.6 | 36.1 ± 3.9 | 37.17 ± 2.71 |
| 30 min after injection | 7.34 ± 0.06 | 98.1 ± 5.3 | 36.2 ± 4.1 | 36.59 ± 1.58 |
| BM-MNC-treated | ||||
| Before surgery | 7.37 ± 0.05 | 96.8 ± 4.7 | 36.3 ± 4.7 | 37.12 ± 1.84 |
| 30 min after surgery | 7.29 ± 0.04 | 97.1 ± 5.1 | 35.1 ± 4.0 | 36.76 ± 1.18 |
| 30 min after injection | 7.33 ± 0.04 | 97.7 ± 4.4 | 36.2 ± 3.9 | 37.09 ± 2.21 |
BM-MNC, bone marrow mononuclear cells.
Fig. 1.
Migration of microglia and BM-MNCs in vivo. Immunofluorescence staining for BrdU-labeled cells revealed that both microglia (A) and BM-MNCs (B) migrated to the boundary zone of the injured cortex in rats after infusion via the tail vein on day 3 after pMCAO. There are more CD45-positive cells (D) around the infarcted area than CD34 cells (C) (n = 4 rats per group). Scale bar = 50 μm.
Fig. 2.
The number of transplanted microglia and BM-MNCs in vivo. There are more BrdU/double-stained CD45 and Iba-1 cells than BrdU/double-stained CD34 around the infarcted area at 3 days after pMCAO (A–I). Scale bar = 50 μm.
Fig. 3.
The morphologic characteristics of transplanted primary microglia in vivo. The transplanted primary microglia did not show a ramified or an activated/amoeboid morphology at 3 days after pMCAO and only a few of them show a ramified or an activated/amoeboid morphology from 7 days after pMCAO. More infused primary microglia can be detected to show a ramified or an activated/amoeboid morphology (A–I). Scale bar = 50 μm.
3.2. The effect of cell transplantation on brain water content
Brain water content was significantly greater in rats that underwent pMCAO than in sham-operated rats. Comparison of the sham-operated, untreated, vehicle-treated, microglia-treated, and BM-MNC-treated rats by one-way ANOVA showed a significant difference among the groups (F = 7.37; P < 0.001). Post hoc analysis revealed that treatment with BM-MNCs, but not microglia, significantly reduced brain water content compared to that in untreated and vehicle-treated rats at 3 days after pMCAO (both P < 0.05; Fig. 4).
Fig. 4.
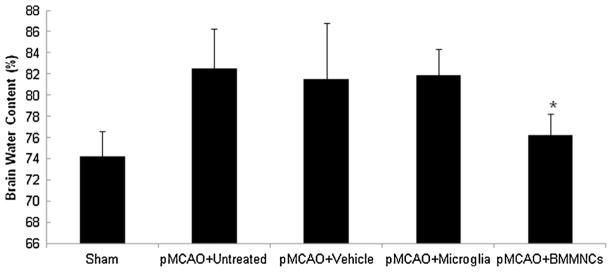
Effect of BM-MNC and microglia treatment on brain water content. Statistical analysis showed significant difference among the five groups (F = 7.37, n = 6 rats per group, P < 0.001). Post hoc analysis revealed that only BM-MNC treatment produced a significant reduction in brain water content. *P < 0.05 vs. untreated, vehicle-treated, and microglia-treated groups.
3.3. The effect of cell transplantation on infarct volume
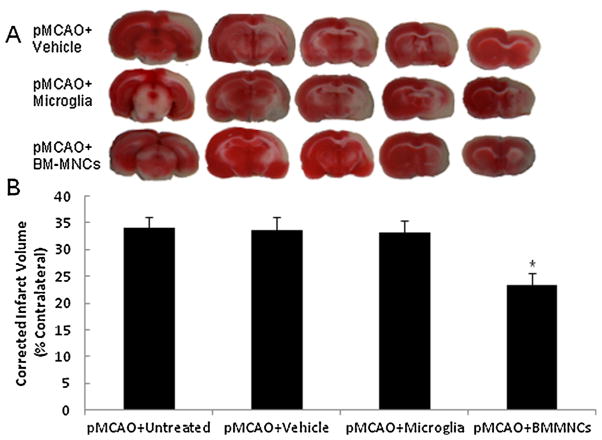
Rats treated with BM-MNCs had a significantly smaller infarction size than did untreated, vehicle-treated, and microglia-treated rats 14 days after pMCAO (F = 30.80, P < 0.001; Fig. 5A and B). In contrast, treatment with microglia had no effect on infarct volume after stroke (P > 0.05).
