Abstract
Background:
The Wnt signaling pathway is a conserved pathway and plays a crucial role in regulating trophoblast functions. Abnormal expression of the Wnt pathway may result in the dysfunction of the trophoblast that can contribute to the pathogenesis of preeclampsia (PE). However, published data regarding the association between Wnt pathway and PE in human pregnancy is rare.
Objective:
The aims of this study were to investigate the expression pattern of Wnt2 and secreted frizzled-related protein 4 (sFRP4) in the third trimester human placenta and to evaluate the relationship between changes in placental Wnt2 and sFRP4 expression and severe PE.
Methods:
The expression of Wnt2 and sFRP4 in normal and severe PE placentas was examined using immunohistochemistry (IHC), real-time polymerase chain reaction, and Western blot.
Results:
Compared to the controls, the relative expression of Wnt2 messenger RNA was remarkably downregulated in the PE placentas, while there was no significant difference in sFRP4 between the 2 groups. The IHC indicated that Wnt2 and sFRP4 were expressed predominantly in the villous syncytiotrophoblast and the extravillous trophoblast, whereas Wnt2 in the control group showed higher staining intensity than in the PE group, and sFRP4 in the PE group had a higher staining intensity than in the control group. Furthermore, the results of the Western blots were consistent with the IHC.
Conclusions:
The Wnt signaling pathway was detected in human third trimester placentas, and the decreased placental expression of Wnt2 and increased placental expression of sFRP4 may be associated with the pathogenesis of severe PE.
Keywords: severe preeclampsia, Wnt2, sFRP4, placenta
Introduction
Hypertensive disease of pregnancy is a pregnancy-specific disorder affecting 6% to 8% of pregnancies worldwide.1 Preeclampsia (PE) is the most common form of hypertensive disease during pregnancy, and it occurs in 3% to 4% of the pregnancies.2 The PE, especially severe PE, remains a major cause of fetal and maternal morbidity and mortality. The PE occurs only during pregnancy, and its clinical symptoms rapidly abate after delivery (including removal of the placenta). The placenta is the direct interface between the fetus and the mother during pregnancy, so the placenta must play a central or initiating role in this disorder.3 Although shallow placental implantation and placental ischemia caused by incomplete trophoblast invasion and impaired spiral arterial remodeling are thought to be the major cause of PE, the pathogenesis of PE is largely unknown.4–7 A recent study demonstrated the complex number of Wnt pathway ligands and receptors in the placenta, with 14 of the 19 known Wnt ligands and 8 frizzled receptors expressed in the human placenta, suggesting that the Wnt signaling pathway plays an important role in placentation and placental development.8
The Wnt signaling pathway is a conserved pathway that has been identified in animals from hydra to humans. During development, the Wnt pathway plays diverse roles in governing cell fate, proliferation, migration, and polarity. In adults, the Wnt pathway functions in homeostasis, and inappropriate activation of the Wnt pathway is implicated in a variety of cancers.9 Wnt signals are transduced through at least 3 distinct intracellular signaling pathways, including the canonical “Wnt/β-catenin” pathway, the “Wnt/Ca2+” pathway and the “Wnt/polarity” pathway.10 The canonical Wnt/β-catenin pathway has been intensely studied and has intricate functions in reproduction. Signaling through this pathway depends on the levels of β-catenin in the cell. In the absence of Wnt, β-catenin is targeted for degradation by a multiprotein destruction complex; Wnt signaling antagonizes the destruction complex, leading to the accumulation of β-catenin and translocation to the nucleus followed by the activation of target genes.
Wnt2 is a secreted glycoprotein that is one of the canonical Wnt ligands. Recently, studies have demonstrated that Wnt2 is overexpressed in various human cancers, including esophageal, gastric, colorectal, breast, lung, cervical, and malignant glioma.11–16 Knockdown of Wnt2 with monoclonal antibodies, small interfering RNA, or pharmacological inhibitors can induce apoptosis in human cancer cells overexpressing Wnt2 and inhibit proliferation, invasion, and angiogenesis and ultimately suppress tumor growth in vitro and in vivo.17–19 Previous studies have provided evidence that Wnt2 acts as an angiogenic factor. Exogenous Wnt2 induces endothelial cells that lack Wnt2 to undergo proliferation, sprouting, and angiogenesis, and Wnt2 was found to be expressed in densely vascularized murine malignant tumors and healing wounds.20 A study by Monkley et al showed that mice deficient in Wnt2, generated by gene targeting, displayed runting and lower birth weight, and approximately 50% died perinatally. Histological analysis revealed alterations in the size and structure of the placentas. The placental defects were primarily associated with impaired placental angiogenesis; there was an apparent decrease in the number of fetal capillaries and an increase in the amount of fibrinoid material in the Wnt2 mutant placentas.21 Therefore, these results suggest that Wnt2 is required for the proper vascularization of the mouse placenta, and the placental defects in Wnt2-deficient mice result in a reduction in birth weight and perinatal lethality.
