Abstract
This study was designed to show whether placental relaxin (RLN), its receptor (RXFP1), or insulin-like peptide 4 (INSL4) might have altered expression in patients with placenta accreta. The baseline expression of their genes through gestation (n = 34) was quantitated in the placental basal plate (BP) and villous trophoblast (TR), and compared to their expression in placenta accreta (n = 6). The proteins were also immunolocalized and quantitated in the accreta tissues. The messenger RNAs (mRNAs) of matrix metalloproteinase 9, -2, and tissue inhibitors of matrix metalloproteinase (TIMP)-1 were also measured. Results demonstrated that the BP and TR expressed low levels of RLN/RXFP1 and INSL4 through gestation. In accreta, increased RLN gene and protein in BP were associated with antepartum bleeding whereas INSL4 expression decreased throughout the TR. There were no changes in mRNAs for MMPs, but TIMP-1 was increased only in the invasive TR.
Keywords: placenta accreta, relaxin, RXFP1, INSL4, basal plate, trophoblast
Introduction
Normal pregnancy is dependent upon normal placental development. During the first trimester, extravillous trophoblast (TR) cells invade into the inner one-third of the decidualized uterine endometrium.1 Inadequate invasion of placental intermediate TR into maternal spiral arteries results in the development of intrauterine growth restriction and/or preeclampsia.2 Placenta accreta is often used as a general term and is defined as the abnormal adherence of chorionic villi to the uterine myometrium3 resulting in catastrophic postpartum hemorrhage. The incidence of placenta accreta has increased and is estimated to be between 1/533 and 1/1000 pregnancies.4 The main risk factor for its development is a history of a prior cesarean delivery and the continued rise in cesarean section rates worldwide ensures that accreta will remain a serious clinical problem. However, the pathogenesis of placenta accreta is unknown, but the proposed hypotheses include primary deficiency of the decidua, abnormal maternal vascular remodeling, excessive TR invasion, or a combination thereof.5 A recent review suggests that it is a 20th century iatrogenic disease secondary to uterine damage. This damage may be caused by almost any type of iatrogenic endometrial disturbance and is likely to be present prior to the pregnancy.6
Abnormal trophoblastic implantation and invasion is a regular finding in abdominal and ectopic pregnancies, where there is no normal endometrium to transform into decidua. There is good evidence that placenta accreta arises from the failure of formation of a normal decidua.7 However, the placenta is formed by interactions between maternal and fetal cells, each directed by different genomes.8 Early on, fetal TRs invade the veins and stroma of maternal tissues, allowing the placenta to grow into the uterine cavity. The attachment site of the placenta onto the uterus is at the decidua basalis or basal plate (BP) with a layer of extracellular matrix, termed Nitabuch fibrinoid lying between the placenta and the uterus. Without a normal BP and Nitabuch fibrinoid, the villous TR has direct access to the maternal myometrium. However, accreta may be total, partial, or focal. In the latter, there can be an area of abnormal adherence with no decidua next to an area of normal decidua and BP.9 This suggests a focal TR/decidual antagonism existing at the molecular level, with the decidua having an impact on trophoblastic differentiation and invasion. Abnormal vascular remodeling and neovascularization is certainly a key feature in accreta development. Vascular endothelial growth factor (VEGF), placental growth factor, and their receptors are well-recognized angiogenic factors in normal pregnancy. The expression of VEGF and its receptors in TRs also regulate the secretion of many proteins and hormones by autocrine/paracrine mechanisms. Indeed, significantly higher expression of VEGF and lower expression of soluble VEGF (sVEGF)-2 receptors are present in placental lysates of patients with accreta compared to those of normal controls, suggesting that increased VEGF and decreased sVEGF-2 induce neovascularization during the development of accreta.10
The insulin-like growth factors are a family of structurally similar peptides essential for fetal growth and development.11 There are 3 genes for human relaxin (RLN; H1, H2, and H3) located on chromosome 9 (H1 and H2)12 and 19 (H3).13 Both RLN1 and RLN2 are expressed in the human decidua and placenta, while RLN2 is the circulating form of the hormone produced primarily by the corpus luteum and is the major form produced by the decidua. Expression of RLN3 appears to be confined to the nervous system. Human RLN1 and 2 bind to the decidua and chorionic cytotrophoblast of the fetal membranes, showing these cells are the principal sites of RLN action in the uterus.14 Increased expression of RLN is associated with the preterm premature rupture of the fetal membranes,15 , 16 by increasing local production of matrix metalloproteinases (MMPs) and cytokines.17 Relaxin also increases the expression of VEGF from normal human endometrial cells, suggesting a role in neovascularization and angiogenesis and has been associated with menometrorrhagia in women.18 The RLN receptor is a leucine-rich repeat G-protein-coupled receptor also known as the relaxin family protein receptor 1 (RXFP1) and binds RLN 1 and 2 with high affinity.14 The RXFP1 is well expressed in the fetal membranes especially at preterm, with relatively low levels expressed in the placenta.19 Insulin-like peptide 4 (INSL4) was identified and its gene localized to chromosome 9, close to those of RLN1 and RLN2. 20 It was shown to be expressed in the placenta and a role suggested in the regulation of early placental and fetal development.21 Its protein (also called early placental insulin-like factor, EPIL) has been associated with the highly invasive properties of c-erbB-2-positive breast cancer cells and higher levels of expression caused more aggressive invasion of basement membranes and stromal tissue.22
The process whereby trophoblastic cells implant and invade into the uterine decidua involves the degradation and remodeling of the extracellular matrix by the MMPs.23 These enzymes are expressed by the amnion, chorionic cytotrophoblast, decidua, placental BP, umbilical cord, and villous TR.24 Specifically, the gelatinases MMP-2 and MMP-9 are well expressed in the placenta and are involved in TR invasion. The activities of these enzymes are regulated by the tissue inhibitors of matrix metalloproteinases (TIMPs) that are coexpressed with the MMPs, suggesting that the invasive properties of the cytotrophoblast depend upon the balance of the MMPs and TIMPs.25
Here, we hypothesized that altered expression of RLN/RXFP1 and INSL4 by the maternal and/or fetal cells of the BP and TR results in the abnormal adherence of the TR, resulting in its excessive invasion into the myometrium. Therefore, the first aim of this study was to quantitate their baseline gene expression in the villous TR and BP during gestation, as these data were unavailable. The second aim was to extend this to patients with placenta accreta, in order to compare expression of their genes and proteins in the BP (when focally present) and in both the invading and noninvading areas of TR. In addition, a potential mechanism of action of these peptides was sought by measurement of MMP-9, MMP-2, and TIMP-1 gene expression in the same samples.
