Abstract
The anamorphic genus Phoma was subdivided into nine sections based on morphological characters, and included teleomorphs in Didymella, Leptosphaeria, Pleospora and Mycosphaerella, suggesting the polyphyly of the genus. Recent molecular, phylogenetic studies led to the conclusion that Phoma should be restricted to Didymellaceae. The present study focuses on the taxonomy of excluded Phoma species, currently classified in Phoma sections Plenodomus, Heterospora and Pilosa. Species of Leptosphaeria and Phoma section Plenodomus are reclassified in Plenodomus, Subplenodomus gen. nov., Leptosphaeria and Paraleptosphaeria gen. nov., based on the phylogeny determined by analysis of sequence data of the large subunit 28S nrDNA (LSU) and Internal Transcribed Spacer regions 1 & 2 and 5.8S nrDNA (ITS). Phoma heteromorphospora, type species of Phoma section Heterospora, and its allied species Phoma dimorphospora, are transferred to the genus Heterospora stat. nov. The Phoma acuta complex (teleomorph Leptosphaeria doliolum), is revised based on a multilocus sequence analysis of the LSU, ITS, small subunit 18S nrDNA (SSU), β-tubulin (TUB), and chitin synthase 1 (CHS-1) regions. Species of Phoma section Pilosa and allied Ascochyta species were determined to belong to Pleosporaceae based on analysis of actin (ACT) sequence data. Anamorphs that are similar morphologically to Phoma and described in Ascochyta, Asteromella, Coniothyrium, Plectophomella, Pleurophoma and Pyrenochaeta are included in this study. Phoma-like species, which grouped outside the Pleosporineae based on a LSU sequence analysis, are transferred to the genera Aposphaeria, Paraconiothyrium and Westerdykella. The genera Medicopsis gen. nov. and Nigrograna gen. nov. are introduced to accommodate the medically important species formerly known as Pyrenochaeta romeroi and Pyrenochaeta mackinnonii, respectively.
Taxonomic novelties:
New genera: Medicopsis Gruyter, Verkley & Crous, Nigrograna Gruyter, Verkley & Crous, Paraleptosphaeria Gruyter, Verkley & Crous, Subplenodomus Gruyter, Verkley & Crous. New species: Aposphaeria corallinolutea Gruyter, Aveskamp & Verkley, Paraconiothyrium maculicutis Verkley & Gruyter. New combinations: Coniothyrium carteri (Gruyter & Boerema) Verkley & Gruyter, C. dolichi (Mohanty) Verkley & Gruyter, C. glycines (R.B. Stewart) Verkley & Gruyter, C. multiporum (V.H. Pawar, P.N. Mathur & Thirum.) Verkley & Gruyter, C. telephii (Allesch.) Verkley & Gruyter, Heterospora (Boerema, Gruyter & Noordel.) Gruyter, Verkley & Crous, H. chenopodii (Westend.) Gruyter, Aveskamp & Verkley, H. dimorphospora (Speg.) Gruyter, Aveskamp & Verkley, Leptosphaeria errabunda (Desm.) Gruyter, Aveskamp & Verkley, L. etheridgei (L.J. Hutchison & Y. Hirats.) Gruyter, Aveskamp & Verkley, L. macrocapsa (Trail) Gruyter, Aveskamp & Verkley, L. pedicularis (Fuckel) Gruyter, Aveskamp & Verkley, L. rubefaciens (Togliani) Gruyter, Aveskamp & Verkley, L. sclerotioides (Sacc.) Gruyter, Aveskamp & Verkley, L. sydowii (Boerema, Kesteren & Loer.) Gruyter, Aveskamp & Verkley, L. veronicae (Hollós) Gruyter, Aveskamp & Verkley, Medicopsis romeroi (Borelli) Gruyter, Verkley & Crous, Nigrograna mackinnonii (Borelli) Gruyter, Verkley & Crous, Paraconiothyrium flavescens (Gruyter, Noordel. & Boerema) Verkley & Gruyter, Paracon. fuckelii (Sacc.) Verkley & Gruyter, Paracon. fusco-maculans (Sacc.) Verkley & Gruyter, Paracon. lini (Pass.) Verkley & Gruyter, Paracon. tiliae (F. Rudolphi) Verkley & Gruyter, Paraleptosphaeria dryadis (Johanson) Gruyter, Aveskamp & Verkley, Paralept. macrospora (Thüm.) Gruyter, Aveskamp & Verkley, Paralept. nitschkei (Rehm ex G. Winter) Gruyter, Aveskamp & Verkley, Paralept. orobanches (Schweinitz: Fr.) Gruyter, Aveskamp & Verkley, Paralept. praetermissa (P. Karst.) Gruyter, Aveskamp & Verkley, Plenodomus agnitus (Desm.) Gruyter, Aveskamp & Verkley, Plen. biglobosus (Shoemaker & H. Brun) Gruyter, Aveskamp & Verkley, Plen. chrysanthemi (Zachos, Constantinou & Panag.) Gruyter, Aveskamp & Verkley, Plen. collinsoniae (Dearn. & House) Gruyter, Aveskamp & Verkley, Plen. confertus (Niessl ex Sacc.) Gruyter, Aveskamp & Verkley, Plen. congestus (M.T. Lucas) Gruyter, Aveskamp & Verkley, Plen. enteroleucus (Sacc.) Gruyter, Aveskamp & Verkley, Plen. fallaciosus (Berl.) Gruyter, Aveskamp & Verkley, Plen. hendersoniae (Fuckel) Gruyter, Aveskamp & Verkley, Plen. influorescens (Boerema & Loer.) Gruyter, Aveskamp & Verkley, Plen. libanotidis (Fuckel) Gruyter, Aveskamp & Verkley, Plen. lindquistii (Frezzi) Gruyter, Aveskamp & Verkley, Plen. lupini (Ellis & Everh.) Gruyter, Aveskamp & Verkley, Plen. pimpinellae (Lowen & Sivan.) Gruyter, Aveskamp & Verkley, Plen. tracheiphilus (Petri) Gruyter, Aveskamp & Verkley, Plen. visci (Moesz) Gruyter, Aveskamp & Verkley, Pleospora fallens (Sacc.) Gruyter & Verkley, Pleo. flavigena (Constantinou & Aa) Gruyter & Verkley, Pleo. incompta (Sacc. & Martelli) Gruyter & Verkley, Pyrenochaetopsis pratorum (P.R. Johnst. & Boerema) Gruyter, Aveskamp & Verkley, Subplenodomus apiicola (Kleb.) Gruyter, Aveskamp & Verkley, Subplen. drobnjacensis (Bubák) Gruyter, Aveskamp & Verkley, Subplen. valerianae (Henn.) Gruyter, Aveskamp & Verkley, Subplen. violicola (P. Syd.) Gruyter, Aveskamp & Verkley, Westerdykella capitulum (V.H. Pawar, P.N. Mathur & Thirum.) de Gruyter, Aveskamp & Verkley, W. minutispora (P.N. Mathur ex Gruyter & Noordel.) Gruyter, Aveskamp & Verkley. New names: Pleospora angustis Gruyter & Verkley, Pleospora halimiones Gruyter & Verkley.
Key words: coelomycetes, Coniothyriaceae, Cucurbitariaceae, Leptosphaeriaceae, Melanommataceae, molecular phylogeny, Montagnulaceae, Phaeosphaeriaceae, Pleosporaceae, Sporormiaceae, taxonomy, Trematosphaeriaceae
INTRODUCTION
The anamorphic genus Phoma includes many important plant pathogens. The taxonomy of Phoma has been studied intensively in the Netherlands for more than 40 years resulting in the development of a generic concept as an outline for identification of Phoma species (Boerema 1997). In this concept species of the genus Phoma are classified based on their morphological characters into nine sections: Phoma, Heterospora, Macrospora, Paraphoma, Peyronellaea, Phyllostictoides, Pilosa, Plenodomus and Sclerophomella (Boerema 1997). The species placed in each of the sections were systematically described culminating in the publication of the “Phoma Identification Manual” (Boerema et al. 2004), which contained the descriptions of 223 specific and infra-specific taxa of Phoma, and more than 1000 synonyms in other coelomycetous genera. The classification of the Phoma species in sections based on morphology is artificial (Boerema et al. 2004), and several species can be classified in more than one section as they reveal multiple “section-specific” characters.
A large, well-studied Phoma culture collection that includes more than 1100 strains of Phoma resulted from the extensive morphological studies conducted on Phoma in The Netherlands. That culture collection is the basis of an intensive molecular phylogenetic study of the genus Phoma, which commenced in 2006. Molecular studies of species of Phoma prior to the onset of this project concentrated on the development of molecular detection methods for specific, important plant pathogenic Phoma species, such as Ph. macdonaldii, Ph. tracheiphila, Stagonosporopsis cucurbitacearum (as Ph. cucurbitacearum) and Boeremia foveata (as Ph. foveata) (Aveskamp et al. 2008). The phylogeny of the type species of the nine Phoma sections and morphologically similar coelomycetes was determined utilising the sequence data of the large subunit 28S nrDNA (LSU) and the small subunit 18S nrDNA (SSU) regions (de Gruyter et al. 2009). Results of that study demonstrated that the type species of the nine Phoma sections all grouped in Pleosporales. The type species of five Phoma sections, Phoma, Phyllostictoides, Sclerophomella, Macrospora and Peyronellaea and similar genera, grouped in a distinct clade in Didymellaceae. The type species of the remaining four Phoma sections, Heterospora, Paraphoma, Pilosa and Plenodomus, clustered in several clades outside Didymellaceae based on the LSU and SSU sequence analysis leading to the conclusion that these species should be excluded from Phoma (de Gruyter et al. 2009, Aveskamp et al. 2010).
The molecular phylogeny of the Phoma species in Didymellaceae was determined in a subsequent study (Aveskamp et al. 2010) and, as the phylogenetic placement of the sectional type species already suggested, included species mainly from sections Phoma, Phyllostictoides, Sclerophomella, Macrospora and Peyronellaea. The molecular phylogeny of 11 Phoma species classified in Phoma section Paraphoma based on their setose pycnidia was investigated using LSU and SSU sequences (de Gruyter et al. 2010) and this section was highly polyphyletic, with species clustering mainly in Phaeosphaeriaceae and Cucurbitariaceae.
The purpose of the present study was to clarify the molecular phylogeny of the Phoma species currently classified in sections Plenodomus and Pilosa, along with Phoma species which were determined to be distantly related to the generic type species Ph. herbarum in previous molecular studies. Additionally, phoma-like isolates of coelomycetes currently classified in Ascochyta and Coniothyrium and clustering outside the Didymellaceae (de Gruyter et al. 2009, Aveskamp et al. 2010) are included in this study along with a number of phoma-like species that do not belong to Pleosporineae.
In the present study, the initial focus was to determine the molecular phylogeny of Phoma betae (teleom. Pleospora betae) and Ph. lingam (teleom. Leptosphaeria maculans), type species of the Phoma sections Pilosa and Plenodomus, respectively, at the generic rank based on the sequence data of the LSU and the SSU regions. In a subsequent study, the sequence data of both the LSU and the ITS regions were used for a revised classification of the Phoma species currently classified in Phoma section Plenodomus. Only a limited number of the species currently classified in this section have a confirmed Leptosphaeria teleomorph.
The Phoma acuta species complex was subject of a more detailed study. The teleomorph of Ph. acuta is Leptosphaeria doliolum, type species of the genus Leptosphaeria. A multilocus analysis of sequence data of the SSU, LSU, ITS, β-tubulin (TUB), and chitin synthase 1 (CHS-1) regions was performed. The phylogeny of Phoma species of section Pilosa, with a Pleospora teleomorph (Pleosporaceae) was studied utilising actin (ACT) sequence data.
Phoma-like species currently attributed to the genera Aposphaeria, Asteromella, Coniothyrium, Phoma, Plenodomus, Pleurophoma and Pyrenochaeta, which could not be classified in the Pleosporineae based on their molecular phylogeny, were included in a LSU sequence analysis. All Phoma taxa that are unrelated to Didymellaceae and treated in this paper are redisposed to other genera.
A further aim of this study was to establish a single nomenclature for well-resolved anamorph-teleomorph relationships as discussed by Hawksworth et al. (2011). In cases where one anamorph-teleomorph generic relation is involved in a monophyletic lineage, one generic name was chosen based on priority and the other named teleomorph or anamorph state is treated as a synonym. Similar approaches towards single nomenclature have been employed in Botryosphaeriales (Crous et al. 2006, 2009a, b, Phillips et al. 2008), Pleosporales (Aveskamp et al. 2010), and Hypocreales (Lombard et al. 2010a, b, c, Chaverri et al. 2011, Gräfenhan et al. 2011, Schroers et al. 2011).
MATERIALS AND METHODS
Isolate selection, culture studies and DNA extraction
The generic abbreviations used in this study are: Ascochyta (A.), Coniothyrium (C.), Heterospora (H.), Leptosphaeria (L.), Paraconiothyrium (Paracon.), Paraleptosphaeria (Paralep.), Phoma (Ph.), Plenodomus (Plen.), Pleospora (Pleo.), Pyrenochaeta (Py.), Subplenodomus (Subplen.) and Westerdykella (W.). The isolates included in this study were obtained from the culture collections of the Centraalbureau voor Schimmelcultures, Utrecht, The Netherlands (CBS-KNAW) and the Dutch National Plant Protection Organization, Wageningen, The Netherlands (PD) (Table 1). The freeze-dried isolates were revived overnight in 2 mL malt/peptone (50 % / 50 %) liquid medium and subsequently transferred and maintained on oatmeal agar (OA) (Crous et al. 2009c). The isolates, which were stored at -196 °C, were directly transferred to OA. Cultures growing on OA and malt extract agar (MEA) (Crous et al. 2009c) were studied morphologically as described in detail by Boerema et al. (2004). The genomic DNA isolation was performed using the Ultraclean Microbial DNA isolation kit (Mo Bio Laboratories, Carlsbad, California) according to the instructions of the manufacturer. All DNA extracts were diluted 10 × in milliQ water and stored at 4 °C before use.
Table 1.
Isolates used in this study and their GenBank accession numbers. Name changes and newly generated sequences are indicated in bold.
| Species name, final identification | Former identification | CBS no. | Other no. | ITS | SSU | LSU | ACT | TUB | CHS-1 | Host, substrate | Country |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Aposphaeria corallinolutea sp. nov. | Pleurophoma sp. | CBS 131286 | PD 83/367 | JF740329 | Kerria japonica (Rosaceae) | Netherlands | |||||
| Pleurophoma sp. | CBS 131287 | PD 83/831 | JF740330 | Fraxinus excelsior (Oleaceae) | Netherlands | ||||||
| Aposphaeria populina | CBS 543.70 | EU754130 | Populus canadensis (Salicaceae) | Netherlands | |||||||
| Pyrenochaeta sp. | CBS 350.82 | JF740265 | Picea abies (Pinaceae) | Germany | |||||||
| Pleurophoma sp. | CBS 130330 | PD 84/221 | JF740328 | Cornus mas (Cornaceae) | Netherlands | ||||||
| Beverwykella pulmonaria | CBS 283.53 | ATCC 32983, IFO 6800 | GU301804 | Fagus sylvatica (Fagaceae) | Netherlands | ||||||
| Byssothecium circinans | CBS 675.92 | ATCC 52767, ATCC 52678, IMI 266220 | AY016357 | Medicago sativa (Fabaceae) | USA | ||||||
| Chaetodiplodia sp. | Chaetodiplodia sp. | CBS 453.68 | JF740115 | Halimione portulacoides (Chenopodiaceae) | Netherlands | ||||||
| Chaetosphaeronema hispidulum | CBS 216.75 | EU754045 | EU754144 | Anthyllis vulneraria (Fabaceae) | Germany | ||||||
| Cochliobolus sativus | DAOM 226212 | DQ677995 | DQ678045 | (Poaceae) | Unknown | ||||||
| Coniothyrium carteri comb. nov. | Phoma carteri | CBS 101633 | PD 84/74 | JF740180 | GQ387593 | Quercus sp. Fagaceae) | Netherlands | ||||
| Phoma carteri | CBS 105.91 | JF740181 | GQ387533 | GQ387594 | Quercus robur (Fagaceae) | Germany | |||||
| Coniothyrium dolichi comb. nov. | Pyrenochaeta dolichi | CBS 124143 | IMI 217261 | JF740182 | GQ387610 | Dolichos biforus (Fabaceae) | India | ||||
| Pyrenochaeta dolichi | CBS 124140 | IMI 217262 | JF740183 | GQ387550 | GQ387611 | Dolichos biforus (Fabaceae) | India | ||||
| Coniothyrium glycines comb. nov. | Phoma glycinicola | CBS 124455 | IMI 294986 | JF740184 | GQ387536 | GQ387597 | Glycine max (Fabaceae) | Zambia | |||
| Phoma glycinicola | CBS 124141 | PG-1 | JF740185 | GQ387598 | Glycine max (Fabaceae) | Zimbabwe | |||||
| Coniothyrium multiporum comb. nov. | Phoma multipora | CBS 501.91 | PD 83/888 | JF740186 | GU238109 | Unknown | Egypt | ||||
| Phoma multipora | CBS 353.65 | IMI 113689, ATCC 16207, HACC 164 | JF740187 | JF740268 | Saline soil | India | |||||
| Coniothyrium palmarum | CBS 400.71 | AY720708 | EU754054 | EU754153 | Chamaerops humilis (Arecaceae) | Italy | |||||
| Coniothyrium telephii comb. nov. | Phoma septicidalis | CBS 188.71 | JF740188 | GQ387538 | GQ387599 | Air | Finland | ||||
| Phoma septicidalis | CBS 856.97 | JF740189 | GQ387539 | GQ387600 | Mineral wool | Finland | |||||
| Phoma septicidalis | CBS 101636 | PD 86/1186 | JF740190 | GQ387540 | GQ387601 | Glycine max (Fabaceae) | Zimbabwe | ||||
| Cucurbitaria berberidis, anam. Pyrenochaeta berberidis | CBS 363.93 | JF740191 | GQ387545 | GQ387606 | Berberis vulgaris (Berberidaceae) | Netherlands | |||||
| Didymella exigua | CBS 183.55 | EU754056 | EU754155 | Rumex arifolius (Polygonaceae) | France | ||||||
| Didymella lycopersici, anam. Boeremia lycopersici | CBS 378.67 | JF740097 | GU237950 | Lycopersicon esculentum (Solanaceae) | Netherlands | ||||||
| Falcisormispora lignatilis | BCC 21118 | GU371827 | Elaeis guineensis (Arecaceae) | Thailand | |||||||
| Herpotrichia juniperi | CBS 200.31 | DQ678080 | Juniperus nana (Cupressaceae) | Switzerland | |||||||
| Heterospora chenopodii comb. nov. | Phoma heteromorphospora | CBS 448.68 | FJ427023 | EU754088 | EU754187 | Chenopodium album (Chenopodiaceae) | Netherlands | ||||
| Phoma heteromorphospora | CBS 115.96 | PD 94/1576 | JF740227 | EU754188 | Chenopodium album (Chenopodiaceae) | Netherlands | |||||
| Heterospora dimorphospora comb. nov. | Phoma dimorphospora | CBS 345.78 | PD 76/1015 | JF740203 | GU238069 | Chenopodium quinoa (Chenopodiaceae) | Peru | ||||
| Phoma dimorphospora | CBS 165.78 | PD 77/884 | JF740204 | JF740098 | JF740281 | Chenopodium quinoa (Chenopodiaceae) | Peru | ||||
| Leptosphaeria conoidea | Leptosphaeria conoidea, anam. Phoma doliolum | CBS 616.75 | ATCC 32813, IMI 199777, PD 74/56 | JF740201 | JF740099 | JF740279 | Lunaria annua (Brassicaceae) | Netherlands | |||
| Leptosphaeria conoidea, anam. Phoma doliolum | CBS 125977 | PD 82/888 | JF740202 | JF740280 | Senecio sp. (Asteraceae) | Netherlands | |||||
| Leptosphaeria doliolum | Leptosphaeria doliolum subsp. doliolum var. doliolum, anam. Phoma acuta subsp. acuta | CBS 505.75 | PD 75/141 | JF740205 | GQ387515 | GQ387576 | JF740126 | JF740144 | JF740162 | Urtica dioica (Urticaceae) | Netherlands |
| Leptosphaeria doliolum subsp. errabunda, anam. Phoma acuta subsp. errabunda | CBS 541.66 | PD 66/221 | JF740206 | JF740284 | JF740127 | JF740145 | JF740163 | Rudbeckia sp. (Asteraceae) | Netherlands | ||
| Phoma acuta subsp. acuta f.sp. phloxis | CBS 155.94 | PD 77/80 | JF740207 | JF740282 | JF740128 | JF740146 | JF740164 | Phlox paniculata (Polemoniaceae) | Netherlands | ||
| Phoma acuta subsp. acuta f.sp. phloxis | CBS 125979 | PD 78/37 | JF740208 | JF740283 | JF740129 | JF740147 | JF740165 | Phlox paniculata (Polemoniaceae) | Netherlands | ||
| Leptosphaeria doliolum subsp. doliolum var. doliolum, anam. Phoma acuta subsp. acuta | CBS 504.75 | PD 74/55 | JF740209 | JF740130 | JF740148 | JF740166 | Urtica dioica (Urticaceae) | Netherlands | |||
| Leptosphaeria doliolum subsp. doliolum var. doliolum, anam. Phoma acuta subsp. acuta | CBS 130000 | PD 82/701 | JF740210 | JF740131 | JF740149 | JF740167 | Urtica dioica (Urticaceae) | Netherlands | |||
| Leptosphaeria errabunda comb. nov. | Leptosphaeria doliolum subsp. errabunda, anam. Phoma acuta subsp. errabunda | CBS 617.75 | ATCC 32814, IMI 199775, PD 74/201 | JF740216 | JF740289 | JF740132 | JF740150 | JF740168 | Solidago sp. (hybrid) (Asteraceae) | Netherlands | |
| Leptosphaeria doliolum subsp. errabunda, anam. Phoma acuta subsp. errabunda | CBS 125978 | PD 74/61 | JF740217 | JF740290 | JF740133 | JF740151 | JF740169 | Delphinium sp. (Ranunculaceae) | Netherlands | ||
| Leptosphaeria doliolum subsp. errabunda, anam. Phoma acuta subsp. errabunda | CBS 129999 | PD 78/569 | JF740218 | JF740134 | JF740152 | JF740170 | Aconitum sp. (Ranunculaceae) | Netherlands | |||
| Leptosphaeria doliolum subsp. errabunda, anam. Phoma acuta subsp. errabunda | CBS 129998 | PD 84/462 | JF740219 | JF740135 | JF740153 | JF740171 | Gailardia (Asteraceae) | Netherlands | |||
| Leptosphaeria doliolum subsp. errabunda, anam. Phoma acuta subsp. errabunda | CBS 129997 | PD 78/631 | JF740220 | JF740136 | JF740154 | JF740172 | Achillea millefolium (Apiaceae) | Netherlands | |||
| Leptosphaeria etheridgei comb. nov. | Phoma etheridgei | CBS 125980 | DAOM 216539, PD 95/1483 | JF740221 | JF740291 | Populus tremuloides (Salicaceae) | Canada | ||||
| Leptosphaeria macrocapsa comb. nov. | Phoma macrocapsa | CBS 640.93 | PD 78/139 | JF740237 | JF740304 | JF740138 | JF740156 | JF740174 | Mercurialis perennis (Euphorbiaceae) | Netherlands | |
| Leptosphaeria pedicularis comb. nov. | Phoma pedicularis | CBS 126582 | PD 77/710 | JF740223 | JF740293 | Gentiana punctata (Gentianaceae) | Switzerland | ||||
| Phoma pedicularis | CBS 390.80 | PD 77/711 | JF740224 | JF740294 | JF740137 | JF740155 | JF740173 | Pedicularis sp. (Scrophulariaceae) | Switzerland | ||
| Leptosphaeria rubefaciens comb. nov. | Phoma rubefaciens | CBS 387.80 | IMI 248432, ATCC 42533, PD 78/809 | JF740242 | JF740311 | Tilia (×) europea (Malvaceae) | Netherlands | ||||
| Phoma rubefaciens | CBS 223.77 | JF740243 | JF740312 | Quercus sp. (Fagaceae) | Switzerland | ||||||
| Leptosphaeria sclerotioides comb. nov. | Phoma sclerotioides | CBS 144.84 | CECT 20025, PD 82/1061 | JF740192 | JF740269 | Medicago sativa (Fabaceae) | Canada | ||||
| Phoma sclerotioides | CBS 148.84 | PD 80/1242 | JF740193 | JF740270 | Medicago sativa (Fabaceae) | Canada | |||||
| Leptosphaeria slovacica | Leptosphaeria slovacica, anam. Phoma leonuri | CBS 389.80 | PD 79/171 | JF740247 | JF740101 | JF740315 | Balota nigra (Lamiaceae) | Netherlands | |||
| Leptosphaeria slovacica, anam. Phoma leonuri | CBS 125975 | PD 77/1161 | JF740248 | JF740316 | Balota nigra (Lamiaceae) | Netherlands | |||||
| Leptosphaeria sydowii comb. nov. | Phoma sydowii | CBS 385.80 | PD 74/477 | JF740244 | JF740313 | JF740139 | JF740157 | JF740175 | Senecio jacobaea (Asteraceae) | UK | |
| Phoma sydowii | CBS 125976 | PD 84/472 | JF740245 | JF740314 | JF740140 | JF740158 | JF740176 | Senecio jacobaea (Asteraceae) | Netherlands | ||
| Phoma sydowii | CBS 297.51 | JF740246 | JF740141 | JF740159 | JF740177 | Papaver rhoeas (Papaveraceae) | Switzerland | ||||
| Leptosphaeria veronicae comb. nov. | Phoma veronicicola | CBS 145.84 | CECT 20059, PD 78/273 | JF740254 | JF740320 | JF740142 | JF740160 | JF740178 | Veronica chamaedryoides (Scrophulariaceae) | Netherlands | |
