Abstract
A critical role of proinflammatory mediators including cytokines, prostaglandins, and extracellular matrix remodeling enzymes in the processes of human labor and delivery, at term and preterm, has been demonstrated. In nongestational tissues, apelin plays an important role in a number of physiologic processes, including the regulation of inflammation. However, the role and regulation of apelin and the apelin receptor (APJ) in human gestational tissues are not known. The aims of this study were to determine the effect of (i) preterm and term labor on apelin and APJ expression in human gestational tissues and (ii) apelin small interfering RNA (siRNA) knockdown in human primary amnion cells on prolabor mediators. Human placenta and fetal membranes were collected from term nonlaboring women and women after spontaneous labor and delivery. Preterm and term spontaneous labor were associated with significantly lower apelin expression in fetal membranes. On the other hand, there was no effect of labor on APJ expression and no effect of term labor on placental apelin or APJ expression. Transfection of primary amnion cells with apelin siRNA was associated with significantly increased interleukin (IL)-1β-induced IL-6 and IL-8 release and cyclooxygenase-2 messenger RNA (mRNA) expression and resultant prostaglandin E2 and prostaglandin F2α release. There was no effect of apelin siRNA on matrix metalloproteinase (MMP)-9 mRNA expression and pro MMP-9 release. In summary, human labor downregulates apelin expression in human fetal membranes. Furthermore, a role of apelin in the regulation of proinflammatory and prolabor mediators in human fetal membranes is supported by our studies.
Keywords: apelin, human labor, fetal membranes, cytokines, prostaglandins
Introduction
The single most important complication contributing to poor pregnancy and neonatal outcome is premature birth, accounting for 75% of perinatal mortality and more than 50% of long-term morbidity.1,2 Together with the fiscal costs of caring for the affected infants in neonatal intensive care units, these deficits impose a serious burden on health systems, families, and society at large.3 Improving our understanding of the underlying mechanisms of human labor is crucial in developing effective treatments to reduce the incidence of preterm labor and thus improve neonatal outcome.
The same terminal events occur in term or preterm labor, myometrial contractility, cervical ripening, and decidual/membrane activation. Preterm birth occurs when these effector pathways are induced irrespective of fetal development. Examples are the activation of effector pathways as a consequence of intrauterine infection, uteroplacental ischemia, or excessive myometrial distension.4 Evidence shows an important role in the formation of contraction associated proteins; inflammatory mediators (eg, cytokines); phospholipid metabolites (eg, prostaglandins); and the induction of extracellular matrix (ECM) remodeling in the processes of human labor, both at term and preterm.5–9
In nongestational tissues, there is some evidence to show that apelin regulates inflammation.10–13 Apelin is the endogenous ligand of the orphan G-protein-coupled receptor, APJ14,15 that is widely expressed in humans.16,17 The apelin gene encodes a 77-amino acid preproprotein that is processed to generate bioactive peptides consisting of 36, 17, or 13 amino acids (apelin 36, apelin 17, and apelin 13, respectively).14,18 Apelin is an angiogenic factor required for normal blood vessel growth and endothelial cell proliferation.19 However, apelin and APJ have also been detected in avascular cells, including intestinal epithelial cells,20 suggesting functional roles distinct from regulation of vascular function. More recently, apelin has been identified in human placenta,21,22 and high concentrations have been demonstrated in umbilical plasma samples.23 However, surprisingly little is known about the role of the apelin/APJ system in human pregnancy.
To our knowledge, the expression and regulation of apelin and APJ and the functions of apelin in human gestational tissues have not been published. In this study, the effect of human labor, at preterm and term, on apelin and APJ expression will be investigated. Further, we will use apelin small interfering RNA (siRNA) knockdown in primary amnion cells to determine its effects on interleukin (IL)-1β-induced cytokine, prostaglandin, and protease expression and release.
Materials and Methods
Tissue Collection
Human placenta and attached fetal membranes were obtained (with the Research Ethics Committee of Mercy Health and Aged Care approval) from consenting women who were of normal body mass index (BMI), 20 to 25 kg/m2, at their first antenatal visit and delivered healthy, singleton infants at preterm and term. Tissues were obtained within 15 minutes of delivery.
Term Studies
The groups were (i) term before labor undergoing elective cesarean section (indications for cesarean section were breech presentation and/or previous cesarean section; n = 6 patients) and (ii) term after spontaneous labor, spontaneous membrane rupture, and normal vaginal delivery (n = 6 patients). Clinical details of the patients are detailed elsewhere.24 The mean gestational age at birth for the nonlaboring groups was 38.7 ± 0.2 weeks and for the after labor group it was 39.3 ± 0.3 weeks.
Placental lobules (cotyledons) were obtained from various locations of the placenta; the basal plate and chorionic surface were removed from the cotyledon, and villous tissue was obtained from the middle cross section. Placental tissue was blunt dissected to remove visible connective tissue and calcium deposits. For the term labor study, fetal membranes from the nonlaboring group, samples were obtained from the supracervical site (SCS). Identification of the SCS was performed as we have previously detailed.24,25 Briefly, Bonneys blue dye was introduced through the cervix prior to cesarean section. Upon delivery of the placenta, a blue mark was obvious on the chorion facing membrane where the dye had been applied. In the after labor group, fetal membranes from the site of membrane rupture as we have previously described24; amnion and underlying choriodecidua were collected from along the line of fetal membrane rupture. For these samples, hematoxylin and eosin was used to confirm the absence of decidua.
Preterm Studies
The groups were (i) preterm no labor: cesarean section with no labor (n = 9) and (ii) preterm labor: after spontaneous labor and normal vaginal delivery (n = 8). All placentas collected from preterm gestations were swabbed for microbiological culture investigations and histopathological examination. Patients with chorioamnionitis were excluded from the analyses. Women with preeclampsia, preexisting diabetes, asthma, multiple pregnancies, and fetuses with chromosomal abnormalities were also excluded. Indications for preterm delivery (in the absence of labor) were placenta previa, placental abruption, antepartum hemorrhage, or Rhesus isoimmunization. For the preterm labor study, fetal membranes from both the nonlaboring and after labor preterm groups were obtained 2 cm from the periplacental edge.
Tissue samples were fixed and paraffin embedded for immunohistochemical analysis or snap frozen in liquid nitrogen and immediately stored at −80°C for apelin and APJ messenger RNA (mRNA) and protein analysis by qualitative reverse transcriptase polymerase chain reaction (qRT-PCR) and Western blotting.
Immunohistochemistry
To determine the localization of apelin and APJ in placenta and fetal membranes, immunohistochemistry was performed as described previously.26 Mouse monoclonal anti-apelin (WH0008862M1; Sigma-Aldrich, St Louis, Missouri) and rabbit polyclonal anti-APJ (SAB4500683, Sigma-Aldrich) were used at 0.5 and 1 μg/mL concentrations, respectively. Negative control slides, where primary antibody was replaced with normal rabbit or mouse immunoglobulin G serum, were also included.
