Abstract
Objectives
The objective of this study was to test the hypothesis that gastric bypass surgery (GBS) would favorably impact cardiac remodeling and function.
Background
GBS is increasingly used to treat severe obesity, but there are limited outcome data.
Methods
We prospectively studied 423 severely obese patients undergoing GBS and a reference group of severely obese subjects that did not have surgery (n = 733).
Results
At a 2-year follow up, GBS subjects had a large reduction in body mass index compared with the reference group (−15.4 ± 7.2 kg/m2 vs. −0.03 ± 4.0 kg/m2; p < 0.0001), as well as significant reductions in waist circumference, systolic blood pressure, heart rate, triglycerides, low-density lipoprotein cholesterol, and insulin resistance. High-density lipoprotein cholesterol increased. The GBS group had reductions in left ventricular (LV) mass index and right ventricular (RV) cavity area. Left atrial volume did not change in GBS but increased in reference subjects. In conjunction with reduced chamber sizes, GBS subjects also had increased LV midwall fractional shortening and RV fractional area change. In multivariable analysis, age, change in body mass index, severity of nocturnal hypoxemia, E/E', and sex were independently associated with LV mass index, whereas surgical status, change in waist circumference, and change in insulin resistance were not.
Conclusions
Marked weight loss in patients undergoing GBS was associated with reverse cardiac remodeling and improved LV and RV function. These data support the use of bariatric surgery to prevent cardiovascular complications in severe obesity.
Keywords: bariatric surgery, cardiac remodeling, diabetes, echocardiography, hypertension, left atrial volume, left ventricular hypertrophy, myocardial contraction, obesity
Obesity is associated with increased risks of developing heart failure (1) and atrial fibrillation (2) and with an increased overall risk of death, mainly from cardiovascular causes (3–7). Obese subjects commonly have evidence of cardiac remodeling, including left ventricular (LV) hyper- trophy (8–16) and left atrial (LA) enlargement (16,17). Increased LV mass and LA volume each predict increased risk of heart failure, atrial fibrillation, and death in population studies (2,18–22). Thus, adverse cardiac remodeling might contribute to the high cardiovascular morbidity and mortality in obesity.
Bariatric surgery is increasingly being used to treat severe obesity (23). Despite several calls for outcome data from this procedure (24), most published studies assessing cardiovascular changes after gastric bypass surgery (GBS) are relatively small and have 1 year or less of follow-up, and only a few have control or reference groups. We hypothesized that marked weight loss achieved via GBS would be associated with reversal of the unfavorable cardiac remodeling and subclinical contractile dysfunction that is seen in severe obesity. We prospectively tested this hypothesis in a large cohort of patients who met criteria for GBS (25). Patients undergoing GBS or nonsurgical therapy (reference) were studied at baseline (visit 1) and again approximately 2 years after enrollment (visit 2).
Methods
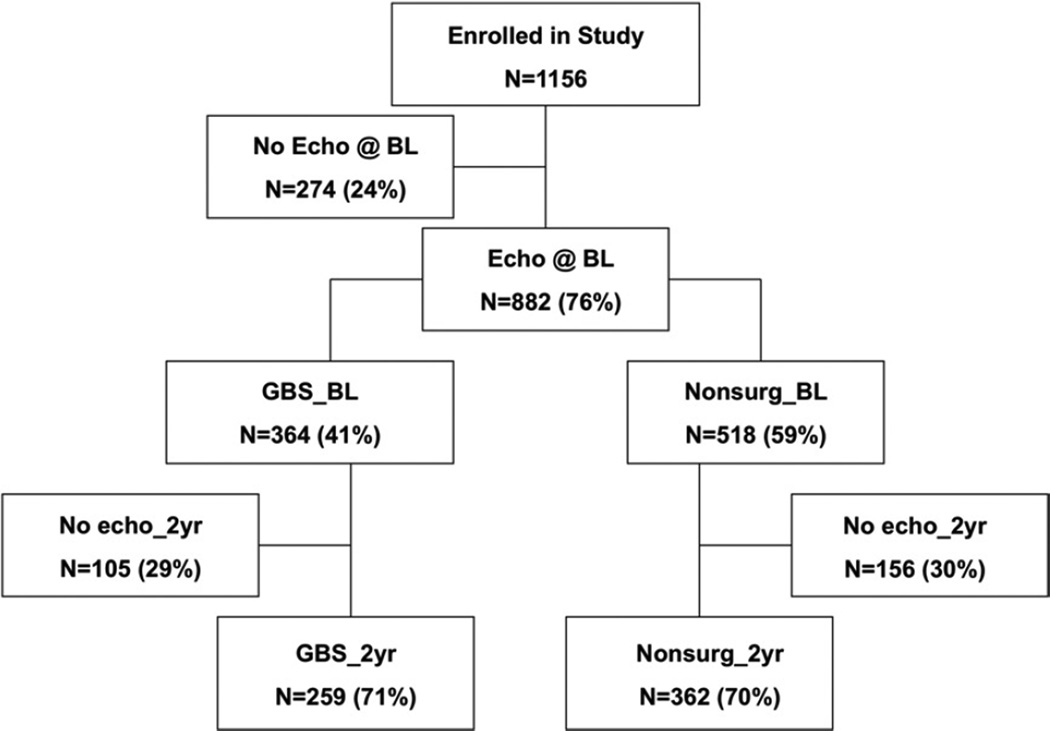
The University of Utah Institutional Review Board approved this study. All subjects gave informed consent. Severely obese subjects who met criteria for bariatric surgery were recruited into a prospective study examining the cardiovascular effects of weight loss achieved by Roux en Y GBS. The study design has been described previously (25). Three groups of subjects: 1) GBS (n = 423); 2) subjects who sought GBS, but the procedure was not covered by their insurance policy (n = 412); and 3) obese subjects not seeking GBS who were randomly selected from a population database (n = 321). The third group was included to help overcome potential bias related to socioeconomic class. By study design, echocardiograms were not performed on a subset of patients (n = 274) from the overall cohort (25). These subjects had demographics and clinical characteristics similar to those who had echocardiograms done except for mildly higher blood pressures (Online Appendix Table 1). The study group in this report consisted of 797 consecutive subjects with echocardiogram windows that were suitable for quantification of LV mass or LA volume. The numbers of patients included at each visit are shown in Figure 1.