Secreted frizzled-related protein 4 (sFRP4) is a member of a family of secreted proteins sharing homology with the extracellular domain of frizzled proteins but lacking the transmembrane and intracellular components that are necessary for signaling transduction. It antagonizes Wnt signaling by competitively binding to Wnt or to their receptors in the plasma membrane.22 sFRP4 functions as a suppressor of tumor growth via inhibition of the Wnt signaling pathway23; downregulation or deletion of sFRP4 has been reported in several cancers and cell lines such as endometrial cancer, ovarian cancer, lung cancer, and others.24–25 A previous study in mice demonstrated that sFRP4 messenger RNA (mRNA) expression increased markedly in the nonproliferative basal zone trophoblasts, but not in the rapidly growing labyrinth zone. Increased expression of sFRP4 is associated with reduced placental growth in normal placentas. Maternal dexamethasone treatment, which has previously been shown to reduce placental growth and increase apoptosis in placenta, further increased the expression of sFRP4 mRNA in both the basal and the labyrinth zones of the placenta.26 sFRP4 can inhibit endothelial cell proliferation, migration, and the development of sprouts and the pseudopodia as well as disrupt the stability of endothelial rings. In addition, sFRP4 blocked the effect of vascular endothelial growth factor on endothelial cells. Furthermore, sFRP4 could induce apoptotic events in endothelial cells by increasing cellular levels of reactive oxygen species.27 These data suggest that sFRP4 might be a potent proapoptotic protein and angiogenesis inhibitor.
Trophoblasts invade and migrate to the maternal endometrium, even the shallow myometrium, in the process of normal pregnancy, and there are considerable similarities to the process of malignant tumor infiltration of the surrounding tissues and distant metastases. It is thought that abnormal trophoblast invasion and failed remodeling of the maternal spiral arteries contribute to the pathogenesis of PE. We speculated that abnormal expression of the Wnt signaling pathway in placentas might be involved in the pathogenesis of PE, but little information was available for human pregnancies. Hence, real-time polymerase chain reaction (RT-PCR), immunohistochemistry (IHC), and Western blot were performed to examine the expression patterns of Wnt2 and sFRP4 in both human third trimester placentas with severe PE and normal controls.
Materials and Methods
Study Population
A total of 30 women with severe PE and 30 women with normal pregnancies were recruited from the Third Affiliated Hospital of Zhengzhou University between October 2010 and December 2011. The criteria for diagnosis of severe PE were strictly based on the American Congress of Obstetricians and Gynecologists criteria (2002). Patients with chronic hypertension, diabetes mellitus, renal disease, polycystic ovarian syndrome, fetal malformations, and multiple gestations were excluded from this study. This study was approved by the Human Ethics Committees of the Third Affiliated Hospital of Zhengzhou University. Informed consent was obtained from all the women participating in the study.
Sample Collection
As soon as possible after delivery (within 15 minutes), 5 samples of placenta, 2 × 2 × 1 cm in size, were taken, 1 from the central portion and 1 from each quadrant (at 3, 6, 9 and 12 o’clock) of the maternal surface, and placenta sampling included full-thickness placental blocks. Areas with macroscopic evidence of necrosis, infarction, and calcification were avoided when collecting the samples. Blood in the tissues was removed with sterile filter paper. A portion of the sample was fixed in 10% formalin for IHC, and the other portion of the sample was immediately frozen in liquid nitrogen and stored at −80°C.
Quantitative RT-PCR
Total RNA was extracted from the 60 placental tissues using Trizol Reagent as detailed in the manufacturers’ protocols (Invitrogen, Carlsbad, California). The RNA integrity was assessed via ethidium bromide staining of the nucleic acids before agarose gel electrophoresis. Complementary DNA (cDNA) was prepared using the Reverse Transcriptase M-MLV kit (TaKaRa, Dalian, China) following the manufacturer’s instructions. The expression levels of Wnt2 and sFRP4 in the 2 groups were investigated by quantitative RT-PCR (qRT-PCR) on an Applied Biosystems 7500 (ABI, Foster City, California) with the Ultra SYBR Mixture With ROX (CWBIO, Beijing, China). Human glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an internal control. The reactions consisted of 10 μL of Ultra SYBR Mixture, 7 μL of dH2O, and 1 μL of each primer and cDNA. The sequences of the Wnt2 primers used were 5′-GGGTCCTACTCCGAAGTAG-3′(sense) and 5′-CCTTGGCTACAGGCCCTG-3′(anti-sense), and the amplified fragment length was 94 base pair (bp). The sequences of the specific primers for sFRP4 were 5′-GTGGCGCTCAAGGATGATG-3′ (sense) and 5′-CTCTTCCCACTGTATGGATC-3′ (antisense), and the fragment length was 88 bp. For GAPDH, the specific primers were 5′-TCGTGGAAGGACTCATGACC-3′ (sense) and 5′-AGGGATGATGTTCTGGAGAG-3′ (anti-sense; 116 bp). The cycling conditions were denaturation at 95°C for 10 minutes and subsequent cycling (35 times) at 95°C for 15 seconds then 60°C for 1 minute. Melt curve analysis was then conducted by raising the temperature from 60°C to 95°C at 0.2°C intervals to ensure amplification of the desired product. All samples were run in triplicate, and the results were standardized against the expression of GAPDH. Calculation of the signals was performed using the 2−ΔΔCT method; briefly, the threshold cycle (CT) is defined as the cycle at which the first fluorescent signal reaches statistical significance above background. For each individual condition, ΔCT (the difference between CTWnt2/sFRP4 and CTGAPDH) values are calculated, representing normalization to the housekeeping gene. Subsequently, the ΔΔCT values are calculated indicating normalization to controls. The amount of target normalized to an endogenous reference and relative to the normal control is then given by 2−ΔΔCT.