| Phoma veronicicola | CBS 126583 | PD 74/227 | JF740255 | JF740321 | JF740143 | JF740161 | JF740179 | Veronica ‘Shirley Blue’ (Scrophulariaceae) | Netherlands | ||
| Massarina eburnea | H 3953, HHUF 26621, JCM 14422 | AB521718 | AB521735 | Fagus sylvatica (Fagaceae) | UK | ||||||
| Massarina eburnea | CBS 473.64 | ETH 2945 | GU296170 | GU301840 | Fagus sylvatica (Fagaceae) | Switzerland | |||||
| Medicopsis romeroi comb. nov. | Pyrenochaeta romeroi | CBS 252.60 | ATCC 13735, FMC 151, UAMH 10841 | EU754108 | EU754207 | Human, maduromycosis | Venezuela | ||||
| Pyrenochaeta romeroi | CBS 122784 | PD 84/1022 | EU754208 | Hordeum vulgare (Gramineae) | Unknown | ||||||
| Melanomma pulvis-pyrius | CBS 371.75 | GU301845 | Wood | France | |||||||
| CBS 400.97 | DQ678020 | DQ678072 | Fagus sp. (Fagaceae) | Belgium | |||||||
| Neophaeosphaeria filamentosa | CBS 102202 | BPI 802755 | JF740259 | GQ387516 | GQ387577 | Yucca rostrata (Agavaceae) | Mexico | ||||
| Neosetophoma samarorum | CBS 138.96 | PD 82/653 | GQ387517 | GQ387578 | Phlox paniculata (Polemoniaceae) | Netherlands | |||||
| Neottiosporina paspali | CBS 331.37 | EU754073 | EU754172 | Paspalum notatum (Poaceae) | USA | ||||||
| Nigrogana mackinnonii comb. nov. | Pyrenochaeta mackinnonii | CBS 674.75 | FMC 270 | GQ387552 | GQ387613 | Human, black grain mycetoma | Venezuela | ||||
| Pyrenochaeta mackinnonii | CBS 110022 | GQ387614 | Human, mycetoma | Mexico | |||||||
| Paraconiothyrium flavescens comb. nov. | Phoma flavescens | CBS 178.93 | PD 82/1062 | GU238075 | Soil | Netherlands | |||||
| Paraconiothyrium fuckelii comb. nov. | Coniothyrium fuckelii | CBS 797.95 | GU238204 | GU237960 | Rubus sp. (Rosaceae) | Denmark | |||||
| Paraconiothyrium fusco-maculans comb. nov. | Plenodomus fusco-maculans | CBS 116.16 | EU754197 | Malus sp. (Rosaceae) | USA | ||||||
| Paraconiothyrium lini comb. nov. | Phoma lini | CBS 253.92 | PD 70/998 | EU238093 | Wisconsin tank | Netherlands | |||||
| Paraconiothyrium maculicutis sp. nov. | Pleurophoma pleurospora | CBS 101461 | IMI 320754, UTHSC 87-144 | EU754200 | Human, cutaneous lesions | USA | |||||
| Paraconiothyrium minitans | CBS 122788 | PD 07/03486739 | EU754074 | EU754173 | Unknown | UK | |||||
| CBS 122786 | PD 99/1064-1 | EU754174 | Clematis sp. (Ranunculaceae) | Netherlands | |||||||
| Paraconiothyrium tiliae comb. nov. | Asteromella tiliae | CBS 265.94 | EU754139 | Tilia platyphyllos (Tiliaceae) | Austria | ||||||
| Paraleptosphaeria dryadis comb. nov. | Leptosphaeria dryadis | CBS 643.86 | JF740213 | GU301828 | Dryas octopetala (Rosaceae) | Switzerland | |||||
| Paraleptosphaeria macrospora comb. nov. | Phoma macrospora | CBS 114198 | UPSC 2686 | JF740238 | JF740305 | Rumex domesticus (Chenopodiaceae) | Norway | ||||
| Paraleptosphaeria nitschkei comb. nov. | Leptosphaeria nitschkei | CBS 306.51 | JF740239 | JF740308 | Cirsium spinosissimum (Asteraceae) | Switzerland | |||||
| Paraleptosphaeria orobanches comb. nov. | Phoma korfii | CBS 101638 | PD 97/12070 | JF400230 | JF740299 | Epifagus virginiana (Orobanchaceae) | USA | ||||
| Paraleptosphaeria praetermissa comb. nov. | Leptosphaeria praetermissa | CBS 114591 | JF740241 | JF740310 | Rubus idaeus (Rosaceae) | Sweden | |||||
| Paraphaeosphaeria michoti | CBS 652.86 | ETH 9483 | GQ387520 | GQ387581 | Typha latifolia (Typhaceae) | Switzerland | |||||
| Paraphoma radicina | CBS 111.79 | IMI 386094, PD 76/437 | EU754092 | EU754191 | Malus sylvestris (Rosaceae) | Netherlands | |||||
| Phaeosphaeria nodorum | CBS 110109 | EU754076 | EU754175 | Lolium perenne (Gramineae) | Denmark | ||||||
| Phoma herbarum | CBS 615.75 | FJ427022 | EU754087 | EU754186 | Rosa multiflora (Rosaceae) | Netherlands | |||||
| Phoma paspali | CBS 560.81 | PD 92/1569 | GU238227 | G238124 | Paspalum dilatum (Poaceae) | New Zealand | |||||
| Plenodomus agnitus comb. nov. | Leptosphaeria agnita, anam. Phoma agnita | CBS 121.89 | PD 82/903 | JF740194 | JF740271 | Eupatorium cannabinum (Asteraceae) | Netherlands | ||||
| Leptosphaeria agnita, anam. Phoma agnita | CBS 126584 | PD 82/561 | JF740195 | JF740272 | Eupatorium cannabinum (Asteraceae) | Netherlands | |||||
| Plenodomus biglobosus comb. nov. | Leptosphaeria biglobosa | CBS 119951 | JF740198 | JF740102 | JF740274 | Brassica rapa (Brassicaceae) | Netherlands | ||||
| CBS 127249 | DAOM 229269 | JF740199 | JF740275 | Brassica juncea (Brassicaceae) | France | ||||||
| Plenodomus chrysanthemi comb. nov. | Phoma vasinfecta, synanam. Phialophora chrysanthemi | CBS 539.63 | JF740253 | GU238230 | GU238151 | Chrysanthemum sp. (Asteraceae) | Greece | ||||
| Plenodomus collinsoniae comb. nov. | Leptosphaeria collinsoniae | CBS 120227 | JCM 13073, MAFF 239583 | JF740200 | JF740276 | Vitis coignetiae (Vitaceae) | Japan | ||||
| Plenodomus confertus comb. nov. | Leptosphaeria conferta, anam. Phoma conferta | CBS 375.64 | AF439459 | JF740277 | Anacyclus radiatus (Asteraceae) | Spain | |||||
| Plenodomus congestus comb. nov. | Leptosphaeria congesta, anam. Phoma congesta | CBS 244.64 | AF439460 | JF740278 | Erigeron canadensis (Asteraceae) | Spain | |||||
| Plenodomus enteroleucus comb. nov. | Phoma enteroleuca var. enteroleuca | CBS 142.84 | PD 81/654, CECT20063 | JF740214 | JF740287 | Catalpa bignonioides (Bignoniaceae) | Netherlands | ||||
| Phoma enteroleuca var. enteroleuca | CBS 831.84 | JF740215 | JF740288 | Triticum aestivum (Poaceae) | Germany | ||||||
| Plenodomus fallaciosus comb. nov. | Leptosphaeria fallaciosa | CBS 414.62 | ETH 2961 | JF740222 | JF740292 | Satureia montana (Lamiaceae) | France | ||||
| Plenodomus hendersoniae comb. nov. | Phoma intricans | CBS 113702 | UPSC 1843 | JF740225 | JF740295 | Salix cinerea (Salicaceae) | Sweden | ||||
| Phoma intricans | CBS 139.78 | JF740226 | JF740296 | Pyrus malus (Rosaceae) | Netherlands | ||||||
| Plenodomus influorescens comb. nov. | Phoma enteroleuca var. influorescens | CBS 143.84 | PD 78/883, CECT 20064 | JF400228 | JF740297 | Fraxinus excelsior (Oleaceae) | Netherlands | ||||
| Phoma enteroleuca var. influorescens | PD 73/1382 | JF400229 | JF740298 | Lilium sp. (Liliaceae) | Netherlands | ||||||
| Plenodomus libanotidis comb. nov. | Leptosphaeria libanotis | CBS 113795 | UPSC 2219 | JF400231 | JF740300 | Seseli libanotis (Apiaceae) | Sweden | ||||
| Plenodomus lindquistii comb. nov. | Leptosphaeria lindquistii, anam. Phoma macdonaldii | CBS 386.80 | PD 77/336 | JF400232 | JF740301 | Helianthus annuus (Asteraceae) | former Yugoslavia | ||||
| Leptosphaeria lindquistii, anam. Phoma macdonaldii | CBS 381.67 | JF400233 | JF740302 | Helianthus annuus (Asteraceae) | Canada | ||||||
| Plenodomus lingam | Leptosphaeria maculans, anam. Phoma lingam | CBS 275.63 | MUCL 9901, UPSC 1025 | JF400234 | JF740103 | JF740306 | Brassica sp. (Brassicaceae) | UK | |||
| Leptosphaeria maculans, anam. Phoma lingam | CBS 260.94 | PD 78/989 | JF400235 | JF740307 | JF740116 | Brassica oleracea (Brassicaceae) | Netherlands | ||||
| Leptosphaeria maculans, anam. Phoma lingam | CBS 147.24 | JF740117 | Unknown | Unknown | |||||||
| Plenodomus lupini comb. nov. | Phoma lupini | CBS 248.92 | PD 79/141 | JF740236 | JF740303 | Lupinus mutabilis (Fabaceae) | Peru | ||||
| Plenodomus pimpinellae comb. nov. | Leptosphaeria pimpinellae, anam. Phoma pimpinellae | CBS 101637 | PD 92/41 | JF740240 | JF740309 | Pimpinella anisum (Apiaceae) | Israel | ||||
| Plenodomus tracheiphilus comb. nov. | Phoma tracheiphila | CBS 551.93 | PD 81/782 | JF740249 | JF740104 | JF740317 | Citrus limonium (Rutaceae) | Israel | |||
| Phoma tracheiphila | CBS 127250 | PD 09/04597141 | JF740250 | JF740318 | Citrus sp. (Rutaceae) | Italy | |||||
| Plenodomus visci comb. nov. | Plectophomella visci | CBS 122783 | PD 74/1021 | JF740256 | EU754096 | EU754195 | Viscum album (Viscaceae) | France | |||
| Plenodomus wasabiae | Phoma wasabiae | CBS 120119 | FAU 559 | JF740257 | JF740323 | Wasabia japonica (Brassicaceae) | Taiwan | ||||
| Phoma wasabiae | CBS 120120 | FAU 561 | JF740258 | JF740324 | Wasabia japonica (Brassicaceae) | Taiwan | |||||
| Pleomassaria siparia | CBS 279.74 | AY004341 | Betula verrucosa (Betulaceae) | Netherlands | |||||||
| Pleospora angustis nom. nov. | Leptosphaeria clavata | CBS 296.51 | JF740122 | Unknown | Switzerland | ||||||
| Pleospora betae | Pleospora betae, anam. Phoma betae | CBS 523.66 | PD 66/270, IHEM 3915 | EU754080 | EU754179 | JF740118 | Beta vulgaris (Chenopodiaceae) | Netherlands | |||
| Pleospora betae, anam. Phoma betae | CBS 109410 | PD 77/113 | EU754178 | JF740119 | Beta vulgaris (Chenopodiaceae) | Netherlands | |||||
| Pleospora calvescens | Pleospora calvescens, anam. Ascochyta caulina | CBS 246.79 | PD 77/655 | EU754032 | EU754131 | JF740120 | Atriplex hastata (Chenopodiaceae) | Germany | |||
| Pleospora calvescens, anam. Ascochyta caulina | CBS 343.78 | JF740121 | Atriplex hastata (Chenopodiaceae) | Netherlands | |||||||
| Pleospora chenopodii | Ascochyta hyalospora | CBS 206.80 | PD 74/1022 | JF740095 | JF740266 | JF740109 | Chenopodium quinoa (Chenopodiaceae) | Bolivia | |||
| Pleospora calvescens, anam. Ascochyta caulina | CBS 344.78 | PD 68/682 | JF740110 | Atriplex hastata (Chenopodiaceae) | Netherlands | ||||||
| Pleospora fallens comb. nov. | Phoma fallens | CBS 161.78 | LEV 1131 | JF740106 | Olea europaea (Oleaeceae) | New Zealand | |||||
| Phoma glaucispora | CBS 284.70 | PD 97/2400 | JF740107 | Nerium oleander (Apocynaceae) | Italy | ||||||
| Pleospora flavigena comb. nov. | Phoma flavigena | CBS 314.80 | PD 91/1613 | JF740108 | Water | Romania | |||||
| Pleospora halimiones nom. nov. | Ascochyta obiones | CBS 432.77 | IMI 282137 | JF740096 | JF740267 | JF740113 | Halimione portulacoides (Chenopodiaceae) | Netherlands | |||
| Ascochyta obiones | CBS 786.68 | JF740114 | Halimione portulacoides (Chenopodiaceae) | Netherlands | |||||||
| Pleospora herbarum | CBS 191.86 | IMI 276975 | GU238232 | GU238160 | JF740123 | Medicago sativa (Fabaceae) | India | ||||
| Pleospora incompta comb. nov. | Phoma incompta | CBS 467.76 | JF740111 | Olea europaea (Oleaeceae) | Greece | ||||||
| Phoma incompta | CBS 526.82 | JF740112 | Olea europaea (Oleaeceae) | Italy | |||||||
| Pleospora typhicola | Pleospora typhicola, anam. Phoma typharum | CBS 132.69 | JF740105 | JF740325 | JF740124 | Typha angustifolia (Typhaceae) | Netherlands | ||||
| Pleospora typhicola, anam. Phoma typharum | CBS 602.72 | JF740125 | Typha sp. (Typhaceae) | Netherlands | |||||||
| Pleurophoma pleurospora | Pleurophoma sp. | CBS 116668 | JF740326 | Citysus scoparius (Fabaceae) | Netherlands | ||||||
| Pleurophoma sp. | CBS 130329 | PD 82/371 | JF740327 | Lonicera sp. (Caprifoliaceae) | Netherlands | ||||||
| Preussia funiculata | CBS 659.74 | GU296187 | GU301864 | Soil | Senegal | ||||||
| Pseudorobillarda phragmitis | CBS 398.61 | IMI 070678 | EU754203 | Phragmitis australis (Poaceae) | UK | ||||||
| Pyrenochaeta cava | CBS 257.68 | IMI 331911 | JF740260 | EU754100 | EU754199 | Wheat field soil | Germany | ||||
| Pyrenochaeta lycopersici | CBS 267.59 | JF740261 | GQ387551 | GQ387612 | Lycopersicon esculentum (Solanaceae) | Netherlands | |||||
| Pyrenochaeta nobilis | CBS 407.76 | EU930011 | EU754107/DQ898287 | EU754206 | Laurus nobilis (Lauraceae) | Italy | |||||
| Pyrenochaetopsis leptospora | CBS 101635 | PD 71/1027 | JF740262 | GQ387566 | GQ387627 | Secale cereale (Poaceae) | Europe | ||||
| Pyrenochaetopsis pratorum comb. nov. | Phoma pratorum | CBS 445.81 | PDDCC 7049, PD 80/1254 | JF740263 | GU238136 | Lolium perenne, leaf (Poaceae) | New Zealand | ||||
| CBS 286.93 | PD 80/1252 | JF740264 | JF740331 | Dactylis glomerata (Poaceae) | New Zealand | ||||||
| Pyrenophora tritici-repentis | OSC 100066 | AY544716 | AY544672 | (Poaceae) | Italy | ||||||
| Roussoella hysterioides | CBS 125434 | HH 26988 | AB524622 | Sasa kurilensis (Poaceae) | Japan | ||||||
| Setomelanomma holmii | CBS 110217 | GQ387572 | GQ387633 | Picea pungens (Pinaceae) | USA | ||||||
| Setophoma terrestris | CBS 335.29 | GQ387526 | GQ387587 | Allium sativum (Alliaceae) | USA | ||||||
| Splanchnonema platani | CBS 221.37 | DQ678013 | DQ678065 | Platanus occidentalis (Platanaceae) | USA | ||||||
| Sporormiella minima | CBS 524.50 | DQ678003 | DQ678056 | Dung of goat | Panama | ||||||
| Stagonosporopsis cucurbitacearum | CBS 133.96 | GU238234 | GU238181 | Cucurbita sp. (Cucurbitaceae) | New Zealand | ||||||
| Subplenodomus apiicola comb. nov. | Phoma apiicola | CBS 285.72 | JF740196 | GU238040 | Apium graveolens var. rapaceum (Umbelliferae) | Germany | |||||
| Phoma apiicola | CBS 504.91 | PD 78/1073 | JF740197 | JF740273 | Apium graveolens (Umbelliferae) | Netherlands | |||||
| Subplenodomus drobnjacensis comb. nov. | Phoma drobnjacensis | CBS 269.92 | PD 88/896 | JF740211 | JF740100 | JF740285 | Eustoma exaltatum (Gentianaceae) | Netherlands | |||
| Phoma drobnjacensis | CBS 270.92 | PD 83/650 | JF740212 | JF740286 | Gentiana makinoi ‘Royal Blue’ (Gentianaceae) | Netherlands | |||||
| Subplenodomus valerianae comb. nov. | Phoma valerianae | CBS 630.68 | PD 68/141 | JF740251 | GU238150 | Valeriana phu (Valerianaceae) | Netherlands | ||||
| Phoma valerianae | CBS 499.91 | PD 73/672 | JF740252 | JF740319 | Valeriana officinalis (Valerianaceae) | Netherlands | |||||
| Subplenodomus violicola comb. nov. | Phoma violicola | CBS 306.68 | FJ427054 | GU238231 | GU238156 | Viola tricolor (Violaceae) | Netherlands | ||||
| Phoma violicola | CBS 100272 | FJ427055 | JF740322 | Viola tricolor (Violaceae) | New Zealand | ||||||
| Thyridaria rubronotata | CBS 419.85 | GU301875 | Acer pseudoplatanus (Aceraceae) | Netherlands | |||||||
| Trematosphaeria pertusa | CBS 122368 | FJ201990 | Fraxinus excelsior (Oleaceae) | France | |||||||
| Westerdykella capitulum comb. nov. | Phoma capitulum | CBS 337.65 | PD 91/1614, ATCC 16195, HACC 167, IMI 113693 | GU238054 | Saline soil | India | |||||
| Westerdykella minutispora comb. nov. | Phoma minutispora | CBS 509.91 | PD 77/920 | GU238108 | Saline soil | India | |||||
| Westerdykella ornata | CBS 379.55 | GU301880 | Mangrove mud | Mozambique |
PCR and sequencing
For nucleotide sequence comparisons, partial regions of SSU, LSU and ITS, as well as part of the ACT, TUB and CHS-1 genes were amplified. The SSU region was amplified with the primers NS1 and NS4 (White et al. 1990) and the LSU region was amplified with the primers LR0R (Rehner & Samuels 1994) and LR7 (Vilgalys & Hester 1990). The ITS and TUB regions were amplified as described by Aveskamp et al. (2009) using the primer pair V9G (de Hoog & Gerrits van den Ende 1998) and ITS4 (White et al. 1990) for the ITS and the BT2Fw and BT4Rd primer pair (Woudenberg et al. 2009) for the TUB locus. The ACT and CHS-1 regions were amplified using the primer pairs ACT-512F / ACT-783R and CHS-354R / CHS-79F (Carbone & Kohn 1999). The amplification reactions were performed and analysed as described by de Gruyter et al. (2009).
Sequencing of the PCR amplicons was conducted using the same primer combinations, although the primer LR5 (Vilgalys & Hester 1990) was used as an additional internal sequencing primer for LSU. The sequence products were purified using Sephadex columns (Sephadex G-50 Superfine, Amersham Biosciences, Roosendaal, Netherlands) and analysed with an ABI Prism 3730XL Sequencer (Applied Biosystems) according to the manufacturer’s instructions. Consensus sequences were computed from both forward and reverse sequences using the Bionumerics v. 4.61 software package (Applied Maths, Sint-Martens-Latem, Belgium) and were lodged with GenBank. All sequences of reference isolates included in this study were obtained from GenBank (Table 1).
Phylogenetic analyses
To determine the phylogeny of Phoma betae and Ph. lingam at rank, the SSU and LSU sequence data of two isolates were aligned with the sequences of 46 reference isolates in the Pleosporales that were obtained from GenBank (Table 1), 14 of which were classified in the Pleosporaceae or Leptosphaeriaceae. The phylogeny of Phoma section Plenodomus was determined with the combined data set of LSU and ITS sequences of 87 isolates, including 53 isolates currently classified in Leptosphaeria and Phoma section Plenodomus. Phoma apiicola, Ph. dimorphospora, Ph. heteromorphospora, Ph. lupini, Ph. valerianae, Ph. vasinfecta and Ph. violicola classified in Phoma sections Phoma or Heterospora (Boerema et al. 2004) grouped in previous molecular phylogenetic studies outside Didymellaceae (de Gruyter et al. 2009, Aveskamp et al. 2010), and are therefore treated here.
In the study of the Leptosphaeria doliolum complex, that includes the subspecies of Ph. acuta, viz. subsp. acuta, errabunda and also Ph. acuta subsp. acuta f. sp. phlogis, a phylogenetic analysis was performed utilising the ITS, ACT, TUB, CHS-1 sequences of 18 isolates. Phoma macrocapsa, Ph. sydowii and Ph. veronicicola being closely related to this species complex were included.
The species concept of phoma-like anamorphs in Pleosporaceae was determined by alignments of the ACT sequences of 15 isolates and five reference isolates. Phoma fallens, Ph. glaucispora and Ph. flavigena were also included. These species were originally classified in Phoma sect. Phoma (de Gruyter & Noordeloos 1992, de Gruyter et al. 1998). However, a molecular phylogenetic study demonstrated that these species grouped in a clade representing Leptosphaeriaceae and Pleosporaceae (Aveskamp et al. 2010). Sequence data were compared with those of isolates currently classified in the genera Phoma, Ascochyta and Coniothyrium, as well as isolates of Leptosphaeria clavata and the generic type species Pleospora herbarum. Phoma incompta is the only species classified in Phoma section Sclerophomella, which proved to be unrelated to Didymellaceae (Aveskamp et al. 2010).
The phoma-like species that could not be attributed to Pleosporineae (Zhang et al. 2009) were studied with the LSU sequences of 40 isolates, including 20 reference isolates representing the anamorph genera Beverwykella, Neottiosporina, Paraconiothyrium, as well as the teleomorph genera Byssothecium, Falciformispora, Herpotrichia, Melanomma, Paraphaeosphaeria, Pleomassaria, Preussia, Roussoella, Splanchnonema, Sporormiella, Thyridaria, Trematosphaeria and Westerdykella. Four Phoma species were included which are currently described in Phoma section Phoma, viz. Ph. capitulum, Ph. flavescens, Ph. lini, and Ph. minutispora (de Gruyter & Noordeloos 1992, de Gruyter et al. 1993). In addition, the human pathogens Pyrenochaeta romeroi and Py. mackinnonii, which could not be classified in a recent study dealing with phoma-like species with setose pycnidia (de Gruyter et al. 2010), were included.
The multiple alignments were automatically calculated by the BioNumerics software package, but manual adjustments for improvement were made by eye where necessary. For multilocus alignments, the phylogenetic analyses were done for each dataset individually, and where similar tree topologies were obtained, an analysis was performed on the combined alignment of all the gene regions in the multilocus alignment. Neighbour-Joining (NJ) distance analyses were conducted using PAUP (Phylogenetic Analysis Using Parsimony) v. 4.0b10 (Swofford 2003) with the uncorrected “p”, Jukes-Cantor and Kimura 2-parameter substitution models. The robustness of the trees obtained was evaluated by 1000 bootstrap replications. A Bayesian analysis was conducted with MrBayes v. 3.1.2 (Huelsenbeck & Ronqvist 2001) in two parallel runs, using the default settings but with the following adjustments: the GTR model (trees 1-3, 5) with gamma-distributed rate and the HKY+ γ-model (tree 4) were selected for the partitions using the Findmodel freeware (http://hcv.lanl.gov/content/hcv-db/findmodel/findmodel.html), and a MCMC heated chain was set with a “temperature” value of 0.05. The number of generations and sample frequencies were set at 5 million and 10 (trees 3-5) or 100 (trees 1, 2) respectively and the run was automatically stopped as soon as the average standard deviation of split frequencies reached below 0.01. The resulting trees were printed with TreeView v. 1.6.6 (Page 1996) and alignments and trees were deposited into TreeBASE (www.treebase.org).
RESULTS
The data for the aligned sequence matrices for the trees obtained in the different studies are provided below. In the case that alignments of multiple loci are involved, the topologies of the obtained trees for each locus were compared by eye to confirm that the overall tree topology of the individual datasets were similar to each other and to that of the tree obtained from the combined alignment. The NJ analyses with the three substitution models showed similar tree topologies and were congruent to those obtained in the Bayesian analyses. The results of the molecular phylogenetic analyses are supplied below; the summarised additional ecology and distribution data of the taxa involved were adopted from Boerema et al. (2004), where the references to original literature are provided.
Phylogeny of Phoma lingam and Ph. betae, the type species of Phoma sections Plenodomus and Pilosa (Pleosporineae)
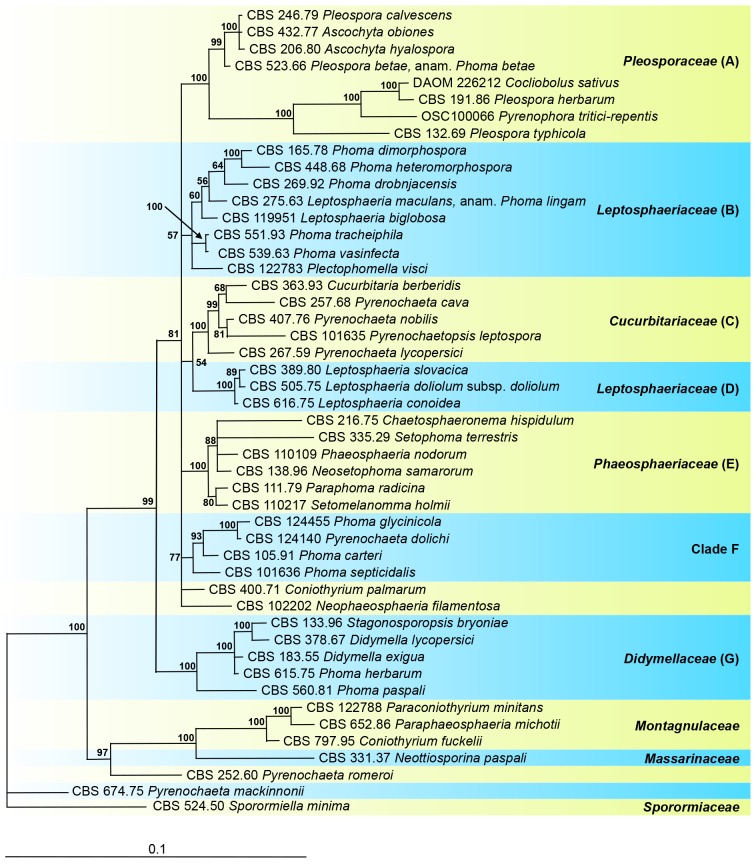
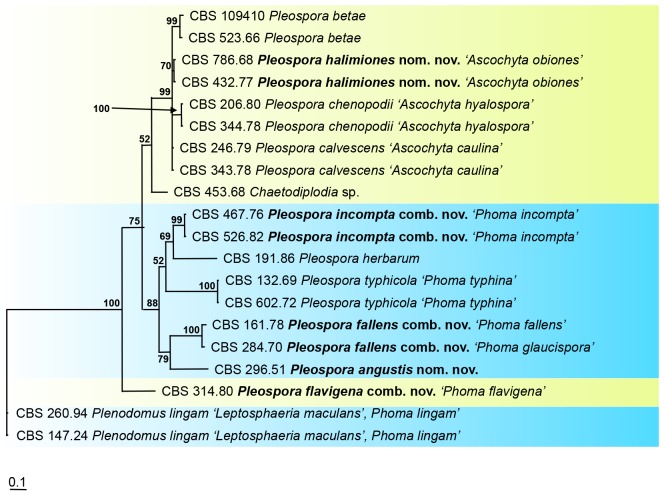
The aligned sequence matrix obtained for the SSU and LSU regions had a total length of 2 671 nucleotide characters, 1 367 and 1 304 respectively. In the alignment, an insertion in the SSU at the positions 478-832 was observed for the cultures CBS 216.75, CBS 165.78, CBS 138.96, CBS 331.37 and CBS 674.75. This insertion was excluded from further phylogenetic analyses. The combined dataset used in the analyses included 48 taxa and contained 2 316 characters with 101 and 213 unique site patterns for SSU and LSU, respectively. The tree (Fig. 1) was rooted to Sporormiella minima (CBS 524.50). The Bayesian analysis resulted in 6 5442 trees after 3 272 000 generations, from which the burn-in was discarded and the consensus tree and posterior probabilities were calculated based on 56 028 trees (Fig. 1).