Materials and Methods
Patient Selection and Tissue Collection
Placentas were collected anonymously from Kapiolani Medical Center for Women and Children (Honolulu, Hawaii) from 2010 to 2011. The patients were divided as follows: first trimester pregnancy terminations 10 to 13 weeks of gestation (n = 7); second trimester terminations 14 to 22 weeks of gestation (n = 8); 30 to 35-week preterm deliveries without preterm premature rupture of membranes (PPROM; n = 5); 30- to 35-week preterm vaginal deliveries complicated by PPROM (n = 3); term elective (>37 weeks of gestation); cesarean sections without labor (n = 6); and term normal spontaneous vaginal deliveries (n = 5). Gestational ages were determined by the patient’s last menstrual period and/or ultrasound and none had any clinical chorioamnionitis. None of the terminations were for a medical condition and all were young healthy women. There was no drug use by any patient. This information was recorded on a data sheet without any identifiable patient health information by the patient’s attending physician and the data sheet was subsequently destroyed. All tissues were examined histologically by a pathologist to eliminate any tissue with a pathology not identified clinically. Tissues from 6 patients with placenta accreta were obtained (30-36 weeks of gestation at time of delivery/hysterectomy) and all of these patients had placenta previa. These patients were matched as closely as possible to similar gestational-age controls from the gestation study. Some clinical details of both sets of the patients are shown in Table 1 and were obtained from the attending physician. For the study of gene expression through gestation, BP and TR biopsies (30 mg) were obtained from intact placental tissues (terminations) or from the center of each placental disk near cord insertion. For the accreta samples, the uterus with the placenta in situ was obtained immediately following cesarean hysterectomy and was bivalved. The BP and TR from areas of normal placentation were each biopsied (30 mg). Additionally TR over sites of invasive accreta (without BP) were biopsied. Tissues were placed in RNAlater and stored at −20°C until used. Full thickness sections over areas of both normal placentation and accreta were also placed into 10% buffered formalin. Archived tissue blocks from cesarean hysterectomy specimens for placenta accreta (n = 5) were also obtained from the Department of Pathology at Kapiolani Medical Center (Dr Karen Thompson) over regions of accreta and normal placentation and used for comparative immunohistological studies. These were not included in the immunohistochemical quantitation. The study protocol was reviewed and deemed to be exempt from regulations by the Hawaii Pacific Health Research Institute which is the institutional review board for Kapiolani Medical Center for Women and Children (Honolulu, Hawaii), the primary teaching hospital for the University of Hawaii Department of Obstetrics/Gynecology and Women’s Health.
Table 1.
Gestational Age and Delivery Indications for Patients With Placenta Accreta (n = 6) and Gestational Age-Matched Controls (n = 6).
| Category | Gestational Age (weeks) | Delivery Indication |
|---|---|---|
| Pair 1 | ||
| Accreta 1 | 34 | Scheduled CH |
| Control | 34 | PTL, VD |
| Pair 2 | ||
| Accreta 2 | 34 | Scheduled CH |
| Control | 35 | PTL, VD |
| Pair 3 | ||
| Accreta 3 | 34 | Scheduled CH |
| Control | 32 | PTL, VD |
| Pair 4 | ||
| Accreta 4 | 33 | Emergent CH, bleeding |
| Control | 32 | PTL, VD |
| Pair 5 | ||
| Accreta 5 | 30 | Emergent CH, bleeding |
| Control | 32 | Induction, cHTN, DM |
| Pair 6 | ||
| Accreta 6 | 36 | LD, unplanned CH, PPH |
| Control | 37 | LD, CS |
Abbreviations: CH, cesarean hysterectomy; VD, vaginal delivery; CS, cesarean section; PTL, preterm labor; LD, labor dystocia; cHTN, chronic hypertension; DM, diabetes mellitus; PPH, postpartum hemorrhage.
Isolation of RNA and Quantitative Real-Time PCR
Tissues stored in RNAlater were thawed, and 2 mg from the BP and TR were flash frozen in liquid nitrogen and pulverized with a BioPulverizer (BioSpec Products Inc, Bartletville, Oklahoma). The resulting powders were used for total RNA extraction with the RNeasy Fibrous MiniKit (QIAGEN Inc, Valencia, California). RNA quality was assessed using the Agilent Bioanalyzer 2100 (Agilent Technologies, Santa Clara, California). Template complementary DNA was prepared by reverse transcription in a total volume of 20 µL containing 1× PCR buffer II, 5.5 mmol/L magnesium chloride, 500 µmol/L each deoxynucleotide triphosphatases, 2.5 µmol/L random hexamers, 0.4 U/µL RNase inhibitor, 2.5 U/µL Multiscribe reverse transcriptase (Applied Biosystems Inc, Foster City, California), and 400 ng total RNA. This was incubated for 10 minutes at 25°C, 120 minutes at 37°C, and 5 minutes at 95°C.
For quantitative polymerase chain reaction (PCR), primers for RLN, RXFP1, INSL4, MMP-2, MMP-9, and TIMP-1 and the housekeeping gene 18S were purchased from Applied Biosystems (TaqMan Assays on Demand). These are proprietary and designed to span the exon junctions. They are of the highest specificity and sensitivity possible, as determined by the manufacturer and remain the “gold standard” of quality of any gene expression technology to date. In order to minimize carryover PCR contamination, universal master mix II (2×) with AmpErase uracil N-glycosylase (Applied Biosystems) was used. The ABI protocol for a PCR was used and the cycles of incubation were 1 cycle at 50°C for 2 minutes, 1 cycle at 95°C for 10 minutes, followed by 40 cycles at 95°C for 15 seconds, and 60°C for 1 minute using the StepOne Real-Time PCR System (Applied Biosystems). Each reaction was performed in triplicate, and the data were analyzed as described in the ABI User’s Bulletin no. 2 (http://www3.appliedbiosystems.com/cms/groups/mcb_support/documents/generaldocuments/cms_040980.pdf). Results were normalized to the expression of 18S in each sample and shown as mean ± standard deviation (SD) of relative gene expression. It should be noted that the levels of 18S differed between the BP and the TR, but none of the genes of interest here were compared between these tissues. Since 18S levels could potentially change during gestation, we plotted the raw data for the genes of interest and confirmed that normalization did not alter the data as shown.
Immunohistochemistry and Quantitation
Tissue samples were removed from formalin, paraffin embedded, and the sections were cut (5 μm) and placed onto the slides. These sections were then deparaffinized, hydrated, and rinsed in distilled water. Antigen retrieval was performed by heating the slides in sodium citrate buffer (10 mmol/L, pH 6.0) for 30 minutes. The slides were then treated for 20 minutes with 0.3% hydrogen peroxide to reduce endogenous peroxidase activity. Nonspecific binding was blocked with 2.5% normal horse serum for 20 minutes and the sections incubated with either rabbit polyclonal antibodies (immunoglobulin G [IgG]) to human RLN (8 µg/mL; Calbiochem/EMD Biosciences, San Diego, California) or RXFP1 (10 µg/mL; MBL International Corporation, Woburn, Massachusetts). The negative controls were nonimmune IgG at the relevant concentrations (DAKO North American Inc, Carpenteria, California). A monoclonal mouse anti-human INSL4 IgG (44 µg/mL; EPIL 41 directed to the 88-108 C-region of Pro-EPIL, a generous gift from Dr D. Bellet, Universite Paris Descartes, Laboratoire d’Immunologie, Paris, France) was used for 1 hour or a respective control nonimmune IgG at the same concentration as primary antibody (DAKO North American Inc). Cellular localization was determined using the ImmPRESS system (Vector Labs, Burlingame, California). Because RLN immunolocalized to both decidual cells and intermediate TR in the BP, anti-HLA-G was used as a marker for intermediate TR on consecutive tissue sections.26 This allowed identification of both cell types, intermediate TR by positive staining and decidua by exclusion of staining. The primary antibody was a monoclonal mouse anti-human HLA-G (10 µg/mL, Abcam, Cambridge, Massachusetts). After washing, the slides were incubated with ImmPRESS reagent for 30 minutes and 3,3-diaminobenzidine (DAB) substrate solution for 5 minutes for color development. Slides were rinsed, counterstained with Gill hematoxylin, cleared, and mounted in ProTexx mounting media (Allegiance Healthcare, Honolulu, Hawaii). The primary antibodies used for RLN and RXFP1 were commercially available and immunolocalized to these proteins in the tissues in an identical way to the previously published work.15 , 16 , 19 The specificity of the monoclonal antibody to INSL4 was evaluated and confirmed by Dr Bellet.
