Abstract
Background
Trastuzumab is associated with improvements in overall survival (OS) among patients with HER2-positive metastatic breast cancer (MBC); however disease course and patterns of care in individual patients are highly variable.
Methods
113 HER2-positive patients diagnosed with MBC from 1999 to 2005 who received trastuzumab-based therapy were retrospectively identified to allow for a minimum of 5 years of follow-up time. Median OS and median duration of therapy were determined using Kaplan–Meier methodology and group comparisons were based on the log-rank test. Hazard ratios (HR) were obtained using a Cox proportional hazards model.
Results
Median OS was 3.5 years (95% CI 3.0–4.4) from time of initiation of first therapy in the metastatic setting. On univariate analysis, central nervous system (CNS) disease at first recurrence was associated with a shorter OS compared with liver and/or lung metastases or other sites (CNS: 1.9 years CI 0.1–5.9, liver/lung: 3.2 years CI 2.5–4.2, other: 4.6 years CI 2.7–8.0; p = 0.05), however, this was not predictive of survival outcome in multivariate analysis. CNS metastases developed in 62 (55%) patients by the time of death or last follow-up. Median duration of therapy was similar up to 6 lines of treatment, and ranged from 5.2 months to 7.2 months.
Conclusions
The natural history of HER2-positive MBC has evolved with trastuzumab-based therapy with median OS now exceeding 3 years. CNS disease is a major problem with continued risk of CNS progression over time. Patients demonstrate clinical benefit to multiple lines of HER2-directed therapy.
Keywords: Breast cancer, Central nervous system, HER2 Metastases, Trastuzumab
Introduction
Trastuzumab, the monoclonal antibody to the extracellular domain of tyrosine kinase HER2 (HER-2/neu, ERBB2), has been routinely administered to patients with HER2-amplified metastatic breast cancer (MBC) since its FDA approval in October 1998. While improvements in systemic control and overall survival have been reported with the use of trastuzumab in the metastatic setting,1–5 there is marked variability in clinical outcomes including a large range in median survival of 2–4 years.1,5–8 Furthermore, it has been suggested that the hazard rate of death in HER2-positive patients may be bimodal, leading to the hypothesis that patients with short-and long-term survival may be fundamentally different.9
Inconsistency in patient outcomes may be secondary to differences in therapy administration and regimen selection. Evidence of continued efficacy of trastuzumab after tumor progression on a trastuzumab-based regimen has lead to serial administration of trastuzumab in clinical practice.10–12 Additionally, lapatinib combined with capecitabine is approved for patients with advanced HER2-amplified disease, and other HER2-targeted drugs are in the pipeline.13–15 Data on the practice patterns associated with the use of sequential HER2-targeted agents are limited. It is necessary to reexamine the clinical course of HER2-amplified MBC, as outcomes reported in the pre-trastuzumab era may no longer accurately reflect the typical patient treated today.
The identification of predictive markers for better outcomes may aid in optimizing treatment approaches and guide prognostication in this patient population. For example, a well described phenomenon is the high incidence of central nervous system (CNS) metastases in patients treated with trastuzumab.16–18 We hypothesized that those individuals with early development of CNS disease will have a worse prognosis compared to patients with other sites of metastases. Additionally, other clinical predictors may divide patients into short- and long-term survivors and help explain the variability in reported patient survival and hazard death rates.
In this retrospective study, we aimed to characterize the patterns of care, clinical outcomes and predictors of survival in patients with recurrent HER2-positive breast cancer who were treated with a trastuzumab-based regimen at Dana-Farber Cancer Institute (DFCI).