Fig. 1.
The phylogeny of Phoma lingam and Phoma betae, the type species of Phoma sections Plenodomus and Pilosa, based on the strict consensus tree from a Bayesian analysis of 48 LSU/SSU sequences. The Bayesian posterior probabilities are given at the nodes. The tree was rooted to Sporormiella minima (CBS 524.50).
The families that belong to Pleosporineae, represented by the species grouping in clades A-G, clustered in a strongly supported clade (99 % posterior probability). Clade A, representing those species classified in Pleosporaceae, was strongly supported (100 %) and included two subclades. Pleospora betae (anam. Ph. betae), clustered with Pleospora calvescens (anam. Ascochyta caulina), A. obiones and A. hyalospora; all recorded as pathogens on Chenopodiaceae. The generic type species Pleospora herbarum, a plurivorous species, grouped with Cochliobolus sativus, Pyrenophora tritici-repentis and Pleospora typhicola (anam. Ph. typhina), all recorded from Poaceae. Clade B includes Leptosphaeria maculans (anam. Ph. lingam) and clustered with Leptosphaeria biglobosa. In clade B also other important plant pathogens of Phoma section Plenodomus can be found, such as Ph. tracheiphila, Ph. vasinfecta, Ph. drobnjacensis, and Plectophomella visci. Phoma heteromorphospora, type species of Phoma section Heterospora (Boerema et al. 1997) and Ph. dimorphospora also grouped in this Leptosphaeria clade, in congruence with previous findings (de Gruyter et al. 2009, Aveskamp et al. 2010).
Leptosphaeria doliolum (anam. Ph. acuta), type species of the genus Leptosphaeria, is found in Clade D, clustering with L. conoidea and L. slovacica. Leptosphaeria doliolum and its relatives comprise a sister clade C with species classified in Cucurbitariaceae, including Cucurbitaria berberidis, the three Pyrenochaeta species, Py. cava, Py. lycopersici and Py. nobilis, and Pyrenochaetopsis leptospora.
Phaeosphaeria nodorum and its relatives Neosetophoma samarorum, Setophoma terrestris, Chaetosphaeronema hispidulum, Paraphoma radicina and Setomelanomma holmii, represent Phaeosphaeriaceae in clade E as has previously been found (de Gruyter et al. 2009, 2010).
A distinct clade F includes Ph. glycinicola, Ph. carteri, Ph. septicidalis, and the taxonomic confusing species Pyrenochaeta dolichi (Grondona et al. 1997). The position of Coniothyrium palmarum and Neophaeosphaeria filamentosa could not be clarified, but both species are also treated below in a phylogeny including close relatives based on ITS and LSU regions (Fig. 2). Didymella exigua, type species of the genus Didymella, and Ph. herbarum represent Didymellaceae, and clustered in a well-supported clade (G) in congruence with previous studies (de Gruyter et al. 2009, 2010, Aveskamp et al. 2010). The molecular phylogeny of species which group in this analysis outside of Pleosporineae in Montagnulaceae, Massarinaceae and Sporormiaceae were further analysed utilising LSU sequence data of a broader range of taxa (Fig. 5).
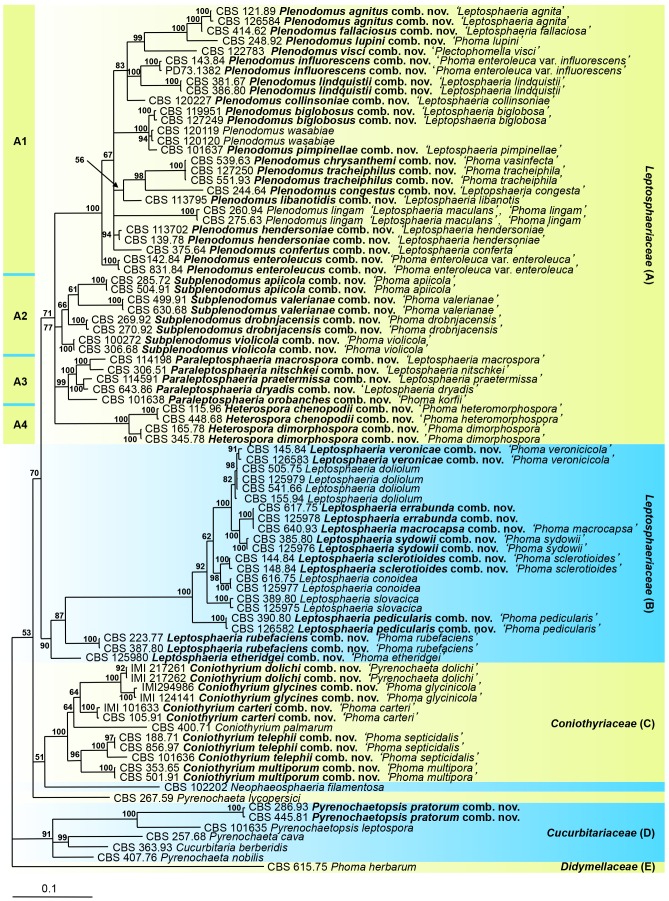
Fig. 2.
The phylogeny of Phoma section Plenodomus and Leptosphaeria, based on the strict consensus tree from a Bayesian analysis of 87 LSU/ITS sequences. The Bayesian posterior probabilities are given at the nodes. The tree was rooted to Phoma herbarum (CBS 615.75).
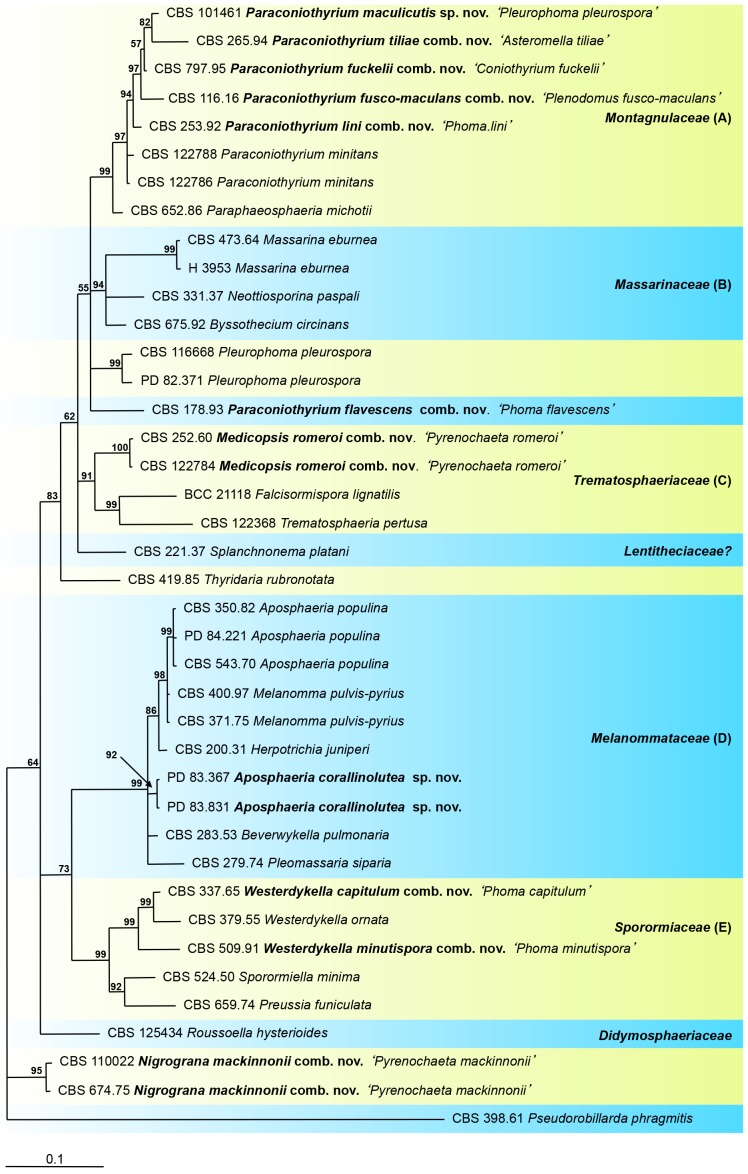
Fig. 5.
LSU The phylogeny of phoma-like isolates excluded from the Pleosporineae, based on the strict consensus tree from a Bayesian analysis of 40 LSU sequences. The Bayesian posterior probabilities are given at the nodes. The tree was rooted to Pseudorobillarda phragmitis (CBS 398.61).
Phoma section Plenodomus and close allies
The aligned sequence matrix obtained for the LSU and ITS regions had a total length of 1 921 nucleotide characters, 1 332 and 589 respectively. The combined dataset used in the analyses included 87 taxa and contained 1921 characters with 298 and 118 unique site patterns for LSU and ITS respectively. The tree (Fig. 2) was rooted to Ph. herbarum (CBS 615.75), the representative isolate of the type species of Phoma (Boerema et al. 2004). The Bayesian analysis resulted in 100 002 trees after 5 000 000 generations, from which the burn-in was discarded and the consensus tree and posterior probabilities were calculated based on 90 930 trees (Fig. 2).
The species currently classified in Leptosphaeria and Phoma section Plenodomus grouped in clades A and B representing Leptosphaeriaceae, including the type species Ph. lingam and Leptosphaeria doliolum, respectively. Isolates of the taxa that represent Cucurbitariaceae, Cucurbitaria berberidis and its related species Pyrenochaeta cava, Py. nobilis, Py. lycopersici and Pyrenochaetopsis leptospora, clustered in a distinct clade D only distantly related to Leptosphaeriaceae. This finding agrees with a recent study (de Gruyter et al. 2010). Phoma pratorum clustered with Pyrenochaetopsis leptospora.
Leptosphaeria biglobosa grouped in a subclade A1 with Ph. wasabiae, the cause of black rot disease on Wasabia japonica (Brassicaceae) and Ph. pimpinellae, a necrotroph on Pimpinella anisum (Apiaceae). Leptosphaeria maculans, considered as closely related to the L. biglobosa complex, proved to be more distantly related in clade A1. In this subclade, other important pathogens can be found, such as Ph. tracheiphila, a quarantine organism on Citrus spp. (Rutaceae), Ph. vasinfecta, a pathogen on Chrysanthemum spp. (Asteraceae), L. lindquistii (anam. Ph. macdonaldii), a worldwide pathogen on Helianthus annuus (Asteraceae) and Ph. lupini, a seed borne pathogen known from Lupinus spp. (Fabaceae). Subclade A1 also comprises both varieties of Ph. enteroleuca, opportunistic pathogens on deciduous trees and shrubs, and the necrotrophic species L. agnita (anam. Ph. agnita), Ph. congesta (both recorded on Asteraceae), Ph. conferta (mainly on Brassicaceae), L. hendersoniae (on Salicaceae), L. fallaciosa, L. collinsoniae (mainly on Lamiaceae) and L. libanotis (on Apiaceae). Plectophomella visci, recorded from leaves of Viscum album (Viscaceae), also clustered in the Leptosphaeriaceae. The genus Plenodomus is re-introduced here to accommodate the species in subclade A1, which are allied to Ph. lingam.
Subclade A2 comprises pathogenic species often causing leaf spots such as Ph. apiicola on Apium graveolens (Apiaceae), Ph. drobnjacensis (on Gentianaceae), Ph. violicola (on Violaceae) as well as the necrotrophic species Ph. valerianae, on Valeriana spp. (Valerianaceae). Phoma apiicola and Ph. valerianae were classified in Phoma section Phoma, and Ph. violicola was classified in Phoma sect. Peyronellaea; however, the relationship of these species in Leptosphaeriaceae is clearly demonstrated (Fig. 2), and therefore the species are transferred to the new genus Subplenodomus. These results are in congruence with a recent study where Ph. violicola, Ph. apiicola and Ph. valerianae clustered in a clade representing both Leptosphaeriaceae and Pleosporaceae (Aveskamp et al. 2010).
Four Leptosphaeria species, L. macrospora (soil) and the necrotrophic species L. nitschkei (on Asteraceae), L. praetermissa, on Rubus idaeus (Rosaceae) and L. dryadis, on Dryas spp. (Rosaceae) grouped in a subclade A3 and are transferred here to a new genus Paraleptosphaeria. Phoma korfii also clustered in this subclade. The European species Ph. heteromorphospora, type species of Phoma section Heterospora, and the American counterpart Ph. dimorphospora, both pathogens on Chenopodiaceae, grouped in a distinct subclade A4. Phoma sect. Heterospora is raised to generic rank to accommodate both species in Leptosphaeriaceae.
Clade B comprises necrotrophic species related to the type species L. doliolum (anam. Ph. acuta). The phylogeny of this species complex, and the closely related species Ph. veronicicola, Ph. macrocapsa and Ph. sydowii, is treated below. The necrotrophic species Ph. sclerotioides, L. conoidea (anam. Ph. doliolum), L. slovacica (anam. Ph. leonuri) and Ph. pedicularis also proved to be related. The species Ph. rubefaciens and Ph. etheridgei also belong to clade B, but these species, both recorded on trees, are more distantly related.
The Phoma species in clades A and B are in majority currently described as anamorphs of the genus Leptosphaeria, or belong to Phoma section Plenodomus. These Phoma anamorphs are only distantly related to the type species Ph. herbarum and its relatives in Didymellaceae, and therefore these species described in section Plenodomus are excluded from the genus Phoma. Clade C is more distantly related to Leptosphaeriaceae and comprises species that are related to Coniothyrium palmarum in Coniothyriaceae. Two subclades are recognised in clade C: Ph. glycinicola, Py. dolichi and Ph. carteri group with the generic type species C. palmarum, whereas two isolates of Ph. septicidalis group with Ph. multipora. The teleomorph Neophaeosphaeria filamentosa clustered basal to this clade. Clade D includes the genera Cucurbitaria, Pyrenochaetopsis and Pyrenochaeta, which represent Cucurbitariaceae. This finding is in congruence with previous studies (de Gruyter et al. 2010).
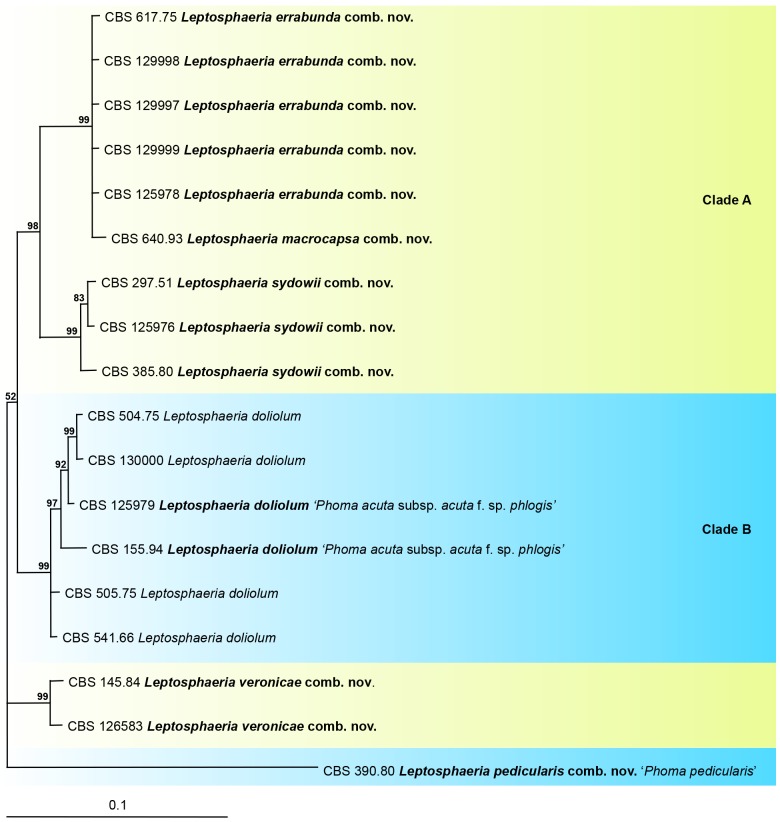
Phylogeny of the Leptosphaeria doliolum complex
The aligned sequence matrix obtained for the ITS, ACT, TUB and CHS-1 regions had a total length of 1 345 nucleotide characters; ITS 522, ACT 240, TUB 332 and CHS-1 251, respectively. The combined dataset used in the analyses included 18 taxa and contained 1 345 characters with 98 unique site patterns. The tree (Fig. 3) was rooted to “Ph. pedicularis” (CBS 390.80). The Bayesian analysis resulted in 6 002 trees after 30 000 generations, from which the burn-in was discarded and the consensus tree and posterior probabilities were calculated based on 3 341 trees.
Fig. 3.
The phylogeny of the Leptosphaeria doliolum complex, based on the strict consensus tree from a Bayesian analysis of 18 ITS/ACT/TUB/CHS-1 sequences. The Bayesian posterior probabilities are given at the nodes. The tree was rooted to Leptosphaeria pedicularis comb. nov. (CBS 390.80).
The phylogenetic tree revealed two clades with high posterior probabilities, 98 and 99 % respectively, clade A with Ph. acuta subsp. errabunda and Ph. macrocapsa, and clade B with Ph. acuta subsp. acuta (anamorph of Leptosphaeria doliolum) and Ph. acuta subsp. acuta f. sp. phlogis. Phoma sydowii, a necrotroph on Asteraceae, Senecio spp. in particular, proved to be closely related to Ph. acuta subsp. errabunda. The isolate CBS 297.51 preserved as Ph. acuta is similar to Ph. sydowii, a synonym of L. sydowii, see below. Phoma veronicicola, as a necrotroph specifically occurring on Veronica spp. (Scrophulariaceae), also proved to be related to Leptosphaeria doliolum.
Phylogeny of Phoma section Pilosa
The aligned sequence matrix obtained for the ACT region had a total length of 252 nucleotide characters (20 taxa), and contained 165 unique sites. The tree was rooted to Ph. lingam (CBS 147.24 and CBS 260.94). The Bayesian analysis resulted in 34 802 trees after 174 000 generations, from which the burn-in was discarded, and the consensus tree and posterior probabilities were calculated based on 11 728 trees (Fig. 4).
Fig. 4.
The phylogeny of phoma-like anamorphs in the Pleosporaceae based on the strict consensus tree from a Bayesian analysis of 20 ACT sequences. The Bayesian posterior probabilities are given at the nodes. The tree was rooted to Plenodomus lingam (CBS 147.24, CBS 260.94).
The phylogenetic tree representing the Pleosporaceae includes Ph. betae, type species of Phoma section Pilosa. This section is characterised by producing pycnidia that are covered by mycelial hairs. Phoma betae clearly groups with other pycnidial fungi pathogenic on Chenopodiaceae, including Ascochyta obiones, A. hyalospora and A. caulina and Chaetodiplodia sp. All species produce similar hairy pycnidia, but are classified in Ascochyta or Coniothyrium due to conidial septation, or brown pigmentation of conidia, respectively.
A subclade comprises the cosmopolitan Pleospora herbarum and related species. The species involved are associated with various hosts or substrates. The most closely related Ph. incompta is a specific pathogen on Olea europea (Oleaceae). Phoma incompta was classified in Phoma section Sclerophomella because of its thick-walled pycnidia (de Gruyter & Noordeloos 1992, Boerema & de Gruyter 1998). The pycnidial characters of Ph. incompta, pycnidia covered with mycelial hairs and with an indistinct ostiole visible as a pallid spot (de Gruyter & Noordeloos 1992) however, agrees with those of Ph. betae and Ph. typhina.
Phoma fallens proved to be closely related to Ph. glaucispora in keeping with the similar in vitro characters, especially the low growth-rate and the size and shape of its conidia (Boerema et al. 2004). Both species originate from southern Europe, and have been associated with spots on fruits and leaves of Olea europea, or leaf spots on Nerium oleander, respectively. An isolate preserved as Leptosphaeria clavata, CBS 259.51, proved to be closely related. The origin of the isolate, deposited by E. Müller, is unknown; however, it is likely that the isolate was obtained from Poaceae, Triticum vulgare or Dactylis glomerata (Müller 1950). Phoma flavigena, once isolated from water and also recorded from southern Europe, proved to be more distantly related in Pleosporaceae.
Phylogeny of phoma-like anamorphs excluded from the suborder Pleosporineae
The aligned sequence matrix obtained for the LSU regions had a total length of 808 nucleotide characters, with 208 unique site patterns. The phylogenetic tree (Fig. 5) was rooted to Pseudorobillarda phragmitis (CBS 398.61). The Bayesian analysis resulted in 48 402 trees after 242 000 generations, from which the burn-in was discarded and the consensus tree and posterior probabilities were calculated based on 24 876 trees.
Clade A includes the reference isolates of the teleomorph Paraphaeosphaeria and the anamorph Paraconiothyrium classified in Montagnulaceae. This teleomorph/anamorph relation agrees with previous molecular phylogenetic studies (Verkley et al. 2004, Damm et al. 2008, de Gruyter et al. 2009). Other phoma-like species in this clade are Ph. lini, Plenodomus fusco-maculans, Pleurophoma pleurospora (CBS 101461) and Asteromella tilliae. Phoma lini, a saprobe frequently recorded on dead stems of Linum spp., was described in Phoma section Phoma (de Gruyter et al. 1993). Re-examination of the conidia revealed that they are hyaline and thin-walled; however, also darker, greenish to yellowish coniothyrium-like conidia were observed. The conidiogenous cells are phoma-like, doliiform to ampulliform.
The isolate Asteromella tiliae (CBS 265.94) clearly represents a species of Paraconiothyrium, and therefore, the teleomorph name Didymosphaeria petrakiana, Didymosphaeriaceae, is probably incorrect. It was already mentioned by Butin & Kehr (1995) that “considering the taxonomical placement of the teleomorph, the authors were informed about forthcoming taxonomic changes”.
The morphological characters of the isolate CBS 101461, considered as representing the generic type species Pleurophoma pleurospora, resembles Paraconiothyrium as was previously discussed (de Gruyter et al. 2009). The sterile ex-type strain of Plenodomus fusco-maculans, CBS 116.16, recorded from Malus sp., also grouped with the Paraconiothyrium isolates.
Coniothyrium fuckelii clustered in the Paraphaeosphaeria/Paraconiothyrium clade, in agreement with previous studies (Damm et al. 2008, Aveskamp et al. 2010), and therefore, the species is transferred to the genus Paraconiothyrium. Two phoma-like species obtained from Citysus scoparius and Lonicera sp. respectively (CBS 116668 and CBS 130329), cluster near Montagnulaceae and Massarinaceae. The morphological characters of the species are typical for Pleurophoma pleurospora. The taxonomic position of both isolates at familial rank could not be determined. The morphology of Phoma flavescens proved to be most similar to that of Paraconiothyrium, it definitely does not belong to Phoma, and therefore the species is transferred to Paraconiothyrium. Sequence data of additional species clustering nearby are required to resolve the current classification of Ph. flavescens. None of the phoma-like anamorphs included in this study grouped in clade B, which represents Massarinaceae.
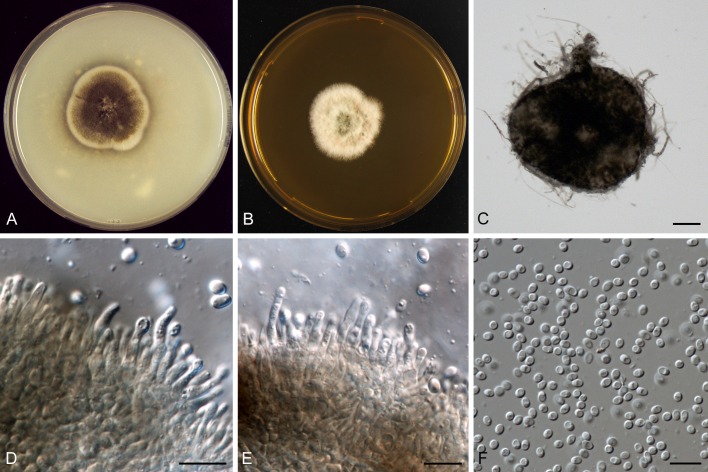
Clade C includes the recently assigned ex-epitype strain of Trematosphaeria pertusa, isolate CBS 122368 (Zhang et al. 2008) and Falcisformispora lignatilis. Both T. perusa and F. lignatilis represent Trematosphaeriaceae (Suetrong et al. 2009). A second isolate preserved as Trematosphaeria pertusa, CBS 400.97, proved to be only distantly related, and clustered in clade D with Aposphaeria populina and Melanomma pulvis-pyrius in Melanommataceae. This isolate is considered as an incorrect identification (Mugambi & Huhndorf 2009), and we consider this sterile isolate as representative of Melanomma pulvis-pyrius. Clade C also comprises the human pathogen Pyrenochaeta romeroi. This species certainly does not belong to Pyrenochaeta (de Gruyter et al. 2010) and therefore, we describe the new genus Medicopsis in Trematosphaeriaceae to accommodate this species.
A well-supported clade D represents the Melanommataceae and includes Melanomma pulvis-pyrius, Herpotrichia juniperi and Beverwijkella pulmonaria, in congruence with Zhang et al. (2009). There were four phoma-like isolates present in the collections of CBS and PD, i.e. CBS 350.82, PD 83/367, PD 83/831 and PD 84/221, which could not be identified according to their morphological characters. The isolates were preserved as Pleurophoma spp. This study demonstrates that two strains represent Aposphaeria populina, whereas the other two strains represent the new species described here as Aposphaeria corallinolutea. Further studies in Melanommataceae are needed to clarify the phylogeny of Aposphaeria in Melanommataceae.
Sporormiaceae (clade E) is represented by Sporormiella minima and Preussia funiculata. Phoma capitulum and Ph. minutispora, well-defined soil-borne fungi from Asia, group in this clade. Both species are related with the anamorph Westerdykella ornata, and therefore the species are transferred to Westerdykella in Sporormiaceae.
