Abstract
Preeclampsia is a pregnancy-specific multisystem disorder characterized by hypertension and proteinuria. Accentuated maternal hyperlipidemia, especially high serum levels of oxidized low-density lipoprotein (oxLDL), is one of the features of preeclampsia. We previously reported that lectin-like oxidized LDL receptor 1 (LOX-1) expression was decreased in preeclamptic placentas. Here, we show that decreased LOX-1 expression is associated with low expression of adenosine triphosphate-binding cassette transporter A1 (ABCA1) in the placenta. The ABCA1 mediates cellular efflux of cholesterol, and liver X receptors (LXRs) are its predominant transcriptional regulators. Both ABCA1 and LXR expressions were significantly lower in preeclamptic placentas than those in normal controls. Oxidized LDL upregulated ABCA1 expression, while LOX-1 blockade resulted in the alleviation of increasing ABCA1 messenger RNA in JAR cells. These results suggest that low LOX-1 expression may lead to insufficient oxLDL uptake, thereby contributing to reduced LXR activation and decreased ABCA1 expression in preeclamptic placentas.
Keywords: preeclampsia, oxidized LDL, LOX-1, ABCA1, LXR
Introduction
Preeclampsia is a pregnancy-specific disorder, clinically characterized by De Novo development of hypertension and proteinuria.1 Complicating 2% to 8% of pregnancies, preeclampsia is a major cause of maternal and neonatal morbidity and mortality.2 Although the etiology of preeclampsia remains enigmatic, it is generally agreed that the placenta plays a critical role in the pathogenesis of this disorder.
One of the striking changes that occur in lipid metabolism during normal pregnancy is maternal hyperlipidemia. This feature is observed more noticeably in women with preeclampsia. Indeed, serum lipid levels such as triglycerides, low-density lipoproteins (LDLs), and small dense LDLs, which are susceptible to oxidation, are higher in women with preeclampsia than those in normal pregnant women.3,4 In addition, oxidized LDL (oxLDL) is also increased in the serum of women with preeclampsia.5,6
Adenosine triphosphate-binding cassette transporter A1 (ABCA1) is a membrane transporter that mediates cellular efflux of cholesterol and phospholipids to lipid-poor apolipoprotein A1, the precursor of high-density lipoprotein (HDL). The ABCA1 is highly expressed in the human placenta7,8 and is thought to play a central role in cholesterol metabolism. Importantly, placental malformation and intrauterine growth restriction were observed in ABCA1 null mice.9 The predominant transcriptional regulator of ABCA1 is the liver X receptor (LXR). The LXRs are nuclear receptors that modulate the expression of genes involved in cholesterol and lipid metabolism in response to changes in cellular cholesterol status.10 Two isotypes have been identified, LXRα is mainly expressed in the liver, adipose tissue, and macrophages, while LXRβ is ubiquitously expressed in human tissues. Both LXRs are activated by oxysterols, oxidized derivatives of cholesterol.10
We previously reported that lectin-like oxidized LDL receptor 1 (LOX-1), a major oxLDL scavenger receptor was decreased in preeclamptic placentas.11 The LOX-1 is responsible for the binding and internalization of oxLDL. However, it is presently unclear whether reduced LOX-1 affects ABCA1 expression through LXR in preeclamptic placentas. We hypothesized that LOX-1 and oxLDL may be involved in the regulation of ABCA1 expression in the placenta. The aim of this study was to clarify the relationship between LOX-1 and ABCA1 in the placenta, particularly in the context of preeclampsia.
Materials and Methods
Patients
Totally, 20 women with singleton pregnancy were enrolled in this study (Table 1). In all, 10 women had normal pregnancies, while 10 women were complicated with preeclampsia. Women with chronic hypertension and renal disease or other pregnancy complications were excluded from this study. Preeclampsia was defined as maternal systolic blood pressure ≥140 mm Hg and/or diastolic blood pressure ≥90 mm Hg in 2 consecutive measurements at least 6 hours apart, and proteinuria ≥300 mg/24 h after 20 weeks of gestation. We calculated the number of standard deviations (SDs) depending on the appropriate normal mean for gestational age in Japanese singleton pregnancies and this was expressed as delta neonatal weight (SD).
Table 1.
