Abstract
Background
Vascular calcifications (VCs) are a cardiovascular risk factor in patients affected by chronic kidney disease and after kidney transplantation (KTx). We evaluated the prevalence of VCs at the abdominal aortic site in KTx patients at the time of transplantation and 1 year after KTx, exploring the possibly associated factors.
Methods
In 107 transplanted patients, the following parameters were evaluated at the first and twelfth month after KTx: the aortic calcification index (ACI), fibroblast growth factor 23, osteoprotegerin (OPG), fetuin A, and clinical and biochemical parameters. Patients were followed up for 2 years after KTx.
Results
At the time of KTx, 60% of patients had some degree of VC (ACI>0), whereas 40% had no VC. One year after KTx, VCs worsened in 26% of patients, whereas in 74%, VCs remained stable or improved. The progression of VC was observed almost exclusively in patients with a positive ACI score at the first month. At the multivariate analysis, serum calcium, OPG, and estimated glomerular filtration rate were the only variables independently associated with the progression of VC.
Conclusions
VCs at the aortic site are frequent in KTx patients, and in a significant percentage of them, they tend to progress even in the short time. High levels of serum calcium and OPG are significantly associated with the progression of VCs. Whether these associations are based on a cause-effect relationship and their correction might impact on the calcification process could be ascertained by prospective interventional studies.
Keywords: Kidney transplantation, Vascular calcification, Osteoprotegerin, Calcium, FGF-23
Patients with chronic kidney diseases (CKD) have a mortality rate that is exceedingly higher than the general population, with cardiovascular disease representing the leading cause of death (1). Vascular calcifications (VCs), a very frequent finding in CKD patients, are also highly associated with global and cardiovascular mortality (2). Moreover, patients with a faster progression of VC fare even worse than patients with slower progression (3).
Even after a successful kidney transplantation (KTx), CKD patients still have an increased cardiovascular risk compared with the general population (4, 5), with VCs being related to cardiovascular mortality (6). All published studies have assessed coronary artery calcifications (CACs), using computed tomography techniques (electron beam computed tomography or multislice computed tomography) (7–14). These methods, although reliable and precise, are not easily available in every clinical facility, expose patients to a significant burden of x-rays, and are relatively expensive.
The first aim of our study was to assess VCs at the abdominal aortic site using a semiquantitative score (aortic calcification index [ACI]) already used in CKD patients (15).
