Abstract
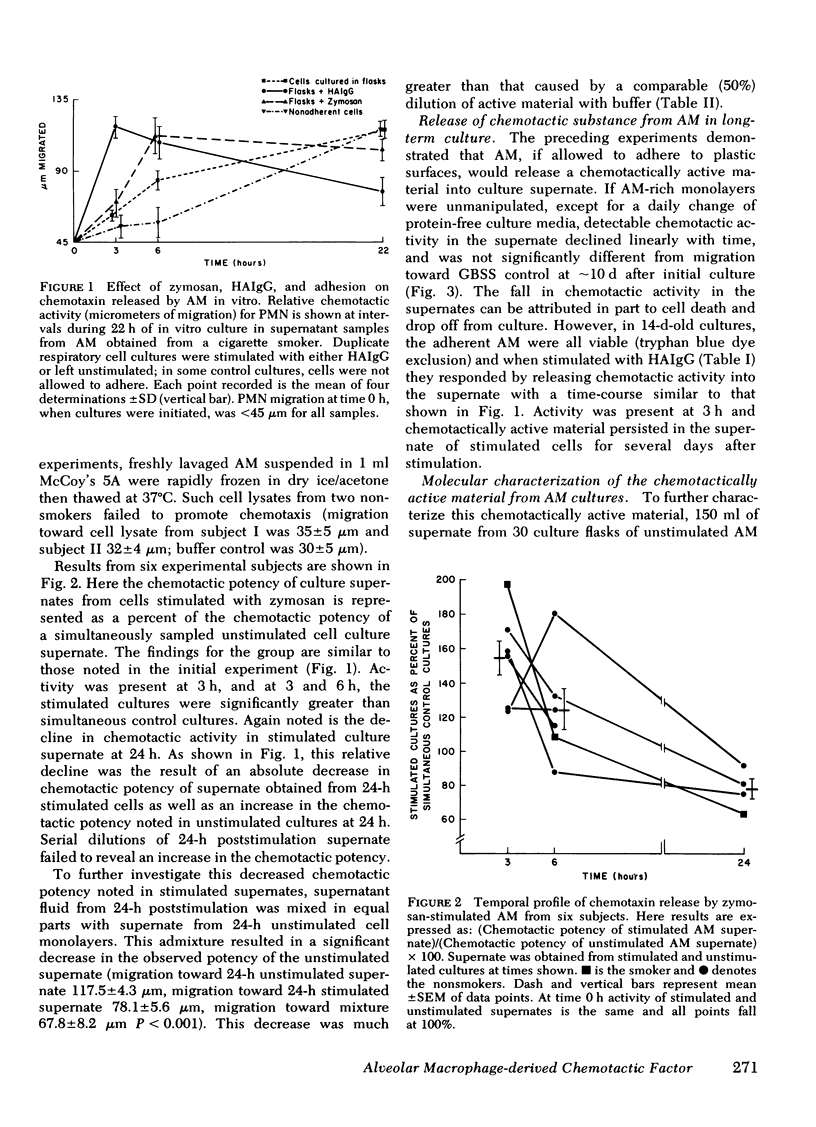
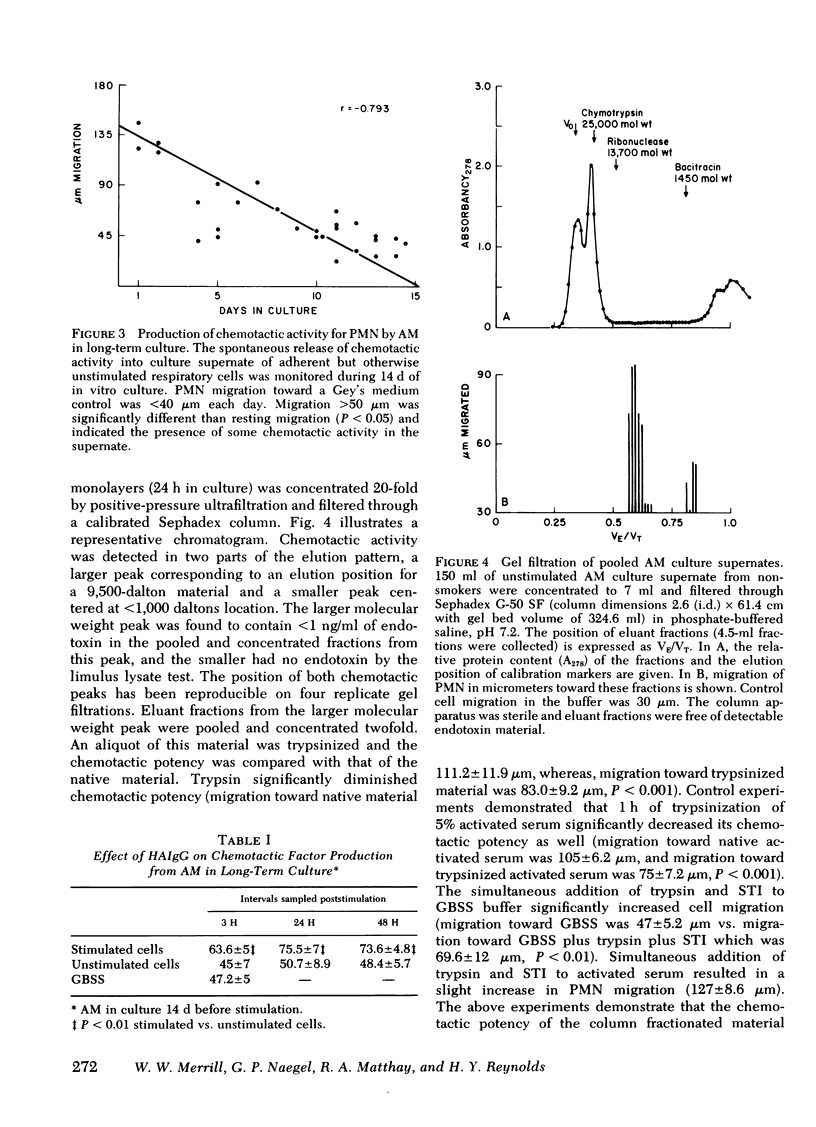
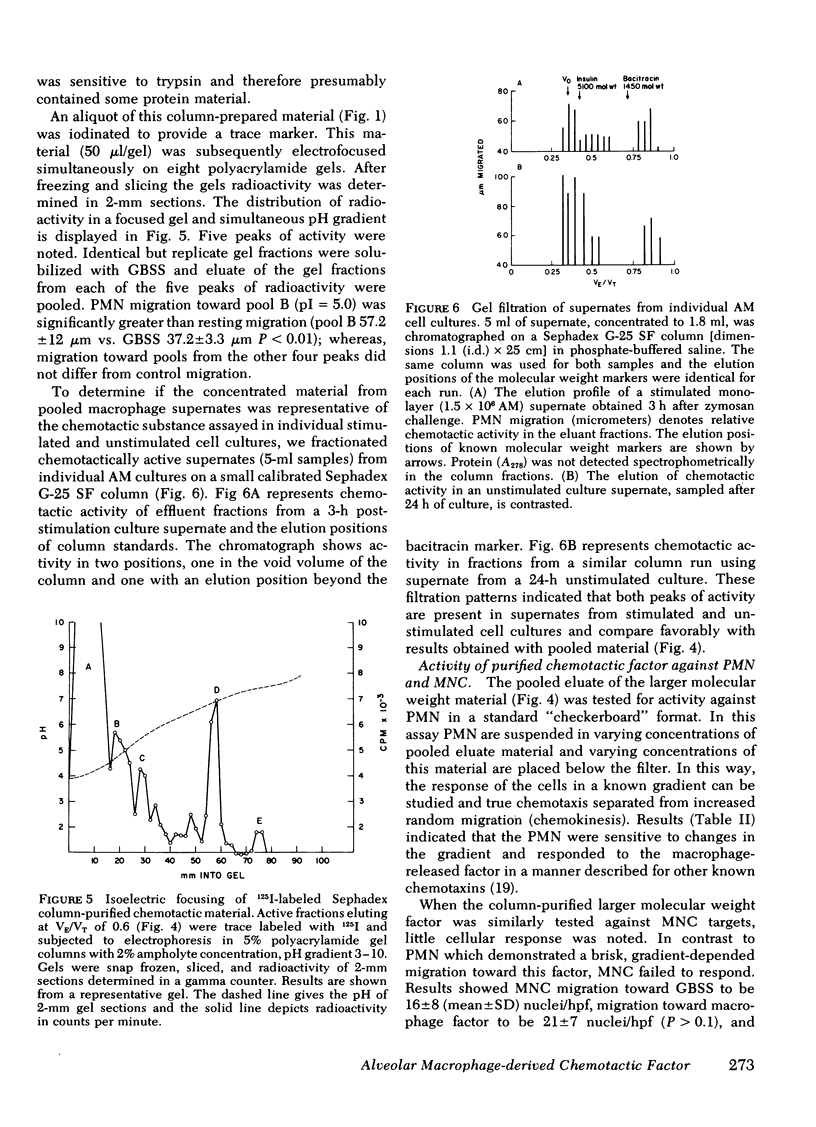
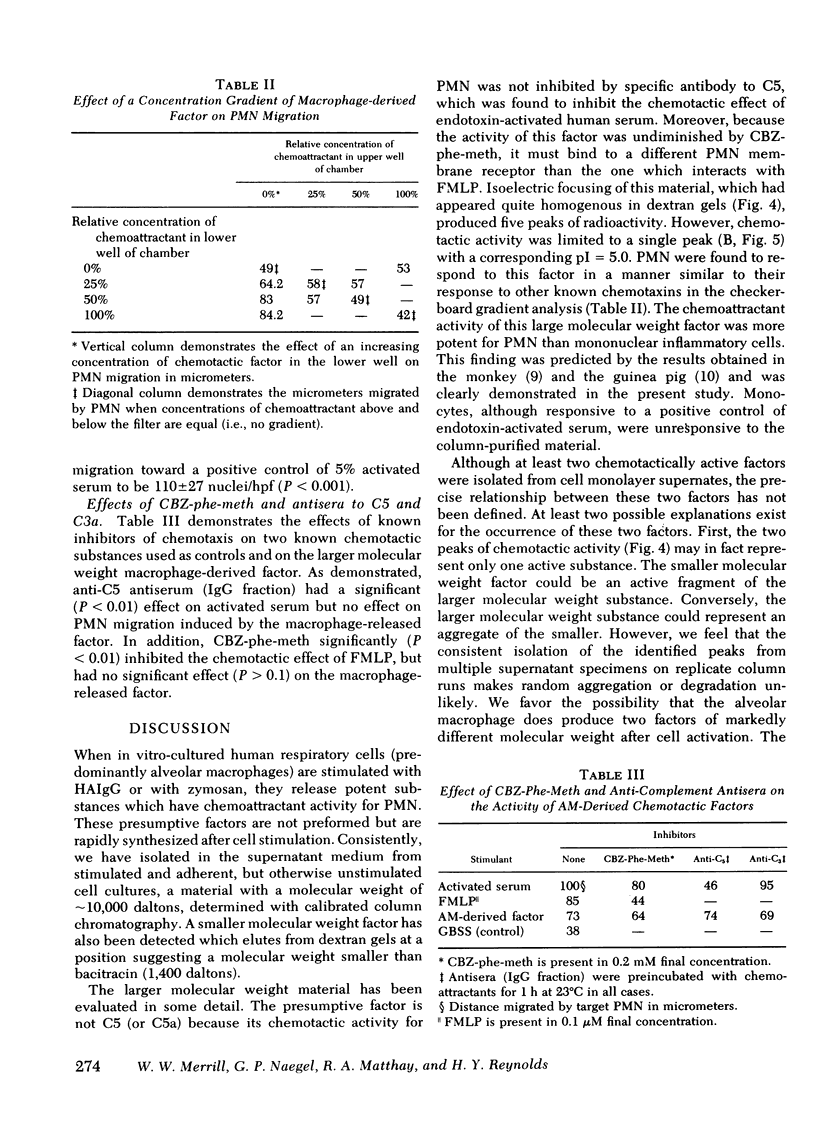
Alveolar macrophages are the initial phagocytic cells that encounter foreign material and particulates deposited in the terminal airways. We have examined a mechanism by which these cells, after phagocytic challenge, may control or amplify the inflammatory response in lung parenchyma. Normal human alveolar macrophages (AM) were studied from eight subjects. With in vitro culture, AM produced and released two substances into culture media which have potent chemoattractant activity for blood polymorphonuclear granulocytes (PMN) and negligible activity for mononuclear cells. Release of these factors is maximally stimulated by aggregated human immunoglobulin (Ig)G or zymosan particles; however, simple adhesion of the macrophages to plastic surfaces is also sufficient to stimulate release of these chemotactic substances. The larger substance (10,000 daltons) is immunologically distinct from C5a and interacts with a different PMN membrane receptor than that known to exist for formyl-methionyl-leucyl-phenylalanine. Its chemotactic activity is sensitive to the enzymatic effect of trypsin. Although producing a single elution peak on gelfiltration chromatography, electrofocusing in polyacrylamide gels yielded five peaks of radioactivity. Chemotactic activity was localized to a fraction with a pI = 5.0. The smaller molecular weight substance has been less well characterized. Thus, the human AM can produce at least two factors which attract PMN and this capability may augment the local inflammatory response in the lung.