Immunohistochemistry
The tissues were fixed in 10% buffered formalin at room temperature and embedded in paraffin. Consecutive serial sections (4 μm thick) of the same blocks were cut for IHC analysis. Briefly, after deparaffinization and hydration to distilled water, antigen recapture was performed on all tissue sections using microwave treatment in 10 mmol/L sodium citrate buffer, pH 6.0, for 20 minutes and then incubated with 3% hydrogen peroxide for 15 minutes at 37°C to block endogenous peroxide activity. The sections were incubated for 2 hours at 37°C with a rabbit antihuman Wnt2 monoclonal antibody (ab109222, Abcam Inc, Cambridge, Massachusetts) at a dilution of 1:250, a goat antihuman sFRP4 polyclonal antibody (ab122905, Abcam) at a dilution of 1:100, and a mouse anti-human MHC I HLA-G Monoclonal Antibody (MEM-G/1; MA1-19219, Thermo scientific, Rockford, California) at a dilution of 1:100. Negative control sections were incubated for 2 hours at 37°C with phosphate-buffered solution (PBS). They then underwent a second incubation with a 2-step IHC detection reagent (GBI, WA, California). Reaction products were visualized by immersing slides in a 3,3-diaminobenzidine tetrahydrochloride substrate kit (ZSGB-BIO, Beijing, China). The sections were counterstained in hematoxylin for 5 minutes, dehydrated, cleared, and cover slipped. The slides were examined using an inversion fluorescence microscope (OLYMPUS IX-71, Tokyo, Japan) and Image-pro Express software. Immunostaining of Wnt2 and sFRP4 in the placenta were graded on a semiquantitative scale: 0, no staining/no color; 1, weak staining/pale brown color; 2, moderate staining/dark brown color; 3, strong staining/brownish-black color. The intensity of immunostaining of each slides was evaluated by 2 pathologists separately.
Western Blot
The placental tissues were homogenized on ice using radioimmunoprecipitation assay lysate buffer according to the manufacturer’s instructions (Solarbio, Beijing, China). The protein concentrations were measured using the bicinchoninic acid assay (Sangon, Shanghai, China). The proteins were denatured by boiling for 10 minutes at 100°C in 4×sample loading buffer. For Western blot analyses, equal amounts of protein lysate (100 μg) were loaded and separated by 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis. After electrophoresis, the proteins were transferred onto nitrocellulose membranes using a semi-dry blotting apparatus. The membranes were blocked for 1 hour in 5% (w/v) nonfat milk in Tris-buffered saline with 0.1% Tween. After blocking, the membranes were incubated overnight at 4°C with a rabbit antihuman Wnt2 monoclonal antibody (1:500; ab109222, Abcam), a goat antihuman sFRP4 polyclonal antibody (1:500; ab122905, Abcam), and a rabbit antihuman β-actin polyclonal antibody (1:1000; bs-0061R, Biosynthesis Biotechnology Co, Beijing, China). After a 1-hour treatment (37°C) with the corresponding horseradish peroxidase (HRP)-conjugated secondary antibodies (1:5000; Dingguo, Beijing, China), the protein bands were revealed by the Pro-Light HRP chemiluminescence detection reagent (TIANGEN, Beijing, China). After exposure in a darkroom, the protein bands could be observed on x-ray film. Quantification of the protein bands on the films was performed by densitometric analysis using a Gel logic 2000 image system (Eastman Kodak Co, NY, California).
Statistical Analysis
Statistical analysis was performed using the SPSS version 17.0 software package. All data were expressed as the mean ± standard deviation. Statistical differences were calculated using Student t test when the data from the 2 groups were normally distributed or a nonparametric Mann-Whitney U test if the data distribution of any group was skewed. For IHC, the intensity of staining in placental tissues between the 2 groups was compared using the chi-square (χ2) test. A P value < .05 was considered statistically significant.
Results
Clinical Data Between Patients With Severe PE and Normal Controls
When we compared variables (such as age, body mass index [BMI] at delivery, fasting blood glucose, and parity) between the patients for the severe PE and control groups, we found no significant differences. We did find that the BMI before pregnancy and systolic and diastolic blood pressure in women with severe PE was significantly higher than in the healthy pregnant women, and the birth weight and gestational age at delivery in the PE group were significantly lower than those of the control group (Table 1).
Table 1.
Clinical Data Between Patients With Severe PE and Normal Controls.
| Variables | Severe PE Group | Normal Control Group | P Value |
|---|---|---|---|
| Age, years | 28.8 ± 5.6 | 30.9 ± 3.7 | .071 |
| Gestational age at delivery, week | 35.3 ± 2.6 | 38.6 ± 1.0 | <.001 |
| BMI before pregnancy, kg/m2 | 23.5 ± 3.2 | 21.9 ± 2.7 | .038 |
| BMI at delivery (kg/m2 | 30.5 ± 4.3 | 28.7 ± 3.8 | .074 |
| Systolic blood pressure, mm Hg | 160.1 ± 11.5 | 113.7 ± 8.3 | <.001 |
| Diastolic blood pressure, mm Hg | 107.6 ± 10.5 | 75.5 ± 7.4 | <.001 |
| Birthweight, g | 2108.8 ± 709.9 | 3368.8 ± 340.7 | <.001 |
| Fasting blood glucose, mmol/L | 4.8 ± 1.3 | 4.7 ± 0.7 | .530 |
| Number of pregnancies | 2.1 ± 1.4 | 2.3 ± 1.2 | .360 |
| Number of children | 0.5 ± 0.6 | 0.6 ± 0.7 | .460 |
Abbreviation: BMI, body mass index; PE, preeclampsia.
Wnt2 and sFRP4 mRNA Expression in the Placentas of Women With Severe PE and Healthy Pregnancies
In the qRT-PCR reaction, the shapes of the melt curves had prominent peaks (data not shown). At the end of the reaction, the samples were assessed by 3% agarose gel electrophoresis with an ethidium bromide nucleic acid stain, and the lengths of the amplified fragments were consistent with the original design (data not shown). When compared to women with normal pregnancy, the expression of Wnt2 mRNA is significantly downregulated in the severe PE group (P = .003; Figure 1A). However, no significant difference was observed in sFRP4 expression between the 2 groups (P = .794; Figure 1B).
