SUMMARY
OBJECTIVE
To investigate spatial patterns of the incidence of pulmonary tuberculosis (TB) and its relationship with socio-economic status in Vitoria, Espirito Santo, Brazil.
DESIGN
In a 4-year, retrospective, territory-based surveillance study of all new pulmonary TB cases conducted in Vitoria between 2002 and 2006, spatial patterns of disease incidence were compared using spatial clustering statistics (Anselin’s local indicators of spatial association [LISA] and Getis-Ord Gi* statistics), smoothed empirical Bayes estimates and model-predicted incidence rates. Spatial Poisson models were fit to examine the relationship between socio-economic status and TB incidence.
RESULTS
A total of 651 TB cases were reported across 78 neighborhoods, with rates ranging from 0 to 129 cases per 100 000 population. Moran’s I indicated strong spatial autocorrelation among incidence rates (0.399, P < 0.0001), and four areas of high incidence were identified by LISA and Gi* statistics. Smoothed spatial empirical Bayes estimates demonstrate that two of these areas range from 70 to 90 cases/100 000, while the other two range from 40 to 70 cases/100 000. TB incidence and socioeconomic status had a significant curvilinear relationship (P = 0.02).
CONCLUSIONS
Data derived from these spatial statistical tools will help TB control programs to allocate TB resources to those populations most at risk of increasing TB rates and to target areas where TB control efforts need to be concentrated.
Keywords: tuberculosis, prevention control, epidemiologic surveillance, spatial distribution
BRAZIL’S Ministry of Health (MoH) has estimated that over the last two decades more than 42 million people have become infected with Mycobacterium tuberculosis, and that there have been 16 million cases of tuberculosis (TB) and 112 000 deaths due to TB.1,2 More recently, there has been growing concern about the emergence of multidrug resistance in the community, stimulating local government health departments to prioritize the improvement of their TB control programs.
Traditionally, TB control efforts are primarily directed towards the detection and treatment of individuals with active TB. Treatment of latently infected contacts of pulmonary TB cases and targeted screening of groups at high risk of infection are important but secondary objectives.3,4 Although these methods have had varying degrees of success, the continuing high TB rates indicate that there is a need to study how TB control programs can be improved.
In 2004, the Brazilian National Tuberculosis Control Program (NTP) designated 315 cities as priority cities for surveillance, control and prevention of TB.5 Before 2004, the NTP was a vertical system, starting with the National TB Center, then the county and district level control programs and, finally, the specialized TB dispensaries. In 2004, the Brazilian MoH implemented a new policy incorporating TB services into the basic family heath units, which are located throughout Brazil and are the primary public health providers for the majority of Brazilians.1,5
Over the past 10 years TB incidence has remained unchanged in Espirito Santo State, Brazil.3,6 One hypothesis for this lack of success is that although DOTS has been implemented in the family health units, little has been done to actively detect TB in high-risk populations. In 2000, the University of Espirito Santo established a laboratory network to improve TB diagnosis in collaboration with the State health department using standardized training of laboratory personnel and use of culture for the diagnosis of TB suspects. As a part of this strategy, an information system based on Lotus Notes™, entitled TB-Notes (IBM, Armonk, NY, USA), was created. This network includes four municipalities that comprise 70% of TB cases in the State, including Vitoria, the State capital.
Although TB is not distributed uniformly throughout Vitoria, the NTP does not provide services based on those areas with the greatest incidence. As rates in Vitoria vary considerably between neighborhoods (in 2005, TB ranged from 26 to 84 per 100 000 population),6 a uniform approach to TB control is not the most efficient or effective approach. Recent studies have suggested problems with treating an entire region with one control strategy, rather than targeting high-risk areas with more effective control measures.6–12
Research to evaluate the spatial distribution of TB and to identify high-risk areas is limited,6,10 especially in developing countries.13 While it is known that control efforts are best designed when areas of high incidence are known, it is also important to identify areas where rates are abnormally high given underlying risk factors. In particular, territory-based surveillance systems have demonstrated that the distribution of endemic diseases is also determined by social processes that are intrinsically related to the space where they occur.6 Tools in spatial statistics have advanced our understanding of the geographic distribution of disease and improved the focus of public health actions.8–13 In this study, we investigated the inner city spatial patterns of pulmonary TB incidence and the relationship between TB and socio-economic status, and used these data to identify those areas in the city of Vitoria that are at risk of highest TB incidence.
