Abstract
Background:
Increased knowledge of anaerobic bacteria in the development of periodontal diseases has led to new treatment strategies aiming primarily at suppression or elimination of specific periodontal pathogens. Over the last few decades, green tea has been subjected to many scientific and medical studies.
Aim:
The purpose of the present study was to assess the effect of green tea catechins on the red complex organisms using Polymerase Chain Reaction for microbiological analysis.
Materials and Methods:
A split mouth study was conducted, in which a total of 20 subjects were included. Green tea catechin as local drug delivery was placed at study sites. Clinical parameters namely probing pocket depth (PPD), gingival index (GI), plaque index (PI) were recorded. Sub-gingival plaque samples were collected, and red complex micro-organisms were studied using PCR. Clinical and microbiological parameters were recorded at baseline, 1st, and 5th week after treatment.
Results:
The results showed statistically significant difference in PPD, GI, and PI and significant reduction of red complex organisms from baseline to 1st week and baseline to 5th week in both study and control groups (P < 0.001). Intergroup comparison between study and control group was statistically insignificant for PPD, PI, and GI. A significantly greater reduction in Tannerella forsythus (Tf) at 1st week and 5th week and Porphyromonas gingivalis (Pg) at 1st week was observed in study group when compared to control group.
Conclusion:
Green tea catechin can be used as an effective local drug delivery along with scaling and root planing in treatment of chronic periodontitis.
Keywords: Chronic periodontitis, green tea, polymerase chain reaction, red complex organisms
INTRODUCTION
Tea is the most consumed drink in the world after water. Over the last few decades, green tea has been subjected to many scientific and medical studies. Since ancient times, green tea has been considered by the traditional Chinese medicine as a healthful beverage. Increasing interest in its health benefits has led to the inclusion of green tea in the group of beverages with functional properties.
Non-surgical periodontal therapy has long been documented to preserve the natural dentition by achieving and maintaining a healthy periodontium.[1] Green tea contains a number of bioactive chemicals. It is particularly rich in flavonoids, including catechins, and their derivatives, such as (−)-epigallocatechin-3- gallate (EGCG), (−)- epigallocatechin (EGC), (−)-epicatechin-3-gallate (ECG), (−)-epicatechin. These polyphenolic compounds, the most abundant of which is epigallocatechin gallate (EGCG), are thought to contribute to the beneficial effects ascribed to tea.[2] Green tea has the ability to reduce symptoms of periodontal disease due to the presence of the antioxidant catechin.[2]
Periodontitis is an infectious disease of bacterial etiology, immunological response, and tissue destruction.[3] Studies have shown that the catechin has an inhibitory effect on the collagenase activity and suggests that it may be useful for prevention of periodontal diseases.[2] Numerous studies have shown that the green tea extracts can be used in the form of local drug delivery system[4] at a concentration of 1.0 mg/ml.
Apart from its effect on the putative periodontal pathogens, green tea is also known to have beneficial effects against dental caries.[5] Hence, in this study, the efficacy of green tea catechin was analyzed as a local drug delivery system for the treatment of periodontal pockets as an adjunct to scaling and root planing.
MATERIALS AND METHODS
Experimental design and patient selection
It was a randomized and placebo-controlled clinical trial, where a split mouth design was followed in each subject, 2 sites in the contra-lateral quadrants with probing pocket depths with 5 mm and more at baseline were chosen.
During the initial phase, all the subjects were explained about proper home-care techniques and monitored during study period and received full mouth scaling and root planing (SRP). The patients were then randomly divided into 2 groups; a study group and a control group. All subjects received microbiological and clinical monitoring at baseline, 1st week, and 5th week respectively. The study was approved by the ethical committee of Maratha Mandal's Nathajirao G. Halgekar Institute of Dental Sciences and Research Center, Belgaum. The research objectives were explained to the patients, who then signed an informed consent form.
Subject population and inclusion/exclusion criteria
A total of 20 subjects with untreated chronic periodontitis were selected by one of the author from the population referred to the department of periodontics. Detailed medical, periodontal, and dental histories were obtained. All the eligible subjects were informed of the nature, potential risks, and benefits of the study.
