Abstract
Background:
The α2-adrenoreceptor agonist, dexmedetomidine, provides excellent sedation with minimal cardiovascular instability or respiratory depression and may be a useful adjunct to facilitate smooth tracheal extubation.
Materials and Methods:
Fifty American Society of Anesthesiologists grade I-II patients, aged 20-45 years, scheduled for elective general surgical, urological and gynecological surgeries were studied after randomization into two groups. Group A and B, received an intravenous infusion of dexmedetomidine 0.75 mcg/kg or placebo respectively, over 15 minutes before anticipated time of end of surgery, in a double blind manner. Anesthesia techniques were standardized. Heart rate, systolic, diastolic, mean arterial pressures were recorded while starting injection, at 1, 3, 5, 10, 15 minutes after starting injection, during extubation, at 1, 3, 5 minutes after extubation, and thereafter every 5 minutes for 30 minutes. Quality of extubation was evaluated on a 5 point scale and postoperative sedation on a 6 point scale. Any event of laryngospasm, bronchospasm, desaturation, respiratory depression, vomiting, hypotension, undue sedation was noted.
Results:
Heart rate, systolic, diastolic, mean arterial pressures were significantly higher in group B (P < 0.05). Extubation quality score of majority of patients was 2 in group A and 3 in group B. Sedation score of most patients was 3 in group A and 2 in group B. Bradycardia and hypotension incidences were higher in group A. One patient in group A, two patients in group B had vomiting. No patient had any other side effects.
Conclusion:
Dexmedetomidine 0.75 mcg/kg administered 15 minutes before extubation, stabilizes hemodynamics and facilitates smooth extubation.
Keywords: α2-adrenoreceptor agonist, dexmedetomidine, extubation, hemodynamics
Introduction
Tracheal extubation is the discontinuation of an artificial airway when the indications for its placement like airway obstruction, protection of airway, suctioning, ventilatory failure and hypoxemia no longer exist. For a smooth extubation, there should be no straining, movement, coughing, breath holding or laryngospasm. Extubation at light levels of anesthesia or sedation can stimulate reflex responses via tracheal and laryngeal irritation. Various agents like lidocaine, opioids, esmolol, calcium channel blockers,[1,2] magnesium sulphate and propofol have been shown to attenuate these responses, but they all have limitations and side effects. Dexmedetomidine, an α2-adrenoreceptor agonist with a distribution half-life of approximately 6 minutes has been successfully used for attenuating the stress response to laryngoscopy.[3,4] Currently, dexmedetomidine is indicated for intensive care unit sedation in mechanically ventilated patients and for sedation of non intubated patients before or during surgical and other procedures.[5]
We hypothesized that dexmedetomidine might be a useful agent to attenuate the response to extubation as it provides sedation, hemodynamic stability and does not depress respiration. We designed this prospective, randomized, double blind trial to determine if dexmedetomidine can serve as an effective alternative to the commonly used agents for blunting the hemodynamic response to tracheal extubation. The aim of the study was to evaluate the effect of dexmedetomidine on hemodynamic and recovery responses during extubation, quality of extubation and postoperative sedation.
Materials and Methods
This study was carried out on 50 patients (25 in each group) between 20 to 45 years of age of either sex belonging to American Society of Anesthesiologists (ASA) physical status I and II and scheduled for elective general surgical, urological and gynecological surgeries. Patients with cardiovascular or respiratory disorders, diabetes, hypertension, obesity, difficult airway, medications that effect heart rate (HR) or blood pressure (BP), pregnant, currently breast feeding women, history of sleep apnea and those for emergency procedures were excluded.
Institutional board approval was taken and written informed consent was taken from each patient. Pre-anesthetic check up was conducted and a detailed history and complete physical examination recorded. Routine investigations like complete blood picture, blood grouping/typing, blood urea and serum creatinine were done. Using computer generated random allocation, patients were divided into two groups (25 patients in each group). The enrolling investigator prepared the drug solution to be given before extubation and had no role in patient’s assessment.
Routine anesthetic technique was used using propofol, fentanyl, vecuronium, nitrous oxide-oxygen and halothane. Standard monitoring with electrokardiography (EKG), pulse oximetry (SpO2) and noninvasive BP was done. About 15 minutes before the estimated time of end of surgery, inhalation agent was cut off and patients in each group received the specified solution intravenously over 15 minutes.
Patients in group A received dexmedetomidine 0.75 mcg/kg intravenous (IV) in 100 ml normal saline (NS) over 15 minutes while in group B, patients received 100 ml NS over 15 minutes.
