Abstract
Background:
The green tea extract is a naturally occurring product having beneficial effects that counteract with the pathobiological features of periodontitis and diabetes mellitus. Hence, the present study was aimed at incorporation of green tea extract into hydroxylpropyl methylcellulose and investigates its efficacy in chronic periodontitis patients associated with and without diabetes mellitus.
Materials and Methods:
For the in vitro study, formulation of green tea strips and placebo strips, and analysis of drug release pattern from the green tea strips at different time intervals were performed. For the in vivo study, 50 patients (20-65 years), including 25 systemically healthy patients with chronic periodontitis (group 1) and 25 diabetic patients with chronic periodontitis (group 2) were enrolled. In each patient, test and control sites were identified for the placement of green tea and placebo strips, respectively. Gingival Index (GI), Probing Pocket Depth (PPD), and Clinical Attachment Level (CAL) were examined at baseline, first, second, third, and fourth weeks. Microbiological analysis for Porphyromonas gingivalis and Aggregatibacter actinomycetemcomitans was performed at baseline and fourth week.
Results:
The in vitro study showed 10.67% green tea release at 30 min; thereafter, a slow release was noted till 120 min.
In vivo study:
Both groups showed significant reduction in GI scores at the test sites. Group 1 showed significant (P < 0.001) PPD reduction at different time intervals at the test sites. However, group 2 showed significant reduction from baseline (5.30 ± 0.70) to fourth week (3.5 ± 0.97). Statistically significant gain in CAL at the test sites was observed both in group 1 (1.33 mm) and group 2 (1.43 mm). The prevalence of P. gingivalis in group 1 test sites was significantly reduced from baseline (75%) to fourth week (25%).
Conclusions:
Local drug delivery using green tea extract could be used as an adjunct in the treatment of chronic periodontitis in diabetic and non-diabetic individuals.
Keywords: Antimicrobial, antioxidant, controlled clinical trial, diabetes, green tea extract, local drug delivery, periodontitis
INTRODUCTION
Periodontal disease is a microbe-induced chronic inflammatory condition affecting periodontium. The incidence and progression of this disease is related to substantial increase in gram-negative anaerobic rods. Among them, Porphyromonas gingivalis and Aggregatibacter actinomycetemcomitans are strongly implicated in periodontitis.[1]
Apart from bacteria, tissue destruction can be enhanced by uncontrolled host immune response to infection. Diabetes is one such condition that affects multitude of tissues and organs including periodontium. It alters the host immuno-inflammatory responses, such as upregulation of inflammatory cell phenotype, elevation of proinflammatory cytokines, increased collagenase activity, and production of reactive oxygen species, thus enhancing the possibility of risk and prevalence of periodontitis.[2,3,4,5,6,7]
The periodontal therapy is directed at controlling the bacterial flora, which has often proven to be difficult by mechanical depuration alone than expected, thus providing a rationale for the adjunctive use of chemotherapeutic agents.[8,9] Various locally delivered antimicrobials have been used aiming at inhibition of the pathogens or modulation of the inflammatory response, which include tetracycline, metronidazole, doxycycline, minocycline, and chlorhexidine.[10,11,12,13,14,15]
Of late, many natural products are used for the treatment of periodontitis. Green tea (Camellia sinensis) extract is one among them which has various therapeutic effects such as antioxidant,[16] anti-collagenase,[17] anti-inflammatory,[18] anticaries,[19] antifungal, antiviral,[20] and antibacterial effects.[21] Even in the field of medicine, epidemiological and experimental observations have suggested anti-obesity,[22,23] anticarcinogenic,[24] cardioprotective effects,[25] anti-diabetic activity,[26,27,28] and also causing inhibition of bone resorption and increase in bone mineral density.[16]
Hence, in the present study, an attempt was made to evaluate the effect of green tea extract as a local antimicrobial agent using hydroxypropyl methylcellulose (HPMC) on chronic periodontitis patients associated with and without diabetes mellitus, using clinical and microbiological parameters.
MATERIALS AND METHODS
In vitro study
Preparation of the strips
Green tea catechin strips were prepared by the method described by Hirasawa et al.[21] In brief, 3.876 g of HPMC and 0.204 g of green tea extract (Geltec Pvt. Ltd, Bengaluru, India) were taken in a clean mortar. Both were ground to a fine powder with pestle, blending the contents by grinding. The entire quantity of the above mixture was carefully transferred to a 500-ml beaker. The mixture was first made to a paste by adding little ethanol. The paste was diluted further by addition of ethanol with simultaneous stirring, ensuring that the constituents were evenly dispersed. A total of 160 ml ethanol was added to the mixture and stirring was done for 1 h.
