Abstract
Persistent viral infections are the result of a series of connected events that culminate in diminished immunity and the inability to eliminate infection. By building our understanding of how distinct components of the immune system function both individually and collectively in productive vs. abortive responses, new potential therapeutic targets can be developed to overcome immune dysfunction and thus fight persistent infections. Using lymphocytic choriomeningitis virus (LCMV) as a model of a persistent virus infection and drawing parallels to persistent human viral infections such as human immunodeficiency virus (HIV) and hepatitis C virus (HCV), we describe the cellular relationships and interactions that determine the outcome of initial infection and highlight immune targets for therapeutic intervention to prevent or treat persistent infections. Ultimately, these findings will further our understanding of the immunologic basis of persistent viral infection and likely lead to strategies to treat human viral infections.
Introduction
Persistent viral infections such as HIV, hepatitis B virus (HBV) and hepatitis C virus (HCV) cause a tremendous disease burden with more than 500 million people infected worldwide. The establishment of a persistent infection is the result of a myriad of factors mediated by both the virus (e.g. viral mutation, escape from immune recognition, viral tropism) and host (e.g., suppressive factors, apoptosis, excessive immune activation). While viruses have evolved mechanisms to modulate and escape host immunity, the interactions and interplay of innate and adaptive immune responses are critical aspects that determine viral persistence. The immune system is composed of a network of cell types that reciprocally regulate each other to determine the scope and direction of the immune response (Figure 1). Understandably, however, laboratories most often focus their research on one immune population to facilitate experimentation. Yet, the functions and interactions between multiple immune cell populations contribute to the outcome of the viral infection. Understanding these interactions will foster both a better understanding of viral infection and illuminate potential points for therapeutic intervention.
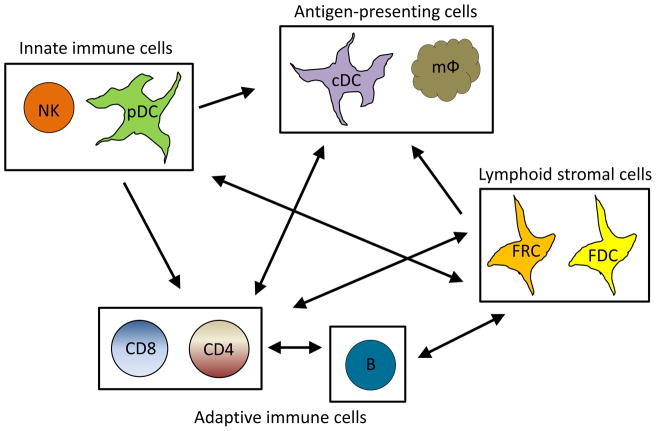
Figure 1. Relationship of immune cell populations during viral infection.
Simplified schematic of the relationships between important cell populations during persistent viral infection. During viral infection, a number of cellular interactions occur that are important in determining the scope and direction of the immune response. Plasmacytoid dendritic cells (pDC) detect viral antigen and release type I IFN which is essential in activation myeloid dendritic cells (mDC), macrophages (mφ), CD4 T cells (CD4) and CD8 T cells (CD8). Antigen presenting cells, as well as pDCs, are necessary for CD4 and CD8 T cell activation and the interactions of these cell types is controlled by chemokines secreted by fibroblastic reticular cells (FRCs). FRCs additionally secrete T cell survival factors. Follicular dendritic cells (FDCs) serve a similar role for B cells and coordinate B cell migration and B and CD4 T cell interactions. CD4 T cell help is necessary for a number of immune cell types, including CD8 T cells, mDCs, B cells (B) and FRCs. Conversely, natural killer cells (NK) may regulate the size of key immune populations.
Viruses employ a variety of methods to establish persistence, however, two main strategies have been noted. Some viruses, such as those of the Herpesviridae family, generate a latent/reactivating infection in which the virus lies dormant within host cells to escape immune surveillance. Infection with these viruses are characterized by long periods with little or no viral activity interspersed with periods of reactivation when viral activity reemerges but is quickly controlled by the immune system. Comparatively, viral infections such as HIV, Hepatitis B virus (HBV), hepatitis C virus (HCV), and lymphocytic choriomeningitis virus (LCMV) have sustained viremia. In addition to virus-encoded evasion strategies, from an immunologic standpoint, persistence of these viruses is maintained primarily by immune dysregulation and suppression (Wherry, 2011; Wilson and Brooks, 2010) and thus, interfering with immune cell activity is necessary for orchestrating anti-viral responses and clearing viral infection. Such chronic infections are characterized by high levels of viral replication, chronic immune activation, lymphoid disorganization, heightened/sustained expression of negative immune regulatory factors and dysfunctional/attenuated T and B cell responses (Wherry, 2011) (Table 1). While viruses in both categories impair immunity, latent viral infections are generally well-controlled. Consequently, this review will focus on the latter strategy of chronic viral infection because of the especially heavy impact on immune function and the high morbidity and mortality of these diseases. We will discuss several important host immune players (Figure 1) as highlighted by the study of lymphocytic choriomeningitis virus (LCMV) to showcase the importance of understanding the network of immunological events that occur during persistent infection and relate these findings to chronic viral infections in humans.
Table 1.
Cellular behaviors observed during persistent viral infection
| Plasmacytoid DCs |
|
| myeloid DCs |
|
| CD4 T cells |
|
| CD8 T cells |
|
| B cells |
|
| Lymphoid stromal cells |
|
LCMV: a prototypic model of immunity to viral infection
LCMV infection of its natural host, Mus musculus, has served as the prototypic system to explore host-virus interactions and identify key determinants of viral clearance or persistence. The LCMV system has led to many seminal virological and immunological discoveries that have subsequently been applied to human immune responses and infections. The great advantages of LCMV are the simplicity of its genome (four genes) and that it is non-lytic, allowing study of structural, cellular, and biochemical changes of the host’s immune response without the complication of direct viral cytopathicity. A unique attribute of the LCMV system is the ability to directly compare immune responses to two genetically similar viral variants, Armstrong53b (ARM) and Clone 13 (Cl-13), that respectively establish acute and persistent infections by inducing very different immunological outcomes (Ahmed et al., 1984). ARM induces a robust T cell response that clears the infection by 10 days post infection. In contrast, Cl-13 replicates to much higher titers, inducing multiple host-based suppressive pathways, thereby generating a systemic persistent infection that remains viremic and replicates in the majority of tissues for 60–90 days, at which point the virus remains in the CNS for extended periods of time and the kidney for the life of the host (Ahmed et al., 1984; Fahey and Brooks, 2010a; Lauterbach et al., 2007). Although human viruses are distinct from LCMV, the immune events that occur during persistent LCMV are similar to what is observed in HIV (Wilson and Brooks, 2010). Hence, it is becoming clear that prolonged virus infection and sustained viremia institute distinct immunologic programs that are conserved across species (Youngblood et al., 2012).
Initiating adaptive immunity: a central role for DCs
DCs are a heterogeneous population of sentinel cells that sample antigens that are subsequently presented on the DC surface to naïve T cells to initiate adaptive immune responses. These antigen-presenting cells (APC) are essential to initiate the immune response as well as determine its direction, quality and quantity (Pulendran, 2004). Three principal groups of DCs are found within the spleens and lymph nodes of mice: 1) myeloid CD11c+CD11b+CD8α− DCs (CD8α− DCs), 2) myeloid CD11c+CD11b+CD8α+ DCs (CD8α+ DCs), and 3) plasmacytoid DCs (pDCs; CD11cmidB220+Siglec-H+). pDCs are the rarest DC type and, upon viral infection, secrete high levels of type I interferons (IFN-I) after recognition of viral nucleic acids by toll-like receptors (TLR) 7 or 9 (Gilliet et al., 2008). IFN-I are critical for the initial control of virus infection and abrogation of TLR-signaling in pDCs diminishes IFN-I production and potentially the ability to control persistent Cl-13 infection (Blasius et al., 2012; Cervantes-Barragan et al., 2012), although the exact contribution of IFN-I by pDC in viral clearance remains controversial (Wang et al., 2012). Because IFN-I is important in fighting viral infection, disruption of pDC function is common in persistence. pDCs are targets of Cl-13 (Bergthaler et al., 2010; Macal et al., 2012) and HIV (Fitzgerald-Bocarsly and Jacobs, 2010). HIV interferes with pDC function by delaying the production of IFNα (Turk et al., 2013) and pDC capacity to produce IFNα is decreased during chronic HIV and HBV infection (Ladell et al., 2013; Mendoza et al., 2013). The decrease in IFNα may negatively impact DC maturation and function and T cell activation (Cervantes-Barragan et al., 2012; Frenz et al., 2010; Le Bon et al., 2003). IFN-I is important for activation and/or expansion of naive CD4 and CD8 T cells (Havenar-Daughton et al., 2006; Welsh et al., 2012) and pDCs are integral to this process (Cervantes-Barragan et al., 2012).
CD8α− and CD8α+ DCs are classical DC subsets that activate naïve T cells by presenting antigen on major histocompatibility complex (MHC) I and MHC II, in conjunction with costimulatory ligands. CD8α+ DCs are the most efficient for cross-presentation, whereby extracellular antigen is taken up and presented on MHC I to CD8 T cells (Iyer et al., 2012). Similar to pDCs, CD8α− DCs are infected at high rates during Cl-13 infection (Ng and Oldstone, 2012; Sevilla et al., 2000) and, productive DC infection is likely necessary for establishing Cl-13 persistence(Popkin et al., 2011). Mice with DCs that are unable to produce infectious virus do not generate a persistent Cl-13 infection and clear the virus in a similar manner to LCMV-ARM infection (Sullivan et al., 2011); comparably ARM only minimally infects DCs. Although HIV uses different mechanisms, HIV also utilizes DCs to establish and maintain infection (Wu and KewalRamani, 2006). DCs disseminate HIV by either direct infection or by transporting virus via the DC-specific lectin DC-SIGN to HIV-specific T cells in lymph nodes (Wu and KewalRamani, 2006), a phenomenon that has also been observed in HCV infection (Frenz et al., 2010). Beyond viral dissemination, persistent viral infection may also interfere with DC function. Myeloid DCs isolated from HIV and HBV patients have reduced capacity to stimulate T cells (Fitzgerald-Bocarsly and Jacobs, 2010; Ladell et al., 2013). Cl-13 infection decreases cell-surface expression of MHC I, MHC II, and T cell costimulatory molecules, CD80, CD86, and CD40 in both CD8α− and CD8α+ DCs which may interfere with their capacity to present antigen and stimulate naïve T cells (Sevilla et al., 2004). The decrease in DC function may be further compounded by inhibition of DC differentiation and maturation (Sevilla et al., 2004). Future studies are important to identify the fundamental outcomes of DC infection during persistent infection and how they lead to distinct aspects of immune dysfunction.
