Abstract
Organic solute transporter α-beta (OSTα-OSTβ) is a unique heteromeric transporter localized to the basolateral membrane of epithelial cells involved in sterol transport. It is believed to be the primary bile acid efflux transporter in the intestine of mammals and is therefore essential to bile acid homeostasis and the enterohepatic circulation. First described in the evolutionarily primitive small skate, Leucoraja erinacea, this facilitated transporter requires expression of both subunits for its function. It can transport a variety of bile acids, as well as estrone 3-sulfate, dehydroepiandrosterone 3-sulfate, digoxin and prostaglandin E2. Expression of both subunits is variable between species and tissues; in humans high expression is noted in the liver, small intestine, kidney, testis, and adrenal gland. OSTα-OSTβ is directly regulated by the bile acid sensing nuclear receptor, farnesoid X receptor (FXR). Furthermore, it is part of the complex regulatory pathway that controls bile acid synthesis and homeostasis. Hepatic OSTα-OSTβ is up-regulated in cholestasis in both humans and rodents, where it appears to play a protective role. Additional studies are necessary to determine its role in liver injury, bile acid malabsorption, and lipid and glucose metabolism, as well as a potential protective role for kidney OSTα-OSTβ in cholestasis.
Keywords: Bile acid homeostasis, ileal bile acid transporter, adaptive regulation, nuclear receptor
One of the primary roles of the liver is to synthesize and secrete bile acids into the biliary system for ultimate delivery to the intestine for solubilization and absorption of dietary lipids. In the terminal portion of the small intestine ~95% of these secreted bile acids are reabsorbed by the enterocyte and are then excreted and recycled back to the liver. Although the apical transporter, apical sodium-dependent bile acid transporter (ASBT, SLC10A2), responsible for uptake of bile acids into the enterocyte was identified in 1994,1 the basolateral transporter which controls the excretion back into the mesenteric blood has been elusive. Possible candidates included tASBT and MRP3, but these two failed to meet criteria believed to be necessary for this key membrane protein. In 2001 Wang et al2 cloned a heteromeric organic solute transporter from the liver of an evolutionarily ancient vertebrate, the small skate Leucoraja erinacea, that was later shown to have mammalian orthologues.3 This transporter was named organic solute transporter α-beta, Ostα-Ostβ, and was subsequently shown to be the primary ileal basolateral bile acid transporter.4
Subsequent characterization of Ostα-Ostβ revealed that in addition to its key role in the enterohepatic circulation of bile acids, this transporter is essential to the regulation of bile acid homeostasis in the intact organism. As will be discussed later in this chapter, Ostα-Ostβ can be positively regulated by bile acids themselves through the nuclear receptor, FXR, as well as other transcription factors. Therefore, this novel transporter has the potential to be a key player in cholestatic liver injury and a candidate for therapeutic interventions. Recent work suggests that renal Ostα-Ostβ may play a hepatoprotective role in obstructive liver injury when down regulated.5
CHARACTERISTICS OF Ostα-Ostβ
In 2001 Wang et al2 used a liver cDNA library from the small skate, Leucoraja erinacea, and Xenopus laevis oocytes to screen for novel organic solute transporters. Taurocholate transport activity was noted in the presence of two distinct gene products, subsequently named Ostα and Ostβ. Skate Ostα encodes for a protein of 352 amino acids and seven putative transmembrane domains, whereas skate Ostβ is predicted to contain 182 amino acids and is a single membrane spanning protein. Human OSTα and mouse Ostα share 83% amino acid identity with each other and 41% amino acid identity with skate Ostα. Human OSTβ shares 63% amino acid identity with mouse Ostβ and only 25% amino acid identity with skate Ostβ. Expression of both subunits is required for transport and mammalian orthologues can functionally complement the original skate proteins despite the differences in amino acid homology.3 Human OSTα-OSTβ can transport estrone 3-sulfate, digoxin and protaglandin E2, as well as taurocholate, but not estradiol 17β-D-glucuronide or p-aminohippurate.3 The substrate specificity is similar between human and mouse.3 This transporter is sodium-independent, is not sensitive to pH, ATP depletion, or Na+, K+ or Cl- gradients.6 Rather, it is a facilitated transporter that is capable of transporting organic solutes in either direction, depending on the substrate gradient.6
OSTα-OSTβ has been localized to many human tissues, including small and large intestine, testis, kidney, liver, and adrenal glands, and expression levels of the individual subunits is quite variable between tissues and between species.3,6 Most notably both subunits are readily detectable in human liver, but expression is very low in livers from rodents, where they are primarily found associated with the cholangiocyte.3,6 Analysis of expressed sequence tag counts in humans has confirmed that OSTα and OSTβ are most abundant in the steroid rich organs, such as liver, intestine, kidney, testis, mammary gland, uterus, prostate and thyroid.7 In mice and rats the expression is highest in the small intestine and the kidney, where its distribution mimics that of the bile acid uptake transporter, Asbt. Ballatori et al6 have postulated that rodents may have higher intestinal expression of Ostα-Ostβ because they require a higher rate of dietary sterol absorption than humans. Interestingly, human small intestine does not appear to demonstrate the prominent gradient of distribution from duodenum to ileum that has been found in the mouse and this may be due to differences in the pool of conjugated bile acid between human and mouse.8 In all tissues, Ostα-Ostβ is localized to the basolateral membrane of the epithelium, presumably acting as the primary organic solute efflux transporter. In endocrine organs it may function to transfer steroid hormones between tissues and blood.7,9