HR, systolic BP and diastolic BP were recorded at the start of bolus drug injection and thereafter at 1, 3, 5, 10 and 15 minutes. Residual neuromuscular blockade was reversed with neostigmine 0.05 mg/kg and atropine 0.02 mg/kg IV. When patients’ spontaneous respirations were considered sufficient and patients were able to obey simple commands, suction of throat was done and trachea was extubated. The anesthesiologist performing the extubation was blinded to the study drugs. HR, systolic BP and diastolic BP were recorded at the time of extubation and thereafter at 1, 3 and 5 minutes after extubation followed by every 5 minutes for 30 minutes. Occurrence of any event like laryngospasm, bronchospasm, desaturation, respiratory depression, vomiting, hypotension, bradycardia or undue sedation was noted.
Hypotension was defined as a decrease in systolic BP of more than 30 mmHg from baseline or a mean arterial pressure of less than 60 mmHg and was corrected with IV fluids and if required, with small dose of mephentermine 3 mg IV. Bradycardia was defined as a HR of less than 60/minute and was corrected, if associated with hemodynamic instability, with atropine 0.6 mg IV.
Quality of extubation was evaluated based on cough immediately after extubation, using a 5 point rating scale (Extubation Quality Score):[6] 1 = no coughing, 2 = smooth extubation, minimal coughing (1 or 2 times), 3 = moderate coughing (3 or 4 times), 4 = severe coughing (5-10 times) and straining, 5 = poor extubation, very uncomfortable (laryngospasm and coughing >10 times).
Postoperative sedation was evaluated on a 6 point scale (Ramsay Scale):[7] 1 = Anxious or agitated and restless or both, 2 = Cooperative, oriented and tranquil, 3 = Drowsy but responds to commands, 4 = Asleep, brisk response to light glabellar tap or loud auditory stimulus, 5 = Asleep, sluggish response to light glabellar tap or loud auditory stimulus, 6 = Asleep and unarousable.
Power of study was calculated assuming a 15% change in HR and BP between baseline and extubation. According to statistical power analysis, 23 patients per treatment group were needed to get a 90% power in detecting a 15% difference between treatment groups with a 5% type I error. Assuming a 5% dropout rate, the final sample size was set at 50 patients (25 patients per group). The parameters were recorded and data was entered into Statistical Package for Social Sciences (SPSS 15.0). Statistical analysis was done using paired-samples t-test for between-group comparisons. The χ2 test or the Fisher exact test was used to analyze extubation quality, sedation scores, and adverse events. P value <0.05 was considered as statistically significant.
Results
The patients in the two groups were comparable for age, weight and male:female ratio, ASA physical status, amount of halothane used, duration of surgery and the difference between the two groups was not statistically significant (P > 0.05) [Table 1].
Table 1.
Demographic profile of two groups
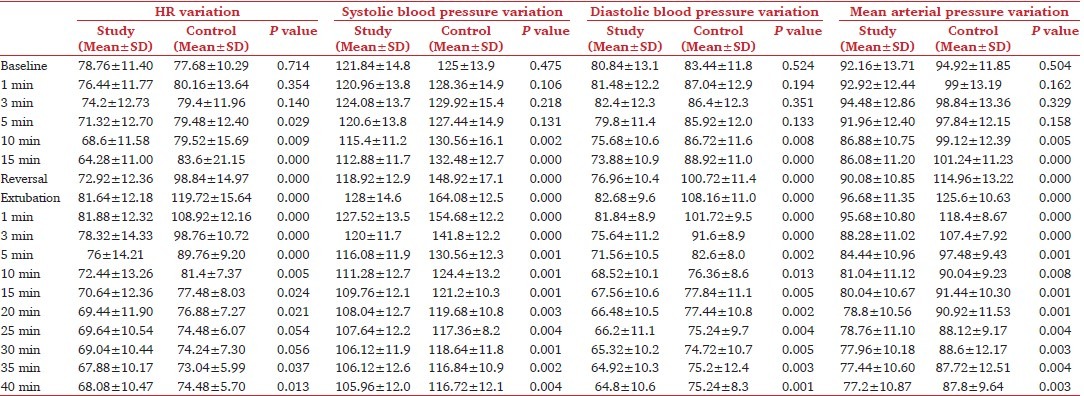
We observed a statistically significant difference (P < 0.05) in HR between the two groups from 5 minutes after starting administration of the agent till 20 minutes after extubation [Table 2]. A statistically significant difference was observed in systolic BP between the two groups (P < 0.05) from 10 minutes after start of administration of the agent and continued till the time observations were made [Table 2]. Our study showed a significant difference in diastolic BP between the two groups (P < 0.05) from 10 minutes after starting the administration of the agent and continued till the time observations were made [Table 2]. The mean arterial pressure between the two groups showed statistically significant difference (P < 0.05) from 10 minutes after starting administration of the agent and continued till the time observations were made [Table 2].