Fig. 5.
Treatment with BM-MNCs reduces infarct volume after pMCAO. (A) Representative images of TTC-stained brain slices from vehicle-treated (top), microglia-treated rats (middle) and BM-MNC-treated rats (bottom) at 14 days post-pMCAO. (B) Quantification shows that the infarct volume of BM-MNC-treated rats was significantly smaller than that of untreated, vehicle-treated, and microglia-treated rats (F = 30.80, n = 6 rats per group, P < 0.001). *P < 0.05 vs. any other group.
3.4. The effect of cell transplantation on neurologic function
No functional deficits were observed in rats prior to the pMCAO procedure. In addition, sham-operated rats exhibited no change in motor function across the 14-day assessment period, confirming that the surgical procedure did not affect neurologic function (P > 0.05). Rats in the four pMCAO groups all exhibited significant increases in neurologic deficit score after occlusion. Repeated measures ANOVA revealed a significant difference among the four groups (F value for tests of between-subject effect = 9.33, P < 0.001). BM-MNC-treated rats demonstrated significantly less neurologic deficit than did untreated, vehicle-treated, and microglia-treated rats on day 7 and 14 after pMCAO (P < 0.05, Fig. 6). Microglial treatment did not affect neurologic function up to day 14 after pMCAO (P > 0.05, Fig. 6).
Fig. 6.
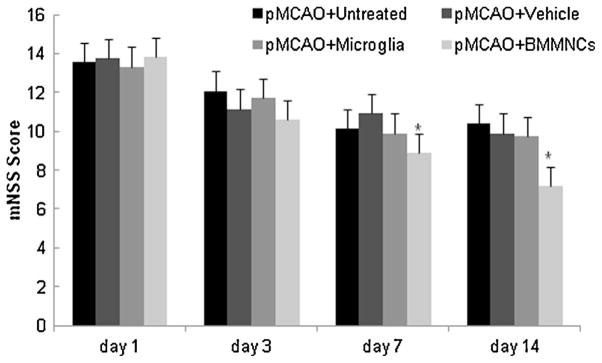
Treatment with BM-MNCs reduces neurologic deficit after pMCAO. ANOVA revealed a significant difference in neurologic function among untreated, vehicle-treated, microglia-treated, and BM-MNC-treated groups on days 1, 3, 7 and 14 after pMCAO (F value for tests of between-subject effect = 9.33, P < 0.001). Treatment with BM-MNCs significantly improved neurologic function on day 14 compared to that in untreated, vehicle-treated, and microglia-treated groups (n = 10 rats per group). *P < 0.05 vs. all other groups.
4. Discussion
In this study, we investigated the effect of transplanted BM-MNCs on infarct volume, brain water content, and functional outcome after permanent cerebral ischemia in rats and compared the effect with that of transplanted primary microglia. We found that BrdU-labeled microglia and BM-MNCs can be detected in the boundary zone of the infarct, illustrating that the transplanted cells can penetrate the blood–brain barrier. Furthermore, BM-MNC-treated rats showed substantial reductions in brain water content and infarct volume (% contralateral hemisphere) compared to those in vehicle- and microglia-treated rats. Neurologic deficit scores also were significantly lower in BM-MNC-treated rats than in vehicle-and microglia-treated rats. In contrast, treatment with transplanted primary microglia did not affect outcome after pMCAO.