Gene Silencing of Apelin With siRNA in Primary Amnion Cells
Primary amnion epithelial cells were used to investigate the effect of siRNA-mediated gene silencing of apelin on prolabor mediators. For these studies, amnion was obtained from women who delivered healthy, singleton infants at term (between 38 and 39.3 weeks of gestation) undergoing elective cesarean section, in the absence of labor. Amnion was obtained approximately 2 cm from the periplacental edge. Primary amnion epithelial cells were prepared as previously described27,28 and incubated in Dulbecco Modified Eagle Medium (DMEM)/F12 enriched with 10% fetal calf serum, 100 U/mL penicillin G, and 100 μg/mL streptomycin. Cells at approximately 50% confluence were transfected using SilenceMag reagent according to manufacturer guidelines (Oz Biosciences, France). Cells were transfected with 200 nmol/L apelin (siRNA ID: s16926) or nonspecific siRNA (Ambion, Austin, Texas) in DMEM for 72 hours. The medium was then replaced with DMEM with or without 1 ng/mL IL-1β,27,29,30 and the cells were incubated at 37°C for an additional 24 hours. The cells were collected and stored at −80°C until assayed for mRNA expression by qRT-PCR and protein expression by Western blotting as detailed below. Media was collected and stored at −80°C until assayed for cytokine, prostaglandin, and matrix metalloproteinase (MMP) release as detailed below. Six independent experiments were performed.
RNA Extraction and qRT-PCR
Total RNA was extracted from cells and tissues using TriReagent according to manufacturer’s instructions (Sigma-Aldrich). RNA concentration and purity were measured using a NanoDrop ND1000 spectrophotometer (Thermo Scientific, Pittsburgh, Pennsylvania). RNA quality and integrity were determined via the A260/A280 ratio and agarose gel electrophoresis. RNA (1 μg for tissue and 0.25 μg for cells) was converted to complementary DNA (cDNA) using the SuperScript VILO cDNA synthesis kit (Invitrogen, Carlsbad, California) according to the manufacturer’s instructions. The cDNA was diluted 50-fold, and 4 μL of this was used to perform RT-PCR using Sensimix Plus SYBR green (Quantace, Alexandria, New South Wales, Australia) and 100 nmol/L of predesigned and validated primers (QuantiTect Primer Assays, Qiagen, Germantown, Maryland). The primers were apelin (GT0004775), APJ (QT00221592), glyceraldehyde-3-phosphate dehydrogenase (GAPDH; QT01192646), interleukin (IL)-6 (QT00083720), IL-8 (QT00000322), cyclooxygenase (COX)-2 (QT00040586), and MMP-9 (QT00040040). The RT-PCR was performed using a CFX384 real-time PCR detection system from Bio-Rad Laboratories (Hercules, California). Average gene C T values were normalized to the average GAPDH Ct values of the same cDNA sample. Fold differences were determined using the comparative C T method and expressed relative to IL-1β-treated controls.
Western Blotting
Assessment of protein expression was analyzed by Western blotting. Forty micrograms of protein were separated onto 10% polyacrylamide gels (Bio-Rad Laboratories) and transferred to polyvinylidene difluoride. Blots were incubated with 1 μg/mL mouse monoclonal anti-apelin (WH0008862M1, Sigma-Aldrich) and 2 μg/mL rabbit polyclonal anti-APJ (SAB4500683, Sigma-Aldrich) diluted in blocking buffer (5% skim milk/Tris-buffered saline and Tween 20 [0.05%]) for 24 hours at 4°C. Membranes were viewed and analyzed using the Chemi-Doc system (Bio-Rad). Semiquantitative analysis of the relative density of the bands in Western blots was performed using Quantity One 4.2.1 image analysis software (Bio-Rad). Data were corrected for background, normalized to β-actin expression, and expressed as the ratio of the controls.
Cytokine and Prostaglandin Assays
The release of IL-6 and IL-8 was performed by sandwich enzyme-linked immunosorbent assay according to the manufacturer’s instructions (Invitrogen). The release of prostaglandin E2 (PGE2) and PGF2α into the incubation medium was assayed using a commercially available competitive enzyme immunoassay kit according to the manufacturer’s specifications (Kookaburra Kits from Sapphire Bioscience, Redfern, New South Wales, Australia). Results were normalized to total protein using bicinchoninic acid protein assay (Pierce, Rockford, Illinois) and expressed relative to IL-1β-treated controls.
Gelatin Zymography
Assessment of enzymes of ECM weakening and rupture (MMP-9) was performed by gelatin zymography as previously described.31,32 Gels were viewed and analyzed using the Chemi-Doc system. Quantitative analysis of the relative density was performed using Quantity One 4.2.1 image analysis software. Data were expressed relative to IL-1β-treated controls.
Statistical Analysis
All statistical analyses were done using GraphPad Prism (GraphPad Software, La Jolla, California). Two sample comparisons in Figure 2 were analyzed by a Student t test. For Figures 3 to 5, the data were normalized to each respective patient’s control as the response to IL-1β between patients varied greatly. Statistics was performed on the normalized data unless otherwise specified. Analysis was performed using a 1-way analysis of variance (using least significant difference correction to discriminate among the means). Statistical significance was ascribed to P value <.05. Data were expressed as mean ± standard error of the mean.
Figure 2.