The information available on the rate of VC progression after KTx is limited (7–10, 12–14). The second aim of our investigation was to assess the progression rate of VCs in the first year after KTx.
Finally, since previous studies addressed the role of some cytokines in the VC process in KTx patients (7, 14, 16, 17), we have also assessed the levels of three proteins potentially involved in mineral metabolism (MM) (osteoprotegerin [OPG], fetuin A, and fibroblast growth factor 23 [FGF–23]), evaluated at different times during the first year after KTx, exploring their possible association with the VC process and its progression.
RESULTS
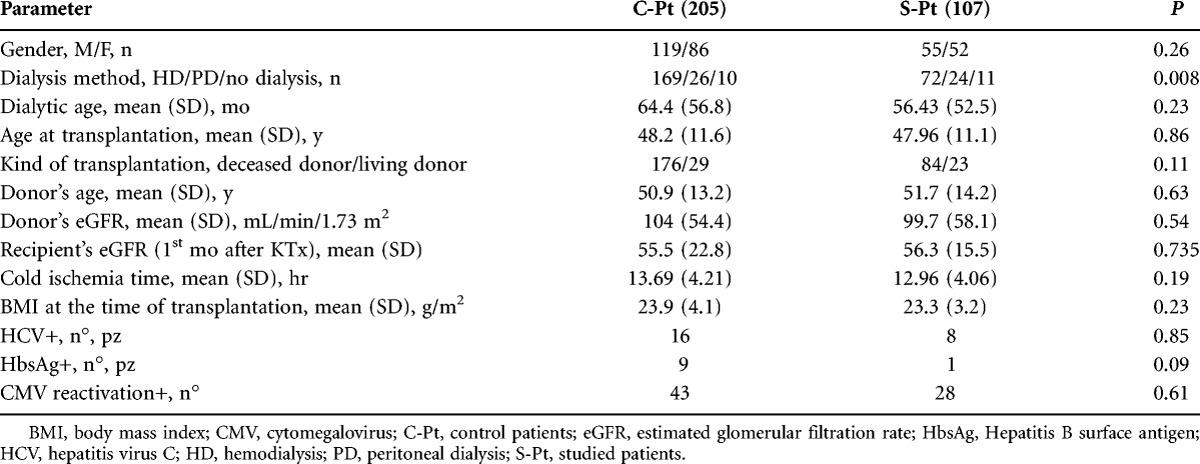
Table 1 summarizes the main characteristics of the studied patients (S-Pt), compared with other patients transplanted in the same period (C-Pt). At the time of KTx, most S-Pt were on hemodialysis, 24 were on peritoneal dialysis, and 11 had not received dialysis at all (preemptive transplantation). No statistically significant difference for any other parameter was observed between S-Pt and C-Pt, except for a higher prevalence of peritoneal dialysis and preemptive KTx in the S-Pt group. All patients received induction immunosuppressive (IS) therapy with basiliximab (98%), antithymocyte globulin (2%), and steroids (100%). Maintenance IS therapy was mainly based on steroid (95%), mycophenolate mofetil/mycophenolate acid (95%), FK506 (83%), cyclosporine A (13%), sirolimus (11%).
TABLE 1.
Comparison between control (C-Pt) and studied patients (S-Pt)
The mean ACI score at the first and the twelfth month was 5.32 and 5.75, respectively (median, 3 and 3; range, 0–22 and 0–23, respectively), which were not statistically different.
Sixty of our transplanted patients also underwent a computed tomographic evaluation of CACs 1 month after KTx. Mean (SD) CAC score in S-Pt was 287.80 (737.14); median, 40.15; and range, 0–5744. Logarithmic-transformed CAC score values were highly correlated with the ACI measured according to Kauppila method (P<0.0001). The evaluation of the concordance between ACI and CAC negative and positive values gave a mild concordance value (Cohen’s kappa, k=0.57).
Table 2 compares the clinical and biochemical parameters in KTx patients with some degree of VC (ACI-1 pos, 60%) and patients without VC (ACI-1 neg, 40%) at the first month.
TABLE 2.
Comparison between patients without (ACI-1 neg) or with (ACI-1 pos) aortic calcifications 1 month after transplantation
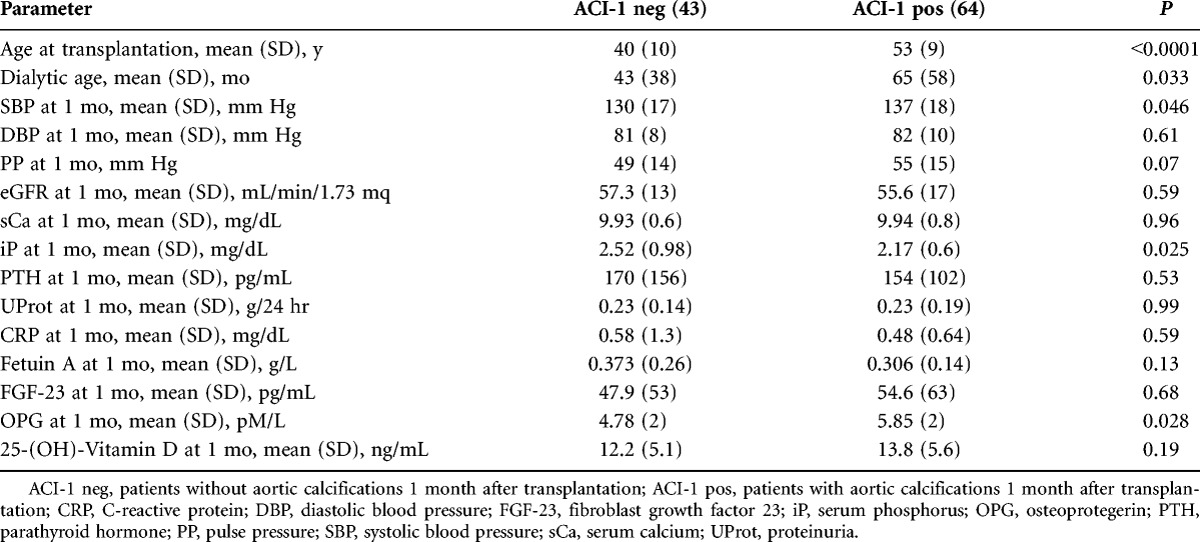
Patients ACI-1 pos had a longer dialysis vintage, were older, and received a kidney from an older donor. No difference was found regarding renal function (estimated glomerular filtration rate [eGFR], proteinuria/24 hr), MM parameters except for a lower phosphorus (iP) and higher serum OPG levels in ACI-1 pos patients.