Methods
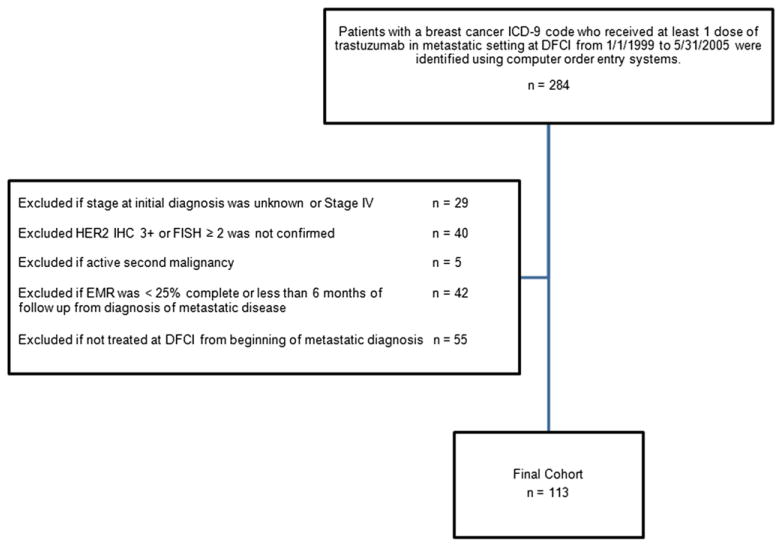
The study was approved by the Institutional Review Board at the Dana-Faber/Harvard Cancer Center. DFCI patients with a breast cancer International Classification of Diseases, 9th Edition (ICD-9) code who received at least one dose of trastuzumab for recurrent disease from January 1, 1999 to May 31, 2005 were identified using computer order entry systems. The end date was chosen to allow for 5 years of potential follow-up time. A total of 284 patients was initially identified (Fig. 1). Patients were included in the analysis if they had an initial diagnosis of stage 0–3 breast cancer and pathology records of the primary or metastatic biopsy confirmed HER2-positive disease as defined by an immunohistochemistry (IHC) score 3+ or a florescence in situ hybridization (FISH) ≥2.0. Patients may have received trastuzumab in the adjuvant or neo-adjuvant setting. All patients were treated at DFCI for their recurrent disease; however, patients may have been treated at another institution for their primary breast cancer diagnosis. Patients were excluded if 1) a second active malignancy was identified 2) the electronic medical record was <25% complete or there was inadequate follow-up of <6 months from diagnosis of metastatic disease 3) patients received treatment for their metastatic disease prior to presentation to DFCI.
Fig. 1.
Identification of patients used in study. Abbreviations: ICD-9, International Classification of Diseases 9th Edition; DFCI, Dana-Farber Cancer Institute; IHC, immunohistochemistry; FISH, florescence in situ hybridization; EMR, electronic medical record.
A total of 113 patients was available for analysis. Follow-up information was available through August 31, 2010. Medical records were reviewed for the following information: date, stage (American Joint Committee on Cancer, Seventh Edition), pathology of initial breast cancer, previous adjuvant regimens, date of diagnosis of metastatic disease, pathology of metastatic biopsy when available, site(s) and dates of initial and subsequent recurrence, date of CNS recurrence, type and date of metastatic therapy, vital status, date of death (or last follow-up). Date of death was confirmed through the Social Security Death Index. A site of breast cancer recurrence was defined as an organ in which a metastatic lesion was identified (i.e. bone, liver, lung/pleura, CNS, abdomen, breast/chest wall, other). If multiple lesions were seen in one organ, this site was counted only once.
Since an accurate evaluation of time to progression requires radiologic assessment of tumor size by RECIST19 criteria at pre-specified time points, duration of therapy was used as a proxy of clinical benefit of a metastatic regimen because it most closely reflects the amount of time a patient was clinically stable. Duration of therapy was defined as time from initiation of therapy until date of initiation of subsequent regimen. A single metastatic regimen was defined as a chemotherapy agent, a hormonal-based therapy, a HER2-targeted agent or any combination of the aforementioned. Patients on a trastuzumab-based therapy in combination with chemotherapy, who subsequently stopped chemotherapy and continued on single-agent trastuzumab, were recorded as receiving a single regimen. Patients on a HER2-based therapy in combination with a chemotherapy agent or a hormonal-based therapy were considered to have switched to a new regimen if the chemotherapy and/or hormonal agent were changed. For patients who were still on therapy at the time of analysis, times were censored at the date of last on-treatment visit.
Statistical analysis
Survival time was defined as time from start of first-line meta-static therapy to death from any cause. Times were censored at the date of last clinic follow-up. Survival and duration of therapy were evaluated using Kaplan–Meier methods. In univariate analyses, group comparisons were based on the log-rank test. Estimates of the hazard rate and 95% CI are based on the life table method. Variables observed to be associated with survival in univariate analysis were analyzed further in multivariable analysis. Hazard ratios and 95% CI were obtained using Cox proportional hazards models. Adjustments in the Cox model included potential confounders such as age at first recurrence, year first-line therapy was initiated, hormone receptor status (ER and/or PR positive versus ER and PR negative) and disease-free interval (<2 years, ≥72 years), defined as time from initial diagnosis to metastatic diagnosis. To examine the role of selection bias due to unavailability of routine HER2 testing prior to 1999, the Cox model also included adjustment for era of initial diagnosis (<1999, ≥1999). The data were reanalyzed by era of initial diagnosis to assess differences in patient and tumor characteristics and survival. To describe the timing of CNS disease, the distribution of CNS disease was examined over intervals of time among patients who were alive for at least 3 years after the start of first-line metastatic therapy. Tests were two-sided at the 0.05 level. Analyses were performed using SAS v9.2.