Lymphoid environment and lymphocyte activation
To initiate immune responses, APCs travel to secondary lymphoid organs (SLOs) such as lymph nodes, spleen, Peyer’s patches, and tonsils. Within SLOs, naive T cells interact with APCs, encounter soluble signals and antigens, and undergo activation. These interactions are spatially and temporally coordinated by fibroblastic reticular cells (FRCs), a specialized non-hematopoietic stromal found within T cell zones of SLOs (Mueller and Germain, 2009). FRCs are the foundation for the reticular network which compartmentalizes T cell zones, transports antigen and small proteins, and provides a surface for the adhesion, migration and interaction of the innate and adaptive immune cells (Mueller and Germain, 2009). FRCs induce migration of DCs and naïve T cells by expressing chemokines CCL19 and CCL21 (Mueller and Germain, 2009), which draw these cells to T cell zones where DCs identify antigen-specific T cells (Junt et al., 2008). During acute ARM infection, FRCs transiently downregulate CCL21 in an IFN-γ-dependent manner after infection, thus, slowing movement into the T cell zone (Mueller et al., 2007a). Follicular DCs, a stromal cell population in B cells zones, serve a similar structural/conduit role in B cell zones, in addition to their other critical role in antigen capture and stimulation of the B cell reaction. Follicular DCs downregulate CXCL13, thus abrogating movement into B cell zones (Mueller et al., 2007a). Though transient downregulation of chemokines is hypothesized to reduce competition for resources, control the size of the immune response, and promote egress of anti-viral T cells, this downregulation also affects the generation of T cell responses against co-challenges several days after the initial challenge (Mueller et al., 2007a). This likely occurs during persistent infection (Teijaro et al., 2013) and would hinder the activation of new anti-viral T cells needed to counteract T cell exhaustion, death, viral escape, and secondary infections (Vezys et al., 2006).
In conjunction with coordination and structure, FRCs provide important regulatory signals that control lymphocyte survival, including IL-7, a crucial survival signal for naïve T cells (Siegert and Luther, 2012; Zeng et al., 2012a). Reciprocally, T cells secrete lymphotoxin-β (LT-β), a survival factor for FRCs (Zeng et al., 2012b) and are the main source of IFN-γ which induces FRC expression of MHC II molecule and other molecules that stimulate or suppress T cell responses (Mueller et al., 2007b; Ng et al., 2012). Because the interaction of lymphocytes and stromal cells is important in homeostasis and immune activation, interrupting the integrity of the SLO hampers the immune response. Mice which have abnormal SLO microarchitecture due to deletion of the LT-β receptor gene, have severely diminished cytotoxic T cell (CTL) responses and delayed viral clearance when infected with ARM (Berger et al., 1999). Disruption of lymphoid architecture, in which there is loss of architecture, FRC/follicular DC networks, and/or defined areas for T, B, and other lymphocytes, has been reported with a variety of pathogens, including LCMV, HIV and SIV (Benedict et al., 2006; Tishon et al., 1993; Zeng et al., 2012a). During Cl-13 infection, mice exhibit severe lymphoid disorganization concomitant with loss of T cell function and immune suppression (Mueller et al., 2007b; Teijaro et al., 2013; Tishon et al., 1993). Similarly, lymphoid structure is damaged during HIV and SIV infection as a result of fibrosis, leading to disrupted T cell homeostasis (Zeng et al., 2012a) by limiting T cell access to IL-7-expressing FRCs, thus, causing T cell apoptosis and decreased numbers of naïve T cells (Zeng et al., 2012a). The damage is compounded by the dependency of FRCs on LT-β expressed by CD4 T cells, resulting in a cyclical loss in both cell populations (Zeng et al., 2012a; Zeng et al., 2012b). The loss of T cells and FRCs likely impacts T cell recovery, as decreased FRC loss is associated with better T cell recovery after the initiation of combination antiretroviral therapy (cART) (Zeng et al., 2012a). Administration of IL-7 can improve T cell responses in Cl-13, HIV, and SIV (Leone et al., 2009; Nanjappa et al., 2011), supporting the importance of cellular sources of IL-7 such as FRCs during infection. Overall, the role of lymphoid stromal cells as regulators of immune cell survival, function, and migration indicates that understanding their function and impact on lymphoid environment, specifically during viral infections, is necessary to plan strategies to initiate and maintain behaviors critical to B and T cell responses.
Chronic inflammation, immune activation and type I IFN during persistent infection: Bridging innate and adaptive immunity
Chronic inflammation and immune activation during persistent infection is associated with multiple immunologic dysfunctions, including aberrant T cell activation, cell senescence and depletion, dysfunctional B cell responses and polyclonal B cell activation, alterations in innate immune capacity, disruption of lymphoid architecture and in HIV infection, increased morbidity and mortality (Appay and Sauce, 2008; Chang and Altfeld, 2010; Moir et al., 2011). Importantly, immune activation is strongly associated with and predictive of HIV disease progression even in patients with antiretroviral therapy suppressed viral loads (Moir et al., 2011; Sauce et al., 2013). Mounting evidence suggests that IFN-I are integrally associated with immune activation (Bosinger et al., 2011; Chang and Altfeld, 2010; Hardy et al., 2013), suggesting that targeting this pathway could have a tremendous therapeutic impact to halt and potentially reverse immune deterioration and HIV disease progression. Recently, using LCMV, we established a direct link between chronic IFN-I signaling, immune suppression and viral persistence (Teijaro et al., 2013; Wilson et al., 2013). Like other persistent infections (Appay and Sauce, 2008; Sauce et al., 2013), Cl-13 infection induced significantly higher IFN-I levels and IFN-stimulated gene expression than acute ARM infection (Teijaro et al., 2013; Wilson et al., 2013), consistent with the association between high levels of IFN-I signaling and persistence. Short-term suppression of IFN-I signaling at the onset of infection corrected multiple dysfunctions associated with persistent viral infections, including increased numbers and stimulatory capacity of APCs, reduced expression of negative immune regulators such as the anti-inflammatory cytokine interleukin-10 (IL-10) and inhibitory ligand PD-L1, prevention of lymphoid tissue disruption, and enhanced numbers and polyfunctionality of virus-specific CD4 T cells (Teijaro et al., 2013; Wilson et al., 2013). Interestingly, therapeutic blockade of chronic IFN-I signaling well into persistent infection diminished IL-10 and PD-L1 expression and substantially decreased virus titers in multiple organs, indicating the continued potentiation of the immunosuppressive program by IFN-I signaling and importantly, the ability to disrupt IFN-I signaling to treat persistent infection. Although the mechanisms underlying enhanced immune function through IFN-I blockade are under investigation, control of persistent infection depended on an initial CD4 T cell response and IFN-γ expression (Teijaro et al., 2013; Wilson et al., 2013). In addition to these factors, the myriad of immune alterations that occur when IFN-I signaling is inhibited are complex, inter-dependent and likely all combine in distinct ways to culminate in the enhanced control of persistent LCMV infection. In HIV infection, strategies that indirectly or directly target IFN-I indicate decreased immune activation and immunosuppressive factors, suggesting that inhibiting IFN-I in HIV infection may also have therapeutic benefit (Ries et al., 2012). As discussed above, IFN-I have important antiviral effects and are a well-established treatment for HCV and potentially HIV (Azzoni et al., 2013). Together, these results highlight the duality and temporal nature of IFN-I during viral infection wherein acute signals possess antiviral and immune stimulatory potential, but when infection is not controlled in a timely fashion, prolonged IFN-I signaling leads to multiple immune dysfunctions that facilitate persistent infection. Future exploration into how excessive IFN-I signaling simultaneously activates and suppresses the immune response and the overall balance between its antiviral vs. immunoregulatory effect will lead to important insights into the pathogenesis of persistent virus infections and potential strategies to treat these infections.
T cell responses during persistent viral infections
Control of persistent viral infection depends upon effective anti-viral CD4+ and CD8+ T cell responses (Oldstone, 2006). CD8 T cells (i.e., CTLs) express inflammatory and antiviral cytokines and lyse infected cells. CD4 T cells (i.e., helper T cells) have a myriad of roles that include expression of inflammatory cytokines, DC licensing, optimal activation and maintenance of CTLs and generation of B cell and antibody responses. During acute LCMV-ARM infection, T cell responses are robust and CD8 T cells alone are necessary for viral clearance (Tishon et al., 1995). During Cl-13 infection, the immune environment and T cell responses are initially comparable to those in acute ARM infection (Brooks et al., 2006a; Wilson et al., 2012). However within one to two weeks after infection, LCMV-specific T cells enter an attenuated (i.e., exhausted) state, characterized by an hierarchical loss of proliferative ability, production of key antiviral and immune stimulatory cytokines, and cytolytic activity (Brooks et al., 2005; Wherry, 2011) (Figure 2). This loss of T cell effector activity, first described for LCMV, mirrors the T cell exhaustion observed in HIV (Wilson and Brooks, 2010). The loss of T cell functionality during persistent infection is compounded by the decreased ability of the immune system to mount de novo immune responses against co-infecting pathogens, thereby rendering the host less able to fight infection and potentially form memory T cells (Ahmed et al., 1984; Oldstone et al., 1988). However, in some instances, the ongoing production of IFN-I and/or IFN-γ during persistent infections has been reported to protect the host from secondary infections (Barton et al., 2007; Valentine et al., 2012). Thus, proper generation and maintenance of antiviral activity is paramount to clear the virus infection and functional perturbations can have a profound impact on the outcome of infection.
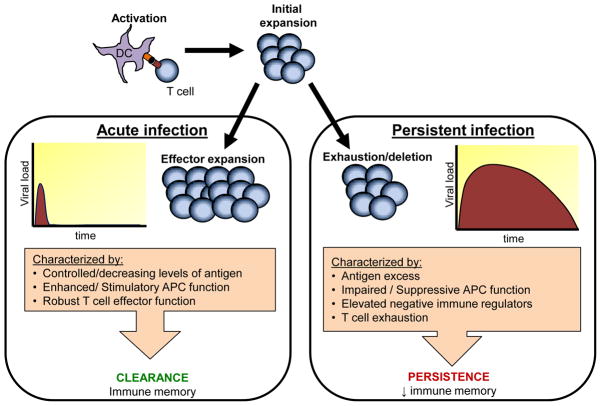
Figure 2. Characteristics of acute vs. persistent viral infection.
Viral infection results in one of two outcomes: clearance or persistence. Clearance of virus in the acute phase is characterized by vigorous expansion of virus-specific T cells with anti-viral activity. Viral persistence is characterized by an initial T cell expansion similar that of an acute infection. However, when virus is not cleared in the acute phase then T cells experience decreasing function (i.e. T cell exhaustion) that may lead to deletion of the cell. The T cell exhaustion is partially caused by the expression of negative immune regulators such as IL-10 and PD-1. Many viruses that cause persistent infection also target DCs by suppressing or subverting their function. Together these factors not only contribute to establishing persistent infection but also hinder the formation of memory responses.
CD8 T cell responses during persistent viral infection
Disrupting the generation of CTLs results in delayed and/or failed virus clearance, and abrogation of CTL function contributes to the inability to control multiple persistent infections (Wherry, 2011). The maintenance of CD8 T cell function during exhaustion as well as the targeting of conserved epitopes necessary for viral function correlates with stronger viral control (Ladell et al., 2013; Mendoza et al., 2013; Vingert et al., 2012). Extensive transcriptional and epigenetic profiling of CD8 T cells of functionally active CTLs vs. “exhausted” CTLs revealed differences in a variety of genes for inhibitory receptors, transcription factors, metabolic pathways, chemotaxins, and migration factors (Doering et al., 2012; Wherry, 2011; Wherry et al., 2007; Youngblood et al., 2012). A key class of factors are negative immune regulators that have been summarized in a recent review (Wherry, 2011). Consequently, we will primarily discuss negative regulators that have been shown by either in vivo gene deletion or antibody neutralization studies to play a major role in viral persistence, programmed death-1 (PD-1) and IL-10. These molecules are upregulated during inflammatory responses to control the immune response and prevent host damage. However, during persistent infections characterized by high viral replication, these molecules, although crucial for limiting immunopathology, also suppress T cell responses, resulting in uncontrolled viral load.