One year after KTx, the percentage of ACI-positive patients was not substantially different from baseline (62%), and only three ACI-1 neg patients developed some calcification (ACI from 0 to 1 in two and from 0 to 2 in one), whereas one patient had an ACI regression from 1 to 0.
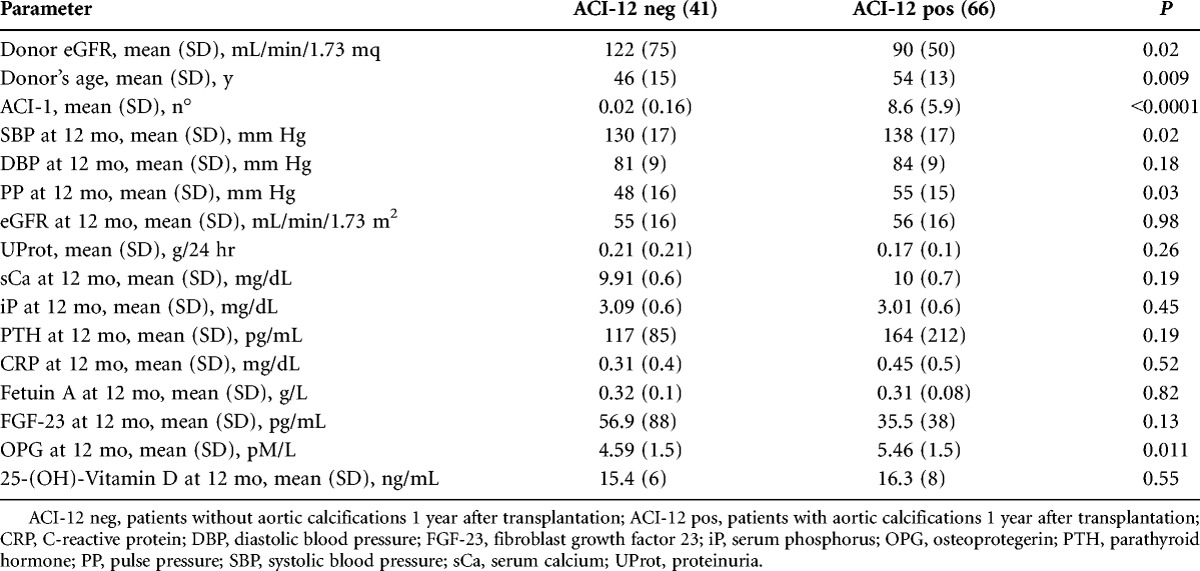
Table 3 shows the main parameters of patients with (ACI-12 pos) or without (ACI-12 neg) aortic calcification at the twelfth month. Again, ACI-12 pos patients had a longer dialysis age (P=0.03), were older (P<0.0001), and received a kidney graft from older donors with a lower eGFR. ACI-12 pos patients also had a higher systolic blood pressure (BP) and pulse pressure. No significant difference was found regarding any renal function and MM-related parameter, except for higher OPG levels in ACI-12 pos patients.
TABLE 3.
Comparison between patients without (ACI-12 neg) and patients with (ACI-12 pos) aortic calcifications 1 year after transplantation
Although there were no significant differences between the percentage of calcified patients at the two observations, 10 patients (9%) lowered the calcification score, 69 (65%) were stable, whereas 28 (26%) increased the calcification score during the first year after KTx.
According to the classification described in the methods, we compared the 79 patients with a stable or lower calcification ACI score (nonprogressing [ACI-NP]) with the remaining 28 progressing patients (ACI-P).
ACI-P patients were older (P=0.012) and had a higher ACI score at the first month (P=0.005), without any other major difference.
