Abstract
Background:
Analgesia and sedation are usually required for the comfort of the patient and surgeon during tympanoplasty surgery done under local anesthesia. In this study, satisfaction scores and effectiveness of sedation and analgesia with dexmedetomidine were compared with a combination of midazolam-fentanyl.
Materials and Methods:
Ninety patients undergoing tympanoplasty under local anesthesia randomly received either IV dexmedetomidine 1 μg kg-1 over 10 min followed by 0.2 μg kg-1h-1 infusion (Group D) or IV midazolam 0.06 mg kg-1 plus IV fentanyl 1 μg kg-1 over 10 min (Group MF) followed by normal saline infusion at 0.2 ml kg-1h-1. Sedation was titrated to Ramsay sedation score (RSS) of three. Vital parameters, rescue analgesics (fentanyl 1 μg kg-1) and sedatives (midazolam 0.01 mg kg-1), patient and surgeon satisfaction scores were recorded.
Results:
Patient and surgeon satisfaction score was better in Group D than Group MF (median interquartile range (IQR) 9 (8-10) vs. 8 (6.5-9.5) and 9 (8.5-9.5) vs. 8 (6.75-9.25), P = 0.0001 for both). Intraoperative heart rate and mean arterial pressure in Group D were lower than the baseline values and the corresponding values in Group MF (P < 0.05). Percentage of patients requiring rescue fentanyl was higher in Group MF than Group D (40% vs. 11.1%, P = 0.01). One patient in Group D while four in Group MF (8.8%) required rescue sedation with midazolam (P > 0.17). Seven patients in Group D had dry mouth vs. none in Group MF (P = 0.006). One patient in Group D had bradycardia with hypotension which was effectively treated.
Conclusion:
Dexmedetomidine is comparable to midazolam-fentanyl for sedation and analgesia in tympanoplasty with better surgeon and patient satisfaction. Hemodynamics need to be closely monitored.
Keywords: Dexmedetomidine, sedation, midazolam fentanyl sedation, monitored anesthesia care, satisfaction scores, surgery, otological
Introduction
Tympanoplasty involves reconstruction of perforated tympanic membrane with or without ossiculoplasty.[1] It is usually done under local anesthesia with sedation under monitored anesthesia care (MAC) or general anesthesia.[2–4] Patients may feel discomfort due to pain, noise due to suction, manipulation of instruments and head-neck position.[5]
Commonly used medications for MAC are benzodiazepines, opioids and propofol.[6] Midazolam with its quick onset, but a relatively long half-life can cause prolonged sedation after repeated administration.[7] Combining midazolam with opioids increases the risk for hypoxemia and apnea.[8,9] The addition of propofol may cause cardio-respiratory depression.[9] Oversedation leading to respiratory depression has been reported to cause patient injuries during MAC.[10]
Dexmedetomidine is a selective α2 receptor agonist with properties of analgesia, sympatholysis and titrating sedation without major respiratory depression.[11–13] It reduces opioid requirements and stress response to surgery ensuring a stable hemodynamic state.[14,15] Dexmedetomidine is increasingly being used as a sedative for MAC for various surgical procedures.[16–19]
This randomized, double-blind study compares dexmedetomidine with a combination of midazolam-fentanyl in patients undergoing tympanoplasty under local anesthesia (LA) with primary end point being the patient satisfaction score. The need of intraoperative rescue analgesics to maintain a cooperative state of the patient was the secondary end point.
Materials and Methods
This prospective, randomized controlled, double-blind study was undertaken after institutional ethics committee approval. Ninety patients of either sex, having American Society of Anesthesiologists (ASA) Grade I/II, aged between 18 and 60 years, undergoing tympanoplasty surgery under local anesthesia were included and written informed consent was obtained from all the participants. Patients with known sensitivity to local anesthetic drug lignocaine, allergy to study drugs, pregnant and lactating females were excluded from the study. Patients on pain perception modifying drugs and those with history of use of any opioid or sedative medications in the week prior to surgery were also excluded. All the patients were examined a day before surgery and were thoroughly investigated according to the institute protocol. They were counseled with regards to sedation, local anesthesia as well as the operative procedure. The visual analogue scale (VAS) (0-10, where 0 indicated no pain while 10 corresponded to maximum pain), was explained to the patient during the preoperative visit. The patients were randomly divided into two equal groups, Group D (dexmedetomidine) and Group MF (midazolam fentanyl) on basis of a computer-generated randomization scheme. The anesthesiologist conducting the case, the patients and the anesthesiologist in the post anesthesia care unit (PACU) were all blinded to group assignment. Data was recorded by a blinded observer and the drugs were prepared by an anesthesiologist who did not participate in patient management or data collection. Two 50-ml syringes, labeled as loading and maintenance were given for each patient. Group D patients had dexmedetomidine 1μg kg-1 and Group MF had midazolam 0.06 mg kg-1 plus fentanyl 1μg kg-1 in their respective loading syringes diluted up to 30 ml of normal saline. Group D had 1μg ml-1 of dexmedetomidine and Group MF had normal saline in their respective maintenance syringes.
