Abstract
Network-based intervention has been a trend of curing systemic diseases, but it relies on regimen optimization and valid multi-target actions of the drugs. The complex multi-component nature of medicinal herbs may serve as valuable resources for network-based multi-target drug discovery due to its potential treatment effects by synergy. Recently, robustness of multiple systems biology platforms shows powerful to uncover molecular mechanisms and connections between the drugs and their targeting dynamic network. However, optimization methods of drug combination are insufficient, owning to lacking of tighter integration across multiple ‘-omics’ databases. The newly developed algorithm- or network-based computational models can tightly integrate ‘-omics’ databases and optimize combinational regimens of drug development, which encourage using medicinal herbs to develop into new wave of network-based multi-target drugs. However, challenges on further integration across the databases of medicinal herbs with multiple system biology platforms for multi-target drug optimization remain to the uncertain reliability of individual data sets, width and depth and degree of standardization of herbal medicine. Standardization of the methodology and terminology of multiple system biology and herbal database would facilitate the integration. Enhance public accessible databases and the number of research using system biology platform on herbal medicine would be helpful. Further integration across various ‘-omics’ platforms and computational tools would accelerate development of network-based drug discovery and network medicine.
Keywords: network-based drug discovery, systems biology, bioinformatics, computational technologies, network medicine
INTRODUCTION
In recent decades, the decreased efficiency of new drug invention has alarmed the pharmaceutical industry, especially by giving considerable investment in research and development [1, 2]. The reductionist approach in medical research can yield only a limited understanding of complicated pathogenesis and multi-target pathologies of systemic diseases, and it has difficulty in identifying relevant interventions to target such complexities. Clearly, bullet-based or mono-target drug intervention cannot effectively combat the complex pathologies of systemic diseases like cancers, cardiovascular diseases and neurodegenerative disorders [2], because those diseases are regulated by complex biological networks and depend on multiple steps of genetic and environmental challenges to progress [1–3]. In clinic, many mono-therapies have been shown to have limited effects or too many adverse effects in long-term treatment for systemic diseases because of the disease nature of natural evolution of feedback loop and pathway redundancy. For example, mono-target treatment in cancer therapy may give adequate time for cancer cells to develop acquired resistance by evolution to the drug [1–3]; while multi-target therapeutics might be more efficacious or less vulnerable to allowing adaptive drug resistance because the biological system is less able to simultaneously compensate for multiple actions produced by two or more drugs [1–3].
Those clinical drugs which have been found with multi-target actions are encouraged to explore new rounds of drug repositioning [2]. One excellent example is metformin. It is a first-line drug for Type II diabetes [2], but recently found to have cancer inhibiting properties [4]. Another example is berberine. This is a classic anti-microbial drug derived from a medical plant that has been also shown to lower cholesterol levels in human [5]. Such examples suggest that currently marketed drugs may have additional, but as yet unknown physiological actions valuable in treating other conditions. Traditional Chinese Medicine (TCM) has been using herbal formulas to treat complex diseases for thousands of years. A herbal formula is often composed of several herbs with multiple chemical ingredients that may have multiple targets and treatment functions which were unknown in the past. Now, it is possible to link the network-based treatment principle of herbal medicine with the pathological target network and optimize the combined-dosage of the essential components. All in all, network-based drug discovery is taking the pharmaceutical industry into a new age where efficient use of systems biology and computational technologies for medicinal herbs investigation will function as a powerful engine for multi-target drug discovery and development of network medicine.
STRATEGIES FOR MULTI-TARGETING
Multi-target intervention drugs have been proven effective and better to those complex diseases than the conventional mono-target drugs that are mostly marketed today. Multi-target drugs can be produced with one single chemical or with a composition of several chemicals; however, most are multi-component and able to comprehensively target the characteristic pathological network of a disease (Table 1).
Table 1:
Examples of multi-target drugs/preparations for treatment of human diseases
| Actions | Diseases | Action characteristics or mechanism | Drugs/preparations | References |
|---|---|---|---|---|
| Anti-viral | AIDS | Targeting different steps in the HIV-1 replication cycle | Highly Active Antiretroviral Therapy (HAART) | [7] |
| Inhibiting HIV-1 entry into cells by interfering with the gp41 six-helix bundle formation, thus blocking HIV-1 fusion. At the same time, inhibiting HIV-1 reverse transcriptase, protease and integrase activities | Tannin-a polyphenolic compound extracted from a Chinese medical herb | [16] | ||
| Anti-microbial | Malaria | Potentiating the anti-microbial action of Berberine by acting as Multi-Drug Resistance (MDR) inhibitor via inhibition of MDR efflux | Berberine, 5′-methoxy-hydnocarpin | [8] |
| Acting at different stages of the asexual parasite cycle | Artemisinin, meflorquine, fansidar | [19] | ||
| Infections | Inhibiting β-lactamase of the bacteria by potassium clavulanate and then preventing degradation of amoxicillin | Augmentin (amoxicillin and clavulanic acid) | [9] | |
| Anti-cancer | Non-Hodgkin’s lymphoma | Reducing toxicity and increasing overall survival by using CHOP than using individual drugs | Combination regimen of ‘CHOP’-Cyclophosphamide, Doxorubicin, Vincristine, Prednisone | [10, 11] |
| Chronic myelogenous leukemia (CML) | Targeting BCR-ABL | Dasatinib, Nilotinib, T315I | [12] | |
| Melanoma | Targeting V600E BRAF mutation and increasing cancer killing efficacy | PLX4032, MEK inhibitor | [12, 13] | |
| Colon cancer | Increasing the anti-tumor potency as well as reducing toxicity of CPT-11 by PHY906 | PHY906-a four herb Chinese medicinal formula and CPT-11 | [25] | |
| Immuno-modulation | Rheumatoid arthritis | Producing synergy in immunotolerance induction by inhibiting PKCθ and augmenting NFAT pathway of T cells | Cocktail preparation for immunotolerance induction | [20] |
| Influencing the pharmacokinetic behavior and metabolism of paeonol by QFGJS, an herbal preparation derived from a Chinese herbal formula | QFGJS and paeonol | [26] | ||
| Improving intestinal transport and absorption of paeoniflorin by sinomenine | Paeoniflroin and sinomenine | [27] | ||
| Reducing acute toxicity of aconitine via alternating its pharmacokinetics by paeoniflroin | Paeoniflroin and aconitine | [28] |
For designing network-based multi-target drugs, the strategies can be classified into three types [6]. The first type is formulating a drug with multiple chemical components that could tackle the multiple major pathogens or pathologies of a disease. For example, antiretroviral triple cocktail therapy for AIDS control can effectively defend against resistance by simultaneously suppressing HIV fusion and interfering with viral protein translation and transcription by using an HIV fusion inhibitor, a protease inhibitor and a reverse transcriptase inhibitor together in one drug. This combined therapy not only reduces a patient’s viral load down to almost an undetectable level but also restores white and red blood cells toward normal levels [7]. A second type of strategy is to design drugs to produce overall therapeutic synergy based on mutual complements, by which one component may perform the major function while others act as adjuvants to enhance efficacy of the major component or reducing toxicity of the drug. Berberine significantly inhibits growth of Staphylococcus aureus and Microcystis aeruginosa when it is used with 5′-methoxy-hydnocarpin (5′-MHC). The latter acts as a multi-drug resistance (MDR) inhibitor and therefore greatly potentiates the antimicrobial effect of the former [8]. Augmentin, one of the most widely used commercial antibiotics is in fact composed of two chemicals, namely amoxicillin and clavulanic acid. Amoxicillin is a β-lactam antibiotic that acts by inhibiting biosynthesis of bacterial cell-wall mucopeptide. Potassium clavulanate, as an adjuvant of the drug, inhibits β-lactamase and prevents the degradation of amoxicillin. With such a combination, optimum anti-bacterial synergy has been successfully produced [9]. The third type of strategy, often found in cancer treatment regimens, is to design a drug that interferes with multiple avenues of pathological cross-talk of cancer cells and thereby reduces chances of the cancer cells developing drug resistance. For instance, a four-drug combination, cyclophosphamide, doxorubicin, vincristine and prednisone, known as ‘CHOP’, has been used to treat non-Hodgkin’s lymphoma with more effective outcomes and less toxicities than any single drug [10, 11]. And, Dasatinib or Nilotinib and T315I inhibitor in combination with Gleevec are proposed to treat chronic myelogenous leukemia (CML) by targeting BCR–ABL fusion proteins [12]. V600E BRAF mutation and MEK activation are commonly found in melanoma; and therefore a combined use of BRAF inhibitor PLX4032 and MEK inhibitor has been used to increase therapeutic efficacy [12, 13].