Clinical Characteristics of the Normal and Patient With Preeclampsia Groups.a
| Normal (n = 10) | PE (n = 10) | P Value | |
|---|---|---|---|
| Patient’s age at delivery, years | 36.8 ± 5.4 (27-44) | 34.1 ± 3.3 (29-39) | NS |
| Primipara, n | 3/10 | 8/10 | – |
| Cesarean section, n | 10/10 | 10/10 | – |
| Gestational age at delivery, weeks | 38 ± 0.9 (37-39) | 35 ± 4.6 (26-40) | <.05 |
| Body mass index at delivery, kg/m2 | 25.4 ± 2.6 (21.2-29.1) | 25.4 ± 3.8 (21.0-33.4) | NS |
| Systolic blood pressure at delivery, mm Hg | 106 ± 7 (90-116) | 173 ± 17 (145-192) | <.0001 |
| Diastolic blood pressure at delivery, mm Hg | 65 ± 9 (52-80) | 105 ± 11 (90-124) | <.0001 |
| Neonatal weight, g | 2902 ± 245 (2494-3296) | 2085 ± 943 (576-3460) | <.05 |
| Delta neonatal weight, SD | −0.12 ± 0.46 (−0.97 - +0.57) | −0.84 ± 0.96 (−2.0 - +0.71) | NS |
Abbreviation: NS, nonsignificant; SD, standard deviation.
a Values are the mean ± SD and (range).
Placental Tissues
Placental villous tissues were obtained from normal pregnancies (n = 10) and preeclamptic pregnancies (n = 10), immediately after cesarean section in the absence of labor, at Kyoto University Hospital, Japan. Villous tissues were collected from the central part of the placenta and were macroscopically free of infarction or calcification. After brief rinsing in saline, these tissues were stored in RNAlater (Ambion, Austin, Texas) at −80°C until RNA extraction. The local Ethics committee of the Graduate School of Medicine, Kyoto University approved the study protocol and written informed consent was obtained from each patient.
Real-Time Quantitative PCR
Total RNA extraction from placental tissues and JAR cells was performed using the RNeasy Mini kit (QIAGEN, Germantown, Maryland) according to the manufacturer’s instructions. The quality and quantity of RNA was measured using an ND-1000 spectrophotometer (Nanodrop, Wilmington, North Carolina). Reverse transcription of 1 µg RNA was performed using the Rever Tra Ace (TOYOBO, Osaka, Japan) according to the manufacturer’s instructions. Primers for the genes examined (Table 2) were designed using GeneFisher 2 software (Bielefeld University Bioinformatics Service, Bielefeld, Germany). Real-time quantitative polymerase chain reaction (RT-qPCR) was performed using SYBR premix Ex TaqII (Takara Bio, Otsu, Japan) on the LightCycler 480 Real-Time PCR system (Roche Diagnostics, Mannheim, Germany) with the following run conditions: 95°C for 30 seconds for initial denaturation followed by 95°C for 5 seconds and 60°C for 30 seconds (40 cycles). For dissociation after PCR amplification, the protocol included slow heating from 60°C to 97°C to ensure amplification specificity. Gene expression was estimated using the comparative crossing point method for relative quantification. All data were normalized using glyceraldehyde 3-phosphate dehydrogenase as an internal control and expressed relative to controls. All samples were run in duplicate and quantitative detection was averaged.
Table 2.
Primer Sequences Used in Real-Time Quantitative PCR.
| Gene | Forward Primer (5′-3′) | Reverse Primer (5′-3′) | Entrez Gene ID |
|---|---|---|---|
| ABCA1 | GGAACAGGCTACTACCTGACCTTGG | ATCGATGGTCAGCGTGTCACTCTC | 19 |
| LXRα | GATCGAGGTGATGCTTCTGG | ACTCGAAGATGGGGTTGATG | 10062 |
| LXRβ | GATCGTGGACTTCGCTAAGCAAGTG | GTCCTTGCTGTAGGTGAAGTCCTTC | 7376 |
| GAPDH | GAGTCAACGGATTTGGTCGTATTGG | GCCATGGGTGGAATCATATTGGAAC | 2597 |
Abbreviations: ABCA1, adenosine triphosphate-binding cassette transporter A1; LXR, liver X receptor; GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
Western Blot
Placental tissues were homogenized in RIPA buffer (50 mmol/L Tris-HCl, pH 8.0, 150 mmol/L sodium chloride, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate, and 1.0% NP-40 substitute) supplemented with cocktail protease inhibitor Complete Mini (Roche Diagnostics). Homogenized tissues were centrifuged at 10 000g for 20 minutes at 4°C, and the supernatant was saved as cytosolic extract from placental tissues. Cells were washed with ice-cold phosphate-buffered saline, and cytosolic protein was extracted in the same manner. Protein concentrations were determined using BCA Protein Assay Kit (Thermo Scientific, Rockford, Illinois). A total of 20 µg of protein was separated on 7.5% sodium dodecyl sulfate-polyacrylamide gels. Separated proteins were transferred onto nitrocellulose membranes, which were blocked with 5% fat-free milk overnight at 4°C. We confirmed an equal amount of protein loading by Ponceau S staining. Membranes were probed with mouse monoclonal antibody against ABCA1 (ab18180; 1:1000; Abcam, Cambridge, UK). Rabbit polyclonal antibody against β-actin (1:5000; Abcam) was used as a loading control. Blots were subsequently incubated with an appropriate secondary antibody (1:10 000; Santa Cruz Biotechnology, Santa Cruz, California). Signals were detected with Western Blotting Substrate Plus (Thermo Scientific) and visualized by the ChemiDoc system (BioRad, Hercules, California).