Cell transplantation therapy is beneficial to the recovery of neurologic function and is considered to have potential as a therapeutic approach for the treatment of cerebrovascular diseases [1,2]. Preclinical studies have shown that bone marrow-derived cells in particular may have great value for the treatment of cerebral ischemia [12,29–31]. Bone marrow-derived cells are composed predominantly of BMSCs and BM-MNCs. As stem cells, BMSCs can differentiate into multiple lineages. Previous research has shown that transplantation of BMSCs significantly reduces the impairment of neurologic function after cerebral ischemia [5–8]. BM-MNCs comprise mainly mesenchymal cells and hematopoietic cells [14]. Previous studies of the therapeutic effects of BM-MNCs have focused mainly on heart disease and have shown BM-MNCs to have great value for the treatment of myocardial infarction and heart failure [20,32,33]. To verify whether BM-MNCs are also beneficial in central nervous system diseases, researchers have investigated whether they can penetrate the blood–brain barrier and whether they are neuroprotective. Priller et al. [34] demonstrated that BM-MNCs are able to migrate into the brains of mice. In addition, BM-MNCs have been observed around the infarct areas in patients with cerebral infarction [35]. These results show that BM-MNCs are able to penetrate the blood–brain barrier in patients with cerebral infarction and support the results of our present study in rats. Such findings can provide the theoretical basis for additional research into the mechanism by which BM-MNCs contribute to recovery after stroke. However, the fate of the transplanted BM-MNCs in the ischemic brain should be investigated in a future study.
Research has shown that some BM-MNCs in the brains of patients with Alzheimer’s disease have microglia-like features [36,37]. Simard et al. [10] found that BM-MNCs can be activated as a branch-like form. Such BM-MNCs, which are also called marrow-derived microglia, have a morphology that resembles that of microglia. In animal models of Alzheimer’s disease, these branch-like cells have been shown to migrate into the central nervous system [38] and to accumulate around and clear beta-amyloid [36].
It has been reported that BM-MNCs can protect neurons and modulate microglia in cell culture models of ischemic stroke [39]. Indeed, the safety and feasibility of autologous BM-MNC therapy have been shown in several small clinical trials for ischemic stroke [15,16]. However, the function of microglia in the pathological process of cerebral ischemia is unclear. We compared the therapeutic effects of BM-MNCs and microglia on ischemic stroke outcomes. The results revealed that BM-MNCs exert neuroprotective effects after permanent cerebral ischemia. Contrary to what Narantuya et al. [17] reported for transient cerebral ischemia, our findings do not support a protective role for transplanted primary microglia in permanent cerebral ischemia. The difference may reside in the stroke model used (transient vs. permanent MCAO) or in the timing of the cell transplantation (48 h vs. 24 h after MCAO) or in the cells transplanted (human microglial cell line vs. primary microglia). Although research suggests that microglia can be activated with an amoeboid morphology and exert neurotoxic effects in the acute phase of stroke, our results revealed that the transplanted primary microglia did not show a ramified or an activated/amoeboid morphology at 3 days after pMCAO and only a few of them begin to show a ramified or an activated/amoeboid morphology from 7 days after pMCAO. The transplanted primary microglia did not affect the stroke outcomes in the acute phase of stroke. Whether transplanted primary microglia can secret some neurotrophic factors and exert neuroprotective effects in the recovery phase of stroke needs to be investigated.
Although previous studies have reported that transplanted BM-MNCs can differentiate into neuron cells, increase the secretion of cytokines and improve neurologic function in animal models of transient cerebral ischemia [12–14], we are the first group to show its neuroprotective effects in the permanent cerebral ischemia model. We provide evidence that direct tail vain injection of BM-MNCs, but not microglia, improves neurologic recovery and reduces the extent of brain damage.
5. Conclusion
The results of this research suggest that transplantation of BM-MNCs, not primary microglia, has potential as a therapeutic approach for cerebral ischemia. If BM-MNCs can be shown to improve recovery in large clinical trials, they may offer an economical and simple treatment for stroke patients. However, the mechanism by which BM-MNCs provide protection is still unclear and warrants additional research.
HIGHLIGHTS.
We treated rats with bone marrow mononuclear cells (BM-MNCs) and microglia after stroke.
The distribution or morphology of transplanted BM-MNCs and microglia was observed in vivo.
We compared the therapeutic effects of BM-MNCs and microglia for stroke.
BM-MNCs improved post-stroke functional outcome and reduced brain water content and lesion volume.