Pyrenochaeta mackinnonii could not be assigned to familial rank. A blast search in GenBank with its LSU sequence suggested a relation with Versicolorisporum triseptum. However, the typical 3-septate conidia of this anamorph are different. Neither could V. triseptum be assigned at familial rank in Pleosporales (Tanaka et al. 2009). We therefore introduce the new genus Nigrograna to accommodate Py. mackinnonii.
TAXONOMY
Leptosphaeriaceae M.E. Barr, Mycotaxon 29: 503. 1987.
Heterospora (Boerema, Gruyter & Noordel.) Gruyter, Verkley & Crous, stat. nov. MycoBank MB564701.
Basionym: Phoma sect. Heterospora Boerema, Gruyter & Noordel., Persoonia 16: 336. 1997.
Type species: Heterospora chenopodii (Westend.) Gruyter, Aveskamp & Verkley, see below (= Phoma heteromorphospora Aa & Kesteren).
Heterospora chenopodii (Westend.) Gruyter, Aveskamp & Verkley, comb. nov. MycoBank MB564702.
Basionym: Phyllosticta chenopodii Westend., Bull. Acad. Roy. Sci. Belgique Ser. 2, 2: 567. 1857; not Phyllosticta chenopodii Sacc., Syll. Fung. 3: 55. 1884 = Phoma exigua Desm. var. exigua; not Plenodomus chenopodii (P. Karst. & Har.) Arx, Verh. Kon. Ned. Akad. Wetensch., Afd. Natuurk., Sect. 2. 51: 72. 1957 ≡ Phoma chenopodiicola Gruyter, Noordel. & Boerema, Persoonia 15: 395. 1993; not Phoma chenopodii Pavgi & U.P. Singh, Mycopathol. Mycol. Appl. 30: 265. 1966. nom. illeg. = Phoma chenopodii S. Ahmad, Sydowia 2: 79. 1948.
≡ Septoria westendorpii G. Winter, Hedwigia 26: 26. 1887. nom. nov.; not Phoma westendorpii Tosquinet, Westend., Bull. Acad. Roy. Sci. Belgique Ser. 2, 2: 564. 1857.
≡ Phoma variospora Aa & Kesteren, Persoonia 10: 268. 1979, nom. nov., nom. illeg. [not Phoma variospora Shreem., Indian J. Mycol. Pl. Pathol. 8: 221. 1979 (“1978”)].
≡ Phoma heteromorphospora Aa & Kesteren, Persoonia 10: 542. 1980, nom. nov.
Specimens examined: Belgium, Beverloo, from leaves of Chenopodium suecicum (album) and Chenopodium urbicum (Chenopodiaceae), no date, G.D. Westendorp, Herb. Crypt. (Ed. Beyaert-Feys), No. 959. BR, holotype of Phyllosticta chenopodii Westend. ex herb. G.D. Westendorp. Netherlands, Baarn, from leaf spots in Chenopodium album, 3 Jul. 1968, H.A. van der Aa, epitype designated here CBS H-16386, culture ex-epitype CBS 448.68; Heelsum, from leaf spots in Chenopodium album, Sep. 1994, J. de Gruyter, CBS 115.96 = PD 94/1576.
Notes: Van der Aa & van Kesteren (1979) provided a nom. nov. since the epithet “chenopodii” was occupied in Phoma. For more details of the taxonomy of the species see van der Aa & van Kesteren (1979). Although Leptosphaeria chenopodii-albi was described from leaves of Chenopodium album (Crane & Shearer 1991) no cultures are available for comparison.
Heterospora dimorphospora (Speg.) Gruyter, Aveskamp & Verkley, comb. nov. MycoBank MB564703.
Basionym: Phyllosticta dimorphospora Speg., Anales Mus. Nac. Buenos Aires 13: 334. 1910.
≡ Phoma dimorphospora (Speg.) Aa & Kesteren, Persoonia 10: 269. 1979.
= Stagonospora chenopodii Peck, Rep. (Annual) New York State Mus. Nat. Hist. 40: 60. 1887 (sometimes erroneously listed as Stag. chenopodii “House”).
Specimens examined: Argentina, La Plata, from leaves of Chenopodium hircinum (Chenopodiaceae), 13 Oct. 1906, C. Spegazzini, Colect. micol. Museo Inst. Spegazzini, No. 11.353, LPS, holotype of Phyllosticta dimorphospora Speg. Lima, from stem of Chenopodium quinoa, 1977, L.J. Turkensteen, CBS 165.78 = PD 77/884. Peru, from lesions in stems of Chenopodium quinoa, 1976, V. Otazu, epitype designated here CBS H-16203, culture ex-epitype CBS 345.78 = PD 76/1015.
Note: For more details of the taxonomy of the species see van der Aa & van Kesteren (1979).
Leptosphaeria Ces. & De Not., Comment. Soc. Crittog. Ital. 1: 234. 1863.
= Leptophoma Höhn., Sitzungsber. Kaiserl. Akad. Wiss., Math.-Naturwiss. Cl., Abt. 1. 124: 73. 1915.
Type species: Leptosphaeria doliolum (Pers.: Fr.) Ces. & De Not., see below.
Note: For full synonymy, including the species listed below, see Crane & Shearer (1991) and Boerema et al. (2004).
Leptosphaeria conoidea (De Not.) Sacc., Fungi Venet. Nov. Vel. Crit. Ser. 2: 314. 1875.
Basionym: Leptosphaeria doliolum var. conoidea De Not., Mycoth. Veneti, No. 76. 1873.
= Leptosphaeria doliolum subsp. pinguicula Sacc., Michelia 2: 598. 1882.
-
= Phoma acuta subsp. amplior Sacc. & Roum., Rev. Mycol. 6: 30. 1884.
≡ Phoma hoehnelii subsp. amplior (Sacc. & Roum.) Boerema & Kesteren, Trans. Brit. Mycol. Soc. 67: 299. 1976.
= Phoma doliolum P. Karst., Meddel. Soc. Fauna Fl. Fenn. 16: 9. 1888.
= Plenodomus microsporus Berl., Bull. Soc. Mycol. France 5: 55. 1889.
Specimens examined: Netherlands, Zaltbommel, from dead stem of Lunaria annua (Brassicaceae), Jan. 1974, G.H. Boerema, CBS 616.75 = ATCC 32813 = IMI 199777 = PD 74/56; Montfoort, Senecio sp. (Asteraceae), 1982, CBS 125977 =PD 82/888.
Leptosphaeria doliolum (Pers.: Fr.) Ces. & de Not., Comment. Soc. Crittog. Ital. 1: 234. 1863.
Basionym: Sphaeria doliolum Pers.: Fr., Icon. Desc. Fung. Min. Cognit. (Leipzig) 2: 39. 1800.
-
= Sphaeria acuta Hoffm.: Fr, Veg. cryptog. 1: 22. 1787. Syst. Mycol. 2: 507. 1823.
≡ Phoma acuta (Hoffm.: Fr.) Fuckel, Jahrb. Nassauischen Vereins Naturk. 23-24: 125. 1870 (as “acutum”).
≡ Leptophoma acuta (Hoffm.: Fr.) Höhn., Sitzungsber. Kaiserl. Akad. Wiss., Math.-Naturwiss. Cl., Abt. 1. 124: 73. 1915.
≡ Plenodomus acutus (Hoffm.: Fr.) Bubák, Ann. Mycol. 13: 29. 1915 [as “(Fuckel)”].
= Phoma phlogis Roum., Rev. Mycol. 6: 160. 1884.
= Phoma hoehnelii var. urticae Boerema & Kesteren, Trans. Brit. Mycol. Soc. 67: 299. 1976.
Specimens examined: Netherlands, from stem of Rudbeckia sp. (Asteraceae), Sep. 1966, M.M.J. Dorenbosch, CBS 541.66 = PD 66/221; from stem of Urtica dioica (Urticaceae), 1974, G.H. Boerema, CBS 504.75 = PD 74/55; Rhenen, from Urtica dioica, Feb. 1975, G.H. Boerema, CBS 505.75 = PD 75/141; Wageningen, from stem of Phlox paniculata (Polemoniaceae), 1977, G.H. Boerema, CBS 155.94 = PD 77/80; from stem of Phlox paniculata, 1978, G.H. Boerema, CBS 125979 =PD 78/37; from stem of Urtica dioica, 1982, G.H. Boerema, CBS 130000 =PD 82/701.
Notes: Isolate CBS 541.66 was preserved as Phoma acuta subsp. errabunda (teleom. Leptosphaeria errabunda, see below); however, the isolate clustered with L. doliolum. Both isolates CBS 155.94 and CBS 125979 were considered as forma specialis “phlogis” (Boerema et al. 1994) of the anamorph Ph. acuta subsp. acuta. The subspecies acuta was created by the differentiation of Phoma acuta subsp amplior Sacc. & Roum., but the latter is a synonym of Ph. doliolum, reclassified here as L. conoidea, see above. Sphaeria acuta Hoffm. was applied as basionym for different anamorphs an a teleomorph of various species of Leptosphaeria leading to a confusing nomenclature. The epitet has been unambiguously tied to Ph. acuta by Boerema & Gams (1995).
Leptosphaeria errabunda (Desm.) Gruyter, Aveskamp & Verkley, comb. nov. MycoBank MB564704.
Basionym: Phoma errabunda Desm., Ann. Sci. Nat., Bot. Ser. 3, 11: 282. 1849.
≡ Phoma acuta subsp. errabunda (Desm.) Boerema, Gruyter & Kesteren, Persoonia 15: 465. 1994.
-
= Leptophoma doliolum Höhn., Sitzungsber. Kaiserl. Akad. Wiss., Math.-Naturwiss. Cl., Abt. 1. 124: 75. 1915 [not Phoma doliolum P. Karst. = Leptosphaeria conoidea (De Not.) Sacc., see above].
≡ Plenodomus doliolum (Höhn.) Höhn., Ber. Deutsch. Bot. Ges. 36: 139. 1918.
≡ Phoma hoehnelii Kesteren, Netherlands J. Pl. Pathol. 78: 116. 1972, nom. nov.
= Leptosphaeria doliolum subsp. errabunda Boerema, Gruyter & Kesteren, Persoonia 15: 466. 1994.
Specimens examined: Netherlands, Leeuwarden, from stem of Delphinium sp. (Ranunculaceae), 1974, CBS 125978 =PD 74/61; Ferwerderadeel, from Solidago sp., hybrid (Asteraceae), Mar. 1974, G.H. Boerema, CBS 617.75 = ATCC 32814 = IMI 199775 = PD 74/201; from stem of Aconitum sp. (Ranunculaceae), CBS 129999 =PD 78/569; from stem of Achillea millefolium (Asteraceae), CBS 129997 =PD 78/631; from Gailardia sp. (Asteraceae), 1984, G.H. Boerema, CBS 129998 =PD 84/462.
Notes: The isolate CBS 617.75 = ATTC 32814 was deposited as the anamorph Ph. hoehnelii var. hoehnelii, but interpreted as L. doliolum subsp. conoidea (Dong et al. 1998). The isolate clustered with L. errabunda in this study.
Leptosphaeria etheridgei (L.J. Hutchison & Y. Hirats.) Gruyter, Aveskamp & Verkley, comb. nov. MycoBank MB564712.
Basionym: Phoma etheridgei L.J. Hutchison & Y. Hirats., Canad. J. Bot. 72: 1425. 1994.
Specimen examined: Canada, Alberta, from bark of gall, on trunck of Populus tremuloides (Salicaceae), Jul. 1989, P. Crane, holotype DAOM 216539, culture exholotype DAOM 216539 = CBS 125980 =PD 95/1483.
Leptosphaeria macrocapsa (Trail) Gruyter, Aveskamp & Verkley, comb. nov. MycoBank MB564713.
Basionym: Phoma macrocapsa Trail, Scott. Naturalist (Perth) 8: 327. 1886.
≡ Plenodomus macrocapsa (Trail) H. Ruppr., Sydowia 13: 20. 1959.
Specimen examined: Netherlands, from stem of Mercurialis perennis (Euphorbiaceae), 1978, G.H. Boerema, CBS 640.93 = PD 78/139.
Leptosphaeria pedicularis (Fuckel) Gruyter, Aveskamp & Verkley, comb. nov. MycoBank MB564714.
Basionym: Phoma pedicularis Fuckel, Reisen Nordpolarmeer 3: 318. 1874 (as “pedicularidis”); not Phoma pedicularis Wehm., Mycologia 38: 319. 1946 (= Phoma herbicola Wehm).
-
= Sphaeronaema gentianae Moesz, Bot Közlem. 14: 152. 1915 (as “Sphaeronema”).
≡ Plenodomus gentianae (Moesz) Petr., Ann. Mycol. 23: 54. 1925.
Specimens examined: Switzerland, Kanton Graubünden, Albulapass, from dead stem of Pedicularis sp. (Scrophulariaceae), 1977, CBS 390.80 = PD 77/711 = ATCC 42535 = IMI 248430; Zürich, from Gentiana punctata (Gentianaceae), 1977, CBS 126582 =PD 77/710.
Leptosphaeria rubefaciens (Togliani) Gruyter, Aveskamp & Verkley, comb. nov. MycoBank MB564715.
Basionym: Phoma rubefaciens Togliani, Ann. Sper. Agr. II, 7: 1626. 1953.
Specimens examined: Switzerland, Zürich, Albis, from twig of Quercus sp. (Fagaceae), Aug. 1976, W. Gams, CBS 223.77. Netherlands, Oploo, from wood of Tilia (×) europaea (Tiliaceae), 1978, G.H. Boerema, CBS 387.80 = ATCC 42533 = IMI 248432 = PD 78/809.
Leptosphaeria sclerotioides (Sacc.) Gruyter, Aveskamp & Verkley, comb. nov. MycoBank MB564716.
Basionym: Phoma sclerotioides Sacc., Fungi Herb. Bruxelles 21. 1892; Syll. Fung. 11: 492. 1895.
= Plenodomus sclerotioides Preuss, Klotzsch. Herb. Vivum Mycol. Sistems Fungorum German., No. 1281. 1849, nom. nud. (no description).
= Plenodomus meliloti Mark.-Let., Bolezni Rast. 16: 195. 1927.
Specimens examined: Canada, British Columbia, from Medicago sativa (Fabaceae), 1980, J. Drew Smith, CBS 148.84 = PD 80/1242; Alberta, from root of Medicago sativa, Mar. 1984, G.H. Boerema, CBS 144.84 = CECT 20025 = PD 82/1061.
Note: Seven varieties of this species have been recognised (Wunsch et al. 2011) in a phylogenetic analysis using 10 loci.
Leptosphaeria slovacica Picb., Sborn. Vysoké Skoly. Zemed. v Brno 7: 7. 1927.
-
= Phoma leonuri Letendre, Revue Mycol. 6: 229. 1884.
≡ Plenodomus leonuri (Letendre) Moesz & Smarods in Moesz, Magyar Bot. Lapok 31: 38. 1932.
Specimens examined: Netherlands, from dead stem of Ballota nigra (Lamiaceae), 1977, CBS 125975 =PD 77/1161; Arnhem, from dead stem of Ballota nigra, 1979, G.H. Boerema, CBS 389.80 = PD 79/171.
Leptosphaeria sydowii (Boerema, Kesteren & Loer.) Gruyter, Aveskamp & Verkley, comb. nov. MycoBank MB564717.
Basionym: Phoma sydowii Boerema, Kesteren & Loer., Trans. Brit. Mycol. Soc. 77: 71. 1981, nom. nov.
-
= Sphaeronaema senecionis Syd. & P. Syd., Ann. Mycol. 3: 185. 1905; not Phoma senecionis P. Syd., Beibl. Hedwigia 38: 136. 1899.
≡ Plenodomus senecionis (Syd. & P. Syd.) Bubák, Ann. Mycol. 13: 29. 1915.
≡ Plenodomus senecionis (Syd. & P. Syd.) Petr., Ann. Mycol. 19: 192. 1921, isonym.
= Plenodomus rostratus Petr., Ann. Mycol. 21: 199. 1923; not Phoma rostrata O’Gara, Mycologia 7: 41. 1915 (not Leptosphaeria rostrata M.L. Far & H.T. Horner, Nova Hedwidgia 15: 250. 1968).
Specimens examined: Switzerland, Kt. Zürich, Zollikon, from Papaver rhoeas (Papaveraceae), Oct. 1949, E. Müller, CBS 297.51. Netherlands, from Senecio jacobaea (Asteraceae), G.H. Boerema, 1984, CBS 125976 =PD 84/472. UK, Scotland, Isle of Lewis, Hebrides, from dead stem of Senecio jacobaea, 1974, R.W.G. Dennis, CBS 385.80 = PD 74/477.
Notes: Leptosphaeria senecionis (Fuckel) G. Winter was suggested as the possible teleomorph (Boerema et al. 2004). Because the teleomorph connection has not been proven, however, we did not include it as a synonym that would have priority as the correct name. The isolate CBS 297.51 was originally identified as L. doliolum var. doliolum.
Leptosphaeria veronicae (Hollós) Gruyter, Aveskamp & Verkley, comb. nov. MycoBank MB564718.
Basionym: Sphaeronaema veronicae Hollós, Ann. Hist.-Nat. Mus. Natl. Hung. 4: 341. 1906.
≡ Phoma veronicicola Boerema & Loer., Trans. Brit. Mycol. Soc. 84: 297. 1985, nom. nov. (not Phoma veronicae Roum., Revue Mycol. 6: 160. 1884).
Specimens examined: Netherlands, from stem of Veronica “Shirley Blue” (Scrophulariaceae), 1974, CBS 126583 =PD 74/227; Huis ter Heide, from dead stem of Veronica chamaedryoides, Mar. 1978, H.A. van Kesteren, neotype CBS H-7632, culture ex-neotype CBS 145.84 = CECT 20059 = PD 78/273.
Paraleptosphaeria Gruyter, Verkley & Crous, gen. nov. MycoBank MB564720.
Pseudothecia immersed, subglobose, solitary or aggregated, thick-walled, pseudoparenchymatous to scleroplectenchymatous, ostiolate, unilocular. Asci bitunicate, broadly ellipsoidal, 8-spored, interascal filaments pseudoparaphyses, Ascospores biseriate, broadly fusiform, transversally 3-5-septate, hyaline to yellow-brownish. Conidiomata pycnidial, globose to subglobose, scleroplectenchymatous, with papillate pore, unilocular. Conidiogenous cells phialidic, ampulliform to doliiform. Conidia hyaline, aseptate, oblong to ellipsoidal. Sclerotia sometimes produced.
Type species: Paraleptosphaeria nitschkei (Rehm ex G. Winter) Gruyter, Aveskamp & Verkley (see below).
Notes: Munk (1957) recognised Leptosphaeria section Para-Leptosphaeria, an invalid taxon, as a heterogenous group. The section was differentiated from Eu-Leptosphaeria, which included the generic type species L. doliolum. Leptosphaeria nitschkei was considered a typical representative of section Eu-Leptosphaeria (Müller & von Arx 1950). However, this molecular phylogeny demonstrates that L. nitschkei is only distantly related to L. doliolum. We introduce Paraleptosphaeria to accomodate L. nitschkei and its relatives. These necrotrophic species are morphologically closely allied to Leptosphaeria. The former classification of Leptosphaeria in sections Eu-Leptosphaeria and Para-Leptosphaeria cannot be upheld from a evolutionary point of view, as two other species attributed to section Eu-Leptosphaeria, namely L. agnita and L. maculans (Munk 1957), were found to group in Plenodomus.
Paraleptosphaeria dryadis (Johanson) Gruyter, Aveskamp & Verkley, comb. nov. MycoBank MB564721.
Basionym: Melanomma dryadis Johanson, Hedwigia 29: 160. 1890.
≡ Leptosphaeria dryadophila Huhndorf, Bull. Illinois Nat. Hist. Surv. 34: 484 (1992), nom. illeg. via nom. superfl.
= Leptosphaeria dryadis Rostr., Bot. Tidsskr. 25: 305. 1903.
Specimen examined: Switzerland, Kt. Ticino, Leventina, Alpe Campolungo, from Dryas octopetala (Rosaceae), 24 July 1980, A. Leuchtmann, CBS 643.86.
Note: An explanation of the nomenclature of Leptosphaeria dryadis has been provided by Chen et al. (2002).
Paraleptosphaeria macrospora (Thüm.) Gruyter, Aveskamp & Verkley, comb. nov. MycoBank MB564722.
Basionym: Leptosphaeria macrospora Thüm. Mycotheca Univ. 1359. 1879, nom. nov.
≡ Metasphaeria macrospora (Fuckel) Sacc., Syll. Fung. 2: 158. 1883.
Replaced synonym: Pleospora macrospora Fuckel, Jahrb. Nassauischen Vereins Naturk. 23-24: 138. 1870, nom. illeg., Art. 53.1. [not Pleospora macrospora (De Not.) Ces. & De Not., Comment. Soc. Crittog. Ital. 1: 218. 1863].
Specimen examined: Norway, Troms, Tromsöya, from Rumex domesticus (Polygonaceae), 20 Aug. 1988, K. & L. Holm, CBS 114198 =UPSC 2686.
Paraleptosphaeria nitschkei (Rehm ex G. Winter) Gruyter, Aveskamp & Verkley, comb. nov. MycoBank MB564723.
Basionym: Leptosphaeria nitschkei Rehm ex G. Winter, Ascomyceten, Fascicle 1, No. 15. 1870, nom. nud. (Flora, Jena und Regensburg 55: 510. 1872).
Specimens examined: Austria, Ötscher in Niederösterreich, c. 4500’, from Cacalia sp. (= Adenostyles sp, Asteraceae), June 1869, Lojka, holotype of Leptosphaeria nitschkei Rehm Ascomyceten 15b, S. Switzerland, Kt. Graubünden, Lü, from Cirsium spinosissimum (Asteraceae), 16 July 1948, E. Müller, epitype designated here CBS H-20822, culture ex-epitype CBS 306.51.
Note: The name Leptosphaeria nitschkei was considered a nom. nud. by Crane and Shearer (1991) who cited Art. 32.1 but gave no further explanation. In Flora, Jena und Regensburg 55: 510. 1872 Rehm refers to additional notes by G. Winter that include a Latin description. Therefore, we consider this name as valid, following Müller (1950) who provided a detailed description in vivo.
Paraleptosphaeria orobanches (Schweinitz: Fr.) Gruyter, Aveskamp & Verkley, comb. nov. MycoBank MB564724.
Basionym: Sclerotium orobanches Schweinitz, Schriften Naturf. Ges. Leipzig 1: 57. 1822: Fr., Syst. Mycol. 2: 257. 1822.
= Phoma korfii Boerema & Gruyter, Persoonia 17: 275. 1999.
Specimen examined: USA, Ringwood Swamp, Lloyd-Cornell, from stem of Epifagus virginiana (Orobanchaceae), 13 Sep. 1995, T. Uturriaga, R.P. Korf, P. Mullin, holotype of Sclerotium orobanches Schweinitz, CUP 63537, culture ex-holotype CBS 101638 =PD 97/12070.
Note: A Phoma synanamorph of Sclerotium orobanches was reported by Yáňez-Morales et al. (1998) and described as Phoma korfii (Boerema & Gruyter 1999).
Paraleptosphaeria praetermissa (P. Karst.) Gruyter, Aveskamp & Verkley, comb. nov. MycoBank MB564725.
Basionym: Sphaeria praetermissa P. Karst., Bidrag Kannedom Finlands Natur Folk 23: 89. 1873.
≡ Leptosphaeria praetermissa (P. Karst.) Sacc., Syll. Fung. 2: 26. 1883.
Specimen examined: Sweden, Dalarna, Folkärna, from Rubus idaeus (Rosaceae), 21 Mar. 1993, K. & L. Holm, CBS 114591.
Plenodomus Preuss, Linnaea 24: 145. 1851.
≡ Phoma sect. Plenodomus (Preuss) Boerema, Kesteren & Loer., Trans. Brit. Mycol. Soc. 77: 61. 1981.
= Diploplenodomus Diedicke, Ann. Mycol. 10: 140. 1912.
= Plectophomella Moesz, Magyar Bot. Lapok 21: 13. 1922.
= Apocytospora Höhn., Mitt. Bot. Lab. TH Wien 1: 43. 1924.
= Deuterophoma Petri, Boll. R. Staz. Patalog. Veget. Roma 9: 396. 1929.
Type species: Plenodomus rabenhorstii Preuss, Linnaea 24: 145. 1851 (dubious synonym, see below) = Plenodomus lingam (Tode: Fr.) Höhn., see below.
Note: For full synonymy of the anamorph names of the species listed below, see Boerema et al. (1994). For additional synonyms of the teleomorph names of the species below that have been recorded on Asteraceous hosts, see Khashnobish et al. (1995).
Plenodomus agnitus (Desm.) Gruyter, Aveskamp & Verkley, comb. nov. MycoBank MB564726.
Basionym: Sphaeria agnita Desm., Ann. Sci. Nat., Bot. Ser. 3, 16: 313. 1851.
≡ Leptosphaeria agnita (Desm.) Ces. & De Not., Comm. Soc. Crittog. Ital. 1: 236. 1863.
= Plenodomus chondrillae Died, Ann. Mycol.. 9: 140. 1911; Krypt.-fl. Brandenburg 9: 236. 1912.
= Phoma agnita Gonz. Frag., Mem. Real Acad. Ci. Barcelona 15: 6. 1920.
Specimens examined: Netherlands, from stem of Eupatorium cannabinum (Asteraceae), 1982, W.M. Loerakker, CBS 126584 =PD 82/561; from stem of Eupatorium cannabinum, 1982, W.M. Loerakker, CBS 121.89 = PD 82/903.
Plenodomus biglobosus (Shoemaker & H. Brun) Gruyter, Aveskamp & Verkley, comb. nov. MycoBank MB564727.
Basionym: Leptosphaeria biglobosa Shoemaker & H. Brun, Canad. J. Bot. 79: 413. 2001.
Specimens examined: France, Le Rheu, from stem of Brassica juncea (Brassicaceae), CBS 127249 =DAOM 229269. Netherlands, from Brassica rapa (Brassicaceae), 2006, R. Veenstra, CBS 119951.
Notes: Leptosphaeria biglobosa was originally described as a less virulent segregate of L. maculans (Shoemaker & Brun 2001). The species, also indicated as Tox0 isolates, has been described from cultivated Brassica species as the cause of upper stem lesions and considered as less damaging than L. maculans (West et al. 2002). However, in Poland L. biglobosa is the predominant cause of these symptoms (Jedryczka et al. 1999, Huang et al. 2005). The current species concept of L. biglobosa is broadly defined with six distinct subclades recognised by multilocus phylogenetic analyses of ITS, β-tubulin and actin sequences (Mendes-Pereira et al. 2003, Vincenot et al. 2008). These subclades are named after the host or geographic origin of the isolates involved. It has been suggested that the clades represent distinct subspecies formed over time by reproductive isolation (Mendes-Pereira et al. 2003). Alignments of the ITS sequences of Ph. wasbiae, Ph. pimpinellae and L. biglobosa isolates were compared with those of the representative strains of the L. biglobosa subclades obtained from GenBank, and both Ph. wasbiae and Ph. pimpinellae grouped in this species complex (unpubl. data). Both species are maintained here, awaiting a redescription of the taxa representing all clades in the L. biglobosa complex.