Cell Culture
The JAR (HTB-144) choriocarcinoma cell line was purchased from the American Type Culture Collection (Manassas, Virginia) and cultured in RPMI medium supplemented with 10% fetal calf serum, 100 U/mL of penicillin, and 100 µg/mL of streptomycin at 37°C in a humidified atmosphere containing 5% CO2. The OxLDL and native LDL (nLDL) were purchased from Intracel (Frederick, Maryland). The JAR cells grown in 48-well plates were treated with 100 µg/mL of oxLDL or nLDL for 3, 6, 9, and 24 hours. Next, after pretreating with 30 µg/mL of TS92, anti-human LOX-1 antibody, or normal human immunoglobulin G (IgG) purchased from R&D Systems (Minneapolis, Minnesota), JAR cells were treated with oxLDL (100 µg/mL) for 9 hours. The TS92 was a kind gift from Dr T. Sawamura, Osaka, Japan. The cells were harvested and mRNA expressions of ABCA1, LXRα, and LXRβ were measured by qPCR. Protein expression of ABCA1 was analyzed by Western blotting. Six experiments were performed in triplicate (n = 6).
Statistical Analysis
The results of normally distributed continuous variables are expressed as mean ± standard error of the mean (range), while those with skewed distribution were expressed as the median value with (interquartile range). Statistical comparisons were performed with the Mann-Whitney U test and 1-way analysis of variance followed by the Tukey test as appropriate using Prism 4.0 (GraphPad Software, La Jolla, California). The values of P <.05 were considered statistically significant.
Results
Patient Characteristics
The clinical characteristics of patients enrolled in this study are shown in Table 1. No patients were habitual smokers. Gestational age at delivery was earlier in the preeclampsia group than that in the normal pregnancy group. Neonatal weight was also lighter in the preeclampsia group than that in the normal pregnancy group. Although delta neonatal weight was larger in the preeclampsia group, it was not statistically significant. Meanwhile, no differences between the groups were observed concerning the age and body mass index of patients at delivery. Among 10 women with preeclampsia, 4 were early-onset (≤34 weeks gestation) preeclampsia and 6 were late-onset (>34 weeks gestation) preeclampsia. All women with preeclampsia were diagnosed with severe preeclampsia according to American College of Obstetricians and Gynecologists criteria.
Expression of ABCA1 in Normal and Preeclamptic Placentas
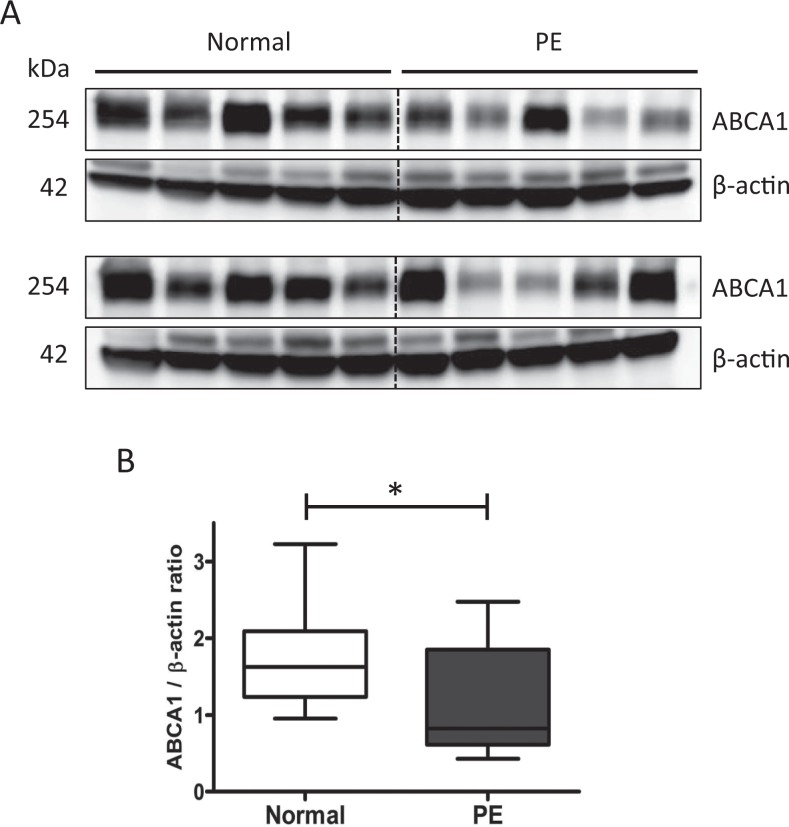
First, we assessed the mRNA expressions of ABCA1, LXRα, and LXRβ in normal and preeclamptic placentas. Both LXRα and LXRβ are predominant upstream regulators of ABCA1. Quantitative RT-PCR analysis showed that mRNA expressions of these genes were significantly lower in preeclamptic placentas than those in normal placentas (Figure 1A and B), indicating that LXR activation was reduced in preeclamptic placentas. Western blot analysis of placental lysates demonstrated that the protein levels of ABCA1 were also significantly reduced in preeclamptic placentas compared to those of the normal controls (Figure 2A and B).