No statistically significant association was found between the VC progression and the class of IS drugs used or the cumulative steroid dose. There was no significant difference in the number of patients receiving Vitamin D therapy between the two groups.
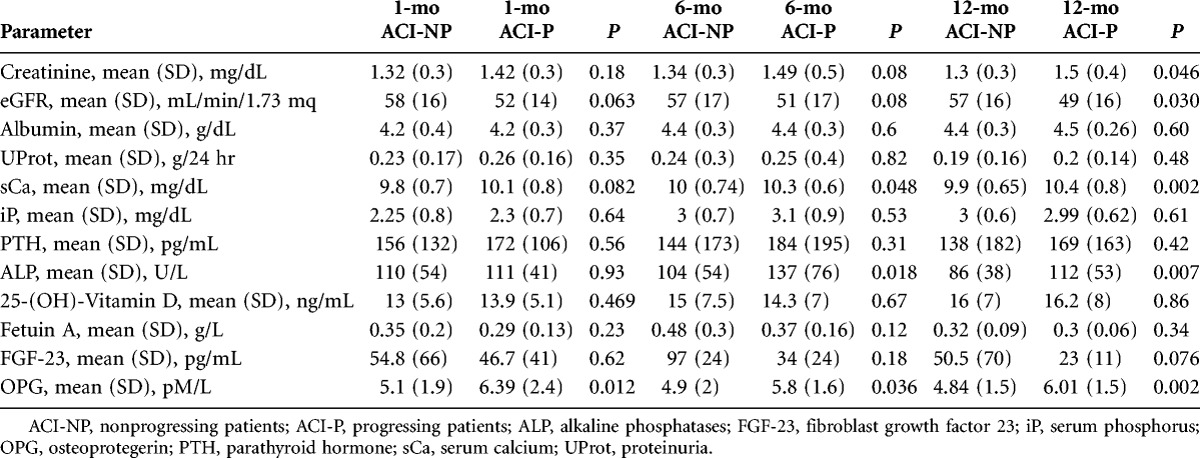
Table 4 summarizes the most important biochemical blood and urinary parameters in ACI-P and ACI-NP. ACI-P patients had a trend toward a worse renal function, at both the first and the sixth month than ACI-NP, and the difference became significant at the twelfth month. No significant difference was found when considering metabolic, inflammatory, and lipid metabolism markers, whereas serum calcium (sCa) and alkaline phosphatases (ALP) were higher in ACI-P patients. OPG was significantly higher in ACI-P as compared with the ACI-NP group at all the observation times, without any significant difference when considering FGF-23 or fetuin A levels.
TABLE 4.
Blood and urinary parameters of nonprogressing (ACI-NP) and progressing (ACI-P) patients
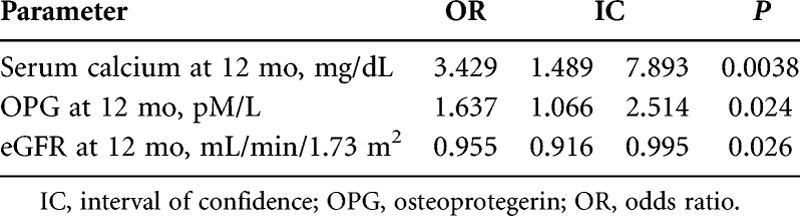
At the logistic regression analysis (Table 5), when the ACI progression (yes/no) was used as the dependent variable and all parameters significantly different at the univariate analysis as the independent variables, sCa, OPG, and eGFR at the twelfth month were the only independent factors associated with ACI progression.
TABLE 5.
Logistic regression for the event: progression of VCs
Two years after KTx, neither eGFR nor proteinuria significantly differ between the two groups. At the second year after KTx, 5 of the 107 S-Pt (4.6%) had a major cardiovascular event (2 ACI-P, 3 ACI-NP); no patient restarted dialysis or died.
DISCUSSION
The cardiovascular risk is exceedingly high in CKD patients both before and even after a well-functioning KTx, with VCs being strongly associated with this clinical outcome (6).
However, the studies dealing with VCs in KTx patients report only data regarding the CAC score, which relies on sophisticated and expensive techniques.