Since not all tissue sections from patients with accreta and controls were of sufficient quality for immunoquantitation, the numbers used were smaller than for the analyses of mRNA. A series of bright field images were acquired between 420 and 700 nm at 20 nm intervals using a multispectral imaging system comprising an Olympus BX51 microscope and a CRI Nuance spectral analyzer (Caliper Life Sciences, Hopkinton, Massachussetts). The images were stacked to create a 3-dimensional image cube. Individual spectral libraries for DAB and hematoxylin were obtained and used to “unmix” the stained specimen into its individual components. Quantitative image analysis of the unmixed data was performed with Nuance software (version 2.10.0). The average signal intensity per pixel from 5 different fields of the tissue section from each patient was collected and the results were expressed as the mean ± SD. This technology circumvents the need for the classical approach to the determination of precision and is unbiased by the observer. For quantitation of immunostaining in the BP, this was carried out over areas of intermediate TR cells, although it is possible that some decidual cells were also included.
Statistical Analysis
Data were analyzed for statistical significance using GraphPad InStat software (GraphPad Software Inc, San Diego, California). Nonparametric analyses were used throughout, which are designed for small sample sizes. A significant result would not be obtained if 1 data point had shifted the mean. Thus, the Kruskal-Wallis nonparametric analysis of variance was used to compare the differences in gene expression through gestation. The Mann-Whitney nonparametric test was used for comparisons of 2 unpaired groups, while multiple comparisons using pairwise comparisons were determined using the Bonferroni-Dunn test. The results were expressed as the mean ± SD and statistical significance was set at P < .05.
Results
The RLN, RXFP1, and INSL4 Gene Expression in BP and TR During Gestation
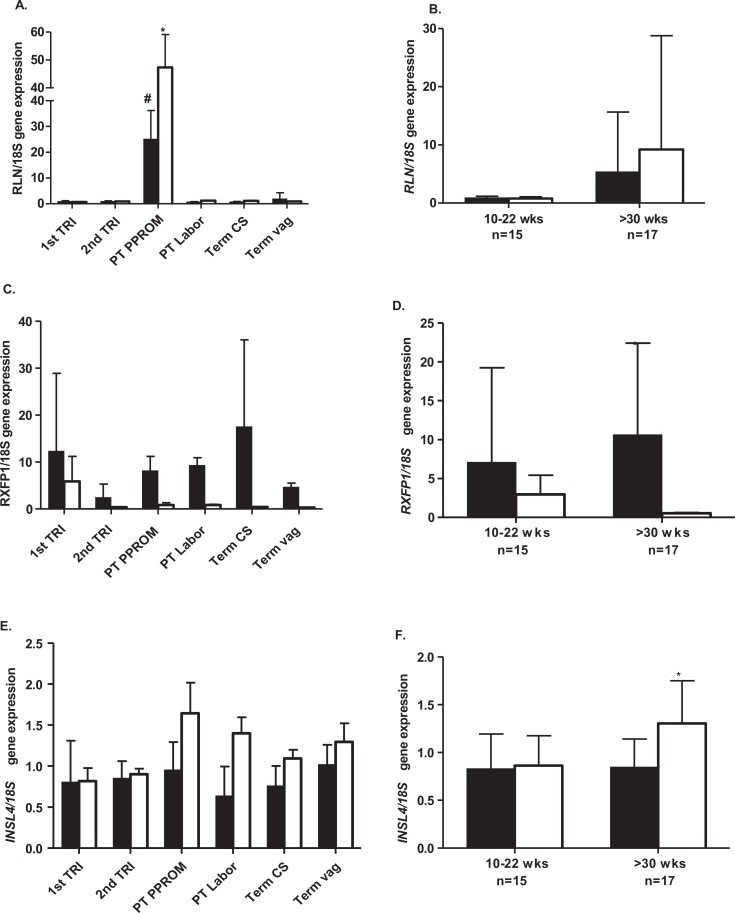
The RLN in both BP and TR was low throughout the gestation (Figure 1A). However, its expression was significantly increased (P < .001) in both BP and TR in pregnancies complicated by PPROM compared to BP and TR from either, the first or second trimester, preterm labor and delivery without PPROM or term delivery, agreeing with previous work from our laboratory.15 , 16 There were no significant changes in RLN expression in either BP or TR over the periods between 10 and 22 weeks or >30 weeks of gestation (Figure 1B). The RXFP1 expression in both BP and TR was highly variable in the first trimester, thereafter declining to low levels in the second trimester. Its expression in the BP but not the TR rose slowly from this time to higher levels at term cesarean delivery before labor, but again showing high variability (Figure 1C). However, there was no increase in RXFP1 in either tissue in patients with PPROM. At term, in patients with vaginal deliveries, RXFP1 expression was low, agreeing with previously published data showing a decline in RXFP1 after labor and delivery.19 There was no significant difference in RXFP1 gene expression in the BP compared to the TR in the first half of gestation, whereas from 30 weeks onward, RXFP1 expression in BP was significantly greater (P < .001) than that of the TR (Figure 1D). There were no significant differences in INSL4 gene expression in the BP or TR as a function of gestational age (Figure 1E). However, its expression was significantly greater (P < .01) in TR after 30 weeks of gestation compared to its expression in the BP over the same period (Figure 1F).
Figure 1.
The RLN, RXFP1, and INSL4 gene expression in the basal plate (BP) and trophoblast (TR) during gestation. The RLN, RXFP1, and INSL4 gene expression (A, C, and E, respectively) in first trimester terminations (10-13 weeks, n = 7), second trimester terminations (14-22 weeks, n = 8), preterm PPROM (30-35 weeks, n = 3), preterm labor and delivery, no PPROM (n = 5), term cesarean section before labor (>37 weeks, n = 6), and term spontaneous vaginal delivery (n = 5). Basal plate black bars and villous TR open bars. The RLN, RXFP1, and INSL4 (B, D, and F, respectively) shown with data combined for early-mid (10-22 weeks) and late (>30 weeks) gestation for BP and TR. A, The RLN expression in both BP and TR in PPROM was significantly greater (#,*P < .001) compared to either first or second trimesters, preterm labor or term vaginal deliveries. B, No significant differences in RLN expression >30 weeks of gestation in either tissue. C, The RXFP1 expression was generally lower in TR than BP throughout gestation. D, Expression was significantly higher >30 weeks (*P < .001) in the BP compared to the TR over this period. E, There were no significant differences in INSL4 expression during gestation, although TR levels were generally higher than BP, especially with preterm labor. F, The INSL4 expression in TR was significantly more >30 weeks of gestation (P < .01) compared to the BP. INSL4 indicates insulin-like peptide 4; PPROM, preterm premature rupture of membranes; RLN, relaxin; RXFP1, relaxin receptor.