The inclusion criteria were as follows, age >35 yrs, probing depth of 5 mm or more, dentate with >20 natural teeth. The exclusion criteria were medically compromised patients, patients who have been administered antibiotic or antimicrobial in the past 6 months, smokers, pregnant, and lactating mother.
Determination of periodontal status
All the participants were evaluated clinically. The following clinical and periodontal parameters were assessed by the same examiner, the plaque index (Silness and Loe 1964), gingival index (Loe and Silness 1963), and probing depth, measured to the nearest millimeter from the gingival margin to the bottom of the pocket was noted using calibrated William's periodontal probe for all measurements.
Collection of plaque samples
Plaque samples were collected from sites of each patient with >5 mm probing depth after isolating the selected sites with sterile cotton rolls. A sterile Gracey curette was inserted into the selected sites in both groups, and the sub-gingival plaque samples were obtained. After collection of plaque samples, they were immediately transferred into a sterile vial containing TE buffer. The plaque samples were stored at 4°C until subsequent analysis.
Catechin strip placement
The Hydroxy propyl cellulose strips (HPC), *containing catechin, were rectangular measuring 2 mm in width, 4 mm in length, and 0.3 mm in thickness and were placed into the selected study sites and covered by Coe-pack to keep the strip in place.
Microbiological assay
One sub-gingival plaque sample was collected from the study and control sites per subject at baseline, 1st week, and 5th week. After the clinical parameters were recorded, the sub-gingival plaque samples were taken and stored at 4°C in a sterile vial containing TE buffer solution. The counts of 3 bacterial species were then determined in each of the samples using PCR analysis.
Statistical analysis
The data so collected were then put for statistical analysis, and the mean values of the observed variables were put to following statistical test. Paired t test, Chi-square test, and Wilcoxon's test were applied, where P < 0.05 was considered as significant.
RESULTS
The present study was conducted to assess the clinical and microbiological efficacy of Green tea catechin local drug delivery system as an adjunct to scaling and root planing in the treatment of patients with chronic periodontitis. The study included 20 patients with a total of 40 sites that were randomly allocated into 2 groups. Each group consisted of 20 sites that were followed up to 5th week. Intragroup and intergroup comparisons for clinical and microbiological parameters were done at baseline, 1st week, and 5th week.
Intragroup comparison: Clinical parameters
Tables 1-3 and Figures 1 and 2 show the mean values of clinical parameters at baseline, 1st week, and 5th week. The reduction in probing depth, gingival index, and plaque index from baseline to 1st week and baseline to 5th week was statistically significant (P < 0.001) in both study and control group.
Table 1.
The mean reduction of probing depth in study and control groups at various intervals
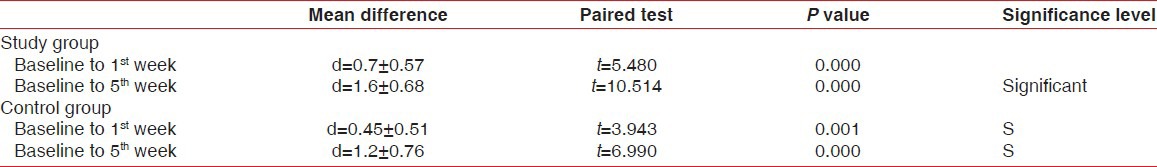
Table 3.
The mean reduction of plaque index in study and control groups at various time intervals

Figure 1.
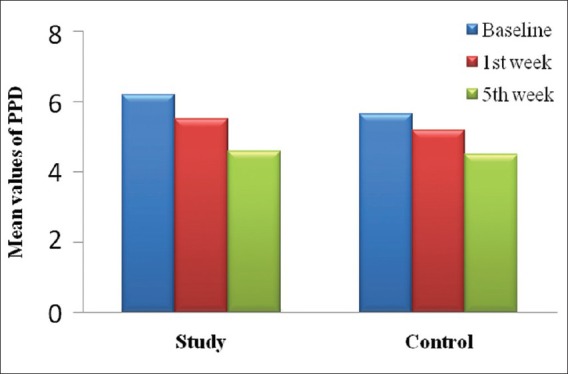
Mean reduction in probing depth from baseline to 5th week in study and control group
Figure 2.
