Abstract
The effect of sleep apnea on the reproductive function of obese men is not entirely elucidated. The objective of this study was to define the effect of sleep apnea on the reproductive hormones and sexual function in obese men. This study included 89 severely obese men with BMI ≥35 kg/m2 considering gastric bypass surgery. Anthropometrics (weight, and BMI), reproductive hormones, and sleep studies were measured. The sexual quality of life was assessed using the Impact of Weight on Quality of Life-Lite questionnaire (IWQOL-Lite). The mean age of our patients was 46.9 ± 11.0 years, the mean BMI was 47.8 ± 8.7 kg/m2 and the mean weight was 337.7 ± 62.4 lb. After correction for age and BMI, means of free testosterone per severity group of sleep apnea were as follows: no or mild sleep apnea 74.4 ± 3.8 pg/ml, moderate sleep apnea 68.6 ± 4.2 pg/ml, and severe sleep apnea 60.2 ± 2.92 pg/ml, P = 0.014. All other parameters of sleep apnea including hypopnea index, percent time below a SpO2 of 90%, and percent time below a SpO2 of 80% were also negatively correlated with testosterone levels after correction for age and BMI. BMI and presence of coronary artery disease decreased the sexual quality of life. Sleep apnea was associated with reduced sexual quality of life. In summary, sleep apnea negatively affects testosterone levels independent of BMI. Severely obese men had decreased sexual quality of life.
INTRODUCTION
Obesity is associated with multiple negative health outcomes including increased risk for diabetes, cardiovascular disorders, and reduced life expectancy (1,2). Obesity in men is also shown to be associated with reduced reproductive potential (3). Obese men have lower total and free testosterone levels and report diminished sexual quality of life (4,5). Multiple and interacting mechanisms are responsible for the alteration in reproductive hormones and sexual function in men. Among these is increased aromatization of the C19 androgens to estradiol, increased central opioid tone that suppresses the hypothalamic pituitary axis and hyperinsulinemia that lowers sex hormone binding protein levels. In addition, anatomic features of obesity result in increased scrotal temperature and sleep apnea that can independently affect reproductive physiology (6). Male sex and obesity are known risk factors for sleep apnea (7). Some studies suggest that sleep apnea may have an independent association with reduced testosterone, sexual dysfunction, and impotence (8–10). However, these studies either had small number of participants or studied the hormonal or sexual response separately (10–13). Other studies show weak or lack of association between sleep apnea and reproductive hormone levels and sexual function after correction for BMI, or showed correlation with some but not all sleep apnea parameters (12,14).
The independent effect of sleep apnea on testosterone levels and male sexual function needs further clarification. The objective of this study is to describe the relation between sleep apnea, testosterone levels, and male sexual quality of life in 89 severely obese men. An adequate understanding of this relation, independent of weight, can help the conceptualization and validation of the results of studies that would look at the effect of correction of sleep apnea on serum testosterone and sexual function in men.
METHODS AND PROCEDURES
Patient population
Our patient population consisted of severely obese men (BMI ≥35 kg/m2) recruited as part of the Utah Obesity Study. This study recruited severely obese subjects who underwent various clinical measures including: a physician interview and detailed medical history, resting electro- and echocardiograms, a submaximal exercise treadmill test and electrocardiogram, pulmonary function, polysomnography, resting metabolic rate, anthropometrics, resting and exercise blood pressure, comprehensive blood chemistry and urinalysis and dietary, quality of life and physical activity questionnaires. Signed informed consent was obtained on all participants and this study was approved by the University of Utah institutional review board. The recruitment criteria are detailed in a previous publication (15). The Utah Obesity Study originally recruited 206 men. Of those, 153 (74.3%) had sufficient stored plasma samples to perform the hormonal analysis. Of the men who had hormonal analysis, 89 (58.2%) had complete diagnostic sleep studies. The participants were examined in the Huntsman General Clinical Research Center, University of Utah Medical Center, Salt Lake City, UT where they were interviewed by one of two hospitalists using a standardized medical history and physical end points questionnaire. Participants had extensive medical screening including metabolic, cardiovascular, and hormonal evaluations. All participants answered detailed questionnaire regarding their medication intake, in particular, none of the participant in this study was on testosterone replacement. The 64 men without sleep studies were similar in demographic, anthropometric, and hormonal characteristics when compared to men who had the sleep studies (data not shown).