The RLN and RXFP1 Gene and Protein Expression in BP and TR of Patients With Placenta Accreta
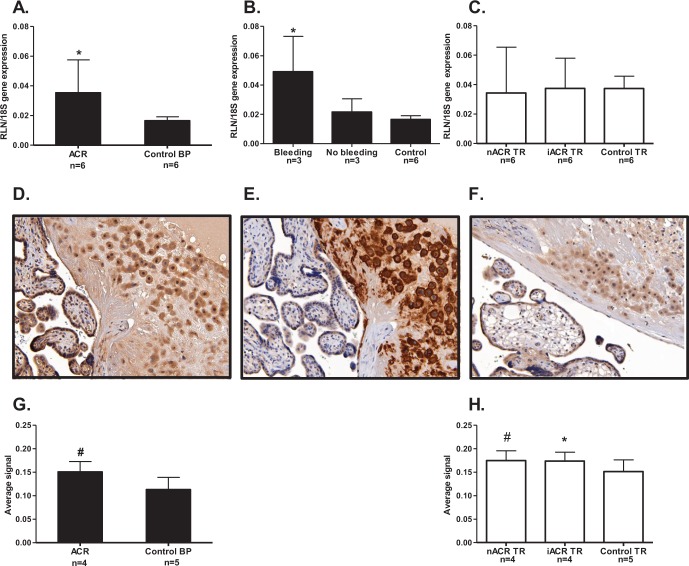
The RLN gene expression was significantly increased (P < .05) in the BP of patients with placenta accreta compared to the BP of control patients at the same gestational ages (Figure 2A). However, when RLN in the BP was compared in cases where emergent hysterectomies were performed for antenatal bleeding (n = 3) in patients with accreta who had planned cesarean hysterectomies (n = 3) or with gestational-matched controls (n = 6), RLN was significantly increased (P < .05) only in the patients with antenatal hemorrhage compared to the other 2 groups (Figure 2B). In contrast, there was no difference in RLN expression in the invasive or noninvasive areas of TR in placenta accreta compared to the TR of controls (Figure 2C). An example of the BP of a patient with accreta at 34 weeks of gestation, immunostained with antibody to RLN is shown in Figure 2D. The negative control is not shown here because the cellular immunolocalization of RLN was identical to that previously published.15,16Thus, RLN was primarily localized to the cells of the intermediate TR, shown in a consecutive section immunostained with the intermediate TR marker HLA-G (Figure 2E). However, RLN also stained the decidual stromal cells in the BP as well as the villous syncytiotrophoblast (Figure 2D). In a control patient delivering at preterm at the same gestational age as the patient with accreta, the RLN staining in the BP was present but less intense (Figure 2F). Photomicrographs of early and mid-gestational tissues are not shown here for simplification, but the cells to which RLN was localized were identical to the images shown from later gestations. When the RLN protein was quantitated in BP and TR from 4 of the same patients with accreta as shown for the mRNA (Figure 1A and C), there was significantly more RLN in the BP (P < .001) compared to the controls (Figure 2G). In addition, the protein in both the noninvasive and invasive areas of TR compared to the controls was significantly increased (P < .01 and P < .05, respectively, Figure 2H). Thus, there was more RLN protein expressed both in the BP and in the villous TR of patients with accreta compared to the controls.
Figure 2.
The RLN gene and protein expression in BP and TR of patients with placenta accreta. The BP is shown as black bars and villous TR open bars. A, RLN gene expression was significantly greater (*P < .05) in the BP (where present) in patients with accreta (ACR, n = 6) compared to normal BP from gestational age-matched controls (n = 6). B, RLN expression in patients with accreta was subdivided according to cases of emergent hysterectomies for antenatal bleeding (n = 3) and those with planned cesarean hysterectomy and no bleeding (n = 3) and age-matched controls (n = 6), RLN was significantly increased (*P < .05) in those with antenatal bleeding compared to the other 2 groups. C, There were no differences in either the areas of noninvasive intermediate TR (nACR, n = 6) or the invasive intermediate TR (iACR, n = 6) compared to TR from gestational age-matched controls (n = 6). D, Example of RLN immunolocalization in the BP and TR of patients with accreta at 34 weeks gestation, showing good staining in the intermediate TR cells and less-intense staining in decidual cells of the BP and syncytiotrophoblast. E, A consecutive section immunostained with an antibody to the intermediate TR marker HLA-G, showing the majority of cells immunostained with anti-RLN in (D) as intermediate TR. F, Example of a section from one of the control patients, showing generally lighter RLN staining. G, Quantitation of immunostaining in the BP showed significantly more (# P < .001) RLN protein in the patients with accreta (n = 4), compared to the age-matched controls (n = 5). H, Quantitation of immunostaining in the TR showed significantly more RLN protein in both noninvasive TR (nACR, n = 4) and invasive TR (iACR, n = 4) of patients with accreta (# P < .01, *P < .05, respectively) compared to the age-matched controls (n = 5). BP indicates basal plate; RLN, relaxin; TR, trophoblast.
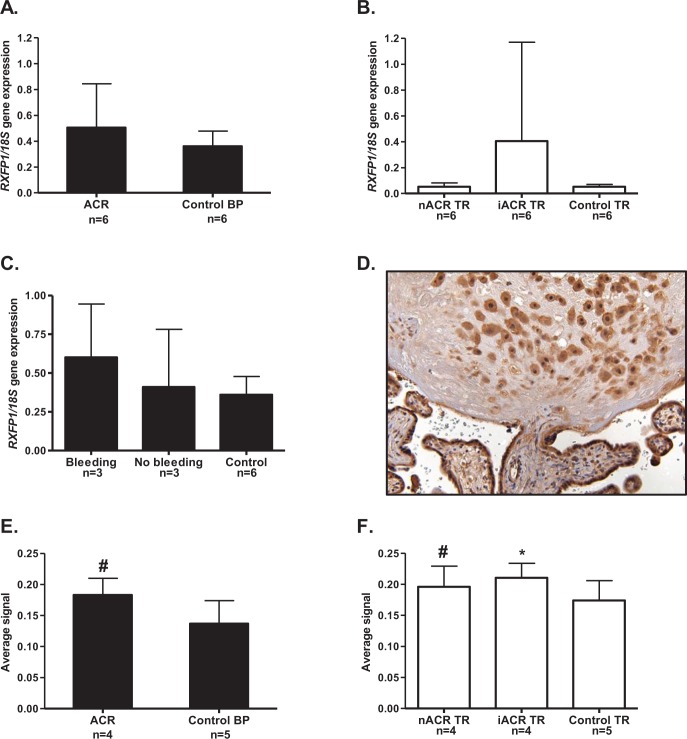
The RXFP1 gene expression did not differ significantly in the BP of patients with accreta compared to the BP of controls (Figure 3A). In the TR of these patients, there were also no differences in either the noninvasive or invasive areas of TR compared to the TR of controls (Figure 3B). However, there was large variability in RXFP1 expressed in the invasive TR, suggesting that greater numbers of samples may have shown significance in this group. When the BP data were subdivided according to cases of emergent hysterectomies for bleeding (n = 3) or planned cesarean hysterectomy without bleeding (n = 3), there were no significant differences (Figure 3C). An example of a section at the junction of the BP and TR from 1 of the patients with accreta is shown immunostained with antibody to RXFP1 in Figure 3D. The negative control is not shown because the cellular localization of RXFP1 was identical to that previously published.19 There was strong staining in the intermediate TR in the BP, identified with HLA-G staining on a consecutive section (not shown) as well as light staining in the decidual cells in the BP. However, there was stronger staining in the villous syncytiotrophoblast. In the early and mid-gestation samples, the immunolocalization of RXFP1 was identical and therefore not shown. When the RXFP1 protein was quantitated in the BP, significantly more was expressed (P < .001) in patients with accreta compared to the controls (Figure 3E). Similarly, quantitation of the immunostaining in both the noninvasive and invasive areas of TR showed significantly greater expression compared to the controls (P < .001 and P < .05, respectively; Figure 3F). These data show a generalized increase in RXFP1 protein expression in the BP and throughout the villous TR in patients with accreta.