The whole contents of the beaker were transferred to a clean reflux flask (250 ml) without any spillage. A clean Teflon magnetic paddle was dropped inside the flask and the condenser was attached. The flask with condenser was placed upon a magnetic stirrer. Stirring was set without splashing of ethanol inside the flask. The stirring process was carried out overnight.
Later, a clean Teflon-coated tray of inner size 11.5 cm × 11.5 cm was placed inside a laminar air flow station. The content of the flask was poured into the tray and allowed for drying. After drying was complete, the sheet was released from the tray aseptically. The dried product in the sheet form was put to the punch die of the required shape and size (4 mm width × 5 mm length with rounded corners on one side). After punching in the strip form, strips of thickness 200-300 μm were selected.
Placebo strips were prepared in a similar manner without adding the green tea extract powder to HPMC powder. The entire operation was performed under aseptic conditions in a clean environment. The strips were individually packed in a medical grade pouch and sterilized by gamma irradiation at 2.5 Mrd.
Estimation of drug release
Drug release pattern was analyzed using UV spectrophotometer. Estimation was carried out in 100 ml of distilled water in a beaker maintained at a temperature of 37 ± 1°C. A sample of 5 ml was collected at regular intervals (30, 60, 90, and 120 min) and checked for peak absorbance at 273 nm. Five milliliters of fresh distilled water was added after removing the sample in order to maintain sink condition. The films were dissolved in ethanol–water mixture, and the total extract present in the film was assayed by measuring the absorbance at 273 nm.
In vivo study
Clinical study design
A randomized, placebo-controlled, parallel-group, single-evaluator–blinded study with split-mouth design was conducted for 4 weeks. The sample size of 50 patients (i.e., 25 patients in each group) was estimated to achieve 90% power to detect a difference of 1.0 between the null hypotheses and alternative mean through Creative Research Systems. Outpatients from both sexes with age limit of 20-65 years were selected from Department of Periodontics, Narayana Dental College, Andhra Pradesh, India from May 2009 to December 2009.
The inclusion criteria were: 1) Patients with chronic periodontitis with pocket depth of >4 mm and radiographic evidence of bone loss bilaterally and 2) those willing to take part in the study and maintain their appointment regularly. The exclusion criteria were: 1) Patients having systemic diseases other than diabetes mellitus; 2) those who have received any topical or systemic antimicrobial treatment or periodontal treatment in the past 6 months; 3) pregnant and lactating mothers; and 4) smokers. The study protocol was reviewed and approved by the institutional and university ethical board.
Criteria for grouping and randomizations
Group 1: 25 systemically healthy patients with chronic periodontitis
Group 2: 25 diabetic patients with chronic periodontitis.
All patients were subjected to supragingival scaling and given oral hygiene instructions. In each patient of both the groups, two contralateral target sites were selected and randomly assigned following simple randomization procedure (computerized random numbers) into test and control sites to receive green tea and placebo strips, respectively.
The green tea and placebo strips were numbered G1-G200 and P1–P200, respectively. After proper isolation of the selected area, a dental hygienist inserted the strips subgingivally with a tweezer at baseline, first, second, and third weeks in test and control sites. Later, the evaluator made recordings of the post-insertion clinical parameters at different intervals without any knowledge of the sites allocated. At the end of the study, decoding was done to compare and relate the data.
Clinical parameters
Gingival Index (GI), Probing Pocket Depth (PPD), and Clinical Attachment Level (CAL) were recorded using periodontal probe (Hu-Friedy, Chicago, United States) at baseline, first, second, third, and fourth weeks. All the parameters were assessed at the test and control sites [Figure 1].
Figure 1.

Study design: Following screening and grouping, test and control sites were selected. Microbial plaque samples were collected and clinical measurements recorded at baseline, and green tea and placebo strips were placed in the test and control sites, respectively. Placement of strips was repeated at first, second, and third weeks after recording the clinical parameters. At the fourth week, clinical parameters and microbial plaque samples were collected
Microbiological analysis
The subgingival plaque was collected using a sterile Gracey curette (Hu-Friedy). The samples were then transferred to Eppendorf vial containing Tris-ethylenediaminetetraacetic acid (EDTA) buffer and sent for microbiological analysis for the detection of A. actinomycetemcomitans, and P. gingivalis by multiplex polymerase chain reaction (PCR) at baseline. Similar procedure was repeated at the end of fourth week. Microbiological analysis was performed for 32 sites (16 control sites and 16 test sites).