Anthropometrics
All men had detailed questionnaires and anthropometric measures at baseline. Height was measured using a Harpenden anthropometer (Holtain, Crymych, UK) to the nearest centimeter. Weight was measured in a hospital gown with a Scaletronix scale (model 5100) (Scaletronix Corporation, Wheaton, IL). The scale has an 800 pound capacity and weighing accuracy of 0.1 kg. BMI was calculated as body weight divided by height squared (kg/m2). Circumferences (Lufkin metal tape) were measured at the waist (at the top of the iliac crest) and hip (at the largest circumference over the buttocks).
Metabolic and hormonal analysis
All study participants underwent a morning venous blood draw after 12 h overnight fasting. The following were measured: total testosterone using electrochemiluminescent immunoassay (Sensitivity (Se) 1.1, coefficient of variation (CV%) 1–6.7, Elecsys Testosterone kit, Roche Diagnostics, Indianapolis, IN, normal values: 300–890 ng/dl), sex hormone-binding globulin using electrochemiluminescent immunoassay (Se 0.01, CV% 1–1.1, Elecsys SHBG kit, Roche Diagnostics, Indianapolis, IN, normal values: 11–80 nmol/l), luteinizing hormone (LH) using electrochemiluminescent immunoassay (Se 0.011, CV% 1.2–1.5, Elecsys LH kit, Roche Diagnostics, Indianapolis, IN, normal values: 1.7–8.6 IU/l), follicle-stimulating hormone using electrochemiluminescent immunoassay (Se 0.0036, CV% 1–1.3, Elecsys FSH kit, Roche Diagnostics, Indianapolis, IN, normal values: 1.5–12.4 IU/l), leptin using enzyme-linked immunosorbent assay (Se 0.5, CV% 11–24.5, Human Leptin ELISA Kit, Linco Research, normal values 0.5–12.7 ng/ml), adiponectin using Enzyme-Linked Immunosorbent Assay (Se 2, CV% 12.5–13.8, Human Adiponectin ELISA Kit (K1001-1), B-Bridge International, Cupertino, CA, normal values: 2–26 µg/ml), C reactive protein using immunoturbidimetric (Se 0.0, CV%: 2.4–6.2, CRPLX Tina-Quant kit, Roche Diagnostics, Indianapolis, IN, normal values: 0.0–0.8 mg/dl) as a marker of inflammation. Free testosterone and estradiol were calculated using the following formula: FH = ([H] − (N × [FH]))/(Kt[SHBG − [H] + N[FH]]) (16) (normal values for free testosterone in males: 47–244 pg/ml). All hormonal analyses were performed at the ARUP Institute for Clinical and Experimental pathology. ARUP is a national reference laboratory located in Salt Lake City, Utah.
Assessment of quality of sexual life
All study participants underwent an initial evaluation that included standardized medical history and physical end points questionnaires. In addition to other questionnaires, the Impact of Weight on Quality of Life-Lite questionnaire (IWQOL-Lite) was administered. The IWQOL-Lite is a validated 31-item self report questionnaire designed to assess the impact of weight on the quality of life in obese individuals (17–19). The IWQOL-Lite assesses five domains: physical function, self esteem, public distress, work and sexual life. The sexual life domain is evaluated with four questions pertaining to (i) lack of enjoyment of sexual activity, (ii) lack of sexual desire, (iii) difficulty with sexual performance, and (iv) avoidance of sexual encounters. Each question has five possible response options: “5 = Always true”, “4 = Usually true”, “3 = Sometimes true”, “2 = Rarely true” and “1 = Never true”. Each of the questions was analyzed separately and an overall score of quality of sexual life was calculated using the scores from each question. A higher score indicates higher dissatisfaction with the quality of sexual life.