So, we focused on the assessment of VCs by lumbar ACI score (15), which is a simple, less expensive, easily repeatable method, characterized by a low x-ray burden. As demonstrated in other cohorts (18), we found a mild significant concordance between the two methodologies in a subgroup of KTx patients.
Aortic Calcifications at Early Assessment
At the first assessment, 60% of the S-Pt had an ACI positive score, quite similar to other studies assessing CACs at the time of KTx (7–11, 13). The presence of VCs was associated with older age, longer dialysis vintage, and higher BP. The association of VCs with age is an expected result in CKD patients, as in the general population (19, 20). It is also expected that the longer the time spent on dialysis, the higher the calcification burden in end stage renal disease patients. The links between higher VC levels and increased BP might be at least twofold because high BP can act as a stressing agent promoting degenerative and calcifying events within the artery wall on one hand, and on the other, particularly systolic BP can be increased by the loss of the elastic compliance of the arterial calcified vessels.
Among the biochemical parameters, only serum OPG and iP were significantly different between the two groups, with ACI-1 pos patients having higher OPG and lower iP values. The possible relationship between OPG and VCs will be discussed later. We might speculate that the unexpected inverse relationship between lower phosphorus levels and the higher ACI score is caused by a greater phosphate load during the hemodialysis period. This might have caused a higher VC burden and higher levels of phosphorus controlling hormones (parathyroid hormone [PTH], FGF-23), which might have been responsible for a more pronounced urine phosphate excretion in the earlier posttransplantation period. It is also worth considering that, at the first month after KTx, most of the biochemical uremic alterations, which could have played a role on the VC process during the hemodialysis period, have been almost completely corrected.
Aortic Calcifications at the Twelfth Month
No major difference in the percentage of ACI positive patients was evident 1 year after KTx as compared with the first month (62% vs. 60%). Most patients free of calcifications at the first observation did not develop any calcification after KTx. These results, in agreement with previous studies (7, 10, 12–14), suggest that, whichever the cause (acquired or genetic), the uncalcified patient at the time of KTx has a low risk of developing VCs, at least in the short term. This might suggest that a strict surveillance is not required for these patients.
ACI score positivity at the twelfth month was associated with the age and eGFR of the donors, the basal ACI score, and also, the factors related to VCs at the first month. Although the relationship with the basal ACI score is counterintuitive, the relationship between the age and the eGFR of donors might be caused by the usual practice of matching by age the recipient with the donor. As a consequence, it is more likely that an older (more calcified) recipient would receive a kidney from an older donor who is more likely to have a less well-functioning kidney.
Aortic Calcification Progression
During the first year after KTx, a progression of VCs was observed in 25% of patients, whereas 10% experienced a reduction of ACI score, and the remaining 65% remained stable. These results are in agreement with published data on CACs (7, 8, 10, 14).
At univariate analysis, older age, sCa, OPG, eGFR, and ALP were significantly related to the VC progression. At variance with previous studies, we did not find any significant association between the progression of VC and inflammatory and lipid metabolism markers.
When all these factors were contemporaneously tested at the same time in a multiple regression model, only sCa, OPG, and eGFR maintained a significant association with the VC progression.
The relationship with eGFR is not unexpected and has been already reported in the literature in KTx (10). This finding is usually believed to be caused by the effect of the metabolic derangements associated with lowering GFR on the VC process. Alternatively, the connection between an older age of both the recipient and the donor might explain a more calcified vascular tree and a worse renal function. However, at variance with this hypothesis, the significance of the relationship between age and VC progression was lost at the multivariate analysis.
We also observed a strong correlation of OPG with the calcification process and its progression. A direct, although not significant, association between OPG and CAC progression has been reported (7). Two further studies demonstrated an association between higher OPG levels and mortality in KTx, but no VC assessment was carried out in these studies (16, 17).
It remains unclear the real role of OPG in the VC process. Experimental studies suggested that OPG might play some regulatory and/or inhibitory role (21), so its increased levels could be interpreted as an attempt to compensate for the ongoing calcification process. However, OPG might increase in conditions of a diffuse calcification process, produced by the calcified vascular cells. In any case, serum OPG levels, being highly associated with both the presence of VCs and their progression, might potentially represent a reliable marker.