Results
Description of study population
Patient and initial tumor characteristics of the 113 patients included in this analysis are shown in Table 1. The mean age at diagnosis was 46 (SD 11). The majority of the patient population was Caucasian (77%) and just over half (57%) had hormone receptor positive disease. The median length of follow-up was 3.6 years (range 0.1–11.5) from the time of metastatic breast cancer diagnosis. At the time of last follow-up, 90 (80%) deaths were confirmed. Median time from initial breast cancer diagnosis to diagnosis of metastatic disease was 2.6 years (range 0.1–15.5). Distant disease was seen in 80% of patients at initial breast cancer recurrence. Adjuvant therapy was administered to the majority of patients (85%). Importantly, only 11 patients were exposed to trastuzumab in the adjuvant or neoadjuvant setting. A disease-free interval ≥2 years was seen in 64% of the studied population.
Table 1.
Patient and tumor characteristics.
| Characteristic | n (%) |
|---|---|
| Overall | 113 |
| Era of initial diagnosis | |
| <1999 | 57 (50%) |
| ≥1999 | 56 (50%) |
| Age at initial diagnosis | |
| <40 years | 38 (34%) |
| ≥40 years | 75 (66%) |
| Stage | |
| 0 | 4 (4%) |
| 1 | 15 (13%) |
| 2 | 49 (43%) |
| 3 | 45 (40%) |
| ER and/or PR status | |
| Positive | 64 (57%) |
| Negative | 49 (43%) |
| Exposure to adjuvant or neoadjuvant trastuzumab | 11 (10%) |
| Exposure to adjuvant hormonal therapy | 50 (44%) |
| Exposure to adjuvant or neoadjuvant chemotherapy | 93 (82%) |
| Age at first recurrence | |
| <50 years | 62 (55%) |
| ≥50 years | 51 (45%) |
| Disease-free interval | |
| <2 years | 41 (36%) |
| ≥2 years | 72 (64%) |
| Site(s) of initial recurrence | |
| Any CNS | 9 (8%) |
| Any liver/lung but no CNS | 66 (58%) |
| Any other site but no CNS liver or lung | 16 (14%) |
| Local/regional only | 22 (19%) |
| Number of distant metastases at initial diagnosis | |
| 0 | 22 (19%) |
| 1 | 42 (37%) |
| ≥2 | 49 (43%) |
| Trastuzumab administered in the 1st line metastatic setting | 73 (65%) |
Abbreviations: ER, estrogen receptor; PR, progesterone receptor; CNS, central nervous system.
Sites of recurrence
The median number of initial site(s) of recurrence was 2 (range 1–5) with a total number of site(s) from metastatic disease diagnosis to last follow-up was 4 (range 1–6). Lung/pleura (55 patients, 49%), bone (46 patients, 38%) and liver (42 patients, 41%) were the most common sites of first recurrence. These locations were also the most common sites of metastases by time of death or last follow-up, with 109 (77%) patients having disease located in the lung/pleura, 102 (72%) patients with metastases in the bone, and 109 (77%) patients with liver tumors.
CNS disease
Only 9 (8%) patients had CNS disease at first diagnosis of metastatic disease, while 62 (55%) patients developed CNS disease in the time period from diagnosis of first recurrence to time of death or last follow-up. Among the 64 patients who were alive at least 3 years after start of first-line metastatic therapy, 30 women were diagnosed with CNS disease. Of 34 patients who survived ≥5 years from initiation of metastatic treatment, 11 had developed metastases to the brain. In patients without CNS relapse at initial diagnosis of recurrence, relatively few brain metastases were diagnosed within the first year after initiation of first-line meta-static therapy and events were dispersed widely over time; the distribution of patients by time of CNS diagnosis is listed in Table 2.
Table 2.
Distribution of CNS metastasis in HER2-positive breast cancer patients over specific time intervals from initiation of first-line metastatic therapy.
| Time interval from initiation of first-line treatment for metastatic diseasea | Total number of patients | Number of patients diagnosed with CNS metastasis
|
|||
|---|---|---|---|---|---|
| ≤1 year | 1–2 years | 2–3 years | >3 years | ||
| Patients alive ≥3 years | 64 | 3 | 10 | 8 | 9 |
| Patients alive ≥5 years | 34 | 2 | 10 | 3 | 5 |
Abbreviations: CNS, central nervous system.
Time of CNS metastasis was defined as the date of earliest diagnosis of CNS disease after the start of first-line therapy for metastatic breast cancer.