The heteromeric nature of OSTα-OSTβ was one of the first surprising findings with this novel transporter and may explain why its discovery was so difficult. Expression cloning clearly determined that functional activity required two separate genes.2 Similar to some G protein-coupled receptors, Ostα is predicted to have seven transmembrane domains and requires an ancillary protein for plasma membrane localization and function.10–12 However, it is still not clear how these two subunits interact, what is the stoichiometry of the interaction, and what role the interaction plays in the function of the intact transporter. Interaction of the β subunit with the α subunit requires the carboxyl terminal, intracellular domain of Ostα12 and appears to stabilize the cellular expression of the transporter.13 The interaction probably occurs in the endoplasmic reticulum, with resultant release to the Golgi where terminal glycosylation of the α subunits occurs prior to trafficking to the plasma membrane.10–12 Thus, the β subunit appears to be acting like a chaperone for the α subunit. Both heterodimers (one Ostα and one Ostβ subunit) and heteromultimers (two Ostα and one Ostβ subunit) have been described13 and the stoichiometry of the functional transporter is still not certain. In addition, although it is clear that surface localization requires the expression of both subunits, it is not known whether transport function requires direct physical interaction between the two subunits.11
REGULATION OF Ostα-Ostβ
One of the most important aspects of OSTα-OSTβ is its ability to be positively regulated by bile acids through the nuclear receptor, farnesoid X receptor (FXR, NR1H4).9,14–16 This nuclear receptor controls bile acid homeostasis by maintaining a fine balance in bile acid synthesis and transport by regulating key genes in the liver, kidney and intestine. Thus, bile acids can repress their own synthesis through binding to FXR in the liver and intestine and stimulating transcription of short heterodimer partner (SHP) and fibroblast growth factor 19 (FGF19), and inhibiting CYP7A1, CYP8B1 and liver receptor homologue 1 (LRH-1).17 Bile acid transporters are also regulated either directly or indirectly by FXR. The uptake transporters, Ntcp and Asbt, are indirectly down-regulated by the transcriptional up-regulation of the repressor Shp by Fxr.18 In contrast, bile acids directly interact with FXR elements (FXRE) in the promoters of OSTα-OSTβ,9,16 Bsep19 and IBABP.20 In the human, two putative IR-1/FXREs have been identified in the OSTα promoter and one in the OSTβ promoter.16 mRNA and protein expression of both subunits can be induced by the FXR agonists chenodeoxycholic acid (CDCA) and GW4064, in various human cell lines, including the hepatocyte lines, HepG2 and Huh7.9,14–16 Reduction of FXR by transfection with FXR-specific siRNAs abolished this agonist induced OSTα-OSTβ expression in Huh7 cells.16 In the mouse, one potential Fxre sequence has been reported in both the Ostα and Ostβ promoters.15 Basal levels of Ostα and Ostβ are lower in ileum from Fxr−/− mice21 and GW4064 treatment of organ culture of adrenal glands from these mice fail to induce Ostα or Ostβ 9. These studies show that FXR is a key regulator of OSTα-OSTβ expression. In the mouse, Lrh is a negative regulator of Ostα-Ostβ.15
An understanding of the critical role for OSTα-OSTβ in the enterohepatic circulation of bile acids and bile acid homeostasis depended upon the development of Ostα deficient mice. These animals demonstrate intestinal hypertrophy and a significantly reduced bile acid pool size, while maintaining normal fecal bile acid excretion.4,22 3H-Taurocholate and 3H-estrone 3-sulfate transport studies in intestinal segments demonstrate that there is a defect in intestinal bile acid absorption in mice lacking Ostα.4,22 In addition, these animals have lower serum levels of cholesterol and triglycerides, but elevated fecal cholesterol excretion.4,22 Interestingly, despite the small bile acid pool size, Cyp7a1, the key regulator of bile acid synthesis in the liver, is decreased. This is probably due to an accumulation of bile acids in the enterocyte, which, in turn, activates Fxr and increases the intestinal synthesis of Fgf15. This hormone then circulates to the liver where it binds to the fibroblast growth factor receptor 4 (FgfR4)/β-klotho complex on the plasma membrane, activating a signal transduction pathway that up-regulates liver Shp and represses Cyp7a1.23 When these mice are fed a cholic acid diet they demonstrate an increase in fecal bile acid excretion compared with the wild-type controls, indicating bile acid malabsorption.4 Despite these metabolic disturbances in the Ostα deficient mouse, these animals are viable and appear to be disease free. In humans, CDCA is the most potent bile acid activator of OSTα-OSTβ expression, unlike the mouse whose bile acid composition is much more hydrophilic. Whether this difference in bile acid composition accounts for why there is higher expression of hepatic OSTα-OSTβ in human liver is not known.
THE ROLE OF OSTα-OSTβ IN CHOLESTASIS
The inability of the liver to secrete bile acids into bile for subsequent release into the intestine leads to the clinical syndrome of cholestasis. The causes of cholestasis may be genetic, mechanical or drug-induced, and the reader is referred to other chapters in this book, as well as to numerous other reviews for a detailed description of cholestatic liver injury.24 Whatever the cause, it is clear from decades of research that when cholestasis occurs the body attempts to minimize injury by altering the synthesis and secretion of toxic products, such as bile acids. In the liver, an adaptive response occurs with all four hepatic phases of bile salt and bilirubin transport and metabolism: Phase 0 transporters for hepatic uptake of bile acids, bilirubin and other organic solutes are down-regulated to prevent them from entering the liver; Phase 1 CYP450 hydroxylation reactions are increased to decrease the toxicity of the bile acids at the same time that bile acid synthesis via CYP7A1 and CYP8B1 is markedly curtailed; Phase 2 metabolic enzymes are activated to increase glurcuronide and sulfate conjugation reactions to increase bile acid solubility; and Phase 3 export pumps are increased, particularly at the basolateral membrane, to export these products back into blood to prevent their retention in the cholestatic liver. Thus, finding OSTα-OSTβ on the basolateral membrane of the hepatocyte provides a potential new member to aid in this latter step of adaptive regulation.