Figure 1.
Messenger RNA expressions of (A) ABCA1, and (B) LXRα and LXRβ in normal and preeclamptic placentas (n = 10 in each group). Values were normalized to those of GAPDH. Data are presented as the median value with interquartile range. *P < .05, **P < .01. ABCA1 indicates adenosine triphosphate-binding cassette transporter A1; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; LXR, liver X receptor.
Figure 2.
Expression of ABCA1 protein in normal and preeclamptic placentas (n = 10 in each group). A, Western blot for ABCA1 in placentas. B, Densitometric analysis of ABCA1 protein expression normalized to β-actin. Data are presented as the median value with interquartile range. * P < .05. ABCA1 indicates adenosine triphosphate-binding cassette transporter A1.
The ABCA1 Upregulation by oxLDL in JAR Cells
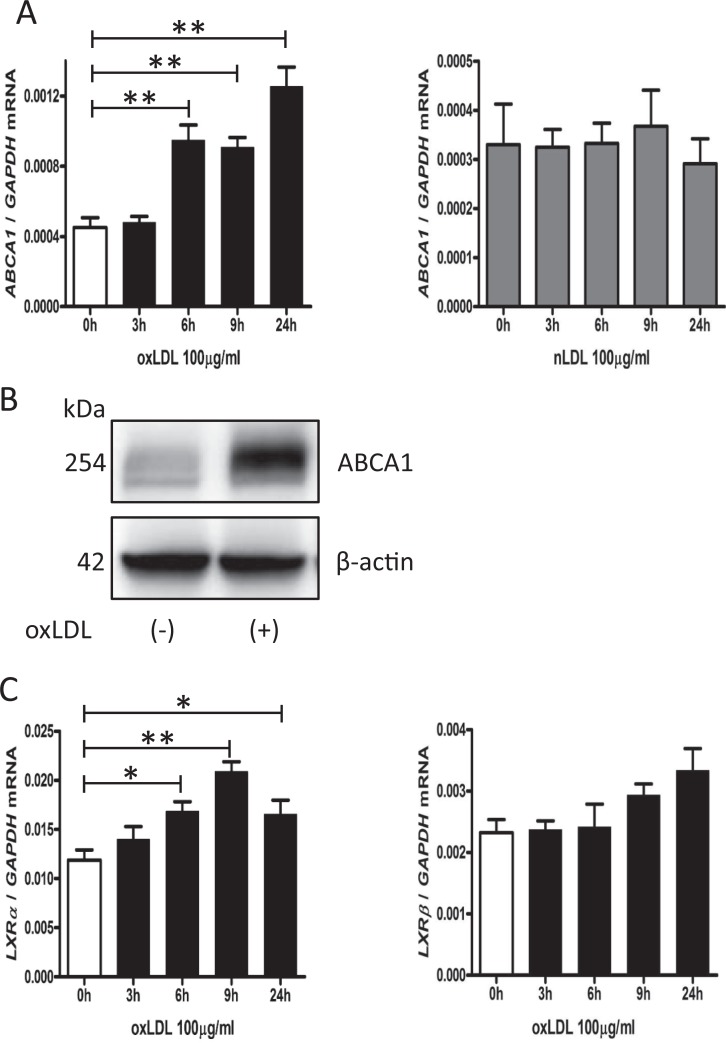
In women with preeclampsia, serum levels of oxLDL are higher than the normal pregnant women.5,6 To investigate the efficacy of oxLDL to ABCA1 gene expression in trophoblast cells, we treated JAR cells with 100 µg/mL of oxLDL or nLDL, as a control, for 3, 6, 9, and 24 hours. The OxLDL treatment significantly increased the expression of ABCA1 mRNA at 6 to 24 hours (Figure 3A, left), while nLDL did not alter ABCA1 mRNA expression (Figure 3A, right). In Western blot analysis, we found increased ABCA1 protein levels in JAR cells treated with oxLDL at 9 hours (Figure 3B). Moreover, oxLDL upregulated LXRα mRNA significantly in a time-dependent manner (Figure 3C, left). Although LXRβ mRNA tended to increase with oxLDL at 9 to 24 hours, it was not statistically significant (Figure 3C, right).
Figure 3.