On arrival in the operation theatre, after confirming adequate starvation, patient’s heart rate, arterial blood pressure, oxygen saturation, respiratory rate and ECG were monitored.(PM-9000Express, Penlon, Abingdon, UK). Intravenous access was secured with 20G cannula and Ringer’s lactate solution at 2 ml kg-1 was started. Oxygen was administered via nasal cannula at 2 L min-1. No sedative premedication was used. Group D (n = 45) received intravenous dexmedetomidine 1 μg kg-1 over 10 min followed by a continuous infusion of 0.2 μg kg-1h-1 using an infusion pump (Infusor 950, Emco, India). Group MF (n = 45) received intravenous midazolam 0.06 mg kg-1 plus intravenous fentanyl 1 μg kg-1 over 10 min followed by continuous infusion of normal saline at 0.2 ml kg-1h-1. During this period the patients were assessed every two minutes using Ramsay sedation score (RSS)[20] (1 = agitated, restless; 2 = cooperative, tranquil; 3 = responds to verbal command while sleeping; 4 = brisk response to gabellar tap or loud voice while sleeping; 5 = sluggish response to gabellar tap or loud voice; 6 = no response to gabellar tap or loud voice). The target end point was a patient having RSS = 3. If the target end point was reached before completing the loading infusion, then the infusion was stopped and noted. After the loading drug infusion if any patient in either of the groups had lesser sedation (a score <3) then bolus IV midazolam 0.01mg kg-1 was administered which was repeated if necessary till RSS was 3. The maintenance infusion in both the groups was commenced immediately, once the loading infusions were stopped. After completing the loading infusion of the drugs and when RSS of 3 was achieved, the blinded ENT surgeon (with a minimum of three years of experience) administered LA using 2% lignocaine with adrenaline (6-7 ml) (1:2,00,000) in the postauricular area to block greater auricular and lesser occipital nerves, in the incisura terminalis to block auriculotemporal nerve and the four quadrants of the external auditory canal. Surgery was commenced after confirming adequate analgesia. Intraoperatively heart rate (HR), mean blood pressure (MAP), respiratory rate and SPO2 were recorded every 2 min during loading infusion of the study drugs and thereafter at 10-min intervals till the end of surgery. Sedation level (RSS) was assessed every 10 min and if RSS <3 IV midazolam 0.01 mg kg-1 was administered as a common rescue sedative in both the groups. The number of rescue doses of midazolam was recorded. Intraoperative pain intensity was evaluated using VAS. Inadequate analgesia was treated with infiltration of 2% lignocaine with adrenaline (2-3 ml) at the surgical site and noted. If the pain was still persistent and VAS >3, then rescue IV fentanyl in the dose of 1μg kg-1 was given. Total number of rescue doses of fentanyl during surgery was recorded. The protocol specified up to a maximum of three rescue doses each of midazolam and fentanyl. At any time, if clinically indicated or if protocol-specified amounts of rescue drugs were reached, the sedation technique was converted to any alternative sedative or anesthetic technique and the study drug was discontinued. The maintenance infusions were discontinued at the time of closure which was approximately 15 min before end of surgery. Adverse events like bradycardia (HR <45 bpm), hypotension (drop in systolic blood pressure >20% of baseline or MAP <60 mmHg), hypertension (an increase in systolic blood pressure or MAP >20% of baseline), bradypnea (RR <8 breaths/min), desaturation (SpO2 < 90%), nausea, vomiting, dry mouth or any other event during or within two hours of the procedure were noted. Bradycardia was treated with intravenous atropine sulphate 0.01mg kg-1, hypotension with fluid replacement and if needed, intravenous ephedrine hydrochloride 5 mg in incremental doses was administered. In case of bradypnea, patient was woken up and was asked to take deep breaths. Desaturation was treated by increasing O2 flow up to 6 liters and if needed, using bag mask ventilation with 10 liters of oxygen.