Figure 3.
The RXFP1 gene and protein expression in BP and TR of patients with placenta accreta. The BP is shown as black bars and villous TR as open bars. A, RXFP1 gene expression in BP of patients with accreta (ACR, n = 6) was no different from that of age-matched control patients (n = 6). B, There were no significant differences in RXFP1 in either areas of noninvasive intermediate TR (nACR) or areas of invasive intermediate TR (iACR) compared to age-matched controls (n = 6). C, In BP, there were no differences in RXFP1 according to whether the patient had an emergent hysterectomy for antenatal bleeding (n = 3) or a planned cesarean hysterectomy for antenatal bleeding (n = 3) compared to the controls (n = 6). D, Example of RXFP1 immunolocalized at the junction of BP and TR in one of the patients with accreta at 34 weeks of gestation, showing good staining in the intermediate TR cells in the BP and villous syncytiotrophoblast. E. Quantitation of the RXFP1 protein in the BP showed significantly more (# P < .001) expression in patients with accreta (ACR, n = 4) compared to the controls (n = 5). F, Significantly more RXFP1 protein was expressed in both the noninvasive (nACR, n = 4) and the invasive (iACR, n = 4) areas of intermediate TR in patients with accreta (# P < .05, *P < .001, respectively) compared to the age-matched controls (n = 5). BP indicates basal plate; RXFP1, relaxin receptor; TR, trophoblast.
The MMP-9, MMP-2, and TIMP-1 Gene Expression in BP and TR of Patients With Placenta Accreta
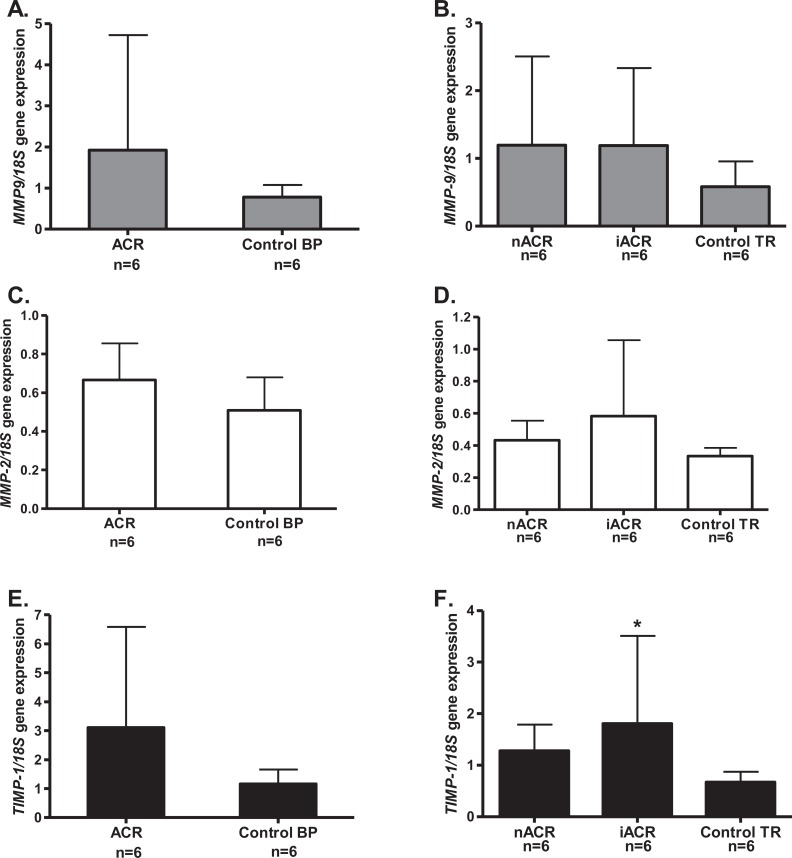
There were no significant differences in the expression of the genes for MMP-9, MMP-2, or TIMP-1 in the BP of patients with accreta, compared to the gestational age-matched controls (Figure 4A, C, and E, respectively). In TR, there was no difference in either the invasive or the noninvasive areas for MMP-9 or MMP-2 expression (Figure 4B and D). However, there was significantly more TIMP-1 expressed (P < .05) in the invasive TR compared to either the noninvasive area of TR of the same patients or the control tissues of the same gestational ages (Figure 4F).
Figure 4.
The MMP-9, MMP-2, and TIMP-1 gene expression in BP and TR of patients with placenta accreta. The MMP-9 grey bars (A and B), MMP-2 open bars (C and D), TIMP-1 black bars (E and F), in BP (A, C and E) and villous TR (B, D, and F). There were no significant differences in either MMP-9 or MMP-2 in the BP of patients with accreta (A and C) or in the noninvasive (nACR, n = 6) or invasive areas of TR (iACR, n = 6) compared to the controls (n = 6; B and D). E, The TIMP-1 expression showed no difference in the BP of patients with accreta (ACR, n = 6) compared to the controls (n = 6). F, TIMP-1 was significantly increased (*P < .05) in the invasive area of TR (iACR, n = 6) compared to either the noninvasive TR (nACR, n = 6) or the age-matched controls (n = 6). BP indicates basal plate; MMP, matrix metalloproteinase; TIMP, tissue inhibitors of matrix metalloproteinase; TR, trophoblast.
