Abstract
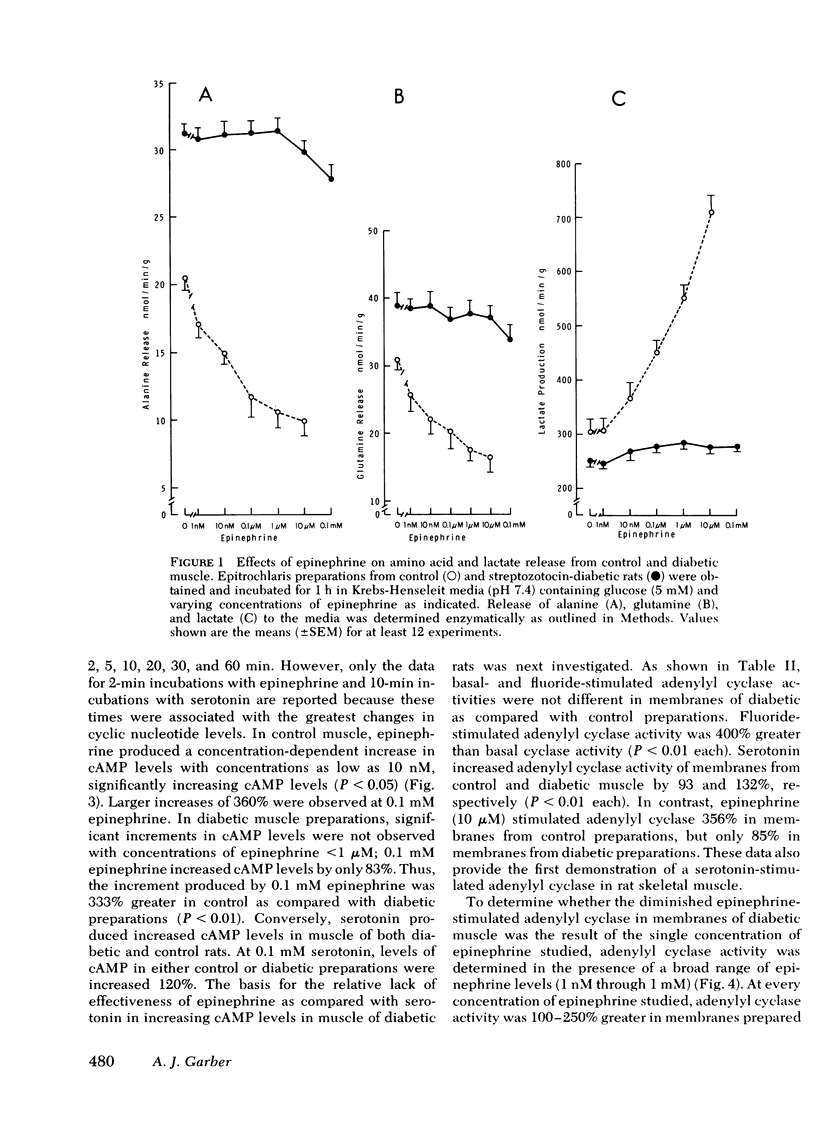
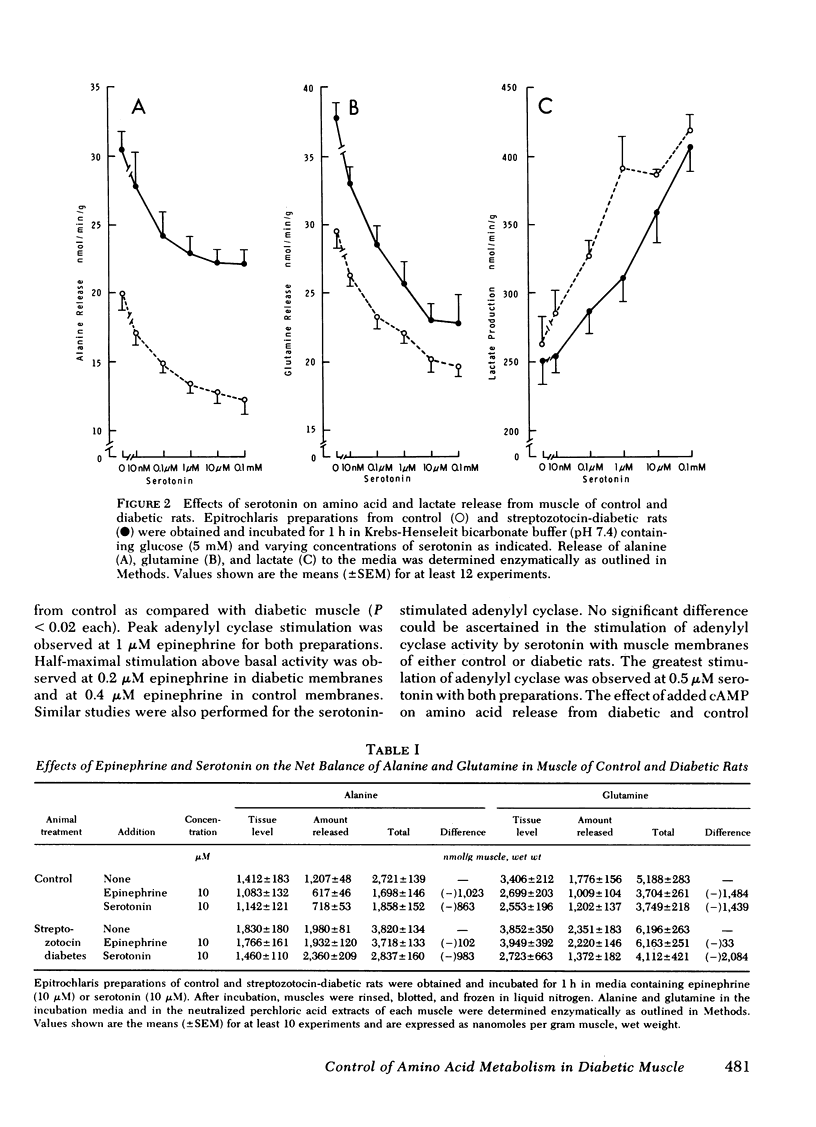
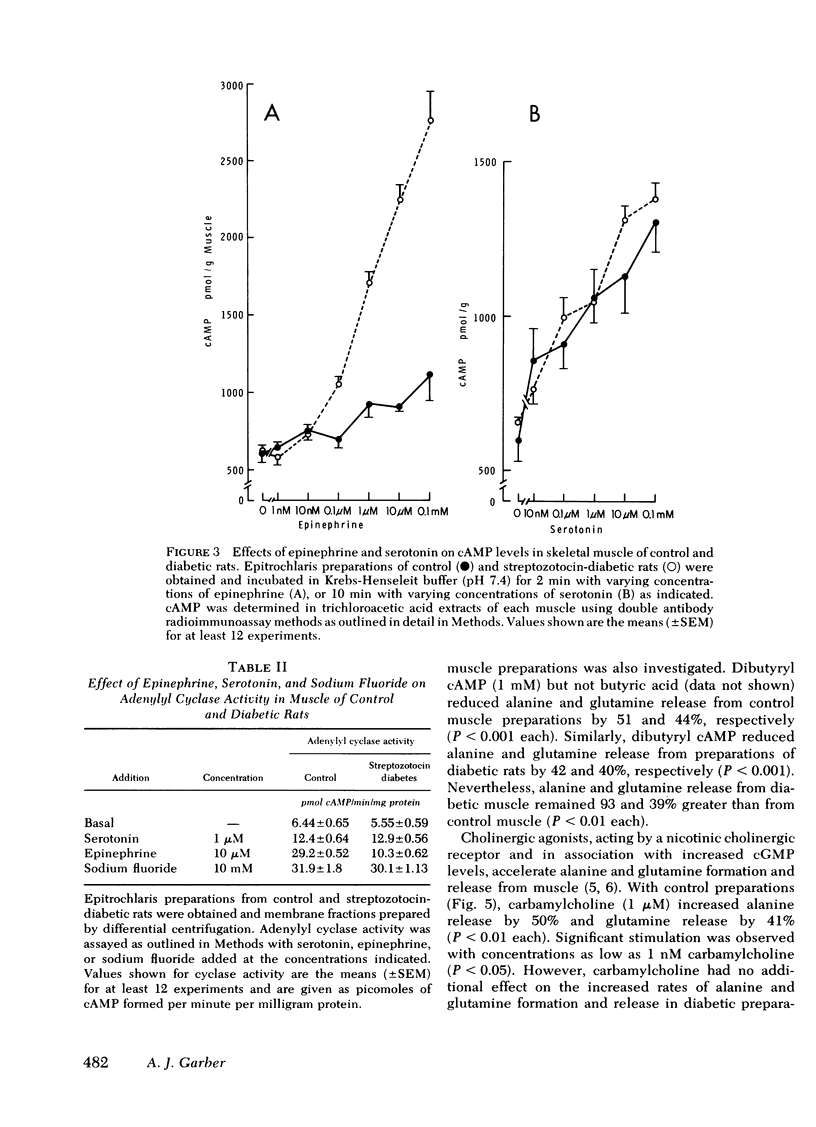
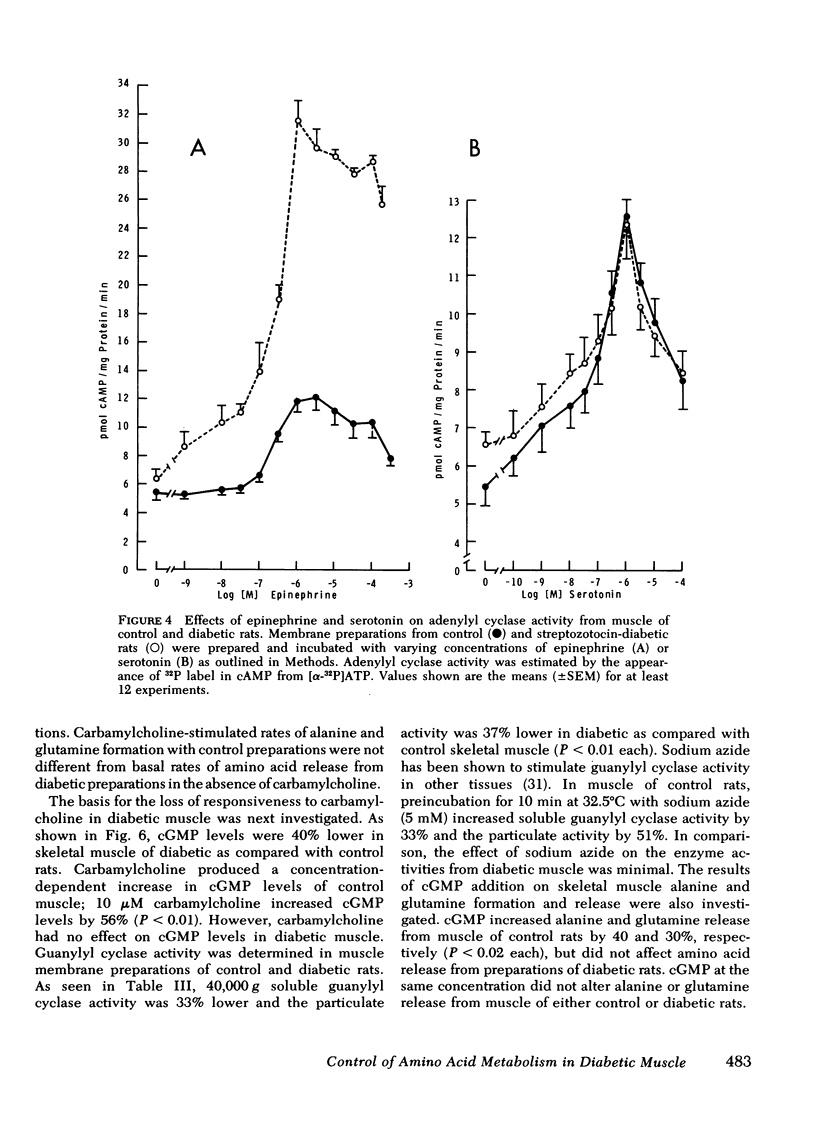
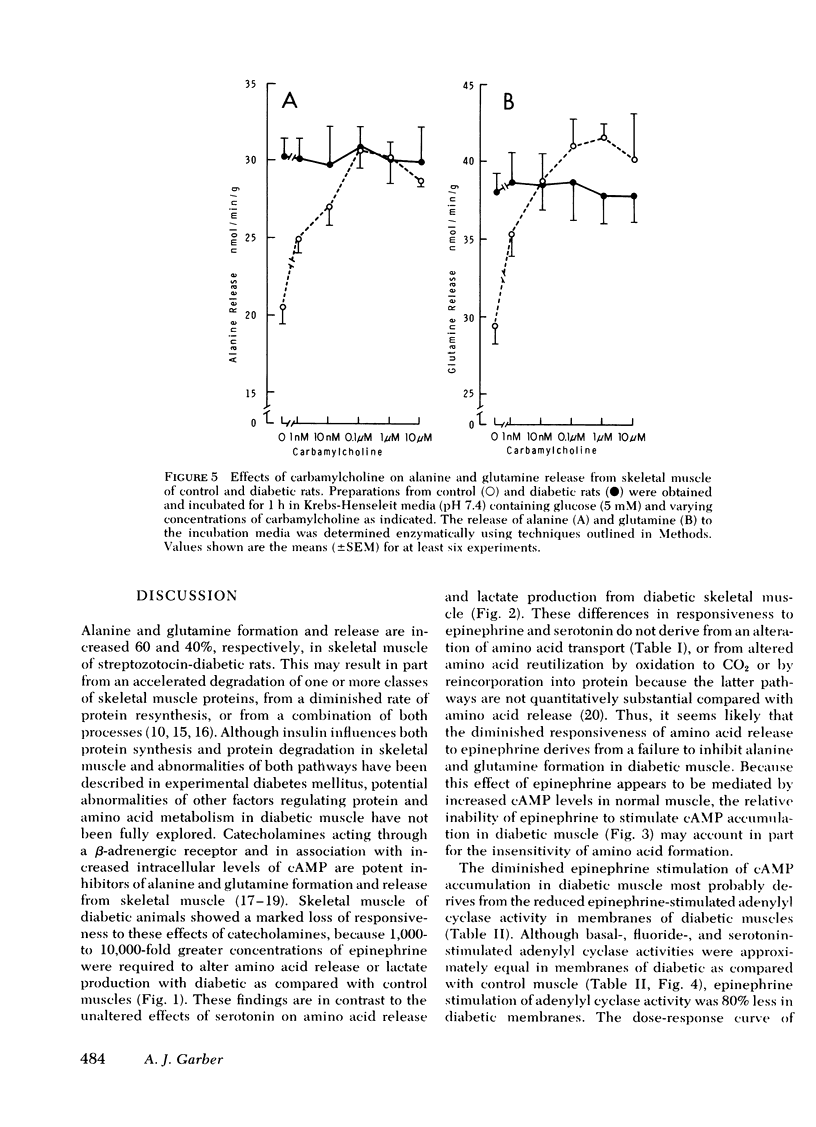
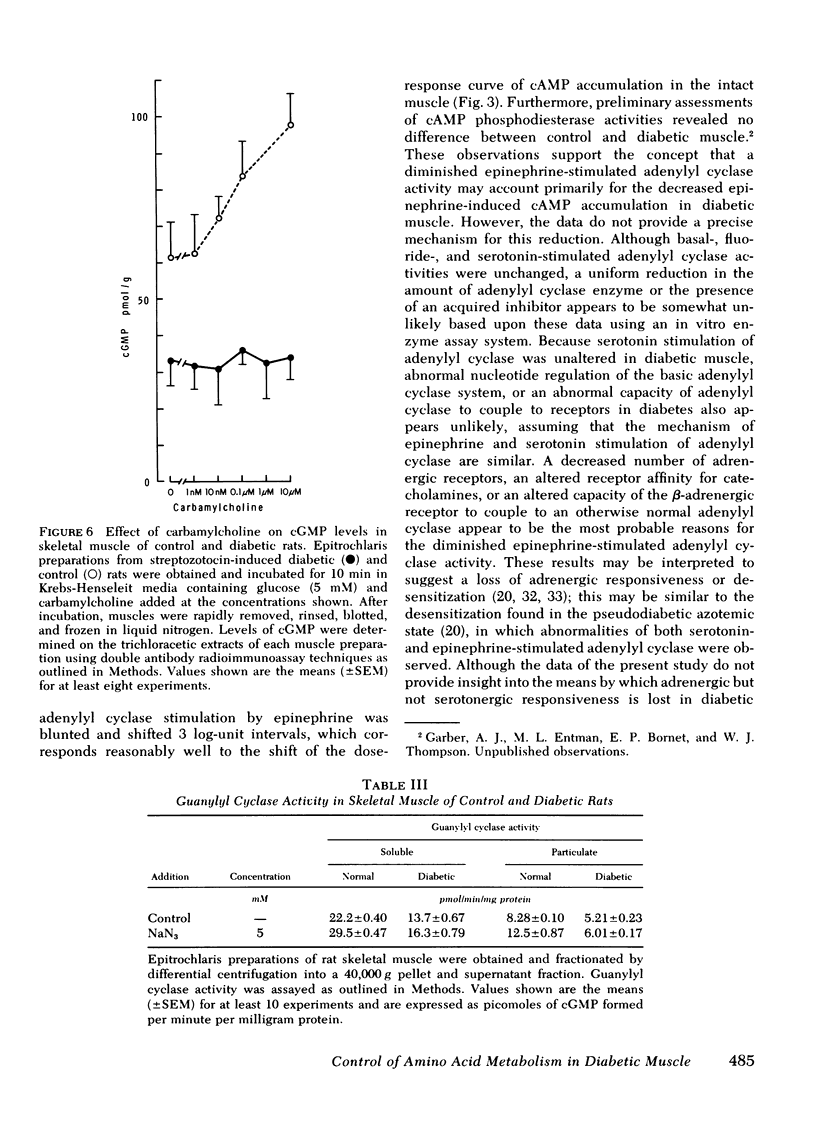
The impact of diabetes on cyclic nucleotide-associated mechanisms regulating skeletal muscle protein and amino acid metabolism was assessed using