Abstract
Heme synthase (ferrochelatase) activity, as determined by the chelation of ferrous iron to protoporphyrin or deuteroporphyrin, is reduced to 10-25% of normal in tissues of patients with protoporphyria. With cultured skin fibroblasts from seven patients with protoporphyria and six normal individuals, the present studies examined the enzymatic defect.
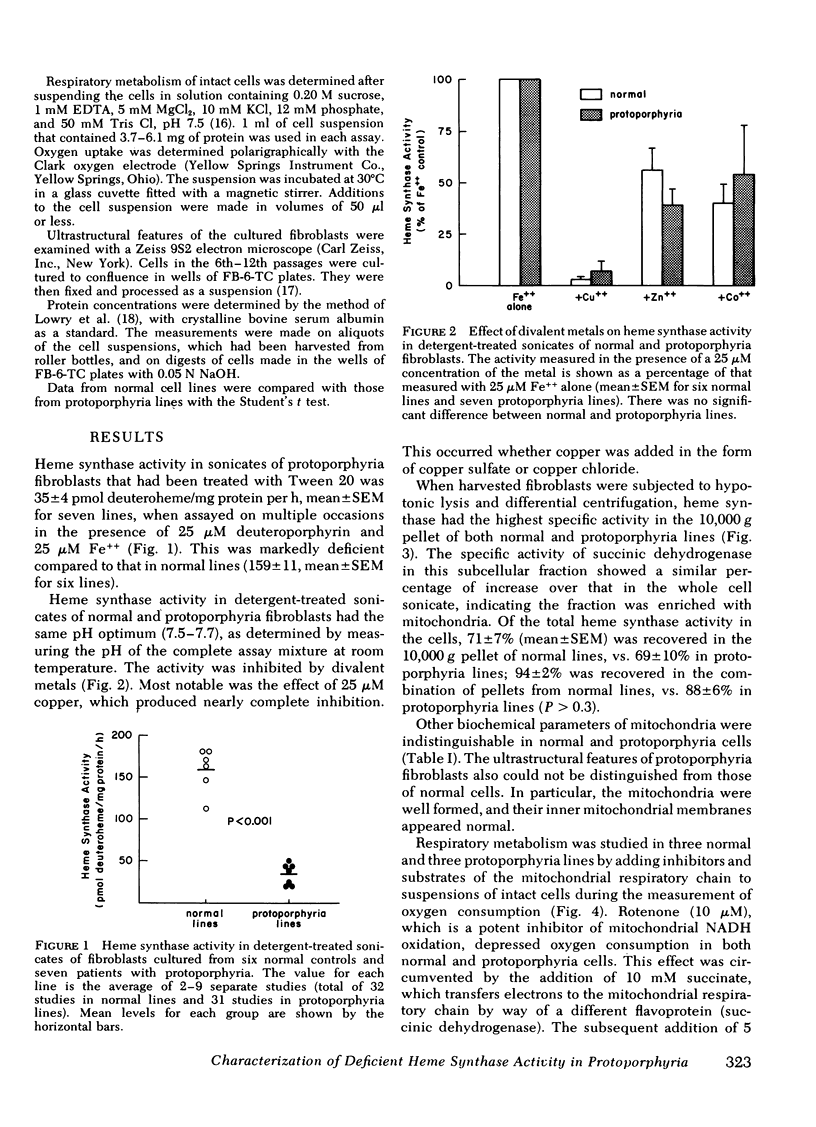
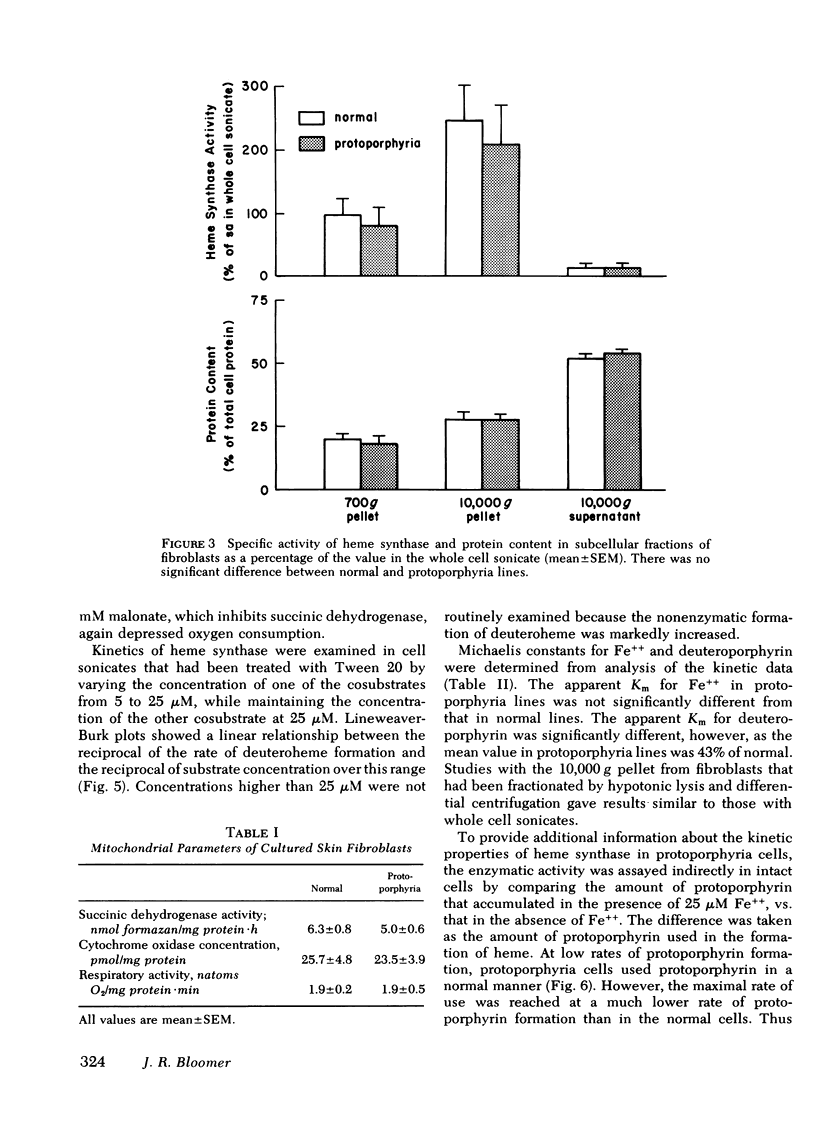
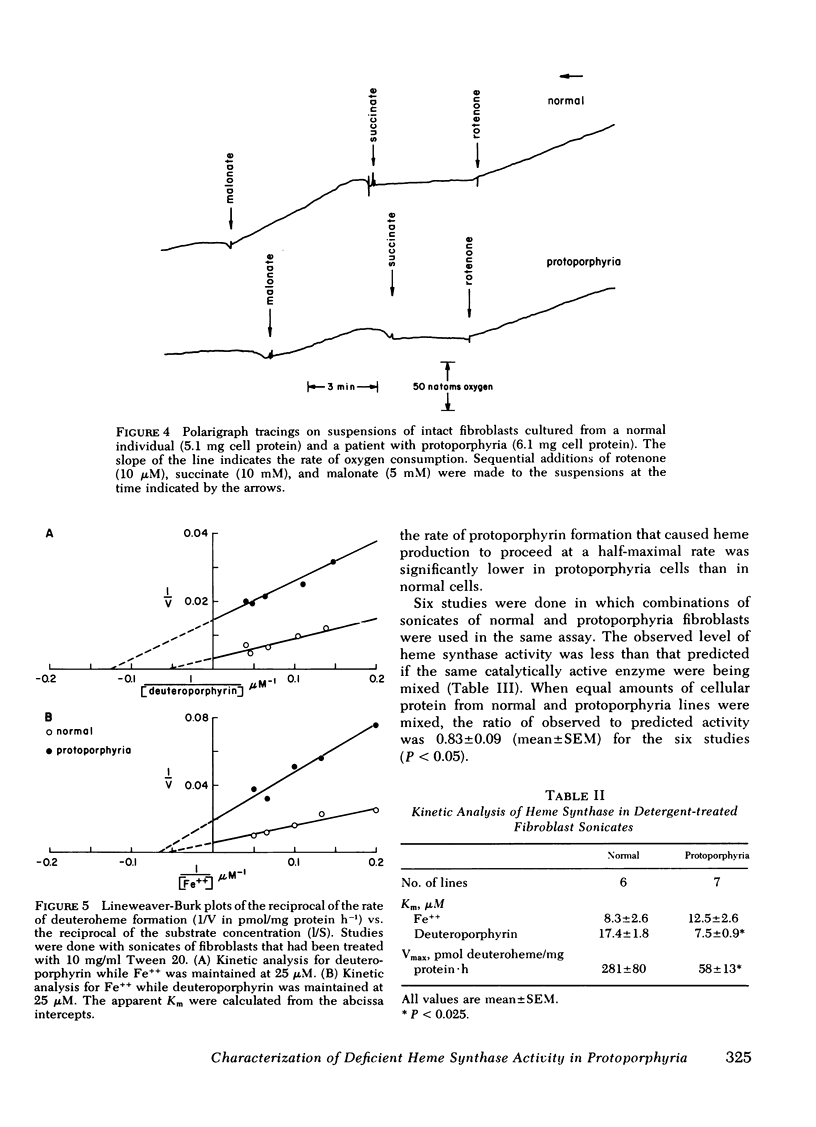
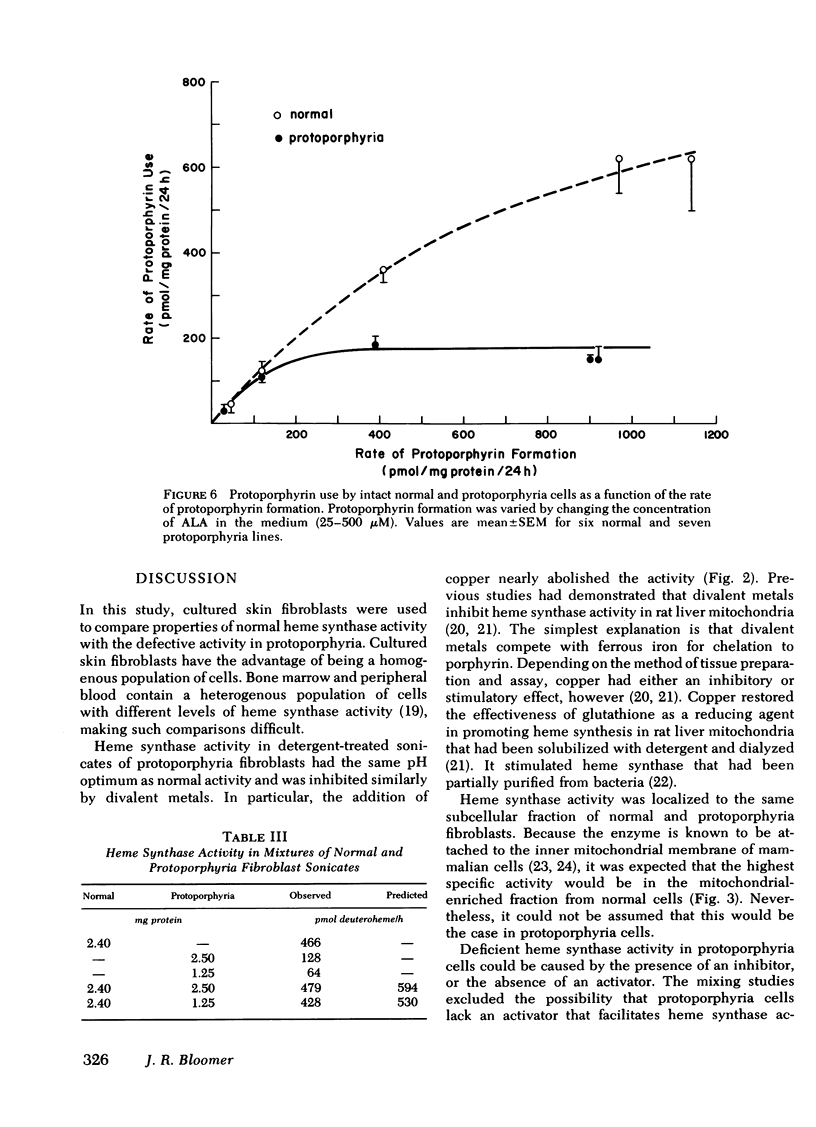
Heme synthase activity in normal and protoporphyria fibroblasts had the same pH optimum, showed similar inhibition by divalent metals, and had the highest specific activity in the mitochondrial-enriched fraction. The ultrastructural features and other biochemical parameters of mitochondria were normal in protoporphyria cells, excluding a general mitochondrial defect. Measurement of the rate of deuteroheme formation at different concentrations of substrate demonstrated a significant reduction in the apparent Km for deuteroporphyrin in detergent-treated sonicates of protoporphyria fibroblasts compared to normal (7.5 ± 0.9 μM, mean ± SEM, vs. 17.4 ± 1.8), as well as a decrease in the velocity of reaction (mean level was 21% of normal). Studies with intact cells, in which heme synthase activity was estimated indirectly, also indicated that the apparent Km for porphyrin substrate was significantly lower in protoporphyria lines.