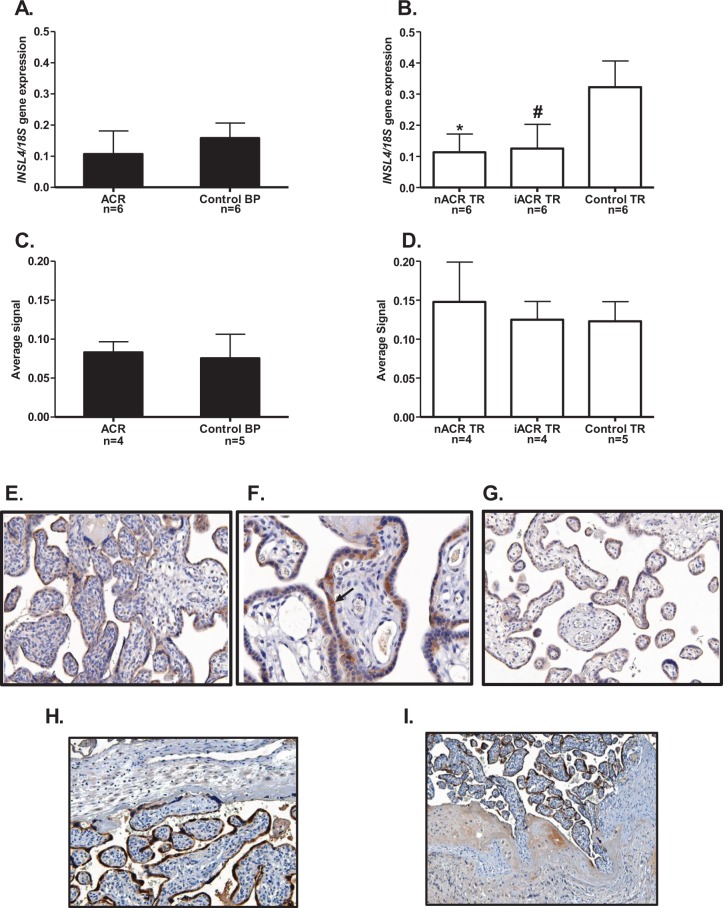
The INSL4 Gene and Protein Expression in BP and TR of Patients With Placenta Accreta
In BP, expression of the INSL4 gene (Figure 5A) and protein (Figure 5C) were not significantly different from the controls. However, INSL4 gene expression in noninvasive and invasive areas of TR of the same patients with accreta was significantly lower (P < .05) compared to TR of the controls (Figure 5B). On the other hand, the INSL4 protein failed to show any significant difference (Figure 5D). An example of the villous TR immunostained with antibody to INSL4 is shown in 1 of the patients with accreta (Figure 5E). The staining was predominantly in the syncytiotrophoblast, although the cytotrophoblast was stained in most placental samples, shown at a higher magnification (see arrow) in Figure 5F. Similar immunolocalization of INSL4 was found in the early and mid-gestational tissues and therefore not shown. A section using control IgG (Figure 5G) had almost no background staining in the villous TR. From one of the patients with accreta at 37 weeks of gestation, 2 sections are shown immunostained with antibody to INSL4, the first (Figure 5H) at the junction of the BP and syncytiotrophoblast shows a focal area where BP was present. Staining in the intermediate TR cells of the BP was light compared to the darker staining in syncytiotrophoblast. In this section, the BP appears to be blocking the invasion of the TR. The second section from the same patient shows an area of accreta lacking any BP and the TR invading into the myometrium (Figure 5I).
Figure 5.
The INSL4 gene and protein expression in BP and TR of patients with placenta accreta. The BP shown as black bars and TR open bars. The INSL4 gene expression in BP (A) and TR (B) and protein in BP (C) and TR (D). Neither INSL4 gene nor protein in BP (A and C, respectively) showed any differences in patients with accreta (ACR, n = 4 or 6) compared to the age-matched controls (n = 5 or 6). In TR, INSL4 (B) was significantly lower in both noninvasive (nACR, n = 6) and invasive TR (iACR, n = 6; *,# P < .05) compared to controls (n = 6). D, The INSL4 protein in TR showed no differences. E, An example of villous TR from a patient with accreta immunostained for INSL4, showing predominant staining in syncytiotrophoblast. F, An example at higher magnification showing staining in the cytotrophoblast as well as syncytiotrophoblast (arrow). G, Control using IgG, showing only light background staining. G, In a patient with accreta at 37 weeks gestation, a section stained for INSL4 showing the junction of the BP and syncytiotrophoblast in a focal area where BP was present. Staining of the intermediate TR cells was light compared to the darker syncytiotrophoblast staining. H, In the same patients with accreta, a focal area of accreta where BP was absent, showing invasion of the intermediate TR into the myometrium. BP indicates basal plate; IgG, immunoglobulin G; INSL, insulin-like peptide; TR, trophoblast.
Discussion
In this study, we have concomitantly measured the expression of the genes for RLN, its receptor (RXFP1), and INSL4 in human BP and TR during pregnancy before studying patients with the important clinical condition of placenta accreta. This approach was necessary because the basic data for comparative purposes was unavailable. Unfortunately, we were unable to include the INSL4 receptor. The INSL4 fails to activate either of the G-protein-coupled receptors RXFP1 or RXFP2 and is likely to have its own, as yet to be identified receptor.27
Immunolocalization of RLN and RXFP1 showed their coexpression in both the maternal and the fetal cells of the placenta (decidual cells, intermediate TR, and syncytiotrophoblast), emphasizing the autocrine/paracrine roles of RLN in these tissues.17 On the other hand, INSL4 was only expressed by fetal cells of the TR, primarily the cytotrophoblast and syncytiotrophoblast, with some expressed by the intermediate TR cells of the BP, agreeing with previous studies.21 This specificity in fetal expression of INSL4 is due to a human endogenous retrovirus element that is used as its primary promoter,28 also explaining why its expression is restricted to the higher primates. Overall, the predominant expression of RLN/RXFP1 and INSL4 in the BP and TR makes them important candidates for potential pathological involvement in placenta accreta.
During gestation, it was not surprising to find that RLN expression was markedly elevated in the patients with PPROM. This had previously been shown in the fetal membranes using different methodologies.15 , 16 Thus, when put together with the data here on TR and BP, there appears to be a generalized increase in intrauterine RLN expression in PPROM. This significant increase in RLN expression in PPROM caused high variability when the data were pooled >30 weeks of gestation to term. On the other hand, this study shows for the first time that RXFP1 expression was unaltered in either the BP or the TR in PPROM. However, in early gestation, it was well expressed in both the TR and the BP, dropping to lower levels until term. Its decline with term vaginal delivery has been previously reported.19 That RXFP1 expression was only increased in the BP >30 weeks of gestation and not in the TR suggests that separate mechanisms control its expression in these 2 tissues over this period. Nothing is currently known about the control of its expression in any tissue. The INSL4 expression during gestation showed no significant differences in the BP or the TR. However, significantly more was expressed in the TR >30 weeks of gestation compared to its expression in the BP, agreeing with its increase in maternal serum over this period.29 However, this may be due to our inclusion of patients with preterm labor, who have been shown to have higher serum INSL4 levels.29 In any event, the higher INSL4 >30 weeks of gestation may be linked to increased placental apoptosis as pregnancy advances,30 since it has been shown that INSL4 increases apoptosis and reduces cell viability.31 These results for RLN/RXFP1 and INSL4 underline the difficulty in obtaining “normal” data during human gestation, since most tissues delivered between 32 and 36 weeks of gestation cannot be considered to be truly normal. However, they were the best that could be obtained under the constraints of using human tissues and were therefore used as baseline data for comparison to the results obtained on tissues from patients with placenta accreta.