Acknowledgments
This work was supported by grants from Medical Science and Technology Research Programs of Henan Province (WKJ2010-2-016), the Overseas Training Program of Henan Province Medical Academic Leaders (2011023), AHA (09BGIA2080137), and NIH (K01AG031926, R01AT007317, R01NS078026). We thank Claire Levine for assistance with this manuscript.
Contributor Information
Jianping Wang, Email: wjpwfy666@126.com.
Jian Wang, Email: jwang79@jhmi.edu.
References
- 1.Burns TC, Steinberg GK. Stem cells and stroke: opportunities, challenges and strategies. Expert Opinion on Biological Therapy. 2011;11:447–61. doi: 10.1517/14712598.2011.552883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bliss TM, Andres RH, Steinberg GK. Optimizing the success of cell transplantation therapy for stroke. Neurobiology of Disease. 2010;37:275–83. doi: 10.1016/j.nbd.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang ZG, Chopp M. Neurorestorative therapies for stroke: underlying mechanisms and translation to the clinic. Lancet Neurology. 2009;8:491–500. doi: 10.1016/S1474-4422(09)70061-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jablonska A, Lukomska B. Stroke induced brain changes: implications for stem cell transplantation. Acta Neurobiologiae Experimentalis (Warsz) 2011;71:74–85. doi: 10.55782/ane-2011-1824. [DOI] [PubMed] [Google Scholar]
- 5.Chen J, Zhang ZG, Li Y, Wang L, Xu YX, Gautam SC, et al. Intravenous administration of human bone marrow stromal cells induces angiogenesis in the ischemic boundary zone after stroke in rats. Circulation Research. 2003;92:692–9. doi: 10.1161/01.RES.0000063425.51108.8D. [DOI] [PubMed] [Google Scholar]
- 6.Zhang J, Li Y, Zhang ZG, Lu M, Borneman J, Buller B, et al. Bone marrow stromal cells increase oligodendrogenesis after stroke. Journal of Cerebral Blood Flow and Metabolism. 2009;29:1166–74. doi: 10.1038/jcbfm.2009.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu Z, Li Y, Zhang RL, Cui Y, Chopp M. Bone marrow stromal cells promote skilled motor recovery and enhance contralesional axonal connections after ischemic stroke in adult mice. Stroke. 2011;42:740–4. doi: 10.1161/STROKEAHA.110.607226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Skvortsova VI, Gubskiy LV, Tairova RT, Povarova OV, Cheglakov IB, Holodenko RV, et al. Use of bone marrow mesenchymal (stromal) stem cells in experimental ischemic stroke in rats. Bulletin of Experimental Biology and Medicine. 2008;145:122–8. doi: 10.1007/s10517-008-0032-7. [DOI] [PubMed] [Google Scholar]
- 9.Henning RJ. Stem cells in cardiac repair. Future Cardiology. 2011;7:99–117. doi: 10.2217/fca.10.109. [DOI] [PubMed] [Google Scholar]
- 10.Simard AR, Soulet D, Gowing G, Julien JP, Rivest S. Bone marrow-derived microglia play a critical role in restricting senile plaque formation in Alzheimer’s disease. Neuron. 2006;49:489–502. doi: 10.1016/j.neuron.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 11.Soulet D, Rivest S. Bone-marrow-derived microglia: myth or reality. Current Opinion in Pharmacology. 2008;8:508–18. doi: 10.1016/j.coph.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 12.Kamiya N, Ueda M, Igarashi H, Nishiyama Y, Suda S, Inaba T, et al. Intra-arterial transplantation of bone marrow mononuclear cells immediately after reperfusion decreases brain injury after focal ischemia in rats. Life Sciences. 2008;83:433–7. doi: 10.1016/j.lfs.2008.07.018. [DOI] [PubMed] [Google Scholar]
- 13.Iihoshi S, Honmou O, Houkin K, Hashi K, Kocsis JD. A therapeutic window for intravenous administration of autologous bone marrow after cerebral ischemia in adult rats. Brain Research. 2004;1007:1–9. doi: 10.1016/j.brainres.2003.09.084. [DOI] [PubMed] [Google Scholar]