NETWORK-BASED DRUG DISCOVERY FROM HERBS
Medicinal herbs are by nature complex, and their efficacy relies on multi-target intervention via their multiple active components. Thus, they can be examined for developing network-based multi-component drugs [14, 15]. The active components of medicinal herbs may have synergistic effect with currently marketed chemical drugs. For instance, a combination of the HIV triple cocktail therapy with Tannin, a polyphenolic fraction derived from a medicinal herb, has been shown to produce a significant, synergistic and long lasting effect in stabilizing HIV virus propagation by tackling the complex multiple components of HIV-1. Tannin suppresses HIV-1 reverse transcriptase, protease and intergrase activities and blocks virus fusion and virus entry to the host cells [16]. Another typical example is artemisinin, an anti-malarial drug purified from the Chinese medicinal herb Qinghao; its discoverer won the Lasker Award in 2011. However, effective treatment relies on combinational use of artemisinin and other chemical drugs. Combined use of artemisinin and chloroquine reverses drug resistance in malaria to against Plasmodium falciparum, the anti-malarial activity of artemisinin was significantly enhanced [17, 18]. In addition, combinational use of meflorquine, fansidar and artemisinin were found to be more effective through influences of multiple stages of the asexual parasite cycle in chloroquine-resistant patients [19]. Recently, we proposed to develop cocktail preparations for immunetolerance induction in T cells by using two chemicals derived from medical plants, of which one targets the PKCθ pathway while another targets the NFAT pathway [20].
Traditional medicinal herbal formulas and individualized treatment concept may provide the major sources for developing multi-target drugs. While traditional medicinal treatment is based on holistic treatment principle, herbal formulas are usually formulated based on long-term experiences of practitioners. Network-based multi-target drugs could be developed from herbal formulas by firstly evaluating the efficacy of the original herbal formula, followed by isolation of the major bioactive components from each herb and then redevelopment of a completely new multi-component formulation composed of the major bioactive components in order to reach a synergistic and optimal combination [6, 21]. In addition, the ‘personalized’ concept of herbal medicine is pivotal element of effective herbal formula design to cure individual patients’ disease pattern. Thus, without selecting the right group of patients, the real effect of herbal formula could not be shown. Recently, we proposed that prescribing tailored-made personalized multi-target regimen specifically targeting individual molecular dynamics network profiles; and new design of personalized randomized clinical trials (PRCTs) are fundamentally important for proving the real clinical treatment efficacy of network-based treatment strategy of herbal medicine [22]. Now, it is also recognized that personal ‘-omics’ profiling may be crucial factor affecting the results of RCTs, thus the recent Crizotinib trial no longer relied on control arm for placebo treatment, drug arm enrollments are based on patients’ genetic makeup by which NSCLC patients with the presence of Crizotinib sensitive EML4–ALK fusion gene mutation are selected [23, 24]. Such patient pre-selection has shortened the FDA approval time for crizontinib. Hence, the revolution of network-based drug discovery can be strengthened by integrating modern systems biology technologies and personalized multi-target treatment concept of herbal medicine.
As pioneer investigators of multi-target action of medicinal herbs, Lam et al. recently demonstrated a four-herb formula (PHY906) can increase anti-tumor activity of CPT-11 and reduce toxicity and side effects in murine colon 38 allograft model [25]. PHY906 contains multiple components that can inhibit multiple inflammatory responses like TNF-α-induced NF-κ-B-mediated transcriptional activity, COX-2 and iNOS enzyme activities, and simultaneously reduce intestinal damage caused by CPT-11. This drug is undergoing clinical trials as an adjuvant remedy with chemotherapy of CPT-11 [25].To elucidate synergy of herbal components, we previously demonstrated that the anti-arthritic herbal formula QFGJS could markedly influence pharmacokinetic behavior and metabolism of paeonol in rats [26], while combinational use of paeoniflroin and sinomenine purified from two individual herbs contained in QFGJS could produce pharmacokinetic synergy in vivo [27]. As such, the bioactive components in QFGJS may interact and then produce synergistic effect for treating arthritis, at least by alternating pharmacokinetic behavior of those components [28].
UTILIZING MULTIPLE ‘-OMICS’ PLATFORMS
Uncover the target network with ‘-omics’ technologies
‘-omics’ technologies have been shown as the most powerful technical platforms for uncovering dynamic correlations within the multi-target networks and drug actions (Table 2). Also, they can provide efficient tools to better define the global picture of disease status and dynamic interaction of pathological targets at the molecular network level; while all of these information can be used for drug design based on network targeting. The ‘-omics’ technologies can also serve as new tools for identifying the target network of a herbal formula.
Table 2:
Current ‘-omics’ platforms in systems biology for elucidating multiple targets and network of human diseases and drug actions
| Platforms | Techniques | Applications | Findings | References |
|---|---|---|---|---|
| Genomic platform | Array comparative genomic hybridization array | Analysis of DNA copy number gain or loss | Global analysis of DNA copy number change across chromosomes between normal and pathological samples | [29] |
| Single cell exome sequencing | Analysis the mutational profiles of the whole exome of intratumoral cells | Spectrumsclear cell renal cell carcinoma (ccRCC) tumor did not contain any significant clonal subpopulations and mutations that had different allele frequencies within the population also had different mutation | [30] | |
| RNAi platform | Multiplex RNAi screening | Analyzing accumulated genetic alternation of loss-of-function phenotypes in vitro | Profiling the essential genes in human mammalian cells by multiplex RNAi screening | [31] |
| Transcriptomic platform | Gene expression array | Discovery of whole genome gene expression profile of a disease | Rheumatoid arthritis (RA) patients diagnosed with TCM Heat or Cold pattern | [32] |
| Gene expression array | Examining the action of Si–Wu–Tang (SWT) in treating women menstrual discomfort, climacteric syndrome, peri- or postmenopausal syndrome and other ostrogen-related diseases | Identifying the nuclear factor erythroid 2-related factor 2 (Nrf2) cytoprotective pathway is the most significantly affected by Si–Wu–Tang (SWT) | [33] | |
| Clinical sample platform | Tissue array/cellular array | Identifying specific cellular components within tissue or single cells | Identifying the proteomic profiles of preeclampsia tissue and normal placenta tissue using recombinant antibody microarrays | [34] |
| Proteomic platform | 2D gel-MS/MS | Detecting global targets and candidate proteins | Identifying proteomic profiles of human pathogenesis for molecular targeting | [35] |
| 2D gel-MS/MS | Detecting network targets response to drug | Identifying a network of 21 differentiated regulated core proteins response to Ganoderic acid D | [36] | |
| Metabolomic platform | GC-MS/MS | Studying the effect of drugs from metabolites | Identifying the differential metabolic profiles of the Xiaoyaosan-treated chronic unpredictable mild stress rats and control rats | [37] |
| LC-MS/MS | Detecting metabolomics markers from serum | Identifying pentol glucuronide as relevant serum biomarkers of epithelium ovarian cancer | [38] | |
| Microbiome platform | Large scale sequencing-based analysis of microbial genomes | Global genomic analysis of human microbiome and earth microbiome | Identification of specific contribution of symbiotic-pathogen in human and earth to host’s pathology and drug metabolism | [39–44] |
| Gut-Microbiota-mediated drug metabolism | Gut-Microbiota-Drug interaction analysis | Identification of new compound K, which is a gingseng metabolite metabolized by human gut microbiota, possess significant stronger cancer prevention activity | [55] | |
| Pharmacogenomics platform | SNP analysis | Identification of subgroup of patients receiving particular types of treatment or drug dosage | Determining a maintenance dose for warfarin based on the CYP2C9 and VKORC1 genotypes | [46] |
| Identification of SNP that is associated to drug treatment outcome | Identification of BIM polymorphic deletion is associated with shorter progression-free survival in NSCLC patients with EGFR activating mutation after TKI therapy | [49] | ||
| Mutation analysis | Identifying a subgroup of patients receiving treatment benefit based on individual mutational profiles | Identifying a subgroup of non-small cell lung cancer patients receiving Gefitinib treatment benefit based on EGFR mutation pattern | [47, 48] | |
| Chemical screening platform | High-throughput screening of natural products for cancer therapy and data collection | High-throughput screening of useful biological active small molecules from natural products | Discovering drugs for cancer therapy and screening bioactive components from medicinal herbs | [50, 51] |
| Herbalomics | Identifying potential benefit and toxicity of the components in herbals | Herbalome chips in which arrays of compounds are screened for their binding to key peptides as well as doing the multi-component multi-target coordination research | [52, 53] |
At the genomic level, the method of array comparative genomic hybridization (aCGH) is used for global analysis of DNA copy number gain or loss across chromosomes between normal and pathological clinical samples [29]. Whole exome sequencing analysis also speeds up the discovery of novel molecular targets at single cell level, the diverse mutational profiles across different single cells of a tumor suggests that multiple drugs are needed to target intratumoral heterogeneity [30]. The RNAi platform profiles gene functions using multiplex RNAi screening and analyzes the accumulated genetic alternations in loss-of-function phenotypes [31]. In the last decade, gene expression microarray platform was widely employed for candidate gene discovery. Recently, differential genomic profiles of blood plasma samples from rheumatoid arthritis (RA) patients who were diagnosed with either ‘Heat’ or ‘Cold’ pattern according to TCM theories were successfully identified, and two distinct genomic network pathways were found in close association with those two patterns [32]. Genomic analysis of the molecular target network of the herbal formula Si–Wu–Tang (SWT) which is traditionally used to treat menstrual discomfort found that the nuclear factor erythroid 2-related factor 2 (Nrf2), a cytoprotective pathway, was significantly affected [33].