Plenodomus chrysanthemi (Zachos, Constantinou & Panag.) Gruyter, Aveskamp & Verkley, comb. nov. MycoBank MB564728.
Basionym: Cephalosporium chrysanthemi Zachos, Constantinou & Panag., Ann. Inst. Phytopath. Benaki, N.S. 55. 1960.
≡ Phialophora chrysanthemi (Zachos, Constantinou & Panag.) W. Gams, Cephalosporium-artige Schimmelpilze (Stuttgart): 207. 1971.
= Phoma vasinfecta Boerema, Gruyter & Kesteren, Persoonia 15: 484. 1994.
Specimen examined: Greece, from Chrysanthemum sp. (Asteraceae), Apr. 1963, D.G. Zachos, holotype CBS H-7576, culture ex-holotype CBS 539.63.
Note: The species was also described as Phoma tracheiphila f. sp. chrysanthemi (Baker et al. 1985).
Plenodomus collinsoniae (Dearn. & House) Gruyter, Aveskamp & Verkley, comb. nov. MycoBank MB564729.
Basionym: Leptosphaeria collinsoniae Dearn. & House, Bull. New York State Mus. Nat. Hist. 233-234: 36. 1921.
Specimen examined: Japan, Osawa river, Komukai, Miyagi, from Vitis coignetiae (Vitaceae), 27 Sep. 2003, Y. Takahashi, CBS 120227 =JCM 13073 = MAFF 239583.
Plenodomus confertus (Niessl ex Sacc.) Gruyter, Aveskamp & Verkley, comb. nov. MycoBank MB564730.
Basionym: Leptosphaeria conferta Niessl ex Sacc., Syll. Fung. 2: 20. 1883.
= Phoma conferta P. Syd. ex Died., Krypt.-fl. Brandenburg 9: 142. 1912.
Specimen examined: Spain, Cais do Tejo, from dead stem of Anacyclus radiatus (Asteraceae), Mar. 1961, M.T. Lucas, CBS 375.64.
Plenodomus congestus (M.T. Lucas) Gruyter, Aveskamp & Verkley, comb. nov. MycoBank MB564731.
Basionym: Leptosphaeria congesta M.T. Lucas, Trans. Brit. Mycol. Soc. 46: 362. 1963.
= Phoma congesta Boerema, Gruyter & Kesteren, Persoonia 15: 461. 1994.
Specimen examined: Spain, Póvoa de Santa Iria, Estremadura, from stem of Erigeron canadensis (Asteraceae), Mar. 1961, M.T. Lucas, holotype of Leptosphaeria congesta M.T. Lucas, dried culture LISE 1638, culture ex-holotype CBS 244.64.
Plenodomus enteroleucus (Sacc.) Gruyter, Aveskamp & Verkley, comb. nov. MycoBank MB564753.
Basionym: Phoma enteroleuca Sacc. var. enteroleuca, Michelia 1: 358. 1878.
Specimens examined: France, Alencon, from Pyrus communis (Rosaceae), 1878, C. C. Gillet, holotype of Phoma enteroleuca var. enteroleuca, Herb. Sacc. ’19’, PAD. Germany, Monheim, from leaf spots of Triticum aestivum (Poaceae), 15 Aug. 1984, M. Hossfeld, CBS H-3684, culture CBS 831.84. Netherlands, Bennekom, from discoloured wood of Catalpa bignonioides (Bignoniaceae), 1981, G.H. Boerema, epitype designated here CBS H-16209, culture ex-epitype CBS 142.84 = PD 81/654 = CECT 20063.
Plenodomus fallaciosus (Berl.) Gruyter, Aveskamp & Verkley, comb. nov. MycoBank MB564732.
Basionym: Leptosphaeria fallaciosa Berl., Bull. Soc. Mycol. France. 5: 43. 1889.
Specimen examined: France, Var, Ste. Baume, from Satureia montana (Lamiaceae), July 1951, E. Müller, CBS 414.62 = ETH 2961.
Plenodomus hendersoniae (Fuckel) Gruyter, Aveskamp & Verkley, comb. nov. MycoBank MB564754.
Basionym: Cucurbitaria hendersoniae Fuckel, Symb. Myc. p. 172. 1870.
≡ Melanomma hendersoniae (Fuckel) Sacc., Syll. Fung. 2: 109. 1883.
≡ Chiajaea hendersoniae (Fuckel) Höhn., Sitzungsber. Kaiserl. Akad. Wiss., Math.-Naturwiss. Cl., Abt. 1. 129: 152. 1920.
≡ Leptosphaeria hendersoniae (Fuckel) L. Holm, Symb. Bot. Upsal. 14: 26. 1957.
= Phoma intricans M.B. Schwarz, Meded. Phytopath. Lab. Willie Commelin Scholten 8: 44. 1922.
Specimens examined: Sweden, Uppland, Jerusalem, from Salix cinerea (Salicaceae), 10 Apr. 1986, K. & L. Holm, CBS 113702 =UPSC 1843. Netherlands, Wilhelminadorp, from bark of Pyrus malus (Rosaceae), June 1977, H.A.Th. van der Scheer, CBS 139.78.
Plenodomus influorescens (Boerema & Loer.) Gruyter, Aveskamp & Verkley, comb. nov. MycoBank MB564755.
Basionym: Phoma enteroleuca var. influorescens Boerema & Loer., Trans. Brit. Mycol. Soc. 84: 290. 1985.
Specimens examined: Netherlands, from Lilium sp. (Liliaceae), 1973, G.H. Boerema, PD 73/1382; Emmeloord, from Fraxinus excelsior (Oleaceae), 1978, J.D. Janse, holotype of Phoma enteroleuca var. influorescens, CBS H-16208, culture ex holotype CBS 143.84 = PD 78/883 = CECT 20064.
Note: The isolate PD 73/1382 is no longer available for study.
Plenodomus libanotidis (Fuckel) Gruyter, Aveskamp & Verkley, comb. nov. MycoBank MB564756.
Basionym: Pleospora libanotidis Fuckel, Jahrb. Nassauischen Vereins Naturk. 27-28: 24. 1873 (as “libanotis”).
≡ Leptosphaeria libanotidis (Fuckel) Sacc., Syll. Fung. 2: 16. 1883 (as “libanotis”).
-
= Phoma sanguinolenta Rostr., Tidsskr. Landokon. 5(7): 384. 1888 (not Phoma sanguinolenta Grove, J. Bot. 23: 164. 1885).
≡ Phoma rostrupii Sacc., Syll. Fung. 11: 490. 1895, nom. nov.
Specimen examined: Sweden, Uppland, Gröna strand, from Seseli libanotis (Apiaceae), 19 May 1987, K. & L. Holm, CBS 113795 =UPSC 2219.
Plenodomus lindquistii (Frezzi) Gruyter, Aveskamp & Verkley, comb. nov. MycoBank MB564757.
Basionym: Leptosphaeria lindquistii Frezzi, Revista Invest. Agropec., Sér. 5, 5: 79. 1968.
= Phoma macdonaldii Boerema, Persoonia 6: 20. 1970.
Specimens examined: Canada, from Helianthus annuus (Asteraceae), 1967, W.C. McDonald, CBS 381.67. Former Yugoslavia, from stem of Helianthus annuus, 1977, A. Maric, CBS 386.80 = PD 77/336.
Note: Strain CBS 381.67 is ex-holotype of Phoma macdonaldii Boerema, pycnidial state of Leptosphaeria lindquistii Frezzi (Boerema 1970).
Plenodomus lingam (Tode: Fr.) Höhn., Sitzungsber. Kaiserl. Akad. Wiss., Math.-Naturwiss. Cl., Abt. 1. 120: 463. 1911.
Basionym: Sphaeria lingam Tode: Fr., Fungi mecklenb. 2: 51. 1791.: Fr., Syst. Mycol. 2: 507. 1823.
≡ Phoma lingam (Tode: Fr.) Desm., Ann. Sci. Nat., Bot. Ser. 3, 11: 281. 1849.
-
= Sphaeria maculans Desm., Ann. Sci. Nat., Bot. Ser. 3, 6: 77. 1846, nom. illeg.
≡ Leptosphaeria maculans (Desm.) Ces. & De Not., Comment. Soc. Crittog. Ital. 1: 235. 1863.
= Plenodomus rabenhorstii Preuss, Linnaea 24: 145. 1851, nom. dub.
Specimens examined: Netherlands, near Goes, from Brassica oleracea (Brassicaceae), 1978, M.M.J. Dorenbosch, CBS 260.94 = PD 78/989. Origin unknown, Mar. 1924, A. Weber, CBS 147.24. UK, from Brassica sp. (Brassicaceae), 1963, B.C. Sutton, CBS 275.63 = MUCL 9901= UPSC 1025.
Notes: The combination Plen. lingam as published by van Höhnel (1911) was preferred over Plen. rabenhorstii Preuss (1851) by Boerema & van Kesteren (1964) because the type material of Plen. rabenhorstii had been lost during the Second World War. Therefore, Plen. rabenhorstii is indicated here as a nomen dubium. Leptosphaeria maculans causes a serious stem base canker (blackleg) on cultivated Brassica spp. (Brassicaceae) in Europe, Australia and North America (West et al. 2001, Fitt et al. 2006).
Plenodomus lupini (Ellis & Everh.) Gruyter, Aveskamp & Verkley, comb. nov. MycoBank MB564758.
Basionym: Phoma lupini Ellis & Everh., Bull. Washburn Lab. Nat. Hist. 1: 6. 1884.
≡ Asteromella lupini (Ellis & Everh.) Petr., Sydowia 9: 495. 1955 (not Phoma lupini N.F. Buchw., Møller, Fungi Faeröes 2: 153. 1958, nom. illeg).
Specimen examined: Peru, Andes region, from stem lesion of Lupinus mutabilis (Fabaceae), May 1992, J. de Gruyter, CBS 248.92 = PD 79/141.
Plenodomus pimpinellae (Lowen & Sivan.) Gruyter, Aveskamp & Verkley, comb. nov. MycoBank MB564759.
Basionym: Leptosphaeria pimpinellae Lowen & Sivan., Mycotaxon 35: 205. 1989.
= Phoma pimpinellae Boerema & Gruyter, Persoonia 17: 278. 1999.
Specimen examined: Israel, Mt Carmel near Kibbutz Oren, from dead stems of Pimpinella anisum (Apiaceae), 9 Dec. 1987, R. Rowen, 523-88 NY, holotype of Leptosphaeria pimpinellae Lowen & Sivan, culture ex-holotype CBS 101637 =PD 92/41.
Plenodomus tracheiphilus (Petri) Gruyter, Aveskamp & Verkley, comb. nov. MycoBank MB564760.
Basionym: Deuterophoma tracheiphila Petri, Boll. Staz. Patol. Veg. Roma 9: 396. 1929.
≡ Bakerophoma tracheiphila (Petri) Cif., Ist. Bot. Reale Univ. Reale Lab. Crittog. Pavia Atti Ser. 5, 5: 307. 1946.
≡ Phoma tracheiphila (Petri) L.A. Kantsch. & Gikaschvili, Trudy Inst. Zasch. Rast. Tibilisi 5: 20. 1948.
Specimens examined: Israel, from Citrus limonium (Rutaceae), Oct. 1993, J. de Gruyter, CBS 551.93 = PD 81/782. Italy, from Citrus sp. (Rutaceae), CBS 127250 =PD 09/04597141.
Note: The species produces a phialophora-like synanamorph.
Plenodomus visci (Moesz) Gruyter, Aveskamp & Verkley, comb. nov. MycoBank MB564761.
Basionym: Plectophomella visci Moesz, Magyar Bot. Lapok 21: 13. 1922.
= Apocytospora visci Höhn., Mitt. Bot. Lab. TH Wien 1: 43. 1924.
Specimen examined: Hungary, Tata-Tóváros, from leaves of Viscum album (Viscaceae), 22 Oct. 1911, G. von Moesz, BP, holotype of Plectophomella visci Moesz. France, from Viscum album, 1974, epitype designated here CBS H-20823, culture ex-epitype CBS 122783 =PD 74/1021.
Notes: Plectophomella visci is the type species of the genus Plectophomella. This genus was accepted by Sutton (1980) based on the eustromatic conidiomata; branched, septate conidiophores, phialidic conidiogenesis and small, hyaline conidia. However, the phylogenetic analyses clearly demonstrated the placement of Plectophomella grouping in the Plenodomus clade and therefore it is treated as a synonym.
Plenodomus wasabiae (Yokogi) J.F. White & P.V. Reddy, Canad. J. Bot. 76: 1920. 1999 (1998).
Basionym: Phoma wasabiae Yokogi, Ann. Phytopathol. Soc. Japan 2: 549. 1933.
Specimens examined: Taiwan, from Wasabia japonica (syn. Eutrema wasabi) (Brassicaceae), A. Rossman, CBS 120119 =FAU 559; from Wasabia japonica, A. Rossman, CBS 120120 =FAU 561.
Subplenodomus Gruyter, Verkley & Crous, gen. nov. MycoBank MB564769.
Etymology: Although the genus resembles Plenodomus in the production of thick-walled pycnidia, the pycnidial cell wall of Subplenodomus often remains pseudoparenchymatous, similar to the pycnidial wall of species of Phoma.
Conidiomata pycnidial, globose to papillate, or with an elongated neck, solitary or aggregated, thin-walled pseudoparenchymatous, or thick-walled scleroplectenchymatous, ostiolate, unilocular. Conidiogenous cells phialidic, ampulliform to doliiform. Conidia hyaline, aseptate, ellipsoid to cylindrical. Chlamydospores sometimes produced, olivaceous, unicellular in chains, or multicellular, dictyosporous-botryoid or forming pseudosclerotioid structures.
Type species: Subplenodomus violicola (P. Syd.) Gruyter, Aveskamp & Verkley (see below)
Subplenodomus apiicola (Kleb.) Gruyter, Aveskamp & Verkley, comb. nov. MycoBank MB564770.
Basionym: Phoma apiicola Kleb., Z. Pflanzenkrankh. 20: 22. 1910.
Specimens examined: Germany, from tuber of Apium graveolens var. rapaceum (Apiaceae), Feb. 1972, Diercks, culture CBS 285.72. Netherlands, from stem base of Apium graveolens, 1978, J. de Gruyter, CBS 504.91 = PD 78/1073.
Subplenodomus drobnjacensis (Bubák) Gruyter, Aveskamp & Verkley, comb. nov. MycoBank MB564771.
Basionym: Phoma drobnjacensis Bubák, Bot. Közlem. 14: 63. 1915
= Pyrenochaeta gentianae Chevassut, Bull. Soc. Mycol. France. 81: 36. 1965.
Specimens examined: Netherlands, from stem base of Gentiana makinoi “Royal Blue” (Gentianaceae), 1983, M.M.J. Dorenbosch, CBS 270.92 = PD 83/650; Naaldwijk, from red-brown root of Eustoma exaltatum (Gentianaceae), 1988, M.M.J. Dorenbosch, CBS 269.92 = PD 88/896.
Subplenodomus valerianae (Henn.) Gruyter, Aveskamp & Verkley, comb. nov. MycoBank MB564772.
Basionym: Phoma valerianae Henn., Nyt Mag. Naturvidensk. 42: 29. 1904.
= Phyllosticta valerianae-tripteris f. minor Unamuno, Mem. Real Soc. Esp. Hist. Nat. 15: 348. 1929.
Specimens examined: Netherlands, Arnhem, from dead stem of Valeriana phu (Valerianaceae), Sep. 1968, G.H. Boerema, CBS 630.68 = PD 68/141; Elburg, from stem base of Valeriana officinalis, 1973, M.M.J. Dorenbosch, culture CBS 499.91 = PD 73/672.
Subplenodomus violicola (P. Syd.) Gruyter, Aveskamp & Verkley, comb. nov. MycoBank MB564774.
Basionym: Phoma violicola P. Syd., Beibl. Hedwigia 38: 137. 1899.
= Phyllosticta violae f. violae-hirtae Allesch. Rabenh.-Fl., Ed. 2, Pilze 6: 156. 1898.
= Phoma violae-tricoloris Died., Ann. Mycol. 2: 179. 1904.
= Phyllosticta violae f. violae-sylvaticae Gonz. Frag., Trab. Mus. Nac. Ci. Nat., Ser. Bot. 7: 35. 1914.
Specimens examined: Netherlands, Baarn, from leaf spot in Viola tricolor, 10 Mar. 1968, H.A. van der Aa, CBS 306.68. New Zealand, Auckland, Henderson, from leaf spot in Viola tricolor (Violaceae), 1997, J. Jury, CBS 100272.
Coniothyriaceae W.B. Cooke. Revista Biol. (Lisbon) 12: 289. 1983.
Coniothyrium carteri (Gruyter & Boerema) Verkley & Gruyter, comb. nov. MycoBank MB564775.
Basionym: Phoma carteri Gruyter & Boerema, Persoonia 17(4): 547. 2002 (“2001”), nom. nov.
Replaced synonym: Pyrenochaeta minuta J.C. Carter, Bull. Illinois Nat. Hist. Surv. 21: 214. 1941 [not Phoma minuta Wehm., Mycologia 38: 318. 1946, nor Phoma minuta Alcalde, Anales Inst. Bot. Cavanilles 10: 235. 1952; not Coniothyrium minutum (Berl.) O. Kuntze, Revis. Gen. Pl. 3: 459. 1898 = Phoma cava, syn. of Pyrenochaeta cava; not Coniothyrium minutum (Died) Petr. & Syd., Feddes Repert. Spec. Nov. Regni Veg. Beih. 42: 349. 1927].
Specimens examined: Germany, isolated from Quercus robur (Fagaceae), 1991, CBS 105.91. Netherlands, from shoot of Quercus sp. (Fagaceae), 1984, M.M.J. Dorenbosch, CBS 101633 =PD 84/74.
Coniothyrium dolichi (Mohanty) Verkley & Gruyter, comb. nov. MycoBank MB564776.
Basionym: Pyrenochaeta dolichi Mohanty, Indian Phytopathol. 11: 85. 1958.
Specimen examined: India, Nani Tal, Sarichuan, from leafspot of Dolichos biflorus (Fabaceae), 20 Oct. 1955, N.N. Mohandy, CBS 124140 =IMI 217262, CBS 124143 =IMI 217261.
Notes: A synanamorph was noted and described as a Coniosporium state based on the dark brown to black, dictyosporous conidia (Mohanty 1958). This synanamorph was considered later as monodictys-like (Grodona et al. 1997).
Coniothyrium glycines (R.B. Stewart) Verkley & Gruyter, comb. nov. MycoBank MB564777.
Basionym: Pyrenochaeta glycines R.B. Stewart, Mycologia 49: 115. 1957.
≡ Phoma glycinicola Gruyter & Boerema, Persoonia 17: 554. 2002 (“2001”), nom. nov., nom. inval. (not Phoma glycines Sawada, Special. Publ. Coll. Agric., Natl. Taiwan Univ. 8: 129. 1959, nom. inval). ≡ Phoma glycines Sawada ex J.K. Bai & G.Z. Lu, Fl. Fungorum Sin. 15: 33. 2003.
Specimens examined: Zambia, on Mt. Makulu, from leaf of Glycine max (Fabaceae), Mar. 1985, J.M. Waller, CBS 124455 =IMI 294986. Zimbabwe, from a leaf of Glycine max (Fabaceae), 2001, C. Lavy, CBS 124141 =PG1.
Coniothyrium multiporum (V.H. Pawar, P.N. Mathur & Thirum.) Verkley & Gruyter, comb. nov. MycoBank MB564778.
Basionym: Phoma multipora V.H. Pawar, P.N. Mathur & Thirum., Trans. Brit. Mycol. Soc. 50: 260. 1967.
≡ Phoma multipora V.H. Pawar & Thirum., Nova Hedwigia 12: 501. 1966, nom. nud.
Specimens examined: Egypt, CBS 501.91 = PD 83/888. India, Bombay, Bandra, from saline soil, 15 Jan. 1958, M.J. Thirumalachar, Isotype CBS H-16492, culture ex-isotype CBS 353.65 = ATCC 16207 = HACC 164 = IMI 113689.
Coniothyrium palmarum Corda, Icon. Fungorum. (Corda) 4: 38. 1840.
≡ Clisosporium palmarum (Corda) Kuntze, Revis. Gen. Pl. 3: 458. 1898.
≡ Microdiplodia palmarum (Corda) Died., Ann. Mycol. 11: 47. 1913.
Specimens examined: Italy, Sardegna, near Dorgali, from a dead petiole of Chamaerops humilis (Arecaceae), Aug. 1970, W. Gams, CBS H-10891-10893, culture CBS 400.71.
Coniothyrium telephii (Allesch.) Verkley & Gruyter, comb. nov. MycoBank MB564779.
Basionym: Pyrenochaeta telephii Allesch., Ber. bayer. bot. Ges. 4: 33. 1896.
≡ Phoma septicidalis Boerema, Versl. Meded. Plantenziektenk. Dienst Wageningen 153 (Jaarb. 1978): 20. 1979, nom. nov. [not Phoma telephii (Vestergr.) Kesteren, Netherlands J. Pl. Pathol. 78: 117. 1972].
Specimens examined: Finland, Helsinki, Asko Kahanpää, obtained from air, Jan. 1971, CBS H-16567, culture CBS 188.71; Oulu, from mineral wool between walls, Dec. 1996, K. Poldmaa, CBS 856.97. Zimbabwe, from leaf of Glycine max (Fabaceae), CBS 101636 =PD 86/1186.
Cucurbitariaceae G. Winter, Rabenh, Krypt.-Fl., Ed 2, 308. 1885.
Neophaeosphaeria filamentosa (Ellis & Everh.) Câmara, M.E. Palm & A.W. Ramaley, Mycol. Res. 107: 519. 2003.
Basionym: Leptosphaeria filamentosa Ellis & Everh., J. Mycol. 4: 76. 1888.
≡ Paraphaeosphaeria filamentosa (Ellis & Everh.) M.E. Barr, Mycotaxon 43: 392. 1992.
Specimen examined: Mexico, from Yucca rostrata (Asparagaceae), Stevens, CBS 102202 =BPI 802755.
Pyrenochaetopsis pratorum (P.R. Johnst. & Boerema) Gruyter, Aveskamp & Verkley, comb. nov. MycoBank MB564780.
Basionym: Phoma pratorum P.R. Johnst. & Boerema, New Zealand J. Bot. 19: 395. 1981.
Specimens examined: New Zealand, Rakura, near Hamilton, from a leaf of Lolium perenne (Poaceae), 1980, P.R. Johnston, isotype CBS H-7625, CBS H-7626, culture CBS 445.81 = PDDCC 7049 = PD 80/1254; Dactylis glomerata (Poaceae), 1980, CBS 286.93 = PD 80/1252.
Pleosporaceae Nitschke, Verh. Naturhist. Vereines Preuss. Rheinl. 26: 74. 1869.
Pleospora angustis Gruyter & Verkley, nom. nov. MycoBank MB564781.
≡ Leptosphaeria clavata A.L. Guyot, Revue Mycol. (Paris) 11: 62. 1946.
≡ Massariosphaeria clavata (A.L. Guyot) Shoemaker & C.E. Babc., Canad. J. Bot. 67: 1582.1989; not Pleospora clavata Gucevič (“as clavatis”), Novosti Sist. Nizsh. Rast. 7: 168. 1970.
Specimen examined: Switzerland, 1951, E. Müller, CBS 296.51.
Notes: The origin of the isolate deposited by E. Müller is unknown; however, it is likely that the isolate was obtained from Poaceae, Triticum vulgare or Dactylis glomerata (Müller 1950). Pleospora clavata Gucevič was obtained from Lonicera alseuosmoides and refers to a different species.
Pleospora betae (Berl.) Nevod., Grib. ross. Exs., No. 247. 1915.
Basionym: Pyrenophora echinella var. betae Berl. Nuovo Giorn. Bot. Ital. 20: 208. 1888.
-
= Pleospora betae Björl., Bot. Not. 1944: 218. 1944. (later homonym), nom. illeg.
≡ Pleospora bjoerlingii Byford, Trans. Brit. Mycol. Soc. 46: 614. 1963, nom. nov.
= Phoma betae A.B. Frank, Z. Rúbenzucker-Ind. 42: 904, tab. 20. 1892.
= Phyllosticta betae Oudem., Ned. Kruidk. Arch. Ser. 2, 2: 181. 1877.
= Gloeosporium betae Dearn. & E.T. Barthol., Mycologia 9: 356. 1917.
Specimens examined: Netherlands, Wageningen, from Beta vulgaris (Chenopodiaceae), Sep. 1966, M.M.J. Dorenbosch, CBS H-16156, culture CBS 523.66 = IHEM 3915 = PD 66/270; from Beta vulgaris, 1977, G.H. Boerema, CBS 109410 =PD 77/113.
Note: The name Phoma betae A.B. Frank has been conserved against Phyllosticta tabifica and any combination based on that name (Shoemaker & Redhead 1999).
Pleospora calvescens (Fr.) Tul. & C. Tul., Selecta Fung. Carpol. (Paris) 2: 266. 1863.
Basionym: Sphaeria calvescens Fr., Ann. Sci. Nat., Bot. Ser. 2, 19: 353. 1843.
≡ Leptosphaeria calvescens (Fr.) Sacc., Syll. fung. 2: 24. 1883.
≡ Pyrenophora calvescens (Fr.) Sacc., Syll. fung. 2: 279. 1883.
-
= Chaetodiplodia caulina P. Karst., Hedwigia 23: 62. 1884.
≡ Ascochyta caulina (P. Karst.) v.d. Aa & Kesteren, Persoonia 10: 271. 1979.
= Microdiplodia henningsii Staritz, Hedwigia 53: 163. 1913.
Specimens examined: Germany, Munkmarsch, from leaf spots in Atriplex hastata (Chenopodiaceae), 20 July 1977, G.H. Boerema, CBS H-8980, culture CBS 246.79 = PD 77/655. Netherlands, Texel, from dead stem of Atriplex hastata, June 1978, H.A. van der Aa, CBS H-8976, culture CBS 343.78.
Note: For additional synonyms see Boerema et al. (1993).
Pleospora chenopodii Ellis & Kellerman, J. Mycol. 4: 26. 1888.
-
= Diplodia hyalospora Cooke & Ellis, Grevillea 7: 5. 1878 (not Pleospora hyalospora Ellis & Everh., Proc. Acad. Nat. Sci. Philadelphia. 42: 238. 1890).
≡ Ascochyta hyalospora (Cooke & Ellis) Boerema, S.B. Mathur & Neerg., Netherlands J. Pl. Pathol. 83: 156. 1977.
= Diplodina ellisii Sacc., Syll Fung. 3: 417. 1884
Specimens examined: Bolivia, isolated from Chenopodium quinoa (Chenopodiaceae), 1974, S.B. Mathur, CBS H-9051, CBS H-9052, culture CBS 206.80 = PD 74/1022. Netherlands, Zoutelande, from Atriplex hastata (Chenopodiaceae), Aug. 1968, H.A. van Kesteren, CBS 344.78 = PD 68/682.
Note: Isolate CBS 344.78 was originally identified as Ascochyta caulina but was identical to Pleospora chenopodii in the present study.