Sleep studies
Limited polysomnography conducted at an elevation consisted of nasal airflow (using a pressure transducer), thoracic and abdominal effort, snoring, activity by biaxel accelerometer, body position, SpO2 and heart rate, was collected using an Embletta Portable Diagnostic System (Broomfield, CO). Participants were studied in a controlled hospital setting initiated between 9:30 and 10:00 pm. Termination of the sleep study occurred between 5:30 and 6:00 am. A single board certified sleep specialist (J.M.W.) analyzed all records to eliminate interscorer variability. Records were scored blindly without knowledge of weight or gender. A central apnea was defined as cessation of airflow for ≥10 s in conjunction with absence of respiratory effort whereas obstructive apnea was defined as an absence of airflow for ≥10 s but continued respiratory effort. Hypopneas were defined as reduction of airflow for ≥10 s in conjunction with a 4% desaturation of SpO2 (20). A respiratory disturbance index RDI (events/h) and a hypopnea index were calculated by dividing the total number of apneas/hypopneas by hours of recording. For the purpose of this study: no sleep apnea was defined as RDI ≤5 events/h, mild sleep apnea 5 < RDI ≤15 events/h, moderate sleep apnea 15 < RDI ≤30 events/h, and severe apnea RDI >30 events/h (21). The Embletta analysis program automatically calculates mean pulse oximeter oxygen saturation (SpO2), percent time below a SpO2 of 90%, and percent time below a SpO2 of 80%.
Statistical analysis
The demographic, anthropometric, hormonal, and sexual satisfaction parameters were reported as mean ± s.e. The unadjusted means were compared using ANOVA. The free and total testosterone levels were then adjusted for age and BMI and were compared using a general linear model. A linear regression model was used to estimate the independent association of the RDI with total score of quality of sexual life after correction for age, BMI, alcohol intake, smoking, coronary artery disease, diabetes, hypertension, marital status, and C reactive protein. Bonferroni correction was used for multiple comparisons. SPSS 16.0 (SPSS, Chicago, IL) was used for statistical analysis.
RESULTS
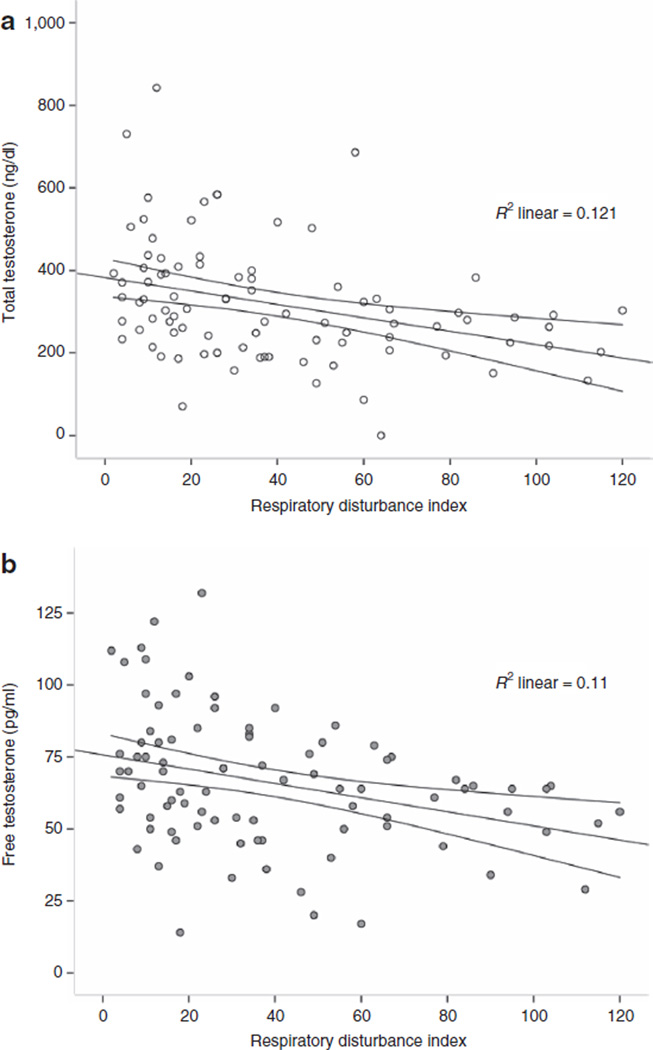
The mean age of our patients was 46.9 ± 11.0 years, the mean BMI was 47.8 ± 8.7 kg/m2, and the mean weight was 337.7 ± 62.4 lb. The incidence of sleep apnea was 93.3%. The distribution of the different degrees of sleep apnea was as follows: 6 (6.7%) patients had no sleep apnea, 19 (21.3%) had mild sleep apnea, 20 (22.5%) had moderate sleep apnea, and 44 (49%) had severe sleep apnea. The distribution of the different demographic, anthropometric, and hormonal characteristics by the degree of the sleep apnea is given in Table 1. This table (1) shows a clear significant association between the severity of sleep apnea and patients’ weight and BMI. BMI correlated better than leptin with sleep apnea. Patients with increased severity of sleep apnea had significantly lower total (P = 0.005) and free testosterone levels (P = 0.005). A scatter showing the correlation between RDI and total and free testosterone level is presented in Figure 1a,b, Pearson’s correlation coefficient: total testosterone: r = −0.34, P = 0.001 and free testosterone: r = −0.53, P = 0.001. This association in the nonadjusted analysis can be attributed, in part, to the know effect of BMI on testosterone. In our study, BMI was negatively correlated with total and free testosterone, Pearson’s correlation coefficient: total testosterone: r = −0.218, P = 0.007 and free testosterone: r = −0.263, P = 0.001.