Figure 1. Flow Chart Showing the Numbers of Subjects With Echocardiographic Examinations at Each Stage of the Study.
Echocardiography
Left ventricular dimensions and volumes were determined from 2-dimensional or M-mode images according to established criteria (26,27). These measurements were used to calculate LV mass using the modified cube formula (28) and the area-length method (26). Global LV systolic function was assessed using LV endocardial fractional shortening and ejection fraction, and myocardial function was assessed with midwall fractional shortening (MWFS) (26,29). Left ventricular hypertrophy was defined using LV mass/height2.7 with sex-specific cut-offs (30). The LA volume was measured in the apical 4-chamber view at end-systole using the method of discs. The LA volume was not indexed to body surface area because this causes underestimation of the relative chamber size in severely obese subjects. Right ventricular (RV) major and minor axis dimensions and systolic and diastolic cavity areas were measured in the apical 4–chamber view. Pulsed wave Doppler measurements were recorded at the medial portion of the mitral annulus to assess the early diastolic myocardial velocity (E'). The ratio of the early diastolic mitral flow velocity (E) to E' was used as an index of LV filling pressure (31). Interobserver and intraobserver variability for the main measurements have been reported previously (16).
Exercise testing
Submaximal exercise testing was performed using a modified Bruce protocol. Exercise was terminated when subjects reached 80% of predicted maximum heart rate because of concerns about the safety of maximal exercise testing in patients with very large body habitus.
Respiratory measures during sleep
Limited overnight polysomnography consisted of nasal airflow (using a pressure transducer), thoracic and abdominal effort, snoring, activity, body position, O2 saturation, and heart rate. Records were scored blindly.
Statistical analysis
Continuous variables are reported as means ± SD. For normally distributed variables, within-group changes from baseline to 2 years were compared using a paired t test, and differences between the groups at each time point (baseline or 2 years) were compared using an unpaired t test. The nonparametric Kruskal-Wallis test was used for between-group comparison, and the Wilcoxon signed-rank sum test was used to examine within-group change for variables that were not normally distributed. To account for incomplete follow-up and missing data, imputation and carry-forward methods were used. Categoric variables are reported as proportions, and differences were compared using the chi-square test. Univariate linear regression was performed using the Pearson correlation. Collinearity between selected variables (body mass index [BMI] and surgical status) was assessed using tolerance and variance inflation factor. Multiple linear regression analysis was used to determine the factors that were associated with each of the 4 main outcome variables (LV mass index, LA volume, LV MWFS, and RV fractional area change) at the 2-year follow-up. For all 3 analyses, the variables used in the initial and final models were chosen based on clinical relevance or a plausible role in a causal pathway that might affect these outcome variables. Two sets of models were constructed. The first (model 1) used changes in clinical parameters between visits 1 and 2 as the input variables, with the absolute value of LV mass index, LA volume, and LV MWFS at visit 2 as the outcome variable. This approach was employed in an effort to minimize the effects of baseline differences between the groups while maintaining the clinical meaning of absolute measures of LV mass, LA volume, and MWFS. The second (model 2) used changes between visit 1 and visit 2 for both input and outcome variables (Online Appendix Tables 2 to 4). The initial variables were the same for both models. In each case, after the initial model was run, backward stepwise regression analysis was used to successively remove variables from the model that were not statistically significant. For analyses in which the mean values of 3 groups were compared, p ≤ 0.01 was considered significant. When only 2 groups were compared, p < 0.05 was considered to be significant.
Results
Clinical characteristics
The clinical characteristics of the study subjects are shown in Table 1. Systolic and diastolic blood pressures, fasting glucose, and glycosylated hemoglobin levels were all at the upper end of the normal range at visit 1. Serum insulin levels were markedly elevated. Patients in the GBS group lost an average of 44 kg (98 lbs) and decreased their BMI by 15.4 kg/m2 (Table 1). In conjunction with this marked degree of weight loss, those in the GBS group had improvements in systolic blood pressure, heart rate, glucose level, insulin level, and homeostasis model assessment of insulin resistance (HOMA IR) at visit 2 compared with baseline and compared with the reference groups (Table 1). At the 2-year follow-up, there were 2 deaths in the GBS group and 2 deaths in the reference group.
Table 1.
Clinical Characteristics of the GBS and Denied-Surgery Reference Subjects n Participating in the Echocardiographic Analysis at Baseline and 2-Year Follow-Up*
| Baseline |
2 Yrs |
|||||
|---|---|---|---|---|---|---|
| Denied Surgery Reference (n = 240) |
Not Seeking Surgery Reference (n = 271) |
GBS (n = 354) |
Denied Surgery Reference (n = 222) |
Not Seeking Surgery Reference (n = 254) |
GBS (n = 338) |
|
| Age (yrs) | 43 ± 11 | 49 ± 11† | 42 ± 11 | 45 ± 11 | 49 ± 11‡ | 45 ± 11 |
| BMI (kg/m2) | 46.1 ± 6.2† | 43.7 ± 6.2† | 47.9 ± 7.0 | 45.4 ± 8.7‡ | 44 ± 7.4‡ | 32.2 ± 7.8§ |
| Weight (kg) | 130 ± 24† | 124.7 ± 24.1† | 136 ± 28 | 126 ± 27‡ | 123.3 ± 26.3‡ | 91 ± 25§ |
| Waist (cm) | 134 ± 16 | 130.4 ± 15† | 137 ± 18 | 132 ± 20‡ | 131.1 ± 18.8‡ | 102 ± 22§ |
| Height (cm) | 168 ± 9 | 168.6 ± 9.5 | 168 ± 9 | 168 ± 9 | 168.3 ± 9.6 | 167 ± 9 |
| Heart rate (beats/min) | 74 ± 12§ | 74.2 ± 10.7 | 74 ± 12 | 68 ± 10‡ | 67 ± 10‡§ | 60 ± 10§ |
| SBP (mm Hg) | 123 ± 17 | 127.5 ± 18 | 123 ± 18 | 123 ± 17‡ | 126.6 ± 19.4‡ | 115 ± 18§ |
| DBP (mm Hg) | 71 ± 10 | 71.8 ± 10.3† | 69 ± 10 | 71 ± 11 | 70.3 ± 9.8 | 69 ± 10 |
| HbA1C (%) | 6.1 ± 1.1 | 5.9 ± 1.1 | 5.9 ± 1.1 | 5.9 ± 0.8‡ | 6.2 ± 0.94‡ | 5.6 ± 0.8 |
| Glucose (mg/dl) | 109 ± 38 | 107 ± 33 | 103 ± 34 | 102 ± 33‡ | 106 ± 35‡ | 83 ± 23§ |
| Plasma insulin (IU) | 17.6 ± 13.2† | 14.0 ± 13.3† | 21.1 ± 8.1 | 19.1 ± 12‡ | 18.2 ± 11.6‡§ | 6.5 ± 7§ |
| HOMA-IR║ | 4.8 ± 4.0 | 3.7 ± 3.8† | 5.4 ± 5.0 | 5.1 ± 4.3‡ | 4.97 ± 4.05‡§ | 1.4 ± 1.5§ |
Data are presented as means (SD).