PD-1, a member of the CD28 receptor family, negatively regulates T cell signaling by binding its cognate ligands PD-L1 and PD-L2 (Sharpe et al., 2007). High levels of PD-1 are expressed on exhausted CD4+ and CD8+ T cells during persistent LCMV infection and blockade of PD-1 signaling via PD-L1 leads to accelerated Cl-13 clearance (Barber et al., 2006). PD-L1 is expressed on many cell types during viral persistence, including hematopoietic and non-hematopoietic cells with the type of cell presenting PD-L1 having a distinct impact on CD8 T cell responses (Mueller et al., 2010). Multiple APCs express PD-L1 during persistent infection and upon interaction with T cells induce and sustain T cell exhaustion (Barber et al., 2006; Wilson et al., 2012). In conjunction, stromal FRCs also negatively regulate activated antiviral T cells via expression of PD-L1 to prevent immunopathology (Mueller et al., 2007b; Mueller et al., 2010; Ng et al., 2012) but, consequently, clearance of infected cells is slowed. Although PD-L1 expression on hematopoietic vs. non-hematopoietic cells play slightly different roles, both contribute to suppressing T cell activity and compromise control of infection.
The discovery of PD-1 as a marker of T cell exhaustion in the LCMV system quickly led to examination of PD-1 in HIV, HCV and HBV infections where PD-1 is also upregulated on virus-specific CD8 T cells and correlates with T cell exhaustion (Yi et al., 2010b). For HIV, in vitro PD-L1 blockade resulted in augmented expansion and cytokine production by HIV-specific T cells (Day et al., 2006; Petrovas et al., 2006) and studies using humanized mice demonstrated enhanced T cell responses and decreased HIV titers following PD-1 blockade (Palmer et al., 2013). Further, PD-L1 blockade in SIV-infected macaques, demonstrated improvements in SIV-specific CD8 T cell and B cell function (Hofmeyer et al., 2011). Similarly, in vitro blockade of PD-L1 improved proliferation and function of HCV-specific CD8 T cells (Fisicaro et al., 2010; Urbani et al., 2008). However, the efficacy of PD-L1 blockade may depend on PD-1 expression level, which correlates with the extent of T cell exhaustion, as HCV-specific CD8 T-cells from chronic HCV patients with high PD-1 expression were less responsive than those with intermediate PD-1 expression to PD-L1 blockade (Blackburn et al., 2008; Nakamoto et al., 2008). The research on the PD-1:PD-L1 pathway is a work in progress but represents a promising target for improving/restoring the function of exhausted T cells.
In combination with PD-1, T cell immunoglobulin and mucin domain-containing molecule-3 (TIM-3) and lymphocyte activation gene (LAG-3), two other negative immune regulators, may be promising targets to treat T cell exhaustion (Wherry, 2011). During LCMV infection, a majority of virus–specific CD8+ T cells coexpress Tim-3 and PD-1, which is associated with increased CD8 T-cell exhaustion when compared to expression of either factor alone (Jin et al., 2010; Jones et al., 2008). Blockade of TIM-3 enhanced the ex vivo function of exhausted CD8 T cells from HIV infection (Jones et al., 2008), and during persistent LCMV infection, simultaneous blockade in vivo of both TIM-3 and PD-1 synergistically improved CD8+ T cell responses and viral control (Jin et al., 2010). LAG-3 blockade or deletion alone does not improve T cell responses or viral control (Richter et al., 2010) but simultaneous blockade of PD-1 and LAG-3 results in synergistic alleviation of T cell exhaustion and viral control (Blackburn et al., 2009), suggesting that LAG-3, along with TIM-3, are sub-dominant suppressive pathways. Thus, the combinatorial regulation of T cell exhaustion by inhibitory receptors is complex, but suggests that it may be possible to specifically interfere with precise signaling pathways to restore desired responses while still maintaining regulation to prevent excessive immunopathology.
T cell expression of these inhibitory receptors is controlled by the transcriptional repressor Blimp-1, a regulator of cell differentiation (Shin et al., 2009), and transcription factors T-bet and eomesodermin (Eomes) that direct T cell fate (Castellino and Germain, 2006a; Pearce et al., 2003). Both Blimp-1 and Eomes are highly expressed in exhausted CD8 T cells in association with PD-1 and other inhibitory receptors (Paley et al., 2012; Shin et al., 2009; Wherry et al., 2007). Conversely, T-bet is reduced in virus-specific CD8+ T cells during chronic HIV and LCMV, and this reduction correlates with T cell dysfunction (Hersperger et al., 2011; Kao et al., 2011). Recently, Paley et al. identified a progenitor or “stem”-like exhausted CD8 T cell that expressed T-bet and was capable of differentiating into Eomes-expressing subset with enhanced virus fighting capacity (Paley et al., 2012). This observation suggests that therapeutic strategies to enhance CD8 T cell responses during persistent infection should additionally focus on amplifying specific subsets of CD8 T cells. How best to modulate transcriptional programs to achieve a desired response and how this affects control of virus replication will be an interesting line of future research.
IL-10 is another key negative immune regulator that is important during multiple persistent virus infections. IL-10 exerts pleiotropic effects on different cell populations and likely with diverse suppressive roles during persistent infection (Ouyang et al., 2011; Wilson and Brooks, 2011). During persistent LCMV-Cl13 infection, IL-10 is significantly upregulated and results in a decrease in virus-specific T cells and a loss of T cell functionality (Brooks et al., 2006b; Ejrnaes et al., 2006). Deletion of the il10 gene or early blockade of the IL-10 receptor (IL-10R) is sufficient to prevent both events (Brooks et al., 2006b; Ejrnaes et al., 2006). Although IL-10 can be expressed by multiple cell-types, one of the main producers of IL-10 during Cl-13 infection is CD8α− CD11b+ DCs (Ng and Oldstone, 2012; Wilson et al., 2012). Comparatively, IL-10 is only minimally expressed by DCs has been observed in ARM-infected mice (Brooks et al., 2006b; Wilson et al., 2012). Interestingly, distinct APC subsets that produce IL-10 also expressed high levels of the other suppressive factors that limit antiviral immunity during persistent infection [e.g., PD-L1, indoleamine 2,3 dioxygenase (IDO)] (Ng and Oldstone, 2012; Wilson et al., 2012) suggesting a potential mechanism whereby expression of multiple immunoregulatory factors on single cells facilitates the simultaneous delivery of numerous inhibitory signals directly to individual T cells in a single interaction. In addition to initiating the suppressive state, IL-10 is also necessary to maintain T cell exhaustion (Brooks et al., 2008b). Antibody blockade of IL-10 signaling during persistent infection restored the function of exhausted T cells and significantly decreased viral titers (Brooks et al., 2008b; Brooks et al., 2006b). Interestingly, PD-L1 and IL-10 signal via independent pathways yet blockade of either pathway alone restores T cell function (Brooks et al., 2008a). Examination of IL-10 in human persistent infections has identified increased levels in HIV (Landay et al., 1996; Navikas et al., 1995), HBV (Peppa et al., 2010), and HCV (Crawford et al., 2011) infection, and IL-10 production has been described in DCs, monocytes, natural killer (NK) cells and non-regulatory T cells (Alter et al., 2010; Brockman et al., 2009; Torheim et al., 2009). Furthermore, IL-10 has been implicated in the lysis of mature DCs by NK cells (Alter et al., 2010) as well as regulation of CD4 T cell death (Clerici et al., 1994; Estaquier et al., 1995) during HIV infection. Although In vivo studies have yet to be performed to assess the efficacy of anti-IL-10 treatment in human chronic viral infections, in vitro blockade of IL-10 leads to improved function of exhausted T cells during HIV infection (Brockman et al., 2009; Landay et al., 1996) and HCV (Kaplan et al., 2008; Rigopoulou et al., 2005). Although data thus far indicates that IL-10 is a key suppressive pathway in persistent infection, further investigation of anti-IL-10 treatment will determine if this is a viable strategy to decrease T cell exhaustion in human chronic infections.
CD4 T cell differentiation during persistent virus infection
CD4 T cells have multiple roles and are essential to support CD8 T cell responses (Battegay et al., 1994; Matloubian et al., 1994). The scope of CD4 T cell help during viral infection is complex, but essential helper functions within persistent viral infection include: (1) sustaining residual function in exhausted CD8 T cells, (2) providing help to B cells to elicit antiviral antibody responses, and (3) licensing of new APCs to sustain antiviral immunity or invoke new antiviral responses (Figure 3) (Fahey and Brooks, 2010b). In response to the levels/combination of antigen stimulation, costimulatory signals and cytokines encountered, CD4 T cells develop into different Th subsets that preferentially stimulate and sustain CD8 T cells (Th1), B cells (T follicular helper; Tfh), suppress T cell activity (Treg), or secrete other key immune driving cytokines (Th17, Th2) (Crotty, 2011; Zhu et al., 2010). Due to their ability to differentially direct and sustain specific immune responses, the type of CD4 T cell response elicited is critical to guide the ensuing response and control infection. While the majority of studies have focused on understanding CD8 T cell dynamics in persistent infection, CD4 T cell responses are an essential correlate of control and/or clearance of multiple persistent virus infections, including HIV and HCV (Day et al., 2003; Gerlach et al., 1999; Grakoui et al., 2003; Rosenberg et al., 1997; Thimme et al., 2001). Thus, identifying the mechanisms through which CD4 T cells modulate and sustain immunity to control persistent infection is highly important and will potentially provide strategies to simultaneously correct multiple immune cell types and functions that are lost during persistent infection.
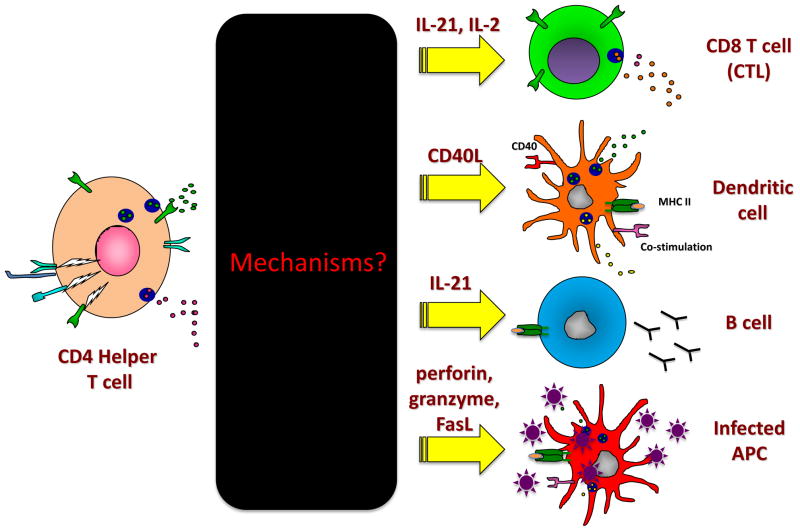
Figure 3. Helper functions of CD4 T cells during persistent virus infection.
Although the functional outcomes of CD4 T cell help to CD8 T cells and B cells are well documented in persistent viral infection, the precise mechanisms of help remain largely an unknown black box. CD4 T cells can help CD8 T cells and B cells through the secretion of cytokines, such as IL-21, IL-2 and likely others awaiting discovery. Furthermore, it is also probable that CD4 T cells can secondarily help antiviral CD8 T cells by continually licensing new dendritic cells, perhaps through CD40/CD40L signaling, cytokines and/or other costimulatory pathways. CD4 T follicular cells are necessary for antiviral antibody production. Cytotoxic CD4 T cells have also been identified in many persistent viral infections and potentially “help” by directly lysing virally infected APCs, such as macrophages in HIV infection.