After the completion of surgery patients were shifted to the PACU and were monitored for hemodynamic parameters, degree of analgesia and adverse events, if any for 2 h. RSS was assessed immediately on arrival in the PACU and every 30 min thereafter till transfer to surgical ward. Requirement of postoperative analgesia was noted. The first rescue dose of analgesic was given at VAS >3 and was documented. Surgeons were asked to grade the surgical conditions as well as their satisfaction with sedation technique on numerical rating scale (NRS) with zero being least satisfied and 10 being most satisfied. Patients were asked to grade their overall satisfaction with the procedure on a similar numerical scale (NRS 0-10) on postoperative Day one in the surgical ward.
The primary end point of our study was the patient satisfaction score using NRS from 0 to 10. Efficacy of the sedation technique was defined as the ability to complete the surgery without any rescue sedatives and analgesics. Safety of the technique was determined based on the frequency of analgesia/sedation-related intra or postoperative adverse events.
Statistical analysis
Data analysis has been done using SPSS Version 16.0. Power analysis was based on the results of a previous study.[17] Sample size calculation was based on a population standard deviation of 1.1 with 80% power and 5% alpha error. To detect a difference in satisfaction score of one between groups, a sample size of 43 patients per group was required.
Hemodynamic and respiratory data were evaluated using unpaired t test for intergroup and paired t-test for within group comparisons. Data not normally distributed was compared using Mann Whitney U test. Categorical data was analyzed using Chi square test. P value less than 0.05 was considered as significant.
Results
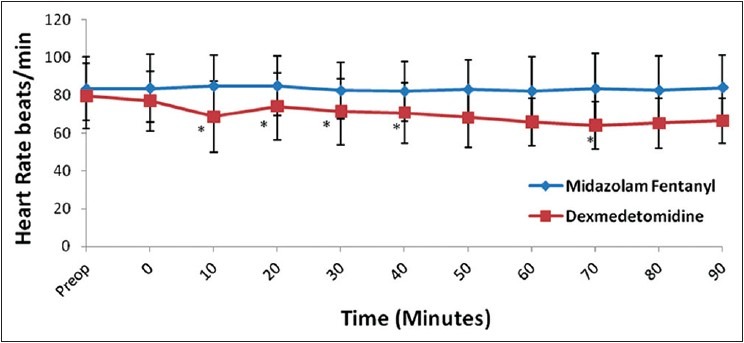
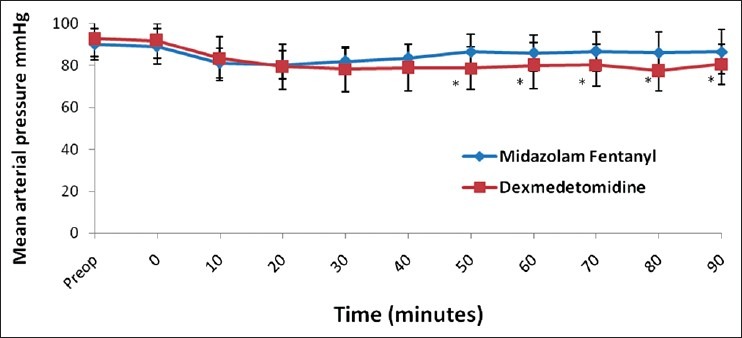
Ninety patients were recruited. All of them underwent their planned surgical procedure and received their allocated study drug. No assigned patients dropped out of the study. The patients’ characteristics and surgical data were comparable between the two groups [Table 1]. There were no differences in baseline measurements of HR and MAP between the two groups, but Group D had significant fall in heart rate (15-20%) (P < 0.001) from 2 min after start of infusion till the end of surgery. In contrast, Group MF had no significant change from baseline till end of surgery (P > 0.05) [Figure 1]. Both the groups had significant reduction in MAP from their respective baseline values, however on analyzing the magnitude of decrease, patients in Group D had a greater fall (10-15%) in comparison to Group MF (5-10%) over a period of time [Figure 2]. Inter-group comparison of MAP at similar time intervals showed no significant difference between the two groups up to the 30th min after start of infusion, subsequent to which Group D had a lower MAP till the end of surgery (P < 0.05). One patient in Group D developed hypotension and bradycardia after completing the loading infusion which was successfully treated with intravenous atropine 0.6 mg and intravenous ephedrine 6 mg. No patient in either group had any episode of hypertension. Respiratory rate [Figure 3] and SpO2 were comparable and within normal limits in both the groups (P > 0.05). There was no episode of desaturation in either group.