p < 0.0 vs. GBS at baseline.
p < 0.01 vs. GBS at 2 years.
p < 0.01 for within-group change from baseline to 2 years.
Data for HOMA-IR are reported as median ± interquartile range; comparisons done with the nonparametric Kruskal-Wallis test for between-group comparisons and the Wilcoxon signed-rank sum test were used to examine within-group change.
BMI = body mass index; DBP = diastolic blood pressure; GBS = gastric bypass surgery; HbA1C = glycosylated hemoglobin; HOMA-IR = homeostasis model assessment of insulin resistance; SBP = systolic blood pressure.
Changes in LV geometry after GBS
The echocardiographic findings at baseline have been reported previously(16). In brief, LV cavity dimensions and volumes were normal, wall thickness was mildly increased, and the majority of subjects had concentric remodeling or concentric LV hypertrophy. At the 2-year follow-up, the subjects in the GBS group had decreases in interventricular septum thickness, posterior wall thickness, relative wall thickness, and LV mass/height2.7 (Table 2). The proportion of patients categorized as having LV hypertrophy decreased to a greater extent in the GBS group than the nonsurgical reference group. The LV diastolic and systolic volumes were slightly higher in the GBS group at baseline but were not different than those in the reference group at the 2-year follow-up. The reduction in mass was related to reductions in cavity volume and decreases in LV wall thickness. Relative wall thickness decreased in the GBS but not the reference subjects. When missing values were filled in by imputation or carry-forward methods, these main conclusions were not changed (Table 2).
Table 2.
Cardiac Geometry and Function at Baseline and 2-Year Follow-Up
| Baseline | 2 Yrs | |||||
|---|---|---|---|---|---|---|
| Variable | Denied Surgery Reference (n = 240) |
Not Seeking Surgery Reference (n = 271) |
GBS (n = 354) |
Denied Surgery Reference (n = 222) |
Not Seeking Surgery Reference (n = 254) |
GBS (n = 338) |
| IVSd (cm) | 1.14 ± 0.24 | 1.18 ± 0.21* | 1.08 ± 0.24 | 1.13 ± 0.24† | 1.16 ± 0.24† | 1.03 ± 0.21‡ |
| PWd (cm) | 1.13 ± 0.22 | 1.17 ± 0.21* | 1.08 ± 0.23 | 1.11 ± 0.23† | 1.14 ± 0.23† | 0.99 ± 0.18‡ |
| LVIDd (cm) | 4.47 ± 0.65 | 4.43 ± 0.62 | 4.58 ± 0.64 | 4.43 ± 0.59 | 4.44 ± 0.6 | 4.44 ± 0.58‡ |
| RWT | 0.53 ± 0.16 | 0.54 ± 0.16* | 0.49 ± 0.15 | 0.52 ± 0.16† | 0.53 ± 0.15† | 0.46 ± 0.12‡ |
| LVMI (g/m2.7) | 44.0 ± 13.0 | 46.5 ± 12.0 | 44.0 ± 12.0 | 44.0 ± 12.0† | 45.1 ± 11.5.0† | 38.0 ± 10.0‡ |
| LVMI (g/m2.7, imputed) | 44.2 ± 10.1 | 46.2 ± 9* | 44.5 ± 11.0 | 41.4 ± 9.1† | 43.7 ± 10† | 38.2 ± 8‡ |
| LVMI (g/m2.7, carry forward) | 43.9 ± 12.6 | 46.5 ± 11.8 | 44.0 ± 12.3 | 42.5 ± 11.4† | 44.9 ± 11.8† | 38.4 ± 10.0‡ |
| LVMI A–L (g/m2.7) | 39.4 ± 11 | 40.1 ± 11.5 | 42.0 ± 11.0‡ | 39.0 ± 7.0* | 39.9 ± 7.5† | 35.0 ± 7.0 |
| Proportion with LV hypertrophy | 67% | 84%* | 64% | 64% | 72%† | 56%‡ |
| Endocardial FS (%) | 35.4 ± 6.6 | 36 ± 6.5 | 34.9 ± 8.1 | 35.5 ± 6.8 | 35.4 ± 6.5 | 35.5 ± 6.3 |
| LV EF (%) | 64 ± 90) | 65.7 ± 8.6 | 63 ± 11 | 65 ± 8 | 64.4 ± 8.3 | 65 ± 8 |
| Midwall FS (%) | 16 ± 3.6 | 15.3 ± 2.9 | 16.0 ± 3.6 | 15.8 ± 3.2† | 15.4 ± 3.1† | 16.7 ± 3.0‡ |
| Left atrial volume (ml) | 53.9 ± 19.1 | 54.1 ± 16 | 55.3 ± 17.0 | 56.7 ± 14.5 | 58.5 ± 16.6‡ | 54.4 ± 15.2 |
| LV diastolic volume (ml) | 92 ± 32 | 91.9 ± 29.3* | 99 ± 32 | 91 ± 28 | 92.1 ± 28.6 | 92 ± 27 |
| LV systolic volume (ml) | 34.6 ± 15 | 31.8 ± 14* | 36.9 ± 18 | 32 ± 12 | 32.5 ± 13.2 | 33 ± 13 |
| Cardiac output (l/min) | 5.7 ± 1.5 | 5.8 ± 1.3 | 5.8 ± 1.4 | 5.3 ± 1.3† | 5.3 ± 1.29† | 4.4 ± 1.1‡ |
| RV minor axis (cm) | 3.1 ± 0.7 | 3.2 ± 0.65 | 3.0 ± 0.6 | 3.3 ± 0.6† | 3.38 ± 0.64† | 3.1 ± 0.6 |
| RV length (cm) | 7.5 ± 0.8 | 7.53 ± 0.82 | 7.6 ± 0.9 | 7.8 ± 0.7†‡ | 7.9 ± 0.81†‡ | 7.7 ± 0.8 |
| RV diastolic area (cm2) | 21.0 ± 5.9 | 21.8 ± 5.3 | 20.7 ± 5.4 | 23.6 ± 5.0†‡ | 23.9 ± 5.7† | 21.1 ± 4.9 |
| RV systolic area (cm2) | 13.1 ± 4 | 13.6 ± 3.7 | 13.0 ± 3.4 | 14.6 ± 3.5†‡ | 14.9 ± 4.1† | 12.6 ± 3.2 |
| RV FAC (%) | 37.0 ± 13 | 38.6 ± 8.5 | 37.0 ± 9.2 | 37.6 ± 9.5 | 37.6 ± 8.4† | 40.3 ± 8.2‡ |
| % SpO2 <90% | 31 ± 6 | 30 ± 7 | 0.31 ± 6 | 31 ± 6† | 30 ± 6† | 25 ± 5 |
p < 0.05 versus GBS at baseline.