Upon Cl-13 infection, CD4 T cells progressively lose the ability to produce key antiviral Th1 cytokines (Fahey et al., 2011). However, CD4 T cells are required to control and eventually resolve persistent infection, indicating that they must retain some helper function to sustain immunity. Indeed, viral persistence results in an alteration or redirection of CD4 T cell responses wherein Th1 cells are progressively transformed into Tfh cells (Brooks et al., 2005; Fahey et al., 2011). The accumulation of Tfh cells was also recently identified in persistent SIV and HIV infection and correlated with increased B cell activation and antibody production (Lindqvist et al., 2012; Petrovas et al., 2012). While this progressive development into B cell helping Tfh cells is critical to sustain the humoral arm of the antiviral response and control infection (Fahey et al., 2011; Harker et al., 2011), the dwindling number of Th1 cells may quantitatively impact CD8 T cell maintenance contributing to the observed CTL exhaustion. Although potentially sacrificing an important branch of antiviral immunity, the skew away from Th1 cells may be a protective mechanism to limit immunopathology in the face of high levels of infected cells during persistent infection.
CD4 T cell help for CD8 T cell responses
CD4 T cells are required throughout viral persistence to sustain CD8 T cell function and control infection, to avert deletion of high-affinity antiviral CTL and to purge an established persistent viral infection (Battegay et al., 1994; Berger et al., 2000; Castellino and Germain, 2006b; Matloubian et al., 1994). Resolution of HCV infection and resistance to re-infection correlates with strong virus-specific CD4 T cell responses that sustain antiviral CD8 T cell activity (Day et al., 2003; Grakoui et al., 2003; Thimme et al., 2001; Urbani et al., 2006). Subsequent loss of CD4 T cell activity after initial control of HCV or LCMV allows the virus to re-emerge and the loss of CD4 T cells is a pivotal mechanism leading to AIDS (Feinberg, 1996; Gerlach et al., 1999; Ou et al., 2001; Schulze Zur Wiesch et al., 2012). Defective CD8 T cell proliferative responses against HIV were augmented in vivo by induction of CD4 T cells in patients with suppressed virus loads (Lichterfeld et al., 2004), demonstrating the desirability of invoking CD4 T cells to control an established persistent infection. Effective CD4 T cell responses are critical to sustain antiviral CD8 T cells to control persistent virus infection and loss of their activity is a critical step in disease progression.
Although the importance of CD4 T cell help to CD8 T cells is well established, the precise mechanisms by which CD4 T cells mediate their helper functions have remained elusive (Figure 3). Recently, a direct role for IL-21 (a common γ-chain cytokine family member) in maintaining CD8 T cell function within persistent LCMV infection was identified (Elsaesser et al., 2009; Frohlich et al., 2009; Yi et al., 2009). Unlike other cytokines, IL-21 expression is enhanced and sustained during prolonged viral replication (Elsaesser et al., 2009; Frohlich et al., 2009; Yi et al., 2009). IL-21 signaling maintained both the number of virus-specific CD8 T cells and their ability to produce antiviral cytokines. Interestingly, despite antigen-specific CD4 T cell numbers being unchanged or even elevated in IL-21R−/− mice, these mice were unable to sustain CD8 T cell responses or control infection (Elsaesser et al., 2009), demonstrating the specific role of IL-21-mediated help. Similarly, enhanced IL-21 expression was observed in HIV infected patients and its levels correlated with relative control of infection (Chevalier et al., 2011; Yue et al., 2010). Ex vivo culture of HIV-specific CD8 T cells with IL-21 led to their expansion and aided their ability to inhibit viral replication in vitro (Chevalier et al., 2011; Yue et al., 2010). In addition to IL-21, loss of signaling by IL-2, a cytokine that largely acts on lymphocytes, also diminished LCMV-specific CD8 T cell responses in vivo (Bachmann et al., 2007) and correlated with loss of HIV-specific CD8 T cells (Lichterfeld et al., 2004). The source of IL-2 is likely CD4 T cells, but may also be derived from other cell types, particularly since CD4 T cell derived IL-2 is greatly diminished in persistent infection (Brooks et al., 2005; Lichterfeld et al., 2004). However, it is clear that multiple cytokines function in combination to sustain CD8 T cells during long-term virus replication and that loss of one cytokine dramatically impacts the maintenance of antiviral CD8 T cells. Ultimately, it will be important to determine the compilation of factors that sustain antiviral CD8 T cells, how they individually contribute to distinct CD8 T cell effector functions and how these effector functions lead to prevention and control of persistent infection.
T follicular helper cells and B cell responses
In addition to CD8 T cells, B cell and antibody responses are required to control persistent infection, and specifically, persistent infections associated with multiple B cell dysfunctions (Hangartner et al., 2006; Moir and Fauci, 2009). Tfh cells are a subset of CD4 T cells essential for providing B cell help. Changes in the dynamics of the Tfh population could lead to many of the observed alterations in B cell activation (Crotty, 2011; Moir and Fauci, 2009). Tfh cells interact with B cells in follicles and germinal centers where B cells mature and differentiate to provide proliferative and survival signals and facilitate affinity maturation and class switch recombination, which improves the function of antibodies produced by B cells (Crotty, 2011). CD4 T cells maintain their B cell helper function throughout persistent viral infection; although, due to intrinsic and extrinsic mechanisms, the help they provide may be attenuated (Cubas et al., 2013; Fahey et al., 2011). Depletion of CD4 T cells after infection or preventing Tfh:B cell interactions abrogated LCMV-specific antibody production and importantly the ability to clear infection (Fahey et al., 2011; Harker et al., 2011), indicating that the continual presence of Tfh and interaction with B cells is required to sustain humoral immunity through viral persistence.
Many persistent viral infections exhibit delayed production of virus-specific neutralizing antibodies and are characterized as having elevated levels of non-specific antibody production (Hangartner et al., 2006; Moir and Fauci, 2009). The elevation in non-specific antibodies correlates with viral load, is CD4 T cell dependent and may arise from the presentation of viral peptides by viral non-specific B cells (Hunziker et al., 2003). The enhancement of Tfh cells in viral persistence most likely fuels this non-specific B cell activation. Indeed, by reducing CD4 T cell numbers in vivo, non-specific antibody levels were decreased while increased neutralizing antibody titers arose earlier in infection (Recher et al., 2004). These data suggest that in some cases the skew towards Tfh could be detrimental, facilitating non-specific antibody activation and augmenting the risk of autoimmune disease.
In addition to effects on CD8 T cells, it is highly probable that IL-21 also influences B cell responses in persistent infection. IL-21 is highly expressed by Tfh (Crotty, 2011; Fahey et al., 2011) and known to promote germinal center B cell formation and the differentiation of plasma cells, which are B cells that produce high levels of antibodies, thus leading to antibody production (Yi et al., 2010a). IL-21−/− and IL-21R−/− mice had a 2–3 fold defect in antibody production during persistent LCMV infection (Elsaesser et al., 2009; Frohlich et al., 2009; Yi et al., 2009), likely due to a direct effect on B cells. Interestingly, although IL-21 is highly expressed by Tfh, it should be noted that upon persistent LCMV infection IL-21 can be produced by both Tfh and non-Tfh CD4 T cells (Fahey et al., 2011), likely accounting for its ability to help both CD8 T cells and B cells, as discussed above. Further research is required to delineate the molecular mechanisms through which IL-21 mediates its effects on B cells and CD8 T cells.
The role of B cells in the control of persistent virus infection is well established (Moir and Fauci, 2009). Yet, the cellular immunology of B cell responses during persistent virus infections is less well characterized in part owing to the difficulty in studying them. Extensive B cell dysfunction is observed during HIV infection (Moir and Fauci, 2009), and during persistent LCMV infection, the highest affinity antibody bearing B cells are physically deleted, likely leading to defects in the B cell repertoire and generation of neutralizing antibodies (Hangartner et al., 2006). One result of B cell dysfunction may be alterations in antibody isotype production and/or glycosylation that could differentially affect Fc receptor binding/usage leading to decreased antibody dependent cellular cytotoxicity (ADCC) by NK cells and antibody dependent cellular phagocytosis (ADCP), both of which have an important impact on clearing virus-infected cells and free virions (Ackerman et al., 2013a; Ackerman et al., 2013b). Further, B cell dysfunction in HIV may hinder the development of broadly neutralizing antibodies which require extensive rounds of affinity maturation to achieve broad neutralization (Klein et al., 2013); this is likely compounded by lymphoid disruption (Zeng et al., 2012a) and the loss of CD4 T cell help (Crotty, 2011). Because of the potential of antibody to neutralize virus and hence prevent persistent infection, passive and genetic based antibody immunotherapies have great appeal (Burton et al., 2012; Law et al., 2008). The recent technologic advances in glycomics and identification of antigen-specific B cells will undoubtedly lead to important insights into B cell dysfunctions, how they contribute to the inability to generate neutralizing antibodies and clear infection and ultimately, how to modulate the B cell response to generate protective and therapeutic antibodies to control persistent infection.
Cytotoxic CD4 T cells
In addition to helping CD8 T cells and B cells to resolve infection, CD4 T cells also have direct cytotoxic function in multiple viral infections (Marshall and Swain, 2011), including persistent infections such as HIV (Norris et al., 2004; Zaunders et al., 2004) and HBV and HCV (Aslan et al., 2006). Much like the mechanisms used by CTLs, CD4 T cells kill target cells via multiple mechanisms, including the release of perforin and granzyme, which act in concert to create pores in the target cell that induce cell death, and by FasL engagement of Fas on the cell surface to induce apoptosis (Marshall and Swain, 2011). Cytotoxic CD4 T cells have MHC class II restricted killing (Jellison et al., 2005), and therefore most likely target APCs. In addition, human CD4 T cells also upregulate MHC class II upon activation (Holling et al., 2002), raising the question of whether they are targets in vivo (Streeck et al., 2013). A recent report identified early CD4 CTL activity as a predictor of enhanced long-term HIV control (Soghoian et al., 2012), suggesting that the ability to generate these CD4 killer cells could prove therapeutically valuable to control persistent virus infections. Although the dissection of the nature of CD4 T cell help in persistent infection remains experimentally challenging, understanding these mechanisms will be invaluable, and once elucidated will allow for derivation of strategies to enhance these functions.
Conclusion
The current understanding of how innate and adaptive immune interactions contribute to persistent viral infection (Table 1) suggests multiple points for potential therapeutic intervention. Currently, ongoing treatment of persistent viral infections is limited to drug targeting. Although in some cases, these therapies are able to eliminate HCV, in most instances and particularly for HIV, they are not a cure and have the problem of long term therapy, cost, and danger of drug resistance. Thus, a combination of anti-viral drug therapy along with therapies to restore immune function would be optimal. The essential requirement of CD4 and CD8 T cells in controlling persistent viral infections and their ability to sustain the cellular and humoral immune responses make them attractive targets of current therapeutic research. Recent evidence for such a possibility is derived from the SIV system in which CMV vectors encoding SIV epitopes are being used to invoke and sustain antiviral CD8 T cells that in many instances effectively control the infection without adjunctive antiretroviral drug therapy (Hansen et al., 2011). Specific induction of CD4 T cells may also be an ideal approach as induction or delivery of virus-specific CD4 T cells can result in enhanced virus-specific CD8 T cells (Aubert et al., 2011; Lichterfeld et al., 2004). Another strategy would be to develop small molecule reagents to inhibit the negative regulatory pathways that maintain T cell exhaustion during persistent infection. As important players in the battle against viral persistence, illumination of methods to maintain CD4 and CD8 T cell function will be critical to devising new vaccine and therapeutic strategies. However, considering the inter-reliance of the immune system, it is likely that effective preventative or restorative therapies will require enhancement of multiple immune subsets (e.g., antiviral CD8 T cells to kill cells, B cell to produce antibody to neutralize free virus and CD4 T cells to sustain both of their activity). Such an integrated approach requires in depth understanding about how persistent infections are established, maintained and eliminated from an immunological standpoint.