epitrochlaris preparations from streptozotocin-induced diabetic rats. 1 nM epinephrine inhibited alanine and glutamine release from control preparations, but no inhibition was observed from diabetic preparations with <0.1 mM. 10 nM epinephrine stimulated lactate production from control muscle but stimulation in diabetic preparations was observed only at 0.1 mM. Serotonin inhibited amino acid release and stimulated lactate production equally in control and diabetic muscle. 0.1 mM epinephrine increased cyclic (c)AMP levels by 360% in control muscles, but these levels were increased only 83% in diabetic muscle. Basal-, fluoride-, and serotonin-stimulated adenylyl cyclase activities were equal in membrane preparations of diabetic and control muscle, but epinephrine-stimulated adenylyl cyclase was reduced by 60% in diabetic muscle. Carbamylcholine stimulation of alanine and glutamine release was blunted in diabetic preparations. Carbamylcholine increased cGMP levels in control but not in diabetic muscle. In diabetic muscle, guanylyl cyclase activity was 65% of control and the stimulation of cyclase activity by sodium azide was less in diabetic than control preparations. Added cGMP stimulated alanine and glutamine release from control, but not from diabetic muscle. These data suggest a loss of adrenergic and cholinergic responsiveness in diabetic muscle. Because amino acid release also showed a decreased responsiveness to added cAMP and cGMP, the presence of other derangements in the mechanism(s) of cyclic nucleotide regulation of muscle amino acid metabolism also seems likely.