Figure 1.
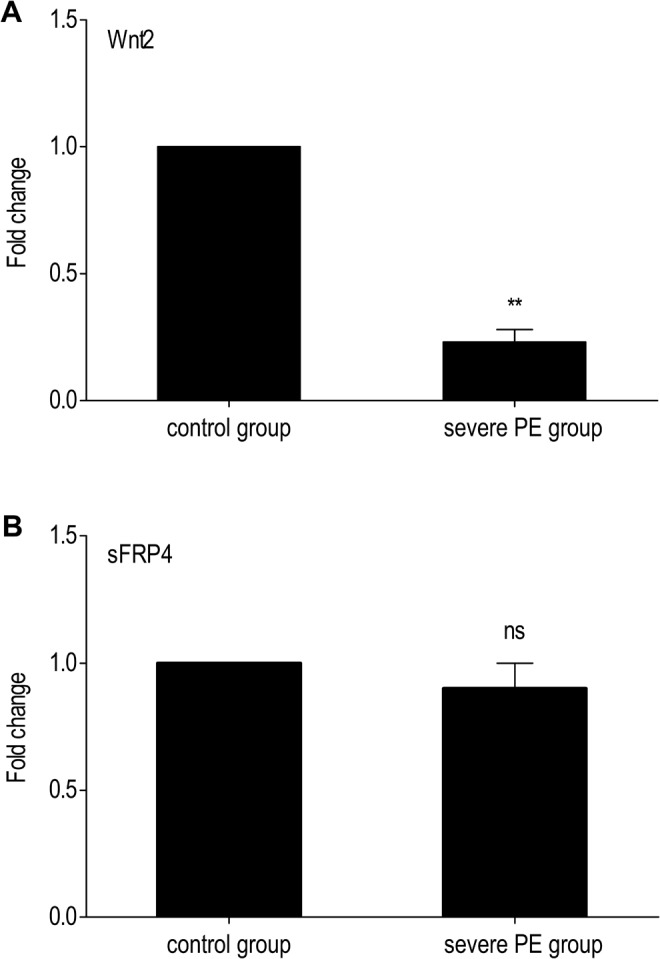
Quantitative real-time polymerase chain reaction (PCR) of Wnt2 and secreted frizzled-related protein 4 (sFRP4) messenger RNA (mRNA) expression in placentas of the 2 groups. A, Compared to the control group, Wnt2 mRNA is downregulated in severe preeclampsia (PE) group. B, No significant difference was detected in sFRP4 mRNA expression between the 2 groups. For comparison of the 2 groups, the values of the control group were arbitrarily set at 1. Bars indicate the mean values ± standard deviation (SD) of the 30 cases performed in triplicate. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as an internal control. **Indicates P value< .01; ns, no significant difference.
Immunostaining of Wnt2 and sFRP4 in Placental Tissues of the 2 Groups
Placental tissue sections were examined by hematoxylin and eosin (H&E) staining before IHC analysis. Immunoreactivity for Wnt2 and sFRP4 was consistently found in the cytomembrane and cytoplasm of cells with the morphological characteristics of the villous syncytiotrophoblast and the extravillous trophoblast (EVT), the serial sections stained with HLA-G confirming the phenotypic characteristic of EVT. The H&E staining was shown in Figure 2A. Image of tissue section stained with PBS was shown in Figure 2B.
Figure 2.
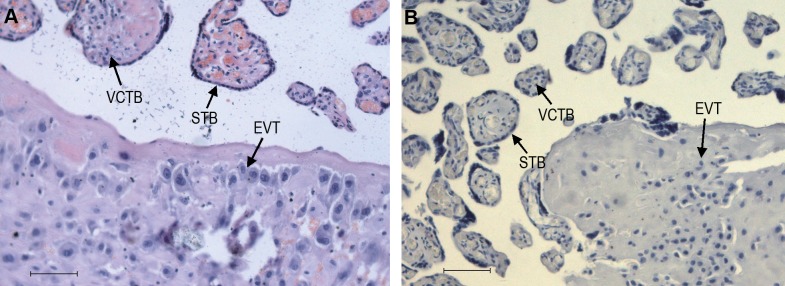
Hematoxylin and eosin (H&E) staining, and PBS staining of placental tissue sections. A, H&E staining. B, PBS staining as negative control. STB indicates syncytiotrophoblast; VCTB, villous trophoblast; EVT, extravillous trophoblast; PBS, phosphate-buffered saline. Original magnification 200×, scale bar = 40 µm.