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson C. L., Grey H. M. Receptors for aggregated IgG on mouse lymphocytes: their presence on thymocytes, thymus-derived, and bone marrow-derived lymphocytes. J Exp Med. 1974 May 1;139(5):1175–1188. doi: 10.1084/jem.139.5.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arend W. P., Mannik M. The macrophage receptor for IgG: number and affinity of binding sites. J Immunol. 1973 Jun;110(6):1455–1463. [PubMed] [Google Scholar]
- Brozna J. P., Senior R. M., Kreutzer D. L., Ward P. A. Chemotactic factor inactivators of human granulocytes. J Clin Invest. 1977 Dec;60(6):1280–1288. doi: 10.1172/JCI108887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- COHN Z. A., WIENER E. THE PARTICULATE HYDROLASES OF MACROPHAGES. II. BIOCHEMICAL AND MORPHOLOGICAL RESPONSE TO PARTICLE INGESTION. J Exp Med. 1963 Dec 1;118:1009–1020. doi: 10.1084/jem.118.6.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen A. B., Cline M. J. The human alveolar macrophage: isolation, cultivation in vitro, and studies of morphologic and functional characteristics. J Clin Invest. 1971 Jul;50(7):1390–1398. doi: 10.1172/JCI106622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREEN G. M., KASS E. H. THE ROLE OF THE ALVEOLAR MACROPHAGE IN THE CLEARANCE OF BACTERIA FROM THE LUNG. J Exp Med. 1964 Jan 1;119:167–176. doi: 10.1084/jem.119.