Full text
PDF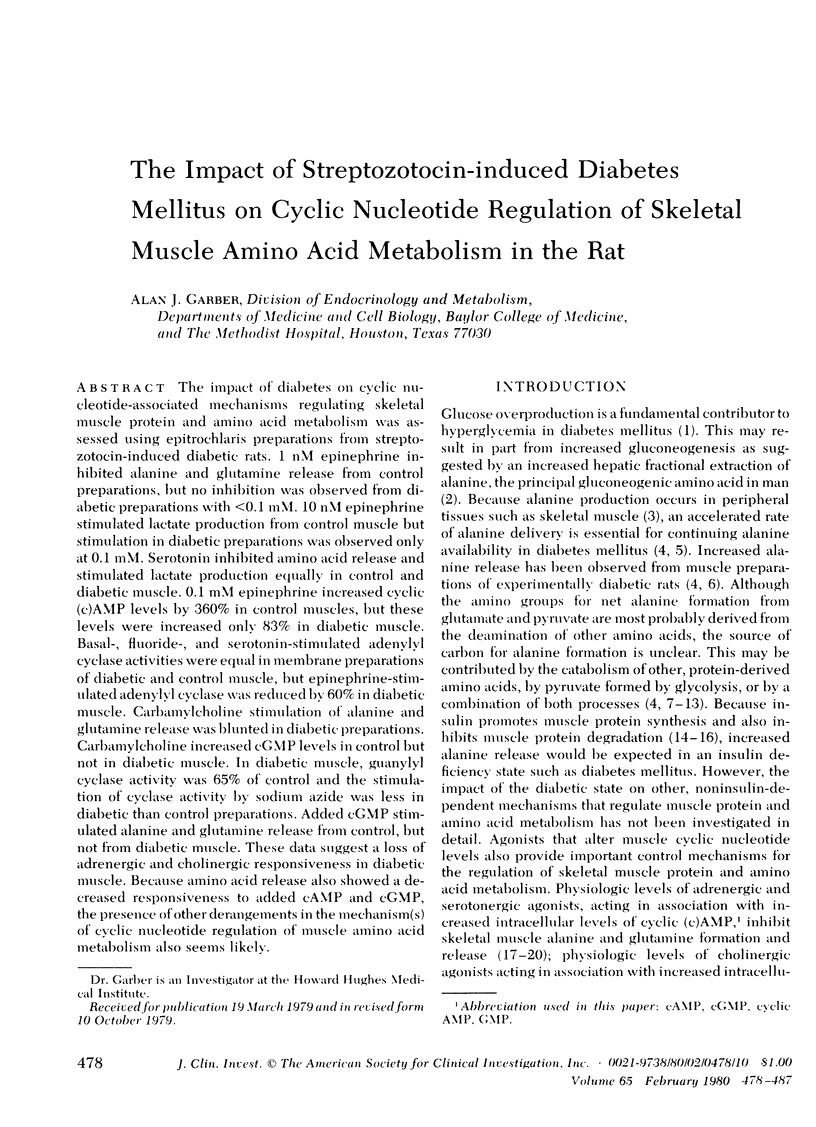



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arner P., Ostman J. Abnormalities in the adrenergic control and the rate of lipolysis in isolated human subcutaneous adipose tissue in diabetes mellitus. Diabetologia. 1976 Dec;12(6):593–599. doi: 10.1007/BF01220636. [DOI] [PubMed] [Google Scholar]
- Bockaert J., Hunzicker-Dunn M., Birnbaumer L. Hormone-stimulated desensitization of hormone-dependent adenylyl cyclase. Dual action of luteninizing hormone on pig graafian follicle membranes. J Biol Chem. 1976 May 10;251(9):2653–2663. [PubMed] [Google Scholar]
- Bowen H. F., Moorhouse J. A. Glucose turnover and disposal in maturity-onset diabetes. J Clin Invest. 1973 Dec;52(12):3033–3045. doi: 10.1172/JCI107502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang T. W., Goldberg A. L. The origin of alanine produced in skeletal muscle. J Biol Chem. 1978 May 25;253(10):3677–3684. [PubMed] [Google Scholar]
- Christensen N. J. Plasma norepinephrine and epinephrine in untreated diabetics, during fasting and after insulin administration. Diabetes. 1974 Jan;23(1):1–8. doi: 10.2337/diab.23.1.1. [DOI] [PubMed] [Google Scholar]
- Frandsen E. K., Krishna G. A simple ultrasensitive method for the assay of cyclic AMP and cyclic GMP in tissues. Life Sci. 1976 Mar 1;18(5):529–541. doi: 10.1016/0024-3205(76)90331-3. [DOI] [PubMed] [Google Scholar]
- Garber A. J., Cryer P. E., Santiago J. V., Haymond M. W., Pagliara A. S., Kipnis D. M. The role of adrenergic mechanisms in the substrate and hormonal response to insulin-induced hypoglycemia in man. J Clin Invest. 1976 Jul;58(1):7–15. doi: 10.1172/JCI108460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garber A. J., Entman M. L., Birnbaumer L. Cholinergic stimulation of skeletal muscle alanine and glutamine formation and release. Evidence for mediation by a nicotinic cholinergic receptor and guanosine 3':5'-monophosphate. J Biol Chem. 1978 Nov 10;253(21):7924–7930. [PubMed] [Google Scholar]
- Garber A. J., Harari Y., Entman M. L. Cholinergic stimulation of alanine and glutamine formation and release from skeletal muscle. J Biol Chem. 1978 Nov 10;253(21):7918–7923. [PubMed] [Google Scholar]
- Garber A. J. Inhibition of serotonin of amino acid release and protein degradation in skeletal muscle. Mol Pharmacol. 1977 Jul;13(4):640–651. [PubMed] [Google Scholar]
- Garber A. J., Karl I. E., Kipnis D. M. Alanine and glutamine synthesis and release from skeletal muscle. I. Glycolysis and amino acid release. J Biol Chem. 1976 Feb 10;251(3):826–835. [PubMed] [Google Scholar]
- Garber A. J., Karl I. E., Kipnis D. M. Alanine and glutamine synthesis and release from skeletal muscle. II. The precursor role of amino acids in alanine and glutamine synthesis. J Biol Chem. 1976 Feb 10;251(3):836–843. [PubMed] [Google Scholar]