Target network could also be explored on proteins and protein–protein interactions level. A tissue array platform using recombinant antibody microarrays is often used to identify proteomic profiles and validate molecular targets from clinical samples in a multiplexed manner [34]. This platform can be also used for validating research information collected from genomic studies. The mass spectrometry-based proteomic platform is robust that it has been widely used for identifying correlations between proteomic profiles and pathogenesis of certain human illnesses. Currently, proteomic analysis is increasingly applied for identifying molecular network targets and response markers sets affected by herbal medicine [35]. For example, Yue et al. [36] performed MALDI-MS/MS analysis of an interactome map and identified a network of 21 differentiated regulated core proteins which are responsible for mediating apoptotic response to Ganoderic acid D treatment in Hela cells. MS-based technology has also been used in metabolomics studies. Gao et al. compared the metabolomics profiles between a control group and a Xiaoyaosan-treated group of rats subjected to chronic, unpredictable mild stress using gas chromatography coupled with mass spectrometry. Results showed significant changes in metabolomics like glycine, glucose and hexadecanoic acid which are related to the disturbance of amino acid metabolism, energy metabolism and glycometabolism [37]. In another study, serum metabolomics analysis has identified a panel of disease biomarkers [38] which provides possibilities for tracing corresponding regulatory kinase networks of relevant drugs. One disadvantage of metabolomics, however, is that results are easily be affected by diet, and the technology and database capacity are not sufficiently large for deeper analysis at this moment. Nevertheless, connections between proteomic and metabolomics profiles should not be neglected, particularly because integration of proteomic and metabolomics platforms is essential for identifying the network targets of multi-component drugs and the metabolism of herbal will eventually affect treatment efficacy. The dynamic interaction of the Gut Microbiota was shown to interact with one another and with human immune system that can influence the phenotype and treatment outcome of diseases including susceptibility to influenza, retrovirus transmission and colon cancer [39–42]. The influence of symbiotic-pathogen could be better investigated with the advanced large scale sequencing projects such as the Human Microbiome Project [43] and the Earth Microbiome Project [44].
Speed up multi-target drug discovery by integration
Individual system biology platform is robust but not informative enough to link drug response with personalized ‘-omics’ profile. Tighter integration across different systems biology platforms could provide mutual-support and validate data as well as eliminate irrelevant ‘noise’ for elucidating the trans-omics connections, and speed up multi-target drug discovery.
By linking the pharmacological actions of the tested drug with population genetics, integrated systems biology platforms can lead to better drug performance in individuals. For example, integrative research on drug responses and genomic connections in pharmacogenomics has led to a number of identifications, measurements and evaluations of the genetic networks responsible for drug treatment at personalized level [45]. For example, CYP2C9 and VKORC1 genotypes, which were found to be the responding biomarkers for better determining the maintenance dosage of Warfarin, a difficult-to-dose anticoagulant, led to a strong awareness for the need of personalized prescription [46]. This led to awareness of the need for personalizing prescriptions of this drug. Indeed, it is important to identify genetic factors associated with the therapeutic responses as well as the toxic susceptibilities of the tested or prescribed drug. For lung cancer, Gefitinib is a kinase inhibitor that inhibits epidermal growth factor receptor (EGFR) signaling in non-small cell lung cancer (NSCLC). However, in cancer molecular targeting therapy, the results of Gefitinib clinical trials were negative initially because none of these trials selected patients based on their EGFR kinase dependence. The real clinical response was proven only after the identification of subgroups of patients based on molecular analysis of EGFR mutation status [47, 48]. Furthermore, recent germline mutation study revealed that polymorphic deletion of BIM in Eastern Asian is common and BIM has a central role in Gefitinib-induced apoptosis in NSCLC harboring activating EGFR mutations. Patients with BIM deletion had a significantly shorter progression-free survival time after TKI treatment, the findings implies that germline mutation or SNP database should also be included for integrative analysis for final drug prescription decision [49].
Traditionally, high-throughput screening of natural products libraries was used for cancer drug discovery by either a cell-based or in vitro screening method [50, 51]. Recently, by combining a traditional chemical screening platform with network-based genomic and proteomic technologies, ‘herbalome projects’ have been launched. Chips with arrays of compounds were screened for their binding affinity to key peptides, then the binding affinity database was analyzed to link the correlations between active components contained in herbs and their multi-target network actions [52, 53]. The ultimate chemical structure or forms of active components of herbal medicine may vary after enzymatic digestion and pH challenges after passing through the digestion tract of host and with the gut microbiota [54]. Thus, a breakthrough idea of biotransformation of herbal will generate new sets of in vivo mimic herbal libraries by digesting the original herbal formula with different known human microbiota or perhaps microbiota of individual to reach personalized medicine need, these biotransformed new structures may be the new active multi-target drug components. For example, it was recognized that the gut microbiota-mediated metabolism of ginsenosides in ginseng may alter the pharmacokinetics of ginseng and enhance their biologically activity. The newly generated gingseng metabolite, which is called Compound K, possesses significantly stronger cancer prevention activity than the parent compounds [55]. Overall, better integration of the data derived from multiple ‘-omics’ technologies can lead to optimization of the treatment by taking advantage of molecular synergy [56].
TIGHT-INTEGRATION WITH COMPUTATIONAL TOOLS
Computational models for multi-target drug design in network perspective
‘-omics’ technologies have produced massive amounts of data, and the databases keep growing. Such data is useful, however, only if we have computational tools that can find and integrate relevant information. Hence, development of bioinformatics resources or computational tools has become critically important for screening these vast databases for effective drug combinations in a cost-effective and timely manner. The computational and mathematical models currently available for network-based multi-component drug design in both western medicine and herbal medicine are summarized in Table 3.