p < 0.05 versus GBS at 2 years.
p < 0.01 for within-group change from baseline at 2 years.
EF = ejection fraction; FAC = fractional area change; FS = fractional shortening; IVSd = interventricular septum thickness in diastole; LV = left ventricular; LVIDd = LV internal dimension in diastole; LVMI = LV mass index (g/m27); PWd = posterior wall thickness in diastole; RV = right ventricular; RWT = relative wall thickness; SpO2 = percent of sleep with O2 saturation <90%. Other abbreviations as in Table 1.
Changes in LA size after GBS
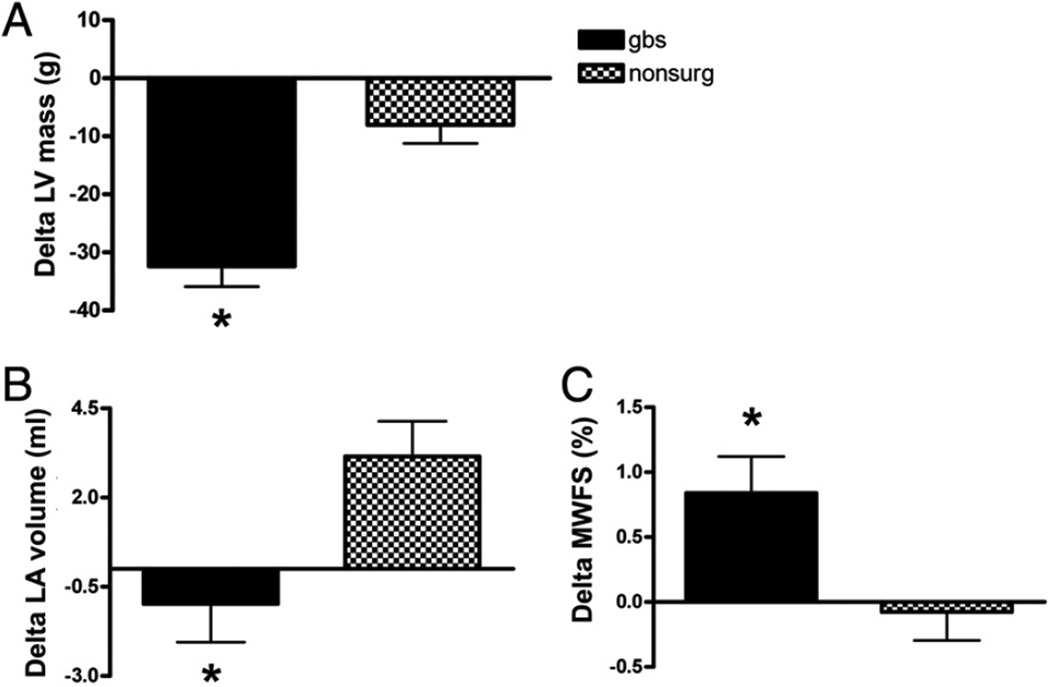
The net change in LA volume at 2 years was a nonsignificant decrease in the surgical group and an increase in the nonsurgical reference group (Table 2, Fig. 2). Although the absolute changes were modest, there was a relative increase of 4.1 ml in the nonsurgical group compared with the GBS group at 2 years (p < 0.01). These changes are similar to those in a smaller study examining LA volume after GBS (32).
Figure 2. Changes iln Cardiac Geometry and Function Between Visit 1 (Baseline) and Visit 2 (~2-Year Follow-Up) in the GBS and Nonsurgical Control Groups.
(A) Change in absolute left ventricular (LV) mass. (B) Change in left atrial (LA) volume.
(C) Change in midwall fractional shortening (MWFS); other abbreviations as in Figure 1.
Changes in RV size after GBS
At baseline, there were no differences in RV size between GBS and nonsurgical subjects, except for RV minor axis dimension, which was slightly smaller in GBS subjects (Table 2). At the 2-year follow-up, all measurements of RV size, with the exception of RV annulus dimension, were smaller in GBS compared with nonsurgical subjects (Table 2). All measures of RV size except RV annulus dimension correlated positively with BMI at visit 2. The strongest correlation (r = 0.40; p < 0.0001) was for RV systolic area. RV fractional area change correlated inversely with BMI (r = −0.14; p = 0.006).