- Garber A. J., Karl I. E., Kipnis D. M. Alanine and glutamine synthesis and release from skeletal muscle. IV. beta-Adrenergic inhibition of amino acid release. J Biol Chem. 1976 Feb 10;251(3):851–857. [PubMed] [Google Scholar]
- Garber A. J. The regulation of skeletal muscle alanine and glutamine formation and release in experimental chronic uremia in the rat: subsensitivity of adenylate cyclase and amino acid release to epinephrine and serotonin. J Clin Invest. 1978 Sep;62(3):633–641. doi: 10.1172/JCI109170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein L., Newsholme E. A. The formation of alanine from amino acids in diaphragm muscle of the rat. Biochem J. 1976 Feb 15;154(2):555–558. doi: 10.1042/bj1540555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferson L. S., Li J. B., Rannels S. R. Regulation by insulin of amino acid release and protein turnover in the perfused rat hemicorpus. J Biol Chem. 1977 Feb 25;252(4):1476–1483. [PubMed] [Google Scholar]
- Karl I. E., Garber A. J., Kipnis D. M. Alanine and glutamine synthesis and release from skeletal muscle. III. Dietary and hormonal regulation. J Biol Chem. 1976 Feb 10;251(3):844–850. [PubMed] [Google Scholar]
- Kimura H., Mittal C. K., Murad F. Activation of guanylate cyclase from rat liver and other tissues by sodium azide. J Biol Chem. 1975 Oct 25;250(20):8016–8022. [PubMed] [Google Scholar]
- Krishna G., Krishnan N. A rapid method for the assay of guanylate cyclase. J Cyclic Nucleotide Res. 1975;1(6):293–302. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laudat M. H., Pairault J. An impaired response of adenylate cyclase to stimulation by epinephrine in adipocyte plasma membranes from genetically obese mice (ob/ob). Eur J Biochem. 1975 Aug 15;56(2):583–589. doi: 10.1111/j.1432-1033.1975.tb02265.x. [DOI] [PubMed] [Google Scholar]
- Lee S. H., Davis E. J. Carboxylation and decarboxylation reactions. Anaplerotic flux and removal of citrate cycle intermediates in skeletal muscle. J Biol Chem. 1979 Jan 25;254(2):420–430. [PubMed] [Google Scholar]
- Levilliers J., Pairault J., Lecot F., Tournemolle A., Laudat M. H. Adenosine 3':5'-monophosphate and guanosine 3':5'-monophosphate: levels and cyclase activities in liver and adipose tissue from diabetic mice (db/db). Eur J Biochem. 1978 Aug 1;88(2):323–330. doi: 10.1111/j.1432-1033.1978.tb12453.x. [DOI] [PubMed] [Google Scholar]
- Li J. B., Jefferson L. S. Effect of isoproterenol on amino acid levels and protein turnover in skeletal muscle. Am J Physiol. 1977 Feb;232(2):E243–E249. doi: 10.1152/ajpendo.1977.232.2.E243. [DOI] [PubMed] [Google Scholar]