Table 1.
Patient characteristics and operative data. Data expressed as Mean (SD) or number (proportion)
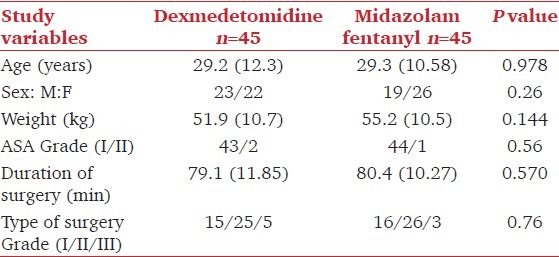
Figure 1.
Changes in heart rate over a period of time. Time ‘0’ start of study drug infusion. Data expressed as mean (Standard deviation) *P< 0.05
Figure 2.
Changes in mean arterial pressure over a period of time. Time ‘0’ start of study drug infusion. Data expressed as mean (Standard deviation) *P< 0.05
Figure 3.
Changes in respiratory rate over a period of time. Time ‘0’ start of study drug infusion. Data expressed as mean (Standard deviation)
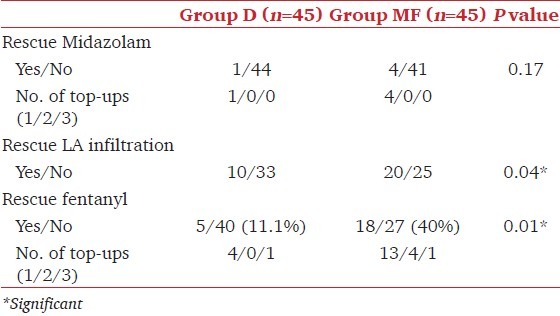
All the patients in both the groups reached RSS of 3 at the end of loading dose infusion, no additional supplementation was required. Two patients each in both the groups required stopping the loading dose infusion in the 8th minute as they had reached the target RSS of 3. In Group D, median (range) dose of dexmedetomidine was 65 μg (41-98) whereas those in Group MF received 3.3 mg (2.2-5.7) of midazolam and 55 μg (37-95) of fentanyl. During surgery, only one patient in Group D required rescue sedation with midazolam when RSS <3 in contrast to four (8.8%) patients in Group MF, though the difference was not significant (P = 0.17). No patient in either group had RSS >3 at any point during surgery. Ten patients in Group D required rescue local anesthetic infiltration in contrast to 20 in Group MF (P = 0.04). In Group MF, significantly more number of patients required rescue fentanyl with 13 patients requiring one dose, four patients requiring two doses and one patient requiring three doses. In contrast only five patients in Group D required rescue analgesic (four patients requiring one dose and one patient requiring three doses of fentanyl) (P = 0.01) [Table 2].
Table 2.
Rescue sedatives and analgesics. Data expressed as number (proportion)
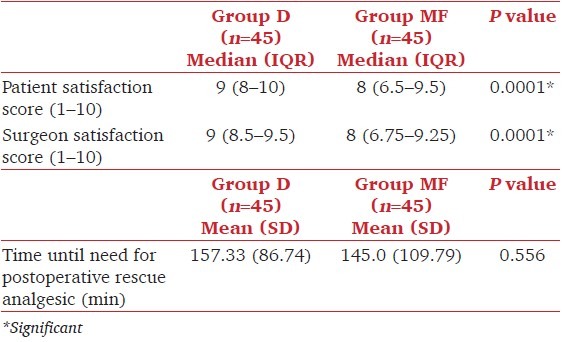
Immediately upon arrival into the recovery room, all the patients were able to obey commands. At the end of 30 min patients in both the groups had reached RSS of 2. Time until need for postoperative analgesic was comparable in both the groups (P = 0.556) [Table 3]. Average patients’ satisfaction with sedation and analgesia was higher in Group D than Group MF (P = 0.0001) [Table 3]. Similarly, surgeons’ satisfaction with patients’ sedation and surgical conditions was higher in Group D than in Group MF (P = 0.0001). In the postoperative period one patient in Group MF had nausea and vomiting which was symptomatically treated. One had shivering and seven patients in Group D (7.8%) had dryness of mouth in contrast to none in Group MF (P = 0.006).