Table 1.
Comparison of means of demographic, anthropometric, and hormonal parameters between the different sleep apnea severity groups (unadjusted means ± s.e., ANOVA)
| No sleep apnea (n = 6) |
Mild sleep apnea (n = 19) |
Moderate sleep (n = 20) |
Severe sleep apnea (n = 44) |
P value | |
|---|---|---|---|---|---|
| Demographic and anthropometric parameters | |||||
| Age (years) | 39.7 ± 4.21 | 46.8 ± 1.94 | 46.1 ± 1.75 | 46.2 ± 1.45 | 0.30 |
| BMI (kg/m2) | 41.9 ± 1.97 | 44.4 ± 1.95 | 47.8 ± 1.67 | 49.0 ± 1.07 | 0.028a |
| Weight (lb) | 295.3 ± 18.48 | 314.4 ± 10.96 | 343.4 ± 11.25 | 351.4 ± 7.70 | 0.006a |
| Neck (cm) | 44.1 ± 1.13 | 47.0 ± 0.56 | 46.5 ± 0.51 | 47.4 ± 0.91 | 0.33 |
| Waist (cm) | 130.4 ± 4.55 | 134.2 ± 3.07 | 143.4 ± 3.35 | 145.2 ± 2.06 | 0.005a |
| Hip (cm) | 128.5 ± 2.07 | 130.5 ± 3.09 | 136.9 ± 3.47 | 140.4 ± 2.17 | 0.03a |
| Hormonal parameters | |||||
| CRP | 0.43 ± 0.098 | 0.61 ± 0.133 | 0.41 ± 0.059 | 0.61 ± 0.072 | 0.34 |
| LH | 4.4 ± 0.61 | 4.8 ± 0.43 | 4.3 ± 0.61 | 4.4 ± 0.45 | 0.93 |
| FSH | 3.0 ± 0.67 | 5.3 ± 0.85 | 5.2 ± 1.02 | 4.9 ± 0.58 | 0.62 |
| Total testosterone (ng/dl) | 390.0 ± 72.36 | 396.3 ± 34.67 | 333.5 ± 33.18 | 270.1 ± 17.73 | 0.005a,b |
| Sex hormone-binding globulin (nmol/l) | 25.8 ± 7.32 | 30.8 ± 3.51 | 25.6 ± 3.02 | 24.1 ± 2.45 | 0.48 |
| Free testosterone (pg/ml) | 80.7 ± 9.68 | 76.2 ± 5.32 | 68.7 ± 6.03 | 58.5 ± 2.75 | 0.009a,b |
| Leptin (ng/ml) | 8.9 ± 1.24 | 7.8 ± 1.67 | 7.7 ± 0.755 | 6.9 ± 0.58 | 0.68 |
| Adeponectin (µg/ml) | 47.6 ± 6.06 | 48.4 ± 10.61 | 43.4 ± 4.14 | 50.1 ± 2.99 | 0.81 |
CRP, C reactive protein; FSH, follicle-stimulating hormone; LH, luteinizing hormone.