METHODS
Study design and site
A 4-year, retrospective, territory-based surveillance study of all new pulmonary TB cases was conducted in Vitoria City between 2002 and 2006. Vitoria, the capital of Espirito Santo State, has a population of 300 000 and an area of 93 381 km2, divided between insular and continental regions. The city is comprised of 78 municipalities (neighborhoods), with a population density that varies from 6.18 to 236.01/km2.14 Using geographic information systems (GIS), TB cases were geo-coded to the neighborhood level and surveillance records were reviewed to avoid misclassification.
Socio-economic status
The index of quality of urban municipality (IQU) was computed for each neighborhood. The IQU, developed by the Instituto Pólis (São Paulo, SP, Brazil),14 is a simple arithmetic mean with 11 variables and a range between 0 and 1, with 1 being the highest level. The IQU was based on the 2000 census, and classifies neighborhoods according to four dimensions: 1) educational dimension: percentage (%) of illiteracy in persons aged >15 years; percentage (%) of heads of households with <4 years of education; percentage (%) of persons responsible for households with ≥15 years of education; 2) environmental dimension: percentage (%) of houses with adequate water supply; percentage (%) of houses with adequate sewage network; percentage (%) of houses with adequate public garbage collection; 3) house dimension: mean number of persons living in the household; mean number of bathrooms in the household; 4) monetary dimension: mean income of household heads in minimum monthly salary (US$200.00); percentage (%) of households with at least two minimum salaries; percentage (%) of households with more than 10 minimal salaries. For this analysis, the IQU was used to represent socioeconomic status, a more commonly used term.
Spatial analysis
Incidence rates were calculated for each neighborhood, using neighborhood census data as denominators and reported TB cases as the numerator. We chose the term ‘incidence rate’ rather than ‘case detection rate’ for easier comparison with the terminology used by the Brazilian NTP. Global spatial correlation was assessed using Moran’s Index (I), while priority TB control neighborhoods were identified by Anselin’s local indicators of spatial autocorrelation (LISAs) and the Getis-Ord Gi*-statistic using raw rates.8,9,11 LISA statistics were run using eight conceptualizations of spatial relationship: inverse distance, inverse distance squared, 2000 m threshold zone of indifference, first-order polygon contiguity (defined in ArcGIS 9.3), and first- and second-order definitions of rook and queen contiguity (defined in GeoDa 0.9.5). The Gi* statistic used the first four conceptualizations listed above. These 12 results were combined to identify the areas most likely to be centers (or hollows) of disease incidence. As indicated, the data were analyzed in ArcGIS and GeoDa.
In addition to clustering statistics applied to raw rates, smoothed disease rates using global and spatial empirical Bayes estimates were computed to provide a better understanding of the true disease burden and community risk. These methods reduce noise in the rates caused by different population sizes and increase our ability to discern systematic patterns in the spatial variation of the underlying risk.7,11,15 Global empirical Bayes (GEBayes) produces smoothed rates that account for the mean across all neighborhoods, while smoothed spatial empirical Bayes estimates (SEBayes, also known as local empirical Bayes) account for the mean among spatial neighbors. TerraView 3.1.4 was used to compute GEBayes and SEBayes estimates (Instituto Nacional de Pesquisas Espaciais, São José dos Campos, Brazil; http://www.dpi.inpe.br/terraview).