Statistical analysis
Data shown are from three separate experiments (in vitro, in vivo, and microbiological) that were analyzed statistically by descriptive statistics, independent-samples t-test, paired samples t-test, and repeated-measures analysis of variance (ANOVA). Contingency coefficient (CC) test was used to analyze the association between the occurrence of microorganisms at baseline and fourth week postoperatively. All the statistical methods were carried out through SPSS for Windows (version 16.0).
RESULTS
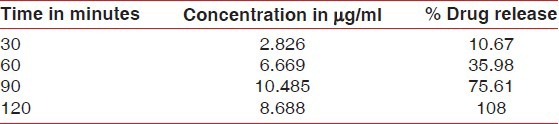
The in vitro release pattern of the green tea catechin strips at different time intervals (30, 60, 90, and 120 min) was evaluated, and the amount of drug released was 2.826 μg/ml (10.67%), 6.669 μg/ml (35.98%), 10.485 μg/ml (75.61%), and 8.688 μg/ml (108%), respectively [Table 1].
Table 1.
In vitro release pattern of the green tea catechin strips at different time intervals
In vivo study: One patient from the non-diabetic group (n = 24) and two patients from the diabetic group (n = 23) missed the appointments after baseline assessment and were excluded from the study.
When GI scores were compared between the groups at different intervals and at study sites, they showed statistically high significance (P < 0.001), whereas no significance was observed when the comparison was made between diabetic and non-diabetic groups [Figure 2].
Figure 2.
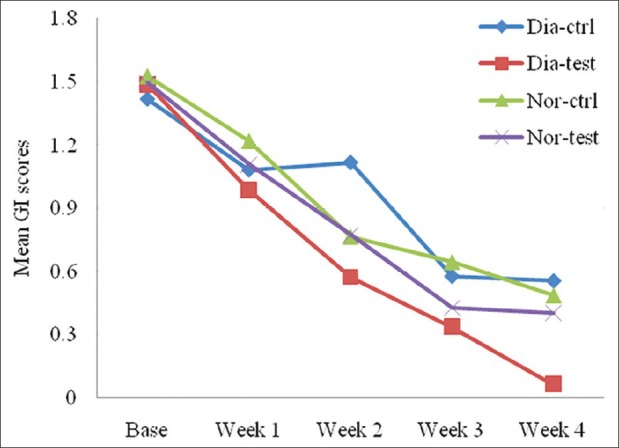
Comparison of gingival index scores between non-diabetic (n = 24) and diabetic (n = 23) groups at different intervals
On comparison of PPD between the groups at different intervals, high significance (P < 0.001) was observed from baseline to the end of fourth week. However, the quantum of change varied in different groups, showing high significance in the non-diabetic group [Figure 3].
Figure 3.
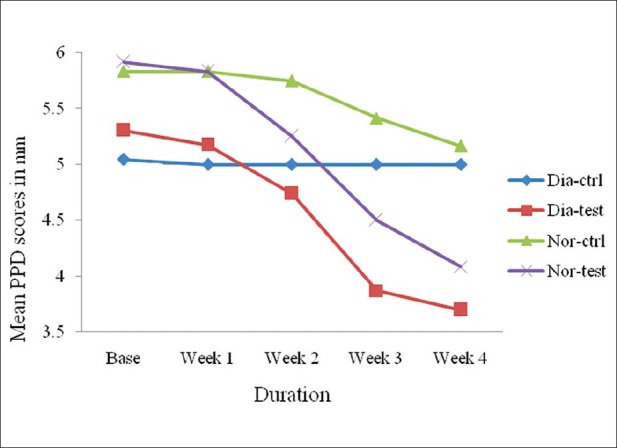
Comparison of probing pocket depth between non-diabetic and diabetic groups at different intervals
Comparison of CAL between the groups at different intervals showed statistically significant difference (P < 0.001) from baseline to fourth week. The quantum of change varied in different groups, whereas with respect to the comparison between the groups, high significant change was observed with respect to the diabetic group [Figure 4].
Figure 4.