These data show that heme synthase in protoporphyria fibroblasts has markedly reduced catalytic activity despite an increased affinity for porphyrin substrate. This could be caused by either a change in the enzyme protein, or an alteration of its micro-environment.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Becker D. M., Viljoen J. D., Katz J., Kramer S. Reduced ferrochelatase activity: a defect common to porphyria variegata and protoporphyria. Br J Haematol. 1977 Jun;36(2):171–179. doi: 10.1111/j.1365-2141.1977.tb00637.x. [DOI] [PubMed] [Google Scholar]
- Bloomer J. R., Bonkowsky H. L., Ebert P. S., Mahoney M. J. Inheritance in protoporphyria. Comparison of haem synthetase activity in skin fibroblasts with clinical features. Lancet. 1976 Jul 31;2(7979):226–228. doi: 10.1016/s0140-6736(76)91027-8. [DOI] [PubMed] [Google Scholar]
- Bloomer J. R., Brenner D. A., Mahoney M. J. Study of factors causing excess protoporphyrin accumulation in cultured skin fibroblasts from patients with protoporphyria. J Clin Invest. 1977 Dec;60(6):1354–1361. doi: 10.1172/JCI108895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonkowsky H. L., Bloomer J. R., Ebert P. S., Mahoney M. J. Heme synthetase deficiency in human protoporphyria. Demonstration of the defect in liver and cultured skin fibroblasts. J Clin Invest. 1975 Nov;56(5):1139–1148. doi: 10.1172/JCI108189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottomley S. S., Tanaka M., Everett M. A. Diminished erythroid ferrochelatase activity in protoporphyria. J Lab Clin Med. 1975 Jul;86(1):126–131. [PubMed] [Google Scholar]