Finally, TB incidence was modeled as a function of socio-economic status to investigate the importance of social risk factors. We used a random effects Poisson model with a log-link function for the outcome (number of TB cases), municipality census population as the offset (population at risk), and an exponential semivariogram to model the variance of the random effects to account for spatially correlated observations. An exponential spatial variogram is a covariance function between two observations that depends on the distance, dij, between them.16 The model was fit via Gaussian Adaptive Quadrature to ensure efficiency and accuracy of estimates.17,18 To evaluate the relationship between socio-economic status and TB, a curvilinear relationship up to a cubic term for IQU was evaluated (quadratic and cubic terms were first centered about the IQU mean) as well as values for latitude and longitude that help control for small-scale spatial variation. We used a supervised backward elimination method to select model variables using both the Akaike’s information criterion (AIC) and Bayesian information criterion (BIC) statistics as criteria (smaller values considered better fit) with the full model written:
Each beta estimate is interpreted as the increase in log-incidence for a one unit increase in its respective variable, where b1, b2 and b3 are the beta parameters for the IQU (linear, quadratic, cubic), and b4 and b5 are beta parameters for latitude and longitude, respectively. The model was fit using PROC GLIMMIX in SAS 9.2 (SAS Institute, Cary, NC, USA). Thematic maps are presented to show the distribution of raw rates, the high/low incidence clusters, smoothed disease rates, model-predicted rates, and disease clusters of the model predicted rates.
The study was approved by the Institutional Review Boards at Universidade Federal do Espírito Santo.
RESULTS
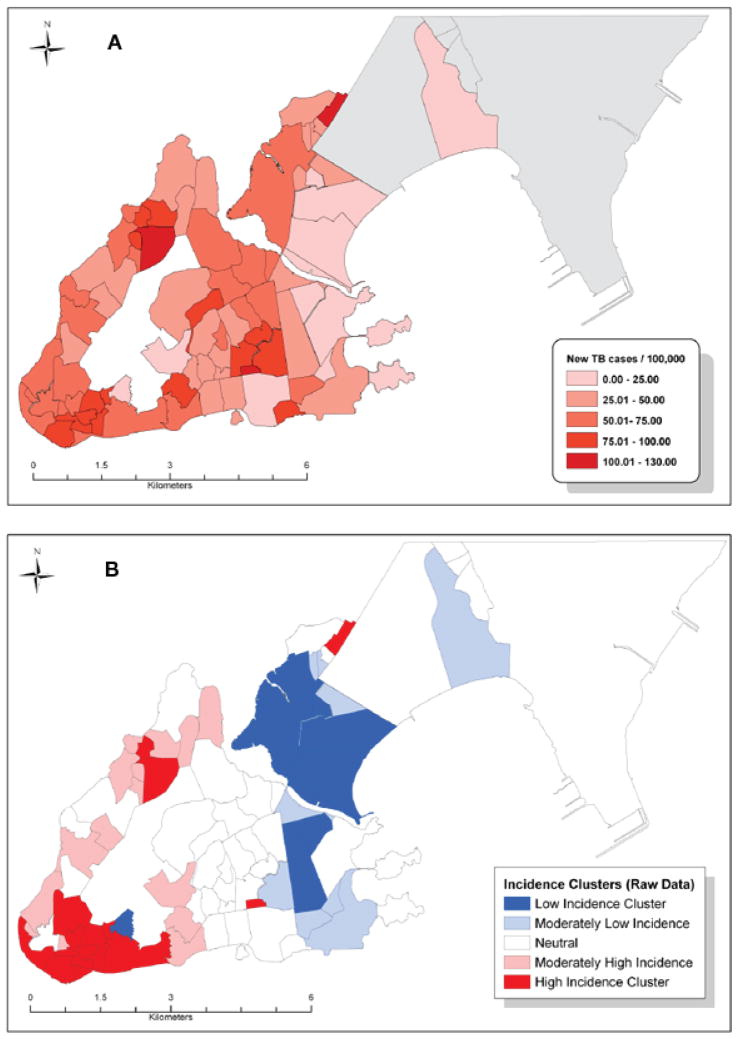
Between 2002 and 2006, 651 new cases of pulmonary TB were reported in Vitoria, with heterogeneity across the 78 neighborhoods ranging from 0 to 129 cases/100 000 (Figure 1A). The mean IQU was 0.58 (standard deviation = 0.124), with a range of 0.34 to 0.84 and high inner-city inequality. Figure 1A shows some areas with high-incidence rate clustering, although areas of clustered neighborhoods were not directly connected. The areas in the North and East coast of the municipality had the lowest incidence rates during this period. Moran’s I (0.399, P < 0.0001) indicated strong spatial autocorrelation between incidence rates in the city. LISA and Gi* statistics computed on the raw data identify four areas of high TB incidence, with rates ranging from 100 to 130 cases/100 000 and three areas of low TB incidence (0–50 cases/100 000; Figure 1B). Neighborhoods in the high-incidence clusters (in red) all share common TB risk factors: low-income, slums and overcrowding.