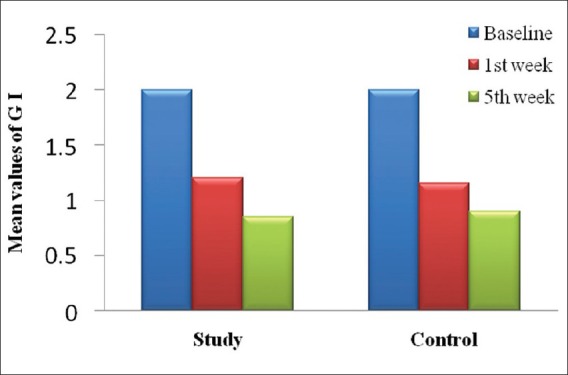
Mean reduction of gingival index from baseline to 5th week in study and control group
Table 2.
The mean reduction of gingival index in study and control groups at various intervals
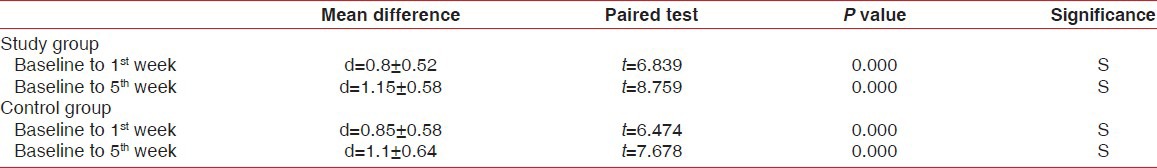
Intergroup comparison of clinical parameters
Tables 4-6 depict difference in the probing depths, gingival index, and plaque index between study and control group at baseline, 1st week, and 5th week, and also reduction in the clinical parameters from baseline to 5th week was compared between study group and control group.
Table 4.
Comparison of reduction in the probing depth between study and control groups at various time intervals

Table 6.
Comparison of reduction in the plaque index between study and control groups at various time intervals

Table 5.
Comparison of reduction of gingival Index between study and control groups at various time intervals
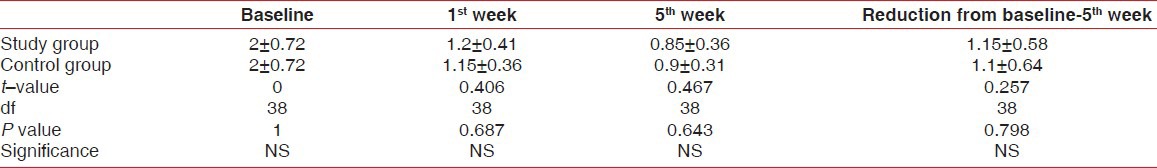
The differences in mean probing depth, gingival index, and plaque index between study and control group at various intervals were statistically insignificant. Similarly, the comparison of reduction in clinical parameters from baseline to 5th week showed that there was no statistically significant difference between study and control group.
Microbiological analysis
The number of red complex organisms namely Treponema denticola (Td), Tannerella forsythia (Tf), Porphyromonas gingivalis (Pg) were analyzed at baseline, 1st week, and 5th week in both the groups.
Intergroup comparison of Td, Tf, and Pg organisms in study group and control groups at baseline, 1st week, and 5th week showed that there was statistically significant reduction in Tf at 1st week and 5th week and Pg at 1st week from baseline in study group as compared to control group [Table 7, Figures 3 and 4].
Table 7.
Inter group comparison of Td, Tf, and Pg organism at Baseline, 1st week, and 5th week in study and control groups
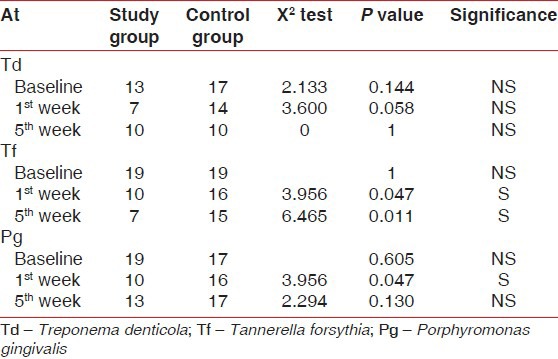
Figure 3.