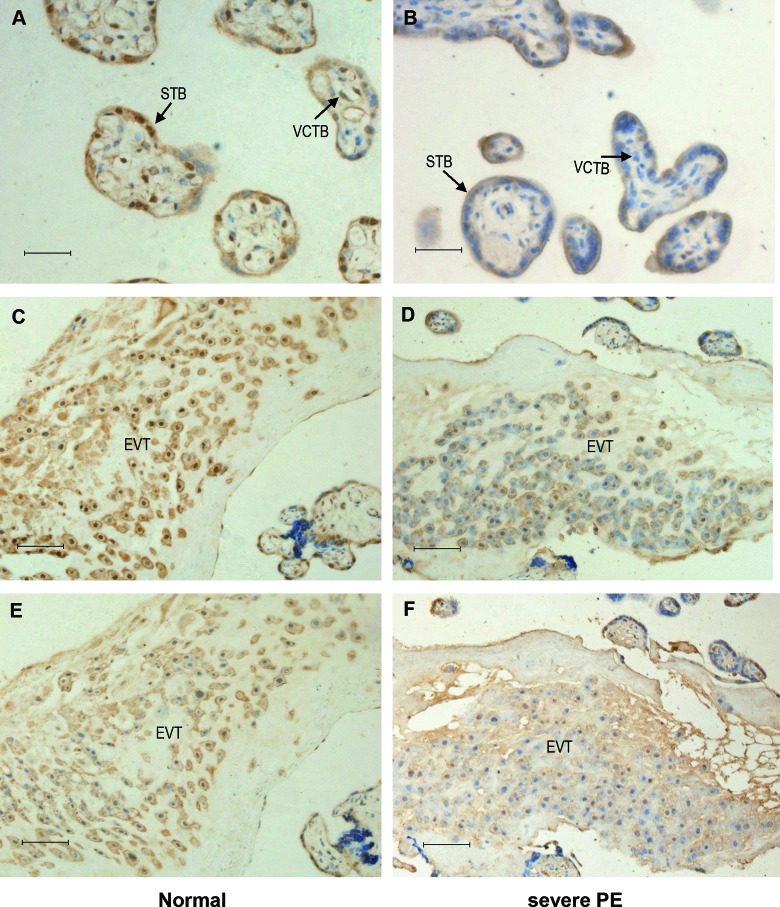
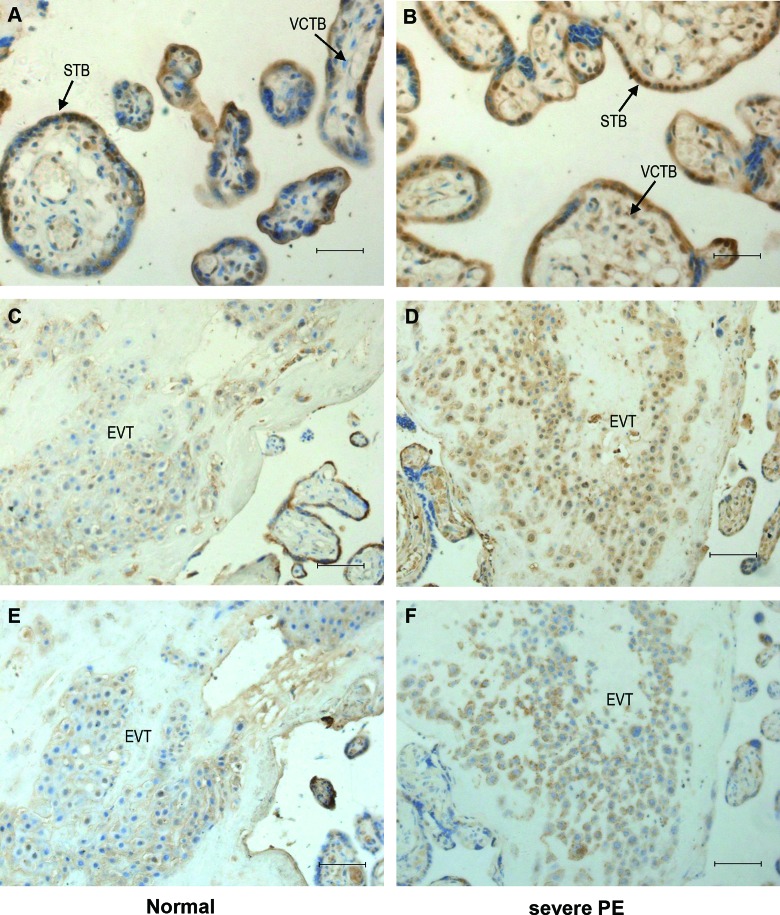
Wnt2 and sFRP4 immunostaining were examined in tissue sections from 40 placentas (n = 20 for each group). Wnt2 showed a stronger staining intensity in the control group (Figure 3A and C) than in the PE group (Figure 3B and D), Figure 3E and F was serial sections of C and D, which stained with HLA-G. The immunostaining of sFRP4 in the control group (Figure 4A and C) was reduced compared to the PE group (Figure 4B and D), Figure 4E and F was serial sections of C and D, which stained with HLA-G. Tables 2 and 3 summarize the results that are categorized by the intensity of immunostaining. Intense immunostaining for Wnt2 in placentas was weaker in PE group than the normal controls (χ2 = 13.2; P < .001), while intense immunostaining for sFRP4 was more frequently present in PE placentas than the controls (χ2 = 9.9; P = .002).
Figure 3.
Immunostaining of Wnt2 in placental tissue sections of the 2 groups. Wnt2 immunostaining is mainly localized in the syncytiotrophoblast and the extravillous trophoblast. The immunostaining of Wnt2 were reduced in severe preeclamptic placentas (B and D) compared to the normal placentas (A and C). E and F, Serial sections of C and D stained with HLA-G. STB indicates syncytiotrophoblast; VCTB, villous trophoblast; EVT, extravillous trophoblast. Original magnification 400× for A and B, 200× for C-F. Scale bar = 20 µm for A and B, scale bar = 40 µm for C-F.
Figure 4.
Immunostaining of sFRP4 in placental tissue sections of the 2 groups. Immunoreactivity for sFRP4 was consistently found in the villous syncytiotrophoblast and the extravillous trophoblast. Immunostaining of sFRP4 were more frequently present in severe PE (B and D) versus control placentas (A and C). E and F, Serial sections of C and D stained with HLA-G. STB indicates syncytiotrophoblast; VCTB, villous trophoblast; EVT, extravillous trophoblast; PE, preeclampsia; sFRP4, secreted frizzled-related protein 4. Original magnification 400× for A and B, 200× for C-F. Scale bar = 20 µm for A and B, scale bar = 40 µm for C-F.