Changes in LV and RV function after GBS
Left ventricular endocardial fractional shortening and LV ejection fraction were not different between the GBS group and the nonsurgical group at baseline, and these measures did not change between the 2 visits in either group (Table 2). However, myocardial function as estimated by MWFS decreased slightly in the reference subjects (not significant) and increased in the GBS subjects at 2 years (Table 2, Fig. 2). The difference between the groups was significant at 2 years. Midwall fractional shortening had a modest positive correlation with exercise capacity (r = 0.12; p = 0.013). Calculated cardiac output decreased significantly at 2 years in the GBS group but did not change in the control group (Table 2). Right ventricular systolic function, as assessed by fractional area change, was higher in the GBS group than the control group at the-2 year follow-up.
Changes in LV filling pressures after GBS
The ratio of E to E' is a noninvasive index of LV filling pressures (31). At the beginning of the study (2001), tissue Doppler was not in widespread use. At 2 years after enrollment, E/E' was slightly lower in the GBS than the control subjects (7.7 ± 2.5 vs. 8.3 ± 3.0). This suggested a modest improvement of LV relaxation rate, as shown in other studies (33,34), and/or a lowering of resting LV filling pressures after GBS-induced weight loss. Even this small change may be clinically meaningful because E/E' had a significant negative correlation with exercise duration at the 2-year visit (r = −0.16; p = 0.002).
Factors associated with changes in cardiac geometry and function
When changes from baseline were considered, the factors that were independently associated with LV mass index at visit 2 were change in BMI, age, and change in diastolic blood pressure (Table 3). Surgical status, change in waist circumference, and change in HOMA IR were not associated with LV mass index. Surgical status and change in BMI were not collinear based on formal testing. Moreover, the main findings of the multivariable analysis were unchanged if surgical status was left out of the initial model. When change in LV mass was used as the outcome variable (model 2, Online Appendix Table 2), change in BMI and change in LA volume were independently associated. Left atrial volume at 2 years was associated with sex, age, and change in diastolic blood pressure (Table 4). In model 2 (Online Appendix Table 3), change in cardiac output and change in percent of sleep time with O2 saturation <90% were independently associated with change in LA volume. The parameters that were independently associated with MWFS at 2 years were age and change in LV mass index (Table 5). In model 2 (Online Appendix Table 4), only change in BMI was independently associated with change in MWFS. Clinical factors associated with RV fractional area change at 2 years were age and severity of nocturnal hypoxemia (Table 6).
Table 3.
Factors Independently Associated With LVMI at Visit 2 in Multivariable Analysis
| Variable | Standardized Coefficient (β) |
t Statistic | p Value |
|---|---|---|---|
| Change in BMI | 0.29 | 7.54 | <0.0001 |
| Age | 0.24 | 6.23 | <0.0001 |
| Change in DBP | 0.13 | 2.89 | 0.0040 |
LVMI = left ventricular mass/height27; other abbreviations as in Table 1.
Table 4.
Factors Independently Associated With Left Atrial Volume at Visit 2 in Multivariable Analysis
| Variable | Standardized Coefficient (β) |
t Statistic | p Value |
|---|---|---|---|
| Sex | −0.27 | −6.48 | <0.0001 |
| Age | 0.17 | 4.14 | <0.0001 |
| Change in DBP | − 0.08 | −2.04 | 0.0423 |
Abbreviations as in Table 1.
Table 5.
Factors Independently Associated With LV Midwall FS at Visit 2 in Multivariable Analysis
| Variable | Standardized Coefficient (β) |
t Statistic | p Value |
|---|---|---|---|
| Age | −0.237 | −3.18 | 0.0018 |
| Change in LVMI | −0.176 | −2.35 | 0.0197 |
| Change in E/E' | 0.109 | 1.47 | 0.1439 |
E = early diastolic mitral flow velocity; E′ = early diastolic myocardial velocity; other abbreviations as in Table 2.
Table 6.
Factors Independently Associated With RV Fractional Area Change at Visit 2 in Multivariable Analysis
| Variable | Standardized Coefficient (β) |
t Statistic | p Value |
|---|---|---|---|
| % of sleep with SpO2 <90% | −0.184 | −2.83 | 0.0050 |
| Age | −0.121 | −2.18 | 0.0298 |
Abbreviations as in Table 2
Discussion
In this large prospective study, we found that marked weight loss occurring via GBS was associated with stabili- zation or partial reversal of the major geometric and functional cardiac changes associated with severe obesity. Continued obesity tended to be associated with modest progression of remodeling. The improvements in cardiac geometry were present 2 years after surgery—a time point that is typically near the nadir of weight loss. This study expands on the findings of smaller studies, many of which had shorter durations of follow-up (35,36). Given the powerful prognostic significance of increased LV mass and LA volume, our data suggest that favorable cardiac remodeling could represent one possible mechanism by which GBS improves survival in severe obesity (37).
Reduction in LV mass after GBS
The reduction in LV mass after GBS in our study (− 13.6%) is comparable to that reported in 13 patients who had cardiac magnetic resonance imaging before and 1 year after GBS (− 14%) (38). The decrease in LV mass after GBS appears to be larger than the decrement in LV mass (approximately −8%) seen in patients who lost weight by diet (−9.9 kg) (33). In this latter study, as in most studies of diet-induced weight loss, weight regain commenced at approximately 6 months after initiation of the diet. Weight regain was associated with an increase in LV mass. The durability of the favorable changes in cardiac remodeling after GBS may be advantageous when compared with changes occurring with lifestyle modification alone.
Not surprisingly, multiple factors that could affect LV mass change concomitantly in patients who lose large amounts of weight. Interestingly, change in BMI was the anthropometric variable most significantly correlated with LV mass (Table 3, Online Appendix Table 2). Whereas other studies have emphasized visceral adiposity as a risk factor for LV remodeling (39,40), our data did not show an independent relationship between LV mass index and waist circumference when BMI was included in the multivariable model. These findings suggest that generalized obesity may be a more important driver of cardiac hypertrophy than central obesity. All subjects in this severely obese group had increased waist circumference, potentially explaining a lack of relationship between waist circumference and LV mass. In contrast to cardiac hypertrophy, visceral adiposity, rather than overall body size, seems to more strongly influence the development of metabolic syndrome and atherosclerosis (41). One mechanism that may link larger body size to increased LV mass is the need for higher cardiac output. Although we found that cardiac output decreased after GBS (Table 2), cardiac output did not have an independent relationship with LV mass in multivariable analysis. This suggests the effect of cardiac output is mediated, at least in part, through interaction with other factors. Change in cardiac output was an independent predictor of change in LA volume (Online Appendix Table 3). It seems intuitive that the reduction in LV mass in the GBS group could be related to the lowering of blood pressure. However, change in blood pressure was significantly associated with absolute LV mass (model 1) but not change in LV mass (model 2). Moreover, even when controlling for blood pressure, change in BMI retained an independent association with LV mass index in both models. Surgical status (yes/no) was not independently associated with improved LV mass. Thus, our data suggest that the improvements in cardiac geometry following GBS are more likely due to weight loss and not some other aspect of bypassing the distal stomach and duodenum (e.g., changes in hormones secreted from the gastrointestinal tract). The direct effects of decreasing body size, in conjunction with several other beneficial hemodynamic changes occurring in the setting of weight loss, can favorably impact LV remodeling. Similar factors are likely associated with favorable changes in RV size and function.