To further our understanding of immunological events during persistent infections, research is needed to illuminate several key areas. First, studies are needed to determine whether DC/APC functions can be fostered to boost generation of new immune responses in the presence of immune suppressive programming during persistent infection. Second, it is necessary to better identify how lymphocyte trafficking and interactions change during chronic viral infections and the effect on the generation and maintenance of anti-viral responses. Identifying these interactions and how to influence them will be an important leap forward in understanding how to prevent or “fix” the abnormal immune responses generated during chronic infections. Third, we need a better understanding of CD4 T cell regulation and differentiation during viral infection and the effects on sustaining and directing both antiviral CD8 and B cell responses. Specifically, studies are needed to further elucidate precise mechanisms and factors that comprise CD4 T cell help during persistent infection, the optimal Th response to elicit and the distinct Th cell fate decisions that lead to increased or decreased control of persistent virus replication. Lastly, examination of how IFN-I controls the multiple immune parameters and how those events control CD4 T cell responses will lend better insight into causes and control of viral persistence. By identifying these immune interactions and learning how to influence them, the field of immunology will take an important leap forward in understanding how to prevent or overcome immune exhaustion and optimally tailor immune response to eliminate persistent infections.
Acknowledgments
D.G. Brooks, C.T. Ng and M.B.A. Oldstone are supported by the National Institutes of Health (AI085043 and AI082975 (D.G.B.); AI009484 (C.T.N. and M.B.A.O.)) and L.M. Snell is supported by a Training Grant from the Fonds de la recherche en santé du Québec.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ackerman ME, Crispin M, Yu X, Baruah K, Boesch AW, Harvey DJ, Dugast AS, Heizen EL, Ercan A, Choi I, et al. Natural variation in Fc glycosylation of HIV-specific antibodies impacts antiviral activity. J Clin Invest. 2013a;123:2183–2192. doi: 10.1172/JCI65708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ackerman ME, Dugast AS, McAndrew EG, Tsoukas S, Licht AF, Irvine DJ, Alter G. Enhanced Phagocytic Activity of HIV-Specific Antibodies Correlates with Natural Production of Immunoglobulins with Skewed Affinity for FcgammaR2a and FcgammaR2b. J Virol. 2013b;87:5468–5476. doi: 10.1128/JVI.03403-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed R, Salmi A, Butler LD, Chiller JM, Oldstone MB. Selection of genetic variants of lymphocytic choriomeningitis virus in spleens of persistently infected mice. Role in suppression of cytotoxic T lymphocyte response and viral persistence. J Exp Med. 1984;160:521–540. doi: 10.1084/jem.160.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alter G, Kavanagh D, Rihn S, Luteijn R, Brooks D, Oldstone M, van Lunzen J, Altfeld M. IL-10 induces aberrant deletion of dendritic cells by natural killer cells in the context of HIV infection. J Clin Invest. 2010;120:1905–1913. doi: 10.1172/JCI40913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appay V, Sauce D. Immune activation and inflammation in HIV-1 infection: causes and consequences. J Pathol. 2008;214:231–241. doi: 10.1002/path.2276. [DOI] [PubMed] [Google Scholar]
- Aslan N, Yurdaydin C, Wiegand J, Greten T, Ciner A, Meyer MF, Heiken H, Kuhlmann B, Kaiser T, Bozkaya H, et al. Cytotoxic CD4 T cells in viral hepatitis. J Viral Hepat. 2006;13:505–514. doi: 10.1111/j.1365-2893.2006.00723.x. [DOI] [PubMed] [Google Scholar]
- Aubert RD, Kamphorst AO, Sarkar S, Vezys V, Ha SJ, Barber DL, Ye L, Sharpe AH, Freeman GJ, Ahmed R. Antigen-specific CD4 T-cell help rescues exhausted CD8 T cells during chronic viral infection. Proc Natl Acad Sci U S A. 2011;108:21182–21187. doi: 10.1073/pnas.1118450109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azzoni L, Foulkes AS, Papasavvas E, Mexas AM, Lynn KM, Mounzer K, Tebas P, Jacobson JM, Frank I, Busch MP, et al. Pegylated Interferon alfa-2a monotherapy results in suppression of HIV type 1 replication and decreased cell-associated HIV DNA integration. J Infect Dis. 2013;207:213–222. doi: 10.1093/infdis/jis663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann MF, Wolint P, Walton S, Schwarz K, Oxenius A. Differential role of IL-2R signaling for CD8+ T cell responses in acute and chronic viral infections. Eur J Immunol. 2007;37:1502–1512. doi: 10.1002/eji.200637023. [DOI] [PubMed] [Google Scholar]
- Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- Barton ES, White DW, Cathelyn JS, Brett-McClellan KA, Engle M, Diamond MS, Miller VL, Virgin HWt. Herpesvirus latency confers symbiotic protection from bacterial infection. Nature. 2007;447:326–329. doi: 10.1038/nature05762. [DOI] [PubMed] [Google Scholar]
- Battegay M, Moskophidis D, Rahemtulla A, Hengartner H, Mak TW, Zinkernagel RM. Enhanced establishment of a virus carrier state in adult CD4+ T-cell-deficient mice. J Virol. 1994;68:4700–4704. doi: 10.1128/jvi.68.7.4700-4704.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedict CA, De Trez C, Schneider K, Ha S, Patterson G, Ware CF. Specific remodeling of splenic architecture by cytomegalovirus. PLoS Pathog. 2006;2:e16. doi: 10.1371/journal.ppat.0020016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger DP, Homann D, Oldstone MB. Defining parameters for successful immunocytotherapy of persistent viral infection. Virology. 2000;266:257–263. doi: 10.1006/viro.1999.0074. [DOI] [PubMed] [Google Scholar]
- Berger DP, Naniche D, Crowley MT, Koni PA, Flavell RA, Oldstone MB. Lymphotoxin-beta-deficient mice show defective antiviral immunity. Virology. 1999;260:136–147. doi: 10.1006/viro.1999.9811. [DOI] [PubMed] [Google Scholar]
- Bergthaler A, Flatz L, Hegazy AN, Johnson S, Horvath E, Lohning M, Pinschewer DD. Viral replicative capacity is the primary determinant of lymphocytic choriomeningitis virus persistence and immunosuppression. Proc Natl Acad Sci U S A. 2010;107:21641–21646. doi: 10.1073/pnas.1011998107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn SD, Shin H, Freeman GJ, Wherry EJ. Selective expansion of a subset of exhausted CD8 T cells by alphaPD-L1 blockade. Proc Natl Acad Sci U S A. 2008;105:15016–15021. doi: 10.1073/pnas.0801497105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn SD, Shin H, Haining WN, Zou T, Workman CJ, Polley A, Betts MR, Freeman GJ, Vignali DA, Wherry EJ. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat Immunol. 2009;10:29–37. doi: 10.1038/ni.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasius AL, Krebs P, Sullivan BM, Oldstone MB, Popkin DL. Slc15a4, a gene required for pDC sensing of TLR ligands, is required to control persistent viral infection. PLoS Pathog. 2012;8:e1002915. doi: 10.1371/journal.ppat.1002915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosinger SE, Sodora DL, Silvestri G. Generalized immune activation and innate immune responses in simian immunodeficiency virus infection. Curr Opin HIV AIDS. 2011;6:411–418. doi: 10.1097/COH.0b013e3283499cf6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockman MA, Kwon DS, Tighe DP, Pavlik DF, Rosato PC, Sela J, Porichis F, Le Gall S, Waring MT, Moss K, et al. IL-10 is up-regulated in multiple cell types during viremic HIV infection and reversibly inhibits virus-specific T cells. Blood. 2009;114:346–356. doi: 10.1182/blood-2008-12-191296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks DG, Ha SJ, Elsaesser H, Sharpe AH, Freeman GJ, Oldstone MB. IL-10 and PD-L1 operate through distinct pathways to suppress T-cell activity during persistent viral infection. Proc Natl Acad Sci U S A. 2008a;105:20428–20433. doi: 10.1073/pnas.0811139106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks DG, Lee AM, Elsaesser H, McGavern DB, Oldstone MB. IL-10 blockade facilitates DNA vaccine-induced T cell responses and enhances clearance of persistent virus infection. J Exp Med. 2008b;205:533–541. doi: 10.1084/jem.20071948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks DG, McGavern DB, Oldstone MB. Reprogramming of antiviral T cells prevents inactivation and restores T cell activity during persistent viral infection. J Clin Invest. 2006a;116:1675–1685. doi: 10.1172/JCI26856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks DG, Teyton L, Oldstone MB, McGavern DB. Intrinsic functional dysregulation of CD4 T cells occurs rapidly following persistent viral infection. J Virol. 2005;79:10514–10527. doi: 10.1128/JVI.79.16.10514-10527.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks DG, Trifilo MJ, Edelmann KH, Teyton L, McGavern DB, Oldstone MB. Interleukin-10 determines viral clearance or persistence in vivo. Nat Med. 2006b;12:1301–1309. doi: 10.1038/nm1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton DR, Ahmed R, Barouch DH, Butera ST, Crotty S, Godzik A, Kaufmann DE, McElrath MJ, Nussenzweig MC, Pulendran B, et al. A Blueprint for HIV Vaccine Discovery. Cell Host Microbe. 2012;12:396–407. doi: 10.1016/j.chom.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellino F, Germain RN. Cooperation between CD4+ and CD8+ T cells: when, where, and how. Annu Rev Immunol. 2006a;24:519–540. doi: 10.1146/annurev.immunol.23.021704.115825. [DOI] [PubMed] [Google Scholar]
- Castellino F, Germain RN. Cooperation between CD4+ and CD8+ T cells: when, where, and how. Annu Rev Immunol. 2006b;24:519–540. doi: 10.1146/annurev.immunol.23.021704.115825. [DOI] [PubMed] [Google Scholar]
- Cervantes-Barragan L, Lewis KL, Firner S, Thiel V, Hugues S, Reith W, Ludewig B, Reizis B. Plasmacytoid dendritic cells control T-cell response to chronic viral infection. Proc Natl Acad Sci U S A. 2012;109:3012–3017. doi: 10.1073/pnas.1117359109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang JJ, Altfeld M. Innate immune activation in primary HIV-1 infection. J Infect Dis. 2010;202(Suppl 2):S297–301. doi: 10.1086/655657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevalier MF, Julg B, Pyo A, Flanders M, Ranasinghe S, Soghoian DZ, Kwon DS, Rychert J, Lian J, Muller MI, et al. HIV-1-specific interleukin-21+ CD4+ T cell responses contribute to durable viral control through the modulation of HIV-specific CD8+ T cell function. J Virol. 2011;85:733–741. doi: 10.1128/JVI.02030-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clerici M, Sarin A, Coffman RL, Wynn TA, Blatt SP, Hendrix CW, Wolf SF, Shearer GM, Henkart PA. Type 1/type 2 cytokine modulation of T-cell programmed cell death as a model for human immunodeficiency virus pathogenesis. Proc Natl Acad Sci U S A. 1994;91:11811–11815. doi: 10.1073/pnas.91.25.11811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford A, Angelosanto JM, Nadwodny KL, Blackburn SD, Wherry EJ. A role for the chemokine RANTES in regulating CD8 T cell responses during chronic viral infection. PLoS Pathog. 2011;7:e1002098. doi: 10.1371/journal.ppat.1002098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crotty S. Follicular helper CD4 T cells (TFH) Annu Rev Immunol. 2011;29:621–663. doi: 10.1146/annurev-immunol-031210-101400. [DOI] [PubMed] [Google Scholar]