- 14.Brenneman M, Sharma S, Harting M, Strong R, Cox CS, Jr, Aronowski J, et al. Autologous bone marrow mononuclear cells enhance recovery after acute ischemic stroke in young and middle-aged rats. Journal of Cerebral Blood Flow and Metabolism. 2010;30:140–9. doi: 10.1038/jcbfm.2009.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Savitz SI, Misra V, Kasam M, Juneja H, Cox CS, Jr, Alderman S, et al. Intravenous autologous bone marrow mononuclear cells for ischemic stroke. Annals of Neurology. 2011;70:59–69. doi: 10.1002/ana.22458. [DOI] [PubMed] [Google Scholar]
- 16.Friedrich MA, Martins MP, Araujo MD, Klamt C, Vedolin L, Garicochea B, et al. Intra-arterial infusion of autologous bone marrow mononuclear cells in patients with moderate to severe middle cerebral artery acute ischemic stroke. Cell Transplantation. 2012;21(Suppl 1):S13–21. doi: 10.3727/096368912x612512. [DOI] [PubMed] [Google Scholar]
- 17.Narantuya D, Nagai A, Sheikh AM, Masuda J, Kobayashi S, Yamaguchi S, et al. Human microglia transplanted in rat focal ischemia brain induce neuroprotection and behavioral improvement. PLoS ONE. 2010;5:e11746. doi: 10.1371/journal.pone.0011746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yenari MA, Kauppinen TM, Swanson RA. Microglial activation in stroke: therapeutic targets. Neurotherapeutics. 2010;7:378–91. doi: 10.1016/j.nurt.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang J. Preclinical and clinical research on inflammation after intracerebral hemorrhage. Progress in Neurobiology. 2010;92:463–77. doi: 10.1016/j.pneurobio.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fukushima S, Varela-Carver A, Coppen SR, Yamahara K, Felkin LE, Lee J, et al. Direct intramyocardial but not intracoronary injection of bone marrow cells induces ventricular arrhythmias in a rat chronic ischemic heart failure model. Circulation. 2007;115:2254–61. doi: 10.1161/CIRCULATIONAHA.106.662577. [DOI] [PubMed] [Google Scholar]
- 21.Levi G, Patrizio M, Bernardo A, Petrucci TC, Agresti C. Human immunodeficiency virus coat protein gp120 inhibits the beta-adrenergic regulation of astroglial and microglial functions. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:1541–5. doi: 10.1073/pnas.90.4.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ni M, Aschner M. Neonatal rat primary microglia: isolation, culturing, and selected applications. Current Protocols in Toxicology. 2010;43(Unit 12.17) doi: 10.1002/0471140856.tx1217s43. (Chapter 12) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilms H, Sievers J, Rickert U, Rostami-Yazdi M, Mrowietz U, Lucius R. Dimethyl-fumarate inhibits microglial and astrocytic inflammation by suppressing the synthesis of nitric oxide, IL-1beta, TNF-alpha and IL-6 in an in-vitro model of brain inflammation. Journal of Neuroinflammation. 2010;7:30. doi: 10.1186/1742-2094-7-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jiang C, Wang J, Li X, Liu C, Chen N, Hao Y. Progesterone exerts neuroprotective effects by inhibiting inflammatory response after stroke. Inflammation Research. 2009;58:619–24. doi: 10.1007/s00011-009-0032-8. [DOI] [PubMed] [Google Scholar]
- 25.Zan L, Wu H, Jiang J, Zhao S, Song Y, Teng G, et al. Temporal profile of Src, SSeCKS, and angiogenic factors after focal cerebral ischemia: correlations with angiogenesis and cerebral edema. Neurochemistry International. 2011;58:872–9. doi: 10.1016/j.neuint.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang J, Jiang C, Liu C, Li X, Chen N, Hao Y. Neuroprotective effects of progesterone following stroke in aged rats. Behavioural Brain Research. 2010;209:119–22. doi: 10.1016/j.bbr.2010.01.026. [DOI] [PubMed] [Google Scholar]