Comparison of clinical attachment level between non-diabetic and diabetic groups at different intervals
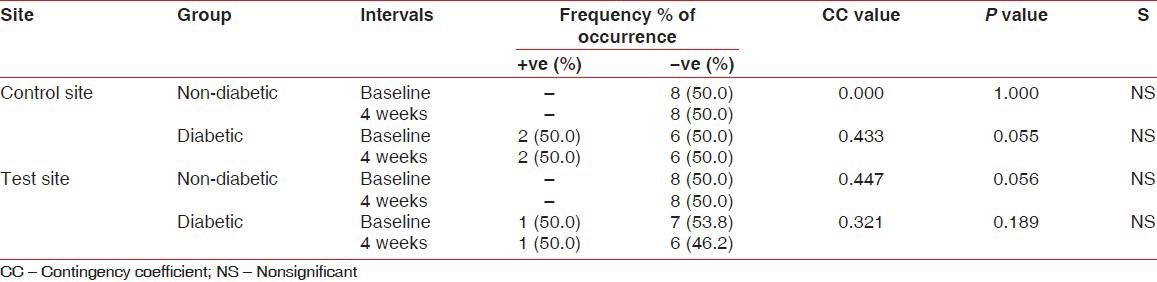
When the comparison of occurrence of P. gingivalis was made in the non-diabetic group, a significant association was observed in the incidence with the duration, which was statistically significant at 5% level (CC = 0.44, P < 0.046) in the test site, whereas no significance was found in the control site. In the diabetic group, there was no significant association at the control site (CC = 0.43, P < 0.055) and test site (CC = 0.32, P < 0.189). However, five sites showed incidence for the organism at baseline and only two sites were positive at the end of fourth week in the test site [Table 2].
Table 2.
Comparison of occurrence of P. gingivalis at different sites in non-diabetic and diabetic groups and at baseline and 4-week intervals

When the occurrence of A. actinomycetemcomitans was compared in both groups, no significant association was observed [Table 3].
Table 3.
Comparison of occurrence of A. actinomycetemcomitans at different sites in the non-diabetic and diabetic groups and at baseline and 4-week intervals
DISCUSSION
The in vitro study showed 10.67% of green tea release at 30 min, thereafter a slow release of the remaining green tea, and then at 120 min, it ended with complete release leading to maximum availability of the drug, which was in accordance with the reports of Hirasawa et al.,[21] Noguchi et al.,[29] and Soskolne.[30]
The in vivo study was conducted for 4 weeks period because healing of periodontium following nonsurgical therapy demonstrates an initial gain of clinical attachment at 3 weeks. Healing of a new dentogingival junction takes at least 4 weeks and no further gain in clinical attachment occurs in the subsequent 3 months.[31,32]
Both the groups in our study showed significant reduction in gingival inflammation at the test sites, similar to that reported by Hirasawa et al.[21] This may be due to the well-controlled antioxidant effect scavenging the increased reactive oxygen and nitrogen species[33,34,35] at the diseased periodontal sites.[36,37,38,39] Also, (−)-epigallocatechin gallate (EGCG) of green tea inhibits lipooxygenases and cyclooxygenases (COX), which are responsible for the production of prostaglandin E2 (PGE2).[40,42] EGCG also inhibits the mRNA expression of COX-2, matrix metalloproteinase (MMP)-1, and interleukin (IL)-1, -6, and -8 by the cultured cells.[43]
The diabetic and non-diabetic groups showed significant PPD reduction similar to that reported by Hirasawa et al.[21] Faster reduction in PPD was observed in the non-diabetic group than in the diabetic group. This may be due to the inhibitory effect of green tea catechins on cysteine proteinases (Arg-gingipain and Lys-gingipain) of P. gingivalis[44] and protein tyrosine phosphatase of P. intermedia,[45] which are considered as potent virulence factors in the development of periodontitis.[46,47,48] This may also be because of absorption of green tea into the epithelial cells in the subgingival pocket, thus inhibiting the growth of black pigmented rods responsible for periodontal disease.
Gain in CAL at the test sites was 1.33 mm in the non-diabetic group and 1.43 mm in the diabetic group, consistent with the report of Yun et al.[49] and Makimura et al.[17] They observed that green tea catechins can inhibit collagenase activity and prevent alveolar bone resorption, and this may help in preventing periodontal disease progression.[50]
Even though bacterial culture is the “gold standard” microbiological assay, it is now accepted that PCR technology provides a more sensitive means of detection, as compared to the culture technique for putative bacterial species.[51] Hence, the microbiological assessment was performed using multiplex PCR showing the incidence of P. gingivalis and A. actinomycetemcomitans.
In the present study, the prevalence of P. gingivalis in the non-diabetic test sites was significantly reduced from baseline (75%) to fourth week (25%), suggesting the antimicrobial effects of green tea catechins, whereas in the control sites, it remained unchanged. In the diabetic test sites, there was no statistically significant association; however, 71.4% showed incidence for the organism at baseline and only 28.6% were positive at the fourth week. These results indicate that green tea catechin was effective in treating P. gingivalis infection. Moreover, reduction in P. gingivalis at the positive sites was accompanied by clinical improvement.