In these patients with placenta accreta, both RLN gene and protein were increased in the BP compared to the controls. Most importantly, by subdividing the patients with accreta according to antepartum bleeding necessitating emergent cesearan hysterectomies, RLN was only increased in those with bleeding. In addition, the RLN protein, but not the gene, was increased in both the noninvasive and invasive areas of TR in the patients with accreta compared to the controls. Little is known about the relative half-lives of RLN mRNA and its protein, but they may be quite different, and may also differ in the BP and TR. There was no increase in RXFP1 gene expression in patients with accreta, although its protein was significantly increased. Thus, both RLN and RXFP1 proteins were significantly increased in the BP and TR in patients with accreta. In a clinical trial of women receiving RLN treatment, significantly more had menometrorrhagia than women receiving a placebo.32 This is consistent with the hypothesis that RLN stimulates neovascularization in the endometrial lining of the uterus by stimulating expression of VEGF, inducing new blood vessel formation.18 Also, sustained RLN administration in rats increases MMP-2, MMP-9, and nitric oxide production by the endothelium.33 Nitric oxide inhibits platelet aggregation and thrombus formation by affecting cyclic guanosine monophosphate (cGMP) signaling by cGMP-dependent kinase I.34 Indeed, a bioactivity assay for RLN has been based upon its inhibition of platelet aggregation.35 Thus, a potential mechanism of action of the increased RLN in these tissues in placenta accreta suggests that RLN may be the cause of the excessive bleeding in these patients. Because RLN also increases the expression of the MMPs,17 we sought changes here in MMP-2 and MMP-9 gene expression in the same samples. The MMP-2 and MMP-9 were chosen because it has been suggested that the invasive ability of TR may be regulated primarily by these gelatinases.36 However, due to the large variability in their expression and the small numbers of samples available, this failed to reach significance. In addition, all accreta samples were collected >30 weeks of gestation, after the time in which TR invasion had occurred and this may also have affected these results. However, TIMP-1 expression was increased, but only in the invasive area of the TR in the accreta samples compared to the controls. It has been suggested that the trophoblastic invasion of the decidua may be regulated by both invasion-promoting factors such as the MMPs and the invasion-inhibiting factors (TIMPs) throughout the pregnancy and at the site of invasion.36 Thus, this highly specific location suggests a very local effect in the invading TR. These results need to be repeated with larger numbers of patients and extended by measurements of enzyme activities.
In this study, we have shown that the measurements of both gene expression and protein levels in the same samples do not always agree. The results for INSL4 differed from RLN and RXFP1, since expression of its gene was significantly lower than controls in both invasive and noninvasive areas of TR in placenta accreta, but there were no changes in the levels of its protein. The translation and processing of INSL4 differs from that of RLN and most RLN-like peptides. The INSL4 protein may be much longer and potentially a single chain product, making it more similar to the insulin-like growth factors.37 On the other hand, since RLN is also made by the TR and is successfully processed, it has been pointed out that INSL4 should also be cleaved in vivo.27 The use of quantitative immunolocalization also depends on the particular antigenic determinants of INSL4 measured with the antibody we used. The INSL4 protein may be processed in different ways by different cells and this needs further study with a range of antibodies. Clearly, more work is needed to understand the complexities of INSL4 posttranslational processing in these reproductive tissues before firm conclusions can be drawn.
This study had limitations; no tissues were collected before 10 weeks of gestation since obtaining intact placental tissue is difficult at these gestational ages. The initiation of abnormal placentation occurs prior to 10 weeks of gestation38 and a study of TR and BP prior to this may have provided more insights into early RLN/RXFP1 and INSL4 expression. However, this study also had several strengths; all tissues were obtained immediately after delivery or hysterectomy ensuring minimal RNA degradation and tissues from patients with clinical chorioamnionitis were discarded. In addition, the study of both invasive and noninvasive areas of placenta in patients with accreta provided an internal control for each patient, with additional controls from patients without accreta at the same gestational ages.
In summary, this study has shown increased RLN gene and protein and RXFP1 protein expression in the BP and TR of patients with accreta. This novel finding suggests that RLN may be the cause of excessive antepartum bleeding by increasing angiogenesis and vasodilation and by decreasing platelet aggregation. Decreased INSL4 gene expression in both the invasive and the noninvasive areas of TR in patients with placenta accreta suggests a generalized effect in the placenta. In contrast, the increased TIMP-1 only in the invasive area of TR suggests that there may be a localized response to control the invasive properties by inhibiting the active MMPs.
Acknowledgments
The authors acknowledge the physicians, nurses, and staff of Kapiolani Medical Center for Women and Children who helped in identifying patients for this study. We particularly thank Dr D. Bellet for the very generous gift of antibody to INSL4. We also acknowledge Jim Davis, PhD for his assistance with statistical analyses.
Footnotes
Authors’ Note: This study has been presented in part at the Society for Maternal Fetal Medicine Annual Meeting, Dallas, February 6-11, 2012.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: the National Institutes of Health, National Center for Research Resources (5P20RR024206) and the Department of Obstetrics, Gynecology and Women’s Health, University of Hawaii, Honolulu, HI, USA.
References
- 1. Kemp B, Kertschanska S, Kadyrov M, Rath W, Kaufmann P, Huppertz B. Invasive depth of extravillous trophoblast correlates with cellular phenotype: a comparison of intra- and extrauterine implantation sites. Histochem Cell Biol. 2002;117(5):401–414. [DOI] [PubMed] [Google Scholar]
- 2. Ness R, Sibai B. Shared and disparate components of the pathophysiologies of fetal growth restriction and preeclampsia. Am J Obstet Gynecol. 2006;195(1):40–49. [DOI] [PubMed] [Google Scholar]
- 3. Belfort MA. Publications committee, society for maternal-fetal medicine. Placenta accreta. Am J Obstet Gynecol. 2010;203(5):430–439. [DOI] [PubMed] [Google Scholar]
- 4. Silver RM, Landon MB, Rouse DJ, et al. Maternal morbidity associated with multiple repeat cesarean deliveries. Obstet Gynecol. 2006;107(6):1226–1232. [DOI] [PubMed] [Google Scholar]