Table 3:
The newly developed computational tools or models for optimizing intervention of multi-targets drugs and elucidating interactive mechanisms among multiple dynamic targets and networks
| Computational tools | Models | Applications | Findings | References |
|---|---|---|---|---|
| Algorithm-based | Systematic combination screening | Combination optimization | Statically analyzing drug efficacy by denoting matrix of scores across 435 possible two-component combinations of 30 compounds, three optimized drug combination were found | [60] |
| Stochastic search algorithm using Gur Game | Combination optimization | Closed-loop control of cellular functions using combinatory drugs | [61, 62] | |
| Medicinal algorithmic combinational screen (MACS) | Combination optimization | Identifying a combination of four drugs from 72 combinations that are the most effective to kill 8 non-small cell lung cancer | [63] | |
| Extensive search algorithm model for examining the quantitative composition-activity relationship (QCAR) of herbal formulae | Combination optimization | Optimizing a combination regimen of three components of TCM formula Shenmai and Qi–Xue–Bing–Zhi–Fang | [67, 68] | |
| Algorithm-based computational program link with Steiner Tree method | Multi-layer correlation analysis of trans-omics | Linking the gene expression array and proteomic data to expand the understanding of the underlying cellular mechanism | [69, 70] | |
| Network-based | Network-based study on three drug combinational analysis using combination index | Multi-target mechanistic study | Identifying six core proteins from a protein network which responds to the Chinese herbal preparation, Realgar-Indigo Naturalis Formula-RIF | [72] |
| Integrative multiple systems biology platforms | Multi-target mechanistic study | Identifying the key pathways underlying the synergistic effects of combined imatinib and arsenic sulfide | [73] | |
| Network target-based identification of multicomponent synergy (NIMS) model | Solving a stochastic relationship of drugs and combination optimization | Transferring the relations between drugs to the interactions among their targets of a specific disease network and prioritizing synergistic pairs from 63 manually collected agents for a disease instanced by angiogenesis | [74] | |
| Network-based multi-target estimation by combining docking scores | Combination optimization | Screening anticoagulant activities of a series of argatroban intermediates and eight natural products based on affinity predictions | [76] | |
| Distance-based Mutual Information Model (DMIM) | Combination optimization and target network deduction | Optimizing dosage of two ingredients derived from a Chinese herbal formula Liu–Wei–Di–Huang (LWDH), the actions of LWDH was deducted to be associated with interaction with cancer pathways and neuro-endocrine-immune pathways | [77] | |
| Systematically target network analysis | Disease crosstalk and herbal mechanism of action analysis | Ingredients of anti-Alzheimer’s disease (AD) herbs interact closely with therapeutic targets that showed crosstalk with multiple diseases. Furthermore, pathways of Ca2+ equilibrium maintaining, upstream of cell proliferation and inflammation were densely targeted by the herbal ingredients | [78] |
The systematic experimental design and analysis using algorithm-based computational program aid identification of an optimized drug combination and the core disease causative pathways. Algorithm is used to step by step calculate a function and perform data process effectively based on a finite list of well-defined instructions [57]. Algorithm can be applied for drug combination optimization using the definition of synergism. For example, Loewe additivity and Bliss additivism were commonly used to distinguish drug additive and synergistic effects [58, 59]. Bliss additivism models can predict the combined response C for two single compounds with effect A and B. Loewe additivity is measured based on the combinational index which is dose-based and applies only to activity levels achieved by the single agents [60]. The highest single agents (HAS) model is also applied to measure the larger effects produced of combination than the effects of single agent. Using these three standard reference models, the best combination across 435 possible two-component combination of 30 compounds were systematically screened using an algorithm to design all possible combinational doses of multiple drugs, three optimized drug combinations were discovered [60].
Algorithm with other computational method, such as closed-loop control, can be used to decide and minimize the number of drug combinations in a screening protocol. For example, actions of the drug cocktails were systematically analyzed using closed-loop control of cellular functions guided by a stochastic search algorithm. The algorithm can minimize the required number of trial experiments from tens of iterations out of one hundred thousand possible trials [61]. NIH3T3 cells were stimulated by a drug mixture from a predetermined set of concentrations, then the anti-viral activity is measured and the results were interpreted with a stochastic search algorithm (Gur Game) to determine the next drug combination to be tested in the next iterative cycle. Gur Game is a classic algorithm used to score a new combination by rewarding or penalizing it after comparing with the anti-viral effect of the original drug combination [61, 62].
Zinner et al. developed another drug combination screening model called Medicinal Algorithmic Combinational Screen (MACS) and have screened 72 combinations of arbitrary size and formed a 19-element drug pool across four generations. The combination of fenretinide, suberoylanilide hydroxamic acid and bortezomib was found to be most effective in killing eight NSCLC cell lines [63].This method is based on assessing synergy of dose combinations using combination index calculated by the Chou–Talalay equation [64–66]. The fitness values were calculated based on the observation that each drug dosed at IC10 added to a cocktail could cause an increase or decrease inhibition by percentage, then the fitness value was subtracted or added by that percentage, respectively [63]. The optimal number of drugs in a combination was determined when the fitness value reach maximum after comparing the parent drug combination with the random alterations of the generation [63].
Obviously, the application of algorithm for multi-target drug optimization in herbal medicine is yet limiting, the above models should be encouraged to be used for optimizing the number of and the dose of herbal components. Recently, Cheng et al. [67] attempted to use method based on lattice experimental design and multivariate regression with extensive search algorithm model for examining the quantitative composition-activity relationship (QCAR) of the Chinese medicinal formula Shenmai. The algorithm is designed based on three factor simplex lattice method to optimize the dosage of the three key components (PD, PT and OP) in Shenmai Formula. Ten groups of mice were treated with the proportion of the three components designed with the three factor simplex lattice method, and their levels of cardioprotective activity were determined accordingly. The data were subjected to fit an equation which can predict the properties of all possible combinations. The optimal proportion of PD, PT and OP was found to be 21.6, 39.2 and 39.2%, respectively [67]. Similarly, they have also used the method to optimize the proportion of two active components of Qi–Xue–Bing–Zhi–Fang [68].
Synergy usually can be achieved with lower dose of individual component but overall may exhibit new form of toxicity or side-effect, therefore algorithm-based combination screen is useful but is still limited. Only by cooperation with network analysis could show the overall phenotypic effect of the drugs. Combining use of algorithm with Steiner Tree can elucidate the connection between trans-omics platforms in order to generate a valid disease network. For example, using an algorithm model solving the Steiner tree problem can integrate the data set of gene expression array and proteomic studies by analyzing the highest degree of correlations and associations of both mRNA and protein expression data. The optimal and possible core disease causative pathways could be identified by calculating the probability of the highest correlation and shortest path [69, 70]. The generated disease network information will help herbal cocktails design in network perspective.
Hence, network-based computational tools are increasingly applied in herbal synergy study through mathematical modeling of specific biological processes or pathways to study global cellular effects of multi-target drugs or multi-component therapies [71]. Wang et al. [72] demonstrated the multi-target actions and the synergy of the Chinese herbal formula Realgar-Indigo Naturalis Formula (RIF) for treating acute promyelocytic leukemia (APL) by performing small-scale combinational study using Chou and Talalay combination index method, and identified the three main active components of RIF and their interactive behavior of six core proteins in mediating the cancer inhibiting effect. Chen’s team later on applied multiple-omics technologies and demonstrated that combinational use of Imatinib and the toxic herbal remedy arsenic sulfide exerted stronger therapeutic effect in a BCR/ABL-positive mouse model of chronic myeloid leukemia (CML) than a single remedy [73]. The key pathway network underlying this synergistic effect was identified by integrating cDNA array, proteome, phosphoproteome and transcriptome profiles in K562 cells [73].