Table 2.
Immunohistochemical Analysis of Wnt2 Expression in Patients With Severe PE and Normal Controls.
| Wnt2 Immunostaining | No Staining | Weak Staining | Moderate Staining | Strong Staining |
|---|---|---|---|---|
| Control group (n = 20) | 0 | 4 | 13 | 3 |
| Severe PE group (n = 20) | 3 | 12 | 5 | 0 |
Abbreviation: PE, preeclampsia.
Table 3.
Immunohistochemical Analysis of sFRP4 Expression in Patients With Severe PE and Normal Controls.
| sFRP4 Immunostaining | No Staining | Weak Staining | Moderate Staining | Strong Staining |
|---|---|---|---|---|
| Control Group (n = 20) | 4 | 12 | 4 | 0 |
| Severe PE Group (n = 20) | 1 | 5 | 12 | 2 |
Abbreviation: PE, preeclampsia.
Wnt2 and sFRP4 Protein Expression in Placental Tissues of the 2 Groups
Wnt2 and sFRP4 protein expressions in placental tissue were determined by Western blot, 20 from normal and 20 from PE placentas. β-Actin expression was used as an internal control for each sample. Western blot analysis demonstrated that the relative expression of Wnt2 in the severe PE group was significantly reduced compared to the control group (P = .037), while the expression of sFRP4 in the severe PE group was increased compared to the control group (P = .001; Figure 5).
Figure 5.
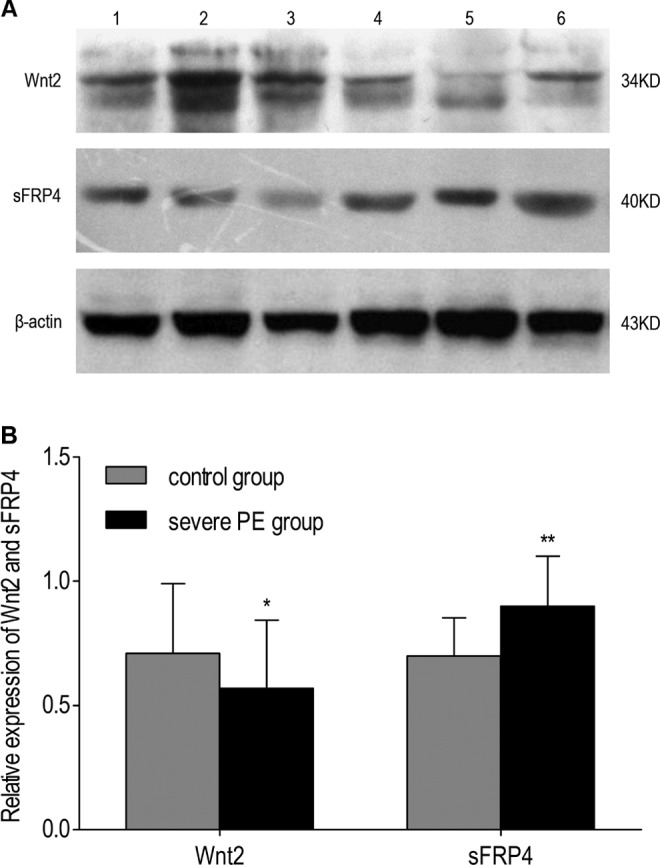
The protein levels of Wnt2 and sFRP4 in the placentas by Western blot. A, Representative examples of Wnt2 and sFRP4 in placentas from normal pregnancies and cases of severe preeclampsia, 1-3, normal control; 4-6, severe PE. B, Quantification of Wnt2 and sFRP4 Western blot data obtained from the 2 groups (n = 20 for each group). Bars represent the mean values; error bars indicate SD. *P value <.05; **P value <.01. PE indicates preeclampsia; SD, standard deviation; sFRP4, secreted frizzled-related protein 4.
Discussion
There are numerous etiological theories of PE, and its pathogenesis has not been elucidated. Maternal obesity at conception and high gestational weight gains have been implicated in the risk of pregnancy complications.28,29 The data from our study show that the prepregnancy BMI in the severe PE group was significantly higher than in the normal control group. Interestingly, no statistical differences were observed in the BMI at delivery between the 2 groups. The fact that the gestational age at delivery was lower in patients with severe PE than in women with normal pregnancies may explain this phenomenon.
In the present study, we identified the localization of the Wnt2 protein in the placental tissues using immunohistochemical staining. Here, we show that Wnt2 was predominantly expressed in the villous syncytiotrophoblast and the EVTs, whereas Wnt2 showed a stronger staining intensity in the control group than in the PE group. Wnt2 is a secreted glycoprotein that acts through autocrine or paracrine modes.30 The high level of expression of Wnt2 in the syncytiotrophoblast rather than the villous cytotrophoblast is consistent with the protein being secreted because the syncytiotrophoblast is directly exposed to the maternal blood. The EVTs are mainly present in the maternal–fetal interface of human placentas and are known as an invasive trophoblast subtype. Early in normal placental development, the EVTs invade the uterine spiral arteries of the deciduas and myometrium. These invasive trophoblasts replace the endothelial cells of the maternal spiral arteries, transforming them from high-resistance vessels to high-caliber capacitance vessels capable of providing adequate placental perfusion. In the PE placenta, the invasion of EVTs into the maternal decidua and myometrium is shallow and limited, and endovascular invasion is nearly absent.4 The decreased expression of Wnt2 in placentas may have certain correlations with PE, whether Wnt2 regulated EVT invasion requires further functional experiments.