- Cubas RA, Mudd JC, Savoye AL, Perreau M, van Grevenynghe J, Metcalf T, Connick E, Meditz A, Freeman GJ, Abesada-Terk G, Jr, et al. Inadequate T follicular cell help impairs B cell immunity during HIV infection. Nat Med. 2013 doi: 10.1038/nm.3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, Reddy S, Mackey EW, Miller JD, Leslie AJ, DePierres C, et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443:350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- Day CL, Seth NP, Lucas M, Appel H, Gauthier L, Lauer GM, Robbins GK, Szczepiorkowski ZM, Casson DR, Chung RT, et al. Ex vivo analysis of human memory CD4 T cells specific for hepatitis C virus using MHC class II tetramers. J Clin Invest. 2003;112:831–842. doi: 10.1172/JCI18509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doering TA, Crawford A, Angelosanto JM, Paley MA, Ziegler CG, Wherry EJ. Network analysis reveals centrally connected genes and pathways involved in CD8+ T cell exhaustion versus memory. Immunity. 2012;37:1130–1144. doi: 10.1016/j.immuni.2012.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ejrnaes M, Filippi CM, Martinic MM, Ling EM, Togher LM, Crotty S, von Herrath MG. Resolution of a chronic viral infection after interleukin-10 receptor blockade. J Exp Med. 2006;203:2461–2472. doi: 10.1084/jem.20061462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsaesser H, Sauer K, Brooks DG. IL-21 is required to control chronic viral infection. Science. 2009;324:1569–1572. doi: 10.1126/science.1174182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estaquier J, Idziorek T, Zou W, Emilie D, Farber CM, Bourez JM, Ameisen JC. T helper type 1/T helper type 2 cytokines and T cell death: preventive effect of interleukin 12 on activation-induced and CD95 (FAS/APO-1)-mediated apoptosis of CD4+ T cells from human immunodeficiency virus-infected persons. J Exp Med. 1995;182:1759–1767. doi: 10.1084/jem.182.6.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahey LM, Brooks DG. Opposing positive and negative regulation of T cell activity during viral persistence. Curr Opin Immunol. 2010a;22:348–354. doi: 10.1016/j.coi.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahey LM, Brooks DG. Opposing positive and negative regulation of T cell activity during viral persistence. Curr Opin Immunol. 2010b;22:348–354. doi: 10.1016/j.coi.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahey LM, Wilson EB, Elsaesser H, Fistonich CD, McGavern DB, Brooks DG. Viral persistence redirects CD4 T cell differentiation toward T follicular helper cells. J Exp Med. 2011;208:987–999. doi: 10.1084/jem.20101773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg MB. Changing the natural history of HIV disease. Lancet. 1996;348:239–246. doi: 10.1016/s0140-6736(96)06231-9. [DOI] [PubMed] [Google Scholar]
- Fisicaro P, Valdatta C, Massari M, Loggi E, Biasini E, Sacchelli L, Cavallo MC, Silini EM, Andreone P, Missale G, et al. Antiviral intrahepatic T-cell responses can be restored by blocking programmed death-1 pathway in chronic hepatitis B. Gastroenterology. 2010;138:682–693. 693 e681–684. doi: 10.1053/j.gastro.2009.09.052. [DOI] [PubMed] [Google Scholar]
- Fitzgerald-Bocarsly P, Jacobs ES. Plasmacytoid dendritic cells in HIV infection: striking a delicate balance. J Leukoc Biol. 2010;87:609–620. doi: 10.1189/jlb.0909635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenz T, Waibler Z, Hofmann J, Hamdorf M, Lantermann M, Reizis B, Tovey MG, Aichele P, Sutter G, Kalinke U. Concomitant type I IFN receptor-triggering of T cells and of DC is required to promote maximal modified vaccinia virus Ankara-induced T-cell expansion. Eur J Immunol. 2010;40:2769–2777. doi: 10.1002/eji.201040453. [DOI] [PubMed] [Google Scholar]
- Frohlich A, Kisielow J, Schmitz I, Freigang S, Shamshiev AT, Weber J, Marsland BJ, Oxenius A, Kopf M. IL-21R on T cells is critical for sustained functionality and control of chronic viral infection. Science. 2009;324:1576–1580. doi: 10.1126/science.1172815. [DOI] [PubMed] [Google Scholar]
- Gerlach JT, Diepolder HM, Jung MC, Gruener NH, Schraut WW, Zachoval R, Hoffmann R, Schirren CA, Santantonio T, Pape GR. Recurrence of hepatitis C virus after loss of virus-specific CD4(+) T-cell response in acute hepatitis C. Gastroenterology. 1999;117:933–941. doi: 10.1016/s0016-5085(99)70353-7. [DOI] [PubMed] [Google Scholar]
- Gilliet M, Cao W, Liu YJ. Plasmacytoid dendritic cells: sensing nucleic acids in viral infection and autoimmune diseases. Nature reviews Immunology. 2008;8:594–606. doi: 10.1038/nri2358. [DOI] [PubMed] [Google Scholar]
- Grakoui A, Shoukry NH, Woollard DJ, Han JH, Hanson HL, Ghrayeb J, Murthy KK, Rice CM, Walker CM. HCV persistence and immune evasion in the absence of memory T cell help. Science. 2003;302:659–662. doi: 10.1126/science.1088774. [DOI] [PubMed] [Google Scholar]
- Hangartner L, Zinkernagel RM, Hengartner H. Antiviral antibody responses: the two extremes of a wide spectrum. Nat Rev Immunol. 2006;6:231–243. doi: 10.1038/nri1783. [DOI] [PubMed] [Google Scholar]
- Hansen SG, Ford JC, Lewis MS, Ventura AB, Hughes CM, Coyne-Johnson L, Whizin N, Oswald K, Shoemaker R, Swanson T, et al. Profound early control of highly pathogenic SIV by an effector memory T-cell vaccine. Nature. 2011;473:523–527. doi: 10.1038/nature10003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy GA, Sieg S, Rodriguez B, Anthony D, Asaad R, Jiang W, Mudd J, Schacker T, Funderburg NT, Pilch-Cooper HA, et al. Interferon-alpha is the primary plasma type-I IFN in HIV-1 infection and correlates with immune activation and disease markers. PLoS One. 2013;8:e56527. doi: 10.1371/journal.pone.0056527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harker JA, Lewis GM, Mack L, Zuniga EI. Late interleukin-6 escalates T follicular helper cell responses and controls a chronic viral infection. Science. 2011;334:825–829. doi: 10.1126/science.1208421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havenar-Daughton C, Kolumam GA, Murali-Krishna K. Cutting Edge: The direct action of type I IFN on CD4 T cells is critical for sustaining clonal expansion in response to a viral but not a bacterial infection. J Immunol. 2006;176:3315–3319. doi: 10.4049/jimmunol.176.6.3315. [DOI] [PubMed] [Google Scholar]
- Hersperger AR, Martin JN, Shin LY, Sheth PM, Kovacs CM, Cosma GL, Makedonas G, Pereyra F, Walker BD, Kaul R, et al. Increased HIV-specific CD8+ T-cell cytotoxic potential in HIV elite controllers is associated with T-bet expression. Blood. 2011;117:3799–3808. doi: 10.1182/blood-2010-12-322727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmeyer KA, Jeon H, Zang X. The PD-1/PD-L1 (B7-H1) pathway in chronic infection-induced cytotoxic T lymphocyte exhaustion. J Biomed Biotechnol. 2011;2011:451694. doi: 10.1155/2011/451694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holling TM, van der Stoep N, Quinten E, van den Elsen PJ. Activated human T cells accomplish MHC class II expression through T cell-specific occupation of class II transactivator promoter III. J Immunol. 2002;168:763–770. doi: 10.4049/jimmunol.168.2.763. [DOI] [PubMed] [Google Scholar]
- Hunziker L, Recher M, Macpherson AJ, Ciurea A, Freigang S, Hengartner H, Zinkernagel RM. Hypergammaglobulinemia and autoantibody induction mechanisms in viral infections. Nat Immunol. 2003;4:343–349. doi: 10.1038/ni911. [DOI] [PubMed] [Google Scholar]
- Iyer SS, Chatraw JH, Tan WG, Wherry EJ, Becker TC, Ahmed R, Kapasi ZF. Protein energy malnutrition impairs homeostatic proliferation of memory CD8 T cells. J Immunol. 2012;188:77–84. doi: 10.4049/jimmunol.1004027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jellison ER, Kim SK, Welsh RM. Cutting edge: MHC class II-restricted killing in vivo during viral infection. J Immunol. 2005;174:614–618. doi: 10.4049/jimmunol.174.2.614. [DOI] [PubMed] [Google Scholar]
- Jin HT, Anderson AC, Tan WG, West EE, Ha SJ, Araki K, Freeman GJ, Kuchroo VK, Ahmed R. Cooperation of Tim-3 and PD-1 in CD8 T-cell exhaustion during chronic viral infection. Proc Natl Acad Sci U S A. 2010;107:14733–14738. doi: 10.1073/pnas.1009731107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones RB, Ndhlovu LC, Barbour JD, Sheth PM, Jha AR, Long BR, Wong JC, Satkunarajah M, Schweneker M, Chapman JM, et al. Tim-3 expression defines a novel population of dysfunctional T cells with highly elevated frequencies in progressive HIV-1 infection. J Exp Med. 2008;205:2763–2779. doi: 10.1084/jem.20081398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junt T, Scandella E, Ludewig B. Form follows function: lymphoid tissue microarchitecture in antimicrobial immune defence. Nat Rev Immunol. 2008;8:764–775. doi: 10.1038/nri2414. [DOI] [PubMed] [Google Scholar]
- Kao C, Oestreich KJ, Paley MA, Crawford A, Angelosanto JM, Ali MA, Intlekofer AM, Boss JM, Reiner SL, Weinmann AS, et al. Transcription factor T-bet represses expression of the inhibitory receptor PD-1 and sustains virus-specific CD8+ T cell responses during chronic infection. Nat Immunol. 2011;12:663–671. doi: 10.1038/ni.2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan DE, Ikeda F, Li Y, Nakamoto N, Ganesan S, Valiga ME, Nunes FA, Rajender Reddy K, Chang KM. Peripheral virus-specific T-cell interleukin-10 responses develop early in acute hepatitis C infection and become dominant in chronic hepatitis. Journal of hepatology. 2008;48:903–913. doi: 10.1016/j.jhep.2008.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein F, Diskin R, Scheid JF, Gaebler C, Mouquet H, Georgiev IS, Pancera M, Zhou T, Incesu RB, Fu BZ, et al. Somatic mutations of the immunoglobulin framework are generally required for broad and potent HIV-1 neutralization. Cell. 2013;153:126–138. doi: 10.1016/j.cell.2013.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladell K, Hashimoto M, Iglesias MC, Wilmann PG, McLaren JE, Gras S, Chikata T, Kuse N, Fastenackels S, Gostick E, et al. A molecular basis for the control of preimmune escape variants by HIV-specific CD8+ T cells. Immunity. 2013;38:425–436. doi: 10.1016/j.immuni.2012.11.021. [DOI] [PubMed] [Google Scholar]