Left atrial volume decreased slightly but not significantly in patients undergoing GBS as compared with the nonsurgical patients in whom LA volume increased over time. Left atrial volume at visit 2 was related to age, sex, and changes in blood pressure, but not waist circumference or surgical status. In addition, change in LA volume was related to change in cardiac output and severity of nocturnal hypoxemia. Clearly there are complex interrelationships between cardiac geometry and the many physiologic and hemodynamic factors that change after GBS.
Systolic function after GBS
Even though LV endocardial fractional shortening and LV ejection fraction were normal at rest in our subjects, MWFS, a better index of myocardial function in hypertrophied hearts (16), was reduced. Other investigators have found similar mild changes in myocardial function in obese subjects using tissue Doppler or strain imaging (8,9). Although it is unknown if these subtle abnormalities of systolic dysfunction progress over time or lead to clinical heart failure in the absence of coronary artery disease, it is still encouraging to see evidence of improvement after weight loss. Although the magnitude of the changes we observed in systolic function were small, MWFS showed a significant positive correlation with exercise capacity, suggesting that this change could have physiologic relevance.
Age and effects of GBS
More advanced age was significantly associated with worse cardiac remodeling and worse LV and RV function in all of the multivariable analyses. This finding suggests that older age, and presumably longer durations of obesity, could limit some of the beneficial cardiac effects of GBS. Surgical treatment might be more beneficial when offered to younger patients. More investigation will be needed to specifically address this important question.
Study limitations
The GBS and reference groups had baseline differences in several clinical characteristics. Those in the GBS group were younger but had higher BMI and were more insulin resistant. Comparisons of the denied surgery group and the GBS group (which were more similar at baseline) showed similar results to analyses comparing the surgical patients with the combined reference group (Tables 1 and 2). Thus, we do not think these baseline differences affected our main conclusions about the effects of weight loss. In addition, to further minimize baseline differences, we used 1 set of multivariable models that only looked at changes from baseline. Because our study was not randomized, it is possible that selection bias influenced the results. We cannot state with certainty whether the favorable effects we observed resulted purely from weight loss or from some other aspect of GBS. This is potentially important because bypassing the distal stomach and proximal small bowel may induce hormonal or other effects that are different from those purely owing to weight loss. Our data suggest that weight loss is more important than bariatric surgery, per se. To sort this out, it would be ideal to compare the changes achieved after GBS with those in a group who lost comparable amounts of weight via lifestyle modification (e.g., diet and exercise). Unfortunately, this is nearly impossible to do given the widely acknowledged failure of lifestyle modification to produce sustained weight loss in the severely obese population. Changes in plasma volume might also contribute to the LV remodeling. We did not measure plasma volume in our subjects. Cardiac output, which is related to plasma volume, was reduced after GBS. Cardiac output was not independently associated with LV mass but was related to LA volume in multivariable analysis. A factor that might limit the ability to generalize our conclusions is the fact that the majority of our subjects had well-controlled blood pressure and normal fasting glucose and glycosylated hemoglobin levels at baseline (Table 1). Although our patients may have been “healthier” than comparably obese subjects in other geographic areas or of different ethnic backgrounds, the relative good health of our subjects would likely minimize the favorable effects of GBS compared with what might be seen in obese populations with more severe comorbidities. Thus, the beneficial changes after GBS might be even more pronounced in other populations. On the other hand, it is possible that the combination of severe obesity and poorly controlled hypertension or diabetes could lead to irreversible cardiac remodeling and dysfunction that would not improve after GBS. Such effects might explain the high mortality seen after GBS in older men that was reported in one study (42). Additional studies in different patient populations will be required to address these issues.
Perspectives
Our results in a large cohort of severely obese subjects showed that GBS was associated with sustained “reverse” cardiac remodeling and modest improvements in myocardial function. These data help to fill some of the previously identified gaps in bariatric surgical published reports (42). Given the powerful prognostic significance of increased LV mass and LA volume, our findings suggest possible mechanisms by which GBS could lead to enhanced survival in severe obesity (37). Despite these favorable results, continued caution is still warranted because there are potential untoward effects of GBS that may take many years to become manifest (i.e., osteoporosis or micronutrient deficiencies). However, given that cardiovascular disease is the leading cause of excess mortality in the obese population (43), the potential risks are unlikely to outweigh the demonstrable benefits of surgical intervention. In addition to the structural and functional changes in the heart that we report herein, other investigators have estimated that GBS should result in substantial reductions in the risk of developing atherosclerotic coronary vascular disease as a result of improvements in blood pressure, lipid levels, and insulin resistance/diabetes (44).
Supplementary Material
Acknowledgments
We wish to thank our skilled team of sonographers for their important contributions and the study coordinator, Sara Frogley for her efforts.
This study was supported by grants from the National Institutes of Health (T32HL7576, 5R01DK055006), National Center for Research Resources (PHS M01-RR00064), Department of Veterans Affairs, and the Deseret Foundation, LDS Hospital. The authors have reported that they have no relationships to disclose.