In normal liver, transporters at the apical canalicular membrane, such as the bile salt export pump (BSEP) and MRP2, provide the rate-limiting step in the hepatic secretion of bile acids and bilirubin conjugates, respectively. They are abundantly expressed in the normal liver of mammals and rodents and act efficiently in removing bile acids and other potentially toxic products from the hepatocyte. In contrast, basolateral membrane export transporters, such as MRP3, MRP4 and OSTα-OSTβ, are expressed at much lower levels in the normal hepatocyte. However, under cholestatic conditions, these alternative export pathways are called upon to prevent accumulation of hydrophobic bile salts and other toxic products. All three of these transporters have been found to be up-regulated in both human and rodent livers when canalicular secretion is prevented. For example, MRP3 is up-regulated in primary biliary cirrhosis (PBC)25 and in extrahepatic cholestasis due to pancreatic malignancy.26 Increased expression of MRP4 can occur in progressive familial intrahepatic cholestasis type I (PFIC1)27 and late stage biliary atresia,28 as well as stage III and IV PBC.29 OSTα-OSTβ was also found to be up-regulated in these later stages of PBC,14 in late stage biliary atresia,28 and in extrahepatic cholestasis due to pancreatic malignancy26. However, it has been difficult to assess the relative contribution of these three basolateral transporters to adaptive regulation in the various forms of cholestatic liver injury. Although there is overlap in the substrate specificities between these three transporters, each of them may be necessary for the liver to fully compensate for the lack of canalicular secretion and the resulting increase in Phase 2 conjugation reactions. For example, MRP3 has a higher affinity for glucuronidated conjugates and may be important for bilirubin glucuronide excretion,30 whereas MRP4 can transport sulfated conjugates but probably does not transport glucuronides31 and OSTα-OSTβ transports bile acids and other sterols.3
Because of the difficulties in determining temporal changes in transporter expression in human liver injury, much of our understanding of these adaptive changes has relied on animal models of cholestasis. These include obstructive models produced by common bile duct ligation (BDL), models of inflammation induced by endotoxin (LPS), hormone induced models (estrogen), and cholangiopathies induced by drugs such as α-napthylthiocyanate (ANIT). For example, we previously demonstrated that rodents subjected to BDL had elevated expression of Ostα and Ostβ in the liver, and that this increase was associated with hepatocytes and not the proliferating bile ducts.14 In addition, mice treated with ANIT showed elevated hepatic Ostβ mRNA levels.32 Although these animal studies have been very informative, it is clear that transporter expression and regulation in rodents are different than in humans. As discussed earlier, the basal expression of hepatic OSTα-OSTβ in humans is much higher than we see in rat and mouse. Expression of Mrp3 in rat liver is very low, but is highly up-regulated in obstructive cholestasis.33–35 In the mouse, however, basal liver Mrp3 expression is higher and is not greatly up-regulated.36–38 MRP3 expression in human liver appears to more closely resemble the rat.39 Despite these differences, current understanding of the relative importance of these salvage pathways is based on studies in mice genetically deficient in these membrane proteins.4,22,40 Mrp3-deficient mice were used to establish that this basolateral transporter is important in the hepatic export of glucuronides and monoanionic bile acids.36 However, after bile duct ligation in these mice, the liver injury was no different in the Mrp3−/− mouse compared with the wild-type control, suggesting that other mechanisms exist for eliminating the accumulation of toxic products in these animals.36,38 In contrast, when BDL was performed in Mrp4 null mice, there was an increase in liver necrosis and serum ALT, and a decrease in serum bile acids in association with an increase in hepatic Mrp3 and Ostα-Ostβ expression.41 However, despite these adaptive responses, in the absence of Mrp4, efficient export of toxic bile acids was prevented, suggesting a critical role for Mrp4 as a hepatic efflux transporter.
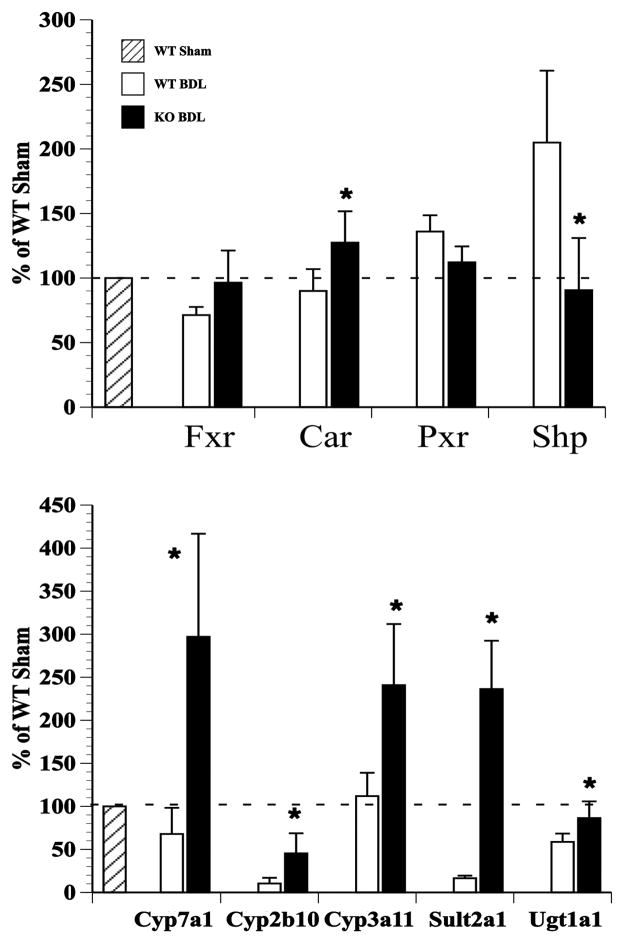
In contrast, and quite surprisingly, when Ostα-Ostβ deficient mice were subjected to BDL, the liver injury was attenuated.5 These mice had lower serum levels of bile acids, ALT, and γ-glutamyl transpeptidase than the wild-type BDL controls (Table 1) and less evidence of liver fibrosis.5 In addition, they had significantly less bile acid retained in the liver. Although this may be expected due to the smaller bile acid pool size (previously reported to be 10–35% of the size of the pool in wild-type mice4,22), these animals had increased urinary bile acid excretion, especially in the form of bile alcohol sulfates, suggesting involvement of the renal excretory pathway (Table 1). In addition, the Ostα-Ostβ deficient mice had an ~5-fold increase in biliary bilirubin after BDL and a significant increase in hepatic Mrp2 protein expression. These animals demonstrate higher mRNA levels of Cyp7a1 and Bsep in an apparent attempt to increase their bile acid pool. These effects could be due to a decrease in the hepatic Cyp7a1 repressor, Shp, which is down-regulated by the lack of sufficient Fgf15 circulating from the intestine due to the absence of bile acids in the intestine following bile duct ligation. Cholestatic Ostα-deficient mice show increases in the mRNA levels of Phase I enzymes, Cyp2b10 and Cyp3a11, and Phase II enzymes, Sult2a1 and Ugt1a1, as compared with their ligated wild-type controls (Fig. 1). Both sham and BDL Ostα-Ostβ mice have elevated levels of mRNA for the nuclear receptor, constitutive androstrane receptor (Car), but not for Fxr or pregnane X receptor (Pxr). Car is a xenobiotic sensing receptor and plays a significant role in regulation of bile acid detoxifying enzymes, Mrp4, and Sult2a1.42–44 Thus, it appears that Car may be regulating many of the adaptive, protective changes that are occurring in the cholestatic Ostα-Ostβ mouse. Interestingly, when mice lacking Car were subjected to BDL, they showed greater liver damage than their wild-type controls, again suggesting that Car activation is critical to protective responses in cholestasis.45 Activators of CAR have also been used to treat cholestatsis in humans. Herbal medicines that are CAR ligands, such as Yin Shi Huang or Yin Chin, have been used to treat neonatal jaundice.46,47 Phenobarbital, a CAR agonist, has also been used to treat PBC and Crigler Najjar type 248,49 and can reduce bile acid and bilirubin levels in the serum. However, it is not used in clinical practice due to adverse side effects. PXR shares many of the same properties as CAR, and it is possible that in humans PXR, rather than CAR, plays a greater role in cholestasis. Rifampacin, a potent PXR agonist, is used in humans to treat symptoms of pruritus in PBC and other cholestatic liver disorders.50 In late stage atresia, children had higher levels of mRNA for OSTα, OSTβ, MRP4 and PXR, and a poor prognosis was associated with lower expression of PXR and CAR.28
Table 1.