- Landay AL, Clerici M, Hashemi F, Kessler H, Berzofsky JA, Shearer GM. In vitro restoration of T cell immune function in human immunodeficiency virus-positive persons: effects of interleukin (IL)-12 and anti-IL-10. J Infect Dis. 1996;173:1085–1091. doi: 10.1093/infdis/173.5.1085. [DOI] [PubMed] [Google Scholar]
- Lauterbach H, Truong P, McGavern DB. Clearance of an immunosuppressive virus from the CNS coincides with immune reanimation and diversification. Virol J. 2007;4:53. doi: 10.1186/1743-422X-4-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law M, Maruyama T, Lewis J, Giang E, Tarr AW, Stamataki Z, Gastaminza P, Chisari FV, Jones IM, Fox RI, et al. Broadly neutralizing antibodies protect against hepatitis C virus quasispecies challenge. Nat Med. 2008;14:25–27. doi: 10.1038/nm1698. [DOI] [PubMed] [Google Scholar]
- Le Bon A, Etchart N, Rossmann C, Ashton M, Hou S, Gewert D, Borrow P, Tough DF. Cross-priming of CD8+ T cells stimulated by virus-induced type I interferon. Nat Immunol. 2003;4:1009–1015. doi: 10.1038/ni978. [DOI] [PubMed] [Google Scholar]
- Leone A, Picker LJ, Sodora DL. IL-2, IL-7 and IL-15 as immuno-modulators during SIV/HIV vaccination and treatment. Curr HIV Res. 2009;7:83–90. doi: 10.2174/157016209787048519. [DOI] [PubMed] [Google Scholar]
- Lichterfeld M, Kaufmann DE, Yu XG, Mui SK, Addo MM, Johnston MN, Cohen D, Robbins GK, Pae E, Alter G, et al. Loss of HIV-1-specific CD8+ T cell proliferation after acute HIV-1 infection and restoration by vaccine-induced HIV-1-specific CD4+ T cells. J Exp Med. 2004;200:701–712. doi: 10.1084/jem.20041270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindqvist M, van Lunzen J, Soghoian DZ, Kuhl BD, Ranasinghe S, Kranias G, Flanders MD, Cutler S, Yudanin N, Muller MI, et al. Expansion of HIV-specific T follicular helper cells in chronic HIV infection. J Clin Invest. 2012;122:3271–3280. doi: 10.1172/JCI64314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macal M, Lewis GM, Kunz S, Flavell R, Harker JA, Zuniga EI. Plasmacytoid dendritic cells are productively infected and activated through TLR-7 early after arenavirus infection. Cell Host Microbe. 2012;11:617–630. doi: 10.1016/j.chom.2012.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall NB, Swain SL. Cytotoxic CD4 T cells in antiviral immunity. J Biomed Biotechnol. 2011;2011:954602. doi: 10.1155/2011/954602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matloubian M, Concepcion RJ, Ahmed R. CD4+ T cells are required to sustain CD8+ cytotoxic T-cell responses during chronic viral infection. J Virol. 1994;68:8056–8063. doi: 10.1128/jvi.68.12.8056-8063.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendoza D, Migueles SA, Rood JE, Peterson B, Johnson S, Doria-Rose N, Schneider D, Rakasz E, Trivett MT, Trubey CM, et al. Cytotoxic capacity of SIV-specific CD8(+) T cells against primary autologous targets correlates with immune control in SIV-infected rhesus macaques. PLoS Pathog. 2013;9:e1003195. doi: 10.1371/journal.ppat.1003195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moir S, Chun TW, Fauci AS. Pathogenic mechanisms of HIV disease. Annu Rev Pathol. 2011;6:223–248. doi: 10.1146/annurev-pathol-011110-130254. [DOI] [PubMed] [Google Scholar]
- Moir S, Fauci AS. B cells in HIV infection and disease. Nat Rev Immunol. 2009;9:235–245. doi: 10.1038/nri2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller SN, Germain RN. Stromal cell contributions to the homeostasis and functionality of the immune system. Nature reviews Immunology. 2009;9:618–629. doi: 10.1038/nri2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller SN, Hosiawa-Meagher KA, Konieczny BT, Sullivan BM, Bachmann MF, Locksley RM, Ahmed R, Matloubian M. Regulation of homeostatic chemokine expression and cell trafficking during immune responses. Science. 2007a;317:670–674. doi: 10.1126/science.1144830. [DOI] [PubMed] [Google Scholar]
- Mueller SN, Matloubian M, Clemens DM, Sharpe AH, Freeman GJ, Gangappa S, Larsen CP, Ahmed R. Viral targeting of fibroblastic reticular cells contributes to immunosuppression and persistence during chronic infection. Proc Natl Acad Sci U S A. 2007b;104:15430–15435. doi: 10.1073/pnas.0702579104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller SN, Vanguri VK, Ha SJ, West EE, Keir ME, Glickman JN, Sharpe AH, Ahmed R. PD-L1 has distinct functions in hematopoietic and nonhematopoietic cells in regulating T cell responses during chronic infection in mice. J Clin Invest. 2010;120:2508–2515. doi: 10.1172/JCI40040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamoto N, Kaplan DE, Coleclough J, Li Y, Valiga ME, Kaminski M, Shaked A, Olthoff K, Gostick E, Price DA, et al. Functional restoration of HCV-specific CD8 T cells by PD-1 blockade is defined by PD-1 expression and compartmentalization. Gastroenterology. 2008;134:1927–1937. 1937 e1921–1922. doi: 10.1053/j.gastro.2008.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanjappa SG, Kim EH, Suresh M. Immunotherapeutic effects of IL-7 during a chronic viral infection in mice. Blood. 2011;117:5123–5132. doi: 10.1182/blood-2010-12-323154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navikas V, Link J, Persson C, Olsson T, Hojeberg B, Ljungdahl A, Link H, Wahren B. Increased mRNA expression of IL-6, IL-10, TNF-alpha, and perforin in blood mononuclear cells in human HIV infection. J Acquir Immune Defic Syndr Hum Retrovirol. 1995;9:484–489. [PubMed] [Google Scholar]
- Ng CT, Nayak BP, Schmedt C, Oldstone MB. Immortalized clones of fibroblastic reticular cells activate virus-specific T cells during virus infection. Proc Natl Acad Sci U S A. 2012;109:7823–7828. doi: 10.1073/pnas.1205850109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng CT, Oldstone MB. Infected CD8alpha-dendritic cells are the predominant source of IL-10 during establishment of persistent viral infection. Proc Natl Acad Sci U S A. 2012;109:14116–14121. doi: 10.1073/pnas.1211910109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris PJ, Moffett HF, Yang OO, Kaufmann DE, Clark MJ, Addo MM, Rosenberg ES. Beyond help: direct effector functions of human immunodeficiency virus type 1-specific CD4(+) T cells. J Virol. 2004;78:8844–8851. doi: 10.1128/JVI.78.16.8844-8851.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldstone MB. Viral persistence: parameters, mechanisms and future predictions. Virology. 2006;344:111–118. doi: 10.1016/j.virol.2005.09.028. [DOI] [PubMed] [Google Scholar]
- Oldstone MB, Salvato M, Tishon A, Lewicki H. Virus-lymphocyte interactions. III. Biologic parameters of a virus variant that fails to generate CTL and establishes persistent infection in immunocompetent hosts. Virology. 1988;164:507–516. doi: 10.1016/0042-6822(88)90565-x. [DOI] [PubMed] [Google Scholar]
- Ou R, Zhou S, Huang L, Moskophidis D. Critical role for alpha/beta and gamma interferons in persistence of lymphocytic choriomeningitis virus by clonal exhaustion of cytotoxic T cells. J Virol. 2001;75:8407–8423. doi: 10.1128/JVI.75.18.8407-8423.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang W, Rutz S, Crellin NK, Valdez PA, Hymowitz SG. Regulation and functions of the IL-10 family of cytokines in inflammation and disease. Annu Rev Immunol. 2011;29:71–109. doi: 10.1146/annurev-immunol-031210-101312. [DOI] [PubMed] [Google Scholar]
- Paley MA, Kroy DC, Odorizzi PM, Johnnidis JB, Dolfi DV, Barnett BE, Bikoff EK, Robertson EJ, Lauer GM, Reiner SL, et al. Progenitor and terminal subsets of CD8+ T cells cooperate to contain chronic viral infection. Science. 2012;338:1220–1225. doi: 10.1126/science.1229620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer BE, Neff CP, Lecureux J, Ehler A, Dsouza M, Remling-Mulder L, Korman AJ, Fontenot AP, Akkina R. In vivo blockade of the PD-1 receptor suppresses HIV-1 viral loads and improves CD4+ T cell levels in humanized mice. J Immunol. 2013;190:211–219. doi: 10.4049/jimmunol.1201108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce EL, Mullen AC, Martins GA, Krawczyk CM, Hutchins AS, Zediak VP, Banica M, DiCioccio CB, Gross DA, Mao CA, et al. Control of effector CD8+ T cell function by the transcription factor Eomesodermin. Science. 2003;302:1041–1043. doi: 10.1126/science.1090148. [DOI] [PubMed] [Google Scholar]
- Peppa D, Micco L, Javaid A, Kennedy PT, Schurich A, Dunn C, Pallant C, Ellis G, Khanna P, Dusheiko G, et al. Blockade of immunosuppressive cytokines restores NK cell antiviral function in chronic hepatitis B virus infection. PLoS Pathog. 2010;6:e1001227. doi: 10.1371/journal.ppat.1001227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovas C, Casazza JP, Brenchley JM, Price DA, Gostick E, Adams WC, Precopio ML, Schacker T, Roederer M, Douek DC, et al. PD-1 is a regulator of virus-specific CD8+ T cell survival in HIV infection. J Exp Med. 2006;203:2281–2292. doi: 10.1084/jem.20061496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovas C, Yamamoto T, Gerner MY, Boswell KL, Wloka K, Smith EC, Ambrozak DR, Sandler NG, Timmer KJ, Sun X, et al. CD4 T follicular helper cell dynamics during SIV infection. J Clin Invest. 2012;122:3281–3294. doi: 10.1172/JCI63039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popkin DL, Teijaro JR, Sullivan BM, Urata S, Rutschmann S, de la Torre JC, Kunz S, Beutler B, Oldstone M. Hypomorphic mutation in the site-1 protease mbtps1 endows resistance to persistent viral infection in a cell-specific manner. Cell Host Microbe. 2011;9:212–222. doi: 10.1016/j.chom.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulendran B. Modulating vaccine responses with dendritic cells and Toll-like receptors. Immunol Rev. 2004;199:227–250. doi: 10.1111/j.0105-2896.2004.00144.x. [DOI] [PubMed] [Google Scholar]
- Recher M, Lang KS, Hunziker L, Freigang S, Eschli B, Harris NL, Navarini A, Senn BM, Fink K, Lotscher M, et al. Deliberate removal of T cell help improves virus-neutralizing antibody production. Nat Immunol. 2004;5:934–942. doi: 10.1038/ni1102. [DOI] [PubMed] [Google Scholar]
- Richter K, Agnellini P, Oxenius A. On the role of the inhibitory receptor LAG-3 in acute and chronic LCMV infection. Int Immunol. 2010;22:13–23. doi: 10.1093/intimm/dxp107. [DOI] [PubMed] [Google Scholar]
- Ries M, Pritschet K, Schmidt B. Blocking type I interferon production: a new therapeutic option to reduce the HIV-1-induced immune activation. Clin Dev Immunol. 2012;2012:534929. doi: 10.1155/2012/534929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigopoulou EI, Abbott WG, Haigh P, Naoumov NV. Blocking of interleukin-10 receptor--a novel approach to stimulate T-helper cell type 1 responses to hepatitis C virus. Clin Immunol. 2005;117:57–64. doi: 10.1016/j.clim.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Rosenberg ES, Billingsley JM, Caliendo AM, Boswell SL, Sax PE, Kalams SA, Walker BD. Vigorous HIV-1-specific CD4+ T cell responses associated with control of viremia. Science. 1997;278:1447–1450. doi: 10.1126/science.278.5342.1447. [DOI] [PubMed] [Google Scholar]