Furthermore, the topological properties of biological network was used based on the integrated Network Target-based Identification of Multicomponent Synergy (NIMS) model which can transfer correlations between drugs to the interactions among their molecular targets [74]. NIMS were performed based on two elements, Topology Score (TS) and Agent Score (AS) which were used for evaluating agent interactions with the biological targets [75]. TS is derived from the topological features of the background network related to certain disease condition while AS is used to quantify the effects of two agents on disease phenotype by text mining on OMIM. NIMS synergy was calculated by multiplying TS with AS. The higher the synergy score, the greater probability of synergy of the drug combination [74].
Also, a biological network-based multi-target computational estimation scheme was used for screening anticoagulant activity of a series of argatroban intermediates and eight natural products based on affinity predictions from their multi-target docking scores and network efficiency analysis [76]. This scheme has been evolved from the traditional single agent virtual screening method which relies on evaluating binding affinity of the agent to single target, on the other hand, this model has focused on a network screening strategy based on phenotypic data of drug molecules against a complex disease by general network estimation.
Li et al. has established another method called Distance-based Mutual Information Model (DMIM) to find the target network and optimal ingredient and dosage of a Chinese herbal formula Liu–Wei–Di–Huang (LWDH), a new anti-angiogenic herbal combination composing of Vitexicarpin and Timosaponin A-III was discovered. Herb network was constructed by DMIM from 3865 Collateral-related herbal formulae and the action of LWDH formula was deduced from a co-module, the action of LWDH was found to be related to the cancer pathways and neuro-endocrine-immune pathways [77].
Recently, we have also presented a systematically target network analysis framework by integrating bioinformatics databases, and used this model to explore the mechanism of anti-Alzheimer’s disease (AD) herb ingredients searched by a large-scale text mining of PubMed and the clinical trial database. Our results indicate that ingredients of anti-AD herbs also interact closely with a number of reported therapeutic targets which are associated crosstalk to other diseases such as inflammation, cancer and diabetes. Furthermore, pathways of Ca2+ equilibrium maintaining, upstream of cell proliferation and inflammation were densely targeted by the anti-AD herbal ingredients [78].
Challenges of interpreting and integrating ‘-omics’ and herbal databases
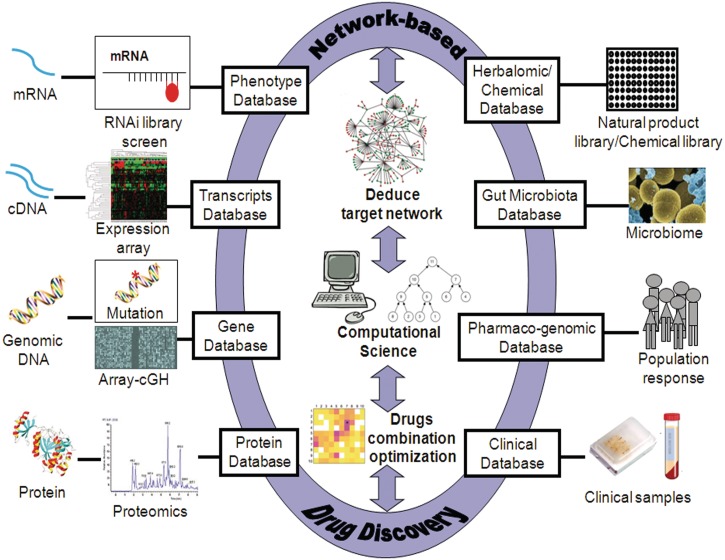
Expanding the translational capacity of ‘-omics’ platforms for network-based drug discovery depends on developing relevant computational tools to interpret databases and correlations across genomics, transcriptomics, proteomics, metabolomics, microbiome, pharmacogenomics and clinical samples (Figure 1). Such computational tools would largely facilitate translational research by high-throughput identification and fast optimization of regimes of the network-based multi-target drugs with multiple ‘-omics’ platforms. The application of system biology and computational models starts to be realized to be essential to uncover the potentials and optimize the use of herbal medicine. However, three major challenges need to be overcome. First, the accuracy of these models largely depends on the reliability of the constructed network. As the network is actually highly dynamic and completely cross-linked, there is still large space for quantitative prediction of synergistic combination based on static network [69, 70]. Specifically, the sensitivity, accuracy and reproducibility are needed to be improved significantly. The ‘-omics’ data sometimes are difficult to be reproduced which are mainly due to biological variations and variation of ‘capture time slot’ of the samples [69, 70]. Also, the choice of models, strains and different drug exposure time from the data sets generated from different laboratories lead to variations [79]. To solve this, it is critical for the industry, regulatory agencies and academic institutions to standardize ‘omics’ methods to reach a consensus about the reliability and interpretation of endpoints [79], where Connectivity MAP (CMAP) has started a nice example of this (http://www.broadinstitute.org/cmap/). Second, while target information is highly important for calculating the effects of a drug combination, the multiple targeting profiles for a natural compound are often limited. For examples, only one database mentioned 78 protein targets for 2597 natural compounds, which obviously needs further updating [80]. Recently, we have developed the Herbal Ingredient Target (HIT) database and TCM-Information Database (TCM-ID) by textmining on literature and books, but the databases are not completed, which need to be further expanded [81, 82]. At the current stage, we have to rely on the ‘-omics’ data, such as RNA expression or protein expression, to deduce the targeted pathways and networks of a prescribed or tested herbal remedy. Significant increase in herbal research is needed to provide sufficient data for filling the gap of our databases. Third, the terminology and topology of herbal medicine have not been standardized and the herbal databases are not all open-accessed and thus could not be integrated into the interlinked data with the public available databases [83]. For examples, many herbals are termed with different languages depending on different countries, thus direct extraction of structured data from biomedical texts cannot be achieved [84, 85]. The difference in ontology/taxonomy of herbal which sometimes causes misunderstanding and confusion in application, and may cause serious drug poisoning due to misuse of the herb [86]. Semantic technologies and linked data initiate a ‘bridge’ linking traditional medicines and modern pharmaceutical researches, emphasizing on combining information using standard representative languages and address the heterogeneous data integration problem [86–88]. However, the existing databases about herbal targets must be further made publicly accessible and interlinked for broader integration. Terminology and standardization of herbals should be unified based on discussion with international regulatory agencies, such as International Standardization Organization (ISO) committee to achieve consensus from governments, academics and industry among different member countries.
Figure 1:
A conceptual diagram of integrating systems biology platforms using computational tools for network-based drug discovery.
CONCLUDING REMARKS
Mono-target therapy often fails to treat chronic diseases that are often induced by multiple pathogenic factors and often involve multiple pathological changes, it is necessary to expand drug discovery toward multi-target approach. Multiple system biology platforms have been used for discovery of molecular targets in network and personalized perspective. Further integration of multiple ‘-omics’ technologies and databases would aid design of optimal network-based multi-target drugs as well as betterment of translational research. The current attempt of using various algorithm-based and network-based computational tools for optimizing molecular synergy of herbal formula and elucidating their target network seems promising, which would also be a promise way for developing network-based multi-component drugs. However, tighter integration of herbal database with various available ‘omics’ platforms is needed. The major challenges in interpreting multiple databases are the lack of reliability, depth and visualization. Standardization of ‘-omics’ methodology, normalization of the terminologies of herbs and herbal formulas and expanding research on herbal synergy with system biology will improve the quantity and quality of the databases, thus making integration more easily be achieved. Resources input on open-accessed TCM databases will also expand the depth. Along with further development of new computational tools, such as Semantic web to integrate ‘omics’ databases with herbal target databases, more accurate algorithms to quantitatively predict the synergistic effects of multi-component drug with multi-targets actions will provide the necessary, powerful engine for building a bridge for TCM and network-based drug discovery. Taken together, we believed the network-based drug discovery will be accelerated by integrating multiple ‘-omics’ and computational technologies together with clinical and translational research knowledge.