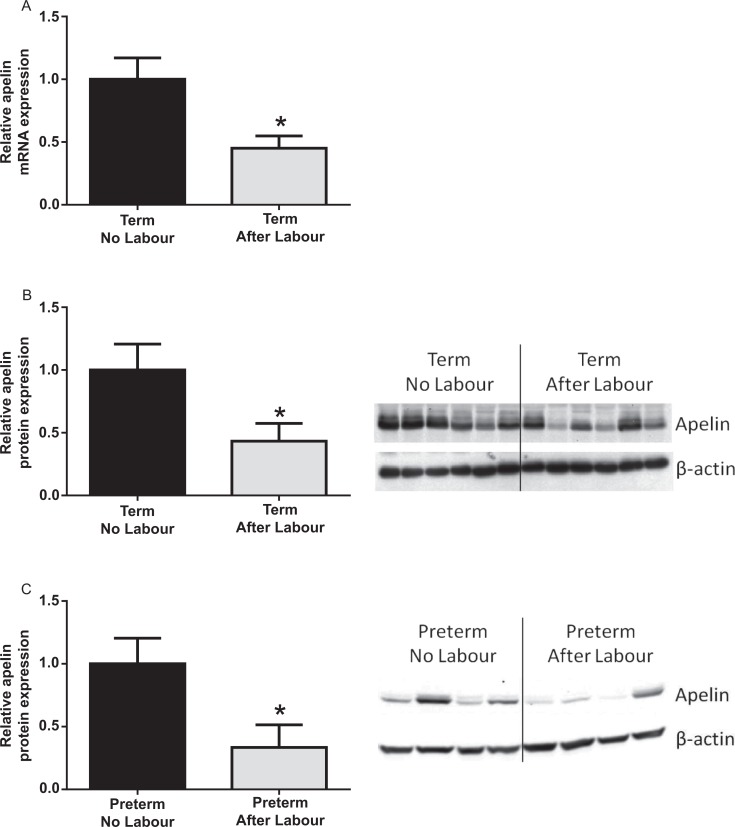
Effect of human labor on apelin expression in human fetal membranes. A and B, Human fetal membranes were obtained from women not in labor at term cesarean section and women after term spontaneous labor onset and delivery (n = 6 per group). A, Gene expression was analyzed by qRT-PCR and normalized to GAPDH mRNA expression. Relative apelin mRNA expression is displayed as mean ± SEM. *P < .05 versus no labor (Student t test).B, Western blot and quantitation for apelin. Expression levels were confirmed by densitometry. Data were displayed as the mean ± SEM. *P < .05 versus no labor (Student t test). C, Human fetal membranes were obtained from preterm nonlaboring women (n = 9) and women after spontaneous preterm labor and delivery (n = 8). Apelin protein expression was analyzed by Western blotting, and displayed as apelin expression normalized to β-actin loading control. *P < .05 versus no labor (Student t test). Representative Western blot of 4 patients per group is shown. GAPDH indicates glyceraldehyde-3-phosphate dehydrogenase; mRNA, messenger RNA; qRT-PCR, qualitative reverse transcriptase polymerase chain reaction; SEM, standard error of the mean.
Figure 3.
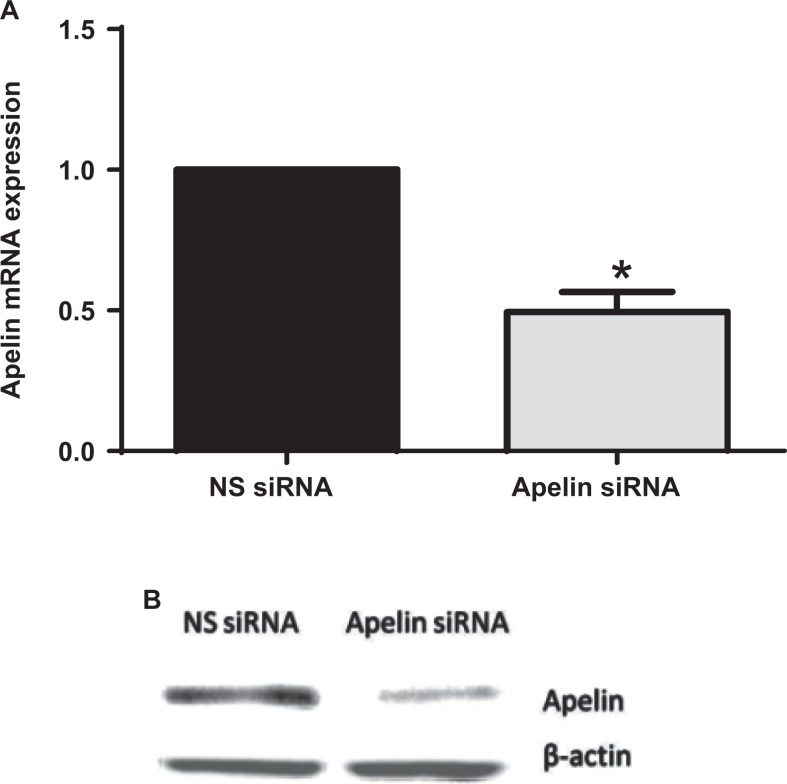
Efficiency of apelin knockdown in primary amnion cells. Human primary amnion cells were transfected with or without 200 nmol/L apelin or NS siRNA (n = 6 patients) for 96 hours. A, Apelin mRNA expression was normalized to GAPDH mRNA expression and the fold change was calculated relative to NS siRNA transfected cells. Data were displayed as mean ± SEM. *P < .05 versus *P < .05 versus NS siRNA (paired sample comparison). B, Representative Western blot for apelin. GAPDH indicates glyceraldehyde-3-phosphate dehydrogenase; mRNA, messenger RNA; NS siRNA, nonspecific small interfering RNA; SEM, standard error of the mean.
Figure 4.
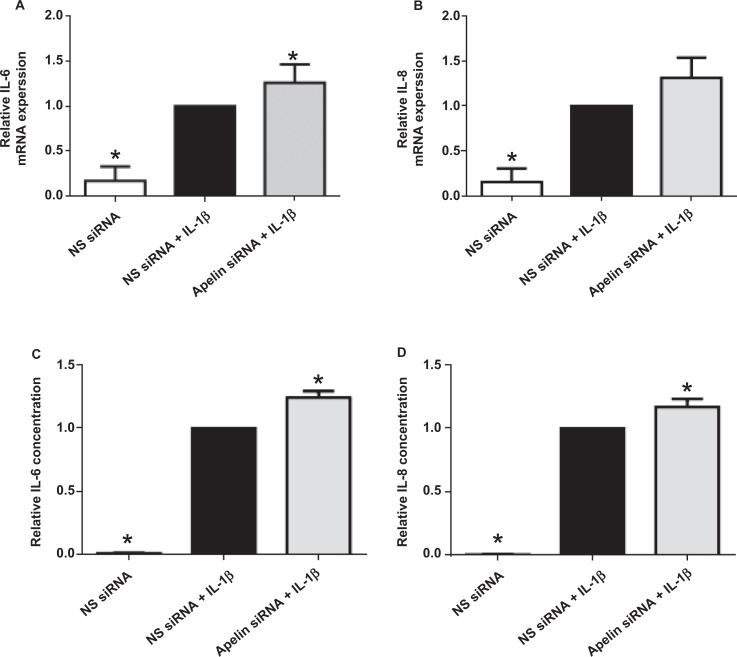
Effect of apelin knockdown on proinflammatory cytokines. Human primary amnion cells were transfected with 200 nmol/L apelin or NS siRNA. After 72 hours of transfection, cells were incubated in the absence or presence of 1 ng/mL of IL-1β for 24 hours (n = 6 patients). A and B, IL-6 and IL-8 gene expression was analyzed by qRT-PCR, normalized to GAPDH mRNA expression and the fold change was calculated relative to IL-1β stimulated NS siRNA transfected cells. Data were displayed as mean ± SEM. *P < .05 versus IL-1β stimulated NS siRNA transfected cells (1-way ANOVA). C and D, IL-6 and IL-8 concentration in the incubation medium was analyzed by ELISA and the fold change was calculated relative to IL-1β stimulated NS siRNA transfected cells. Each bar represents the mean ± SEM. *P < .05 versus IL-1β stimulated NS siRNA transfected cells (1-way ANOVA). ANOVA indicates analysis of variance; ELISA, enzyme-linked immunosorbent assay; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; IL, interleukin; mRNA, messenger RNA; NS siRNA, nonspecific small interfering RNA; qRT-PCR, qualitative reverse transcriptase polymerase chain reaction; SEM, standard error of the mean.