The incidence of A. actinomycetemcomitans in both the groups was not significant in accordance with the reports of Ali et al.,[52] Conrads et al.,[53] and Umeda et al.[54] The baseline data indicated that A. actinomycetemcomitans was present in 12.5% of the sampled sites. This could be because A. actinomycetemcomitans and P. gingivalis are rarely detected in the same periodontal site as reported by Yoshida et al.[52,55]
However, the short-term trial period and early dissolution of the strips may warrant further interventions to consider the long-term benefits of green tea as a local drug therapy.
CONCLUSION
Green tea catechin strip can be used as an adjunct to scaling and root planing in the treatment of chronic periodontitis both in diabetic and non-diabetic patients. Its continuous application on a daily basis may be a useful and practical method in the treatment of periodontal disease.
ACKNOWLEDGMENT
The authors thank Department of Biochemistry and Microbiology for the timely help during the study.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Kushiyama M, Shimazaki Y, Murakami M, Yamashita Y. Relationship between intake of Green tea and Periodontal disease. J Periodontol. 2009;80:372–7. doi: 10.1902/jop.2009.080510. [DOI] [PubMed] [Google Scholar]
- 2.Golub LM, Schneir M, Ramamurthy NS. Enhanced collagenase activity in diabetic rat gingiva: In Vitro and In Vivo evidence. J Dent Res. 1998;57:520–5. doi: 10.1177/00220345780570032101. [DOI] [PubMed] [Google Scholar]
- 3.Gustke CJ. Treatment of periodontitis in the diabetic patient: A critical review. J Clin Periodontol. 1999;26:133–7. doi: 10.1034/j.1600-051x.1999.260301.x. [DOI] [PubMed] [Google Scholar]
- 4.Rees TD. Periodontal management of the patient with Diabetes mellitus. Periodontol 2000. 2000;23:63–72. doi: 10.1034/j.1600-0757.2000.2230105.x. [DOI] [PubMed] [Google Scholar]
- 5.Campus G, Salem A, Uzzau S, Baldoni E, Tonolo G. Diabetes and Periodontal Disease: A case-control study. J Periodontol. 2005;76:418–25. doi: 10.1902/jop.2005.76.3.418. [DOI] [PubMed] [Google Scholar]
- 6.Mealey B, Ocampo GL. Diabetes mellitus and periodontal disease. Periodontol 2000. 2007;44:127–53. doi: 10.1111/j.1600-0757.2006.00193.x. [DOI] [PubMed] [Google Scholar]
- 7.Nishimura F, Iwamoto Y, Soga Y. The Periodontal host response with diabetes. Periodontol 2000. 2007;43:245–53. doi: 10.1111/j.1600-0757.2006.00171.x. [DOI] [PubMed] [Google Scholar]
- 8.Cugini MA, Haffajee AD, Smith C, Kent RL, Jr, Socransky SS. The effect of scaling and root planing on the clinical and microbiological parameters of periodontal diseases: 12-month results. J Clin Periodontol. 2000;27:30–6. doi: 10.1034/j.1600-051x.2000.027001030.x. [DOI] [PubMed] [Google Scholar]
- 9.Jorgensen MG, Safarian A, Daneshmand N, Keim RJ, Slots J. Initial antimicrobial effect of controlled-release doxycycline in subgingival sites. J Periodontal Res. 2004;39:315–9. doi: 10.1111/j.1600-0765.2004.00742.x. [DOI] [PubMed] [Google Scholar]
- 10.Kornman KS. Controlled-release local delivery antimicrobials in periodontics: Prospects for the future. J Periodontol. 1993;64:782–91. doi: 10.1902/jop.1993.64.8s.782. [DOI] [PubMed] [Google Scholar]
- 11.Greenstein G, Polson A. The role of local drug delivery in the management of periodontal disease: A comprehensive review. J Periodontol. 1998;69:507–20. doi: 10.1902/jop.1998.69.5.507. [DOI] [PubMed] [Google Scholar]
- 12.Soskolne WA. Subgingival delivery of Therapeutic agents in the treatment of Periodontal diseases. Crit Rev Oral Biol Med. 1997;8:164–74. doi: 10.1177/10454411970080020501. [DOI] [PubMed] [Google Scholar]