- 5. Tantbirojn P, Crum C, Parast M. Pathophysiology of placenta creta: the role of decidua and extravillous trophoblast. Placenta. 2008;29(7):639–645. [DOI] [PubMed] [Google Scholar]
- 6. Jauniaux E, Jurkovic D. Placenta accreta: pathogenesis of a 20th century iatrogenic uterine disease. Placenta. 2012;33(4):244–251. [DOI] [PubMed] [Google Scholar]
- 7. Bernirschke K, Kaufmann P, Baergen R. Pathology of the Human Placenta. 5th ed New York, NY; Springer; 2006. [Google Scholar]
- 8. Craven C, Chedwick L, Ward K. Placental basal plate formation is associated with fibrin deposition in decidual veins at sites of trophoblastic cell invasion. Am J Obstet Gyneceol. 2002;186(2):291–296. [DOI] [PubMed] [Google Scholar]
- 9. Stanek J, Drummond Z. Occult placenta accreta: the missing link in the diagnosis of abnormal placentation. Pediatr Dev Pathol. 2007;10(4):266–273. [DOI] [PubMed] [Google Scholar]
- 10. Tseng J, Chou M. Differential expression of growth-, angiogenesis- and invasion-related factors in the development of placenta accreta. Taiwan J Obstet Gynecol. 2006;45(2):100–106. [DOI] [PubMed] [Google Scholar]
- 11. Han V, Basset N, Walton J, Challis J. The expression of insulin-like growth factor (IGF) and IGF-binding protein (IGRBP) genes in the human placenta and membranes: evidence for IGF-IGFBP interactions at the feto-maternal interface. J Clin Endocrinol Metab. 1996;81(7):2680–2693. [DOI] [PubMed] [Google Scholar]
- 12. Crawford R, Hudson P, Shine J, Niall H, Eddy R, Shows T. Two human relaxin genes are on chromosome 9. EMBO J. 1984;3(10):2341–2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bathgate R, Samuel C, Burazin T, et al. Human relaxin gene 3 (H3) and the equivalent mouse relaxin (M3) gene. Novel members of the relaxin peptide family. J Biol Chem. 2002;277(2):1148–1157. [DOI] [PubMed] [Google Scholar]
- 14. Garibay-Tupas J, Maaskant R, Greenwood F, Bryant-Greenwood G. Characteristics of the binding of 32P-labelled human relaxins to the human fetal membranes. J Endocrinol. 1995;145(3):441–448. [DOI] [PubMed] [Google Scholar]
- 15. Bogic L, Yamamoto S, Millar L, Bryant-Greenwood G. Developmental regulation of the human relaxin genes in the decidua and placenta: overexpression in the preterm premature rupture of the fetal membranes. Biol Reprod. 1997;57(4):908–920. [DOI] [PubMed] [Google Scholar]
- 16. Tashima L, Yamamoto S, Yasuda M, Millar L, Bryant-Greenwood G. Decidual relaxins: gene and protein up-regulation in preterm premature rupture of the membranes by complementary DNA arrays and quantitative immunocytochemistry. Am J Obstet Gynecol. 2002;187(3):785–797. [DOI] [PubMed] [Google Scholar]
- 17. Bryant-Greenwood G, Kern A, Yamamoto S, Sadowsky D, Novy M. Relaxin and the human fetal membranes. Reprod Sci. 2007;14(suppl 8):42–45. [DOI] [PubMed] [Google Scholar]
- 18. Unemori EN, Erikson ME, Rocco SE, et al. Relaxin stimulates expression of vascular endothelial growth factor in normal human endometrial cells in vitro and is associated with menometrorrhagia in women. Hum Reprod. 1999;14(3):800–806. [DOI] [PubMed] [Google Scholar]
- 19. Lowndes Amano A, Yamamoto S, Bryant-Greenwood G. The human relaxin receptor (LGR7): expression in the fetal membranes and placenta. Placenta. 2006;27(6-7):610–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chassin D, Laurent A, Janneau J, Berger R, Bellet D. Cloning of a new member of the insulin gene superfamily (INSL4) expressed in human placenta. Genomics. 1995;29(2):465–470. [DOI] [PubMed] [Google Scholar]
- 21. Laurent A, Rouillac C, Delezoide A, et al. Insulin-like 4 (INSL4) gene expression in human embryonic and trophoblastic tissues. Mol Reprod Dev. 1998;51(2):123–129. [DOI] [PubMed] [Google Scholar]
- 22. Brandt B, Roetger A, Bidart J, et al. Early placenta insulin-like growth factor (pro-EPIL) is overexpressed and secreted by c-erbB-2-positive cells with high invasion potential. Cancer Res. 2002;62(4):1020–1024. [PubMed] [Google Scholar]
- 23. Cohen M, Meisser A, Bischof P. Metalloproteinases and human placenta invasiveness. Placenta. 2006;27(8):783–793. [DOI] [PubMed] [Google Scholar]
- 24. Demir-Weusten A, Seval Y, Kaufmann P, Demir R, Yucel G, Huppertz B. Matrix metalloproteinases-2, -3 and -9 in human term placenta. Acta Histochem. 2007;109(5):403–412. [DOI] [PubMed] [Google Scholar]
- 25. Tarrade A, Goffin F, Munaut C, et al. Effect of matrigel on human extravillous trophoblasts differentiation: modulation of protease pattern gene expression. Biol Reprod. 2002;67(5):1628–1637. [DOI] [PubMed] [Google Scholar]
- 26. Moser G, Orendi K, Gauster M, Siwetz M, Helige C, Huppertz B. The art of identification of extravillous trophoblast. Placenta. 2011;32(2):197–199. [DOI] [PubMed] [Google Scholar]
- 27. Lin F, Otvos L, Jr , Kumagai J, Tregear GW, Bathgate RA, Wade JD. Synthetic human insulin 4 does not activate the G-protein coupled receptors LGR7 or LGR8. J Pept Sci. 2004;10(5):257–264. [DOI] [PubMed] [Google Scholar]
- 28. Bieche I, Laurent A, Laurendaeau I, et al. Placenta-specific INSL4 expression is mediated by a human endogenous retrovirus element. Biol Reprod. 2003;68(4):1422–1429. [DOI] [PubMed] [Google Scholar]
- 29. Bruni L, Luisi S, Ferretti C, et al. Changes in the maternal serum concentration of proearly placenta insulin-like growth factor peptides in normal vs abnormal pregnancy. Am J Obstet Gynecol. 2007;197(6):606.e1–e4. [DOI] [PubMed] [Google Scholar]
- 30. Smith S, Baker P, Symonds E. Placental apoptosis in normal human pregnancy. Am J Obstet Gynecol. 1997;177(1):57–65. [DOI] [PubMed] [Google Scholar]
- 31. Millar L, Streiner N, Webster L, et al. Early placental insulin-like protein (INSL4 or EPIL) in placental and fetal membrane growth. Biol Reprod. 2005;73(4):695–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Seibold JR, Korn J, Simms R, et al. Controlled trial of recombinant human relaxin (rhRLXN) in diffuse scleroderma (DS). Arthritis Rheum. 1997;40:S123. [Google Scholar]
- 33. Conrad K, Shroff S. Effects of relaxin on arterial dilation, remodeling and mechanical properties. Curr Hypertens Rep. 2011;13(6):409–420. [DOI] [PubMed] [Google Scholar]
- 34. Antl M, von Bruhl M, Eiglsperger C, et al. IRAG mediates NO/cGMP-dependent inhibition of platelet aggregation and thrombus formation. Blood. 2007;109(2):552–559. [DOI] [PubMed] [Google Scholar]
- 35. Bani D, Nistri S, Cinci L, et al. A novel, simple bioactivity assay for relaxin based on inhibition of platelet aggregation. Regul Pept. 2007;144(1-3):10–16. [DOI] [PubMed] [Google Scholar]
- 36. Isaka K, Usuda S, Ito H, et al. Expression and activity of matrix metalloproteinase 2 and 9 in human trophoblasts. Placenta. 2003;24(1):54–64. [DOI] [PubMed] [Google Scholar]
- 37. Bullesbach E, Schwabe C. Synthesis and conformational analysis of the insulin-like 4 gene product. J Pept Res. 2001;57(1):77–83. [DOI] [PubMed] [Google Scholar]
- 38. Kay H, Nelson D, Wang Y. The Placenta. From Development to Disease. 1st ed Chichester, UK: Blackwell Publishing Ltd, 2011:20–36. [Google Scholar]