Figure 5.
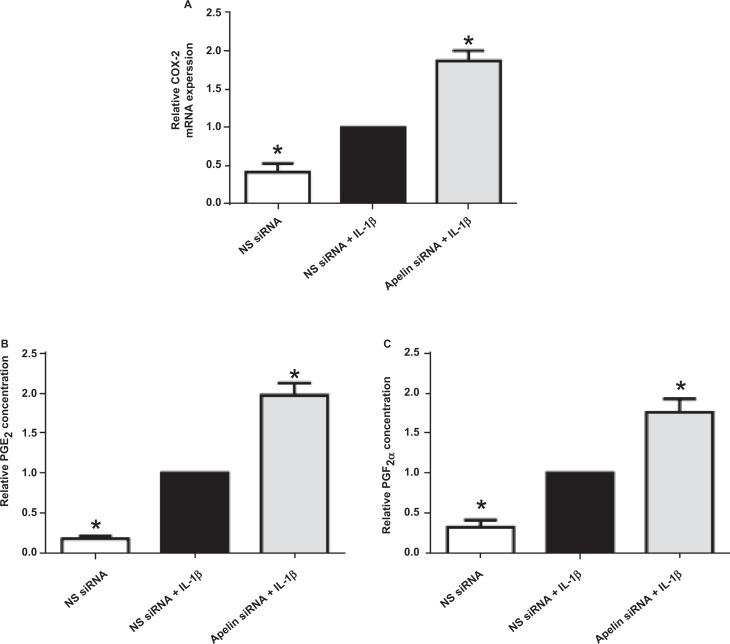
Effect of apelin knockdown on COX-2-prostaglandin pathway. Human primary amnion cells were transfected with 200 nmol/L of apelin or NS siRNA. After 72 hours of transfection, cells were incubated in the absence or presence of 1 ng/mL of IL-1β for 24 hours (n = 6 patients). A, COX-2 gene expression was analyzed by qRT-PCR, normalized to GAPDH mRNA expression and the fold change was calculated relative to IL-1β stimulated NS siRNA transfected cells. Data were displayed as mean ± SEM. *P < .05 versus IL-1β stimulated NS siRNA transfected cells (1-way ANOVA). B and C, PGE2 and PGF2α concentrations in the incubation medium were analyzed by EIA and the fold change was calculated relative to IL-1β stimulated NS siRNA transfected cells. Each bar represents the mean ± SEM. *P < .05 versus IL-1β-stimulated NS siRNA transfected cells (1-way ANOVA). ANOVA indicates analysis of variance; COX-2, cyclooxygenase 2; EIA, enzyme immunoassay; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; IL, interleukin; mRNA, messenger RNA; NS siRNA, nonspecific small interfering RNA; PGE2, prostaglandin E2; PGF2α, prostaglandin F2α; qRT-PCR, qualitative reverse transcriptase polymerase chain reaction; SEM, standard error of the mean.
Results
Localization of Apelin and APJ in Placenta and Fetal Membranes
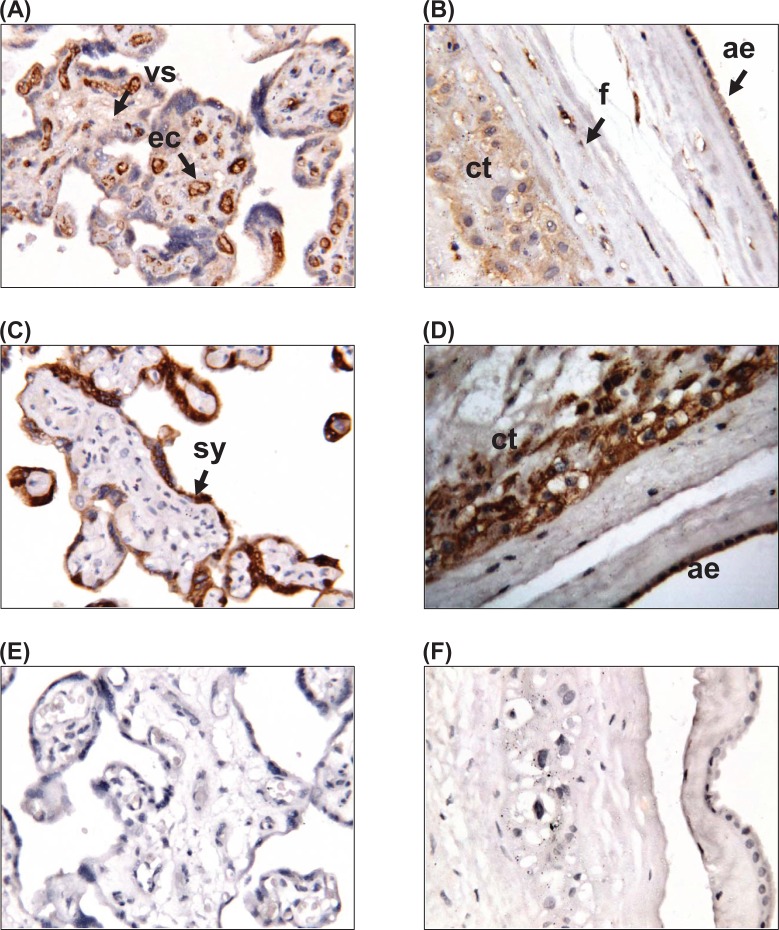
Immunohistochemistry was used to localize apelin and APJ expression in human placenta and fetal membranes at term (Figure 1). Apelin was expressed in the villous stroma and the endothelial cells of the placenta, with no staining observed in the syncytiotrophoblast layer (Figure 1A). In the fetal membranes, apelin was localized to the chorionic cytotrophoblasts, amnion epithelium, and fibroblasts cells of the connective tissue layer (Figure 1B). Placenta exhibited APJ staining only in the syncytiotrophoblast layer; APJ was not present within the villous stroma or endothelial cells (Figure 1C). In the fetal membranes, APJ was expressed in the chorionic cytotrophoblasts and amnion epithelium (Figure 1D). No staining was present in the negative controls in placenta (Figure 1E) and fetal membranes (Figure 1F).