ANOVA, statistically significant at the level of 0.05.
Severe sleep apnea compared to mild sleep apnea (after Bonferroni correction).
Figure 1.
The correlation between respiratory disturbance index with (a) total and (b) free testosterone levels in the study population. Linear fit line with 95% confidence interval of the mean. (Pearson’s correlation coefficient: total testosterone: r = −0.34, P = 0.001 and free testosterone: r = −0.53, P = 0.001).
The severity of sleep apnea was found to be negatively correlated with testosterone levels after correction for age and BMI, Table 2. Because of the low number of patients in the group of “no sleep apnea”, it was combined with the group of mild sleep apnea in the adjusted analysis. The adjusted means of total testosterone (mean ± s.e.) per severity group of sleep apnea were as follows: No or mild sleep apnea: 386.51 ± 27.34 ng/dl, moderate sleep apnea 331.9 ± 30.11 ng/dl, and severe sleep apnea 275 ± 20.50 ng/dl, P = 0.002. The adjusted means of free testosterone per severity group of sleep apnea were as follows: no or mild sleep apnea: 74.4 ± 3.8 pg/ml, moderate sleep apnea: 68.6 ± 4.2 pg/ml, and severe sleep apnea: 60.2 ± 2.92 pg/ml, P = 0.014. Our analysis showed that an increase of 10 events in the RDI will result in ~2 pg/ml reduction in free testosterone levels. After correction for age and BMI, other parameters of sleep apnea including hypopnea index, percent time below a SpO2 of 90%, and percent time below a SpO2 of 80% were also negatively correlated with total and free testosterone levels. Mean SpO2 was positively correlated with total and free testosterone levels, Table 2.
Table 2.
Linear regression models predicting testosterone levels and including each of the sleep study parameters after correcting for BMI, age, and alcohol intake
| Each model included age, BMI, alcohol intake, and one of factors below: |
Standardized β coefficients measuring the independent contribution to the reduction in testosterone (P value) |
|
|---|---|---|
| Free testosterone |
Total testosterone |
|
| Respiratory disturbance index | −0.269a (0.004) | −0.303a (0.004) |
| Hypopnea index | −0.233a (0.011) | −0.247a (0.018) |
| Average SpO2 | 0.298a (0.002) | 0.262a (0.015) |
| Percent time SpO2 <90% | −0.280a (0.002) | −0.222a (0.048) |
| Percent time SpO2 <80% | −0.229a (0.013) | −0.217a (0.038) |
Each of the sleep study parameters was included in a separate model.
Statistically significant at the level of 0.05.
In this study, BMI correlated negatively with quality of sexual life. Partial correlation coefficients for BMI and parameters of quality of sexual life after correcting for age were as follows: total score of quality of sexual life: r = 0.399 (P < 0.001), not enjoying sexual activity: r = 0.339 (P < 0.001), having little sexual desire: r = 0.346 (P < 0.001), difficulty with sexual performance: r = 0.409 (P < 0.001) and avoiding sexual encounters: r = 0.344 (P < 0.001). In all categories of sleep apnea, patients reported an overall severe reduction in their quality of sexual life evidenced by the values of the total score and the individual parameters of the quality of sexual life, Table 3. The manual for the IWQOL-Lite report a score of 1–2 in the quality of sexual life section for nonobese men. All categories of sleep apnea in this study were associated with higher score denoting lower satisfaction with sexual life. However, the severity of sleep apnea (compared to other obesity-related factors) did not correlate with the quality of sexual life as measured by the IWQOL-Lite. Among all the factors, BMI (P = 0.001) and presence of coronary artery disease (P = 0.036) were the strongest predictors of decreased quality of sexual life.
Table 3.