- 13.Drisko CH. The use of locally-delivered doxycycline in the treatment of periodontitis: Clinical results. J Clin Periodontol. 1998;25:947–52. doi: 10.1111/j.1600-051x.1998.tb02396.x. [DOI] [PubMed] [Google Scholar]
- 14.Garnett S, Johnson L, Drisko CH. Two multi-center studies evaluating locally delivered doxycycline hyclate, placebo control, oral hygiene, and scaling and root planing in the treatment of periodontitis. J Periodontol. 1999;70:490–503. doi: 10.1902/jop.1999.70.5.490. [DOI] [PubMed] [Google Scholar]
- 15.Meinberg TA, Barnes CM, Dunning DG, Reinhardt RA. Comparison of conventional periodontal maintenance versus scaling and root planing with subgingival minocycline. J Periodontol. 2002;73:167–72. doi: 10.1902/jop.2002.73.2.167. [DOI] [PubMed] [Google Scholar]
- 16.Higdon JV, Frei B. Tea catechins and polyphenols: Health effects, metabolism, and antioxidant functions. Crit Rev Food Sci Nutr. 2003;43:89–143. doi: 10.1080/10408690390826464. [DOI] [PubMed] [Google Scholar]
- 17.Makimura M, Hirasawa M, Kobayashi K, Indo J, Sakanaka S, Taguchi T, et al. Inhibitory effect of tea catechins on collagenase activity. J Periodontol. 1993;64:630–6. doi: 10.1902/jop.1993.64.7.630. [DOI] [PubMed] [Google Scholar]
- 18.Inaba H, Tagashira M, Honma D, Kanda T, Kou Y, Ohtake Y, et al. Identification of Hop Polyphenolic components which inhibit prostaglandin-E 2 production by gingival epithelial cells stimulated with periodontal pathogen. Biol Pharm Bull. 2008;31:527–30. doi: 10.1248/bpb.31.527. [DOI] [PubMed] [Google Scholar]
- 19.Otake S, Makimura M, Kuroki T, Nishihara Y, Hirasawa M. Anticaries effects of polyphenolic compounds from Japanese Green tea. Caries Res. 1991;25:438–43. doi: 10.1159/000261407. [DOI] [PubMed] [Google Scholar]
- 20.Lee MJ, Lambert JD, Prabhu S, Meng X, Lu H, Maliakal P, et al. Delivery of tea polyphenols to the oral cavity by green tea leaves and black tea extract. Cancer Epidemiol Biomarkers Prev. 2004;13:132–7. doi: 10.1158/1055-9965.epi-03-0040. [DOI] [PubMed] [Google Scholar]
- 21.Hirasawa M, Takada K, Makimura M, Otake S. Improvement of periodontal status by green tea catechin using a local delivery system: A clinical pilot study. J Periodontal Res. 2002;37:433–8. doi: 10.1034/j.1600-0765.2002.01640.x. [DOI] [PubMed] [Google Scholar]
- 22.Maron DJ, Lu GP, Cai NS, Wu ZG, Li YH, Chen H, et al. Cholesterol-lowering effect of a theaflavin-enriched green tea extract: A randomized controlled trial. Arch Intern Med. 2003;163:1448–53. doi: 10.1001/archinte.163.12.1448. [DOI] [PubMed] [Google Scholar]
- 23.Wolfram S, Wang Y, Thielecke F. Anti-obesity effects of green tea: From bedside to bench. Mol Nutr Food Res. 2006;50:176–87. doi: 10.1002/mnfr.200500102. [DOI] [PubMed] [Google Scholar]
- 24.Chow SH, Hakim IA, Vining DR, Crowell JA, Ranger-Moore J, Chew WM, et al. Effects of dosing condition on the oral bioavailability of green tea catechins after single-dose administration of Polyphenon-E in Healthy individuals. Clin Cancer Res. 2005;11:4627–33. doi: 10.1158/1078-0432.CCR-04-2549. [DOI] [PubMed] [Google Scholar]
- 25.Nagao T, Hase T, Takimitsu I. A Green tea extract high in catechins reduces Body fat and cardiovascular risks in Human body. Obesity. 2007;15:1473–83. doi: 10.1038/oby.2007.176. [DOI] [PubMed] [Google Scholar]
- 26.Miura T, Koike T, Ishida T. Antidiabetic activity of Green tea (Thea Sinensisn L.) In genetically Type-2 diabetic mice. J Health Sci. 2005;51:708–10. [Google Scholar]