Serum parameters, hepatic and urinary bile acids of Ostα +/+ and Ostα −/− mice.
| Ostα +/+ sham | Ostα −/− sham | Ostα +/+ BDL | Ostα −/− BDL | |
|---|---|---|---|---|
| Serum ALT (U/L) | 4.8 ± 1.3 | 4.8 ± 1.2 | 56.1 ± 26.6+ | 31.8 ± 2.2# |
| Serum γGT (U/L) | 7.6 ± 1.7 | 10.8 ± 5.0 | 71.6 ± 31.2+ | 17.8 ± 3.3* |
| Serum bile acids (μM) | 12.5 ± 12.4 | 11.9 ± 9.0 | 2023 ± 647+ | 371 ± 133#* |
| Serum bilirubin (mg/dL) | 0.11 ± 0.11 | 0.18 ± 0.13 | 23.1 ± 9.1+ | 6.1 ± 1.8#* |
| Hepatic bile acids (μM) | 10.5+11.2 | ND | 130.2+36.3+ | 68.6+12.0#* |
| Urinary bile acids (μM) | 0 | 0 | 132.9 ± 25.5 | 316.7 ± 104.5* |
| Urinary bile alcohol sulfates (μM) | BD | BD | 57 ± 53 | 258 ± 242 |
Data represent mean ± SD of n = 4–6. ALT, aminotransferase; γGT, γ-glutamyl-transpeptidase; BD, below detection limit; ND, Not done
p < 0.001, Ostα+/+ Sham VS Ostα+/+ BDL
p < 0.001, Ostα−/− Sham VS Ostα−/− BDL
p < 0.005, Ostα+/+ BDL VS Ostα−/− BDL
%p<0.05, Ostα+/+ Sham VS Ostα−/− Sham
Figure 1.
The nuclear receptor Car and Phase I and II enzymes are up-regulated in the liver after bile duct ligation in Ostα −/− mice. Quantitation RT-PCR results demonstrate that mRNA level for the nuclear receptor Car, but not Fxr or Pxr, is up-regulated after BDL in Ostα −/− mouse liver compared with wildtype BDL mice. mRNA for the Car target genes Cyp2b10, Cyp3a11, Sult2a1 and Ugt1a1 are also up-regulated compared with the wildtype BDL liver. Down-regulation of the repressor Shp and up-regulation of Cyp7a1 may result in an increase in the bile acid pool in these cholestatic mice. n = 4–6/group; *p<0.05
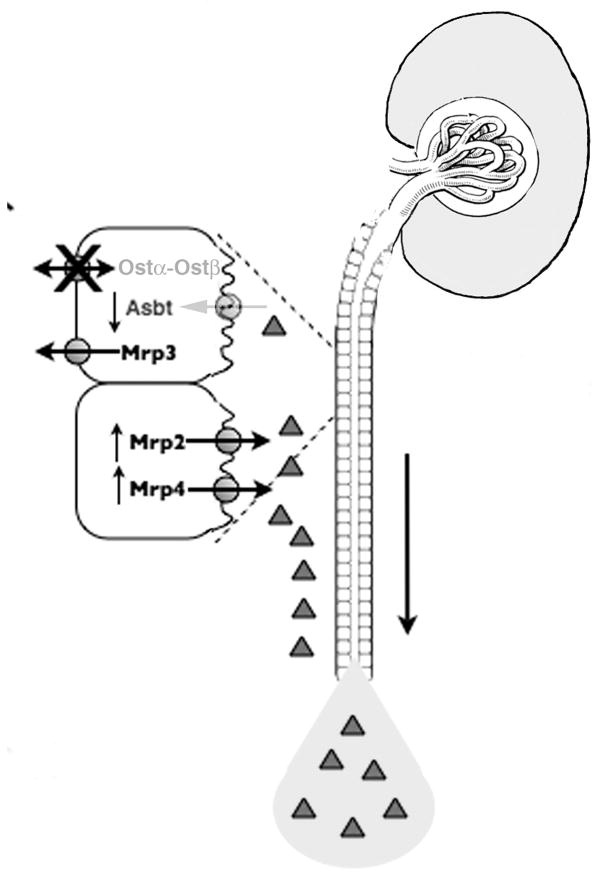
The surprising finding of attenuated liver injury in bile duct ligated Ostα-deficient mice led us to investigate the possible role that the renal excretory pathway may play in this protection. Although urinary bile acid excretion increases in cholestasis,51,52 the ligated Ostα-deficient mice excreted ~ 3 times the level of urinary bile acids than their wild-type bile duct ligated controls, with a significant increase in bile alcohol sulfates (Table 1). Analysis of the expression of membrane transporters in the kidney provided an explanation for the increases in urinary bile acids as illustrated in Fig. 2. The primary effect is the inability for urinary bile acids to be reabsorbed by the proximal tubule because of the lack of Ostα-Ostβ on the basolateral membrane along with a significant down-regulation of the apically located bile acid transporter, Asbt. At the same time, the apical membrane export transporters, Mrp2 and Mrp4, are up-regulated so that any bile acids that manage to be transported into the proximal tubule epithelial cell are excreted effectively into the urine. The clinical implications of these findings are that inhibition of renal OSTα-OSTβ by genetic or drug manipulation might provide a novel therapeutic means of reducing the accumulation of bile acids in cholestatic liver injury.