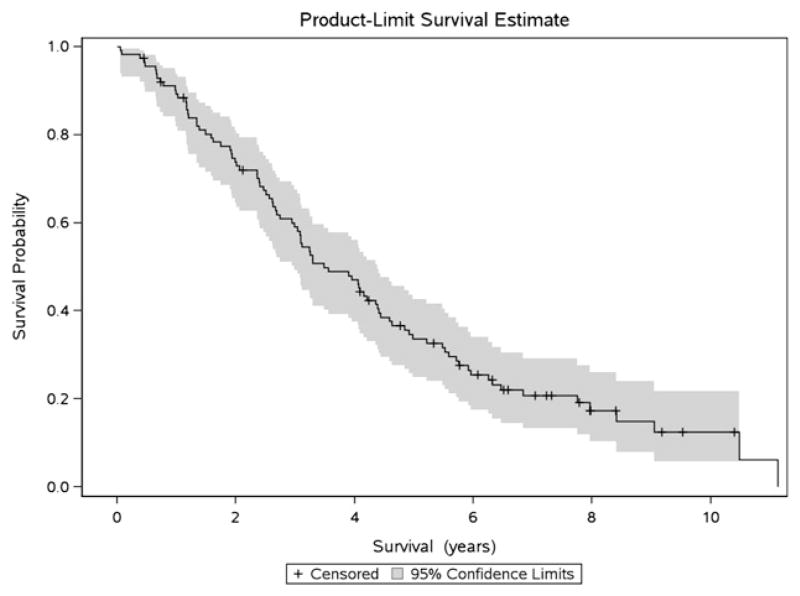
Overall survival
The median overall survival (OS) was 3.5 years (CI 3.0–4.4; Fig. 2). Deaths occurred continuously over time and did not follow a bimodal distribution, with similar HR for death 1, 3 and 5 years (Fig. 3). In univariate analyses, differences in OS were not observed by era of initial diagnosis, age at first recurrence, hormone receptor status, exposure to any adjuvant therapy or administration of trastuzumab in the first-line metastatic setting (Table 3); however, due to sample size this analysis may be underpowered to draw significant conclusions. OS was shorter in patients with a disease-free interval of <2 years (3.0 years, CI 1.6–3.9) compared to ≥2 years (4.4 years, CI 3.1–5.5; p = 0.03). Patients with ≥2 distant sites of disease at diagnosis of recurrence also demonstrated worse outcomes (2.6 years, CI 2.0–3.3) compared to patients with only 1 site (4.1 years, CI 3.2–6.0) or local recurrence only (4.6 years, CI 2.7–8.4, p = 0.01). CNS disease at first recurrence was associated with a shorter OS compared to patients with liver and/or lung metastases or other sites (CNS: 1.9 years CI 0.1–5.9, liver/lung: 3.2 years CI 2.5–4.2, other: 4.6 years CI 2.7–8.0; p = 0.05). These variables were not associated with survival in multivariate analysis.
Fig. 2.
Kaplan–Meier product-limit overall survival estimate for all patients in cohort.
Fig. 3.
Hazard function of death for all patients in cohort.
Table 3.
Predictors of overall survival from initiation of first-line metastatic therapy to death or last follow-up for all patients in cohort.
| Variable | n | Deaths | Univariate
|
Multivariatec
|
||
|---|---|---|---|---|---|---|
| Median OS (95% CI)a | pb | Hazard ratio (95% CI) | p | |||
| Overall | 113 | 90 | 3.5 (3.0–4.4) | |||
| Era of initial diagnosis | ||||||
| <1999 | 57 | 50 | 4.2 (3.1–5.2) | 0.47 | ||
| ≥1999 | 56 | 40 | 3.1 (1.9–4.2) | |||
| Age at first recurrence | ||||||
| <50 years | 62 | 51 | 4.1 (2.9–4.6) | 0.39 | ||
| ≥50 years | 51 | 39 | 3.2 (2.6–4.6) | |||
| ER and/or PR status | ||||||
| Positive | 64 | 55 | 4.2 (3.1–5.0) | 0.39 | ||
| Negative | 49 | 35 | 3.0 (1.7–4.1) | |||
| Site(s) of initial recurrence | ||||||
| Any CNS | 9 | 8 | 1.9 (0.1–5.9) | 0.05 | 2.1 (0.7–6.0) | 0.18 |
| Any liver/lung but no CNS | 66 | 57 | 3.2 (2.5–4.2) | 1.4 (0.7–2.9) | 0.34 | |
| Any other site but no CNS liver or lung | 16 | 12 | 4.6 (2.7–8.0) | 0.9 (0.4–2.3) | 0.91 | |
| Local/regional only | 22 | 13 | 4.6 (2.7–8.4) | Reference | ||
| Number of distant metastases at diagnosis of recurrent disease | ||||||
| 0 | 22 | 13 | 4.6 (2.7–8.4) | 0.01 | Referenced | |
| 1 | 42 | 33 | 4.1 (3.2–6.0) | |||
| ≥2 | 49 | 44 | 2.6 (2.0–3.3) | 1.5 (0.9–2.7) | 0.14 | |
| Disease-free intervale | ||||||
| <2 years | 41 | 33 | 3.0 (1.6–3.9) | 0.03 | ||
| ≥2 years | 72 | 57 | 4.4 (3.1–5.5) | |||
| Exposure to adjuvant trastuzumab | ||||||
| Yes | 11 | 10 | 2.0 (1.0–4.6) | 0.10 | ||
| No | 102 | 80 | 3.9 (3.1–4.4) | |||
| Exposure to adjuvant hormonal therapy | ||||||
| Yes | 50 | 39 | 3.9 (2.6–5.5) | 0.74 | ||
| No | 63 | 51 | 3.5 (2.7–4.4) | |||
| Exposure to adjuvant or neoadjuvant chemotherapy | ||||||
| Yes | 93 | 73 | 3.5 (2.6–4.4) | 0.88 | ||
| No | 20 | 17 | 4.1 (2.7–5.5) | |||
| Trastuzumab administered in the first-line metastatic setting | ||||||
| Yes | 73 | 58 | 3.1 (2.4–4.2) | 0.18 | ||
| No | 40 | 32 | 4.6 (3.1–5.9) | |||
Abbreviations: CI, confidence interval; ER, estrogen receptor; PR, progesterone receptor; CNS, central nervous system.