Figure 1.
Localization of apelin and its receptor APJ in human placenta and fetal membranes. Immunohistochemical localization of (A, B) apelin and (C, D) APJ in (A, C) placenta and (B, D) fetal membranes. (E, F) Negative controls for (E) placenta and (F) fetal membranes. Magnification ×250. Sy indicates syncytiotrophoblast cells; cy, cytotrophoblast cells; ec, endothelial cells; ae, amnion epithelium; cl, connective tissue layer; ct, chorionic trophoblast layer; dec, decidua.
Effect of Human Term Labor on Apelin and APJ Expression in Placenta and Fetal Membranes
To determine the effect of human term labor on apelin and APJ gene and protein expression, qRT-PCR and Western blotting were performed on tissues obtained before and after term labor. Human placenta and fetal membranes (n = 8) were obtained at cesarean section before the onset of labor (no labor) and after spontaneous labor and membrane rupture (after labor). In placenta, the expression of apelin and APJ was not different among the 2 groups (data not shown). However, in fetal membranes, when compared to nonlaboring tissues, apelin gene (Figure 2A) and protein (Figure 2B) expressions were significantly lower after spontaneous labor at term. There was, however, no difference in APJ mRNA and protein expression in fetal membranes obtained from nonlaboring and laboring tissues (data not shown).
Effect of Spontaneous Preterm Labor on Apelin and APJ Expression in Fetal Membranes
Given that term labor significantly decreased apelin expression in fetal membranes, we next sought to examine the effect of spontaneous preterm labor and delivery on apelin and APJ expression. Fetal membranes were obtained from women with (i) preterm no labor, cesarean section with no labor (n = 9) and (ii) preterm labor, after spontaneous labor and normal vaginal delivery (n = 9). Women with preterm deliveries were matched for maternal age, BMI, gravidity, parity, and gestational age. Fetal birth weight was significantly higher in the preterm labor cohort compared to preterm no labor. Western blotting was used to examine the effect of human preterm labor on apelin and APJ protein expression. When compared to fetal membranes from the nonlaboring groups, apelin protein expression was significantly lower after preterm spontaneous labor and delivery (Figure 2C). There was, however, no difference in APJ protein expression obtained from nonlaboring and laboring preterm fetal membranes (data not shown).
Effect of Apelin siRNA Knockdown in Primary Amnion Cells on the Expression of Prolabor Mediators
Having shown that apelin expression is lower after labor, we next sought to determine whether apelin regulates prolabor mediators in fetal membranes. To achieve this, we utilized primary amnion cells where apelin was knocked down by siRNA transfection. Figure 3 demonstrates the effect of apelin siRNA at the mRNA and protein level.
Figures 4 to 6 illustrate the effect of apelin siRNA on IL-1β-induced pro-inflammatory and pro-labor gene expression and release from primary amnion cells. There was no significant effect of apelin knockdown on cytokine gene expression (Figure 4A and B). On the other hand, IL-1β-induced IL-6 and IL-8 release was significantly increased in apelin siRNA transfected cells (Figure 4C and D). Apelin siRNA significantly augmented IL-1β-induced COX-2 mRNA expression (Figure 5A) and resultant PGE2 (Figure 5B) and PGF2α (Figure 5C) levels.
Figure 6.
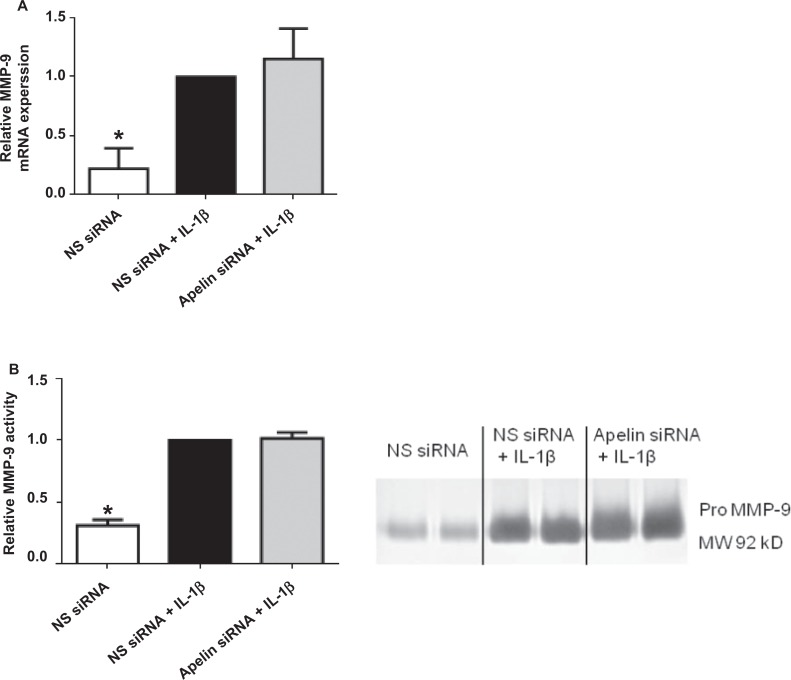
Effect of apelin knockdown on MMP-9. Human primary amnion cells were transfected with 200 nmol/L apelin or NS siRNA. After 72 hours of transfection, cells were incubated in the absence or presence of 1 ng/mL IL-1β for 24 hours (n = 6 patients). A, MMP-9 gene expression was analyzed by qRT-PCR, normalized to GAPDH mRNA expression and the fold change was calculated relative to IL-1β stimulated NS siRNA transfected cells. Data were displayed as mean ± SEM. *P < .05 versus IL-1β stimulated NS siRNA transfected cells (1-way ANOVA). B, Representative zymography and quantitation for pro MMP-9. Expression levels were confirmed by densitometry. The fold change was calculated relative to IL-1β stimulated NS siRNA transfected cells. Each bar represents the mean ± SEM. *P < .05 versus IL-1β stimulated NS siRNA transfected cells (1-way ANOVA). ANOVA indicates analysis of variance; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; IL, interleukin; MMP, metalloproteinase; mRNA, messenger RNA; NS siRNA, nonspecific small interfering RNA; qRT-PCR, qualitative reverse transcriptase polymerase chain reaction; SEM, standard error of the mean.
Assessment for enzymes of ECM weakening and rupture (ie, MMP-9) was performed by qRT-PCR (Figure 6A) and gelatin zymography (Figure 6B). The IL-1β significantly induced the gene expression and protein activity of MMP-9; however, there was no effect of apelin knockdown on MMP-9 expression (Figure 6B). Additionally, there was, however, no effect of IL-1β or apelin siRNA on MMP-2 expression and release (data not shown).