Abbreviations and Acronyms
- BMI
body mass index
- E
early diastolic mitral flow velocity
- E′
early diastolic myocardial velocity
- GBS
gastric bypass surgery
- LV
left ventricular
- MWFS
midwall fractional shortening
- RV
right ventricular
REFERENCES
- 1.Kenchaiah S, Evans JC, Levy D, et al. Obesity and the risk of heart failure. N Engl J Med. 2002;347:305–313. doi: 10.1056/NEJMoa020245. [DOI] [PubMed] [Google Scholar]
- 2.Wang TJ, Parise H, Levy D, et al. Obesity and the risk of new-onset atrial fibrillation. JAMA. 2004;292:2471–2477. doi: 10.1001/jama.292.20.2471. [DOI] [PubMed] [Google Scholar]
- 3.Calle EE, Thun MJ, Petrelli JM, Rodriguez C, Heath CW., Jr Body-mass index mortality in a prospective cohort of U.S adults. N Engl J Med. 1999;341:1097–1105. doi: 10.1056/NEJM199910073411501. [DOI] [PubMed] [Google Scholar]
- 4.Gu D, He J, Duan X, et al. Body weight and mortality among men and women in China. JAMA. 2006;295:776–783. doi: 10.1001/jama.295.7.776. [DOI] [PubMed] [Google Scholar]
- 5.Flegal KM, Graubard BI, Williamson DF, Gail MH. Excess deaths associated with underweight, overweight, and obesity. JAMA. 2005;293:1861–1867. doi: 10.1001/jama.293.15.1861. [DOI] [PubMed] [Google Scholar]
- 6.Adams KF, Schatzkin A, Harris TB, et al. Overweight, obesity, and mortality in a large prospective cohort of persons 50 to 71 years old. N Engl J Med. 2006;355:763–778. doi: 10.1056/NEJMoa055643. [DOI] [PubMed] [Google Scholar]
- 7.Jee SH, Sull JW, Park J, et al. Body-mass index and mortality in Korean men and women. N Engl J Med. 2006;355:779–787. doi: 10.1056/NEJMoa054017. [DOI] [PubMed] [Google Scholar]
- 8.Wong CY, O’Moore-Sullivan T, Leano R, Byrne N, Beller E, Marwick TH. Aterations of left ventricular myocardial characteristics associated with obesity. Circulation. 2004;110:3081–3087. doi: 10.1161/01.CIR.0000147184.13872.0F. [DOI] [PubMed] [Google Scholar]
- 9.Peterson LR, Waggoner AD, Schechtman KB, et al. Aterations in left ventricular structure and function in young healthy obese women: assessment by echocardiography and tissue Doppler imaging. J Am Coll Cardiol. 2004;43:1399–1404. doi: 10.1016/j.jacc.2003.10.062. [DOI] [PubMed] [Google Scholar]
- 10.Li X, Li S, Ulusoy E, Chen W, Srinivasan SR, Berenson GS. Childhood adiposity as a predictor of cardiac mass in adulthood: the Bogalusa Heart Study. Circulation. 2004;110:3488–3492. doi: 10.1161/01.CIR.0000149713.48317.27. [DOI] [PubMed] [Google Scholar]
- 11.Alpert MA, Lambert CR, Panayiotou H, et al. Relation of duration of morbid obesity to left ventricular mass, systolic function, and diastolic filling, and effect of weight loss. Am J Cardiol. 1995;76:1194–1197. doi: 10.1016/s0002-9149(99)80338-5. [DOI] [PubMed] [Google Scholar]
- 12.Lauer MS, Anderson KM, Kannel WB, Levy D. The impact of obesity on left ventricular mass and geometry The Framingham Heart Study. JAMA. 1991;266:231–236. [PubMed] [Google Scholar]
- 13.Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med. 1990;322:1561–1566. doi: 10.1056/NEJM199005313222203. [DOI] [PubMed] [Google Scholar]
- 14.de Simone G, Kizer JR, Chinali M, et al. Normalization for body size and population-attributable risk of left ventricular hypertrophy: the Strong Heart Study. Am J Hypertens. 2005;18:191–196. doi: 10.1016/j.amjhyper.2004.08.032. [DOI] [PubMed] [Google Scholar]
- 15.de Simone G, Devereux RB, Roman MJ, Alderman MH, Laragh JH. Relation of obesity and gender to left ventricular hypertrophy in normotensive and hypertensive adults. Hypertension. 1994;23:600–606. doi: 10.1161/01.hyp.23.5.600. [DOI] [PubMed] [Google Scholar]
- 16.Avelar E, Cloward TV, Walker JM, et al. Left ventricular hypertrophy in severe obesity: interactions among blood pressure, nocturnal hypoxemia, and body mass. Hypertension. 2007;49:34–39. doi: 10.1161/01.HYP.0000251711.92482.14. [DOI] [PubMed] [Google Scholar]
- 17.Gottdiener JS, Reda DJ, Williams DW, Materson BJ. for the Department of Veterans Affairs Cooperative Study Group on Antihypertensive Agents Left atrial size in hypertensive men: influence of obesity, race and age. J Am Coll Cardiol. 1997;29:651–658. doi: 10.1016/s0735-1097(96)00554-2. [DOI] [PubMed] [Google Scholar]
- 18.Melenovsky V, Borlaug BA, Rosen B, et al. Cardiovascular features of heart failure with preserved ejection fraction versus nonfailing hypertensive left ventricular hypertrophy in the urban Baltimore community: the role of atrial remodeling/dysfunction. J Am Coll Cardiol. 2007;49:198–207. doi: 10.1016/j.jacc.2006.08.050. [DOI] [PubMed] [Google Scholar]
- 19.Takemoto Y, Barnes ME, Seward JB, et al. Usefulness of left atrial volume in predicting first congestive heart failure in patients > or = 65 years of age with well-preserved left ventricular systolic function. Am J Cardiol. 2005;96:832–836. doi: 10.1016/j.amjcard.2005.05.031. [DOI] [PubMed] [Google Scholar]
- 20.Okin PM, Wachtell K, Devereux RB, et al. Regression of electrocardiographic left ventricular hypertrophy and decreased incidence of new-onset atrial fibrillation in patients with hypertension. JAMA. 2006;296:1242–1248. doi: 10.1001/jama.296.10.1242. [DOI] [PubMed] [Google Scholar]
- 21.Cooper RS, Simmons BE, Castaner A, Santhanam V, Ghali J, Mar M. Left ventricular hypertrophy is associated with worse survival independent of ventricular function and number of coronary arteries severely narrowed. Am J Cardiol. 1990;65:441–445. doi: 10.1016/0002-9149(90)90807-d. [DOI] [PubMed] [Google Scholar]