- Brenner D. A., Bloomer J. R. Heme content of normal and porphyric cultured skin fibroblasts. Biochem Genet. 1977 Dec;15(11-12):1061–1070. doi: 10.1007/BF00484497. [DOI] [PubMed] [Google Scholar]
- Brodie M. J., Moore M. R., Thompson G. G., Goldbrrg A., Holti G. Haem biosynthesis in peripheral blood in erythropoietic protoporphyria. Clin Exp Dermatol. 1977 Dec;2(4):381–388. doi: 10.1111/j.1365-2230.1977.tb01579.x. [DOI] [PubMed] [Google Scholar]
- Dailey H. A., Jr Purification and characterization of the membrane-bound ferrochelatase from Spirillum itersonii. J Bacteriol. 1977 Oct;132(1):302–307. doi: 10.1128/jb.132.1.302-307.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAEGER-ARONSEN B. ERYTHROPOIETIC PROTOPORPHYRIA. A NEW TYPE OF INBORN ERROR OF METABOLISM. Am J Med. 1963 Oct;35:450–454. doi: 10.1016/0002-9343(63)90144-x. [DOI] [PubMed] [Google Scholar]
- Jones M. S., Jones O. T. The structural organization of haem synthesis in rat liver mitochondria. Biochem J. 1969 Jul;113(3):507–514. doi: 10.1042/bj1130507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LABBE R. F., HUBBARD N. Metal specificity of the ironprotoporphyrin chelating enzyme from rat liver. Biochim Biophys Acta. 1961 Sep 2;52:130–135. doi: 10.1016/0006-3002(61)90910-6. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Langelaan D. E., Losowsky M. S., Toothill C. Haem synthetase activity of human blood cells. Clin Chim Acta. 1970 Mar;27(3):453–459. doi: 10.1016/0009-8981(70)90298-6. [DOI] [PubMed] [Google Scholar]