Importantly, a strong association between sCa and VC progression was observed. It is well known that hypercalcemia is frequent in KTx patients, associated with a persistent secondary hyperparathyroidism (22). Both VCs and hypercalcemia might be dependent on the persistence of high PTH levels. However, no relationship between PTH and the calcification process was observed in our patients. At variance, high sCa per se might represent a toxic risk factor for the progression of VC. This result might have some clinical relevance because hypercalcemia is a risk factor modifiable by medical or surgical therapies. However, we would need interventional studies, designed to specifically address this point, to definitively confirm this hypothesis.
Clinical Outcomes
The limited number of events makes our results inconclusive as far as the main clinical outcomes (renal function, return to dialysis, and death) are concerned. An enlargement of the studied cohort and a prolongation of the observation period could add some more reliable information.
Strengths and Limitations
The main limitations of this study are the observational design, the relatively low number of patients, and the short follow-up. However, although the absolute number of the S-Pt is relatively limited, it is indeed one of the largest cohorts studied where VC progression in KTx patients has been assessed.
One of the strengths of our study is the use of an easily repeatable method for the assessment of VCs. Second, we assessed the potential role of OPG, fetuin A, and FGF-23 in the VC process after KTx, confirming a prominent role for OPG and suggesting a role for sCa in the VC process and its progression.
Conclusive Remarks
This study shows that VCs are a very frequent finding in patients undergoing KTx.
Even after a well-functioning KTx, the progression of VC in the first year is more frequent than the regression, involving almost exclusively the already calcified patients.
OPG might be considered a reliable marker of the presence of VCs and their progression, and sCa, a modifiable parameter, appears related with the worsening of calcifications.
MATERIALS AND METHODS
Study Cohort Characteristics
One hundred seven KTx patients from the 312 patients transplanted in our unit between June 2004 and June 2010. In fact, we excluded from the study 187 patients for logistic and organizational reasons. The other 18 patients refused to participate in the study.
All the participants signed an informed consent, according to the best clinical and research practice recommendations of our institution, and the study was carried out in accordance with the principles of the Declaration of Helsinki.
Study Design
The medical history of each patient was recorded at the time of KTx. Thereafter, all patients were submitted to a regular out-patient surgery follow-up, for clinical and biochemical evaluation. Any major event was recorded.
At the first and the twelfth month after KTx, the following investigations were assessed:
(1) serum FGF23, OPG, fetuin A, and 25-(OH)-Vitamin D
(2) lateral lumbar x-ray
The main parameters relating to renal function (serum creatinine, proteinuria, and eGFR) and the main clinical events recorded at the twenty-fourth month were also reported in this study.
All blood samples were taken in our renal transplantation unit, with patients fasting for at least 12 hr.
Laboratory Analyses
Renal function parameters
Serum creatinine was determined by means of Jaffe reaction. eGFR was calculated by the simplified Modification of Diet in Renal Disease formula (23). Urinary proteins were measured in 24-hr urines by immunoturbidimetry.
Vitamin D
Serum 25-(OH)-Vitamin D was assessed by an enzyme-immunoassay (Kit EIA AC-57F1 immunodiagnostic system, Boldon, United Kingdom), with a sensitivity threshold of 5 nM/mL (2 ng/mL). This method has been previously validated, comparing it with gold-standard radioimmune assay methodology (24).
Cytokines
Plasma human intact FGF-23 was determined with the enzyme-linked immunosorbent assay (ELISA) method (Kit Immunotopics, Inc., Pantec San Clemente, CA), using two affinity purified goat polyclonal antibodies that detect epitopes within the amino-terminal and the carboxyl-terminal portions of FGF-23.
Fetuin-A level was determined in serum by ELISA using polyclonal antihuman fetuin-A specific antibodies (BioVendor, Brno Řečkovice, Czech Republic).
OPG was evaluated by ELISA using monoclonal antihuman OPG antibody (BioVendor).
The Vitamin D and cytokine assessment was available in 85 patients at basal evaluation and in 91 at the twelfth month.
Aorta Calcification Index
ACI was measured at the abdominal aortic level according to Kauppila et al. (15) in 1997.