Figure 2.
Adaptive regulation of key transporters in the kidney of Ostα −/− mice provide protection from obstructive cholestasis. The increased serum bile acids in BDL mice cannot be efficiently reabsorbed in the kidney because of the decreased expression of apical Asbt and the absence of basolateral Ostα-Ostβ. Furthermore, an up-regulation of the apical export transporters, Mrp2 and Mrp4, efficiently prevents further retention of bile acids in the kidney. Therefore, ~3 fold more bile acids are excreted into the urine and removed from the body after BDL, compared with wild type mice.
OTHER CLINICAL IMPLICATIONS OF OSTα-OSTβ
To date there are no diseases directly associated with OSTα-OSTβ. However, given its importance in enterohepatic circulation and bile acid homeostasis, as well as intestinal lipid absorption, additional studies will be needed to look at its role in bile acid malabsorption, irritable bowel syndromes, enterocolitis, cholelithiasis, and lipid and glucose metabolism. A recent report investigated the role of various ileal bile acid transporters in primary idiopathic bile acid malabsorption (IBAM) which may be responsible for 30–50% of patients with unexplained chronic diarrhea.8 ASBT and IBABP were found to be significantly coexpressed in patients with diarrhea but not in controls. OSTα was found to be associated with caudal-related homeobox factor 2 (CDX2), LRH-1 and FXR in the controls, but not in the patients with diarrhea. Further studies are needed to examine if these correlations could impact diseases of bile acid malabsorption. Furthermore, the concentration of fecal bile acids has been positively correlated with the incidence of colorectal cancer.53 However, the potential role of OSTα-OSTβ in this cancer has yet to be examined. Necrotizing enterocolitis is another severe diarrhea disease seen mostly in premature neonates where the abnormal accumulation of bile acids in the distal small intestine might play a role in its pathogenesis.54 Polymorphisms in OSTα-OSTβ that reduce its functional expression in the ileum could predispose to this and other unexplained diarrheal disorders.
Expression of OSTα-OSTβ has also been examined in obese and non-obese patients with gallstone disease.55 A significant reduction in both mRNA and protein expression of both OSTα and OSTβ was found in normal weight gallstone carriers, but not in controls or in obese gallstone carriers. These changes correlated positively with expression of ASBT, IBABP and FXR, suggesting a role for these proteins in gallstone disease in non-obese patients.
Given our data suggesting a role for Car in regulating Ostα-Ostβ, future studies will need to examine diseases that have been associated with CAR regulation in humans. Activation of CAR and PXR has been shown to alter glucose homeostasis, lipid metabolism, and inflammation.56–58 For example, glucose levels were lowered in patients with non-insulin dependent diabetes after long term treatment with phenobarbital59 and in ob/ob mice after treatment with the Car agonist TCPOBOP.60 Whether OSTα-OSTβ has any potential role in these metabolism disorders awaits further studies.
SUMMARY
OSTα-OSTβ is the primary solute efflux transporter on the basolateral membrane of enterocytes. It is responsible for the secretion of bile acids from the intestine into the systemic circulation where they can then be recycled by the liver. Loss of expression of this heteromeric transporter results in disruption of bile acid homeostasis through a complex interplay of intestinal and hepatic transcription factors. This essential role in the intestine may lead to novel insights in diseases of bile acid malabsorption. Futhermore, OSTα-OSTβ is also present in the liver and has been shown to be up-regulated in cholestatic conditions such as PBC and biliary atresia. Along with the other basolateral membrane proteins, MRP3 and MRP4, OSTα-OSTβ acts to prevent the accumulation of toxic compounds in the liver. Interestingly, a genetic deficiency of Ostα-Ostβ in a model of obstructive cholestasis resulted in a hepatoprotective mechanism. This appears to involve increased urinary excretion of bile acids and raises the possibility of therapeutic interventions to decrease the expression of the renal OSTα-OSTβ. It is obvious that we are just beginning to understand the physiologic and medical significance of this unique transporter and to appreciate the potential role it may play in various diseases. Further studies are needed to explore how it may impact human metabolic disorders involving multiple organ systems.