Quality of sexual life scores across sleep apnea severity groups (unadjusted means ± s.e., ANOVA)
| No sleep apnea | Mild sleep apnea | Moderate sleep apnea |
Severe sleep apnea |
P value | |
|---|---|---|---|---|---|
| Total sexual quality of life score | 10.7 ± 0.94 | 10.0 ± 1.09 | 9.5 ± 0.94 | 9.6 ± 0.63 | 0.90 |
| Not enjoying sexual activity | 2.7 ± 0.26 | 2.6 ± 0.26 | 2.5 ± 0.26 | 2.4 ± 0.18 | 0.80 |
| Have little sexual desire | 2.6 ± 0.34 | 2.4 ± 0.29 | 2.2 ± 0.26 | 2.3 ± 0.18 | 0.81 |
| Difficulty with sexual performance | 2.9 ± 0.38 | 2.7 ± 0.31 | 2.9 ± 0.27 | 2.9 ± 0.18 | 0.94 |
| Avoiding sexual encounters | 2.5 ± 0.27 | 2.3 ± 0.30 | 2.0 ± 0.26 | 2.1 ± 0.17 | 0.71 |
DISCUSSION
This study showed that increased severity of sleep apnea is associated with lower free testosterone levels independent of age and BMI. Correcting for BMI allowed us to control for other obesity-associated factors that can result in low testosterone levels such as estradiol levels, hyperinsulinemia, central hypothalamic suppression, and increased scrotal temperature (3). Moreover, our study showed that all measures of sleep apnea showed a negative correlation with free testosterone including RDI, mean SpO2, percent time below a SpO2 of 90%, and percent time below a SpO2 of 80%. In this population of severely obese men, the quality of sexual life was severely reduced in correlation with BMI and coronary artery disease. Sleep apnea was also associated with reduced quality of sexual life. However, the severity of sleep apnea did not seem to reduce an already low quality of sexual life. In this study, arterial blood gases were not part of patients’ evaluation. This did not allow the assessment of the prevalence of the obesity hypoventilation syndrome. This syndrome has been associated with more advanced metabolic abnormalities when present in association with obstructive sleep apnea (22). The effect of obesity hypoventilation syndrome on hormonal parameters and quality of sexual life scores could not be evaluated.
Grunstein et al. reported in 1989 that sleep apnea (hypopnea index and mean minimal oxygen saturation) was independently associated with lower total and free testosterone levels. Interestingly, in the same study, 43 men with severe sleep apnea who had 3 months of nasal continuous positive airway pressure treatment showed improvement in their sex hormone-binding globulin and total testosterone but not free testosterone levels. Gambineri et al. also showed that the reduction of testosterone levels in 15 obese patients with sleep apnea was independent of BMI or abdominal fatness (11). Luboshitzky et al. studied 10 patients with obstructive sleep apnea who were found to have a significantly lower mean and area under curve testosterone values when compared with controls. After correction for BMI, the difference in mean testosterone levels became non-significant but the difference in area under curve of overnight testosterone remained significant. In the same study, after correcting for BMI, area under curve of LH (but not mean LH) was lower in men with obstructive sleep apnea when compared to control. The authors concluded that central suppression is a possible cause for the lower testosterone levels associated with sleep apnea. In our study, there was no difference in spot LH or follicle-stimulating hormone levels between the different groups of severity of sleep apnea. In a later study, Luboshitzky et al. showed that men with obstructive sleep apnea had a difference in stage 2 and stage 3–4 sleep when compared to controls which correlated with lower LH and testosterone levels (10). This study suggests that the lower testosterone levels associated with sleep apnea can be attributed to differences in sleep patterns and sleep fragmentation (10). In all previously mentioned studies, there was no evaluation of sexual function. Our study did not include electroencephalography monitoring. Subsequently, the relation between sleep stages and sex hormone levels could not be examined.
Other studies reported on the association between sleep apnea and sexual dysfunction in men. Semple et al. in 1984 reported on a man who presented with obesity, sleep apnea, whose reduced testosterone and impotence were reversed after weight loss (13). Schiavi et al. did not find any correlation between sleep disorders or respiratory flow studies and erectile dysfunction in healthy older men (23). Margel et al. showed that severe sleep apnea (RDI >40) was associated with erectile dysfunction and reduced morning erections. The average BMI in this study was 28.1 kg/m2 compared to the average BMI of 47.8 ± 8.71 kg/m2 in our study (24). In a more recent study, Stannek et al. concluded that age had a bigger influence in affecting sexual functioning in men with sleep apnea than the severity of the disease (8). In another recent study that included 401 men who presented for sleep studies, sleep apnea measured as mean SpO2 was correlated with erectile dysfunction after correction for other risk factors for erectile dysfunction including age and BMI. However, a similar correlation was not found for other indexes of sleep apnea (RDI, lowest SpO2, desaturation index, arousal index) and erectile dysfunction (9). Moreover, in this study, men with hypogonadism were excluded, correction was not made for endocrine parameters, and the correlation between erectile dysfunction and mean SpO2 was only found for three of the five aspects of erectile function studied. In particular, sexual desire and overall satisfaction domains were not correlated to sleep apnea. This difference between study results is attributed to the difference in BMI of various study populations and difference in the instruments used to assess the sexual function. The lack of correlation in our study between the severity of sleep apnea and the quality of sexual life can be explained by the already reduced quality of sexual life in this group of severely obese men. Future studies should also include men with normal weight. While the IWQOL-Lite questionnaire is validated to study the overall satisfaction with the quality of sexual life, there is need to address the specific question of the effect of sleep apnea on erectile dysfunction using specific tools such as the international index of erectile function.