Discussion
Apelin has been reported to play a role in a number of physiological and pathological processes. However, although apelin and APJ are constitutively expressed in human placenta,21 there is a paucity of data on the role and regulation of apelin in human gestational tissues. In this article, we report that human preterm and term labor significantly decreased apelin gene expression, but had no effect on APJ expression. Furthermore, an anti-inflammatory role of apelin in the regulation of prolabor mediators was demonstrated in this study. Apelin inhibition, using siRNA, was associated with a modest, but significant increase in the release of proinflammatory cytokines from primary amnion cells. In addition, knockdown of apelin in primary amnion cells was associated with significantly increased COX-2 mRNA expression and subsequent prostaglandin release.
In this study, we confirm the localization of apelin and APJ in human placenta; apelin was present in the vilous stroma and endothelial cells, but not in syncytiotrophoblasts, while APJ was expressed in syncytiotrophoblasts but not the villous stroma or endothelial cells. Cobellis et al reported that apelin was present in placental syncytiotrophoblasts, but this decreased with gestation, with the greater amount of apelin expression in placenta in the first trimester.21 We also report, for the first time, their expression in human fetal membranes. Apelin and APJ were mainly expressed in the chorionic cytotrophoblasts and amnion epithelium.
Pan and colleagues recently reported lower circulating apelin and decreased apelin and APJ mRNA levels in myocardia of rats with sepsis by attenuating inflammatory responses.12 This led to our hypothesis that apelin and APJ expression in human gestational tissues may be downregulated by labor. In support of this original hypothesis, we show that both preterm and term spontaneous labor significantly decreased apelin gene expression in fetal membranes. This suggests that apelin may play a role in the processes of human labor and delivery. Further to this, in primary amnion cells, we found that knockdown of apelin was associated with significantly increased release of the proinflammatory cytokine IL-6, the chemokine IL-8, and COX-2 mRNA expression and subsequent prostaglandin release. Of note, we found that there was a lack of regulation for cytokines at the mRNA level, perhaps suggesting a role for APJ. Alternatively, it may be possible that shorter incubation times are required to see a response at the transcriptional level for these cytokines. Nevertheless, our data demonstrate a novel anti-inflammatory role of apelin in human pregnancy. Indeed, there is an increasing amount of evidence to show that apelin regulates inflammation in various tissues.10–12 Specifically, apelin reduces the expression of inflammatory cytokines and chemokines by macrophages and in nonaneurysmal suprarenal aorta animals10; attenuates pulmonary inflammation in rat pups with neonatal hyperoxic lung injury11; and lowers the content of plasma cytokines monocyte chemoattractant protein 1 and IL-8 in rats with septic shock and in lipopolysaccharide-induced rat peritoneal macrophages.12 In vivo, apelin transgenic mice exhibited a decreased number of CD11b-positive macrophages in a model of ultraviolet B-induced inflammation.13 As labor is considered an inflammatory state, these studies support our hypothesis. It is of interest that in vitro studies show that apelin inhibits human uterine contractility.33 This finding complements our study in that apelin knockdown increases COX-2 expression and subsequent prostaglandin release. Myometrial contractility is one of the terminal events of human labor and further studies would determine whether apelin could be a therapeutic for the management of preterm labor.
It was surprising; however, that loss of apelin was not associated with the changes in MMP activity. Although MMP-2 is constitutively expressed, MMP-9 is the major MMP responsible for gelatinolytic activity at the membranes at term labor as well as pathological labor.34 Apoptosis also seems to play a role in pathological labor, particularly deliveries involving preterm premature rupture of membrane. It has been reported that apelin demonstrates a protective effect on cultured rat bone marrow mesenchymal stem cells against apoptosis35; if these roles could be applied to gestational tissues, then we would expect the loss of apelin to increase apoptotic activity, and MMP-9 activity, leading to membrane rupture. We report no change in MMP-9 activity with apelin knockdown, indicating that apelin does not play a role in membrane rupture during human labor.
To date, apelin is the only endogenous ligand for the APJ receptor36 and being widely expressed in various tissues such as heart, brain, pancreas, and blood vessel,37 apelin is understandably involved in many physiological functions. In the cardiovascular system, administration of apelin decreased the blood pressure, an effect that is mediated via APJ, as apelin injected into APJ-deficient mice showed no change in systolic blood pressure.38 Apelin affects the propagation of action potential and contractility on normal cardiomyocytes, a role that involves APJ in intracellular communication between these cells,39 to induce myocardial contractility. Apelin has also been shown to play an important role in the spinal cord, having a neuroprotective effect against the pathogenesis of amyotrophic lateral sclerosis.40
Apelin interacts directly with APJ receptor to elicit their effects through a number of signaling pathways including mitogen-activated protein kinases (MAPKs)41 and nuclear factor kappa B.42 This is of note as both the pathways have been shown to play important roles in human labor.43,44 It has also shown that angiotensin II receptors type 1 and type 2 (AT1 and AT2) play critical roles in apelin–APJ signaling.45 Activation of AT1 receptor stimulates apelin secretion in Ca2+, protein kinase C, and MAPK kinase-dependent modes, while AT2 receptors inhibit apelin secretion through cyclic adenosine monophosphate- and cyclic guanosine monophosphate-dependent pathways. The APJ is also regulated by AT1 and AT2. The findings of this study suggest that angiotensin receptors may affect the apelin–APJ system; further studies are required to explore this avenue, warranted by the studies showing that AT1 and AT2 are associated with preeclampsia.46,47
An important role for apelin and APJ in embryonic and fetal growth and development has been suggested. Apelin and APJ expression is higher in the placenta of pregnancies complicated by preeclampsia.21 An increase in preeclamptic placental endothelial cells has been observed, suggesting an important role for apelin and APJ in the regulation of placental flow and vasculogenesis. Indeed, recent studies have shown that apelin causes endothelium-dependent vasodilatation by activating nitric oxide synthase and triggering the release of nitric oxide from endothelial cells.48 Further, apelin has potent in vivo endothelium-dependent vasodilator actions in rats; studies were confirmed using apelin receptor knockout mice.49–51 However, the role of the apelin/APJ system in endothelial and vascular function in the placenta needs to be confirmed in future studies.
In this article, we report the novel findings that in fetal membranes, apelin exerts an anti-inflammatory role. We have demonstrated that spontaneous preterm and term labor downregulates apelin expression in human fetal membranes. Furthermore, apelin regulates proinflammatory cytokines and prostaglandins in amnion. These data suggest that apelin may play a role in the processes that regulate the rupture of fetal membranes. This is of importance as approximately one-third of all cases of preterm birth are due to the untimely rupture of the fetal membranes. Further studies are, however, required to determine whether apelin can be used in the management of preterm labor by ensuring inflammatory homeostasis and the maintenance of human parturition.