Figure 1.
A. Distribution of TB cases, Vitoria, Espirito do Santo, 2002–2006. B. High- and low-incidence clusters, Vitoria, 2002–2006. TB = tuberculosis. This image can be viewed online in color at http://www.ingentaconnect.com/content/iuatld/ijtld/2010/00000014/00000011/art00007
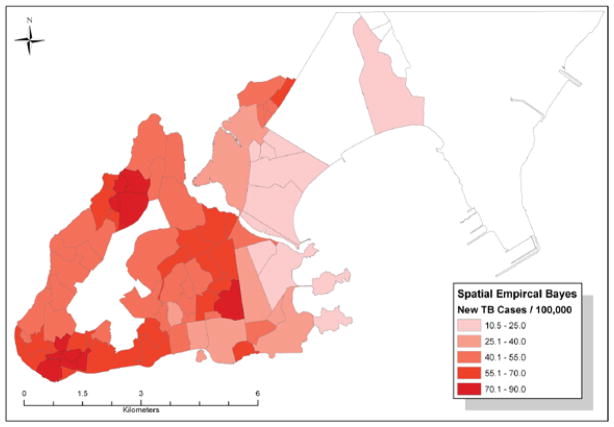
The distribution of smoothed disease rates via GEBayes and SEBayes were nearly identical. SEBayes estimates are displayed in Figure 2. Areas previously identified as high-disease clusters have slightly lower values: the two highest areas have rates ranging from 70 to 90 cases/100 000, while the rates in the other two high-incidence areas range from 40 to 70 cases/100 000. The low-incidence areas remain low (10–40 cases/100 000), except for one neighborhood in the southwest, which has a smoothed rate of 40–55 cases/100000.
Figure 2.
Spatial empirical Bayes estimates of TB cases, Vitoria, 2002–2006. TB = tuberculosis. This image can be viewed online in color at http://www.ingentaconnect.com/content/iuatld/ijtld/2010/00000014/00000011/art00007
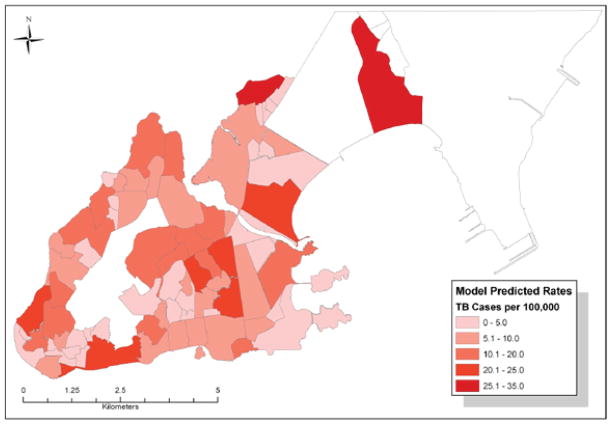
The final Poisson regression model includes up to a cubic term for IQU and latitude, which resulted in an AIC of 373.6 and a BIC of 390.1, and demonstrated good fit to the data (Figure 3). Model estimates are shown in the Table, and predicted values in Figure 4. The linear term for IQU indicates an overall inverse trend between IQU and TB incidence; however, the significant quadratic and cubic terms demonstrate a curvilinear relationship between IQU and TB (Figure 3). An F-test to evaluate the significance of all three IQU terms provided a P value of <0.0001, indicating the importance of the IQU in predicting TB incidence. The inclusion of latitude suggests a significant north-south trend in incidence rates, which may be due to variables not measured in our study. The model-predicted estimates in Figure 4 have a range of 0–35 TB cases/100 000, which is significantly lower than the raw rates in Figure 1A due to model correction of the estimates for the underlying risk caused by IQU and adjustment of these estimates for spatial trends. Clustering statistics (LISA and Gi*) were again computed for these predicted rates to identify areas where TB incidence is higher or lower than expected.