Table 2.
Hemodynamic parameters
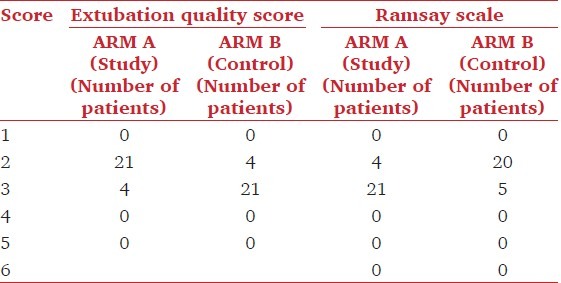
We observed a significant difference in the quality of extubation between the two groups (P = 0.04) [Table 3]. 84 percent of the patients in group A could be extubated smoothly with minimal coughing, whereas 16 percent patients showed moderate coughing at the time of extubation. 84 percent patients in group B showed moderate coughing at the time of extubation, whereas only 16 percent patients could be extubated smoothly. A significant difference in the level of postoperative sedation was observed between the two groups (P = 0.017) [Table 3]. 84 percent of patients in group A were drowsy, but responding to commands with a sedation score of 3 on the Ramsay Scale; whereas, in group B, 80 percent patients were cooperative, oriented and tranquil with a sedation score of 2 on the Ramsay Scale.
Table 3.
Smooth extubation parameters
The incidence of bradycardia and hypotension was higher in group A compared to group B [Table 4]. 13 patients in group A developed bradycardia as compared to only 2 patients in the control group, but, none required treatment. 2 patients in group A developed hypotension; whereas, none of the patients in group B developed hypotension, but, none required vasopressors.
Table 4.
Adverse effects
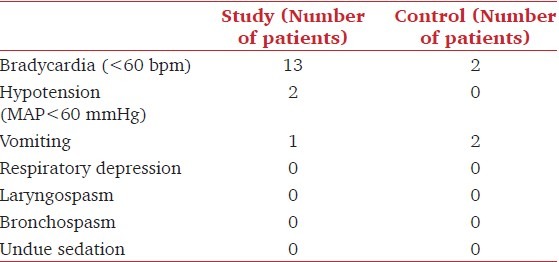
One patient in group A and two in group B had vomiting after extubation. None of the patients in either group had any of the other side effects like respiratory depression, laryngospasm, bronchospasm, undue sedation [Table 4]. No significant difference was observed between the two groups in SpO2 values [Figure 1].
Figure 1.
SpO2 Variation, shows line diagram comparing SpO2 between the two groups at various timings
Discussion
Extubation can be associated with several complications like coughing and respiratory and hemodynamic alterations. These changes are usually transient and well tolerated by most patients, but may be deleterious in certain subgroups of patients.
Dexmedetomidine has been successfully used to attenuate the hemodynamic responses to tracheal intubation. Basing on its characteristics of sedation, hemodynamic stability, and lack of respiratory depression, along with its relatively short half-life and analgesic effects, the present study was conducted to evaluate the effect of dexmedetomidine in a dose of 0.75 mcg/kg on hemodynamic responses during extubation, the quality of extubation, the level of postoperative sedation and the prevalence of complications.