Pleospora fallens (Sacc.) Gruyter & Verkley, comb. nov. MycoBank MB564782.
Basionym: Phoma fallens Sacc., Syll. Fung. 10: 146. 1892.
-
= Phyllosticta glaucispora Delacr., Bull. Soc. Mycol. France 9: 266. 1893.
≡ Phoma glaucispora (Delacr.) Noordel. & Boerema, Versl. Meded. Plantenziektenk. Dienst Wageningen 166 (Jaarb. 1987): 108. 1989 (“1988”).
= Phyllosticta oleandri Gutner, Trudy Bot. Inst. Akad. Nauk S.S.S.R., Ser. 2, Sporov. Rast. 1: 306. 1933.
Specimens examined: Italy, Capri, Villa Jovis, from a leaf spot of Nerium oleander (Apogynaceae), CBS H-16639, culture CBS 284.70 = PD 97/2400. New Zealand, Levin, from leaf spot of Olea europaea (Oleaceae), 1978, G.F. Laundon, CBS 161.78 = LEV 1131.
Pleospora flavigena (Constantinou & Aa) Gruyter & Verkley, comb. nov. MycoBank MB564783.
Basionym: Phoma flavigena Constantinou & Aa, Trans. Brit. Mycol. Soc. 79: 343. 1982.
Specimen examined: Romania, Bucuresti, isolated from water, 1980, K. Fodor, CBS H-1418, holotype of Phoma flavigena Constantinou & Aa, culture ex-holotype CBS 314.80 = PD 91/1613.
Pleospora halimiones Gruyter & Verkley, nom. nov. MycoBank MB564784.
≡ Diplodina obiones Jaap (as “obionis”), Verh. Bot. Vereins Prov. Brandenburg 47: 96. 1905 (not Pleopora obiones P. Crouan & H. Crouan, Fl. Finistère: 22. 1867).
≡ Ascochytula obiones (Jaap) Died., Ann. Mycol. 10: 141. 1912.
≡ Ascochyta obiones (Jaap) P.K. Buchanan, Mycol. Pap. 156: 28 1987.
= Coniothyrium obiones Jaap (as “obionis”), Schriften Naturwiss. Vereins Schleswig-Holstein 14: 29. 1907.
Specimens examined: Netherlands, Texel, from leaf spots in Halimione portulacoides (Chenopodiaceae), 27 Oct. 1968, H.A. van der Aa, CBS H-9127, CBS H-9129, culture CBS 786.68; Texel, De Cocksdorp, from dead stems of Halimione portulacoides, 6 July 1977, H.A. van der Aa, CBS H-9126, CBS H-9125, culture CBS 432.77 = IMI 282137.
Notes: Isolate CBS 453.68 preserved as Chaetodiplodia sp. and also isolated from dying stems and leaf sheaths of Halimione portulacoides on Texel, is not the same as Pleo. halimiones and is probably a different species.
Pleospora herbarum (Pers.) Rabenh., Bot. Zeitung (Berlin) 15: 428. 1857; Klotzschii Herb. Viv. Mycol. 2: no. 547 (1854.)
Basionym: Sphaeria herbarum Pers., Syn. Meth. Fung. 1: 78. 1801.
= Stemphylium herbarum E.G. Simmons, Sydowia 38: 291. 1986 (1985).
Specimen examined: India, Uttar Pradesh, from a leaf of Medicago sativa (Fabaceae), 1986 (isolated in 1983), E.G. Simmons, CBS 191.86 = IMI 276975.
Note: This isolate is the ex-type culture of Stemphylium herbarum.
Pleospora incompta (Sacc. & Martelli) Gruyter & Verkley, comb. nov. MycoBank MB564785.
Basionym: Phoma incompta Sacc. & Martelli, Syll. Fung. 10: 146. 1892.
Specimens examined: Greece, Crete, from branch of Olea europaea (Oleaceae), 1976, N. Malathrakis, CBS H-16394, culture CBS 467.76. Italy, from branch of Olea europaea, Mar. 1982, CBS H-16392, culture CBS 526.82.
Pleospora typhicola (Cooke) Sacc., Syll. Fung. 2: 264. 1883.
Basionym: Sphaeria typhicola Cooke, Grevillea 5: 121. 1877.
≡ Clathrospora typhicola (Cooke) Höhn., Ann. Mycol. 16: 88. 1918.
≡ Pyrenophora typhicola (Cooke) E. Müll., Sydowia 5: 256. 1951.
≡ Macrospora typhicola (Cooke) Shoemaker & C.E. Babc., Canad. J. Bot. 70: 1644. 1992.
-
= Phyllosticta typhina Sacc. & Malbr., Sacc., Michelia 2: 88. 1880.
≡ Phoma typhina (Sacc. & Malbr.) van der Aa & Vanev, A revision of the species described in Phyllosticta: 468. 2002.
= Phoma typharum Sacc., Syll. Fung. 3: 163. 1884.
Specimens examined: Netherlands, Texel, from dead leaves of Typha angustifolia (Typhaceae), 1969, W. Gams, CBS H-16597, culture CBS 132.69; Staverden, from leaf spots of Typha sp., 24 June 1972, G.S. de Hoog, CBS H-16598, culture CBS 602.72.
Phoma-like anamorphs excluded from the suborder Pleosporineae
Montagnulaceae M.E. Barr, Mycotaxon 77: 194. 2001.
Paraconiothyrium Verkley, Stud. Mycol. 50: 327. 2004.
Type species: Paraconiothyrium estuarinum Verkley & M. da Silva, Stud. Mycol. 50: 327. 2004.
Paraconiothyrium flavescens (Gruyter, Noordel. & Boerema) Verkley & Gruyter, comb. nov. MycoBank MB564786.
Basionym: Phoma flavescens Gruyter, Noordel. & Boerema, Persoonia 15(3): 375. 1993.
Specimen examined: Netherlands, Nagele, from soil, rhizosphere of Solanum tuberosum (Solanaceae), CBS 178.93 = PD 82/1062.
Paraconiothyrium fuckelii (Sacc.) Verkley & Gruyter, comb. nov. MycoBank MB564787.
Basionym: Coniothyrium fuckelii Sacc., Nuovo Giorn. Bot. Ital. 8: 200. 1876; Michelia 1: 207. 1878
≡ Clisosporium fuckelii (Sacc.) Kuntze, Revis. Gen. Pl. 3: 458. 1898.
≡ Microsphaeropsis fuckelii (Sacc.) Boerema, 2003, Persoonia 18: 160. 2003.
Specimen examined: Denmark, Geelskov, from a dead stem of Rubus sp. (Rosaceae), 1995, A.M. Dahl-Jensen, CBS 797.95.
Notes: Coniothyrium fuckelii var. sporulosum has been redisposed as Paraconiothyrium sporulosum (Verkley et al. 2004) and it is clearly different from Paraconiothyrium fuckelii (Damm et al. 2008).
Paraconiothyrium fusco-maculans (Sacc.) Verkley & Gruyter, comb. nov. MycoBank MB564788.
Basionym: Phoma fusco-maculans Sacc., Michelia 2: 275. 1881
≡ Plenodomus fusco-maculans (Sacc.) Coons, J. Agric. Res. 5: 714. 1916.
Specimens examined: Italy, Selva, from decorticated wood of Malus pumila (Rosaceae), Oct. 1880, PAD, holotype of Phoma fusco-maculans Sacc. USA, from wood of Malus sp. (Rosaceae), July 1916, G.H. Coons, epitype designated here CBS H-20825, culture ex-epitype CBS 116.16.
Notes: Plenodomus fusco-maculans was discussed by Boerema & Loerakker (1985) and de Gruyter et al. (2010). The holotype of the basionym Aposphaeria fusco-maculans was studied and considered to be Aposphaeria pulviscula (Boerema et al. 1996). However, the description of A. fusco-maculans given by Boerema et al. (1996) fits the generic concept of Paraconiothyrium, in congruence with the molecular phylogeny of the culture CBS 116.16.
Paraconiothyrium lini (Pass.) Verkley & Gruyter, comb. nov. MycoBank MB564789.
Basionym: Phoma lini Pass., Diagn. Funghi Nuovi 4, No. 81. 1890.
Specimen examined: Netherlands, from Wisconsin tank, 1970, CBS 253.92 = PD 70/998.
Paraconiothyrium maculicutis Verkley & Gruyter, sp. nov. MycoBank MB564796. Fig. 6.
Fig. 6.
Paraconiothyrium maculicutis sp. nov. CBS 101461. A-B. Fourteen day old cultures on OA (A) and MA (B). C-D. Pycnidia. E. Phoma-like conidiogenous cells. F-G. Conidia, initially hyaline to pale olivaceous (F), then becoming olivaceous (G). Scale bars: C-D = 20 μm; E = 10 μm; F-G = 5 μm.
Etymology: Latin, cutis = skin; maculae = spots.
Pycnidia in vitro 50-125 μm diam, globose to subglobose, glabrous or with mycelial outgrowth, scattered, non-ostiolate or ostiolate, pycnidial wall made up of 5-7 layers of cells. Conidiogenous cells 1.5-3 × 0.5-2.5 μm, indeterminate or ampulliform to filiform in a later state, up to 10 μm in length. Conidia 1.5-2.5 × 0.5-1.5 μm, ellipsoidal, initially hyaline, then discolouring to olivaceous.
Description in vitro: Colonies on OA 50-52 mm diam after 7 d, margin entire; colony olivaceous buff to greenish olivaceous/grey olivaceous, with greenish olivaceous to pale olivaceous grey, finely floccose to woolly aerial mycelium; reverse smoke-grey to greenish olivaceous, with olivaceous patches. Colonies on MEA 43-44 mm diam after 7 d, margin entire; colony pale olivaceous grey to greenish olivaceous, with isabelline to cinnamon at centre, with compact pale olivaceous grey, finely floccose to woolly aerial mycelium; reverse buff to honey, isabelline to olivaceous near margin. Pycnidia globose to subglobose, olivaceous to brick, finally olivaceous black, scattered, mainly on the agar, 50-125 μm diam, glabrous or with mycelial outgrowth, non-ostiolate or ostiolate, pycnidial wall made up of 5-7 layers of cells. Conidiogenous cells 1.5-3 × 0.5-2.5 μm, ampulliform to filiform in a later state, up to 10 μm in length. Conidia 1.5-2.5 × 0.5-1.5 μm, av. 1 × 2 μm, length/width ratio = 1.5-3.2, av. 2.2, ellipsoidal, initially hyaline, then discolouring to olivaceous. Chlamydospores absent. NaOH spot test: negative. Crystals absent.
Specimen examined: USA, Texas; San Antonio, Fort Sam Houston, from human, cutaneous lesions, 1989, D.P. Dooley, holotype CBS H-20824, culture ex-holotype CBS 101461 =IMI 320754 = UTHSC 87-144.
Notes: Isolate CBS 101461 was identified as Pleurophoma pleurospora (Dooly et al. 1989). However, in vitro data and the molecular phylogeny demonstrate that this isolate does not belong to Pleurophoma pleurospora, see below, and therefore is described as a new species in the genus Paraconiothyrium.
Paraconiothyrium minitans (W.A. Campb.) Verkley, Stud. Mycol. 50: 332. 2004.
Basionym: Coniothyrium minitans W.A. Campb., Mycologia 39: 191. 1947.
Specimens examined: Netherlands, Boskoop, from stem of Clematis sp. (Ranunculaceae), 1999, J. de Gruyter, CBS 122786 =PD 99/1064-1. UK, CBS 122788 =PD 07/03486739.
Paraconiothyrium tiliae (F. Rudolphi) Verkley & Gruyter, comb. nov. MycoBank MB564790.
Basionym: Asteroma tiliae F. Rudolphi, Linnaea 4: 514. 1829.
≡ Asteromella tiliae (F. Rudolphi) Butin & Kehr, Mycol. Res. 99: 1193. 1995, nom. inval., Art. 33.4.
Specimen examined: Austria, Amlach, from a leaf of Tilia platyphyllos (Tiliaceae), 10 Sep. 1993, H. Butin, neotype IMI 362854, lectotype designated here CBS H-20826, culture ex-lectotype CBS 265.94.
Pleurophoma pleurospora (Sacc.) Höhn., Sitzungsber. Kaiserl. Akad. Wiss., Math.-Naturwiss. Cl., Abt. 1. 123: 117. 1914. Fig. 7.
Fig. 7.
Pleurophoma pleurospora. CBS 130329. A-B. Fourteen day old cultures on OA (A) and MA (B). C. Pycnidia. D-H. Conidiogenous cells, septate conidiophores with acropleurogenous conidiogenesis (D-G) or phoma-like (H). I. Conidia. Scale bars: C = 50 μm; D-G, I = 10 μm; H = 5 μm.
Basionym: Dendrophoma pleurospora Sacc., Michelia 2: 97. 1880.
Description in vitro: Colonies on OA 14-18 mm diam after 7 d (18-28 mm after 14 d), margin entire to undulate; colony greenish olivaceous/olivaceous to rosy-buff and sepia, with white, felty aerial mycelium; reverse olivaceous grey to greenish olivaceous/olivaceous. Colonies on MEA 11-16 mm diam after 7 d (19-29 mm after 14 d), colony margin undulate; colony pale olivaceous grey/olivaceous grey to dark mouse-grey with rosy-buff tinges, with white, floccose, compact aerial mycelium, reverse umber/brown olivaceous to olivaceous/olivaceous black. Pycnidia globose to subglobose,olivaceous to olivaceous black, abundant, scattered, mainly on the agar, 30-120 μm diam, solitary or aggregated, covered by mycelial outgrowths or setae-like hyphae, up to 50 μm, non-papillated, without or with ostiole, walls made up of 2-5 layers of cells, outer layer(s) pigmented; conidial exudate not observed. Conidiogenous cells of two types; ampulliform to doliiform, 4-6.5 × 2-5.5 μm, or filiform, septate, branched, acropleurogenous, up to 60 μm long. Conidia 3.5-5.5 × 1.5-2.5 μm, av. 4.5 × 2 μm, length/width ratio = 1.5-3, av. 2.1, cylindrical to oblong, without or with some minute, polar orientated guttules. Chlamydospores absent. NaOH spot test: a weak reddish discolouring may occur on MA, not specific. Crystals absent.
Specimens examined: France, Perpignan, from leaf of Laurus nobilis (Lauraceae), PAD, holotype of Dendrophoma pleurospora Sacc. Netherlands, from wood of Lonicera sp. (Caprifoliaceae), lectotype designated here CBS H-20626, culture ex-lectotype CBS 130329 =PD 82/371; Molenhoek, Heumense Schans, from twig lesions of Cytisus scoparius (Fabaceae), 23 Aug. 2004, G. Verkley & M. Starink, CBS 116668.
Notes: A specimen derived from isolate CBS 130329 is assigned here as lectotype of Pleurophoma pleurospora, the type species of the genus (von Höhnel 1914). The species is known from branches and bare wood of trees and shrubs (Sutton 1980, Boerema et al. 1996) and the isolate from Cytisus scoparius demonstrates that the species also may occur on green twigs. The isolates showed two types of conidiogenesis characteristic for the genus Pleurophoma; phoma-like, ampulliform to doliiform conidiogenous cells, as well as pyrenochaeta-like branched, filiform, septate, acropleurogenous. As a result, species of the genus Pleurophoma can easily be confused with taxa classified in the genera Phoma, Paraphoma, Pyrenochaeta and Pyrenochaetopsis.
Paraphaeosphaeria michotii (Westend.) O.E. Erikss., Arkiv før Botanik 6: 406. 1967.
Basionym: Sphaeria michotii Westend., Bull. Acad. Roy. Sci. Belgique Ser. 2, 7: 87. 1859.
Specimen examined: Switzerland, Kt. Obwalden, from Typha latifolia (Typhaceae), 18 May 1980, A. Leuchtmann, CBS 652.86 = ETH 9483.
Massarinaceae Munk, Friesia 5: 305. 1956.
Byssothecium circinans Fuckel, Bot. Zeitung (Berlin) 19: 251. 1861.
≡ Leptosphaeria circinans (Fuckel) Sacc., Syll. Fung. 2: 88. 1883.
≡ Passeriniella circinans (Fuckel) Sacc., Syll. Fung. 11: 326. 1895.
≡ Trematosphaeria circinans (Fuckel) G. Winter, Rabenh. Krypt.-Fl., ed 1(2): 277. 1887.
≡ Heptameria circinans (Fuckel) Cooke, Grevillea 18: 30. 1889.
-
= Melanomma vindelicorum Rehm, Ber. Nat. Ver. Augsburg: 116. 1881.
≡ Trematosphaeria vindelicorum (Rehm) Sacc., Syll. Fung. 2: 122. 1883.
Specimen examined: USA, South Dakota, from rotten crown of Medicago sativa (Fabaceae), G. Semeniuk, CBS 675.92 = ATCC 52767 = ATCC 52678 = IMI 266220.
Massarina eburnea (Tul. & C. Tul.) Sacc., Syll. Fung. 2: 153. 1883.
Basionym: Massaria eburnea Tul. & C. Tul., Select. Fung. Carpol. (Paris) 2: 239. 1863.
Specimens examined: Switzerland, Zürich, from Fagus sylvatica (Fagaceae), S.K. Bose, CBS 473.64 = ETH 2945. UK, Wales, isolated from dead branch of Fagus sylvatica, HHUF 26621, JCM 14422 = H3953.
Neottiosporina paspali (G.F. Atk.) B. Sutton & Alcorn, Austral. J. Bot. 22: 519. 1974.
Basionym: Stagonospora paspali G.F. Atk., Bull. Cornell Univ. (Science) 3: 33. 1897.
Specimen examined: USA, Florida, from Paspalum notatum (Poaceae), Oct. 1937, R.K. Voorhees, CBS 331.37.
Trematosphaeriaceae Suetrong et al. Cryptogamie Mycol. 32: 347. 2011.
Falciformispora lignatilis K.D. Hyde, Mycol. Res. 96: 27. 1992.
Specimen examined: Thailand, Pinruan Ban Bang, from Elaeis guineensis (Arecaceae), BCC 21118.
Medicopsis Gruyter, Verkley & Crous, gen. nov. MycoBank MB564791.
Etymology: refers to Medi-medica, Latin, -opsis, refers to, Greek. The description of the type species as the cause of a mycetoma suggest this is a human pathogen. However, the mycetoma described was secondary to a wound produced by a thorn of Palito blanco tree, and the species was found later on Hordeum vulgare.
Pycnidia solitary or confluent, on upper surface of the agar, globose to pyriform with elongated neck, setose, ostiolate, olivaceous to olivaceous-black, the wall with pseudoparenchymatal cells. Conidiogenous cells hyaline, phialidic, ampulliform to doliiform, to elongated. Conidia sub-hyaline to yellowish, ellipsoid, aseptate, catenulate.
Type species: Medicopsis romeroi (Borelli) Gruyter, Verkley & Crous (see below).
Medicopsis romeroi (Borelli) Gruyter, Verkley & Crous, comb. nov. MycoBank MB564792.
Basionym: Pyrenochaeta romeroi Borelli, Dermatol. Venez. 1: 326. 1959.
Specimens examined: Venezuela, from human, maduromycosis, no date, D. Borelli, UAMH 2892, holotype of Pyrenochaeta romeroi Borelli, culture ex-holotype CBS 252.60 = ATCC 13735 = FMC 151 = UAMH 10841. Country unknown, from Hordeum vulgare (Poaceae), 1984, M.M.J. Dorenbosch, CBS 122784 =PD 84/1022.
Notes: The species was described as a human pathogen of tropical origin, and it may cause suppurative subcutaneous or deep nonmycetomatous infections, or a subcutaneous phaeohyphomycotic cyst (Badali et al. 2010). However, the species also occurs in plant material.
Trematosphaeria pertusa (Pers.) Fuckel, Jahrb. Nassauischen Vereins Naturk 23-24: 161. 1870.
Basionym: Sphaeria pertusa Pers., Syn. Meth. Fung. 1: 83. 1801.
Specimen examined: France, Deux Sèvres, from bark of a dead stump of Fraxinus excelsior (Oleaceae), 25 Apr. 2004, Jacques Fournier, epitype IFRD 2002, culture ex-epitype CBS 122368.
Note: The epitype IFRD 2002 was designated by Zhang et al. (2008).
Lentitheciaceae Yin. Zhang, C.L. Schoch, J. Fourn., Crous & K.D. Hyde, Stud. Mycol. 64: 93. 2009.
Splanchnonema platani (Ces.) M.E. Barr, Mycotaxon 15: 364. 1982.
Basionym: Sphaeria (Massaria) platani Ces., in Rabenhorst, Klotzschii Herb. Viv. Mycol.: no. 1842. 1854.
Specimen examined: USA, from Platanus occidentalis (Platanaceae), Jan. 1937, C.L. Shear, CBS 221.37.
Note: This taxon was shown by Zhang et al. (2012) to cluster basal to the Lentitheciaceae.
Melanommataceae G. Winter, Rabenh. Krypt.-Fl., ed 1(2): 220 (1885) [as “Melanommeae”]
Aposphaeria corallinolutea Gruyter, Aveskamp & Verkley, sp. nov. MycoBank MB564798. Fig. 8.
Fig. 8.
Aposphaeria corallinolutea sp. nov. CBS 131287. A-B. Fourteen day old cultures on OA (A) and MA (B). C-D. Pycnidia. E-H. Conidiogenous cells. I. Conidia. Scale bars: C = 50 μm; D = 20 μm; E-I = 10 μm.
Etymology: The name refers to the coral coloured colony on OA, and the luteous exudate diffusing into the agar medium.
Pycnidia in vitro 65-215 μm diam, solitary or aggregated to confluent, globose to subglobose, ostiolate or non-ostiolate. Conidiogenous cells 7-9 × 2-4 μm, ampuliform to filiform. Conidia 3-5 × 1-2 μm, ellipsoidal to allantoid, eguttulate or with some small, polar guttules.
Description in vitro: Colonies on OA 13-15 mm diam after 14 d, margin entire to somewhat lobated; colony vinaceous to brick, with white at centre, ochraceous near margin due to a diffusible pigment, with white, felty or poorly developed aerial mycelium; reverse cinnamon to brick. Colonies on MEA 15-20 mm diam after 14 d, margin entire to somewhat lobated; colony white with dull green and grey olivaceous sectors and primrose tinges, with white, felty aerial mycelium; reverse sepia to brown olivaceous, greenish grey at centre, white near margin. Pycnidia globose to subglobose, olivaceous to brick, then olivaceous black, solitary or aggregated, 65-215 μm diam, non-setose or with short setae-like outgrowths up to 25 μm long, with or without distinct ostiole, pycnidial wall consisting of 3-5 layers of cells. Conidiogenous cells 7-9 × 2-4 μm, ampulliform to filiform. Conidia 3-5 × 1-2 μm, av. 4 × 1.5 μm, length/width ratio is 1.7-3.3, av. = 2.5, ellipsoidal to allantoid, eguttulate or with some small, polar guttules. Chlamydospores absent, NaOH test negative. Crystals produced in the agar, small, orange coloured.
Specimens examined: Netherlands, from wood of Fraxinus excelsior (Oleaceae), 1983, M.M.J. Dorenbosch, holotype CBS H-20625, culture ex-holotype CBS 131287 =PD 83/831; from wood of Kerria japonica (Rosaceae), 1983, M.M.J. Dorenbosch, CBS 131286 =PD 83/367.
Aposphaeria populina Died., Krypt.-Fl. Brandenburg 9: 206. 1912 (vol. dated “1915”). Fig. 9.
Fig. 9.
Aposphaeria populina. CBS 543.70. A-B. Fourteen day old cultures on OA (A) and MA (B). C. Pycnidium with mycelial outgrowths (CBS 130330). D-E. Conidiogenous cells. F. Conidia. Scale bars: C = 20 μm; D-E = 10 μm; F = 5 μm.
Description in vitro: Colonies on OA 21-24 mm diam after 7 d (32-37 mm diam after 14 d), margin entire to undulate; colony grey olivaceous/olivaceous to pale luteous/luteous, with white to pale olivaceous grey, finely felty to woolly aerial mycelium; reverse luteous to orange, greenish olivaceous to olivaceous or grey olivaceous/olivaceous grey to iron-grey, a rosy-buff discolouring near margin may occur. Colonies on MEA 16-20 mm diam after 7 d (30-37 mm diam after 14 d), margin entire to undulate; colony pale olivaceous grey with rosy-vinaceous tinges to peach or olivaceous grey, with white, woolly aerial mycelium; reverse saffron to pale olivaceous/olivaceous grey, sometimes with dark vinaceous tinges, rosy-buff near margin. Pycnidia globose to subglobose, olivaceous to olivaceous black, scattered, 55-305 μm diam, glabrous or with mycelial outgrowths, non-ostiolate or ostiolate, pycnidial wall composed of up to 10 layers of cells. Conidiogenous cells 5-11.5 × 1.5-3 μm, ampulliform to filiform. Conidia hyaline, subglobose to ellipsoidal, with 1-3 minute guttules, 1-2 × 1-1.5 μm, av. 1.5 × 1 μm, length/width ratio is 1.0-2.0, av. = 1.4. Chlamydospores and crystals absent, NaOH test negative.
Specimens examined: Germany, Triglitz, from twigs of Populus canadensis (Salicaceae), Mar. 1904. O. Jaap, B, holotype; from branch scars of Picea abies, (Pinaceae), Feb. 1982, H. von Aufess, CBS 350.82. Netherlands, Valkenswaard, from fallen twig of Populus canadensis (Salicaceae), 23 Mar. 1970, H.A. van der Aa, epitype designated here CBS H-9336, culture ex lectotype CBS 543.70; from wood of Cornus mas (Cornaceae), 1984, M.M.J. Dorenbosch, CBS 130330 =PD 84/221.
Beverwykella pulmonaria (Beverw.) Tubaki, Trans. Mycol. Soc. Japan 16: 139. 1975.
Basionym: Papulaspora pulmonaria Beverw., Antonie van Leeuwenhoek 20: 11. 1954.
Specimen examined: Netherlands, Baarn, from submerged leaf in rain water barrel of Fagus sylvatica (Fagaceae), Apr. 1953, A.L. van Beverwijk, culture CBS 283.53 = ATCC 32983 = IFO 6800.
Herpotrichia juniperi (Duby) Petr., Ann. Mycol. 23: 43. 1925.
Basionym: Sphaeria juniperi Duby, Klotzsch. Herb. Vivum Mycol. Sistems Fungorum German., no. 1833. 1854.
Specimen examined: Switzerland, Andermatt, from Juniperus nana (Cupressaceae), Nov. 1931, E. Gäumann, CBS 200.31.
Melanomma pulvis-pyrius (Pers.) Fuckel, Jahrb. Nassauischen Vereins Naturk. 23-24: 160. 1870.
Basionym: Sphaeria pulvis-pyrius Pers., Syn. Meth. Fung. 1: 86. 1801.
Specimens examined: Belgium, from wood of Fagus sp. (Fagaceae), CBS 400.97. France, Vosges, Bot. Garden Le Chitelet, from unidentified decaying wood, CBS 371.75.