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallin J. I., Clark R. A., Frank M. M. Kinetic analysis of chemotactic factor generation in human serum via activation of the classical and alternate complement pathways. Clin Immunol Immunopathol. 1975 Jan;3(3):334–346. doi: 10.1016/0090-1229(75)90020-3. [DOI] [PubMed] [Google Scholar]
- Goetzl E. J., Woods J. M., Gorman R. R. Stimulation of human eosinophil and neutrophil polymorphonuclear leukocyte chemotaxis and random migration by 12-L-hydroxy-5,8,10,14-eicosatetraenoic acid. J Clin Invest. 1977 Jan;59(1):179–183. doi: 10.1172/JCI108617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein E., Lippert W., Warshauer D. Pulmonary alveolar macrophage. Defender against bacterial infection of the lung. J Clin Invest. 1974 Sep;54(3):519–528. doi: 10.1172/JCI107788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green G. M., Jakab G. J., Low R. B., Davis G. S. Defense mechanisms of the respiratory membrane. Am Rev Respir Dis. 1977 Mar;115(3):479–514. doi: 10.1164/arrd.1977.115.3.479. [DOI] [PubMed] [Google Scholar]
- HANKS J. H., WALLACE J. H. Determination of cell viability. Proc Soc Exp Biol Med. 1958 May;98(1):188–192. doi: 10.3181/00379727-98-23985. [DOI] [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- Hunninghake G. W., Gallin J. I., Fauci A. S. Immunologic reactivity of the lung: the in vivo and in vitro generation of a neutrophil chemotactic factor by alveolar macrophages. Am Rev Respir Dis. 1978 Jan;117(1):15–23. doi: 10.1164/arrd.1978.117.1.15. [DOI] [PubMed] [Google Scholar]