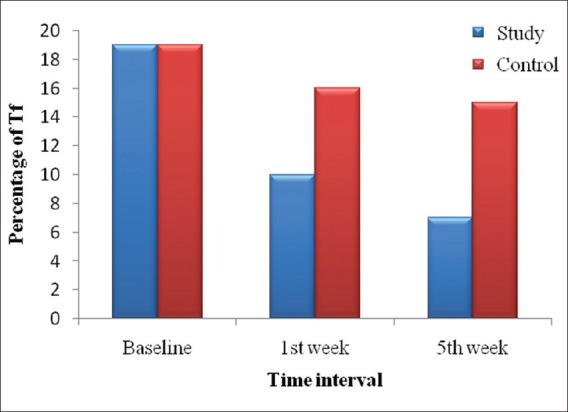
Distribution of Tf organisms present at various time interval in study and control group
Figure 4.
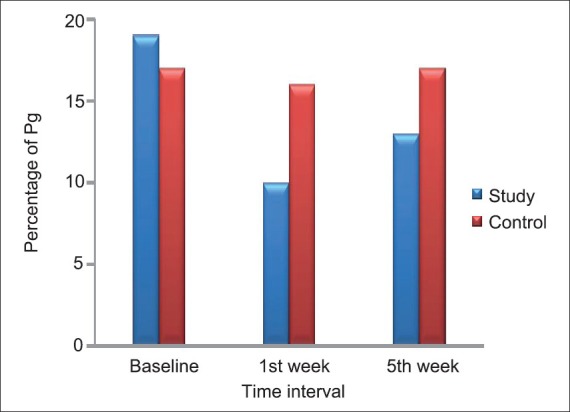
Distribution of Pg organism present at various intervals in study and control group
The Intragroup comparison of mean reduction of red complex organisms in study group and control group showed statistically significant reduction of red complex organisms from baseline to 1st week and 5th week [Table 8].
Table 8.
Intra group comparison of mean reduction of red complex organisms in study group and control group

DISCUSSION
The concept of periodontal disease has changed considerably over the years. The putative pathogens associated with periodontal diseases are susceptible to a variety of antiseptics and antibiotics.[6] Non-surgical periodontal therapy has long been documented to preserve the natural dentition by achieving and maintaining a healthy periodontium.[7] According to the current state of knowledge, species such as the red complex organisms are a few of them; those have shown to play a major role in the pathogenesis of periodontal diseases.[8]
The gold standard of periodontal therapy has been scaling and root planing; however, various adjunctives like local drug delivery have been used in conjunction to this to improve the therapeutic results. Several in vitro studies have suggested that green tea catechins such as (-). epigallocatechin gallate (EGCG), inhibit periodontal pathogens[5] and the destruction of the periodontal tissue.[9,10]
The application of biogradable and bioresorbable polymers in drug delivery and clinical medicine has gained increased attention in recent years.[11] Delivery of green tea catechin has been documented as an effective vehicle for local drug delivery in periodontal pockets by Noguchi et al.[11]
In the present study, a significant decrease in both plaque and gingival index scores was seen, the reduction in PI was 0.90 ± 0.55 and that for GI was 1.15 ± 0.58 from baseline to 5th week. This reduction was in accordance to the results obtained by Knowles et al.[12] and Ramfjord et al.[13]
Results of probing pocket depth (PPD) in control group showed a mean reduction of 1.2 mm (5.6 ± 0.99 to 5.2 ± 1.01) from baseline to 5th week. This was in accordance with the study done by Ryder MI,[14] in which there was a mean reduction in PPD of 1.1 mm at the end of 6 months. Results of PPD in study group showed a mean reduction of 1.6 mm (6.2 ± 1.28 mm to 4.6 ± 1.23 mm) from baseline to 5th week. This was in accordance with the study done by Hirasawa et al.[15] where he used green tea catechins as local drug delivery system for 8 weeks and reported a 1.3 mm reduction in PPD. This reduction in PPD could be attributed to anti-inflammatory role of green tea catechins.[9,16]
A statistical significant reduction in PPD was found from baseline to 5th week in both the groups. The reduction found in PPD was consistent with a reduction of inflammation in the adjacent gingival tissues. This reduction in inflammation and presence of healing in the connective tissue subjacent to the junctional epithelium can be attributed to the property of green tea catechin to inhibit tissue collagenase activity.[17] The seemingly greater closure of pockets could have been enhanced by the possibility of green tea catechin adsorbing onto the mineralized dental structures where it may act as a reservoir of the anti-microbial agent during a period of substantivity.[15]
However, intergroup comparison between study and control group was statistically insignificant for PPD, PI, and GI. This insignificant reduction in clinical parameters may be due to non-standardization of the pocket depths and also due to inaccessibility of instrumentation into the deep pockets that would affect the results.