Table 3.
Patient and surgeon satisfaction scores and time to postoperative rescue analgesics
No major adverse events were observed in this study and no patients had to be converted to an alternative sedative or anesthetic therapy in either of the groups.
Discussion
Dexmedetomidine can be safely and effectively used for procedural sedation and surgeries done under MAC.[15–18,21] It’s use in other ENT surgeries like functional endoscopic sinus surgery (FESS), septoplasty, and thyroplasty under MAC has also been documented.[19,22,23] Middle-ear surgeries pose a different set of challenges for the patient, surgeons and anesthesiologists. Sympathetic stimulation and movements of an anxious patient cause increased bleeding and disturb the fine microscopic nature of the surgery which may even lead to graft failure. The advantages of local anesthesia include testing hearing intraoperatively, immediately detecting complications and a truncated postsurgical emergence. Good patient selection, preoperative counseling and use of appropriate sedation are important factors for success of this surgery under local anesthesia.[2,5]
We chose a loading dose of 1 mcg kg-1 of dexmedetomidine based on previous literature[24,25] and studies.[16,17] Reports suggest that on administration of low or moderate doses and slow rates of infusion of dexmedetomidine, α2 agonist effects are observed but not α1 effect.[12,26–29] In view of its short distribution half-life of 5 min dexmedetomidine necessitates that it be given as a maintenance infusion. We selected a maintenance dose of 0.2 mcg kg-1h-1, because the surgery was essentially done under local anesthesia. Increasing the infusion rate of dexmedetomidine to maintain desired levels of sedation would also confer additional analgesia and probably reduce the number of rescue fentanyl top-ups in Group D. To avoid this we used a fixed maintenance dose. Additional sedatives and analgesics if required were provided using midazolam and fentanyl respectively so that the rescue drugs were common in both the groups. The dose of midazolam 0.06 mg kg-1 was chosen based on a recent study by Eren et al.,[30] that this dose is comparable to dexmedetomidine 1 μg kg-1 in terms of sedation. We aimed to compare equivalent doses of both the drugs to avoid any bias in our results. Also, drugs in both the study groups were targeted to a predefined end point (Ramsay score of 3). Fentanyl was added in the other group as midazolam has no analgesic properties and this combination is conventionally used for MAC in our setup. Dexmedetomidine has both sedative and analgesic properties and has been used as a single agent in many painful procedures.[18,19,22]
The lower HR and MAP in Group D in comparison to the midazolam-fentanyl group could be explained by the markedly decreased sympathetic activity.[25] Also, intraoperatively Group MF had more number of patients who complained of pain which was initially treated with infiltration of lignocaine 2% with adrenaline (when VAS <4). Our findings are similar to other studies where lower HR and MAP were observed in the dexmedetomidine group.[16,17,31,32] These results suggest that dexmedetomidine has clinical advantage over midazolam in providing a better operative field for microscopic surgery. Durmus et al.,[33] have evaluated this property of dexmedetomidine for providing controlled hypotension in general anesthesia for tympanoplasty cases and concluded that it is a useful adjuvant to decrease bleeding when a bloodless surgical field is required.
In the present study, in addition to comparable respiratory rates there was no evidence of bradypnea in either of the groups. Dexmedetomidine is unique in that it does not cause respiratory depression because its effects are not mediated by the Ỳ aminobutyric system.[34] These findings are similar to other studies.[17,31] However, Alhashemi et al.,[16] in their comparative study of dexmedetomidine with midazolam for cataract had observed a higher ventilatory frequency in patients receiving midazolam. They attributed the increased respiratory rate to midazolam causing decreased tidal volume and an increase in the respiratory rate as a compensation to maintain minute ventilation.