- Mahoney M. J., Hart A. C., Steen V. D., Rosenberg L. E. Methylmalonicacidemia: biochemical heterogeneity in defects of 5'-deoxyadenosylcobalamin synthesis. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2799–2803. doi: 10.1073/pnas.72.7.2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazanowska A. M., Neuberger A., Tait G. H. Effect of lipids and organic solvents on the enzymic formation of zinc protoporphyrin and haem. Biochem J. 1966 Jan;98(1):117–127. doi: 10.1042/bj0980117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazanowska A., Dancewicz A. M., Malinowska T., Kowalski E. Chelation of iron and zinc by protoporphyrin catalyzed by mitochondrial preparations. Eur J Biochem. 1969 Feb;7(4):583–587. doi: 10.1111/j.1432-1033.1969.tb19646.x. [DOI] [PubMed] [Google Scholar]
- McKay R., Druyan R., Getz G. S., Rabinowitz M. Intramitochondrial localization of delta-aminolaevulate synthetase and ferrochelatase in rat liver. Biochem J. 1969 Sep;114(3):455–461. doi: 10.1042/bj1140455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassa S., Zalar G. L., Kappas A. Studies in porphyria. VII. Induction of uroporphyrinogen-I synthase and expression of the gene defect of acute intermittent porphyria in mitogen-stimulated human lymphocytes. J Clin Invest. 