The ABCA1 upregulation by oxLDL in JAR cells. A, Time courses of ABCA1 mRNA expression in JAR cells treated with oxLDL (100 µg/mL) or nLDL (100 µg/mL). B, A representative Western blot image for ABCA1 in JAR cells treated with or without oxLDL (100 µg/mL) for 9 hours. C, Time courses of LXRα and LXRβ mRNA expression in JAR cells treated with oxLDL (100 µg/mL). Values were normalized to those of GAPDH. Data are presented as the mean ± standard error of the mean. * P < .05, ** P < .01. Six experiments were performed in triplicate (n = 6). ABCA1 indicates adenosine triphosphate-binding cassette transporter A1; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; LXR, liver X receptor; mRNA, messenger RNA; nLDL, native low-density lipoprotein; oxLDL, oxidized low-density lipoprotein.
Attenuation of Increasing ABCA1 mRNA by LOX-1 Blockade
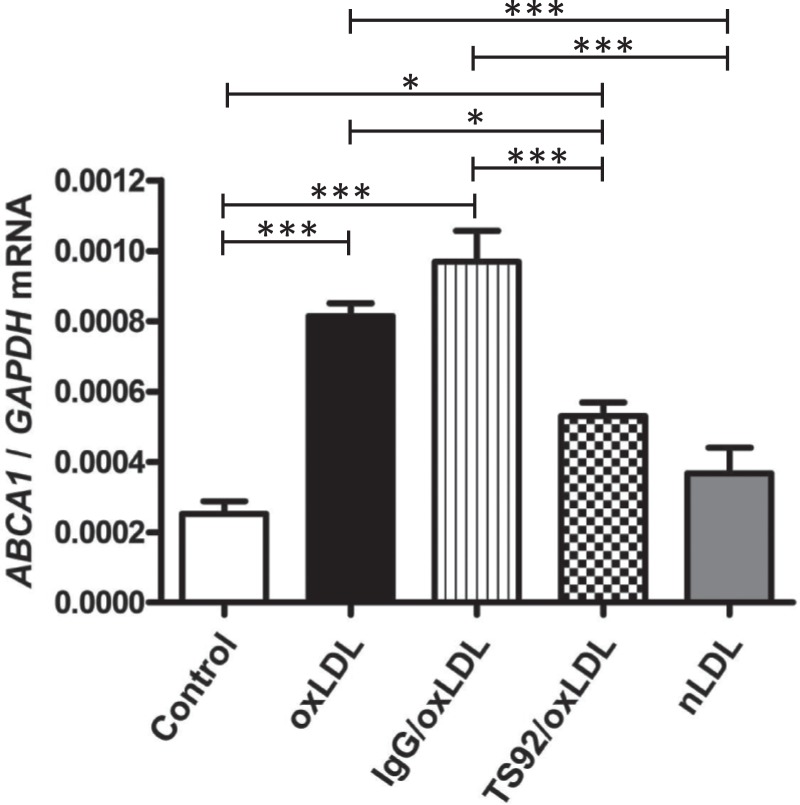
In order to determine the possible involvement of reduced LOX-1 expression in decreased ABCA1 expression in preeclamptic placentas, JAR cells were pretreated with TS92 (30 µg/mL), an anti-human LOX-1 antibody, and then stimulated with oxLDL (100 µg/mL) for 9 hours. In this culture model, TS92 significantly inhibited ABCA1 upregulation induced by oxLDL (Figure 4). Normal human IgG did not affect increased ABCA1 mRNA expression and nLDL did not upregulate ABCA1 mRNA.
Figure 4.
Attenuation of increasing ABCA1 mRNA by LOX-1 blockade. ABCA1 mRNA expression in JAR cells treated with oxLDL (100 µg/mL) in the presence or absence of TS92 (30 µg/mL) or normal human IgG (30 µg/mL), and that in JAR cells treated with nLDL (100 µg/mL) for 9 hours. Values were normalized to those of GAPDH. Data are presented as the mean ± SEM. * P < .05, *** P < .001. Six experiments were performed in triplicate (n = 6). ABCA1 indicates adenosine triphosphate-binding cassette transporter A1; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; IgG, immunoglobulin G; LOX-1, lectin-like oxidized LDL receptor 1; mRNA, messenger RNA; nLDL, native low-density lipoprotein; oxLDL, oxidized low-density lipoprotein; SEM, standard error of the mean.