Figure 3.

Poisson regression predicted TB incidence for IQU, Vitoria, 2002–2006. IQU was used as a measure of socioeconomic status. IQU = index of quality of urban municipality; TB = tuberculosis.
Table.
Spatial Poisson model estimates
| Variable | Range | Beta | 95%CI | P value |
|---|---|---|---|---|
| IQU | 0.35–0.85 | −0.70 | −2.27–0.88 | 0.3895 |
| IQU2 | 0.12–0.72 | −10.42 | −16.76– −4.08 | 0.0019 |
| IQU3 | 0.04–0.62 | −55.11 | −103.24– −6.97 | 0.0279 |
| Latitude | 0–7.5 | −0.06 | −0.10– −0.02 | 0.0033 |
| Constant | −6.88 | −7.76– −6.01 | <0.0001 |
CI = confi dence interval; IQU = index of quality of urban municipality.
Figure 4.
Model of predicted TB incidence, Vitoria, 2002–2006. TB = tuberculosis. This image can be viewed online in color at http://www.ingentaconnect.com/content/iuatld/ijtld/2010/00000014/00000011/art00007
DISCUSSION
Our results show the inequality of TB disease distribution in Vitoria, and indicate areas at risk of a more severe TB epidemic in the future. Neighborhood incidence was highly correlated with a decreasing socioeconomic index, detecting those areas with most severe TB rates.
Poverty and TB have long been linked in the industrialized world.19–23 In the TB epidemic in New York City in the early 1990s, neighborhood poverty was strongly associated with TB incidence, with the association particularly evident in the severely impoverished areas.23 Although this association has been well documented in industrialized countries, where socio-economic status is often intertwined with race and ethnicity, in Brazil it is not so clearly correlated due to the complexity of racial classification.24
The relationship between TB and poverty is multi-faceted. Individuals with lower socio-economic status are at greater risk of becoming infected with TB and reactivation of TB disease; however, much of this risk may be attributable to poorer access to health care services. The probability of being diagnosed and receiving proper treatment is related to the quality of the TB control programs, which are often substandard in areas of lowest socio-economic status.19,23 In a recent editorial, Waaler stated that the health services’ fight against TB should continue with increased intensity, but at the same time they should acknowledge that the eradication of the TB problem is characterized by poverty and inequality worldwide.25
In a study mapping notified cases in Recife, Brazil, significant associations with tuberculosis incidence were found for the average number of inhabitants per household, the existence of families with more than one TB case during the study period, and the presence of retreatment cases.26 These factors impact TB incidence in an area and should be monitored at local level by the TB surveillance system. The authors call for control programs to focus on small areas with high priority for intensive intervention which can be applied using this methodology.26
Although our study did not combine molecular genotyping and GIS technologies, the geographic clusters detected need to be investigated to determine if they are predominantly due to recent transmission or reactivation of latent infection. Molecular genotyping will significantly augment the GIS by further narrowing in on specific recent transmission, as in other studies.9,27,28
Epidemiologic inference using disease incidence or mortality maps assumes that risk estimates displayed in maps are appropriate approximations of actual risk levels in the corresponding geographical areas.9,28–30 A number of methodological developments over the last two decades (used in our analysis) have made this assumption more plausible.8,31
Our data confirmed that empirical Bayes estimates are indeed substantial improvements over crude incidence rate estimates when estimating disease risk in small areas.8,31 It is well-established that Bayes procedures offer a trade-off between bias and variance reduction of the estimates and, particularly in cases where the sample size is small, have been shown to produce a set of point estimates that have good properties in terms of minimizing squared error loss.32 Although our results reveal large differences in incidence across neighborhoods, graphic investigation revealed important differences across the map and defined low- and high-risk areas more clearly.