This study demonstrates that sleep apnea appears to be an independent predictor for low testosterone levels in severely obese men. There is also a general low satisfaction with sexual life in the severely obese men that was not affected further by sleep apnea. Whether improvement in sleep apnea in obese men, independent of weight loss (or bariatric surgery), improves testosterone and satisfaction with sexual life remains to be demonstrated.
ACKNOWLEDGMENTS
This work was supported by a grant (DK-55006) from the National Institute of Diabetes and Digestive and Kidney Diseases and a grant (M01-RR00064) from the National Center for Research Resources.
R.L.K. was a consultant on the National Institutes of Health (NIH) grant and received royalties on the use of Impact of Weight on Quality of Life-Lite questionnaire. A.W.M is the director of the endocrine testing at ARUP laboratories. S.C.H and T.D.A received NIH funds related to this research. T.D.A received lecture fees related to this research.
Footnotes
DISCLOSURE
A.O.H., J.M.W., T.C., and M.G. declared no conflict of interest.
REFERENCES
- 1.Must A, Spadano J, Coakley EH, et al. The disease burden associated with overweight and obesity. JAMA. 1999;282:1523–1529. doi: 10.1001/jama.282.16.1523. [DOI] [PubMed] [Google Scholar]
- 2.Adams KF, Schatzkin A, Harris TB, et al. Overweight, obesity, and mortality in a large prospective cohort of persons 50 to 71 years old. N Engl J Med. 2006;355:763–778. doi: 10.1056/NEJMoa055643. [DOI] [PubMed] [Google Scholar]
- 3.Hammoud AO, Gibson M, Peterson CM, Hamilton BD, Carrell DT. Obesity and male reproductive potential. J Androl. 2006;27:619–626. doi: 10.2164/jandrol.106.000125. [DOI] [PubMed] [Google Scholar]
- 4.Hammoud A, Gibson M, Hunt SC, et al. Effect of Roux-en-Y gastric bypass surgery on the sex steroids and quality of life in obese men. J Clin Endocrinol Metab. 2009;94:1329–1332. doi: 10.1210/jc.2008-1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Østbye T, Kolotkin R, He DT, et al. Sexual functioning among obese adults enrolling in a weight loss study. J Sex Marital Ther. doi: 10.1080/0092623X.2011.564530. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hammoud AO, Gibson M, Peterson CM, Meikle AW, Carrell DT. Impact of male obesity on infertility: a critical review of the current literature. Fertil Steril. 2008;90:897–904. doi: 10.1016/j.fertnstert.2008.08.026. [DOI] [PubMed] [Google Scholar]
- 7.Young T, Palta M, Dempsey J, et al. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–1235. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 8.Stannek T, Hürny C, Schoch OD, Bucher T, Münzer T. Factors affecting self-reported sexuality in men with obstructive sleep apnea syndrome. J Sex Med. 2009;6:3415–3424. doi: 10.1111/j.1743-6109.2009.01486.x. [DOI] [PubMed] [Google Scholar]
- 9.Budweiser S, Enderlein S, Jörres RA, et al. Sleep apnea is an independent correlate of erectile and sexual dysfunction. J Sex Med. 2009;6:3147–3157. doi: 10.1111/j.1743-6109.2009.01372.x. [DOI] [PubMed] [Google Scholar]
- 10.Luboshitzky R, Lavie L, Shen-Orr Z, Herer P. Altered luteinizing hormone and testosterone secretion in middle-aged obese men with obstructive sleep apnea. Obes Res. 2005;13:780–786. doi: 10.1038/oby.2005.88. [DOI] [PubMed] [Google Scholar]