Survival times were evaluated using Kaplan–Meier methods.
Group comparisons in the univariate analysis were based on long-rank test.
Hazard ratios and 95% CI were obtained using a Cox proportional hazard model. Adjustments in model included era of initial diagnosis, age at first recurrence, year of initiation of first-line therapy, ER and/or PR status, and disease-free interval.
Reference value for the multivariate analysis was 0–1 sites of distant metastatic disease at initial diagnosis compared to ≥2 site of distant metastatic disease.
As defined from date of initial diagnosis to diagnosis of recurrent disease.
Patterns of care
The median number of total metastatic regimens was 5 (range 1–16, Fig. 4a). The general treatment approach in the population studied included the use of multiple lines of trastuzumab therapy; with a median number of trastuzumab-based regimens in the metastatic setting of 3 (range 1–12). Four or more HER2-based regimens were given to 34% of patients. Trastuzumab with vinorelbine was the most common metastatic trastuzumab-based therapy with 94 (83%) patients followed by trastuzumab combined with a taxane at 58 (51%) patients. Sixteen (14%) of the patients received lapatinib-based therapy, likely reflecting the time period of the study. Median duration of each line of therapy ranged from a minimum of 5.2 months to a maximum of 7.2 months (Fig. 4b). There did not appear to be a difference in the duration of therapy according to line of treatment, but the absolute number of patients who received each successive line of therapy decreased, therefore limiting any direct comparisons based on line of treatment.
Fig. 4.
a) Distribution of all patients by total number of metastatic regimens administered over the course of the follow-up period. b) Median duration of therapy on each metastatic regimen from the first-line of therapy to up to 6 lines of therapy. Duration of therapy was defined from initiation of treatment to initiation of subsequent regimen; for patients continuing on treatment, times were censored at date of last visit.
Discussion
Trastuzumab-based therapy has improved the survival for patients with HER2-positive metastatic breast cancer.1–5 Studies on the outcomes of this patient population prior to the wide spread use of trastuzumab are likely not applicable in the modern setting, and reexamining the clinical outcomes, practice patterns and predictors of survival in this patient population is essential for prognostication and clinical decision making.
Overall survival was 3.5 years (95% CI 3.0–4.4) and is higher than those demonstrated in the pivotal trastuzumab-based trials.1 Our results are similar to other population-based studies demonstrating a significant improvement in survival with modern regimens;20 suggesting that in the post-trastuzumab era, outcomes of patients with HER2-positive MBC are similar to those with ER-positive, HER2-negative MBC.5 We originally hypothesized that individuals with HER2-amplified MBC would segregate into groups of long- and short-term survivors, based on inherent trastuzumab responsiveness. Instead, the hazard rate of death did not identify unique groups of patients, suggesting that trastuzumab-based therapies may have varied efficacy across the HER2-positive patient population. This exploratory analysis results must be interpreted with caution, as this study was not powered to identify subgroups by hazard rate alone. CNS disease, number of sites of disease and DFI were associated with OS in univariate analysis, however, we did not observe an association after adjustment for potential confounders. Although the study’s ability to detect meaningful associations is limited by sample size, our observations propose that clinical factors alone may not allow for adequate prognostication and speaks to the need for scientific investigation into biologically-based predictors of outcome.