In this report, we also demonstrated that the Wnt2 mRNA and protein levels could be detected in human third trimester placentas by qRT-PCR and Western blot. Sonderegger et al also reported that Wnt2 mRNA is expressed in the human placenta and different trophoblast model systems.8 In mice, various Wnt genes and their secreted antagonists have been detected in the blastocysts during implantation and postimplantation development.31 Accordingly, disrupting the Wnt genes results in different phenotypes of mutant embryos and an impaired frequency of implantation, indicating the diverse functions of Wnt genes during embryogenesis and placental development. Wnt2-deficient mice display placental defects.9 The consistent expression of Wnt2 in the placenta in the rat and human suggests that it has an important, evolutionarily conserved function. We show here that compared to the normal pregnancies, the Wnt2 transcript and protein levels were strikingly decreased in the PE placentas. We speculated that the decrease in Wnt2 expression in the placenta may influence the biological functions of trophoblast cells and the development of PE.
In the placentas, sFRP4 was mainly observed in the syncytiotrophoblasts and EVTs; these observations were consistent with a previous report by White et al32 who found that sFRP4 appears to be associated with apoptosis in trophoblasts. Here, we show that the staining intensity of sFRP4 in the severe PE group is stronger than in the control group. Furthermore, the Western blot results were consistent with IHC. Real-time PCR analysis was performed to investigate the sFRP4 mRNA level in severe PE placentas and normal controls. However, no significant difference was detected between the 2 groups. The transcriptional level of sFRP4 is not consistent with the translational level in our study. There may be 2 reasons, the changes in the protein levels were delayed compared to changes in the mRNA expression or regulations in the posttranscriptional and translational levels. It was reported that sFRP4 is expressed in the pregnant rat uterus, but not in the nonpregnant uterus; the expression of sFRP4 mRNA reached a peak in the uterus on day 12 and then declined.33 It was also reported that sFRP4 mRNA was produced throughout gestation in the macaque placenta; the expression of sFRP4 mRNA was highest on day 50 of gestation (the duration of gestation in the macaque is 165 days).32 Data from the above-mentioned research suggest that the sFRP4 mRNA levels declined in the uterus (rat) and placenta (macaque) with gestational age in the mid-late pregnancy. We speculated that the mean gestational week at delivery of patients with severe PE being lower than the normal pregnant women in this research may be another explanation for the inconsistency between the mRNA and the protein levels of sFRP4. Previous studies have shown that sFRP4 has antiproliferative, proapoptotic, and antiangiogenic properties. In PE, the size of the placenta is usually small, and apoptosis is increased. So, we speculate that the sFRP4 mRNA in the PE placentas may be higher than the controls when there was no difference in the gestational age, and further research is needed.
In conclusion, Wnt2 and sFRP4 are expressed in human third trimester placentas, and the abnormal expression of Wnt2 and sFRP4 may be associated with the pathogenesis of PE. However, we only examined the third trimester placentas due to limitations in human placental studies. Studies of the expression patterns of the Wnt signaling pathways in trophoblast cells of different gestational ages and their roles in complicated pregnancies are warranted to validate our findings.
Footnotes
Authors’ Note: This study was completed in the Research Center of The Third Affiliated Hospital of Zhengzhou University.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: the Innovative Talents Foundation of Henan Province (grant number 20121).
References
- 1. Beaufils M. Pregnancy hypertension. Nephrol Ther. 2010;6(3):200–214. [DOI] [PubMed] [Google Scholar]
- 2. Wang A, Rana S, Karumanchi SA. Preeclampsia: the role of angiogenic factors in its pathogenesis. Physiology (Bethesda). 2009;24(3):147–158. [DOI] [PubMed] [Google Scholar]
- 3. Roberts JM, Escudero C. The placenta in preeclampsia. Pregnancy Hypertens. 2012;2(2):72–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pennington KA, Schlitt JM, Jackson DL, Schulz LC, Schust DJ. Preeclampsia: multiple approaches for a multifactorial disease. Dis Model Mech. 2012;5(1):9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sonderegger S, Pollheimer J, Knofler M. Wnt signalling in implantation, decidualisation and placental differentiation--review. Placenta. 2010;31(10):839–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fitzgerald JS, Germeyer A, Huppertz B, et al. Governing the invasive trophoblast: current aspects on intra- and extracellular regulation. Am J Reprod Immunol. 2010;63(6):492–505. [DOI] [PubMed] [Google Scholar]