The Moran’s I calculation showed there is an important autocorrelation in the city which is relevant to epidemiologic inference for the dynamic of disease transmission. These data will be useful in identifying high-risk areas for TB in Vitoria, thus providing a useful instrument for the structuring of a territory-based surveillance system, and thereby aiding to prioritize population groups for further interventions.9 Moreover, we were able to view the heterogeneity of the disease distribution in the urban space despite the inclusion of many small geographic areas. This makes it possible to plan interventions directed towards specific groups, as presented by the cluster analysis in LISA.7,8
Detection of TB hotspots can aid the TB control program in directing specific interventions towards those populations at greatest risk. Interventions may include targeted intensified case finding, contact investigations and provision of isoniazid preventive therapy. Intensive community case finding has rarely been used in Brazil, but a recent study reported increased detection using a door-to-door case detection approach in a high-incidence slum. Contact investigations have been greatly underutilized in Brazil despite reported successes of 2–4% detection among close contacts of TB cases, similar to studies conducted in other high-risk areas.33–36 Resources and political will for these interventions is currently low in Brazil, despite local successes. Our methodology can detect geographic areas and populations where these interventions may be most beneficial.
GIS is a powerful tool for TB control and prevention in developing countries, and should be used for real-time surveillance and decision making. Although TB has always been endemic in Brazil,37 our analysis emphasizes the connection between poverty and TB and the need to include poverty when determining city-wide TB control measures. Even when controlling for the spatial distribution of poverty, we still identified areas with high transmission. This means that, apart from poverty, there are other factors that cause elevated incidence of disease. Poverty does not explain the whole story. We do not have enough patient-level data to fully understand why these areas are hotspots. It is possible that undetected outbreaks may have increased rates, or that these areas have a high prevalence of social factors leading to increased transmission. However, the strength of our study is that these methods can identify spots where further investigation and interventions can be implemented. Although health departments can do little to alleviate poverty, detecting TB ‘hotspots’ can lead to targeted interventions in these populations.
Acknowledgments
The authors thank W M Ramalho from the Minister of Health in Brazil who contributed towards the study by teaching the use of the Terraview application; the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) by Edital MCT/CNPq/MS-SCTIE-DECIT N° 25/2006 Doenças negligenciadas and National Institute of Health on the ICHORTA Grant No. 5 U2R TW006883-02.
References
- 1.Ministério da Saúde. Guia para tratamento da tuberculose para o Programa de Saúde da Família. Brasília, Brazil: Ministério da Saúde; 2002. [Portuguese] [Google Scholar]
- 2.Ministério da Saúde. Sistema de informações sobre mortalidade. Brasília, Brazil: Ministério da Saúde; [Accessed September 2006]. http://www.datasus.gov.br. [Portuguese] [Google Scholar]
- 3.Ministério da Saúde. Série histórica de doenças de notificação compulsória por UF, 1980–2001. Brasília, Brazil: Ministério da Saúde; [Accessed September 2006]. http://www.funasa.gov.br/epi/epi00.htm. [Portuguese] [Google Scholar]