- 27.Panagiotakos DB, Lionis C, Zeimbekis A, Gelastopoulou K, Papairakleous N, Das UN, et al. Long-Term Tea intake is associated with reduced prevalence of (Type 2) Diabetes Mellitus among Elderly people from Mediterranean Islands: MEDIS Epidemiological Study. Yonsei Med J. 2009;50:31–8. doi: 10.3349/ymj.2009.50.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iso H, Date C, Wakai K, Fukui M, Tamakoshi A. The Relationship between Green tea and total Caffeine intake and Risk for self-reported Type-2 Diabetes among Japanese Adults. Ann Intern Med. 2006;144:554–62. doi: 10.7326/0003-4819-144-8-200604180-00005. [DOI] [PubMed] [Google Scholar]
- 29.Noguchi T, Fukuda M, Ishikawa I. Periodontal treatment by Local drug delivery using Resorbable base material. Adv Dent Res. 1988;2:401–4. doi: 10.1177/08959374880020023701. [DOI] [PubMed] [Google Scholar]
- 30.Soskolne WA, Heasman PA, Stabholz A, Smart GJ, Palmer M, Flashner M, et al. Sustained local delivery of Chlorhexidine in the treatment of Periodontitis: A Multi-center study. J Periodontol. 1997;68:32–8. doi: 10.1902/jop.1997.68.1.32. [DOI] [PubMed] [Google Scholar]
- 31.Mealey B, Oates TW. Diabetes mellitus and periodontal disease. AAP - Commissioned Review. J Periodontol. 2006;77:1289–303. doi: 10.1902/jop.2006.050459. [DOI] [PubMed] [Google Scholar]
- 32.Lowenguth R, Greenstein G. Clinical and microbiological response to nonsurgical mechanical periodontal therapy. Periodontol 2000. 1995;9:14–22. doi: 10.1111/j.1600-0757.1995.tb00052.x. [DOI] [PubMed] [Google Scholar]
- 33.Henning SM, Niu Y, Lee NH. Bioavailability and antioxidant activity of tea flavanols after consumption of green tea, black tea, or a green tea extract supplement. Am J Clin Nutr. 2004;80:1558–64. doi: 10.1093/ajcn/80.6.1558. [DOI] [PubMed] [Google Scholar]
- 34.Horie N, Hirabayashi N, Takahashi Y, Miyauchi Y, Taguchi H, Takeishi K, et al. Synergistic effect of green tea catechins on cell growth and apoptosis induction in gastric carcinoma cells. Biol Pharma Bull. 2005;28:574–9. doi: 10.1248/bpb.28.574. [DOI] [PubMed] [Google Scholar]
- 35.Cabrera C, Artacho R, Gimenez R. Beneficial effects of Green tea- A Review. J Am Col Nutr. 2006;25:79–99. doi: 10.1080/07315724.2006.10719518. [DOI] [PubMed] [Google Scholar]
- 36.Ingman T, Sorsa T, Suomalainen K, Halinen S, Lindy O, Lauhio A, et al. Tetracycline Inhibition and the cellular source of collagenase in Gingival crevicular fluid in different Periodontal diseases. A review article. J Periodontol. 1993;64:82–8. doi: 10.1902/jop.1993.64.2.82. [DOI] [PubMed] [Google Scholar]
- 37.Shapira L, Gordon B, Warbington M, Van Dyke TE, et al. Priming Effect of Porphyromonas gingivalis lipopolysaccharide on superoxide production by Neutrophils from Healthy and Rapidly Progressive Periodontitis subjects. J Periodontol. 1994;65:129–33. doi: 10.1902/jop.1994.65.2.129. [DOI] [PubMed] [Google Scholar]
- 38.Eick S, Pfister W. Comparison of microbial cultivation and a commercial nucleic acid based method for detection of periodontopathogenic species in subgingival plaque samples. J Clin Periodontol. 2002;29:638–44. doi: 10.1034/j.1600-051x.2002.290708.x. [DOI] [PubMed] [Google Scholar]
- 39.Ohnishi T, Bandow K, Kakimoto K, Machigashira M, Matsuyama T, Matsuguchi T. Oxidative stress causes alveolar bone loss in metabolic syndrome model mice with type-2 diabetes. J Periodontal Res. 2009;44:43–51. doi: 10.1111/j.1600-0765.2007.01060.x. [DOI] [PubMed] [Google Scholar]
- 40.Dreosti IE. Bioactive ingredients: Antioxidants and polyphenols in tea. Nutr Rev. 1996;54:S51–8. doi: 10.1111/j.1753-4887.1996.tb03819.x. [DOI] [PubMed] [Google Scholar]