Notes: Phoma-like anamorphs have been reported by Chesters (1938) and Sivanesan (1984), but no anamorphic stage was observed in IFRDCC 2044, CBS 109.77 or CBS 371.75 after culturing 3 mo on PDA (Zhang et al. 2008). CBS 400.97 was preserved as Trematosphaeria pertusa.
Pleomassaria siparia (Berk. & Broome) Sacc., Syll. Fung. 2: 239. 1883.
Basionym: Sphaeria siparia Berk. & Broome, Ann. Mag. Nat. Hist. Ser. 2(9): 321. 1852.
Specimen examined: Netherlands, Uden, from dead branch of Betula verrucosa (Betulaceae), 8 Dec. 1973, W.M. Loerakker, CBS H-258, CBS H-260, culture CBS 279.74.
Sporormiaceae Munk, Dansk Bot. Ark. 17(1): 450. 1957, nom. inval., Art. 36.1.
Preussia funiculata (Preuss) Fuckel, Jahrb. Nassauischen Vereins Naturk. 23-24: 91. 1870 (1869-70).
Basionym: Perisporium funiculatum Preuss, Linnaea 24(1): 143. 1851.
Specimen examined: Senegal, from soil, CBS 659.74.
Sporormiella minima (Auersw.) S.I. Ahmed & Cain, Canad. J. Bot. 50: 449. 1972.
Basionym: Sporormia minima Auersw., Hedwigia 7: 66. 1868.
Specimen examined: Panama, from dung of goat, CBS 524.50.
Westerdykella Stolk, Trans. Brit. Mycol. Soc. 38: 422. 1955.
Type species: Westerdykella ornata Stolk, see below.
Westerdykella capitulum (V.H. Pawar, P.N. Mathur & Thirum) Gruyter, Aveskamp & Verkley, comb. nov. MycoBank MB564801.
Basionym: Phoma capitulum V.H. Pawar, P.N. Mathur & Thirum., Trans. Brit. Mycol. Soc. 50: 261. 1967.
≡ Phoma capitulum V.H. Pawar & Thirum., Nova Hedwigia 12: 502. 1966 (as “capitula”), nom. nud., nom. inval.
-
= Phoma ostiolata V.H. Pawar, P.N. Mathur & Thirum., Trans. Brit. Mycol. Soc. 50: 262. 1967, var. ostiolata.
≡ Phoma ostiolata V.H. Pawar & Thirum., Nova Hedwigia 12: 502. 1966, nom. nud., nom. inval.
-
= Phoma ostiolata var. brunnea V.H. Pawar, P.N. Mathur & Thirum., Trans. Brit. Mycol. Soc. 50: 263. 1967.
≡ Phoma ostiolata var. brunnea V.H. Pawar & Thirum., Nova Hedwigia 12: 502. 1966, nom. nud., nom. inval.
Specimen examined: India, Bandra, Bombay, from saline soil, 15 Jan. 1958, M.J. Thirumalachar, Isotype CBS H-7602, culture ex-isotype CBS 337.65 = ATCC 16195 = HACC 167 = IMI 113693 = PD 91/1614.
Westerdykella minutispora (P.N. Mathur ex Gruyter & Noordel.) Gruyter, Aveskamp & Verkley, comb. nov. MycoBank MB564793.
Basionym: Phoma minutispora P.N. Mathur ex Gruyter & Noordel., Persoonia 15: 75. 1992 (as “collection name” originally also referred to Thirumalachar; = depositor).
Replaced synonym: Phoma oryzae Cooke & Massee, Grevillea 16: 15. 1887 (not Phoma oryzae Catt., Arch. Triennale Bot. Crittog. Pavia 2-3: 118. 1879, nom. illeg).
≡ Phyllosticta oryzae (Cooke & Massee) I. Miyake. J. Coll. Agric. Imp. Univ. Tokyo 2: 252. 1910, nom. illeg.
Specimen examined: India, from saline soil, 1977, M.J. Thirumalachar, CBS H-5941, culture CBS 509.91 = PD 77/920.
Westerdykella ornata Stolk, Trans. Brit. Mycol. Soc. 38: 422. 1955.
Specimen examined: Mozambique, from mangrove mud, CBS 379.55.
Didymosphaeriaceae Munk, Dansk Bot. Ark. 15(2): 128. 1953.
Roussoella hysterioides (Ces.) Höhn., Sitzungsber. Kaiserl. Akad. Wiss., Math.-Naturwiss. Cl., Abt. 1. 128: 563. 1919.
Basionym: Dothidea hysterioides Ces., Atti Accad. Sci. Fis. 8: 24. 1879.
Specimen examined: Japan, Aomori, Shimokita Yagen, from culms of Sasa kurilensis (Poaceae), Y. Ooki, culture CBS 125434 =HH 26988.
Family incertae sedis
Nigrograna Gruyter, Verkley & Crous, gen. nov. MycoBank MB564794.
Etymology: refers to Nigro-, black, Latin, -grana, grains, Latin. The description refers to the black grains produced by the type species.
Pycnidia solitary or rarely confluent, on upper surface or submerged in agar, globose to subglobose or pyriform, with dark brown, septate mycelial outgrowths, with papillate ostioles, olivaceous to olivaceous-black, the wall with pseudoparenchymatous cells. Conidiogenous cells hyaline, phialidic, discrete. Conidia sub-hyaline, brown in mass, aseptate, ellipsoidal.
Type species: Nigrograna mackinnonii (Borelli) Gruyter, Verkley & Crous (see below).
Nigrograna mackinnonii (Borelli) Gruyter, Verkley & Crous, comb. nov. MycoBank MB564795.
Basionym: Pyrenochaeta mackinnonii Borelli, Castellania 4: 230. 1976.
Specimens examined: Mexico, from a mycetoma of a human, Feb. 2002, R. Arenas, CBS 110022; Venezuela, from a black grain mycetoma of human, Aug. 1975, D. Borelli, holotype FMC 270, culture ex-holotype CBS 674.75.
Thyridaria rubronotata (Berk. & Broome) Sacc., Syll. Fung. 2: 141. 1883.
Basionym: Melogramma rubronotatum Berk. & Broome, Ann. Mag. Nat. Hist. Ser. 3(3): 20. 1859.
Specimen examined: Netherlands, Zuidelijk Flevoland, from a dead branch of Acer pseudoplatanus (Aceraceae), 13 Apr. 1985, N. Ernste, CBS H-18824, culture CBS 419.85.
DISCUSSION
The genus Phoma has been shown to be highly polyphyletic and Phoma is now restricted to taxa in the Didymellaceae (de Gruyter et al. 2009, Aveskamp et al. 2010). Phoma anamorphs and phoma-like species in Coniothyriaceae, Leptosphaeriaceae, Melanommataceae, Montagnulaceae, Pleosporaceae, Sporormiaceae and Trematosphaeriaceae are redisposed here as a result of this and previous studies.
The delimitation of Leptosphaeriaceae in Pleosporineae from Cucurbitariaceae, Didymellaceae, Phaeosphaeriaceae and Pleosporaceae agrees with recent studies of phoma-like species in Pleosporales (de Gruyter et al. 2009, Aveskamp et al. 2010, de Gruyter et al. 2010). Cucurbitariaceae is recognised as the fifth family in Pleosporineae in addition to the four families accepted by Zhang et al. (2009), which are Didymellaceae, Leptosphaeriaceae Phaeosphaeriaceae and Pleosporaceae.
The genera Leptosphaeria, Paraleptosphaeria, Plenodomus, Subplenodomus and Heterospora
Plenodomus lingam and L. doliolum, the type species of Plenodomus and Leptosphaeria respectively, were found to be distant genetically, which agrees with findings of previous molecular phylogenetic studies (Jasalavic et al. 1995, Morales et al. 1995, Dong et al. 1998, Câmara et al. 2002, Eriksson & Hawksworth 2003, Wunsch & Bergstrom 2011). In our study the generic type species grouped in sister clades, which represent Leptosphaeria and Plenodomus. Species of Leptosphaeria produce dark brown, 3-septate ascospores, which have been considered the primitive state with more recently evolved species producing ascospores that are paler in colour, longer and narrower, and more than 3-septate (Wehmeyer 1946). This hypothesis is supported by the results obtained in our study. Paraleptosphaeria is distinct but seems to be most closely related to Leptosphaeria producing 3(-5)-septate, yellow/brown or hyaline ascospores. Both genera include only necrotrophic species. Plenodomus and Subplenodomus include necrotrophs and plant pathogens. Ascospores in Plenodomus are 3-7-septate, whereas in Subplenodomus no sexual state has thus far been recorded. The scleroplectenchymatous pycnidial cell wall is typical for Plenodomus, whereas in Subplenodomus the pycnidial cell wall is pseudoparenchymatous. Heterospora is closely allied to Subplenodomus and no sexual state has been recorded for this genus either. The distinctive characterisitics of the genera Heterospora, Leptosphaeria, Paraleptosphaeria, Plenodomus and Subplenodomus are summerised in Table 2. A blast search in GenBank using ITS sequences of five selected species of the Leptosphaeriaceae, namely L. doliolum, L. etheridgei, Plen. lingam, H. dimorphospora and Subplen. drobnjacensis, did not reveal close matches to other teleomorphic or anamorphic genera.
Table 2.
Characteristics of ascospores, mitosporic state and pathogenicity of Leptosphaeria, Paraleptosphaeria, Plenodomus and Subplenodomus in vivo.
| Genus | Ascospores | Mitosporic state | Pathogenicity |
|---|---|---|---|
| Leptosphaeria | Ascospores 3-septate, (dark) brown | Mitosporic state common, pycnidial cell wall usually directly scleroplectenchymatous, conidia mostly aseptate | Necrotrophic |
| Paraleptosphaeria | Ascospores 3-5-septate, hyaline to yellow/brown | Mitosporic state rare, pycnidial cell wall directly scleroplectenchymatous, conidia aseptate | Necrotrophic |
| Plenodomus | Ascospores 3-7-septate, pale yellow to brown | Mitosporic state common, pycnidial cell wall initially pseudoparenchymatous, later scleroplectenchymatous, conidia aseptate | Necrotrophic or plant pathogenic |
| Subplenodomus | No known sexual state | Mitosporic state common, pycnidial cell wall mainly pseudoparenchymatous, conidia aseptate | Necrotrophic or plant pathogenic |
| Heterospora | No known sexual state | Mitosporic state common, pycnidial cell wall pseudoparenchymatous, conidia of two types: small aseptate and large septate | Plant pathogenic |
Plectophomella visci grouped in Plenodomus in this study and in the Leptosphaeriaceae in a previous molecular phylogeny of Phoma and allied anamorph genera (de Gruyter et al. 2009). Plectophomella visci is the type species of Plectophomella (Moesz 1922) and three additional species have been described in the genus. Two species were described from the bark of Ulmus spp., viz. Plectophomella ulmi (basionym Dothiorella ulmi) and Plectophomella concentrica (Redfern & Sutton 1981). Dothiorella ulmi is considered the appropriate name for Plectophomella ulmi (Crous et al. 2004). A third species, Plectophomella nypae, was described from Nypa fruticans (Arecaceae) (Hyde & Sutton 1992). As a result of the transfer of the type species Plectophomella visci to Plenodomus, the taxonomy of both Plectophomella concentrica and P. nypae needs to be reconsidered based on the outcome of a molecular study.
Plenodomus chrysanthemi could not be differentiated from Plen. tracheiphilus based on comparison of their LSU and ITS sequences. Plenodomus vasinfecta was proposed by Boerema et al. (1994) for the species originally described as Phoma tracheiphila f. sp. chrysanthemi (Baker et al. 1985). Because these are part of the Plenodomus clade the name Plenodomus chrysanthemi is proposed with P. tracheiphila f. sp. chrysanthemi and P. vasinfecta as synonyms. Plenodomus chrysanthemi and Plen. tracheiphilus are host specific (Chrysanthemum and Citrus, respectively) and the scleroplectenchymatous conidiomatal wall of Plen. tracheiphilus differentiates this species from Plen. chrysanthemi, where only a parenchymatous wall has been observed (Boerema et al. 1994). The results of this molecular study and the production of a Phialophora synanamorph by both species demonstrate the close relationship of both taxa.
Plenodomus enteroleucus and Plen. influorescens have a similar ecological niche as opportunistic pathogens on woody plants in Europe. Both taxa were formerly described as varieties of Ph. enteroleuca, vars. enteroleuca and influorescens, and could be differentiated only by the fluorescence of var. enteroleuca under black light. However, the molecular phylogeny demonstrates the two varieties are only distantly related and they are raised from varietal status to species rank. The close relation of Plen. wasabiae with Plen. biglobosus agrees with the results of a previous study on the production of Phomalignin A and other yellow pigments, as well as ITS sequence analyses (Pedras et al. 1995).
Subplenodomus apiicola, Subplen. drobnjacensis, Subplen. valerianae and Subplen. violicola all produce pycnidia with an elongated neck, resembling Plenodomus. The pycnidial wall remains usually pseudoparenchymatous. Pycnidia with a scleroplectenchymatous wall are only observed in Subplen. drobnjacensis. Subplenodomus apiicolus, Subplen. drobnjacensis and Subplen. valerianae produce relatively small conidia, up to 4.5 × 2 μm (de Gruyter & Noordeloos 1992) in congruence with many of the Plenodomus species described; however, in contrast Subplen. violicola produces relatively large conidia, up to 11 × 3 μm (Boerema 1993).
The grouping of species of Phoma section Plenodomus based on the host being either herbaceous plants or wood of trees and shrubs (Boerema 1982, Boerema et al. 1994) is not supported by the molecular phylogeny. The grouping of the species into two categories based on the production of pseudoparenchymatous pycnidia that become scleroplectenchymatous pycnidia (type I), versus always scleroplectenchymatous pycnidia (type 2) (Boerema et al. 1981), is partly supported by the molecular phylogeny. In the Leptosphaeria clade most species directly develop scleroplectenchymatous pycnidia, whereas in the Plenodomus clade the pycnidia generally are pseudoparenchymatous and become scleroplectenchymatous.
Heterospora is established for two species of Phoma sect. Heterospora that cluster in the Leptosphaeriaceae, viz H. chenopodii and H. dimorphospora. All other species of Phoma sect. Heterospora are in the Didymellaceae (Aveskamp et al. 2010).
The Leptosphaeria doliolum species complex
The taxonomy of the generic type species Leptosphaeria doliolum and Phoma anamorphs is complex with a number of subspecies and varieties described in literature. Leptosphaeria doliolum subsp. doliolum and L. doliolum subsp. errabunda are morphologically very similar, as well as the anamorphs Ph. acuta subsp. errabunda and Ph. acuta subsp. acuta. It has been suggested that both taxa represent originally American and European counterparts (Boerema et al. 1994). Both subspecies of L. doliolum proved to be closely related in a phylogenetic analysis utilising LSU and ITS. A detailed multilocus phylogenetic study including the ITS, ACT, TUB and CHS genes, however, demonstrated that both subspecies could be clearly differentiated, and represent two subclades in the L. doliolum complex. All species allied with L. doliolum and L. errabunda are necrotrophic species. Surprisingly, L. macrocapsa grouped with the L. errabunda isolates. Leptosphaeria macrocapsa is described as a host-specialised necrotroph on Mercurialis perennis (Euphorbiaceae) in Europe (Boerema et al. 1994). The species is characterised by large pycnidia (Grove, 1935), with a conspicuously broad, long cylindrical neck (Boerema et al. 1994). This is different to the sharply delimited papilla or neck of variable length of the pycnidia of L. errabunda. Leptosphaeria sydowii, a necrotroph on Senecio spp. in particular (Asteraceae), proved to be closely related to L. errabunda. It can be concluded that the Leptosphaeria doliolum complex includes several necrotrophic species, with adapted host specificity.
The genus Coniothyrium
Coniothyrium palmarum is the type species of the genus Coniothyrium. Coniothyrium is characterised by ostiolate pycnidial conidiomata, annellidic conidiogenous cells, the absence of conidiophores, and brown, thick-walled, 0- or 1-septate, verrucose conidia. Coniothyrium is similar morphologically to some species in the genus Microsphaeropsis. However, Microsphaeropsis is characterised by the production of phialidic conidiogenous cells with periclinal thickening, and thin-walled, pale greenish brown conidia.
Coniothyrium, Microsphaeropsis and Paraconiothyrium clearly grouped in different clades in a study of the partial SSU nrDNA (Verkley et al. 2004). In a subsequent study utilising SSU and LSU sequences, the generic type species Microsphaeropsis olivacea grouped in Didymellaceae, whereas Coniothyrium palmarum clustered with the genus Leptosphaeria in Leptosphaeriaceae (de Gruyter et al. 2009). In the present study C. palmarum and its relatives grouped in a distinct clade, which represents Coniothyriaceae. Phoma carteri, Ph. glycinicola, Ph. septicidalis and Pyrenochaeta dolichi grouped in this clade and are transferred to the genus Coniothyrium. The inclusion of these species with setose pycnidia and conidiogenesis with elongated conidiophores expands the morphological circumscription of Coniothyrium. Species with those characters are also found in other genera treated in this paper in the Cucurbitariaceae, Didymellaceae, Phaeosphaeriaceae, Leptosphaeriaceae, Montagnulaceae and Sporormiaceae, indicating convergent evolution.
The Coniothyrium species included here are plurivorous or soil-borne, such as C. palmarum, C. septicidalis and C. multiporum, or are associated with a specific host such as C. carteri on Quercus spp. (Fagaceae), C. glycinicola on Glycine max (Fabaceae) and C. dolichii on Dolichos biflorus (Fabaceae). The species also are diverse geographically.
Coniothyrium palmarum was frequently found associated with leaf spots on Phoenix dactylifera (Arecaceae) in India and Cyprus (Sutton 1980). The C. palmarum isolates regularly used in phylogenetic studies are CBS 758.73, from leaf spots on Phoenix dactylifera in Israel, and CBS 400.71, from a dead petiole of Chaemeropsis humulis (Arecaceae) in Italy. The subtropical distribution of these species is similar to that of the most closely allied C. dolichi and C. glycinicola. Coniothyrium multiporum, recorded from marine soil, also is found in warm regions. Coniothyium carteri, in contrast, is reported from North America and Europe.
Coniothyrium dolichi produces setose pycnidia with hyaline conidia (Mohanty 1958). The conidiogenesis was studied in detail later. phoma-like ampulliform conidiogenous cells as well as conidiogenous cells on filiform, septate conidiophores were found in the same pycnidia leading to confusion regarding the classification of this species in Phoma or Pyrenochaeta (Grodona et al. 1997). This study clearly supports the classification in Coniothyrium. Coniothyrium glycinicola was originally placed in the genus Pyrenochaeta as Py. glycines due to its setose pycnidia (Stewart 1957). The conidiogenesis and hyaline conidia are phoma-like and therefore, it was reclassified as Ph. glycinicola in Phoma sect. Paraphoma (de Gruyter & Boerema 2002). However, in the original description it was noted that the conidia were greenish-yellow in mass (Stewart 1957), resembling Microsphaeropsis or coniothyrium-like conidia. This study clearly supports the classification in Coniothyrium. Coniothyrium carteri produces setose pycnidia with hyaline conidia and therefore, the species was classified in Phoma section Paraphoma (de Gruyter & Boerema 2002). In spite of this similarity, C. carteri was determined to be only distantly related to the generic type species Paraphoma radicina (de Gruyter et al. 2010). Coniothyrium multiporum was described in Phoma section Phoma; however, it proved to be unrelated to Phoma in Didymellaceae (Aveskamp et al. 2010). The conidiogenesis may comprise elongated conidiophores (Pawar et al. 1967). Two isolates originally described as Ph. septicidalis are placed here in Coniothyrium telephii. Other strains deposited as Ph. septicidalis proved to be Pyrenochaeta unguis-hominis (de Gruyter et al. 2010).
The anamorph of the genus Neophaeosphaeria was described as coniothyrium-like, producing pigmented, aseptate conidia from holoblastic, percurrently proliferating conidiogenous cells with conspicuous annellations (Câmara et al. 2003). Although Neophaeosphaeria is related to Coniothyrium based on the molecular data, Neophaeosphaeria probably belongs to a separate phylogenetic clade. The grouping of N. filamentosa with the Coniothyrium species included in this study was poorly supported and N. filamentosa proved to be more distantly related in previous molecular phylogenetic studies (Verkley et al. 2004, Damm et al. 2008, de Gruyter et al. 2010).
Both anamorph genera Cyclothyrium and Cytoplea were considered to be related to Coniothyrium and Microsphaeropsis (Sutton 1980) based on morphological similarities. Cyclothyrium also resembles Paraconiothyrium but produces conidiogenous cells that are more elongated than in most species of Paraconiothyrium and the conidia are almost truncate at the base, or at least they are much less rounded at the base than the conidia of Paraconiothyrium (Verkley et al. 2004). The generic type species Cyclothyrium juglandis, the anamorph of Thyridaria rubronotata, proved to be related to Roussoella hysterioides, teleomorph of Cytoplea (Verkley et al. 2004). Based on present results R. hysterioides could not be assigned to familial rank. The clustering of this species in Massariaceae (Zhang et al. 2009) could not be confirmed. Moreover, Roussoella probably is not a monophyletic genus (Tanaka et al. 2009). Thyridaria rubronotata, the teleomorph of Cyclothyrium juglandis, proved to be related to Massariosphaeria phaeospora but was not assigned to familial rank (Schoch et al. 2009).
Coniothyrium-like anamorphs also have been linked to Mycosphaerella in the past. However, these species were subsequently accommodated in Colletogloeopsis (Cortinas et al. 2006), Readeriella/Kirramyces (Crous et al. 2007) and are now known to be species of Teratosphaeria (Crous et al. 2009b).
The genus Pleospora
Pleospora is a large genus in Pleosporaceae, Pleosporales, and includes important pathogens that occur on both monocotyledons and dicotyledons. Anamorphs of Pleospora s. lat. have been described in various genera of coelomycetes and hyphomycetes as summarised by Zhang et al. (2009, 2012). A delimitation of Pleospora into two sections, Pyrenophora and Eu-Pleospora was made based on the size of fruiting bodies and ascospore septation and colour (Munk 1957). The genus Pyrenophora (Drechslera anamorphs) is recognised at the generic rank. However, Pleospora remains heterogenous (Wehmeyer 1961, Berbee 1996) and molecular phylogenetic studies demonstrated that Pleospora is polyphyletic in Pleosporaceae (Kodsueb et al. 2006, Wang et al. 2007, Inderbitzin et al. 2009). Taxa with a Stemphylium anamorph such as Pleospora sedicola and Pleo. tomatonis, as well as Pleo. halophola with no known anamorph, are closely related to Cochliobolus, whereas Pleo. herbarum and Pleo. ambigua were more distantly related in the Pleosporaceae (Kodsueb et al. 2006, Wang et al. 2007). A phylogenetic study of the genus Massariosphaeria demonstrated the polyphyly in the genera Pleospora, Kirschsteiniothelia, Massarina, Melanomma, Trematosphaeria and Massariosphaeria in the Loculoascomycetes (Wang et al. 2007) and the paraphyletic character of the genus Cochliobolus was demonstrated (Kodsueb et al. 2006, Mugambi & Huhndorf 2009). These findings support the previous speculation by several authors that ascomatal and ascospore morphologies have undergone convergent evolution among Pleosporales (Wang et al. 2007).
Pleospora betae groups ambiguously in Pleosporaceae (Dong et al. 1998). SSU nrDNA sequence data supported the affinity of P. betae to Leptosphaeriaceae. Partial LSU nrDNA data supported the affinity of P. betae to Pleosporaceae (Dong et al. 1998), but bootstrap support values in that study were low. In a multigene phylogenetic study Pleo. betae was found as being basal to Pleosporaceae (Zhang et al. 2009). Our results demonstrate the sister group relationship of Pleo. betae and its relatives to the generic type species Pleo. herbarum.
Pleospora betae has been often confused with Pleo. calvescens as was discussed by Boerema et al. (1987). Both species are pathogens of Chenopodiaceae and are morphologically rather similar and therefore, a phylogenetic relation of both species was inferred (Boerema 1984). In addition Ascochyta hyalospora, originally found on the American continent on Chenopodiaceae, also was supposed to be closely related. Our results demonstrate that Pleo. betae and Pleo. calvescens could be recognised at species rank and confirmed that A. hyalospora is related supporting our transfer to Pleospora as Pleo. chenopodii. The delimitation of both halophytic species Pleo. chenopodii and Pleo. calvescens needs further study; both species could not be clearly differentiated based on the ACT sequences alone. Additional studies are underway to elucidate these species boundaries, in which also the recently described halophyte, Ascochyta manawaorae (Verkley et al. 2010), will be included. Pleospora fallens and Pleo. incompta, formerly described in Phoma sect. Phoma and producing mainly glabrous pycnidia, grouped in the Pleo. herbarum clade. Pleospora typhicola, producing pilose pycnidia, also grouped in this clade.
Phoma-like species excluded from the Pleosporineae
The genus Paraconiothyrium was introduced by Verkley et al. (2004) as the anamorph of Paraphaeosphaeria. The morphological characters of Paraconiothyrium are variable. The conidiomata can be eustromatic to pycnidial, the phialidic conidiogenous cells are discrete or integrated, and the thin-walled conidia are aseptate or septate, smooth-walled or minutely warted, and hyaline to brown in a later stage (Verkley et al. 2004). The morphological characters of Ph. lini and Asteromella tilliae, redisposed here in Paraconiothyrium, fit this description.
Paraconiothyrium fuckelii is a serious plant pathogen of Rosaceae (Horst & Cloyd 2007), but it also is recorded as an opportunistic human pathogen as summarised by de Hoog et al. (2000). The teleomorph is currently known as Leptosphaeria coniothyrium, but this is not likely considering the phylogeny of Leptosphaeriaceae in Pleosporales (Fig 1). The species was also described as Melanomma coniothyrium (Holm 1957); however, Melanomma is more distantly related in Melanommataceae.
Neottiosporina paspali proved to be related to Paraconiothyrium. However, this species is characterised by conidia with an apical appendage (Sutton 1980) and resembles members of Massarinaceae. Pyrenochaeta romeroi is redescribed in the new genus Medicopsis, and its taxonomic position is most close to Trematosphaeriaceae.
Aposphaeria corallinolutea could be recognised as a new species in Melanommataceae. Phoma capitulum and Ph. minutispora (Phoma section Phoma) clustered in the Sporormiaceae, most closely related to the holotype isolate of Westerdykella ornata. Other phoma-like anamorphs have been recorded in Sporormiaceae, such as anamorphs of Sporormia aemulans (≡ Preussia aemulans) and Westerdykella dispersa (≡ Pycnidiophora dispersa) (von Arx & Storm 1967). The in vitro characters of W. capitulum and W. oryzae agree with the in vitro characters of phoma-like anamorphs in the Sporormiaceae summarised by Boerema et al. (2004). The conidia produced are small, mostly 2-3 × 1-2 μm, arising from undifferentiated cells, but sometimes also elongated conidiogenous cells are observed. The colonies, often with a pink-yellow-red discolouration on OA, usually produce little aerial mycelium, whereas pycnidia are often produced in abundance. No matching sequences were found in a blast search in GenBank using the partial LSU sequences of W. capitulum and W. minutispora. Westerdykella minutispora from India was most similar to a sequence of Westerdykella nigra, isolate CBS 416.72, obtained from soil in Pakistan, and W. capitulum was most similar to a sequence of W. dispersa, isolate CBS 297.56, obtained from a seedling of Phlox drummondii, USA. These blast results support the redisposition of both species in the genus Westerdykella.