- ISHIZAKA K., ISHIZAKA T. Biologic activity of aggregated gamma-globulin. II. A study of various methods for aggregation and species differences. J Immunol. 1960 Aug;85:163–171. [PubMed] [Google Scholar]
- Johnson K. J., Anderson T. P., Ward P. A. Suppression of immune complex-induced inflammation by the chemotactic factor inactivator. J Clin Invest. 1977 May;59(5):951–958. doi: 10.1172/JCI108717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazmierowski J. A., Gallin J. I., Reynolds H. Y. Mechanism for the inflammatory response in primate lungs. Demonstration and partial characterization of an alveolar macrophage-derived chemotactic factor with preferential activity for polymorphonuclear leukocytes. J Clin Invest. 1977 Feb;59(2):273–281. doi: 10.1172/JCI108638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Flaherty J. T., Showell H. J., Kreutzer D. L., Ward P. A., Becker E. L. Inhibition of in vivo and in vitro neutrophil responses to chemotactic factors by a competitive antagonist. J Immunol. 1978 Apr;120(4):1326–1332. [PubMed] [Google Scholar]
- Reynolds H. Y., Kazmierowski J. A., Newball H. H. Specificity of opsonic antibodies to enhance phagocytosis of Pseudomonas aeruginosa by human alveolar macrophages. J Clin Invest. 1975 Aug;56(2):376–385. doi: 10.1172/JCI108102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds H. Y. Lung host defenses: a status report. Chest. 1979 Feb;75(2 Suppl):239–242. [PubMed] [Google Scholar]
- Reynolds H. Y., Newball H. H. Analysis of proteins and respiratory cells obtained from human lungs by bronchial lavage. J Lab Clin Med. 1974 Oct;84(4):559–573. [PubMed] [Google Scholar]