- 4.Advisory Council for the Elimination of Tuberculosis. Essential components of a tuberculosis prevention and control program recommendations of the Advisory Council for the Elimination of Tuberculosis. MMWR. 1995;44 (RR-11):1–16. [PubMed] [Google Scholar]
- 5.Conde MB, de Melo FA, Marques AM, et al. BTA Committee on Tuberculosis; BTA guidelines on Tuberculosis Work Group. III Brazilian Thoracic Association guidelines on tuberculosis. J Bras Pneumol. 2009;35:1018–1048. doi: 10.1590/s1806-37132009001000011. [DOI] [PubMed] [Google Scholar]
- 6.Vieira RC, Prado TN, Siqueira MG, Dietze R, Maciel EL. Spatial distribution of new tuberculosis cases in Vitória, state of Espírito Santo, between 2000 and 2005. Rev Soc Bras Med Trop. 2008;41:82–86. doi: 10.1590/s0037-86822008000100017. [DOI] [PubMed] [Google Scholar]
- 7.Assunção RM, Barreto SM, Guerra HL, Sakurai E. Mapas de taxas epidemiológicas: uma abordagem Bayesiana. Cad Saúde Pública. 1998;14:713–723. doi: 10.1590/s0102-311x1998000400013. [Portuguese] [DOI] [PubMed] [Google Scholar]
- 8.Anselin L. Local indicators of spatial association—LISA. Georg Anal. 1995;27:91–115. [Google Scholar]
- 9.Bailey TC, Gatrell AC. Interactive spatial data analysis. Harlow, UK: Longman Scientific & Technical; 1995. [Google Scholar]
- 10.Bishai WR, Graham NM, Harrington S, et al. Molecular and geographic patterns of tuberculosis transmission after 15 years of directly observed therapy. JAMA. 1998;280:1679–1684. doi: 10.1001/jama.280.19.1679. [DOI] [PubMed] [Google Scholar]
- 11.Bailey TC. Spatial statistical methods in health. Cad Saúde Pública. 2001;17:1083–1098. doi: 10.1590/s0102-311x2001000500011. [DOI] [PubMed] [Google Scholar]
- 12.Militino AF, Ugarte MD, Dean CB. The use of mixture models for identifying high risks in disease mapping. Stat Med. 2001;20:2035–2049. doi: 10.1002/sim.821. [DOI] [PubMed] [Google Scholar]
- 13.Verver S, Warren RM, Munch Z, et al. Transmission of tuberculosis in a high incidence urban community in South Africa. Int J Epidemiol. 2004;33:351–357. doi: 10.1093/ije/dyh021. [DOI] [PubMed] [Google Scholar]
- 14.Vitória ES. Prefeitura Municipal. Índice de Qualidade Urbana (IQU): bairros de Vitória; 1991 e 2000. Vitória, ES, Brazil: Co-ordenadoria de Planejamento; 2004. [Accessed September 2010]. http://legado.vitoria.es.gov.br/regionais/indicadores/iqu/iqu.asp. [Portuguese] [Google Scholar]
- 15.Druck S, Carvalho MS, Câmara G, Monteiro AVM, editors. Análise espacial de dados geográficos. Brasília, Brazil: Empresa Brasileira de Pesquisa Agropecuária; 2004. [Portuguese] [Google Scholar]
- 16.Getis A, Ord JK. The analysis of spatial association by use of distance statistics. Geographical Anal. 1992;24:189–206. [Google Scholar]
- 17.Littell RC, Milliken GA, Stroup WW, Wolfinger RD, Schabenberger O. SAS for mixed models. 2. Cary, NC, USA: SAS Institute; 2006. [Google Scholar]
- 18.Pinheiro JC, Chao EC. Efficient LaPlacian and adaptive Gaussian quadrature algorithms for multilevel generalized linear mixed models. J Comput Graph Stat. 2006;15:58–81. [Google Scholar]
- 19.Rom WN, O’Brien R, Spinaci S. Design and support for national tuberculosis programs. In: Rom WN, Garay SM, editors. Tuberculosis. Boston, MA, USA: Little, Brown; 1995. pp. 953–963. [Google Scholar]
- 20.Liestøl K, Tretli S, Tverdal A, Maehlen J. Tuberculin status, socio-economic differences and differences in all-cause mortality: experience from Norwegian cohorts born 1910–1949. Int J Epidemiol. 2009;38:427–434. doi: 10.1093/ije/dyn347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lopez De Fede A, Stewart JE, Harris MJ, Mayfield-Smith K. Tuberculosis in socio-economically deprived neighborhoods: missed opportunities for prevention. Int J Tuberc Lung Dis. 2008;12:1425–1430. [PubMed] [Google Scholar]