1978 Feb;61(2):499–508. doi: 10.1172/JCI108961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawada H., Takeshita M., Sugita Y., Yoneyama Y. Effect of lipid on protoheme ferro-lyase. Biochim Biophys Acta. 1969 Mar 18;178(1):145–155. doi: 10.1016/0005-2744(69)90141-7. [DOI] [PubMed] [Google Scholar]
- Shephard E. H., Hübscher G. Phosphatidate biosynthesis in mitochondrial subfractions of rat liver. Biochem J. 1969 Jun;113(2):429–440. doi: 10.1042/bj1130429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson D. M., Poulson R. Effects of lipids on the activity of ferrochelatase. Biochim Biophys Acta. 1977 Jun 10;482(2):461–469. doi: 10.1016/0005-2744(77)90260-1. [DOI] [PubMed] [Google Scholar]
- Steinman R. M., Cohn Z. A. The interaction of soluble horseradish peroxidase with mouse peritoneal macrophages in vitro. J Cell Biol. 1972 Oct;55(1):186–204. doi: 10.1083/jcb.55.1.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warshaw J. B., Terry M. L. Cellular energy metabolism during fetal development. II. Fatty acid oxidation by the developing heart. J Cell Biol. 1970 Feb;44(2):354–360. doi: 10.1083/jcb.44.2.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Goeij A. F., Christianse K., van Steveninck J. Decreased haem synthetase activity in blood cells of patients with erythropoietic protoporphyria. Eur J Clin Invest. 1975 Sep 12;5(5):397–400. doi: 10.1111/j.1365-2362.1975.tb00470.x. [DOI] [PubMed] [Google Scholar]