The dose of dexmedetomidine ranges from 0.5-1 mcg/kg.[8] A pilot study, using three different doses (0.5 μg/kg, 0.75 μg/kg and 1 μg/kg) of dexmedetomidine, was conducted. In this pilot study, a dose of 1 mcg/kg resulted in hemodynamic instability while a dose of 0.5 mcg/kg gave insignificant results. The present study was therefore, conducted with a dose of 0.75 mcg/kg, although there is evidence in favor of a dose of 0.5 mcg/kg.[9]
Dexmedetomidine activates receptors in the medullary vasomotor center, reducing norepinephrine turnover and decreasing central sympathetic outflow, resulting in alterations in sympathetic function and decreased HR and BP. In the present study, the hemodynamic parameters in the study group were significantly stable during extubation when compared to the placebo group. Dexmedetomidine 0.5 mcg/kg administered 5 minutes before the end of surgery has been shown to stabilize hemodynamics, allow easy extubation, provide a more comfortable recovery and allow early neurological examination following intracranial operations.[6] Dexmedetomidine 0.5 mcg/kg, given 5 minutes before extubation has been found to be more effective than fentanyl 1 mcg/kg in attenuating airway reflex responses to tracheal extubation and maintaining hemodynamic stability without prolonging recovery.[10] In patients undergoing vascular surgery, dexmedetomidine (plasma concentrations in the range of 0.18 to 0.35 ng/ml) attenuated the increase in HR and plasma norepinephrine concentrations during emergence from anesthesia and did not attenuate postoperative increases in HR or BP after emergence from anesthesia or affect intraoperative anesthetic or postoperative analgesic requirements.[11] An infusion of dexmedetomidine started 20 minutes before anesthesia and continued until the start of skin closure in patients undergoing supratentorial brain tumor surgery was found to blunt tachycardic response to intubation and the hypertensive response to extubation.[12]
In vitro studies indicate that α2 stimulation can cause smooth muscle relaxation thereby preventing bronchoconstriction. In our study, most patients in the study group could be extubated smoothly with minimal coughing (Extubation Quality Score 2) when compared to control group, where most patients had moderate cough (Extubation Quality Score 3). Dexmedetomidine 0.5 mcg/kg given as a single-dose bolus before tracheal extubation has been shown to attenuate airway-circulatory reflexes during extubation with no difference between the groups in the incidence of breath holding or desaturation.[13]
Central stimulation of parasympathetic outflow and inhibition of sympathetic outflow from the locus coeruleus in the brainstem plays a prominent role in the sedation and anxiolysis produced by dexmedetomidine. Decreased noradrenergic output from the locus coeruleus allows for increased firing of inhibitory neurons including the γ-amino butyric acid system resulting in anxiolysis and sedation. We found that most patients in study group were drowsy but responding to verbal commands (Ramsay Sedation Scale 3) after extubation when compared to control group, where most patients belonged to Ramsay Sedation Scale 2. Dexmedetomidine 0.25 mcg/kg/hour has been used for sedation during mechanical ventilation in pediatric patients and found to be as effective as midazolam 0.22 mg/kg/hour.[14] The quality of sedation is better and the need for rescue sedation is less with dexmedetomidine use as compared with midazolam and there is no significant adverse effect on hemodynamic or respiratory function.[15]
The activation of α2 adrenoceptors, imidazoline-preferring receptors, or both in the ventrolateral medulla and especially in the solitarius nucleus tract by dexmedetomidine causes bradycardia. In our study, the incidence of bradycardia and hypotension was higher in study group than in control group. Dexmedetomidine 2.5 mcg/kg followed by 0.2-2.5 mcg/kg/hour has been found to reduce HR in patients.[16] A higher frequency of postoperative hypotension has been reported when patient controlled analgesia with dexmedetomidine is administered.[17]
Our study found insignificant difference in the incidence of vomiting between the two groups. However, others have found a higher, though not statistically significant, prevalence of adverse events (i.e., hypotension, bradycardia, and perioperative nausea and vomiting) with use of dexmedetomidine.[18]
In our study, none of the patients in either group developed respiratory depression, laryngospasm, bronchospasm, undue sedation or desaturation. Similar findings have been made by Guler et al.[13] Dexmedetomidine use in morbidly obese patients has been found not to induce respiratory depression at clinical doses although it improved quality of postoperative analgesia.[19]
To conclude, use of dexmedetomidine before extubation attenuates the hemodynamic response to extubation. It enables smooth extubation of the trachea and provides adequate sedation postoperatively. Dexmedetomidine increases the incidence of bradycardia and hypotension, but does not cause side effects like respiratory depression, laryngospasm, bronchospasm, undue sedation and desaturation.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Minogue SC, Ralph J, Lampa MJ. Laryngotracheal topicalization with lidocaine before intubation decreases the incidence of coughing on emergence from general anesthesia. Anesth Analg. 2004;99:1253–7. doi: 10.1213/01.ANE.0000132779.27085.52. [DOI] [PubMed] [Google Scholar]
- 2.Aouad MT, Al-Alami AA, Nasr VG, Souki FG. The effect of low dose remifentanil on responses to the endotracheal tube during emergence from general anesthesia. Anesth Analg. 2009;96:1320–4. doi: 10.1213/ane.0b013e31819b03d8. [DOI] [PubMed] [Google Scholar]
- 3.Bhana N, Goa KL, McClellan KJ. Dexmedetomidine. Drugs. 2000;59:263–8. doi: 10.2165/00003495-200059020-00012. [DOI] [PubMed] [Google Scholar]
- 4.Sulaiman S, Karthekeyan RB, Vakamudi M, Sundar AS, Ravullapalli H, Gandham R. The effects of dexmedetomidine on attenuation of stress response to endotracheal intubation in patients undergoing elective off-pump coronary artery bypass grafting. Ann Card Anaesth. 2012;15:39–43. doi: 10.4103/0971-9784.91480. [DOI] [PubMed] [Google Scholar]
- 5.Grewal A. Dexmedetomidine: New avenues. J Anaesthesiol Clin Pharmacol. 2011;27:297–302. doi: 10.4103/0970-9185.83670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Turan G, Ozgultekin A, Turan C, Dincer E, Yuksel G. Advantageous effects of dexmedetomidine on haemodynamic and recovery responses during extubation for intracranial surgery. Eur J Anaesthesiol. 2008;25:816–20. doi: 10.1017/S0265021508004201. [DOI] [PubMed] [Google Scholar]
- 7.Ramsay MA, Huddleston P, Hamman B, Tai S, Matter G. The patient state index correlates well with the Ramsay sedation score in ICU patients. Anesthesiology. 2004;101:A338. [Google Scholar]
- 8.Dosing Guidelines for Precedex®. [Last retrieved on 2010 Nov 21]. Available from: http://www.precedex.com/wp-content/uploads/2010/02/Dosing_Guide.pdf .