Acknowledgments
The authors gratefully acknowledge the assistance of the Clinical Research Midwives Gabrielle Fleming, Renee Grant, Debra Jinks, and Asha Ferguson, and the Obstetrics and Midwifery staff of the Mercy Hospital for Women for their cooperation.
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Dr Martha Lappas was a recipient of a National Health and Medical Research Council (NHMRC) RD Wright Fellowship (grant no. 454777).
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: funding for the Chemi-Doc system and xMark microplate reader was provided by the Medical Research Foundation for Women and Babies.
References
- 1. Callaghan WM, MacDorman MF, Rasmussen SA, Qin C, Lackritz EM. The contribution of preterm birth to infant mortality rates in the United States. Pediatrics. 2006;118(4):1566–1573. [DOI] [PubMed] [Google Scholar]
- 2. Saigal S, Doyle LW. An overview of mortality and sequelae of preterm birth from infancy to adulthood. Lancet. 2008;371(9608):261–269. [DOI] [PubMed] [Google Scholar]
- 3. Moster D, Lie RT, Markestad T. Long-term medical and social consequences of preterm birth. N Engl J Med. 2008;359(3):262–273. [DOI] [PubMed] [Google Scholar]
- 4. Romero R, Espinoza J, Gonçalves LF, Kusanovic JP, Friel L, Hassan S. The role of inflammation and infection in preterm birth. Semin Reprod Med. 2007;25(1):21–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bowen JM, Chamley L, Keelan JA, Mitchell MD. Cytokines of the placenta and extra-placental membranes: roles and regulation during human pregnancy and parturition. Placenta. 2002;23(4):257–273. [DOI] [PubMed] [Google Scholar]
- 6. Christiaens I, Zaragoza DB, Guilbert L, Robertson SA, Mitchell BF, Olson DM. Inflammatory processes in preterm and term parturition. J Reprod Immunol. 2008;79(1):50–57. [DOI] [PubMed] [Google Scholar]
- 7. Olson DM. The role of prostaglandins in the initiation of parturition. Best Pract Res Clin Obstet Gynaecol. 2003;17(5):717–730. [DOI] [PubMed] [Google Scholar]
- 8. Fata JE, Ho AT, Leco KJ, Moorehead RA, Khokha R. Cellular turnover and extracellular matrix remodeling in female reproductive tissues: functions of metalloproteinases and their inhibitors. Cell Mol Life Sci. 2000;57(1):77–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lappas M, Rice GE. Phospholipase A2 isozymes in pregnancy and parturition. Prostaglandins Leukot Essent Fatty Acids. 2004;70(2):87–100. [DOI] [PubMed] [Google Scholar]
- 10. Leeper NJ, Tedesco MM, Kojima Y, et al. Apelin prevents aortic aneurysm formation by inhibiting macrophage inflammation. Am J Physiol Heart Circ Physiol. 2009;296(5):H1329–H1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Visser YP, Walther FJ, Laghmaniel H, Laarse AV, Wagenaar GT. Apelin attenuates hyperoxic lung and heart injury in neonatal rats. Am J Respir Crit Care Med. 2010;182(10):1239–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pan CS, Teng X, Zhang J, et al. Apelin antagonizes myocardial impairment in sepsis. J Card Fail. 2010;16(7):609–617. [DOI] [PubMed] [Google Scholar]
- 13. Sawane M, Kidoya H, Muramatsu F, Takakura N, Kajiya K. Apelin attenuates UVB-induced edema and inflammation by promoting vessel function. Am J Pathol. 2011;179(6):2691–2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tatemoto K, Hosoya M, Habata Y, et al. Isolation and characterization of a novel endogenous pptide ligand for the human APJ receptor. Biochem Biophys Res Commun. 1998;251(2):471–476. [DOI] [PubMed] [Google Scholar]
- 15. O'Dowd BF, Heiber M, Chan A, et al. A human gene that shows identity with the gene encoding the angiotensin receptor is located on chromosome 11. Gene. 1993;136(1-2):355–360. [DOI] [PubMed] [Google Scholar]
- 16. De Falco M, De Luca L, Onori N, et al. Apelin expression in normal human tissues. In Vivo. 2002;16(5):333–336. [PubMed] [Google Scholar]
- 17. Kleinz MJ, Davenport AP. Emerging roles of apelin in biology and medicine. Pharmacol Ther. 2005;107(2):198–211. [DOI] [PubMed] [Google Scholar]
- 18. Habata Y, Fujii R, Hosoya M, et al. Apelin, the natural ligand of the orphan receptor APJ, is abundantly secreted in the colostrum. Biochim Biophys Acta. 1999;1452(1):25–35. [DOI] [PubMed] [Google Scholar]
- 19. Cox CM, D'Agostino SL, Miller MK, Heimark RL, Krieg PA. Apelin, the ligand for the endothelial G-protein-coupled receptor, APJ, is a potent angiogenic factor required for normal vascular development of the frog embryo. Dev Biol. 2006;296(1):177–189. [DOI] [PubMed] [Google Scholar]
- 20. Han S, Wang G, Qiu S, et al. Increased colonic apelin production in rodents with experimental colitis and in humans with IBD. Regul Pept. 2007;142(3):131–137. [DOI] [PubMed] [Google Scholar]
- 21. Cobellis L, De Falco M, Mastrogiacomo A, et al. Modulation of apelin and APJ receptor in normal and preeclampsia-complicated placentas. Histol Histopathol. 2007;22(1):1–8. [DOI] [PubMed] [Google Scholar]
- 22. Telejko B, Kuzmicki M, Wawrusiewicz-Kurylonek N, et al. Plasma apelin levels and apelin/APJ mRNA expression in patients with gestational diabetes mellitus. Diabetes Res Clin Pract. 2010;87(2):176–183. [DOI] [PubMed] [Google Scholar]
- 23. Ariadne MP, Dimitrios G, Maria B, Stavroula B, Dimitrios H, Despina DB. Circulating apelin concentrations in mother/infant pairs at term. Acta Paediatr. 2007;96(12):1751–1754. [DOI] [PubMed] [Google Scholar]
- 24. Lappas M, Mitton A, Lim R, Barker G, Riley C, Permezel M. SIRT1 is a novel regulator of key pathways of human labor. Biol Reprod. 2011;84(1):167–178. [DOI] [PubMed] [Google Scholar]
- 25. Lappas M, Riley C, Rice GE, Permezel M. Increased expression of ac-FoxO1 protein in prelabor fetal membranes overlying the cervix: possible role in human fetal membrane rupture. Reprod Sci. 2009;16(7):635–641. [DOI] [PubMed] [Google Scholar]