- Sauce D, Elbim C, Appay V. Monitoring cellular immune markers in HIV infection: from activation to exhaustion. Curr Opin HIV AIDS. 2013;8:125–131. doi: 10.1097/COH.0b013e32835d08a9. [DOI] [PubMed] [Google Scholar]
- Schulze Zur Wiesch J, Ciuffreda D, Lewis-Ximenez L, Kasprowicz V, Nolan BE, Streeck H, Aneja J, Reyor LL, Allen TM, Lohse AW, et al. Broadly directed virus-specific CD4+ T cell responses are primed during acute hepatitis C infection, but rapidly disappear from human blood with viral persistence. J Exp Med. 2012;209:61–75. doi: 10.1084/jem.20100388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevilla N, Kunz S, Holz A, Lewicki H, Homann D, Yamada H, Campbell KP, de La Torre JC, Oldstone MB. Immunosuppression and resultant viral persistence by specific viral targeting of dendritic cells. J Exp Med. 2000;192:1249–1260. doi: 10.1084/jem.192.9.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevilla N, McGavern DB, Teng C, Kunz S, Oldstone MB. Viral targeting of hematopoietic progenitors and inhibition of DC maturation as a dual strategy for immune subversion. J Clin Invest. 2004;113:737–745. doi: 10.1172/JCI20243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe AH, Wherry EJ, Ahmed R, Freeman GJ. The function of programmed cell death 1 and its ligands in regulating autoimmunity and infection. Nat Immunol. 2007;8:239–245. doi: 10.1038/ni1443. [DOI] [PubMed] [Google Scholar]
- Shin H, Blackburn SD, Intlekofer AM, Kao C, Angelosanto JM, Reiner SL, Wherry EJ. A role for the transcriptional repressor Blimp-1 in CD8(+) T cell exhaustion during chronic viral infection. Immunity. 2009;31:309–320. doi: 10.1016/j.immuni.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegert S, Luther SA. Positive and negative regulation of T cell responses by fibroblastic reticular cells within paracortical regions of lymph nodes. Front Immunol. 2012;3:285. doi: 10.3389/fimmu.2012.00285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soghoian DZ, Jessen H, Flanders M, Sierra-Davidson K, Cutler S, Pertel T, Ranasinghe S, Lindqvist M, Davis I, Lane K, et al. HIV-specific cytolytic CD4 T cell responses during acute HIV infection predict disease outcome. Sci Transl Med. 2012;4:123ra125. doi: 10.1126/scitranslmed.3003165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streeck H, D’Souza MP, Littman DR, Crotty S. Harnessing CD4(+) T cell responses in HIV vaccine development. Nat Med. 2013;19:143–149. doi: 10.1038/nm.3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan BM, Emonet SF, Welch MJ, Lee AM, Campbell KP, de la Torre JC, Oldstone MB. Point mutation in the glycoprotein of lymphocytic choriomeningitis virus is necessary for receptor binding, dendritic cell infection, and long-term persistence. Proc Natl Acad Sci U S A. 2011;108:2969–2974. doi: 10.1073/pnas.1019304108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teijaro JR, Ng C, Lee AM, Sullivan BM, Sheehan KC, Welch M, Schreiber RD, de la Torre JC, Oldstone MB. Persistent LCMV infection is controlled by blockade of type I interferon signaling. Science. 2013;340:207–211. doi: 10.1126/science.1235214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thimme R, Oldach D, Chang KM, Steiger C, Ray SC, Chisari FV. Determinants of viral clearance and persistence during acute hepatitis C virus infection. J Exp Med. 2001;194:1395–1406. doi: 10.1084/jem.194.10.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tishon A, Borrow P, Evans C, Oldstone MB. Virus-induced immunosuppression. 1. Age at infection relates to a selective or generalized defect. Virology. 1993;195:397–405. doi: 10.1006/viro.1993.1389. [DOI] [PubMed] [Google Scholar]
- Tishon A, Lewicki H, Rall G, Von Herrath M, Oldstone MB. An essential role for type 1 interferon-gamma in terminating persistent viral infection. Virology. 1995;212:244–250. doi: 10.1006/viro.1995.1477. [DOI] [PubMed] [Google Scholar]
- Torheim EA, Ndhlovu LC, Pettersen FO, Larsen TL, Jha AR, Torgersen KM, Kvale D, Nixon DF, Tasken K, Aandahl EM. Interleukin-10-secreting T cells define a suppressive subset within the HIV-1-specific T-cell population. Eur J Immunol. 2009;39:1280–1287. doi: 10.1002/eji.200839002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turk G, Ghiglione Y, Falivene J, Socias ME, Laufer N, Coloccini RS, Rodriguez AM, Ruiz MJ, Pando MA, Giavedoni LD, et al. Early Gag immunodominance of the HIV-specific T-cell response during acute/early infection is associated with higher CD8+ T-cell antiviral activity, and correlates with the preservation of the CD4+ T-cell compartment. J Virol. 2013 doi: 10.1128/JVI.00865-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbani S, Amadei B, Fisicaro P, Tola D, Orlandini A, Sacchelli L, Mori C, Missale G, Ferrari C. Outcome of acute hepatitis C is related to virus-specific CD4 function and maturation of antiviral memory CD8 responses. Hepatology. 2006;44:126–139. doi: 10.1002/hep.21242. [DOI] [PubMed] [Google Scholar]
- Urbani S, Amadei B, Tola D, Pedrazzi G, Sacchelli L, Cavallo MC, Orlandini A, Missale G, Ferrari C. Restoration of HCV-specific T cell functions by PD-1/PD-L1 blockade in HCV infection: effect of viremia levels and antiviral treatment. J Hepatol. 2008;48:548–558. doi: 10.1016/j.jhep.2007.12.014. [DOI] [PubMed] [Google Scholar]
- Valentine L, Potts R, Premenko-Lanier M. CD8+ T cell-derived IFN-gamma prevents infection by a second heterologous virus. J Immunol. 2012;189:5841–5848. doi: 10.4049/jimmunol.1201679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezys V, Masopust D, Kemball CC, Barber DL, O’Mara LA, Larsen CP, Pearson TC, Ahmed R, Lukacher AE. Continuous recruitment of naive T cells contributes to heterogeneity of antiviral CD8 T cells during persistent infection. J Exp Med. 2006;203:2263–2269. doi: 10.1084/jem.20060995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vingert B, Benati D, Lambotte O, de Truchis P, Slama L, Jeannin P, Galperin M, Perez-Patrigeon S, Boufassa F, Kwok WW, et al. HIV controllers maintain a population of highly efficient Th1 effector cells in contrast to patients treated in the long term. J Virol. 2012;86:10661–10674. doi: 10.1128/JVI.00056-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Swiecki M, Cella M, Alber G, Schreiber RD, Gilfillan S, Colonna M. Timing and magnitude of type I interferon responses by distinct sensors impact CD8 T cell exhaustion and chronic viral infection. Cell Host Microbe. 2012;11:631–642. doi: 10.1016/j.chom.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh RM, Bahl K, Marshall HD, Urban SL. Type 1 interferons and antiviral CD8 T-cell responses. PLoS Pathog. 2012;8:e1002352. doi: 10.1371/journal.ppat.1002352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wherry EJ. T cell exhaustion. Nat Immunol. 2011;12:492–499. doi: 10.1038/ni.2035. [DOI] [PubMed] [Google Scholar]
- Wherry EJ, Ha SJ, Kaech SM, Haining WN, Sarkar S, Kalia V, Subramaniam S, Blattman JN, Barber DL, Ahmed R. Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity. 2007;27:670–684. doi: 10.1016/j.immuni.2007.09.006. [DOI] [PubMed] [Google Scholar]
- Wilson EB, Brooks DG. Translating insights from persistent LCMV infection into anti-HIV immunity. Immunol Res. 2010;48:3–13. doi: 10.1007/s12026-010-8162-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson EB, Brooks DG. The role of IL-10 in regulating immunity to persistent viral infections. Curr Top Microbiol Immunol. 2011;350:39–65. doi: 10.1007/82_2010_96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson EB, Kidani Y, Elsaesser H, Barnard J, Raff L, Karp CL, Bensinger S, Brooks DG. Emergence of distinct multiarmed immunoregulatory antigen-presenting cells during persistent viral infection. Cell Host Microbe. 2012;11:481–491. doi: 10.1016/j.chom.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson EB, Yamada DH, Elsaesser H, Herskovitz J, Deng J, Cheng G, Aronow BJ, Karp CL, Brooks DG. Blockade of chronic type I interferon signaling to control persistent LCMV infection. Science. 2013;340:202–207. doi: 10.1126/science.1235208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L, KewalRamani VN. Dendritic-cell interactions with HIV: infection and viral dissemination. Nat Rev Immunol. 2006;6:859–868. doi: 10.1038/nri1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi JS, Cox MA, Zajac AJ. Interleukin-21: a multifunctional regulator of immunity to infections. Microbes and infection/Institut Pasteur. 2010a;12:1111–1119. doi: 10.1016/j.micinf.2010.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi JS, Cox MA, Zajac AJ. T-cell exhaustion: characteristics, causes and conversion. Immunology. 2010b;129:474–481. doi: 10.1111/j.1365-2567.2010.03255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi JS, Du M, Zajac AJ. A vital role for interleukin-21 in the control of a chronic viral infection. Science. 2009;324:1572–1576. doi: 10.1126/science.1175194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngblood B, Wherry EJ, Ahmed R. Acquired transcriptional programming in functional and exhausted virus-specific CD8 T cells. Curr Opin HIV AIDS. 2012;7:50–57. doi: 10.1097/COH.0b013e32834ddcf2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue FY, Lo C, Sakhdari A, Lee EY, Kovacs CM, Benko E, Liu J, Song H, Jones RB, Sheth P, et al. HIV-specific IL-21 producing CD4+ T cells are induced in acute and chronic progressive HIV infection and are associated with relative viral control. J Immunol. 2010;185:498–506. doi: 10.4049/jimmunol.0903915. [DOI] [PubMed] [Google Scholar]
- Zaunders JJ, Dyer WB, Wang B, Munier ML, Miranda-Saksena M, Newton R, Moore J, Mackay CR, Cooper DA, Saksena NK, et al. Identification of circulating antigen-specific CD4+ T lymphocytes with a CCR5+, cytotoxic phenotype in an HIV-1 long-term nonprogressor and in CMV infection. Blood. 2004;103:2238–2247. doi: 10.1182/blood-2003-08-2765. [DOI] [PubMed] [Google Scholar]
- Zeng M, Haase AT, Schacker TW. Lymphoid tissue structure and HIV-1 infection: life or death for T cells. Trends in immunology. 2012a;33:306–314. doi: 10.1016/j.it.2012.04.002. [DOI] [PubMed] [Google Scholar]
- Zeng M, Paiardini M, Engram JC, Beilman GJ, Chipman JG, Schacker TW, Silvestri G, Haase AT. Critical role of CD4 T cells in maintaining lymphoid tissue structure for immune cell homeostasis and reconstitution. Blood. 2012b;120:1856–1867. doi: 10.1182/blood-2012-03-418624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Yamane H, Paul WE. Differentiation of effector CD4 T cell populations (*) Annu Rev Immunol. 2010;28:445–489. doi: 10.1146/annurev-immunol-030409-101212. [DOI] [PMC free article] [PubMed] [Google Scholar]