- Showell H. J., Freer R. J., Zigmond S. H., Schiffmann E., Aswanikumar S., Corcoran B., Becker E. L. The structure-activity relations of synthetic peptides as chemotactic factors and inducers of lysosomal secretion for neutrophils. J Exp Med. 1976 May 1;143(5):1154–1169. doi: 10.1084/jem.143.5.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson M. J., Becker E. L. Measurement of chemotaxis of human polymorphonuclear leukocytes in filters by counting the number of cells in a single plane and comparison with leading front method. J Immunol Methods. 1976;13(2):191–197. doi: 10.1016/0022-1759(76)90158-7. [DOI] [PubMed] [Google Scholar]
- Thompson R. E., Reynolds H. Y., Waxdal M. J. Structural composition of canine secretory component and immunoglobulin A. Biochemistry. 1975 Jul;14(13):2853–2860. doi: 10.1021/bi00684a010. [DOI] [PubMed] [Google Scholar]
- Tobias L. D., Hamilton J. G. The effect of 5,8,11,14-eicosatetraynoic acid on lipid metabolism. Lipids. 1979 Feb;14(2):181–193. doi: 10.1007/BF02533870. [DOI] [PubMed] [Google Scholar]
- Unanue E. R. Secretory function of mononuclear phagocytes: a review. Am J Pathol. 1976 May;83(2):396–418. [PMC free article] [PubMed] [Google Scholar]
- Zigmond S. H., Hirsch J. G. Leukocyte locomotion and chemotaxis. New methods for evaluation, and demonstration of a cell-derived chemotactic factor. J Exp Med. 1973 Feb 1;137(2):387–410. doi: 10.1084/jem.137.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]