- 22.East MA, Jollis JG, Nelson CL, Marks D, Peterson ED. The influence of left ventricular hypertrophy on survival in patients with coronary artery disease: do race and gender matter? J Am Coll Cardiol. 2003;41:949–954. doi: 10.1016/s0735-1097(02)03006-1. [DOI] [PubMed] [Google Scholar]
- 23.Davis MM, Slish K, Chao C, Cabana MD. National trends in bariatric surgery, 1996–2002. Arch Surg. 2006;141:71–74. doi: 10.1001/archsurg.141.1.71. [DOI] [PubMed] [Google Scholar]
- 24.DeMaria EJ. Bariatric surgery for morbid obesity. N Engl J Med. 2007;356:2176–2183. doi: 10.1056/NEJMct067019. [DOI] [PubMed] [Google Scholar]
- 25.Adams TD, Avelar E, Cloward T, et al. Design and rationale of the Utah Obesity Study. A study to assess morbidity following gastric bypass surgery. Contemp Clin Trials. 2005;26:534–551. doi: 10.1016/j.cct.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 26.Gottdiener JS, Bednarz J, Devereux R, et al. American Society of Echocardiography recommendations for use of echocardiography in clinical trials. J Am Soc Echocardiogr. 2004;2017:1086–1119. doi: 10.1016/j.echo.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 27.Palmieri V, Dahlof B, DeQuattro V, et al. Reliability of echocardiographic assessment of left ventricular structure and function: the PRESERVE study. Prospective Randomized Study Evaluating Regression of Ventricular Enlargement. J Am Coll Cardiol. 1999;34:1625–1632. doi: 10.1016/s0735-1097(99)00396-4. [DOI] [PubMed] [Google Scholar]
- 28.Devereux RB, Alonso DR, Lutas EM, et al. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol. 1986;57:450–458. doi: 10.1016/0002-9149(86)90771-x. [DOI] [PubMed] [Google Scholar]
- 29.de Simone G, Devereux RB, Roman MJ, et al. Assessment of left ventricular function by the midwall fractional shortening/end-systolic stress relation in human hypertension. J Am Coll Cardiol. 1994;23:1444–1451. doi: 10.1016/0735-1097(94)90390-5. [DOI] [PubMed] [Google Scholar]
- 30.Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 31.Yu CM, Sanderson JE, Marwick TH, Oh JK. Tissue Doppler imaging a new prognosticator for cardiovascular diseases. J Am Coll Cardiol. 2007;49:1903–1914. doi: 10.1016/j.jacc.2007.01.078. [DOI] [PubMed] [Google Scholar]
- 32.Garza CA, Pellikka PA, Somers VK, et al. Major weight loss prevents long-term left atrial enlargement in patients with morbid and extreme obesity. Eur J Echocardiogr. 2008;9:587–593. doi: 10.1093/ejechocard/jen117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de las Fuentes L, Waggoner AD, Mohammed BS, et al. Effect of moderate diet-induced weight loss and weight regain on cardiovascular structure and function. J Am Coll Cardiol. 2009;54:2376–2381. doi: 10.1016/j.jacc.2009.07.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leichman JG, Wilson EB, Scarborough T, et al. Dramatic reversal of derangements in muscle metabolism and left ventricular function after bariatric surgery. Am J Med. 2008;121:966–973. doi: 10.1016/j.amjmed.2008.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alpert MA, Lambert CR, Terry BE, et al. Effect of weight loss on left ventricular mass in nonhypertensive morbidly obese patients. Am J Cardiol. 1994;73:918–921. doi: 10.1016/0002-9149(94)90829-x. [DOI] [PubMed] [Google Scholar]
- 36.Karason K, Wallentin I, Larsson B, Sjostrom L. Effects of obesity and weight loss on left ventricular mass and relative wall thickness: survey and intervention study. BMJ. 1997;315:912–916. doi: 10.1136/bmj.315.7113.912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Adams TD, Gress RE, Smith SC, et al. Long-term mortality after gastric bypass surgery. N Engl J Med. 2007;357:753–761. doi: 10.1056/NEJMoa066603. [DOI] [PubMed] [Google Scholar]
- 38.Rider OJ, Francis JM, Ali MK, et al. Beneficial cardiovascular effects of bariatric surgical and dietary weight loss in obesity. J Am Coll Cardiol. 2009;54:718–726. doi: 10.1016/j.jacc.2009.02.086. [DOI] [PubMed] [Google Scholar]
- 39.Morricone L, Malavazos AE, Coman C, Donati C, Hassan T, Caviezel F. Echocardiographic abnormalities in normotensive obese patients: relationship with visceral fat. Obes Res. 2002;10:489–498. doi: 10.1038/oby.2002.67. [DOI] [PubMed] [Google Scholar]
- 40.Iacobellis G, Corradi D, Sharma AM. Epicardial adipose tissue: anatomic, biomolecular and clinical relationships with the heart. Nat Clin Pract Cardiovasc Med. 2005;2:536–543. doi: 10.1038/ncpcardio0319. [DOI] [PubMed] [Google Scholar]
- 41.See R, Abdullah SM, McGuire DK, et al. The association of differing measures of overweight and obesity with prevalent atherosclerosis: the Dallas Heart Study. J Am Coll Cardiol. 2007;50:752–759. doi: 10.1016/j.jacc.2007.04.066. [DOI] [PubMed] [Google Scholar]
- 42.Courcoulas AP, Flum DR. Filling the gaps in bariatric surgical research. JAMA. 2005;294:1957–1960. doi: 10.1001/jama.294.15.1957. [DOI] [PubMed] [Google Scholar]
- 43.Bray GA. Medical consequences of obesity. J Clin Endocrinol Metab. 2004;89:2583–2589. doi: 10.1210/jc.2004-0535. [DOI] [PubMed] [Google Scholar]
- 44.Vogel JA, Franklin BA, Zalesin KC, et al. Reduction in predicted coronary heart disease risk after substantial weight reduction after bariatric surgery. Am J Cardiol. 2007;99:222–226. doi: 10.1016/j.amjcard.2006.08.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.