PCR was used to monitor the effects of mechanical and/or chemical therapy on the sub-gingival microflora, as PCR assay has been shown to be highly sensitive and specific for the periodontal pathogens.[18] Green tea catechin has been shown to be effective in altering the flora and acting as an adjunct to scaling and root planing by Cobb et al. and Bruce et al.[19,20] A significantly greater reduction in Tf at 1st week and 5th week and Pg at 1st week was observed in study group when compared to control group [Table 7]. As evident from this study, the results demonstrate clinical and microbiological improvements following the use of green tea catechin chip as an adjunct to scaling and root planing.
Further research with relatively large sample size, longer follow-up period, and use of advanced newer delivery systems are advocated to further study the efficacy of green tea as an effective local drug delivery agent in periodontal therapy.
*- Ambe Phyto Extracts Pvt. Ltd., Hasanpur, Delhi.
ACKNOWLEDGMENT
My sincere thanks to Dr. Kishore. Bhatt, Professor, Department of Molecular Biology, for extending all support in assessing and helping me in my microbiological evaluation, for their constant encouragement, support, patience, and invaluable guidance during the course of my study.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Ishikawa I, Baehni P. Nonsurgical periodontal therapy where do we stand now? Periodontology 2000. 2004;36:9–13. doi: 10.1111/j.1600-0757.2004.03670.x. [DOI] [PubMed] [Google Scholar]
- 2.Goodson JM, Cugini MA, Kent RL, Armitage GC, Cobb CM, Fine D, et al. Multicenter evaluation of tetracycline fiber therapy: II Clinical response. J Periodontol Res. 1991;26:371–9. doi: 10.1111/j.1600-0765.1991.tb02076.x. [DOI] [PubMed] [Google Scholar]
- 3.Greenstein G, Polson A. The role of Local drug delivery in the management of periodontal diseases. A comprehensive review. J Periodontol. 1998;69:507–20. doi: 10.1902/jop.1998.69.5.507. [DOI] [PubMed] [Google Scholar]
- 4.Goodson JM, Hafazee A, Socransky SS. Periodontal therapy by local delivery of tetracycline. J Clin Periodontol. 1979;6:83–92. doi: 10.1111/j.1600-051x.1979.tb02186.x. [DOI] [PubMed] [Google Scholar]
- 5.Sakanaka S, Aizawa M, Kim M, Yamamoto T. Inhibitory effects of green tea polyphenols on growth and cellular adherence of an oral bacterium, Porphyromonas gingivalis. Biosci Biotech Biochem. 1996;60:745–9. doi: 10.1271/bbb.60.745. [DOI] [PubMed] [Google Scholar]
- 6.Hirasawa M, Takada K, Makimura M, Otake S. Improvement of periodontal status by green tea catechin using a local delivery system: A clinical pilot study. J Periodontal Res. 2002;37:433–8. doi: 10.1034/j.1600-0765.2002.01640.x. [DOI] [PubMed] [Google Scholar]
- 7.Claffey N, Polyzosis I, Ziaka P. An overview of nonsurgical and surgical therapy. Periodontology 2000. 2004;36:35–44. doi: 10.1111/j.1600-0757.2004.00073.x. [DOI] [PubMed] [Google Scholar]