Our study demonstrated significantly higher patient and surgeon satisfaction scores with dexmedetomidine suggesting a difference in the quality of sedation of both the drugs.[16] The lower HR and MAP in these patients could have probably resulted in a better surgical field thus attributing to better surgeon satisfaction. Moreover, surgeons are satisfied if there is no patient movement during surgery. Lesser number of patients (11.1%) receiving dexmedetomidine demanded rescue analgesics as compared to the midazolam-fentanyl group (40%). Similar findings have been reported by K. Karaaslan et al.,[32] where Group dexmedetomidine used significantly less rescue tramadol in comparison to Group midazolam when both the drugs were compared in FESS and nasal septoplasties. Analgesic property of α2 agonists like dexmedetomidine with its opiate-sparing properties has been documented,[35] and has been reported in studies conducted in general anesthesia with dexmedetomidine.[36] Other studies have also reported better satisfaction scores with dexmedetomidine[6,16,18,31] However, Zeyneloglu et al.,[37] have reported better satisfaction scores with midazolam-fentanyl combination as compared to dexmedetomidine in extracorporeal shock wave lithotripsy (ESWL) when used alone. The authors also concluded that probably it was not effective as a sole agent in ESWL. In our study in spite of higher satisfaction scores in the dexmedetomidine group, both the drugs were comparable in terms of sedation as none of the patients in either group required additional sedation with propofol or any alternative anesthesia technique.
A possible limitation of this study could be that amnesia scoring and cognitive function testing for psychomotor impairment was not done as early discharge of the patients was not a concern of this study. Midazolam has a potent anterograde amnesic effect and dexmedetomidine also results in memory impairment.[11] However, tympanoplasty in our setup is not a daycare procedure, so this issue was not considered as a part of the study. Another limitation could be that the effects of the drugs were seen only in ASA I/II patients. The effects of α2 agonists on the cardiovascular system may be beneficial in high-risk patients.[28,38] Further studies need to be carried out recruiting high-risk patients.
Conclusion
Dexmedetomidine is a comparable alternative to the combination of midazolam-fentanyl for sedation and analgesia in tympanoplasty surgery under local anesthesia. It is associated with better patient and surgeon satisfaction but with a high incidence of dry mouth. However hemodynamic parameters need to be closely monitored.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Jackson CG. Principles of Temporal bone and Skull Base Surgery. In: Glasscock, editor. Surgery of the Ear. 5th ed. New Delhi: Elsevier India; 2003. pp. 264–6. [Google Scholar]
- 2.Sarmento KM, Jr, Tomita S. Retroauricular tympanoplasty and tympanomastoidectomy under local anesthesia and sedation. Acta Otolaryngol. 2009;129:726–8. doi: 10.1080/00016480802398996. [DOI] [PubMed] [Google Scholar]
- 3.Liang S, Irwin MG. Review of anesthesia for middle ear surgery. Anesthesiol Clin. 2010;28:519–28. doi: 10.1016/j.anclin.2010.07.009. [DOI] [PubMed] [Google Scholar]
- 4.Edussuriya B, Goonasekera CD, Rajapakse M, Rajapakse VP, Jayasooriya D. Middle ear surgery under local anaesthesia and sedation. Ceylon Med J. 1997;42:75–7. [PubMed] [Google Scholar]
- 5.Yung MW. Local anaesthesia in middle ear surgery: Survey of patients and surgeons. Clin Otolaryngol Allied Sci. 1996;21:404–8. doi: 10.1046/j.1365-2273.1996.00814.x. [DOI] [PubMed] [Google Scholar]
- 6.Candiotti KA, Bergese SD, Bokesch PM, Feldman MA, Wisemandle W, Bekker AY. Monitored anesthesia care with dexmedetomidine: A prospective, randomized, double-blind, multicenter trial. Anesth Analg. 2010;110:47–56. doi: 10.1213/ane.0b013e3181ae0856. [DOI] [PubMed] [Google Scholar]