- 7. Sonderegger S, Haslinger P, Sabri A, et al. Wingless (Wnt)-3A induces trophoblast migration and matrix metalloproteinase-2 secretion through canonical Wnt signaling and protein kinase B/AKT activation. Endocrinology. 2010;151(1):211–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sonderegger S, Husslein H, Leisser C, Knofler M. Complex expression pattern of Wnt ligands and frizzled receptors in human placenta and its trophoblast subtypes. Placenta. 2007;28(suppl A):S97–S102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Miller JR. The Wnts. Genome Biol. 2002;3(1):REVIEWS3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Turashvili G, Bouchal J, Burkadze G, Kolar Z. Wnt signaling pathway in mammary gland development and carcinogenesis. Pathobiology. 2006;73(5):213–223. [DOI] [PubMed] [Google Scholar]
- 11. Katoh M. WNT2 and human gastrointestinal cancer (review). Int J Mol Med. 2003;12(5):811–816. [PubMed] [Google Scholar]
- 12. Fu L, Zhang C, Zhang LY, et al. Wnt2 secreted by tumour fibroblasts promotes tumour progression in oesophageal cancer by activation of the Wnt/beta-catenin signalling pathway. Gut. 2011;60(12):1635–1643. [DOI] [PubMed] [Google Scholar]
- 13. Mazieres J, You L, He B, et al. Wnt2 as a new therapeutic target in malignant pleural mesothelioma. Int J Cancer. 2005;117(2):326–332. [DOI] [PubMed] [Google Scholar]
- 14. Park JK, Song JH, He TC, Nam SW, Lee JY, Park WS. Overexpression of Wnt-2 in colorectal cancers. Neoplasma. 2009;56(2):119–123. [DOI] [PubMed] [Google Scholar]
- 15. Wang GX, Zhang ZY, Pu PY, et al. Expression of core components of Wnt2 signaling pathway in gliomas. Zhonghua Bing Li Xue Za Zhi. 2009;38(7):481–482. [PubMed] [Google Scholar]
- 16. Watanabe O, Imamura H, Shimizu T, et al. Expression of twist and wnt in human breast cancer. Anticancer Res. 2004;24(6):3851–3856. [PubMed] [Google Scholar]
- 17. Kuster K, Koschel A, Rohwer N, Fischer A, Wiedenmann B, Anders M. Downregulation of the coxsackie and adenovirus receptor in cancer cells by hypoxia depends on HIF-1alpha. Cancer Gene Ther. 2010;17(2):141–146. [DOI] [PubMed] [Google Scholar]
- 18. You L, He B, Xu Z, et al. An anti-Wnt-2 monoclonal antibody induces apoptosis in malignant melanoma cells and inhibits tumor growth. Cancer Res. 2004;64(15):5385–5389. [DOI] [PubMed] [Google Scholar]
- 19. Gong YD, Dong MS, Lee SB, Kim N, Bae MS, Kang NS. A novel 3-arylethynyl-substituted pyrido[2,3,-b]pyrazine derivatives and pharmacophore model as Wnt2/beta-catenin pathway inhibitors in non-small-cell lung cancer cell lines. Bioorg Med Chem. 2011;19(18):5639–5647. [DOI] [PubMed] [Google Scholar]
- 20. Klein D, Demory A, Peyre F, et al. Wnt2 acts as an angiogenic growth factor for non-sinusoidal endothelial cells and inhibits expression of stanniocalcin-1. Angiogenesis. 2009;12(3):251–265. [DOI] [PubMed] [Google Scholar]
- 21. Monkley SJ, Delaney SJ, Pennisi DJ, Christiansen JH, Wainwright BJ. Targeted disruption of the Wnt2 gene results in placentation defects. Development. 1996;122(11):3343–3353. [DOI] [PubMed] [Google Scholar]
- 22. Bafico A, Gazit A, Pramila T, Finch PW, Yaniv A, Aaronson SA. Interaction of frizzled related protein (FRP) with Wnt ligands and the frizzled receptor suggests alternative mechanisms for FRP inhibition of Wnt signaling. J Biol Chem. 1999;274(23):16180–16187. [DOI] [PubMed] [Google Scholar]
- 23. Carmon KS, Loose DS. Secreted frizzled-related protein 4 regulates two Wnt7a signaling pathways and inhibits proliferation in endometrial cancer cells. Mol Cancer Res. 2008;6(6):1017–1028. [DOI] [PubMed] [Google Scholar]
- 24. Jacob F, Ukegjini K, Nixdorf S, et al. Loss of secreted frizzled-related protein 4 correlates with an aggressive phenotype and predicts poor outcome in ovarian cancer patients. PLoS One. 2012;7(2):e31885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Castro M, Grau L, Puerta P, et al. Multiplexed methylation profiles of tumor suppressor genes and clinical outcome in lung cancer. J Transl Med. Sep 2010;8:86-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hewitt DP, Mark PJ, Dharmarajan AM, Waddell BJ. Placental expression of secreted frizzled related protein-4 in the rat and the impact of glucocorticoid-induced fetal and placental growth restriction. Biol Reprod. 2006;75(1):75–81. [DOI] [PubMed] [Google Scholar]
- 27. Muley A, Majumder S, Kolluru GK, et al. Secreted frizzled-related protein 4: an angiogenesis inhibitor. Am J Pathol. 2010;176(3):1505–1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tsai IH, Chen CP, Sun FJ, Wu CH, Yeh SL. Associations of the pre-pregnancy body mass index and gestational weight gain with pregnancy outcomes in Taiwanese women. Asia Pac J Clin Nutr. 2012;21(1):82–87. [PubMed] [Google Scholar]
- 29. Marshall NE, Guild C, Cheng YW, Caughey AB, Halloran DR. Maternal superobesity and perinatal outcomes. Am J Obstet Gynecol. 2012;206(5):417 e411–e416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Smolich BD, McMahon JA, McMahon AP, Papkoff J. Wnt family proteins are secreted and associated with the cell surface. Mol Biol Cell. 1993;4(12):1267–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kemp C, Willems E, Abdo S, Lambiv L, Leyns L. Expression of all Wnt genes and their secreted antagonists during mouse blastocyst and postimplantation development. Dev Dyn. 2005;233(3):1064–1075. [DOI] [PubMed] [Google Scholar]
- 32. White L, Suganthini G, Friis R, Dharmarajan A, Charles A. Expression of secreted frizzled-related protein 4 in the primate placenta. Reprod Biomed Online. 2009;18(1):104–110. [DOI] [PubMed] [Google Scholar]
- 33. Fujita M, Ogawa S, Fukuoka H, et al. Differential expression of secreted frizzled-related protein 4 in decidual cells during pregnancy. J Mol Endocrinol. 2002;28(3):213–223. [DOI] [PubMed] [Google Scholar]