Discussion
In placental tissue, ABCA1 is localized in villous cytotrophoblast cells,12 the surface of the syncytiotrophoblast membrane,13 and placental endothelial cells.14 Based on this evidence, ABCA1 is believed to be engaged not only in cholesterol homeostasis in the placenta during pregnancy but also in feta-placental cholesterol transport. Indeed, studies of ABCA1 knockout mice revealed aberrant placental development and fetal growth restriction.9 However, whether altered ABCA1 expression in the human placenta is associated with pathological pregnancies, including preeclampsia, has not been explored thoroughly. In the present study, we first found that ABCA1 expression was significantly lower in preeclamptic placentas than those in normal placentas in both mRNA and protein levels. Intriguingly, our results were inconsistent with the previous studies. Plosch et al revealed the upregulation of ABCA1 in early-onset preeclamptic placentas,15 and Albrecht et al reported the unchanged expression of ABCA1 in placentas from women with preeclampsia, while they found decreased ABCA1 expression in those with antiphospholipid syndrome.16 Although the causes of these disparities remain unclear, they might derive from the phenotype of preeclampsia, especially the timing of onset or disease duration of preeclampsia. In addition, we must take into account that our sample number was limited and the gestational age in preeclamptic group ranged from 26 to 40 weeks. Meanwhile, as a pilot study, we confirmed that our preeclamptic placentas were appropriate samples by revealing high expressions of soluble fms-like tyrosine kinase 1 and leptin mRNA (1.89-fold, P < .05; 33.7-fold, P < .001, respectively. Data not shown.), since it is widely acknowledged that these mRNAs were increased in preeclamptic placentas.17,18 Remarkably, Lindegaard et al treated C57Bl/6 mice with an LXR agonist and demonstrated significant upregulation of placental ABCA1 mRNA expression and increased maternal–fetal cholesterol transfer, which is beneficial for some congenital fetal diseases.19 On the other hand, the patients with ABCA1 mutations, known as Tangier disease, present with low levels of HDL and develop premature atherosclerosis.20 Thus, suppression of ABCA1 function in the placenta may lead to maternal aberrant lipid metabolism in preeclampsia or may cause fetal growth restriction.
The LXR is a predominant upstream regulator of ABCA1, and the LXR pathway regulates lipid metabolism and inflammation. We showed that mRNA expressions of LXRs were significantly downregulated in preeclamptic placentas, which is consistent with the report by Weedon-Fekjaer et al.21 Taken together, these results suggest that LXR activation is reduced and can be one of the causes of decreases in ABCA1 expression in preeclamptic placentas. Moreover, LXR has a powerful anti-inflammatory effect that may contribute to antiatherosclerotic potency,22 and many studies have established that an LXR agonist results in the attenuation of atherosclerosis in vivo.23–25 On the other hand, a combined deficiency of LXRα and LXRβ in mice was associated with increased LDL levels and foamy macrophage accumulation in the arterial wall.26 Interestingly, the spiral arteries of preeclamptic placental decidua often exhibit lipid deposition and the involvement of foamy macrophages.27 This phenomenon is called acute atherosis and resembles the early stages of atherosclerotic development. Given these considerations, it is possible that decreasing ABCA1 expression due to disruption of LXR signaling in term placentas is intimately related to the pathophysiology of preeclampsia.
Endogenous LXR ligands are oxysterols (oxidized cholesterol derivatives). Increasing intracellular concentrations of oxysterols subsequently activate LXR and upregulate ABCA1 expression. We demonstrated that ABCA1 expression was increased by oxLDL, not by nLDL in JAR cells. The OxLDL also increased only the LXRα mRNA expression. Whitney et al showed that both natural and synthetic LXR agonists upregulated LXRα, but not LXRβ gene expression in human macrophages, adipocytes, and hepatocytes.28 Moreover, Arai et al found that oxLDL activates LXR in macrophages, and hence, our results suggest that oxLDL acts as an LXR agonist also in trophoblasts.
However, the reason why ABCA1 expression was decreased in preeclamptic placentas in spite of high maternal serum levels of oxLDL remained unexplained. To address this query, we focused on LOX-1, a predominant oxLDL scavenger receptor. In our previous study, we revealed the expressions of scavenger receptors for oxLDL, including LOX-1, were decreased in preeclamptic placentas. In the present study, LOX-1 blockade resulted in the attenuation of increasing ABCA1 mRNA induced by oxLDL. These results robustly suggest that oxLDL-mediated ABCA1 regulation partially depends on LOX-1 expression in the placenta. In other words, insufficient oxLDL uptake due to decreased LOX-1 expression may lead to low ABCA1 expression in preeclamptic placentas (Figure 5). However, we are currently ignorant of precise mechanism by which LOX-1 was decreased in preeclamptic placentas, and thus, further investigation is required.
Figure 5.