- 7.Gan TJ. Pharmacokinetic and pharmacodynamic characteristics of medications used for moderate sedation. Clin Pharmacokinet. 2006;45:855–69. doi: 10.2165/00003088-200645090-00001. [DOI] [PubMed] [Google Scholar]
- 8.Bailey PL, Pace NL, Ashburn MA, Moll JW, East KA, Stanley TH. Frequent hypoxemia and apnea after sedation with Midazolam and fentanyl. Anesthesiology. 1990;73:826–30. doi: 10.1097/00000542-199011000-00005. [DOI] [PubMed] [Google Scholar]
- 9.American Society of Anesthesiologists Task Force on Sedation and Analgesia by Non-Anesthesiologists. Practice guidelines for sedation and analgesia by non-anesthesiologists. Anesthesiology. 2002;96:1004–17. doi: 10.1097/00000542-200204000-00031. [DOI] [PubMed] [Google Scholar]
- 10.Bhananker SM, Posner KL, Cheney FW, Caplan RA, Lee LA, Domino KB. Injury and liability associated with monitored anesthesia care: A closed claims analysis. Anesthesiology. 2006;104:228–34. doi: 10.1097/00000542-200602000-00005. [DOI] [PubMed] [Google Scholar]
- 11.Hall JE, Uhrich TD, Barney JA, Arain SR, Ebert TJ. Sedative, amnestic, and analgesic properties of small-dose dexmedetomidine infusions. Anesth Analg. 2000;90:699–705. doi: 10.1097/00000539-200003000-00035. [DOI] [PubMed] [Google Scholar]
- 12.Ebert TJ, Hall JE, Barney JA, Uhrich TD, Colinco MD. The effects of increasing plasma concentrations of Dexmedetomidine in humans. Anesthesiology. 2000;93:382–94. doi: 10.1097/00000542-200008000-00016. [DOI] [PubMed] [Google Scholar]
- 13.Cortinez LI, Hsu YW, Sum-Ping ST, Young C, Keifer JC, Robertson KM, et al. Dexmedetomidine pharmacodynamics: Part I. Crossover comparison of the respiratory effects of Dexmedetomidine and remifentanil in healthy volunteers. Anesthesiology. 2004;101:1066–76. doi: 10.1097/00000542-200411000-00005. [DOI] [PubMed] [Google Scholar]
- 14.Arain SR, Ebert TJ. The efficacy, side effects, and recovery characteristics of Dexmedetomidine versus propofol when used for intraoperative sedation. Anesth Analg. 2002;95:461–6. doi: 10.1097/00000539-200208000-00042. [DOI] [PubMed] [Google Scholar]
- 15.Abdalla MI, Mansouri FA, Bener A. Dexmedetomidine during local anesthesia. J Anesth. 2006;20:54–6. doi: 10.1007/s00540-005-0351-z. [DOI] [PubMed] [Google Scholar]
- 16.Alhashemi JA. Dexmedetomidine vs. Midazolam for monitored anaesthesia care during cataract surgery. Br J Anaesth. 2006;96:722–6. doi: 10.1093/bja/ael080. [DOI] [PubMed] [Google Scholar]
- 17.Cheung CW, Ying CL, Chiu WK, Wong GT, Ng KF, Irwin MG. A comparison of Dexmedetomidine and Midazolam for sedation in third molar surgery. Anaesthesia. 2007;62:1132–8. doi: 10.1111/j.1365-2044.2007.05230.x. [DOI] [PubMed] [Google Scholar]
- 18.Demiraran Y, Korkut E, Tamer A, Yorulmaz I, Kocaman B, Sezen G, et al. The comparison of Dexmedetomidine and Midazolam used for sedation of patients during upper endoscopy: A prospective, randomized study. Can J Gastroenterol. 2007;21:25–9. doi: 10.1155/2007/350279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goksu S, Arik H, Demiryurek S, Mumbuc S, Oner U, Demiryurek AT. Effects of Dexmedetomidine infusion in patients undergoing functional endoscopic sinus surgery under local anaesthesia. Eur J Anaesthesiol. 2008;25:22–8. doi: 10.1017/S0265021507001317. [DOI] [PubMed] [Google Scholar]
- 20.Ramsay MA, Savege TM, Simpson BR, Goodwin R. Controlled sedation with alphaxone-alphadolone. Br Med J. 1974;2:656–9. doi: 10.1136/bmj.2.5920.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taghinia AH, Shapiro FE, Slavin SA. Dexmedetomidine in aesthetic facial surgery: Improving anesthetic safety and efficacy. Plast Reconstr Surg. 2008;121:269–76. doi: 10.1097/01.prs.0000293867.05857.90. [DOI] [PubMed] [Google Scholar]
- 22.Dogan R, Erbek S, Gonencer HH, Erbek HS, Isbilen C, Arslan G. Comparison of local anaesthesia with Dexmedetomidine sedation and general anaesthesia during septoplasty. Eur J Anaesthesiol. 2010;27:960–4. doi: 10.1097/EJA.0b013e32833a45c4. [DOI] [PubMed] [Google Scholar]