- 26. Lim R, Riley C, Barker G, Rice GE, Lappas M. Human labour is associated with decreased cytoplasmic FoxO4. Placenta. 2012;33(1):52–59. [DOI] [PubMed] [Google Scholar]
- 27. Lee Y, Allport V, Sykes A, Lindstrom T, Slater D, Bennett P. The effects of labour and of interleukin 1 beta upon the expression of nuclear factor kappa B related proteins in human amnion. Mol Hum Reprod. 2003;9(4):213–218. [DOI] [PubMed] [Google Scholar]
- 28. Bennett PR, Rose MP, Myatt L, Elder MG. Preterm labor: stimulation of arachidonic acid metabolism in human amnion cells by bacterial products. Am J Obstet Gynecol. 1987;156(3):649–655. [DOI] [PubMed] [Google Scholar]
- 29. Moore RM, Novak JB, Kumar D, Mansour JM, Mercer BM, Moore JJ. Alpha-lipoic acid inhibits tumor necrosis factor-induced remodeling and weakening of human fetal membranes. Biol Reprod. 2009;80(4):781–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Terzidou V, Blanks AM, Kim SH, Thornton S, Bennett PR. Labor and inflammation increase the expression of oxytocin receptor in human amnion. Biol Reprod. 2011;84(3):546–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lappas M, Permezel M, Rice GE. N-Acetyl-cysteine inhibits phospholipid metabolism, proinflammatory cytokine release, protease activity, and nuclear factor-kappaB deoxyribonucleic acid-binding activity in human fetal membranes in vitro. J Clin Endocrinol Metab. 2003;88(4):1723–1729. [DOI] [PubMed] [Google Scholar]
- 32. Lappas M, Permezel M, Rice GE. 15-Deoxy-Delta(12,14)-prostaglandin J(2) and troglitazone regulation of the release of phospholipid metabolites, inflammatory cytokines and proteases from human gestational tissues. Placenta. 2006;27(11-12):1060–1072. [DOI] [PubMed] [Google Scholar]
- 33. Hehir MP, Morrison JJ. The adipokine apelin and human uterine contractility. Am J Obstet Gynecol. 2012;206(4):359 e1–e5. [DOI] [PubMed] [Google Scholar]
- 34. Weiss A, Goldman S, Shalev E. The matrix metalloproteinases (MMPS) in the decidua and fetal membranes. Front Biosci. 2007;12:649–59. [DOI] [PubMed] [Google Scholar]
- 35. Zeng X, Yu SP, Taylor T, Ogle M, Wei L. Protective effect of apelin on cultured rat bone marrow mesenchymal stem cells against apoptosis. Stem Cell Res. 2012;8(3):357–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tatemoto K, Hosoya M, Habata Y, et al. Isolation and characterization of a novel endogenous peptide ligand for the human APJ receptor. Biochem Biophys Res Commun. 1998;251(2):471–476. [DOI] [PubMed] [Google Scholar]
- 37. Masri B, Knibiehler B, Audigier Y. Apelin signalling: a promising pathway from cloning to pharmacology. Cell Signal. 2005;17(4):415–426. [DOI] [PubMed] [Google Scholar]
- 38. Ishida J, Hashimoto T, Hashimoto Y, et al. Regulatory roles for APJ, a seven-transmembrane receptor related to angiotensin-type 1 receptor in blood pressure in vivo. J Biol Chem. 2004;279(25):26274–26279. [DOI] [PubMed] [Google Scholar]
- 39. Farkasfalvi K, Stagg MA, Coppen SR, et al. Direct effects of apelin on cardiomyocyte contractility and electrophysiology. Biochem Biophys Res Commun. 2007;357(4):889–895. [DOI] [PubMed] [Google Scholar]
- 40. Kasai A, Kinjo T, Ishihara R, et al. Apelin deficiency accelerates the progression of amyotrophic lateral sclerosis. Plos One. 2011;6(8):e23968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Than A, Cheng Y, Foh LC, et al. Apelin inhibits adipogenesis and lipolysis through distinct molecular pathways. Mol Cell Endocrinol. 2012;362(1-2):227–241. [DOI] [PubMed] [Google Scholar]
- 42. Lu Y, Zhu X, Liang GX, et al. Apelin-APJ induces ICAM-1, VCAM-1 and MCP-1 expression via NF-kappaB/JNK signal pathway in human umbilical vein endothelial cells. Amino Acids. 2012;43(5):2125–2136. [DOI] [PubMed] [Google Scholar]
- 43. Lappas M, Michael P, Harry MG, Gregory ER. Nuclear factor kappa B regulation of proinflammatory cytokines in human gestational tissues in vitro. Biol Reprod. 2002;67(2):668–673. [DOI] [PubMed] [Google Scholar]
- 44. Lappas M, Riley C, Lim R, et al. MAPK and AP-1 proteins are increased in term pre-labour fetal membranes overlying the cervix: regulation of enzymes involved in the degradation of fetal membranes. Placenta. 2011;32(12):1016–1025. [DOI] [PubMed] [Google Scholar]
- 45. Than A, Tee WT, Chen P. Apelin secretion and expression of apelin receptors in 3T3-L1 adipocytes are differentially regulated by angiotensin type 1 and type 2 receptors. Mol Cell Endocrinol. 2012;351(2):296–305. [DOI] [PubMed] [Google Scholar]
- 46. Hladunewich MA, Kingdom J, Odutayo A, et al. Postpartum assessment of the renin angiotensin system in women with previous severe, early-onset preeclampsia. J Clin Endocrinol Metab. 2011;96(11):3517–3524. [DOI] [PubMed] [Google Scholar]
- 47. Knock GA, Sullivan MH, McCarthy A, Elder MG, Polak JM, Wharton J. Angiotensin II (AT1) vascular binding sites in human placentae from normal-term, preeclamptic and growth retarded pregnancies. J Pharmacol Exp Ther. 1994;271(2):1007–1015. [PubMed] [Google Scholar]
- 48. Cohen RA, Vanhoutte PM. Endothelium-dependent hyperpolarization. Beyond nitric oxide and cyclic GMP. Circulation. 1995;92(11):3337–3349. [DOI] [PubMed] [Google Scholar]
- 49. Reaux A, De Mota N, Skultetyova I, et al. Physiological role of a novel neuropeptide, apelin, and its receptor in the rat brain. J Neurochem. 2001;77(4):1085–1096. [DOI] [PubMed] [Google Scholar]
- 50. Lee DK, Cheng R, Nguyen T, et al. Characterization of apelin, the ligand for the APJ receptor. J Neurochem. 2000;74(1):34–41. [DOI] [PubMed] [Google Scholar]
- 51. Tatemoto K, Takayama K, Zou MX, et al. The novel peptide apelin lowers blood pressure via a nitric oxide-dependent mechanism. Regul Pept. 2001;99(2-3):87–92. [DOI] [PubMed] [Google Scholar]