ABBREVIATIONS
- ALT
aminotransferase
- ANIT
α-napthylthiocyanate
- ASBT
apical sodium dependent bile salt transporter
- BDL
bile duct ligation
- BSEP
Bile salt export pump
- CAR
constitutive androstane receptor
- CDCA
chenodeoxycholic acid
- CYP
cytochrome
- P450 CDX2
caudal-related homeobox factor 2
- FXR
farnesoid X receptor
- Fgf15/19
fibroblast growth factor 15/19
- FgfR4
fibroblast growth factor receptor 4
- IBABP
intestinal bile acid binding protein
- IBAM
idiopathic bile acid malabsorption
- LRH-1
liver receptor homologue 1
- LPS
lipopolysaccharide
- MRP
multidrug resistance-associated protein
- NTCP
sodium dependent taurocholate co-transporting polypeptide
- OSTα-OSTβ
organic solute transporter
- α-beta PBC
primary biliary cirrhosis
- PFIC1
progressive familial intrahepatic cholestasis type 1
- PXR
pregnane X receptor
- SHP
small heterodimer partner
- Sult2a1
sulfotransferase 2a1
- Ugt1a1
UDP-glucuronosyltransferase 1a1
References
- 1.Wong MH, Oelkers P, Craddock AL, Dawson PA. Expression cloning and characterization of the hamster ileal sodium-dependent bile acid transporter. J Biol Chem. 1994;269(2):1340–1347. [PubMed] [Google Scholar]
- 2.Wang W, Seward DJ, Li L, Boyer JL, Ballatori N. Expression cloning of two genes that together mediate organic solute and steroid transport in the liver of a marine vertebrate. Proc Natl Acad Sci U S A. 2001;98(16):9431–9436. doi: 10.1073/pnas.161099898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seward DJ, Koh AS, Boyer JL, Ballatori N. Functional complementation between a novel mammalian polygenic transport complex and an evolutionarily ancient organic solute transporter, OSTalpha-OSTbeta. J Biol Chem. 2003;278(30):27473–27482. doi: 10.1074/jbc.M301106200. [DOI] [PubMed] [Google Scholar]
- 4.Rao A, Haywood J, Craddock AL, Belinsky MG, Kruh GD, Dawson PA. The organic solute transporter α-β, Ostalpha-Ostbeta, is essential for intestinal bile acid transport and homeostasis. Proc Natl Acad Sci U S A. 2008;105(10):3891–3896. doi: 10.1073/pnas.0712328105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Soroka CJ, Mennone A, Hagey L, Ballatori N, Boyer JL. Organic solute transporter α deficiency enhances renal excretion of bile acids and attenuates cholestasis. Hepatology. 2010;51:181–190. doi: 10.1002/hep.23265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ballatori N, Christian WV, Lee JY, et al. OSTalpha-OSTbeta: a major basolateral bile acid and steroid transporter in human intestinal, renal, and biliary epithelia. Hepatology. 2005;42(6):1270–1279. doi: 10.1002/hep.20961. [DOI] [PubMed] [Google Scholar]
- 7.Ballatori N, Li N, Fang F, Boyer JL, Christian WV, Hammond CL. OST alpha-OST beta: a key membrane transporter of bile acids and conjugated steroids. Front Biosci. 2009;14:2829–2844. doi: 10.2741/3416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Balesaria S, Pell RJ, Abbott LJ, et al. Exploring possible mechanisms for primary bile acid malabsorption: evidence for different regulation of ileal bile acid transporter transcripts in chronic diarrhoea. Eur J Gastroenterol Hepatol. 2008;20(5):413–422. doi: 10.1097/MEG.0b013e3282f41b82. [DOI] [PubMed] [Google Scholar]
- 9.Lee H, Zhang Y, Lee FY, Nelson SF, Gonzalez FJ, Edwards PA. FXR regulates organic solute transporters α and β in the adrenal gland, kidney, and intestine. J Lipid Res. 2006;47(1):201–214. doi: 10.1194/jlr.M500417-JLR200. [DOI] [PubMed] [Google Scholar]
- 10.Dawson PA, Hubbert M, Haywood J, et al. The heteromeric organic solute transporter alpha-beta, Ostalpha-Ostbeta, is an ileal basolateral bile acid transporter. J Biol Chem. 2005;280(8):6960–6968. doi: 10.1074/jbc.M412752200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Soroka CJ, Xu S, Mennone A, Lam P, Boyer JL. N-Glycosylation of the alpha subunit does not influence trafficking or functional activity of the human organic solute transporter alpha/beta. BMC Cell Biol. 2008;9:57. doi: 10.1186/1471-2121-9-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sun A-Q, Balasubramaniyan N, Xu K, et al. Protein-protein interactions and membrane localization of the human organic solute transporter. Am J Physiol Gastrointest Liver Physiol. 2007;292(6):G1586–G1593. doi: 10.1152/ajpgi.00457.2006. [DOI] [PubMed] [Google Scholar]
- 13.Li N, Cui Z, Fang F, Lee JY, Ballatori N. Heterodimerization, trafficking and membrane topology of the two proteins, Ost α and Ost beta, that constitute the organic solute and steroid transporter. Biochem J. 2007;407(3):363–372. doi: 10.1042/BJ20070716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boyer JL, Trauner M, Mennone A, et al. Upregulation of a basolateral FXR-dependent bile acid efflux transporter OSTalpha-OSTbeta in cholestasis in humans and rodents. Am J Physiol Gastrointest Liver Physiol. 2006;290(6):G1124–G1130. doi: 10.1152/ajpgi.00539.2005. [DOI] [PubMed] [Google Scholar]
- 15.Frankenberg T, Rao A, Chen F, Haywood J, Shneider BL, Dawson PA. Regulation of the mouse organic solute transporter α-β, Ostalpha-Ostbeta, by bile acids. Am J Physiol Gastrointest Liver Physiol. 2006;290(5):G912–G922. doi: 10.1152/ajpgi.00479.2005. [DOI] [PubMed] [Google Scholar]
- 16.Landrier J-F, Eloranta JJ, Vavricka SR, Kullak-Ublick GA. The nuclear receptor for bile acids, FXR, transactivates human organic solute transporter-α and -β genes. Am J Physiol Gastrointest Liver Physiol. 2006;290(3):G476–G485. doi: 10.1152/ajpgi.00430.2005. [DOI] [PubMed] [Google Scholar]