- 22.Cantwell MF, McKenna MT, McCray E, Onorato IM. Tuberculosis and race/ethnicity in the United States: impact of socioeconomic status. Am J Respir Crit Care Med. 1998;157 (4 Pt 1):1016–1020. doi: 10.1164/ajrccm.157.4.9704036. [DOI] [PubMed] [Google Scholar]
- 23.Barr RG, Diez-Roux AV, Knirsch CA, Pablos-Méndez A. Neighborhood poverty and the resurgence of tuberculosis in New York City, 1984–1992. Am J Public Health. 2001;91:1487–1493. doi: 10.2105/ajph.91.9.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bastos JL, Dumith SC, Santos RV, et al. Does the way I see you affect the way I see myself? Associations between interviewers’ and interviewees’ ‘color/race’ in southern. Brazil Cad Saude Publica. 2009;25:2111–2124. doi: 10.1590/s0102-311x2009001000003. [DOI] [PubMed] [Google Scholar]
- 25.Waaler HT. Tuberculosis and poverty. Int J Tuberc Lung Dis. 2002;6:745–746. [PubMed] [Google Scholar]
- 26.Souza WV, de Albuquerque MF, Barcellos CC, Ximenes RA, Carvalho MS. Tuberculosis in Brazil: construction of a territorially based surveillance system. Rev Saúde Publica. 2005;39:82–89. doi: 10.1590/s0034-89102005000100011. [Portuguese] [DOI] [PubMed] [Google Scholar]
- 27.McConkey SJ, Williams M, Weiss D, et al. Prospective molecular typing for tuberculosis. Clin Infect Dis. 2002;34:612–619. doi: 10.1086/338785. [DOI] [PubMed] [Google Scholar]
- 28.Yang ZH, Rendon A, Flores A, et al. A clinic-based molecular epidemiologic study of tuberculosis in Monterrey, Mexico. Int J Tuberc Lung Dis. 2001;5:313–320. [PubMed] [Google Scholar]
- 29.Kistemann T, Munzinger A, Dangendorf F. Spatial patterns of tuberculosis incidence in Cologne (Germany) Soc Sci Med. 2002;55:7–19. doi: 10.1016/s0277-9536(01)00216-7. [DOI] [PubMed] [Google Scholar]
- 30.Barr RG, Diez-Roux AV, Knirsch CA, Pablos-Méndez A. Neighborhood poverty and the resurgence of tuberculosis in New York City, 1984–1992. Am J Public Health. 2001;91:1487–1493. doi: 10.2105/ajph.91.9.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Clayton D, Kaldor J. Empirical Bayes estimates of age-standardized relative risks for use in disease mapping. Biometrics. 1987;43:671–681. [PubMed] [Google Scholar]
- 32.Carlin BP, Louis TA, editors. Bayes and empirical Bayes methods for data analysis. New York, NY, USA: Chapman & Hall/CRC; 2000. [Google Scholar]
- 33.Cavalcante SC, Durovni B, Barnes GL, et al. Community-randomized trial of enhanced DOTS for tuberculosis control in Rio de Janeiro, Brazil. Int J Tuberc Lung Dis. 2010;14:203–209. [PMC free article] [PubMed] [Google Scholar]
- 34.Gazetta CE, de Santos ML, Vendramini SH, Poletti NA, Pinto Neto JM, Villa TC. Tuberculosis contact control in Brazil: a literature review (1984–2004) Rev Latino-Am Enfermagem. 2008;16:306–313. doi: 10.1590/s0104-11692008000200021. [serial on the Internet] [DOI] [PubMed] [Google Scholar]
- 35.Gazetta CE, Ruffino-Netto A, Pinto Neto JM, et al. Investigation of tuberculosis contacts in the tuberculosis control program of a medium-sized municipality in the southeast of Brazil in 2002. J Bras Pneumol. 2006;32:559–565. doi: 10.1590/s1806-37132006000600014. [DOI] [PubMed] [Google Scholar]
- 36.Lemos AC, Matos ED, Pedral-Sampaio DB, Netto EM. Risk of tuberculosis among household contacts in Salvador, Bahia. Braz J Infect Dis. 2004;8:424– 430. doi: 10.1590/s1413-86702004000600006. [DOI] [PubMed] [Google Scholar]
- 37.Ruffino-Netto A. Tuberculosis: the neglected calamity. Rev Soc Bras Med Trop. 2002;35:51–58. doi: 10.1590/s0037-86822002000100010. [serial on the Internet] [DOI] [PubMed] [Google Scholar]