Patients in our study received multiple lines of therapy, with a median of 3 (range 1–12) trastuzumab-based regimens in the metastatic setting. Over one third of patients received ≥4 lines of HER2-based therapy in the metastatic setting. There did not appear to be a difference in the duration of therapy according to line of treatment, however, the absolute number of patients who received each successive line of therapy decreased and limits any direct comparisons. It is hypothesized that HER2-positive breast cancer in the post-trastuzumab era may behave differently than the traditional oncologic paradigm in which the likelihood of clinical benefit diminishes with each subsequent line of anti-cancer treatment.21 For example, in patients with triple negative breast cancer, Kassam and colleagues report that the median duration of first-line therapy is close to 3 months but decreases to 1 month on third-line chemotherapy.22 Acknowledging that patients with a good prognosis may be alive longer and may have a higher likelihood of response to multiple sequential regimens, our observation highlights a major clinical challenge in caring for patients with HER2-positive MBC. If the sixth-line therapy has potentially similar efficacy as the second-line therapy, then what clinical and/or biological factors should clinicians use to guide recommendations for additional cancer-directed treatment versus a focus on palliative care?
CNS involvement at initial metastatic presentation was relatively uncommon, occurring in only 8% of patients. Our results are similar to other published work reporting an incidence of CNS disease as first site of relapse of 2–9% in HER2-positive woman compared to 1–2% of patients with HER2-negative disease.17,23 In contrast to other studies where one third of patients have CNS disease at time of death,24 we found that over half (55%) of the patients developed CNS metastases during their disease course. Of note, we report the numbers of patients who developed CNS relapse with continued risk of CNS progression at multiple time intervals after treatment with first-line metastatic therapy. Exposure to trastuzumab appears to lead to an increased risk of CNS metastases, revealing the brain as a sanctuary site for cancer growth due to trastuzumab’s inability to cross the blood brain barrier.25 While our analyses are descriptive in nature and include a small number of patients, these data could suggest a process of continuous seeding of the brain and raise doubts about the success of trials using one-time interventions (e.g. prophylactic cranial irradiation) to prevent brain metastases over a patient’s lifetime.
Our study has several limitations. The results must be interpreted within the confines of a single institution, retrospective study and are intended to be hypothesis generating. Approximately one half of the patients identified were initially diagnosed before 1999 –prior to the start of routine HER2 testing at DFCI –so these patients were deemed HER2-positive based on testing at the time of recurrence rather than at initial diagnosis. There is an unavoidable selection bias in that patients who were initially diagnosed before 1999 (and survived long enough to be tested for HER2 in the metastatic setting) were more likely to be included, while patients with a similar initial diagnosis but a shorter survival time were less likely to be included. To account for this potential bias, patients were subdivided by era of initial diagnosis, and there was no difference observed in patient outcomes when controlling for a diagnosis before or after 1999. We acknowledge that selection bias may be present and controlling for era of diagnosis may not be sufficient to eliminate this bias. We are aware that model estimates obtained here are likely to change over time as the percentage of patients who have HER2 testing at the time of initial diagnosis approaches 100%. On the other hand, the study was conducted within an academic referral center and therefore we purposely excluded patients who were referred after they had received therapy for metastatic breast cancer. This potentially makes our study more generalizable by reducing the potential for referral bias, as one might expect that patients referred for “nth line” chemotherapy trials might be systematically different in prognostic or treatment variables.
As this was not a randomized prospective study, we cannot determine whether continued HER2-directed therapy beyond progression was causally related to the duration of therapy or OS when compared to an approach of cytotoxic chemotherapy alone. However, the EGF104900 trial indicated an improvement in PFS and OS among heavily refractory patients treated with trastuzumab in addition to lapatinib –compared to lapatinib alone –and supports the hypothesis that continued HER2 blockade is of important value.11,26 Additionally, because of our interest in long-term survival outcomes, we did not include patients diagnosed with metastatic disease after 2005. The use of HER2-directed therapy has continued to increase since that time, as has the administration of additional agents (e.g. neratinib, afatinib, TDM1, pertuzumab) as part of clinical trials. Today, sequential HER2-targeted therapies utilized post-progression is common practice and may result in even greater impact on survival. As the options for patients with HER2-positive disease expand, it is likely that the natural history of HER2-amplified breast cancer will continue to evolve.
In summary, our study demonstrates that in the modern setting, post-approval investigation into the outcomes of patients treated with targeted agents by breast cancer subtype is critical in order to fully understand the impact of such drugs on the natural history of breast cancer over time. In our series, the median survival of patients with HER2-amplified metastatic breast cancer now exceeds 3 years, suggesting that patients may continue to benefit from therapies in the third-line setting and beyond. As patients live longer, the incidence of CNS metastases in this patient population is continuing to increase, and remains an important clinical problem. Clinical factors are unfortunately insufficient to adequately predict prognosis in patients, emphasizing that the analysis of metastatic tumor samples from HER2-amplifed patients should be heavily prioritized for further progress in the field.27
Acknowledgments
EMO is supported by Translational Grant No. K12 CA 133250 in experimental therapeutics from the National Cancer Institute.