- 17.Lu TT, Makishima M, Repa JJ, et al. Molecular basis for feedback regulation of bile acid synthesis by nuclear receptors. Mol Cell. 2000;6(3):507–515. doi: 10.1016/s1097-2765(00)00050-2. [DOI] [PubMed] [Google Scholar]
- 18.Denson LA, Sturm E, Echevarria W, et al. The orphan nuclear receptor, shp, mediates bile acid-induced inhibition of the rat bile acid transporter, ntcp. Gastroenterology. 2001;121(1):140–147. doi: 10.1053/gast.2001.25503. [DOI] [PubMed] [Google Scholar]
- 19.Sinal CJ, Tohkin M, Miyata M, Ward JM, Lambert G, Gonzalez FJ. Targeted disruption of the nuclear receptor FXR/BAR impairs bile acid and lipid homeostasis. Cell. 2000;102(6):731–744. doi: 10.1016/s0092-8674(00)00062-3. [DOI] [PubMed] [Google Scholar]
- 20.Grober J, Zaghini I, Fujii H, et al. Identification of a bile acid-responsive element in the human ileal bile acid-binding protein gene. Involvement of the farnesoid X receptor/9-cis-retinoic acid receptor heterodimer. J Biol Chem. 1999;274(42):29749–29754. doi: 10.1074/jbc.274.42.29749. [DOI] [PubMed] [Google Scholar]
- 21.Zollner G, Wagner M, Moustafa T, et al. Coordinated induction of bile acid detoxification and alternative elimination in mice: role of FXR-regulated organic solute transporter-α/β in the adaptive response to bile acids. Am J Physiol Gastrointest Liver Physiol. 2006;290(5):G923–G932. doi: 10.1152/ajpgi.00490.2005. [DOI] [PubMed] [Google Scholar]
- 22.Ballatori N, Fang F, Christian WV, Li N, Hammond CL. Ostalpha-Ostbeta is required for bile acid and conjugated steroid disposition in the intestine, kidney, and liver. Am J Physiol Gastrointest Liver Physiol. 2008;295(1):G179–G186. doi: 10.1152/ajpgi.90319.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Inagaki T, Choi M, Moschetta A, et al. Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis. Cell Metab. 2005;2(4):217–225. doi: 10.1016/j.cmet.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 24.Boyer JL. New perspectives for the treatment of cholestasis: lessons from basic science applied clinically. J Hepatol. 2007;46(3):365–371. doi: 10.1016/j.jhep.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zollner G, Fickert P, Silbert D, et al. Adaptive changes in hepatobiliary transporter expression in primary biliary cirrhosis. J Hepatol. 2003;38(6):717–727. doi: 10.1016/s0168-8278(03)00096-5. [DOI] [PubMed] [Google Scholar]
- 26.Schaap FG, van der Gaag NA, Gouma DJ, Jansen PLM. High expression of the bile salt-homeostatic hormone fibroblast growth factor 19 in the liver of patients with extrahepatic cholestasis. Hepatology. 2009;49(4):1228–1235. doi: 10.1002/hep.22771. [DOI] [PubMed] [Google Scholar]
- 27.Keitel V, Burdelski M, Warskulat U, et al. Expression and localization of hepatobiliary transport proteins in progressive familial intrahepatic cholestasis. Hepatology. 2005;41(5):1160–1172. doi: 10.1002/hep.20682. [DOI] [PubMed] [Google Scholar]
- 28.Chen J, Terada T, Ogasawara K, Katsura T, Inui K-i. Adaptive responses of renal organic anion transporter 3 (OAT3) during cholestasis. Am J Physiol Renal Physiol. 2008;295(1):F247–F252. doi: 10.1152/ajprenal.00139.2008. [DOI] [PubMed] [Google Scholar]
- 29.Zollner G, Wagner M, Fickert P, et al. Expression of bile acid synthesis and detoxification enzymes and the alternative bile acid efflux pump MRP4 in patients with primary biliary cirrhosis. Liver Int. 2007;27(7):920–929. doi: 10.1111/j.1478-3231.2007.01506.x. [DOI] [PubMed] [Google Scholar]
- 30.Hirohashi T, Suzuki H, Takikawa H, Sugiyama Y. ATP-dependent transport of bile salts by rat multidrug resistance-associated protein 3 (Mrp3) J Biol Chem. 2000;275(4):2905–2910. doi: 10.1074/jbc.275.4.2905. [DOI] [PubMed] [Google Scholar]
- 31.Zamek-Gliszczynski MJ, Nezasa K-i, Tian X, et al. Evaluation of the role of multidrug resistance-associated protein (Mrp) 3 and Mrp4 in hepatic basolateral excretion of sulfate and glucuronide metabolites of acetaminophen, 4-methylumbelliferone, and harmol in Abcc3−/− and Abcc4−/− mice. J Pharmacol Exp Ther. 2006;319(3):1485–1491. doi: 10.1124/jpet.106.110106. [DOI] [PubMed] [Google Scholar]
- 32.Cui YJ, Aleksunes LM, Tanaka Y, Goedken MJ, Klaassen CD. Compensatory induction of liver efflux transporters in response to ANIT-induced liver injury is impaired in FXR-null mice. Toxicol Sci. 2009;110(1):47–60. doi: 10.1093/toxsci/kfp094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Donner MG, Keppler D. Up-regulation of basolateral multidrug resistance protein 3 (Mrp3) in cholestatic rat liver. Hepatology. 2001;34(2):351–359. doi: 10.1053/jhep.2001.26213. [DOI] [PubMed] [Google Scholar]
- 34.Ogawa K, Suzuki H, Hirohashi T, et al. Characterization of inducible nature of MRP3 in rat liver. Am J Physiol Gastrointest Liver Physiol. 2000;278(3):G438–G446. doi: 10.1152/ajpgi.2000.278.3.G438. [DOI] [PubMed] [Google Scholar]
- 35.Soroka CJ, Lee JM, Azzaroli F, Boyer JL. Cellular localization and up-regulation of multidrug resistance-associated protein 3 in hepatocytes and cholangiocytes during obstructive cholestasis in rat liver. Hepatology. 2001;33(4):783–791. doi: 10.1053/jhep.2001.23501. [DOI] [PubMed] [Google Scholar]
- 36.Belinsky MG, Dawson PA, Shchaveleva I, et al. Analysis of the in vivo functions of Mrp3. Mol Pharmacol. 2005;68(1):160–168. doi: 10.1124/mol.104.010587. [DOI] [PubMed] [Google Scholar]
- 37.Bohan A, Chen W-S, Denson LA, Held MA, Boyer JL. Tumor necrosis factor alpha-dependent up-regulation of Lrh-1 and Mrp3(Abcc3) reduces liver injury in obstructive cholestasis. J Biol Chem. 2003;278(38):36688–36698. doi: 10.1074/jbc.M304011200. [DOI] [PubMed] [Google Scholar]