Acknowledgments
This project, “Strengthening the Plant Health Infrastructure”, was supported by The Dutch Ministry of Economic Affairs, Agriculture and Innovation. We thank Mrs Trix Merkx and Mrs Karin Rosendahl-Peters for providing the strains from the culture collection of CBS and PD respectively and for their assistance in the deposit of strains. Mrs Arien van Iperen kindly helped us with the deposit of herbarium material. Thanks are due to Marjan Vermaas for her assistance in preparing the photoplates. We are indebted to Machiel E. Noordeloos and the reviewers for critical reading of the manuscript.
REFERENCES
- Aa HA van der, Kesteren HA van. (1979). Some pycnidial fungi occurring on Atriplex and Chenopodium. Persoonia 10: 267–276 [Google Scholar]
- Arx JA von, Storm PK. (1967). Über einige aus dem erdboden isolierte, zu Sporormia, Preussia und Westerdykella gehörende Ascomyceten. Persoonia 4: 407–415 [Google Scholar]
- Aveskamp MM, Gruyter J de, Crous PW. (2008). Biology and recent developments in the systematics of Phoma, a complex genus of major quarantine significance. Fungal Diversity 31: 1–18 [Google Scholar]
- Aveskamp MM, Gruyter J de, Woudenberg JHC, Verkley GJM, Crous PW. (2010). Highlights of the Didymellaceae: A polyphasic approach to characterise Phoma and related pleosporalean genera. Studies in Mycology 65: 1–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aveskamp MM, Verkley GJM, Gruyter J de, Murace MA, Perelló A, Woudenberg JHC, Groenewald JZ, Crous PW. (2009). DNA phylogeny reveals polyphyly of Phoma section Peyronellaea and multiple taxonomic novelties. Mycologia 101: 359–378 [DOI] [PubMed] [Google Scholar]
- Badali H, Chander J, Gulati N, Attri A, Chopra R, Najafzadeh MJ, Chhabra S, Meis JFGM, Hoog GS de. (2010). Subcutaneous phaeohyphomycotic cyst caused by Pyrenochaeta romeroi. Medical Biology 48: 763–768 [DOI] [PubMed] [Google Scholar]
- Baker KF, Davis-Clark LH, Wilhelm S, Snyder WC. (1985). An aggressive vascular-inhabiting Phoma (Phoma tracheiphila f.sp. chrysanthemi nov. f. sp.) weakly pathogenic to Chrysanthemum. Canadian Journal of Botany 63: 1730–1735 [Google Scholar]
- Berbee ML. (1996). Loculoascomycete origins and evolution of filamentous Ascomycete morphology based on 18s rRNA gene sequence data. Molecular Biology and Evollution 13: 462–470 [DOI] [PubMed] [Google Scholar]
- Boerema GH. (1970). Additional notes on Phoma herbarum. Persoonia 6: 15–48 [Google Scholar]
- Boerema GH. (1982). Phoma-soorten van de sectie Plenodomus. Verslagen en Mededelingen Plantenziektenkundige Dienst Wageningen 158 (Jaarboek 1981): 28–30 [Google Scholar]
- Boerema GH. (1984). Mycologisch-taxonomisch onderzoek. Ascochyta’s met lichtbruine conidien die pathogen zijn voor Chenopodiaceae. Verslagen en Mededelingen Plantenziektenkundige Dienst Wageningen 162 (Jaarboek 1983): 31–34 [Google Scholar]
- Boerema GH. (1993). Contributions towards a monograph of Phoma (Coelomycetes) - II. Section Peyronellaea. Persoonia 15: 197–221 [Google Scholar]
- Boerema GH. (1997). Contributions towards a monograph of Phoma (Coelomycetes) - V. Subdivision of the genus in sections. Mycotaxon 64: 321–333 [Google Scholar]
- Boerema GH. and Coworkers (1993). Check-list for scientific names of common parasitic fungi. Libri botanici; Vol. 10 IHW-Verlag, Eching: [Google Scholar]
- Boerema GH, Gams W. (1995). What is Sphaeria acuta Hoffm.: Fr.? Mycotaxon 53: 355–360 [Google Scholar]
- Boerema GH, Gruyter J de. (1998). Contributions towards a monograph of Phoma (Coelomycetes) - VII. Section Sclerophomella: Taxa with thick-walled pseudoparenchymatous pycnidia. Persoonia 17: 81–95 [Google Scholar]
- Boerema GH, Gruyter J de. (1999). Contributions towards a monograph of Phoma (Coelomycetes) - III-Supplement: Additional species of section Plenodomus. Persoonia 17: 273–280 [Google Scholar]
- Boerema GH, Gruyter J de, Kesteren HA van. (1994). Contributions towards a monograph of Phoma (Coelomycetes) - III-1. Section Plenodomus: Taxa often with a Leptosphaeria teleomorph. Persoonia 15: 431–487 [Google Scholar]
- Boerema GH, Gruyter J de, Noordeloos ME. (1997). Contributions towards a monograph of Phoma (Coelomycetes) - IV. Section Heterospora: Taxa with large sized conidial dimorphs, in vivo sometimes as Stagonosporopsis synanamorphs. Persoonia 16: 335–371 [Google Scholar]
- Boerema GH, Gruyter J de, Noordeloos ME, Hamers MEC. (2004). Phoma identification manual. Differentiation of specific and infra-specific taxa in culture. CABI Publishing, Wallingford, UK: [Google Scholar]
- Boerema GH, Kesteren HA van. (1964). The nomenclature of two fungi parasitizing Brassica. Persoonia 3: 17–28 [Google Scholar]
- Boerema GH, Kesteren HA van, Loerakker WM. (1981). Notes on Phoma. Transactions of the British Mycological Society 77: 61–74 [Google Scholar]
- Boerema GH, Loerakker WM. (1985). Notes on Phoma 2. Transactions of the British Mycological Society 84: 289–302 [Google Scholar]
- Boerema GH, Loerakker WM, Hamers MEC. (1987). Check-list for scientific names of common parasitic fungi. Supplement Series 2a (additions and corrections): Fungi on field crops: beet and potato; caraway, flax and oilseed poppy. Netherlands Journal of Plant Pathology 93, Suppl. 1: 1–20 [Google Scholar]
- Boerema GH, Loerakker WM, Hamers MEC. (1996). Contributions towards a monograph of Phoma (Coelomycetes) - III-2. Misapplications of the type species-name and the generic synonyms of section Plenodomus (Excluded species). Persoonia 16: 141–190 [Google Scholar]
- Butin H, Kehr R. (1995). Leaf blotch of lime associated with Asteromella tiliae comb. nov. and the latter’s connection to Didymosphaeria petrakiana. Mycological Research 99: 1191–1194 [Google Scholar]
- Câmara MPS, Palm ME, Berkum P van, O’Neill NR. (2002). Molecular phylogeny of Leptosphaeria and Phaeosphaeria. Mycologia 94: 630–640 [DOI] [PubMed] [Google Scholar]
- Câmara MPS, Ramaley AW, Castlebury LA, Palm ME. (2003). Neophaeosphaeria and Phaeosphaeriopsis, segregates of Paraphaeosphaeria. Mycological Research 107: 516–522 [DOI] [PubMed] [Google Scholar]
- Carbone I, Kohn LM. (1999). A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia 91: 553–556 [Google Scholar]
- Chaverri P, Salgado C, Hirooka Y, Rossman AY, Samuels GJ. (2011). Delimitation of Neonectria and Cylindrocarpon (Nectriaceae, Hypocreales, Ascomycota) and related genera with Cylindrocarpon-like anamorphs. Studies in Mycology 68: 57–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C-Y, David JC, Hsieh WH. (2002). Leptosphaeria dryadis. IMI Descriptions of Fungi and Bacteria 154, Sheet 1533. CABI Bioscience, Egham, Surrey, UK: [Google Scholar]
- Chesters CGC. (1938). Studies on British pyrenomycetes II. A comparative study of Melanomma pulvis-pyrius (Pers.) Fuckel, Melanomma fuscidulum Sacc. and Thyridaria rubro-notata (B. & Br.) Sacc. Transactions of the British Mycological Society 22: 116–150 [Google Scholar]
- Cortinas MN, Burgess T, Dell B, Xu DP, Crous PW, Wingfield BD, Wingfield MJ. (2006). First record of Colletogloeopsis zuluense comb. nov., causing a stem canker of Eucalyptus in China. Mycological Research 110: 229–236 [DOI] [PubMed] [Google Scholar]
- Crane JL, Shearer CA. (1991). A nomenclator of Leptosphaeria V. Cesati & G. de Notaris (Mycota-Ascomycotina-Loculoascomycetes). Illinois Natural history Survey Bulletin 34: 195–355 [Google Scholar]
- Crous PW, Braun U, Groenewald JZ. (2007). Mycosphaerella is polyphyletic. Studies in Mycology 58: 1–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Gams W, Stalpers JA, Robert V, Stegehuis G. (2004). MycoBank: an online initiative to launch mycology into the 21st century. Studies in Mycology 50: 19–22 [Google Scholar]
- Crous PW, Schoch CL, Hyde KD, Wood AR, Gueidan C. (2009a). Phylogenetic lineages in the Capnodiales. Studies in Mycology 64: 17–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Slippers B, Wingfield MJ, Rheeder J, Marasas WOF, Phillips AJL, Alves A, Burgess T, Barber P, Groenewald JZ. (2006). Phylogenetic lineages in the Botryosphaeriaceae. Studies in Mycology 55: 235–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Summerell BA, Carnegie AJ, Wingfield MJ, Hunter GC, Burgess TI, Andijc V, Barber PA, Groenewald JZ. (2009b). Unravelling Mycosphaerella: do you believe in genera? Persoonia 23: 99–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Verkley GJM, Groenewald JZ, Samson RA. (2009c). Fungal Biodiversity. CBS Laboratory Manual Series. Centraalbureau voor Schimmelcultures, Utrecht, Netherlands: [Google Scholar]
- Damm U, Verkley GJM, Crous PW, Fourie PH, Haegi A, Riccioni L. (2008). Novel Paraconiothyrium species on stone fruit trees and other woody hosts. Persoonia 20: 9–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong J, Chen W, Crane JL. (1998). Phylogenetic studies of the Leptosphaeriaceae, Pleosporaceae and some other Loculoascomycetes based on nuclear ribosomal DNA sequences. Mycological Research 102: 151–156 [Google Scholar]
- Dooly DP, Beckius ML, Jeffery BS, McAllister CK, Radentz WH, Feldman AR, Rinaldi MG, Bailey SR, Keeling JH. (1989). Phaeohyphomycotic cutaneous disease caused by Pleurophoma in a cardiac transplant patient. The Journal of Infectious Diseases 159: 503–507 [DOI] [PubMed] [Google Scholar]
- Eriksson OE, Hawksworth DL. (2003). Saccharicola, a new genus for two Leptosphaeria species on sugar cane. Mycologia 95: 426–433 [PubMed] [Google Scholar]
- Fitt BDL, Brun H, Barbetti MJ, Rimmer SR. (2006). World-wide importance of phoma stem canker (Leptosphaeria maculans and L. biglobosa) on oilseed rape (Brassica napus). European Journal of Plant Pathology 114: 3–15 [Google Scholar]
- Gräfenhan T, Schroers H-J, Nirenberg HI, Seifert KA. (2011). An overview of the taxonomy, phylogeny, and typification of nectriaceous fungi in Cosmospora, Acremonium, Fusarium, Stilbella, and Volutella. Studies in Mycology 68: 79–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grondona I, Monte E, Garcia-Acha I, Sutton B. (1997). Pyrenochaeta dolichi: an example of a confusing species. Mycological Research 101: 1404–1408 [Google Scholar]
- Grove WB. (1935). British stem- and leaf-fungi (Coelomycetes) Vol. 1 Sphaeropsidales. Cambridge, UK: Cambridge University Press; [Google Scholar]
- Gruyter J de, Aveskamp MM, Woudenberg JHC, Verkley GJM, Groenewald JZ, Crous PW. (2009). Molecular phylogeny of Phoma and allied anamorph genera: towards a reclassification of the Phoma complex. Mycological Research 113: 508–519 [DOI] [PubMed] [Google Scholar]
- Gruyter J de, Boerema GH. (2002). Contributions towards a monograph of Phoma (Coelomycetes) - VIII. Section Paraphoma: Taxa with setose pycnidia. Persoonia 17 (“2001”): 541–561 [Google Scholar]
- Gruyter J de, Boerema GH, Aa HA van der. (2002). Contributions towards a monograph of Phoma (Coelomycetes) - VI-2. Section Phyllostictoides: Outline of its taxa. Persoonia 18: 1–53 [Google Scholar]
- Gruyter J de, Noordeloos ME. (1992). Contributions towards a monograph of Phoma (Coelomycetes) - I-1. Section Phoma: Taxa with very small conidia in vitro. Persoonia 15: 71–92 [Google Scholar]
- Gruyter J de, Noordeloos ME, Boerema GH. (1993). Contributions towards a monograph of Phoma (Coelomycetes) - I-2. Section Phoma: Additional taxa with very small conidia and taxa with conidia up to 7 mm long. Persoonia 15: 369–400 [Google Scholar]
- Gruyter J de, Noordeloos ME, Boerema GH. (1998). Contributions towards a monograph of Phoma (Coelomycetes) - I-3. Section Phoma: Taxa with conidia longer than 7 mm. Persoonia 16: 471–490 [Google Scholar]
- Gruyter J de, Woudenberg JHC, Aveskamp MM, Verkley GJM, Groenewald JZ, Crous PW. (2010). Systematic reappraisal of species in Phoma section Paraphoma, Pyrenochaeta and Pleurophoma. Mycologia 102: 1066–1081 [DOI] [PubMed] [Google Scholar]
- Hawksworth DL, Crous PW, Redhead SA, Reynolds DR, Samson RA, et al. (2011). The Amsterdam declaration on fungal nomenclature. IMA Fungus 2: 105–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höhnel F von. (1911). Fragmente zur Mykologie XIII (713): Über Leptosphaeria maculans (Desm.) und Sphaeria lingam Tode. Sitzungsberichte der Kaiserlichen Akademie der Wissenschaften in Wien. (Mathematisch-naturwissenschaftliche Klasse (Abteilung I) 120: 458–463 [Google Scholar]
- Höhnel F von. (1914). Fragmente zur Mykologie XVI. (XVI. Mitteilung, Nr. 813 bis 875). Sitzungsberichte der Kaiserlichen Akademie der Wissenschaften in Wien. (Mathematisch-naturwissenschaftliche Klasse (Abteilung I) 123: 49–155 [Google Scholar]
- Holm L. (1957). Études taxonomiques sur les Pléosporacées. Symbolae Botanicae Upsalienses 14: 5–188 [Google Scholar]
- Hoog GS de, Gerrits van den Ende AHG. (1998). Molecular diagnostics of clinical strains of filamentous Basidiomycetes. Mycoses 41: 183–189 [DOI] [PubMed] [Google Scholar]
- Hoog GS de, Guarro J, Gené J, Figueras MJ. (2000). Atlas of Clinical Fungi. 2nd edition Centraalbureau voor Schimmelcultures, Utrecht, Netherlands: [Google Scholar]
- Horst RK, Cloyd R. (2007). Compendium of rose diseases and pests. 2nd edition APS press, MN, USA: [Google Scholar]
- Huang YJ, Fitt BDL, Jedryczka M, Dakowska S, West JS, Gladders P, Steed JM, Li ZQ. (2005). Patterns of ascospore release in relation to phoma stem canker epidemiology in England (Leptosphaeria maculans) and Poland (L. biglobosa). European Journal of Plant Pathology 111: 263–277 [Google Scholar]
- Huelsenbeck JP, Ronquist F. (2001). MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 17: 754–755 [DOI] [PubMed] [Google Scholar]
- Hyde KD, Sutton BC. (1992). Nypaella frondicola gen. et sp. nov., Plectophomella nypae sp. nov. and Pleurophomopsis nypae sp. nov. (Coelomycetes) from intertidal fronds of Nypa fruticans. Mycological Research 96: 210–214 [Google Scholar]
- Inderbitzin P, Mehta YR, Berbee ML. (2009). Pleospora species with Stemphylium anamorphs: a four locus phylogeny resolves new lineages yet does not distinguish among species in the Pleospora herbarum clade. Mycologia 101: 329–339 [DOI] [PubMed] [Google Scholar]
- Jasalavic CA, Morales VM, Pelscher LE, Seguin-Swartz G. (1995). Comparison of nuclear ribosomal DNA sequences from Alternaria species pathogenic to crucifers. Mycological Research 99: 604–614 [Google Scholar]
- Jedryczka M, Fitt BDL, Kachlicki P, Lewartowska E, Balesdent MH, Rouxel T. (1999). Comparison between Polish and United Kingdom populations of Leptosphaeria maculans, cause of stem canker of winter oilseed rape. Zeitschrift für Pflanzenkrankheiten und Pflanzenschutz 106: 608–617 [Google Scholar]
- Khashnobish A, Shearer CA, Crane JL. (1995). Reexamination of species of Leptosphaeria on asteraceous hosts. Mycotaxon 54: 91–106 [Google Scholar]
- Kodsueb R, Dhanasekaran V, Aptroot A, Lumyong S, McKenzie EHC, et al. (2006). The family Pleosporaceae: intergeneric relationships and phylogenetic perspectives based on sequence analyses of partial 28SrDNA. Mycologia 98: 571–583 [DOI] [PubMed] [Google Scholar]
- Lombard L, Crous PW, Wingfield BD, Wingfield MJ. (2010a). Species concepts in Calonectria (Cylindrocladium). Studies in Mycology 66: 1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombard L, Crous PW, Wingfield BD, Wingfield MJ. (2010b). Multigene phylogeny and mating tests reveal three cryptic species related to Calonectria pauciramosa. Studies in Mycology 66: 15–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombard L, Crous PW, Wingfield BD, Wingfield MJ. (2010c). Phylogeny and systematics of the genus Calonectria. Studies in Mycology 66: 31–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendes-Pereira E, Balesdent MH, Brun H, Rouxel T. (2003). Molecular phylogeny of the Leptosphaeria maculans - L. biglobosa species complex. Mycological Research 107: 1287–1304 [DOI] [PubMed] [Google Scholar]
- Moesz G. (1922). Mycologiai közlemények V. (Mykologische Mitteilungen V.). Magyar Botanikai Lapok 21: 5–16 [Google Scholar]
- Mohanty NN. (1958). An undescribed species of Pyrenochaeta on Dolichos biflorus Linn. Indian Phytopathology 8: 85–87 [Google Scholar]
- Morales VM, Jasalavich CA, Pelcher LE, Petrie GA, Taylor JL. (1995). Phylogenetic relationship among several Leptosphaeria species based on their ribosomal DNA sequences. Mycological Research 99: 593–603 [Google Scholar]
- Mugambi GK, Huhndorf SM. (2009). Molecular phylogenetics of Pleosporales: Melanommataceae and Lophiostomataceae re-circumscribed (Pleosporomycetidae, Dothideomycetes, Ascomycota). Studies in Mycology 64: 103–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller E. (1950). Die schweizerischen arten der gattung Leptosphaeria und ihrer verwandten. Sydowia, Annales Mycologici editi in Notitiam Scientiae Mycologicae Universalis. Horn, Austria 4: 185–319 [Google Scholar]
- Müller E, Arx JA von. (1950). Einige aspecte zur systematik pseudosphärialer Ascomyceten. Berichte der Schweizerichen Botanischen Gesellschaft 21: 329–397 [Google Scholar]
- Munk A. (1957). Danish Pyrenomycetes: a preliminary flora. Dansk botanisk Arkiv 17, Dansk Botanisk Forening. Ejnar Munksgaard, Copenhagen, Denmark: [Google Scholar]
- Page RDM. (1996). Treeview: An application to display phylogenetic trees on personal computers. Computer Applications in the Biosciences 12: 357–358 [DOI] [PubMed] [Google Scholar]
- Pawar VH, Mathur PN, Thirumalachar MJ. (1967). Species of Phoma isolated from marine soils in India. Transactions of the British Mycological Society 50: 259–265 [Google Scholar]
- Pedras MSC, Taylor JL, Morales VM. (1995). Phomaligin A and other yellow pigments in Phoma lingam and P. wasabiae. Phytochemistry 38: 1215–1222 [Google Scholar]
- Phillips AJL, Alves A, Pennycook SR, Johnston PR, Ramaley A, et al. (2008). Resolving the phylogenetic and taxonomic status of dark-spored teleomorph genera in the Botryosphaeriaceae. Persoonia 21: 29–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redfern DB, Sutton BC. (1981). Canker and dieback of Ulmus glabra caused by Plectophomella concentrica, and its relationship to P. ulmi. Transactions of the British Mycological Society 77: 381–390 [Google Scholar]
- Rehner SA, Samuels GJ. (1994). Taxonomy and phylogeny of Gliocladium analysed from nuclear large subunit ribosomal DNA sequences. Mycological Research 98: 625–634 [Google Scholar]
- Schoch CL, Crous PW, Groenewald JZ, Boehm EWA, Burgess TI. (2009). A class-wide phylogenetic assessment of Dothideomycetes. Studies in Mycology 64: 1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroers H-J, Gräfenhan T, Nirenberg HI, Seifert KA. (2011). A revision of Cyanonectria and Geejayessia gen. nov., and related species with Fusarium-like anamorphs. Studies in Mycology 68: 115–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoemaker RA, Brun H. (2001). The teleomorph of the weakly aggressive segregate of Leptosphaeria maculans. Canadian Journal of Botany 79: 412–419 [Google Scholar]
- Shoemaker RA, Redhead SA. (1999). Proposals to conserve the names of four species of fungi (Phoma betae, Helminthosporium avenae, Pyrenophora avenae and Pleospora tritici-repentis) against competing earlier synonyms. Taxon 48: 381–384 [Google Scholar]
- Sivanesan A. (1984). The Bitunicate Ascomycetes and their Anamorphs. J. Cramer, Vaduz, Liechtenstein: [Google Scholar]
- Stewart RB. (1957). An undescribed species of Pyrenochaeta on soybean. Mycologia 49: 115–117 [Google Scholar]
- Suetrong S, Schoch CL, Spatafora JW, Kohlmeyer J, Volkmann-Kohlmeyer B. (2009). Molecular systematics of the marine Dothideomycetes. Studies in Mycology 64: 155–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton BC. (1980). The Coelomycetes. Fungi Imperfecti with Pycnidia, Acervuli and Stromata. CMI, Kew, UK: [Google Scholar]
- Swofford DL. (2003). PAUP*: Phylogenetic analysis using parsimony (*and other methods), version 4.0b10. Sinauer Associates, Sunderland, Massachusetts: [Google Scholar]
- Tanaka K, Hirayama K, Yonezawa H, Hatakeyama S, Harada Y, Sano T, Shirouzu T, Hosoya T. (2009). Molecular taxonomy of bambusicolous fungi: Tetraplosphaeriaceae, a new pleosporalean family with Tetraploa-like anamorphs. Studies in Mycology 64: 175–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkley GJM, Silva M da, Wicklow DT, Crous PW. (2004). Paraconiothyrium, a new genus to accommodate the mycoparasite Coniothyrium minitans, anamorphs of Paraphaeosphaeria, and four new species. Studies in Mycology 50: 323–335 [Google Scholar]
- Verkley GJM, Woudenberg JHC, Gruyter J de. (2010). Ascochyta manawaorae Verkley, Woudenberg & de Gruyter, sp. nov. Persoonia 24: 128–129 [Google Scholar]
- Vilgalys R, Hester M. (1990). Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. Journal of Bacteriology 172: 4238–4246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincenot L, Balesdent MH, Li H, Barbetti MJ, Sivasithamparam K, Gout L, Rouxel T. (2008). Occurrence of a new subclade of Leptosphaeria biglobosa in Western Australia. Phytopathology 98: 321–329 [DOI] [PubMed] [Google Scholar]
- Wang HK, Aptroot A, Crous PW, Hyde KD, Jeewon R. (2007). The polyphyletic nature of Pleosporales: an example from Massariosphaeria based on rDNA and RBP2 gene phylogenies. Mycological Research 111: 1268–1276 [DOI] [PubMed] [Google Scholar]
- Wehmeyer LE. (1946). Studies on some fungi of northwestern Wyoming. III. Pleospora and Leptosphaeria. Lloydia. A quarterly Journal of Biological Science, Manasha 9: 203–240 [Google Scholar]
- Wehmeyer LE. (1961). A world monograph of the genus Pleospora and its segregates. Univ. Michigan Press, USA: [Google Scholar]
- West JS, Balesdent MH, Rouxel T, Narcy JP, Huang YJ. (2002). Colonization of winter oilseed rape tissues by A/Tox+ and B/Tox0 Leptosphaeria maculans (phoma stem canker) in France and England. Plant Pathology 51: 311–321 [Google Scholar]
- West JS, Kharbanda PD, Barbetti MJ, Fitt BDL. (2001). Epidemiology and management of Leptosphaeria maculans (phoma stem canker) on oilseed rape in Australia, Canada and Europe. Plant Pathology 50: 10–27 [Google Scholar]
- White TJ, Bruns T, Lee S, Taylor J. (1990). Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: PCR protocols: a guide to methods and applications. Innis MA, Gelfand DH, Sninsky JJ, White TJ, eds. Academic press, San Diego, CA, USA: 315–322 [Google Scholar]
- Woudenberg JHC, Aveskamp MM, Gruyter J de, Spiers AG, Crous PW. (2009). Multiple Didymella teleomorphs are linked to the Phoma clematidina morphotype. Persoonia 22: 56–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wunsch MJ, Bergstrom GC. (2011). Genetic and morphological evidence that Phoma sclerotioides, causal agent of brown root rot of alfalfa, is composed of a species complex. Phytopathology 101: 594–610 [DOI] [PubMed] [Google Scholar]
- Yáňez-Morales M de J, Korf RP, Babcock JF. (1998). Fungi on Epifagus (Orobanchaceae) - I. On Sclerotium orobanches and its Phoma synanamorph. Mycotaxon 67: 275–286 [Google Scholar]
- Zhang Y, Crous PW, Schoch CL, Hyde KD. (2012). Pleosporales. Fungal Diversity 53: 1–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Fournier J, Pointing SB, Hyde KD. (2008). Are Melanomma pulvis-pyrius and Trematosphaeria pertusa congeneric? Fungal Diversity 33: 47–60 [Google Scholar]
- Zhang Y, Schoch CL, Fournier J, Crous PW, Gruyter J de. (2009). Multi-locus phylogeny of the Pleosporales: a taxonomic, ecological and evolutionary reevaluation. Studies in Mycology 64: 85–102 [DOI] [PMC free article] [PubMed] [Google Scholar]