- 27.Wang J, Dore S. Heme oxygenase-1 exacerbates early brain injury after intra-cerebral haemorrhage. Brain. 2007;130:1643–52. doi: 10.1093/brain/awm095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang J, Li Y, Chen J, Yang M, Katakowski M, Lu M, et al. Expression of insulin-like growth factor 1 and receptor in ischemic rats treated with human marrow stromal cells. Brain Research. 2004;1030:19–27. doi: 10.1016/j.brainres.2004.09.061. [DOI] [PubMed] [Google Scholar]
- 29.Bao X, Wei J, Feng M, Lu S, Li G, Dou W, et al. Transplantation of human bone marrow-derived mesenchymal stem cells promotes behavioral recovery and endogenous neurogenesis after cerebral ischemia in rats. Brain Research. 2011;1367:103–13. doi: 10.1016/j.brainres.2010.10.063. [DOI] [PubMed] [Google Scholar]
- 30.Hao Q, Su H, Palmer D, Sun B, Gao P, Yang GY, et al. Bone marrow-derived cells contribute to vascular endothelial growth factor-induced angiogenesis in the adult mouse brain by supplying matrix metalloproteinase-9. Stroke. 2011;42:453–8. doi: 10.1161/STROKEAHA.110.596452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Taguchi A, Zhu P, Cao F, Kikuchi-Taura A, Kasahara Y, Stern DM, et al. Reduced ischemic brain injury by partial rejuvenation of bone marrow cells in aged rats. Journal of Cerebral Blood Flow and Metabolism. 2011;31:855–67. doi: 10.1038/jcbfm.2010.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leistner DM, Schmitt J, Palm S, Klotsche J, Estel S, Fink A, et al. Intracoronary administration of bone marrow-derived mononuclear cells and arrhythmic events in patients with chronic heart failure. European Heart Journal. 2011;32:485–91. doi: 10.1093/eurheartj/ehq430. [DOI] [PubMed] [Google Scholar]
- 33.Suarez de Lezo J, Herrera C, Romero MA, Pan M, Jimenez R, Carmona D, et al. Functional recovery following intracoronary infusion of autologous mononuclear bone marrow cells in patients with chronic anterior myocardial infarction and severely depressed ventricular function. Revista Espanola de Cardiologia. 2010;63:1127–35. doi: 10.1016/s1885-5857(10)70226-7. [DOI] [PubMed] [Google Scholar]
- 34.Priller J, Prinz M, Heikenwalder M, Zeller N, Schwarz P, Heppner FL, et al. Early and rapid engraftment of bone marrow-derived microglia in scrapie. Journal of Neuroscience. 2006;26:11753–62. doi: 10.1523/JNEUROSCI.2275-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barbosa da Fonseca LM, Gutfilen B, Rosado de Castro PH, Battistella V, Gold-enberg RC, Kasai-Brunswick T, et al. Migration and homing of bone-marrow mononuclear cells in chronic ischemic stroke after intra-arterial injection. Experimental Neurology. 2010;221:122–8. doi: 10.1016/j.expneurol.2009.10.010. [DOI] [PubMed] [Google Scholar]
- 36.Lee JK, Jin HK, Bae JS. Bone marrow-derived mesenchymal stem cells reduce brain amyloid-beta deposition and accelerate the activation of microglia in an acutely induced Alzheimer’s disease mouse model. Neuroscience Letters. 2009;450:136–41. doi: 10.1016/j.neulet.2008.11.059. [DOI] [PubMed] [Google Scholar]
- 37.Malm T, Koistinaho M, Muona A, Magga J, Koistinaho J. The role and therapeutic potential of monocytic cells in Alzheimer’s disease. Glia. 2010;58:889–900. doi: 10.1002/glia.20973. [DOI] [PubMed] [Google Scholar]
- 38.Malm TM, Koistinaho M, Parepalo M, Vatanen T, Ooka A, Karlsson S, et al. Bone-marrow-derived cells contribute to the recruitment of microglial cells in response to beta-amyloid deposition in APP/PS1 double transgenic Alzheimer mice. Neurobiology of Disease. 2005;18:134–42. doi: 10.1016/j.nbd.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 39.Sharma S, Yang B, Strong R, Xi X, Brenneman M, Grotta JC, et al. Bone marrow mononuclear cells protect neurons and modulate microglia in cell culture models of ischemic stroke. Journal of Neuroscience Research. 2010;88:2869–76. doi: 10.1002/jnr.22452. [DOI] [PMC free article] [PubMed] [Google Scholar]