- 23.Busick T, Kussman M, Scheidt T, Tobias JD. Preliminary experience with dexmedetomidine for monitored anesthesia care during ENT surgical procedures. Am J Ther. 2008;15:520–7. doi: 10.1097/MJT.0b013e31815ae755. [DOI] [PubMed] [Google Scholar]
- 24.Bhana N, Goa KL, McClellan KJ. Dexmedetomidine. Drugs. 2000;59:263–8. doi: 10.2165/00003495-200059020-00012. [DOI] [PubMed] [Google Scholar]
- 25.Kamibayashi T, Maze M. Clinical uses of alpha2- adrenergic agonists. Anesthesiology. 2000;93:1345–9. doi: 10.1097/00000542-200011000-00030. [DOI] [PubMed] [Google Scholar]
- 26.McCutcheon CA, Orme RM, Scott DA, Davies MJ, McGlade DP. Comparison of Dexmedetomidine versus conventional therapy for sedation and hemodynamic control during carotid endarterectomy performed under regional anesthesia. Anesth Analg. 2006;102:668–75. doi: 10.1213/01.ane.0000197777.62397.d5. [DOI] [PubMed] [Google Scholar]
- 27.Dyck JB, Maze M, Haack C, Vuorilehto L, Shafer SL. The pharmacokinetics and hemodynamic effects of intravenous and intramuscular dexmedetomidine hydrochloride in adult human volunteers. Anesthesiology. 1993;78:813–20. doi: 10.1097/00000542-199305000-00002. [DOI] [PubMed] [Google Scholar]
- 28.Bloor BC, Ward DS, Belleville JP, Maze M. Effects of intravenous Dexmedetomidine in humans. II. Hemodynamic changes. Anesthesiology. 1992;77:1134–42. doi: 10.1097/00000542-199212000-00014. [DOI] [PubMed] [Google Scholar]
- 29.Paris A, Tonner PH. Dexmedetomidine in anaesthesia. Curr Opin Anaesthesiol. 2005;18:412–8. doi: 10.1097/01.aco.0000174958.05383.d5. [DOI] [PubMed] [Google Scholar]
- 30.Eren G, Cukurova Z, Demir G, Hergunsel O, Kozanhan B, Emir NS. Comparison of Dexmedetomidine and three different doses of Midazolam in preoperative sedation. J Anaesthesiol Clin Pharmacol. 2011;27:367–72. doi: 10.4103/0970-9185.83684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Na HS, Song IA, Park HS, Hwang JW, Do SH, Kim CS. Dexmedetomidine is effective for monitored anesthesia care in outpatients undergoing cataract surgery. Korean J Anesthesiol. 2011;61:453–9. doi: 10.4097/kjae.2011.61.6.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Karaaslan K, Yilmaz F, Gulcu N, Colak C, Sereflican M, Kocoglu H. Comparison of dexmedetomidine and midazolam for monitored anesthesia care combined with tramadol via patient-controlled analgesia in endoscopic nasal surgery: A prospective, randomized, double-blind, clinical study. Curr Ther Res Clin Exp. 2007;68:69–81. doi: 10.1016/j.curtheres.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Durmus M, But AK, Dogan Z, Yucel A, Miman MC, Ersoy MO. Effect of Dexmedetomidine on bleeding during tympanoplasty or septorhinoplasty. Eur J Anaesthesiol. 2007;24:447–53. doi: 10.1017/S0265021506002122. [DOI] [PubMed] [Google Scholar]
- 34.Gerlach AT, Dasta JF. Dexmedetomidine: An updated review. Ann Pharmacother. 2007;41:245–52. doi: 10.1345/aph.1H314. [DOI] [PubMed] [Google Scholar]
- 35.Smith H, Elliott J. Alpha (2) receptors and agonists in pain management. Curr Opin Anaesthesiol. 2001;14:513–8. doi: 10.1097/00001503-200110000-00009. [DOI] [PubMed] [Google Scholar]
- 36.Keniya VM, Ladi S, Naphade R. Dexmedetomidine attenuates sympathetoadrenal response to tracheal intubation and reduces perioperative anaesthetic requirement. Indian J Anaesth. 2011;55:352–7. doi: 10.4103/0019-5049.84846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zeyneloglu P, Pirat A, Candan S, Kuyumcu S, Tekin I, Arslan G. Dexmedetomidine causes prolonged recovery when compared with Midazolam/fentanyl combination in outpatient shock wave lithotripsy. Eur J Anaesthesiol. 2008;25:961–7. doi: 10.1017/S0265021508004699. [DOI] [PubMed] [Google Scholar]
- 38.Aantaa R, Jalonen J. Perioperative use of alpha2-adrenoceptor agonists and the cardiac patient. Eur J Anaesthesiol. 2006;23:361–72. doi: 10.1017/S0265021506000378. [DOI] [PubMed] [Google Scholar]