A tighter integration of multiple systems biology platforms should be achieved with the help of computational tools which can connect drug combinations with core target network and elucidate their correlations to overall clinical drug responses. First, expanding the individual platform database is necessary to providing sufficiently large amounts of raw information for statistically analysis. Second, computational modeling can eliminate ‘noise’ via probability calculation which predicts the optimal path from genes to transcripts; and from transcripts to the protein and protein network level. Thirdly, the herbalomic database may provide efficacy information of herbal constituents to specific potential targets network. Gut microbiota database could give information on the herbal metabolites which might be the ultimate active forms of the herbal ingredients. Population response database could provide guidance of the best-fit patient selection to a potential combination regime. Clinical validation could be linked with clinical samples and databases. Finally, optimization of the combinational dose of a designed drug for a candidate target network could be predicted with computational tools and is further experimentally tested by basic research scientists and validated clinically to fasten translational processes.
Key Points.
Network-based drug discovery has come to new era of pharmaceutical industry.
The design of network-based drug could be based on three main strategies: multi-components, mutual complement and multi-targeting.
Multi-target nature of herbal medicine may provide resources to network-based drug discovery.
The robust and high-throughput systems biology platforms could efficiently generate important biological data sets for the development of network-based multi-target drugs.
Tighter integration across the databases from multiple systems biology platforms with computational technologies is essential for breakthrough of the herbal synergy and network-based drug discovery.
FUNDING
This work was supported by Macao Science and Technology Development Fund (project no: 035/2011/A2 and 074/2011/A3).
Acknowledgements
We thank Dr M. Dalhen for carefully proofreading the manuscript and providing valuable comments.
Biographies
Dr Elaine Leung is an Assistant Professor at the State Key Laboratory of Quality Research in Chinese Medicine, Macau University of Science and Technology. She is a molecular biologist. Her major research interest is about identification of novel small molecule inhibitors and molecular targets in lung cancer.
Prof. Zhi-Wei Cao is the Vice-Dean of the School of Life Science and Technology, Tongji University. She is a computational biologist, her major research interest is about using bioinformatics and computational tools to study medicinal herbs and pharmacokinetic properties of drugs.
Prof. Zhi-Hong Jiang is a Professor at the State Key Laboratory of Quality Research in Chinese Medicine, Macau University of Science and Technology. He is an expert in using proteomics, metabolomics and chemical restructuring in natural products research.
Dr Hua Zhou is an Associate Professor at the State Key Laboratory of Quality Research in Chinese Medicine, Macau University of Science and Technology. He is an herbal pharmacologist, and his research interest is in herbal pharmacology and pharmacokinetics.
Prof. Liang Liu is the Vice Rector of Macau University of Science and Technology and the Director of the State Key Laboratory of Quality Research in Chinese Medicine. He is a pioneer researcher in using modern technologies for traditional Chinese medicine research and ISO/TC249 Committee member.
References
- 1.Sams-Dodd F. Target-based drug discovery: is something wrong? Drug Discov Today. 2005;10:139–47. doi: 10.1016/S1359-6446(04)03316-1. [DOI] [PubMed] [Google Scholar]
- 2.Zimmermann GR, Lehar J, Keith CT. Multi-target therapeutics: when the whole is greater than the sum of the parts. Drug Discov Today. 2007;12:34–42. doi: 10.1016/j.drudis.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 3.Schrattenholz A, Soskic V. What does systems biology mean for drug development? Curr Med Chem. 2008;15:1520–8. doi: 10.2174/092986708784638843. [DOI] [PubMed] [Google Scholar]
- 4.Clements A, Gao B, Yeap SH, et al. Metformin in prostate cancer: two for the price of one. Ann Oncol. 2011;22:2556–60. doi: 10.1093/annonc/mdr037. [DOI] [PubMed] [Google Scholar]
- 5.Kong W, Wei J, Abidi P, et al. Berberine is a novel cholesterol-lowering drug working through a unique mechanism distinct from statins. Nat Med. 2004;10:1344–51. doi: 10.1038/nm1135. [DOI] [PubMed] [Google Scholar]
- 6.Tian X, Liu L. Drug discovery enters a new era with multi-target intervention strategy. Chin J Med. 2011;17:1–4. doi: 10.1007/s11655-011-0900-2. [DOI] [PubMed] [Google Scholar]
- 7.Ho DD. Time to hit HIV, early and hard. N Engl J Med. 1995;333:450–1. doi: 10.1056/NEJM199508173330710. [DOI] [PubMed] [Google Scholar]
- 8.Stermitz FR, Lorenz P, Tawara JN, et al. Synergy in a medicinal plant: antimicrobial action of berberine potentiated by 5′-methoxyhydnocarpin, a multidrug pump inhibitor. Proc Natl Acad Sci USA. 2000;97:1433–7. doi: 10.1073/pnas.030540597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Keith CT, Borisy AA, Stockwell BR. Multicomponent therapeutics for networked systems. Nat Rev Drug Discov. 2005;4:71–8. doi: 10.1038/nrd1609. [DOI] [PubMed] [Google Scholar]
- 10.Dixon SJ, Stockwell BR. Drug discovery: engineering drug combinations. Nat Chem Biol. 2010;6:318–9. doi: 10.1038/nchembio.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fisher RI, Gaynor ER, Dahlberg S, et al. Comparison of a standard regimen (CHOP) with three intensive chemotherapy regimens for advanced non-Hodgkin’s lymphoma. N Engl J Med. 1993;328:1002–6. doi: 10.1056/NEJM199304083281404. [DOI] [PubMed] [Google Scholar]
- 12.Kaiser J. Combining targeted drugs to stop resistant tumors. Science. 2011;331:1542–5. doi: 10.1126/science.331.6024.1542. [DOI] [PubMed] [Google Scholar]
- 13.Poulikakos PI, Solit DB. Resistance to MEK inhibitors: should we co-target upstream? Sci Signal. 2011;4:pe16. doi: 10.1126/scisignal.2001948. [DOI] [PubMed] [Google Scholar]
- 14.Hsiao WL, Liu L. The role of traditional Chinese herbal medicines in cancer therapy–from TCM theory to mechanistic insights. Planta Med. 2010;76:1118–31. doi: 10.1055/s-0030-1250186. [DOI] [PubMed] [Google Scholar]
- 15.Zhao J, Jiang P, Zhang W. Molecular networks for the study of TCM pharmacology. Brief Bioinform. 2010;11:417–30. doi: 10.1093/bib/bbp063. [DOI] [PubMed] [Google Scholar]
- 16.Borkow G, Lapidot A. Multi-targeting the entrance door to block HIV-1. Curr Drug Targets. 2005;5:3–15. doi: 10.2174/1568005053174645. [DOI] [PubMed] [Google Scholar]
- 17.Miller LH, Siu X. Artemisinin: discovery from the chinese herbal garden. Cell. 2011;146:855–8. doi: 10.1016/j.cell.2011.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tu Y. The discovery of artemisinin (qinghaosh) and gifts from Chinese medicine. Nat Med. 2011;17:1217–20. doi: 10.1038/nm.2471. [DOI] [PubMed] [Google Scholar]
- 19.Li GQ, Arnold K, Guo XB, et al. Randomised comparative study of mefloquine, qinghaosu, and pyrimethamine-sulfadoxine in patients with falciparum malaria. Lancet. 1984;2:1360–1. doi: 10.1016/s0140-6736(84)92057-9. [DOI] [PubMed] [Google Scholar]
- 20.Li T, Wong VK, Yi XQ, et al. Matrine induces cell anergy in human Jurkat T cells through modulation of mitogen-activated protein kinases and nuclear factor of activated T-cells signaling with concomitant up-regulation of anergy-associated genes expression. Biol Pharma Bull. 2010;33:40–6. doi: 10.1248/bpb.33.40. [DOI] [PubMed] [Google Scholar]