- 11.Gambineri A, Pelusi C, Pasquali R. Testosterone levels in obese male patients with obstructive sleep apnea syndrome: relation to oxygen desaturation, body weight, fat distribution and the metabolic parameters. J Endocrinol Invest. 2003;26:493–498. doi: 10.1007/BF03345209. [DOI] [PubMed] [Google Scholar]
- 12.Luboshitzky R, Aviv A, Hefetz A, et al. Decreased pituitary-gonadal secretion in men with obstructive sleep apnea. J Clin Endocrinol Metab. 2002;87:3394–3398. doi: 10.1210/jcem.87.7.8663. [DOI] [PubMed] [Google Scholar]
- 13.Semple PA, Graham A, Malcolm Y, Beastall GH, Watson WS. Hypoxia, depression of testosterone, and impotence in pickwickian syndrome reversed by weight reduction. Br Med J (Clin Res Ed) 1984;289:801–802. doi: 10.1136/bmj.289.6448.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grunstein RR, Handelsman DJ, Lawrence SJ, et al. Neuroendocrine dysfunction in sleep apnea: reversal by continuous positive airways pressure therapy. J Clin Endocrinol Metab. 1989;68:352–358. doi: 10.1210/jcem-68-2-352. [DOI] [PubMed] [Google Scholar]
- 15.Adams TD, Avelar E, Cloward T, et al. Design and rationale of the Utah obesity study. A study to assess morbidity following gastric bypass surgery. Contemp Clin Trials. 2005;26:534–551. doi: 10.1016/j.cct.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 16.Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab. 1999;84:3666–3672. doi: 10.1210/jcem.84.10.6079. [DOI] [PubMed] [Google Scholar]
- 17.Kolotkin RL, Crosby RD, Williams GR, Hartley GG, Nicol S. The relationship between health-related quality of life and weight loss. Obes Res. 2001;9:564–571. doi: 10.1038/oby.2001.73. [DOI] [PubMed] [Google Scholar]
- 18.Kolotkin RL, Crosby RD, Kosloski KD, Williams GR. Development of a brief measure to assess quality of life in obesity. Obes Res. 2001;9:102–111. doi: 10.1038/oby.2001.13. [DOI] [PubMed] [Google Scholar]
- 19.Kolotkin RL, Crosby RD. Psychometric evaluation of the impact of weight on quality of life-lite questionnaire (IWQOL-lite) in a community sample. Qual Life Res. 2002;11:157–171. doi: 10.1023/a:1015081805439. [DOI] [PubMed] [Google Scholar]
- 20.Redline S, Sanders M. Hypopnea, a floating metric: implications for prevalence, morbidity estimates, and case finding. Sleep. 1997;20:1209–1217. doi: 10.1093/sleep/20.12.1209. [DOI] [PubMed] [Google Scholar]
- 21.Flemons WW. Clinical practice. Obstructive sleep apnea. N Engl J Med. 2002;347:498–504. doi: 10.1056/NEJMcp012849. [DOI] [PubMed] [Google Scholar]
- 22.Piper AJ, Grunstein RR. Obesity hypoventilation syndrome - mechanisms and management. Am J Respir Crit Care Med. 2010 doi: 10.1164/rccm.201008-1280CI. e-pub ahead of print 29 October 2010. [DOI] [PubMed] [Google Scholar]
- 23.Schiavi RC, Mandeli J, Schreiner-Engel P, Chambers A. Aging, sleep disorders, and male sexual function. Biol Psychiatry. 1991;30:15–24. doi: 10.1016/0006-3223(91)90066-u. [DOI] [PubMed] [Google Scholar]
- 24.Margel D, Cohen M, Livne PM, Pillar G. Severe, but not mild, obstructive sleep apnea syndrome is associated with erectile dysfunction. Urology. 2004;63:545–549. doi: 10.1016/j.urology.2003.10.016. [DOI] [PubMed] [Google Scholar]