Footnotes
Conflict of Interest Statement
All other authors have no conflicts of interest.
Ethical approval
The study was approved by the Institutional Review Board at the Dana-Faber/Harvard Cancer Center.
Disclosures
NUL and EPW receive research support from Genentech Inc.
References
- 1.Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344(11):783–92. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 2.Cobleigh MA, Vogel CL, Tripathy D, Robert NJ, Scholl S, Fehrenbacher L, et al. Multinational study of the efficacy and safety of humanized anti-HER2 monoclonal antibody in women who have HER2-overexpressing metastatic breast cancer that has progressed after chemotherapy for metastatic disease. J Clin Oncol. 1999;17(9):2639–48. doi: 10.1200/JCO.1999.17.9.2639. [DOI] [PubMed] [Google Scholar]
- 3.Vogel CL, Cobleigh MA, Tripathy D, Gutheil JC, Harris LN, Fehrenbacher L, et al. Efficacy and safety of trastuzumab as a single agent in first-line treatment of HER2-overexpressing metastatic breast cancer. J Clin Oncol. 2002;20(3):719–26. doi: 10.1200/JCO.2002.20.3.719. [DOI] [PubMed] [Google Scholar]
- 4.Burstein HJ, Kuter I, Campos SM, Gelman RS, Tribou L, Parker LM, et al. Clinical activity of trastuzumab and vinorelbine in women with HER2-overexpressing metastatic breast cancer. J Clin Oncol. 2001;19(10):2722–30. doi: 10.1200/JCO.2001.19.10.2722. [DOI] [PubMed] [Google Scholar]
- 5.Dawood S, Broglio K, Buzdar AU, Hortobagyi GN, Giordano SH. Prognosis of women with metastatic breast cancer by HER2 status and trastuzumab treatment: an institutional-based review. J Clin Oncol. 2010;28(1):92–8. doi: 10.1200/JCO.2008.19.9844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marty M, Cognetti F, Maraninchi D, Snyder R, Mauriac L, Tubiana-Hulin M, et al. Randomized phase II trial of the efficacy and safety of trastuzumab combined with docetaxel in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer administered as first-line treatment: the M77001 study group. J Clin Oncol. 2005;23(19):4265–74. doi: 10.1200/JCO.2005.04.173. [DOI] [PubMed] [Google Scholar]
- 7.Robert N, Leyland-Jones B, Asmar L, Belt R, Ilegbodu D, Loesch D, et al. Randomized phase III study of trastuzumab, paclitaxel, and carboplatin compared with trastuzumab and paclitaxel in women with HER-2-overexpressing metastatic breast cancer. J Clin Oncol. 2006;24(18):2786–92. doi: 10.1200/JCO.2005.04.1764. [DOI] [PubMed] [Google Scholar]
- 8.Chan A. A review of the use of trastuzumab (Herceptin) plus vinorelbine in metastatic breast cancer. Ann Oncol. 2007;18(7):1152–8. doi: 10.1093/annonc/mdl476. [DOI] [PubMed] [Google Scholar]
- 9.Jatoi I, Anderson WF, Jeong J-H, Redmond CK. Breast cancer adjuvant therapy: time to consider its time-dependent effects. J Clin Oncol. 2011;29(17):2301–4. doi: 10.1200/JCO.2010.32.3550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.von Minckwitz G, du Bois A, Schmidt M, Maass N, Cufer T, de Jongh FE, et al. Trastuzumab beyond progression in human epidermal growth factor receptor 2-positive advanced breast cancer: a German breast group 26/breast international group 03–05 study. J Clin Oncol. 2009;27(12):1999–2006. doi: 10.1200/JCO.2008.19.6618. [DOI] [PubMed] [Google Scholar]
- 11.Blackwell KL, Burstein HJ, Storniolo AM, Rugo H, Sledge G, Koehler M, et al. Randomized study of Lapatinib alone or in combination with trastuzumab in women with ErbB2-positive, trastuzumab-refractory metastatic breast cancer. J Clin Oncol. 2010;28(7):1124–30. doi: 10.1200/JCO.2008.21.4437. [DOI] [PubMed] [Google Scholar]
- 12.Olson EM, Lin NU, Dipiro PJ, Najita JS, Krop IE, Winer EP, et al. Responses to subsequent anti-HER2 therapy after treatment with trastuzumab-DM1 in women with HER2-positive metastatic breast cancer. Ann Oncol. 2012;23(1):93–7. doi: 10.1093/annonc/mdr061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Isakoff SJ, Baselga J. Trastuzumab-DM1: building a chemotherapy-free road in the treatment of human epidermal growth factor receptor 2-positive breast cancer. J Clin Oncol. 2011;29(4):351–4. doi: 10.1200/JCO.2010.31.6679. [DOI] [PubMed] [Google Scholar]