- 38.Zelcer N, van de Wetering K, de Waart R, et al. Mice lacking Mrp3 (Abcc3) have normal bile salt transport, but altered hepatic transport of endogenous glucuronides. J Hepatol. 2006;44(4):768–775. doi: 10.1016/j.jhep.2005.07.022. [DOI] [PubMed] [Google Scholar]
- 39.König J, Rost D, Cui Y, Keppler D. Characterization of the human multidrug resistance protein isoform MRP3 localized to the basolateral hepatocyte membrane. Hepatology. 1999;29(4):1156–1163. doi: 10.1002/hep.510290404. [DOI] [PubMed] [Google Scholar]
- 40.Kruh GD, Belinsky MG, Gallo JM, Lee K. Physiological and pharmacological functions of Mrp2, Mrp3 and Mrp4 as determined from recent studies on gene-disrupted mice. Cancer Metastasis Rev. 2007;26(1):5–14. doi: 10.1007/s10555-007-9039-1. [DOI] [PubMed] [Google Scholar]
- 41.Mennone A, Soroka CJ, Cai S-Y, et al. Mrp4−/− mice have an impaired cytoprotective response in obstructive cholestasis. Hepatology. 2006;43(5):1013–1021. doi: 10.1002/hep.21158. [DOI] [PubMed] [Google Scholar]
- 42.Assem M, Schuetz EG, Leggas M, et al. Interactions between hepatic Mrp4 and Sult2a as revealed by the constitutive androstane receptor and Mrp4 knockout mice. J Biol Chem. 2004;279(21):22250–22257. doi: 10.1074/jbc.M314111200. [DOI] [PubMed] [Google Scholar]
- 43.Saini SPS, Sonoda J, Xu L, et al. A novel constitutive androstane receptor-mediated and CYP3A-independent pathway of bile acid detoxification. Mol Pharmacol. 2004;65(2):292–300. doi: 10.1124/mol.65.2.292. [DOI] [PubMed] [Google Scholar]
- 44.Wagner M, Halilbasic E, Marschall H-U, et al. CAR and PXR agonists stimulate hepatic bile acid and bilirubin detoxification and elimination pathways in mice. Hepatology. 2005;42(2):420–430. doi: 10.1002/hep.20784. [DOI] [PubMed] [Google Scholar]
- 45.Stedman CAM, Liddle C, Coulter SA, et al. Nuclear receptors constitutive androstane receptor and pregnane X receptor ameliorate cholestatic liver injury. Proc Natl Acad Sci U S A. 2005;102(6):2063–2068. doi: 10.1073/pnas.0409794102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huang W, Zhang J, Moore DD. A traditional herbal medicine enhances bilirubin clearance by activating the nuclear receptor CAR. J Clin Invest. 2004;113(1):137–143. doi: 10.1172/JCI200418385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yin J, Wennberg RP, Xia YC, Liu JW, Zhou HZ. Effect of a traditional Chinese medicine, yin zhi huang, on bilirubin clearance and conjugation. Dev Pharmacol Ther. 1991;16(1):59–64. [PubMed] [Google Scholar]
- 48.Modica S, Bellafante E, Moschetta A. Master regulation of bile acid and xenobiotic metabolism via the FXR, PXR and CAR trio. Front Biosci. 2009;14:4719–4745. doi: 10.2741/3563. [DOI] [PubMed] [Google Scholar]
- 49.Bloomer JR, Boyer JL. Phenobarbital effects in cholestatic liver diseases. Ann Intern Med. 1975;82(3):310–317. doi: 10.7326/0003-4819-82-3-310. [DOI] [PubMed] [Google Scholar]
- 50.Khurana S, Singh P. Rifampin is safe for treatment of pruritus due to chronic cholestasis: a meta-analysis of prospective randomized-controlled trials. Liver Int. 2006;26(8):943–948. doi: 10.1111/j.1478-3231.2006.01326.x. [DOI] [PubMed] [Google Scholar]
- 51.Schlattjan JH, Winter C, Greven J. Regulation of renal tubular bile acid transport in the early phase of an obstructive cholestasis in the rat. Nephron Physiol. 2003;95(3):49–56. doi: 10.1159/000074330. [DOI] [PubMed] [Google Scholar]
- 52.Stiehl A, Raedsch R, Rudolph G, Gundert-Remy U, Senn M. Biliary and urinary excretion of sulfated, glucuronidated and tetrahydroxylated bile acids in cirrhotic patients. Hepatology. 1985;5(3):492–495. doi: 10.1002/hep.1840050325. [DOI] [PubMed] [Google Scholar]
- 53.Knisely AS, Strautnieks SS, Meier Y, et al. Hepatocellular carcinoma in ten children under five years of age with bile salt export pump deficiency. Hepatology. 2006;44(2):478–486. doi: 10.1002/hep.21287. [DOI] [PubMed] [Google Scholar]
- 54.Halpern MD, Dvorak B. Does abnormal bile acid metabolism contribute to NEC? Semin Perinatol. 2008;32(2):114–121. doi: 10.1053/j.semperi.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Renner O, Harsch S, Strohmeyer A, Schimmel S, Stange EF. Reduced ileal expression of OSTalpha-OSTbeta in non-obese gallstone disease. J Lipid Res. 2008;49(9):2045–2054. doi: 10.1194/jlr.M800162-JLR200. [DOI] [PubMed] [Google Scholar]
- 56.Kakizaki S, Yamazaki Y, Takizawa D, Negishi M. New insights on the xenobiotic-sensing nuclear receptors in liver diseases—CAR and PXR—. Curr Drug Metab. 2008;9(7):614–621. doi: 10.2174/138920008785821666. [DOI] [PubMed] [Google Scholar]
- 57.Rezen T, Tamasi V, Lövgren-Sandblom A, Björkhem I, Meyer UA, Rozman D. Effect of CAR activation on selected metabolic pathways in normal and hyperlipidemic mouse livers. BMC Genomics. 2009;10:384. doi: 10.1186/1471-2164-10-384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stefano F, Sabrina C, Baldelli F, Mencarelli A. Bile acid-activated receptors in the treatment of dyslipidemia and related disorders. Prog Lipid Res. 2009 doi: 10.1016/j.plipres.2009.11.001. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 59.Lahtela JT, Arranto AJ, Sotaniemi EA. Enzyme inducers improve insulin sensitivity in non-insulin-dependent diabetic subjects. Diabetes. 1985;34(9):911–916. doi: 10.2337/diab.34.9.911. [DOI] [PubMed] [Google Scholar]
- 60.Dong B, Saha PK, Huang W, et al. Activation of nuclear receptor CAR ameliorates diabetes and fatty liver disease. Proc Natl Acad Sci U S A. 2009;106(44):18831–18836. doi: 10.1073/pnas.0909731106. [DOI] [PMC free article] [PubMed] [Google Scholar]