Briefly, in a lateral radiograph of the lumbar spine performed in the standing position, calcifications were identified by the presence of densities in the area corresponding to the abdominal aorta, anterior to the lumbar spine. Calcifications of the anterior and posterior walls were considered separately in the aorta segments adjacent to the first four lumbar vertebrae. For each anterior and posterior segment, the calcification score was graded from 0 to 3 and then summed up for a total of 0 to 24.
According to the ACI score, patients were classified as:
(1) ACI at the first month:
a. ACI>1: ACI-1 pos
b. ACI=0: ACI-1 neg
(2) ACI at the twelfth month:
a. ACI>1: ACI-12 pos
b. ACI=0: ACI-12 neg
According to the ACI variations between the two observation times, patients were classified as:
a. ACI-12>ACI-1: progressing patients (ACI-P)
b. ACI-12≤ACI-1: nonprogressing patients (ACI-NP)
Coronary Calcifications
Sixty KTx patients (37 of whom were included in this study) had been assessed for both lumbar ACI score and CAC score by CT evaluation (DSCT Somatom Definition; Siemens Medical Solutions, Malvern, PA) 1 month after KTx. The CAC score was assessed according to the Agatston method (25).
The CAC scores were used in the statistics expressed as logarithmic-transformed values because of their nonnormal distribution.
Statistics
Standard descriptive statistics were used to describe the characteristics of all the groups analyzed. Differences in participant characteristics between groups were tested using Student t tests (confirmed by Wilcoxon–Mann-Whitney test) and Fisher exact tests.
The degree of concordance between ACI and CAC score was calculated with k of Cohen index using two dichotomous variables for negative or positive values for both parameters.
The relationship between ACI progression (yes/no) and the significantly different characteristics in the 107 characterized patients was evaluated with a multivariate logistic regression at any time. In all statistical analyses, significance was set for P values less than 0.05.
Statistical analysis was performed using software Statistica version 9 and SAS 9.2 (SAS Institute, Inc., Cary, North Carolina).
ACKNOWLEDGMENTS
The authors thank Marina Balderacchi, Alessia Centa, and Andrea Centa for the high-quality work that they performed in maintaining the renal transplantation database in our institution.
Footnotes
The authors declare no funding or conflicts of interest.
E-mail: piergiorgio.messa@policlinico.mi.it
M.M. participated in making the research design and writing the paper. A.R. and M.T.G. participated in collecting the data and in the patients’ follow-up. C.A. participated in making the research design and in collecting the data. F.B. performed the statistical analysis of the data. D.C. performed the laboratory analyses. S.V. participated in collecting the data. M.P.R. participated in making the research design and in the laboratory activity. P.M. designed the research, and participated in analyzing the data and writing the paper.
Received 21 January 2013. Revision requested 12 February 2013.
Accepted 20 March 2013.
REFERENCES
- 1. Foley RN, Murray AM, Li S, et al. Chronic kidney disease and the risk for cardiovascular disease, renal replacement, and death in the United States Medicare population, 1998 to 1999. J Am Soc Nephrol 2005; 16: 489. [DOI] [PubMed] [Google Scholar]
- 2. Blacher J, Guérin AP, Pannier B, et al. Arterial calcifications, arterial stiffness, and cardiovascular risk in end-stage renal disease. Hypertension 2001; 38: 938. [DOI] [PubMed] [Google Scholar]
- 3. Budoff MJ, Hokanson JE, Nasir K, et al. Progression of coronary artery calcium predicts all-cause mortality. JACC Cardiovasc Imaging 2010; 3: 1229. [DOI] [PubMed] [Google Scholar]
- 4. Kasiske BL, Maclean JR, Snyder JJ. Acute myocardial infarction and kidney transplantation. J Am Soc Nephrol 2006; 17: 900. [DOI] [PubMed] [Google Scholar]
- 5. Ojo AO. Cardiovascular complications after renal transplantation and their prevention. Transplantation 2006; 82: 603. [DOI] [PubMed] [Google Scholar]