- 21.Liang L, Wong JH, Lou XY. Methodological approach for pharmacological research of Chinese herbal formulas through investigation of the formulas on anti-gastric ulcers in rats. J Tradit Chin Med. 1985;2:50–3. [Google Scholar]
- 22.Liu L, Leung EL, Tian X. Perspective: the clinical trial barriers. Nature. 2011;480:S100. doi: 10.1038/480S100a. [DOI] [PubMed] [Google Scholar]
- 23.Chen R, Mias GI, Li-Pook-Than J, et al. Personal omics profiling reveals dynamic molecular and medical phenotypes. Cell. 2012;148:1293–307. doi: 10.1016/j.cell.2012.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vaidyanathan G. Redefining clinical trials: the age of personalized medicine. Cell. 2012;148:1079–80. doi: 10.1016/j.cell.2012.02.041. [DOI] [PubMed] [Google Scholar]
- 25.Lam W, Bussom S, Guan F, et al. The four-herb Chinese medicine PHY906 reduces chemotherapy-induced gastrointestinal toxicity. Sci Trans Med. 2010;2:45ra59. doi: 10.1126/scitranslmed.3001270. [DOI] [PubMed] [Google Scholar]
- 26.Xie Y, Zhou H, Wong YF, et al. Study on the pharmacokinetics and metabolism of paeonol in rats treated with pure paeonol and an herbal preparation containing paeonol by using HPLC-DAD-MS method. J Pharm Biomed Anal. 2008;46:748–56. doi: 10.1016/j.jpba.2007.11.046. [DOI] [PubMed] [Google Scholar]
- 27.Liu ZQ, Jiang ZH, Chan K, et al. Pharmacokinetic interaction of paeoniflorin and sinomenine: pharmacokinetic parameters and tissue distribution characteristics in rats and protein binding ability in vitro. J Pharmacol Sci. 2005;99:381–91. doi: 10.1254/jphs.fp0050687. [DOI] [PubMed] [Google Scholar]
- 28.Fan YF, Xie Y, Liu L, et al. Paeoniflorin reduced acute toxicity of aconitine in rats is associated with the pharmacokinetic alteration of aconitine. J Ethnopharmacol. 2012;141:701–8. doi: 10.1016/j.jep.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 29.Shinawi M, Cheung SW. The array CGH and its clinical applications. Drug Discov Today. 2008;13:760–70. doi: 10.1016/j.drudis.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 30.Xu X, Hou Y, Yin X, et al. Single-cell exome sequencing reveals single-nucleotide mutation characteristics of a kidney tumor. Cell. 2012;148:886–95. doi: 10.1016/j.cell.2012.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Silva JM, Marran K, Parker JS, et al. Profiling essential genes in human mammary cells by multiplex RNAi screening. Science. 2008;319:617–20. doi: 10.1126/science.1149185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van der Greef J, van Wietmarschen H, Schroen J, et al. Systems biology-based diagnostic principles as pillars of the bridge between Chinese and Western medicine. Planta Med. 2010;76:2036–47. doi: 10.1055/s-0030-1250450. [DOI] [PubMed] [Google Scholar]
- 33.Wen Z, Wang Z, Wang S, et al. Discovery of molecular mechanisms of traditional Chinese medicinal formula Si-Wu-Tang using gene expression microarray and connectivity map. PloS One. 2011;6:e18278. doi: 10.1371/journal.pone.0018278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dexlin-Mellby L, Sandstrom A, Centlow M, et al. Tissue proteome profiling of preeclamptic placenta using recombinant antibody microarrays, proteomics. Clin Appl. 2010;4:794–807. doi: 10.1002/prca.201000001. [DOI] [PubMed] [Google Scholar]
- 35.Qiao YC, A. D, Yin SK, et al. Utilization of the chemical proteomics for the study of Chinese medicines towards modernization. World Sci Technol. 2010;12:502–10. [Google Scholar]
- 36.Yue QX, Cao ZW, Guan SH, et al. Proteomics characterization of the cytotoxicity mechanism of ganoderic acid D and computer-automated estimation of the possible drug target network. Mol Cell Proteomics. 2008;7:949–61. doi: 10.1074/mcp.M700259-MCP200. [DOI] [PubMed] [Google Scholar]
- 37.Gao X, Zheng X, Li Z, et al. Metabonomic study on chronic unpredictable mild stress and intervention effects of Xiaoyaosan in rats using gas chromatography coupled with mass spectrometry. J Ethnopharmacol. 2011;137:690–9. doi: 10.1016/j.jep.2011.06.024. [DOI] [PubMed] [Google Scholar]
- 38.Chen J, Zhang X, Cao R, et al. Serum 27-nor-5beta-cholestane-3,7,12,24,25 pentol glucuronide discovered by metabolomics as potential diagnostic biomarker for epithelium ovarian cancer. J. Proteome Res. 2011;10:2625–32. doi: 10.1021/pr200173q. [DOI] [PubMed] [Google Scholar]
- 39.Clemente JC, Ursell LK, Parfrey LW, et al. The impact of the gut microbiota on human health: an integrative view. Cell. 2012;148:1258–70. doi: 10.1016/j.cell.2012.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ichinohe T, Pang IK, Kumamoto Y, et al. Microbiota regulates immune defense against respiratory tract influenza A virus infection. Proc Natl Acad Sci USA. 2011;108:5354–9. doi: 10.1073/pnas.1019378108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kane M, Case LK, Kopaskie K, et al. Successful transmission of a retrovirus depends on the commensal microbiota. Science. 2011;334:245–9. doi: 10.1126/science.1210718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kostic AD, Gevers D, Pedamallu CS, et al. Genomic analysis identifies association of Fusobacterium with colorectal carcinoma. Genome Res. 2012;22:292–8. doi: 10.1101/gr.126573.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peterson J, Garges S, Giovanni M, et al. The NIH human microbiome project. Genome Res. 2009;19:2317–23. doi: 10.1101/gr.096651.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gilbert JA, Meyer F, Antonopoulos D, et al. Meeting report: the terabase metagenomics workshop and the vision of an Earth microbiome project. Stand Genomic Sci. 2010;3:243–8. doi: 10.4056/sigs.1433550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pirmohamed M. Pharmacogenetics: past, present and future. Drug Discov Today. 2011;16:852–61. doi: 10.1016/j.drudis.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 46.Sconce EA, Khan TI, Wynne HA, et al. The impact of CYP2C9 and VKORC1 genetic polymorphism and patient characteristics upon warfarin dose requirements: proposal for a new dosing regimen. Blood. 2005;106:2329–33. doi: 10.1182/blood-2005-03-1108. [DOI] [PubMed] [Google Scholar]
- 47.Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–39. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 48.Paez JG, Janne PA, Lee JC, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304:1497–500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 49.Cheng EH, Sawyers CL. In cancer drug resistance, germline matters too. Nat Med. 2012;18:494–6. doi: 10.1038/nm.2725. [DOI] [PubMed] [Google Scholar]
- 50.Harvey AL, Cree IA. High-throughput screening of natural products for cancer therapy. Planta Med. 2010;76:1080–6. doi: 10.1055/s-0030-1250162. [DOI] [PubMed] [Google Scholar]
- 51.Zhu Y, Zhang Z, Zhang M, et al. High-throughput screening for bioactive components from traditional Chinese medicine. Comb Chem High Throughput Screen. 2010;13:837–48. doi: 10.2174/138620710793360257. [DOI] [PubMed] [Google Scholar]
- 52.Kang YJ. Herbogenomics: from traditional Chinese medicine to novel therapeutics. Exp Biol Med. 2008;233:1059–65. doi: 10.3181/0802-MR-47. [DOI] [PubMed] [Google Scholar]
- 53.Stone R. Lifting the veil on traditional Chinese medicine. Science. 2008;319:709–10. doi: 10.1126/science.319.5864.709. [DOI] [PubMed] [Google Scholar]
- 54.Li H, Zhou M, Zhao A, et al. Traditional Chinese medicine: balancing the gut ecosystem. Phytother Res. 2009;23:1332–5. doi: 10.1002/ptr.2590. [DOI] [PubMed] [Google Scholar]
- 55.Qi LW, Wang CZ, Du GJ, et al. Metabolism of ginseng and its interactions with drugs. Curr Drug Metab. 2011;12:818–22. doi: 10.2174/138920011797470128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gertsch J. Botanical drugs, synergy, and network pharmacology: forth and back to intelligent mixtures. Planta Med. 2011;77:1086–98. doi: 10.1055/s-0030-1270904. [DOI] [PubMed] [Google Scholar]