- 41.Kirkwood KL, Cirelli JA, Rogers JE, Giannobile MV. Novel host response therapeutic approaches to treat periodontal diseases. Periodontol 2000. 2007;43:294–315. doi: 10.1111/j.1600-0757.2006.00166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kou Y, Inaba H, Kato T, Tagashira M, Honma D, Kanda T, et al. Inflammatory responses of gingival epithelial cells stimulated with Porphyromonas gingivalis vesicles are inhibited by Hop-associated polyphenols. J Periodontol. 2008;79:174–80. doi: 10.1902/jop.2008.070364. [DOI] [PubMed] [Google Scholar]
- 43.Ahmed S, Wang N, Lalonde M, Goldberg VM, Haqqi TM. Green Tea Polyphenol Epigallocatechin-3-gallate (EGCG) differentially inhibits Interleukin-1 induced expression of Matrix Metalloproteinase-1 and -13 in Human Chondrocytes. J Pharmacol Exp Ther. 2004;308:767–73. doi: 10.1124/jpet.103.059220. [DOI] [PubMed] [Google Scholar]
- 44.Okamoto M, Sugimoto A, Leung KP, Nakayama K, Kamaguchi A, Maeda N, et al. Inhibitory effect of green tea catechins on cysteine proteinases in Porphyromonas gingivalis. Oral Microbiol Immunol. 2004;19:118–20. doi: 10.1046/j.0902-0055.2003.00112.x. [DOI] [PubMed] [Google Scholar]
- 45.Hirasawa M, Takada K, Otake S. Inhibition of acid production in dental plaque bacteria by green tea catechins. Caries Res. 2006;40:265–70. doi: 10.1159/000092236. [DOI] [PubMed] [Google Scholar]
- 46.Haffajee AD, Socransky SS. Microbial etiological agents of destructive periodontal diseases. Periodontol 2000. 1994;5:78–111. doi: 10.1111/j.1600-0757.1994.tb00020.x. [DOI] [PubMed] [Google Scholar]
- 47.Holt SC, Kesavalu L, Walker S, Genco CA. Virulence factors of Porphyrornonas gingivalis. Periodontol 2000. 1999;20:168–238. doi: 10.1111/j.1600-0757.1999.tb00162.x. [DOI] [PubMed] [Google Scholar]
- 48.Newman MG, Takei HH, Klokkevold PR, Carranza FA. Fermin A Carranza. 10th ed. Netherlands: Elsevier; 2006. Carranza's Clinical Periodontology; pp. 228–50. [Google Scholar]
- 49.Yun JH, Pang EK, Kim CS, Yoo YJ, Cho KS, Chai JK, et al. Inhibitory effects of green tea polyphenol (–)- epigallocatechin gallate on the expression of matrix metalloproteinase-9 and on the formation of osteoclasts. J Periodontal Res. 2004;39:300–7. doi: 10.1111/j.1600-0765.2004.00743.x. [DOI] [PubMed] [Google Scholar]
- 50.Yun JH, Kim CS, Cho KS, Chai JK, Kim CK, Choi SH, et al. (-)-Epigallocatechin gallate induces apoptosis, via caspase activation, in osteoclasts differentiated from RAW 264.7 cells. J Periodontal Res. 2007;42:212–8. doi: 10.1111/j.1600-0765.2006.00935.x. [DOI] [PubMed] [Google Scholar]
- 51.Okada M, Hayashi F, Nagasaka N. PCR detection of 5 putative periodontal pathogens in dental plaque samples from children 2 to 12 years of age. J Clin Periodontol. 2001;28:576–82. doi: 10.1034/j.1600-051x.2001.028006576.x. [DOI] [PubMed] [Google Scholar]
- 52.Ali RW, Lie T, Skaug N. Early effects of Periodontal therapy on the detection frequency of four putative Periodontal pathogens in adults. J Periodontol. 1992;63:540–7. doi: 10.1902/jop.1992.63.6.540. [DOI] [PubMed] [Google Scholar]
- 53.Conrads G, Mutters R, Fischer J, Brauner A, Lütticken R, Lampert F. PCR reaction and Dot-Blot Hybridization to monitor the distribution of Oral Pathogens within plaque samples of Periodontally healthy individuals. J Periodontol. 1996;67:994–1003. doi: 10.1902/jop.1996.67.10.994. [DOI] [PubMed] [Google Scholar]
- 54.Umeda M, Miwa Z, Takeuchi Y, Ishizuka M, Huang Y, Noguchi K, et al. The distribution of periodontopathic bacteria among Japanese children and their parents. J Periodontal Res. 2004;39:398–404. doi: 10.1111/j.1600-0765.2004.00754.x. [DOI] [PubMed] [Google Scholar]
- 55.Yoshida Y, Suzuki N, Nakano Y, Shibuya K, Ogawa Y, Koga T. Distribution of Actinobacillus actinomycetemcomitans serotypes and Porphyromonas gingivalis in Japanese adults. Oral Microbiol Immunol. 2003;18:135–9. doi: 10.1034/j.1399-302x.2003.00034.x. [DOI] [PubMed] [Google Scholar]