- 9.McGee JP, Maroof M, Passannante AN, Isenberg AV, Maroof BM. Use of dexmedetomidine for smooth recovery from general anesthesia after ECT, a case series. Anesthesiology. 2007;107:A844. [Google Scholar]
- 10.Aksu R, Akýn A, Bicer C, Esmaoglu A, Tosun Z, Boyacý A. Comparison of the effects of dexmedetomidine versus fentanyl on airway reflexes and hemodynamic responses to tracheal extubation during rhinoplasty: A double-blind, randomized, controlled study. Curr Ther Res Clin Exp. 2009;70:209–20. doi: 10.1016/j.curtheres.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Talke P, Chen R, Thomas B, Aggarwall A, Gottlieb A, Thorborg P, et al. The hemodynamic and adrenergic effects of perioperative dexmedetomidine infusion after vascular surgery. Anesth Analg. 2000;90:834–9. doi: 10.1097/00000539-200004000-00011. [DOI] [PubMed] [Google Scholar]
- 12.Tanskanen PE, Kytta JV, Randell TT, Aantaa RE. Dexmedetomidine as an anesthetic adjuvant in patients undergoing intracranial tumor surgery: A double-blind, randomized and placebo-controlled study. Br J Anaesth. 2006;97:658–65. doi: 10.1093/bja/ael220. [DOI] [PubMed] [Google Scholar]
- 13.Guler G, Akin A, Tosun Z, Eskitascoglu E, Mizrak A, Boyaci A. Single-dose dexmedetomidine attenuates airway and circulatory reflexes during extubation. Acta Anesthesiol Scand. 2005;49:1088–91. doi: 10.1111/j.1399-6576.2005.00780.x. [DOI] [PubMed] [Google Scholar]
- 14.Tobias JD, Berkenbosch JW. Sedation during mechanical ventilation in infants and children: Dexmedetomidine versus midazolam. South Med J. 2004;97:451–5. doi: 10.1097/00007611-200405000-00007. [DOI] [PubMed] [Google Scholar]
- 15.Koroglu A, Demirbilek S, Teksan H, Sagir O, But AK, Ersoy MO. Sedative, haemodynamic and respiratory effects of dexmedetomidine in children undergoing magnetic resonance imaging examination: Preliminary results. Br J Anaesth. 2005;94:821–4. doi: 10.1093/bja/aei119. [DOI] [PubMed] [Google Scholar]
- 16.Venn RM, Grounds RM. Comparison between dexmedetomidine and propofol for sedation in the intensive care unit: Patient and clinician perceptions. Br J Anaesth. 2001;87:684–90. doi: 10.1093/bja/87.5.684. [DOI] [PubMed] [Google Scholar]
- 17.Sadhasivam S, Boat A, Mahmoud M. Comparison of patient-controlled analgesia with and without dexmedetomidine following spine surgery in children. J Clin Anesth. 2009;21:493–501. doi: 10.1016/j.jclinane.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 18.Karaaslan K, Yilmaz F, Gulcu N, Colak C, Sereflican M, Kocoglu H. Comparison of dexmedetomidine and midazolam for monitored anesthesia care combined with tramadol via patient-controlled analgesia in endoscopic nasal surgery: A prospective, randomized, double-blind, clinical study. Curr Ther Res Clin Exp. 2007;68:69–81. doi: 10.1016/j.curtheres.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ramsay MA. Bariatric surgery: The role of dexmedetomidine. Semin Anesth Perioper Med Pain. 2006;25:51–6. [Google Scholar]