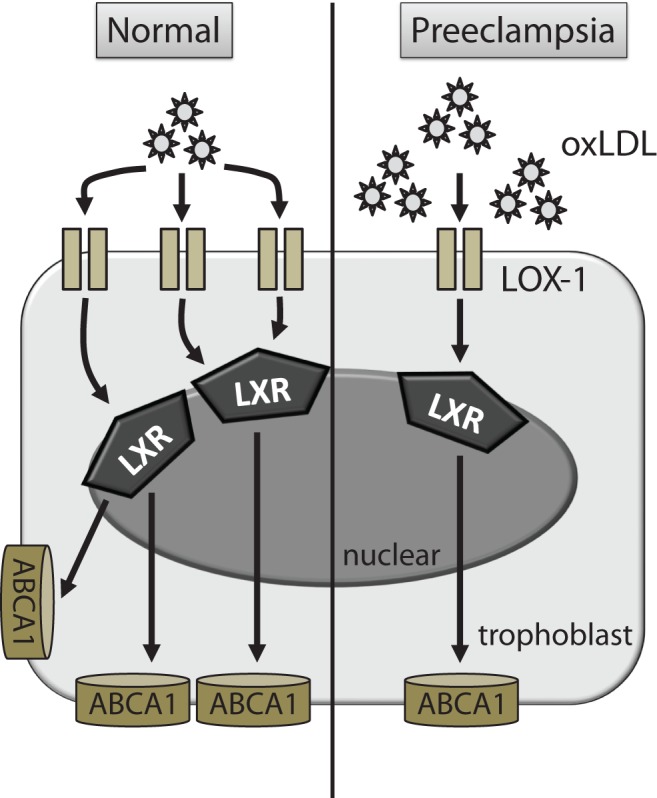
Scheme of oxLDL-mediated signaling pathways in trophoblasts. The enhancement in ABCA1 expression following increased oxLDL is suppressed in preeclamptic placentas. ABCA1 indicates adenosine triphosphate-binding cassette transporter A1; oxLDL, oxidized low-density lipoprotein.
Conclusion
In conclusion, to the best of our knowledge, this is the first study to reveal decreased ABCA1 expression in both mRNA and protein levels as well as mRNA expression of LXRs in preeclamptic placentas. Moreover, we demonstrated that oxLDL upregulated ABCA1 expression, while LOX-1 blockade resulted in the alleviation of increasing ABCA1 mRNA in JAR cells. These results strongly suggest that low LOX-1 expression may lead to insufficient oxLDL uptake, thereby contributing to reduced LXR activation and decreases in ABCA1 expression in preeclamptic placentas. Our findings provided new insight into the pathophysiology of preeclampsia particularly in the context of lipid metabolism.
Acknowledgments
We are greatly indebted to Dr Tatsuya Sawamura for his kind gift of anti-LOX-1 antibody (TS92) and informative guidance. We also thank Ms Akiko Abe for her secretarial and technical assistance.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: in part by Grants-in-Aid for Scientific Research from the Ministry of Education, Science, Culture and Sports, Japan (No. 21592096, 22591822, and 23791833) and by a grant from the Smoking Research Foundation.
References
- 1. Sibai B, Dekker G, Kupferminc M. Pre-eclampsia. Lancet. 2005;365(9461):785–799. [DOI] [PubMed] [Google Scholar]
- 2. Steegers EA, von Dadelszen P, Duvekot JJ, Pijnenborg R. Pre-eclampsia. Lancet. 2010;376(9741):631–644. [DOI] [PubMed] [Google Scholar]
- 3. Potter JM, Nestel PJ. The hyperlipidemia of pregnancy in normal and complicated pregnancies. Am J Obstet Gynecol. 1979;133(2):165–170. [DOI] [PubMed] [Google Scholar]
- 4. Sattar N, Bendomir A, Berry C, Shepherd J, Greer IA, Packard CJ. Lipoprotein subfraction concentrations in preeclampsia: pathogenic parallels to atherosclerosis. Obstet Gynecol. 1997;89(3):403–408. [DOI] [PubMed] [Google Scholar]
- 5. Uzun H, Benian A, Madazli R, Topcuoglu MA, Aydin S, Albayrak M. Circulating oxidized low-density lipoprotein and paraoxonase activity in preeclampsia. Gynecol Obstet Investig. 2005;60(4):195–200. [DOI] [PubMed] [Google Scholar]
- 6. Branch DW, Mitchell MD, Miller E, Palinski W, Witztum JL. Pre-eclampsia and serum antibodies to oxidised low-density lipoprotein. Lancet. 1994;343(8898):645–646. [DOI] [PubMed] [Google Scholar]
- 7. Nishimura M, Naito S. Tissue-specific mRNA expression profiles of human ATP-binding cassette and solute carrier transporter superfamilies. Drug Metab Pharmacokinet. 2005;20(6):452–477. [DOI] [PubMed] [Google Scholar]
- 8. Langmann T, Mauerer R, Zahn A, et al. Real-time reverse transcription-PCR expression profiling of the complete human ATP-binding cassette transporter superfamily in various tissues. Clin Chem. 2003;49(2):230–238. [DOI] [PubMed] [Google Scholar]
- 9. Christiansen-Weber TA, Voland JR, Wu Y, et al. Functional loss of ABCA1 in mice causes severe placental malformation, aberrant lipid distribution, and kidney glomerulonephritis as well as high-density lipoprotein cholesterol deficiency. Am J Pathol. 2000;157(3):1017–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Janowski BA, Willy PJ, Devi TR, Falck JR, Mangelsdorf DJ. An oxysterol signalling pathway mediated by the nuclear receptor LXR alpha. Nature. 1996;383(6602):728–731. [DOI] [PubMed] [Google Scholar]
- 11. Chigusa Y, Tatsumi K, Kondoh E, et al. Decreased lectin-like oxidized LDL receptor 1 (LOX-1) and Low Nrf2 activation in placenta are involved in preeclampsia. J Clin Endocrinol Metab. 2012;97(10): E1862–1870. [DOI] [PubMed] [Google Scholar]