- 8.Ramseier CA, Kinney JS, Herr AE, Braun T, Sugai JV, Shelburne CV, et al. Identification of pathogen and host-response markers correlated with periodontal disease. J Periodontol. 2009;80:436–46. doi: 10.1902/jop.2009.080480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yun JH, Pang EK, Kim CS, Yoo YJ, Cho KS, Chai JK, et al. Inhibitory effects of green tea polyphenol (–)-epigallocatechin gallate on the expression of matrix metalloproteinase-9 and on the formation of osteoclasts. J Periodontal Res. 2004;39:300–7. doi: 10.1111/j.1600-0765.2004.00743.x. [DOI] [PubMed] [Google Scholar]
- 10.Yun JH, Kim CS, Cho KS, Chai JK, Kim CK, Choi SH. (-)-Epigallocatechin gallate induces apoptosis, via caspase activation, in osteoclasts differentiated from RAW 264.7 cells. J Periodontal Res. 2007;42:212–8. doi: 10.1111/j.1600-0765.2006.00935.x. [DOI] [PubMed] [Google Scholar]
- 11.Noguchi T, Fukunda M, Ishikawa I. Periodontal treatment by local drug delivery using Resorbable base material. Adv Dent Res. 1988;2:401–4. doi: 10.1177/08959374880020023701. [DOI] [PubMed] [Google Scholar]
- 12.Knowles JW, Burgett FG, Nissle RR, Shick RA, Morrison EC, Ramfjord SP. Results of periodontal treatment related to pocket depth and attachment level. Eight years. J Periodontal. 1979;50:225–33. doi: 10.1902/jop.1979.50.5.225. [DOI] [PubMed] [Google Scholar]
- 13.Ramfjord SP, Caffesse RG, Morrision EC, Hill RW, Kerry GJ, Appleberry EA, et al. Four modalities of periodontal treatment compared over 3 years. J Clin Periodontol. 1987;14:445–52. doi: 10.1111/j.1600-051x.1987.tb02249.x. [DOI] [PubMed] [Google Scholar]
- 14.Ryder MI, Pons B, Adams D, Beiswanger B, Blanco V, Bogle G, et al. Effects of smoking on local drug delivery of controlled-release doxycycline as compared to scaling and root planing. J Periodontol. 1999;26:683–91. doi: 10.1034/j.1600-051x.1999.261008.x. [DOI] [PubMed] [Google Scholar]
- 15.Hirasawa M, Takada K, Makimura M, Otake S. Improvement of periodontal status by green tea catechin using a local delivery system: A clinical pilot study. J Periodontal Res. 2002;37:433–8. doi: 10.1034/j.1600-0765.2002.01640.x. [DOI] [PubMed] [Google Scholar]
- 16.Cabrera C, Artacho R, Gimenez R. Beneficial effects of Green tea - A Review. J Am Col Nutr. 2006;25:79–99. doi: 10.1080/07315724.2006.10719518. [DOI] [PubMed] [Google Scholar]
- 17.Hong J, Smith TJ, Ho CT, August DA, Yang CS. Effects of purified green and black tea polyphenols on cyclooxygenase- and lipoxygenase-dependent metabolism of arachidonic acid in human colon mucosa and colon tumor tissues. Biochem Pharmacol. 2001;62:1175–83. doi: 10.1016/s0006-2952(01)00767-5. [DOI] [PubMed] [Google Scholar]
- 18.Erick S, Pfister W. Comparison of microbial cultivation and a commercial PCR based method for detection of periodontopathogenic species in subgingival plaque samples. J Clin Periodontal. 2002;29:638–44. doi: 10.1034/j.1600-051x.2002.290708.x. [DOI] [PubMed] [Google Scholar]
- 19.Cobb CM. Non surgical pocket therapy. Mechanical. Ann Periodontal. 1996;1:443–90. doi: 10.1902/annals.1996.1.1.443. [DOI] [PubMed] [Google Scholar]
- 20.Bruce LP, Willam FA. American academy of periodontology. Treatment of gingivitis and periodontitis (Position paper) J Periodontol. 1997;68:1246–53. [PubMed] [Google Scholar]