- 57.Harel D, Feldman YA. Algorithmics: The Spirit of Computing. 3rd edn. Pearson Education; 2004. [Google Scholar]
- 58.Fitzgerald JB, Schoeberl B, Nielsen UB, et al. Systems biology and combination therapy in the quest for clinical efficacy. Nat Chem Biol. 2006;2:458–66. doi: 10.1038/nchembio817. [DOI] [PubMed] [Google Scholar]
- 59.Goldoni M, Johansson C. A mathematical approach to study combined effects of toxicants in vitro: evaluation of the Bliss independence criterion and the Loewe additivity model. Toxicol In Vitro. 2007;21:759–69. doi: 10.1016/j.tiv.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 60.Borisy AA, Elliott PJ, Hurst NW, et al. Systematic discovery of multicomponent therapeutics. Proc Natl Acad Sci USA. 2003;100:7977–82. doi: 10.1073/pnas.1337088100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wong PK, Yu F, Shahangian A, et al. Closed-loop control of cellular functions using combinatory drugs guided by a stochastic search algorithm. Proc Natl Acad Sci USA. 2008;105:5105–10. doi: 10.1073/pnas.0800823105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yoon BJ. Enhanced stochastic optimization algorithm for finding effective multi-target therapeutics. BMC Bioinformatics. 2011;12(Suppl 1):S18. doi: 10.1186/1471-2105-12-S1-S18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zinner RG, Barrett BL, Popova E, et al. Algorithmic guided screening of drug combinations of arbitrary size for activity against cancer cells. Mol Cancer Ther. 2009;8:521–32. doi: 10.1158/1535-7163.MCT-08-0937. [DOI] [PubMed] [Google Scholar]
- 64.Chou TC. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res. 2010;70:440–6. doi: 10.1158/0008-5472.CAN-09-1947. [DOI] [PubMed] [Google Scholar]
- 65.Chou TC, Motzer RJ, Tong Y, et al. Computerized quantitation of synergism and antagonism of taxol, topotecan, and cisplatin against human teratocarcinoma cell growth: a rational approach to clinical protocol design. J Natl Cancer Inst. 1994;86:1517–24. doi: 10.1093/jnci/86.20.1517. [DOI] [PubMed] [Google Scholar]
- 66.Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 67.Wang Y, Yu L, Zhang L, et al. A novel methodology for multicomponent drug design and its application in optimizing the combination of active components from Chinese medicinal formula Shenmai. Chem Biol Drug Des. 2010;75:318–24. doi: 10.1111/j.1747-0285.2009.00934.x. [DOI] [PubMed] [Google Scholar]
- 68.Wang Y, Wang X, Cheng Y. A computational approach to botanical drug design by modeling quantitative composition-activity relationship. Chem Biol Drug Des. 2006;68:166–72. doi: 10.1111/j.1747-0285.2006.00431.x. [DOI] [PubMed] [Google Scholar]
- 69.Huang SS, Fraenkel E. Integrating proteomic, transcriptional, and interactome data reveals hidden components of signaling and regulatory networks. Sci Signal. 2009;2:ra40. doi: 10.1126/scisignal.2000350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lan A, Smoly IY, Rapaport G, et al. ResponseNet: revealing signaling and regulatory networks linking genetic and transcriptomic screening data. Nucleic Acids Res. 2011;39:W424–9. doi: 10.1093/nar/gkr359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yan H, Zhang B, Li S, et al. A formal model for analyzing drug combination effects and its application in TNF-alpha-induced NFkappaB pathway. BMC Syst Biol. 2010;4:50. doi: 10.1186/1752-0509-4-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang L, Zhou GB, Liu P, et al. Dissection of mechanisms of Chinese medicinal formula Realgar-Indigo naturalis as an effective treatment for promyelocytic leukemia. Proc Natl Acad Sci USA. 2008;105:4826–31. doi: 10.1073/pnas.0712365105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang QY, Mao JH, Liu P, et al. A systems biology understanding of the synergistic effects of arsenic sulfide and Imatinib in BCR/ABL-associated leukemia. Proc Natl Acad Sci USA. 2009;106:3378–83. doi: 10.1073/pnas.0813142106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li S, Zhang B, Zhang N. Network target for screening synergistic drug combinations with application to traditional Chinese medicine. BMC Syst Biol. 2011;5(Suppl 1):S10. doi: 10.1186/1752-0509-5-S1-S10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.van Driel MA, Bruggeman J, Vriend G, et al. A text-mining analysis of the human phenome. Eur J Hum Genet. 2006;14:535–42. doi: 10.1038/sj.ejhg.5201585. [DOI] [PubMed] [Google Scholar]
- 76.Li Q, Li X, Li C, et al. A network-based multi-target computational estimation scheme for anticoagulant activities of compounds. PloS One. 2011;6:e14774. doi: 10.1371/journal.pone.0014774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Li S, Zhang B, Jiang D, et al. Herb network construction and co-module analysis for uncovering the combination rule of traditional Chinese herbal formulae. BMC Bioinformatics. 2010;11(Suppl 11):S6. doi: 10.1186/1471-2105-11-S11-S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sun Y, Zhu R, Ye H, et al. Towards a bioinformatics analysis of anti-Alzheimer’s herbal medicines from a target network perspective. Brief Bioinformatics. 2012 doi: 10.1093/bib/bbs025. In press, doi:10.1093/bib/bbs025. [DOI] [PubMed] [Google Scholar]
- 79.Ouedraogo M, Baudoux T, Stevigny C, et al. Review of current and “omics” methods for assessing the toxicity (genotoxicity, teratogenicity and nephrotoxicity) of herbal medicines and mushrooms. J Ethnopharmacol. 2012;140:492–512. doi: 10.1016/j.jep.2012.01.059. [DOI] [PubMed] [Google Scholar]
- 80.Ehrman TM, Barlow DJ, Hylands PJ. Phytochemical databases of Chinese herbal constituents and bioactive plant compounds with known target specificities. J Chem Inf Model. 2007;47:254–63. doi: 10.1021/ci600288m. [DOI] [PubMed] [Google Scholar]
- 81.Chen X, Zhou H, Liu YB, et al. Database of traditional Chinese medicine and its application to studies of mechanism and to prescription validation. Br J Pharmacol. 2006;149:1092–103. doi: 10.1038/sj.bjp.0706945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ye H, Ye L, Kang H, et al. HIT: linking herbal active ingredients to targets. Nucleic Acids Res. 2011;39:D1055–9. doi: 10.1093/nar/gkq1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sams-Dodd F. Optimizing the discovery organization for innovation. Drug Discov Today. 2005;10:1049–56. doi: 10.1016/S1359-6446(05)03539-7. [DOI] [PubMed] [Google Scholar]
- 84.Fang YC, Huang HC, Chen HH, et al. TCMGeneDIT: a database for associated traditional Chinese medicine, gene and disease information using text mining. BMC Complement Altern Med. 2008;8:58. doi: 10.1186/1472-6882-8-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang Y, Yu Z, Jiang Y, et al. Automatic symptom name normalization in clinical records of traditional Chinese medicine. BMC Bioinformatics. 2010;11:40. doi: 10.1186/1471-2105-11-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Samwald M, Dumontier M, Zhao J, et al. Integrating findings of traditional medicine with modern pharmaceutical research: the potential role of linked open data. Chin Med. 2010;5:43. doi: 10.1186/1749-8546-5-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chen H, Mao Y, Zheng X, et al. Towards semantic e-science for traditional Chinese medicine. BMC Bioinformatics. 2007;8(Suppl 3):S6. doi: 10.1186/1471-2105-8-S3-S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Manning M, Aggarwal A, Gao K, et al. Scaling the walls of discovery: using semantic metadata for integrative problem solving. Brief Bioinformatics. 2009;10:164–76. doi: 10.1093/bib/bbp007. [DOI] [PubMed] [Google Scholar]