- 14.Geyer CE, Forster J, Lindquist D, Chan S, Romieu CG, Pienkowski T, et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med. 2006;355(26):2733–43. doi: 10.1056/NEJMoa064320. [DOI] [PubMed] [Google Scholar]
- 15.Burstein HJ, Sun Y, Dirix LY, Jiang Z, Paridaens R, Tan AR, et al. Neratinib, an irreversible ErbB receptor tyrosine kinase inhibitor, in patients with advanced ErbB2-positive breast cancer. J Clin Oncol. 2010;28(8):1301–7. doi: 10.1200/JCO.2009.25.8707. [DOI] [PubMed] [Google Scholar]
- 16.Bendell JC, Domchek SM, Burstein HJ, Harris L, Younger J, Kuter I, et al. Central nervous system metastases in women who receive trastuzumab-based therapy for metastatic breast carcinoma. Cancer. 2003;97(12):2972–7. doi: 10.1002/cncr.11436. [DOI] [PubMed] [Google Scholar]
- 17.Lin NU, Winer EP. Brain metastases: the HER2 paradigm. Clin Cancer Res. 2007;13(6):1648–55. doi: 10.1158/1078-0432.CCR-06-2478. [DOI] [PubMed] [Google Scholar]
- 18.Freedman RA, Bullitt E, Sun L, et al. A phase II study of sagopilone (ZK 219477; ZK-EPO) in patients with breast cancer and brain metastases. Clin Breast Cancer. 2011;11(6):376–83. doi: 10.1016/j.clbc.2011.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1. 1) Eur J Cancer. 2009;45(2):228–47. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 20.Chia SK, Speers CH, D’Yachkova Y, Kang A, Malfair-Taylor S, Barnett J, et al. The impact of new chemotherapeutic and hormone agents on survival in a population-based cohort of women with metastatic breast cancer. Cancer. 2007;110(5):973–9. doi: 10.1002/cncr.22867. [DOI] [PubMed] [Google Scholar]
- 21.Olson EM. Maximizing human epidermal growth factor receptor 2 inhibition: a new oncologic paradigm in the era of targeted therapy. J Clin Oncol. 2012;30(14):1712–4. doi: 10.1200/JCO.2011.40.2545. [DOI] [PubMed] [Google Scholar]
- 22.Kassam F, Enright K, Dent R, Dranitsaris G, Myers J, Flynn C, et al. Survival outcomes for patients with metastatic triple-negative breast cancer: implications for clinical practice and trial design. Clin Breast Cancer. 2009;9(1):29–33. doi: 10.3816/CBC.2009.n.005. [DOI] [PubMed] [Google Scholar]
- 23.Chien AJ, Rugo H. Emerging treatment options for the management of brain metastases in patients with HER2-positive metastatic breast cancer. Breast Cancer Res Treat. 2012;137(1):1–12. doi: 10.1007/s10549-012-2328-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin NU, Eierman W, Greil R, Campone M, Kaufman B, Steplewski K, et al. Randomized phase II study of lapatinib plus capecitabine or lapatinib plus topotecan for patients with HER2-positive breast cancer brain metastases. J Neurooncol. 2011;105(3):613–20. doi: 10.1007/s11060-011-0629-y. [DOI] [PubMed] [Google Scholar]
- 25.Musolino A, Ciccolallo L, Panebianco M, Fontana E, Zanoni D, Bozzetti C, et al. Multifactorial central nervous system recurrence susceptibility in patients with HER2-positive breast cancer. Cancer. 2011;117(9):1837–46. doi: 10.1002/cncr.25771. [DOI] [PubMed] [Google Scholar]
- 26.Blackwell K, Burstein H, Sledge G, Stein S, Ellis C, Casey M, et al. Updated survival analysis of a randomized study of lapatinib alone or in combination with trastuzumab in women with HER2-positive metastatic breast cancer progressing on trastuzumab therapy. San Antonio breast conference symposium; San Antonio, TX. 2009. [Google Scholar]
- 27.Olson EM, Lin NU, Krop IE, Winer EP. The ethical use of mandatory research biopsies. Nat Rev Clin Oncol. 2011;8(10):620–5. doi: 10.1038/nrclinonc.2011.114. [DOI] [PMC free article] [PubMed] [Google Scholar]