- 6. Nguyen PT, Henrard S, Coche E, et al. Coronary artery calcification: a strong predictor of cardiovascular events in renal transplant recipients. Nephrol Dial Transplant 2010; 25: 3773. [DOI] [PubMed] [Google Scholar]
- 7. Bargnoux AS, Dupuy AM, Garrigue V, et al. Evolution of coronary artery calcifications following kidney transplantation: relationship with osteoprotegerin levels. Am J Transplant 2009; 9: 2571. [DOI] [PubMed] [Google Scholar]
- 8. Mazzaferro S, Pasquali M, Taggi F, et al. Progression of coronary artery calcification in renal transplantation and the role of secondary hyperparathyroidism and inflammation. Clin J Am Soc Nephrol 2009; 4: 685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bubenicek P, Kautznerova D, Sotornik I, et al. Coronary calcium score in renal transplant recipients. Nephron Clin Pract 2009; 112: c1. [DOI] [PubMed] [Google Scholar]
- 10. Schankel K, Robinson J, Bloom RD, et al. Determinants of coronary artery calcification progression in renal transplant recipients. Am J Transplant 2007; 7: 2158. [DOI] [PubMed] [Google Scholar]
- 11. Rosas SE, Mensah K, Weinstein RB, et al. Coronary artery calcification in renal transplant recipients. Am J Transplant 2005; 5: 1942. [DOI] [PubMed] [Google Scholar]
- 12. Moe SM, O’Neill KD, Reslerova M, et al. Natural history of vascular calcification in dialysis and transplant patients. Nephrol Dial Transplant 2004; 19: 2387. [DOI] [PubMed] [Google Scholar]
- 13. Oschatz E, Benesch T, Kodras K, et al. Changes of coronary calcification after kidney transplantation. Am J Kidney Dis 2006; 48: 307. [DOI] [PubMed] [Google Scholar]
- 14. Maréchal C, Coche E, Goffin E, et al. Progression of coronary artery calcification and thoracic aorta calcification in kidney transplant recipients. Am J Kidney Dis 2012; 59: 258. [DOI] [PubMed] [Google Scholar]
- 15. Kauppila LI, Polak JF, Cupples LA, et al. New indices to classify location, severity and progression of calcific lesions in the abdominal aorta: a 25-year follow-up study. Atherosclerosis 1997; 132: 245. [DOI] [PubMed] [Google Scholar]
- 16. Hjelmesaeth J, Ueland T, Flyvbjerg A, et al. Early posttransplant serum osteoprotegerin levels predict long-term (8-year) patient survival and cardiovascular death in renal transplant patients. J Am Soc Nephrol 2006; 17: 1746. [DOI] [PubMed] [Google Scholar]
- 17. Svensson M, Dahle DO, Mjøen G, et al. Osteoprotegerin as a predictor of renal and cardiovascular outcomes in renal transplant recipients: follow-up data from the ALERT study. Nephrol Dial Transplant 2012; 27: 2571. [DOI] [PubMed] [Google Scholar]
- 18. Bellasi A, Ferramosca E, Muntner P, et al. Correlation of simple imaging tests and coronary artery calcium measured by computed tomography in hemodialysis patients. Kidney Int 2006; 70: 1623. [DOI] [PubMed] [Google Scholar]
- 19. McCullough PA, Sandberg KR, Dumler F, et al. Determinants of coronary vascular calcification in patients with chronic kidney disease and end-stage renal disease: a systematic review. J Nephrol 2004; 17: 205. [PubMed] [Google Scholar]
- 20. Amann K. Media calcification and intima calcification are distinct entities in chronic kidney disease. Clin J Am Soc Nephrol 2008; 3: 1599. [DOI] [PubMed] [Google Scholar]
- 21. Van Campenhout A, Golledge J. Osteoprotegerin, vascular calcification and atherosclerosis. Atherosclerosis 2009; 204: 321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Messa P, Cafforio C, Alfieri C. Calcium and phosphate changes after renal transplantation. J Nephrol 2010; 23 (Suppl 16): S175. [PubMed] [Google Scholar]
- 23. Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 1999; 130: 461. [DOI] [PubMed] [Google Scholar]
- 24.Immunodiagnostic Systems. http://www.idsplc.com/int/products/25-hydroxy-vitamin-d-eia-ac-57f1#description Accessed November 6, 2012.
- 25. Agatston AS, Janowitz WR, Hildner FJ, et al. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol 1990; 15: 827. [DOI] [PubMed] [Google Scholar]