- 12. Bhattacharjee J, Ietta F, Giacomello E, et al. Expression and localization of ATP binding cassette transporter A1 (ABCA1) in first trimester and term human placenta. Placenta. 2010;31(5):423–430. [DOI] [PubMed] [Google Scholar]
- 13. Aye IL, Waddell BJ, Mark PJ, Keelan JA. Placental ABCA1 and ABCG1 transporters efflux cholesterol and protect trophoblasts from oxysterol induced toxicity. Biochim Biophys Acta. 2010;1801(9):1013–1024. [DOI] [PubMed] [Google Scholar]
- 14. Stefulj J, Panzenboeck U, Becker T, et al. Human endothelial cells of the placental barrier efficiently deliver cholesterol to the fetal circulation via ABCA1 and ABCG1. Circulation Res. 2009;104(5):600–608. [DOI] [PubMed] [Google Scholar]
- 15. Plosch T, Gellhaus A, van Straten EM, et al. The liver X receptor (LXR) and its target gene ABCA1 are regulated upon low oxygen in human trophoblast cells: a reason for alterations in preeclampsia? Placenta. 2010;31(10):910–918. [DOI] [PubMed] [Google Scholar]
- 16. Albrecht C, Soumian S, Tetlow N, et al. Placental ABCA1 expression is reduced in primary antiphospholipid syndrome compared to pre-eclampsia and controls. Placenta. 2007;28(7):701–708. [DOI] [PubMed] [Google Scholar]
- 17. Poston L. Leptin and preeclampsia. Semin Reprod Med. 2002;20(2):131–138. [DOI] [PubMed] [Google Scholar]
- 18. Maynard SE, Min JY, Merchan J, et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Investig. 2003;111(5):649–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lindegaard ML, Wassif CA, Vaisman B, et al. Characterization of placental cholesterol transport: ABCA1 is a potential target for in utero therapy of Smith-Lemli-Opitz syndrome. Hum Mol Genet. 2008;17(23):3806–3813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bodzioch M, Orso E, Klucken J, et al. The gene encoding ATP-binding cassette transporter 1 is mutated in Tangier disease. Nat Genet. 1999;22(4):347–351. [DOI] [PubMed] [Google Scholar]
- 21. Weedon-Fekjaer MS, Johnsen GM, Anthonisen EH, et al. Expression of liver X receptors in pregnancies complicated by preeclampsia. Placenta. 2010;31(9):818–824. [DOI] [PubMed] [Google Scholar]
- 22. Joseph SB, Castrillo A, Laffitte BA, Mangelsdorf DJ, Tontonoz P. Reciprocal regulation of inflammation and lipid metabolism by liver X receptors. Nat Med. 2003;9(2):213–219. [DOI] [PubMed] [Google Scholar]
- 23. Terasaka N, Hiroshima A, Koieyama T, et al. T-0901317, a synthetic liver X receptor ligand, inhibits development of atherosclerosis in LDL receptor-deficient mice. FEBS Lett. 2003;536(1-3):6–11. [DOI] [PubMed] [Google Scholar]
- 24. Levin N, Bischoff ED, Daige CL, et al. Macrophage liver X receptor is required for antiatherogenic activity of LXR agonists. Arterioscler Thromb Vasc Biol. 2005;25(1):135–142. [DOI] [PubMed] [Google Scholar]
- 25. Peng D, Hiipakka RA, Reardon CA, Getz GS, Liao S. Differential anti-atherosclerotic effects in the innominate artery and aortic sinus by the liver X receptor agonist T0901317. Atherosclerosis. 2009;203(1):59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Schuster GU, Parini P, Wang L, et al. Accumulation of foam cells in liver X receptor-deficient mice. Circulation. 2002;106(9):1147–1153. [DOI] [PubMed] [Google Scholar]
- 27. Robertson WB, Khong TY, Brosens I, De Wolf F, Sheppard BL, Bonnar J. The placental bed biopsy: review from three European centers. Am J Obstet Gynecol. 1986;155(2):401–412. [DOI] [PubMed] [Google Scholar]
- 28. Whitney KD, Watson MA, Goodwin B, et al. Liver X receptor (LXR) regulation of the LXRalpha gene in human macrophages. J Biol Chem. 2001;276(47):43509–43515. [DOI] [PubMed] [Google Scholar]