- Mickey J., Tate R., Lefkowitz R. J. Subsensitivity of adenylate cyclase and decreased beta-adrenergic receptor binding after chronic exposure to (minus)-isoproterenol in vitro. J Biol Chem. 1975 Jul 25;250(14):5727–5729. [PubMed] [Google Scholar]
- Mukherjee C., Caron M. G., Lefkowitz R. J. Catecholamine-induced subsensitivity of adenylate cyclase associated with loss of beta-adrenergic receptor binding sites. Proc Natl Acad Sci U S A. 1975 May;72(5):1945–1949. doi: 10.1073/pnas.72.5.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odessey R., Khairallah E. A., Goldberg A. L. Origin and possible significance of alanine production by skeletal muscle. J Biol Chem. 1974 Dec 10;249(23):7623–7629. [PubMed] [Google Scholar]
- Pain V. M., Garlick P. J. Effect of streptozotocin diabetes and insulin treatment on the rate of protein synthesis in tissues of the rat in vivo. J Biol Chem. 1974 Jul 25;249(14):4510–4514. [PubMed] [Google Scholar]
- Ruderman N. B., Berger M. The formation of glutamine and alanine in skeletal muscle. J Biol Chem. 1974 Sep 10;249(17):5500–5506. [PubMed] [Google Scholar]
- Shepherd R. E., Malbon C. C., Smith C. J., Fain J. N. Lipolysis and adenosine 3':5'-monophosphate metabolism in isolated white fat cells from genetically obese-hyperglycemic mice (ob/ob). J Biol Chem. 1977 Oct 25;252(20):7243–7248. [PubMed] [Google Scholar]
- Snell K., Duff D. A. The release of alanine by rat diaphragm muscle in vitro. Biochem J. 1977 Feb 15;162(2):399–403. doi: 10.1042/bj1620399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner A. L., Pagliara A. S., Chase L. R., Kipnis D. M. Radioimmunoassay for cyclic nucleotides. II. Adenosine 3',5'-monophosphate and guanosine 3',5'-monophosphate in mammalian tissues and body fluids. J Biol Chem. 1972 Feb 25;247(4):1114–1120. [PubMed] [Google Scholar]
- Steiner A. L., Parker C. W., Kipnis D. M. Radioimmunoassay for cyclic nucleotides. I. Preparation of antibodies and iodinated cyclic nucleotides. J Biol Chem. 1972 Feb 25;247(4):1106–1113. [PubMed] [Google Scholar]
- Vesely D. L., Castro A., Levey G. S. Decreased rat hepatic guanylate cyclase activity in streptozotocin-induced diabetes mellitus. Diabetes. 1977 Apr;26(4):308–313. doi: 10.2337/diab.26.4.308. [DOI] [PubMed] [Google Scholar]
- WOOL I. G., KRAHL M. E. Incorporation of C14-amino acids into protein of isolated diaphragms: an effect of insulin independent of glucose entry. Am J Physiol. 1959 May;196(5):961–964. doi: 10.1152/ajplegacy.1959.196.5.961. [DOI] [PubMed] [Google Scholar]
- Wahren J., Felig P., Cerasi E., Luft R. Splanchnic and peripheral glucose and amino acid metabolism in diabetes mellitus. J Clin Invest. 1972 Jul;51(7):1870–1878. doi: 10.1172/JCI106989. [DOI] [PMC free article] [PubMed] [Google Scholar]



