Abstract
Pseudocercospora is a large cosmopolitan genus of plant pathogenic fungi that are commonly associated with leaf and fruit spots as well as blights on a wide range of plant hosts. They occur in arid as well as wet environments and in a wide range of climates including cool temperate, sub-tropical and tropical regions. Pseudocercospora is now treated as a genus in its own right, although formerly recognised as either an anamorphic state of Mycosphaerella or having mycosphaerella-like teleomorphs. The aim of this study was to sequence the partial 28S nuclear ribosomal RNA gene of a selected set of isolates to resolve phylogenetic generic limits within the Pseudocercospora complex. From these data, 14 clades are recognised, six of which cluster in Mycosphaerellaceae. Pseudocercospora s. str. represents a distinct clade, sister to Passalora eucalypti, and a clade representing the genera Scolecostigmina, Trochophora and Pallidocercospora gen. nov., taxa formerly accommodated in the Mycosphaerella heimii complex and characterised by smooth, pale brown conidia, as well as the formation of red crystals in agar media. Other clades in Mycosphaerellaceae include Sonderhenia, Microcyclosporella, and Paracercospora. Pseudocercosporella resides in a large clade along with Phloeospora, Miuraea, Cercospora and Septoria. Additional clades represent Dissoconiaceae, Teratosphaeriaceae, Cladosporiaceae, and the genera Xenostigmina, Strelitziana, Cyphellophora and Thedgonia. The genus Phaeomycocentrospora is introduced to accommodate Mycocentrospora cantuariensis, primarily distinguished from Pseudocercospora based on its hyaline hyphae, broad conidiogenous loci and hila. Host specificity was considered for 146 species of Pseudocercospora occurring on 115 host genera from 33 countries. Partial nucleotide sequence data for three gene loci, ITS, EF-1α, and ACT suggest that the majority of these species are host specific. Species identified on the basis of host, symptomatology and general morphology, within the same geographic region, frequently differed phylogenetically, indicating that the application of European and American names to Asian taxa, and vice versa, was often not warranted.
Taxonomic novelties:
New genera - Pallidocercospora Crous, Phaeomycocentrospora Crous, H.D. Shin & U. Braun; New species - Cercospora eucommiae Crous, U. Braun & H.D. Shin, Microcyclospora quercina Crous & Verkley, Pseudocercospora ampelopsis Crous, U. Braun & H.D. Shin, Pseudocercospora cercidicola Crous, U. Braun & C. Nakash., Pseudocercospora crispans G.C. Hunter & Crous, Pseudocercospora crocea Crous, U. Braun, G.C. Hunter & H.D. Shin, Pseudocercospora haiweiensis Crous & X. Zhou, Pseudocercospora humulicola Crous, U. Braun & H.D. Shin, Pseudocercospora marginalis G.C. Hunter, Crous, U. Braun & H.D. Shin, Pseudocercospora ocimi-basilici Crous, M.E. Palm & U. Braun, Pseudocercospora plectranthi G.C. Hunter, Crous, U. Braun & H.D. Shin, Pseudocercospora proteae Crous, Pseudocercospora pseudostigmina-platani Crous, U. Braun & H.D. Shin, Pseudocercospora pyracanthigena Crous, U. Braun & H.D. Shin, Pseudocercospora ravenalicola G.C. Hunter & Crous, Pseudocercospora rhamnellae G.C. Hunter, H.D. Shin, U. Braun & Crous, Pseudocercospora rhododendri-indici Crous, U. Braun & H.D. Shin, Pseudocercospora tibouchinigena Crous & U. Braun, Pseudocercospora xanthocercidis Crous, U. Braun & A. Wood, Pseudocercosporella koreana Crous, U. Braun & H.D. Shin; New combinations - Pallidocercospora acaciigena (Crous & M.J. Wingf.) Crous & M.J. Wingf., Pallidocercospora crystallina (Crous & M.J. Wingf.) Crous & M.J. Wingf., Pallidocercospora heimii (Crous) Crous, Pallidocercospora heimioides (Crous & M.J. Wingf.) Crous & M.J. Wingf., Pallidocercospora holualoana (Crous, Joanne E. Taylor & M.E. Palm) Crous, Pallidocercospora konae (Crous, Joanne E. Taylor & M.E. Palm) Crous, Pallidoocercospora irregulariramosa (Crous & M.J. Wingf.) Crous & M.J. Wingf., Phaeomycocentrospora cantuariensis (E.S. Salmon & Wormald) Crous, H.D. Shin & U. Braun, Pseudocercospora hakeae (U. Braun & Crous) U. Braun & Crous, Pseudocercospora leucadendri (Cooke) U. Braun & Crous, Pseudocercospora snelliana (Reichert) U. Braun, H.D. Shin, C. Nakash. & Crous, Pseudocercosporella chaenomelis (Y. Suto) C. Nakash., Crous, U. Braun & H.D. Shin; Typifications: Epitypifications - Pseudocercospora angolensis (T. Carvalho & O. Mendes) Crous & U. Braun, Pseudocercospora araliae (Henn.) Deighton, Pseudocercospora cercidis-chinensis H.D. Shin & U. Braun, Pseudocercospora corylopsidis (Togashi & Katsuki) C. Nakash. & Tak. Kobay., Pseudocercospora dovyalidis (Chupp & Doidge) Deighton, Pseudocercospora fukuokaensis (Chupp) X.J. Liu & Y.L. Guo, Pseudocercospora humuli (Hori) Y.L. Guo & X.J. Liu, Pseudocercospora kiggelariae (Syd.) Crous & U. Braun, Pseudocercospora lyoniae (Katsuki & Tak. Kobay.) Deighton, Pseudocercospora lythri H.D. Shin & U. Braun, Pseudocercospora sambucigena U. Braun, Crous & K. Schub., Pseudocercospora stephanandrae (Tak. Kobay. & H. Horie) C. Nakash. & Tak. Kobay., Pseudocercospora viburnigena U. Braun & Crous, Pseudocercosporella chaenomelis (Y. Suto) C. Nakash., Crous, U. Braun & H.D. Shin, Xenostigmina zilleri (A. Funk) Crous; Lectotypification - Pseudocercospora ocimicola (Petr. & Cif.) Deighton; Neotypifications - Pseudocercospora kiggelariae (Syd.) Crous & U. Braun, Pseudocercospora lonicericola (W. Yamam.) Deighton, Pseudocercospora zelkovae (Hori) X.J. Liu & Y.L. Guo.
Key words: Capnodiales, Cercospora, cercosporoid, Mycosphaerella, Mycosphaerellaceae, Paracercospora, Pseudocercosporella, Multi-Locus Sequence Typing (MLST), systematics
INTRODUCTION
Until recently, Pseudocercospora was treated as an anamorphic genus linked to Mycosphaerella (Mycosphaerellaceae, Capnodiales), along with approximately 30 other anamorphic genera (Crous 2009). The separation of the Mycosphaerella complex into families (Crous et al. 2007a, 2009b) and genera (Crous et al. 2009c) based on DNA sequence data and morphology had substantial implications for Pseudocercospora. Pseudocercospora is now recognised as a holomorphic genus in its own right, several species of which have mycosphaerella-like teleomorphs, for example, Pseudocercospora fijiensis and its mycosphaerella-like teleomorph that cause black leaf streak of banana (Arzanlou et al. 2008). The name Mycosphaerella is restricted to species with Ramularia anamorphs (Verkley et al. 2004, Crous et al. 2009c, Koike et al. 2011), with Ramularia being an older name than Mycosphaerella. A single generic name is now used for species of Pseudocercospora (Hawksworth et al. 2011, Wingfield et al. 2011), in compliance with the recently accepted changes to the International Code of Nomenclature for algae, fungi and plants (ICN) adoped during the Botanical Congress in Sydney in 2011, in particular, the abolishment of Article 59 dealing with pleomorphic fungi.
Species of Pseudocercospora are well recognised as plant pathogens, endophytes or saprobes, with some used as biological control agents of weeds (Den Breeÿen et al. 2006). They occur on a large number of plants, many of which are important ornamentals or food crops including fruits, cereals and commercially propagated forest trees (Fig. 1). An early hypothesis was that the majority of Pseudocercospora species were strictly host specific. Later studies have reported that a few species occur on different hosts belonging to a single plant family (Deighton 1976, 1979), although DNA data or inoculation studies to support wider host ranges has often been lacking.
Fig. 1.
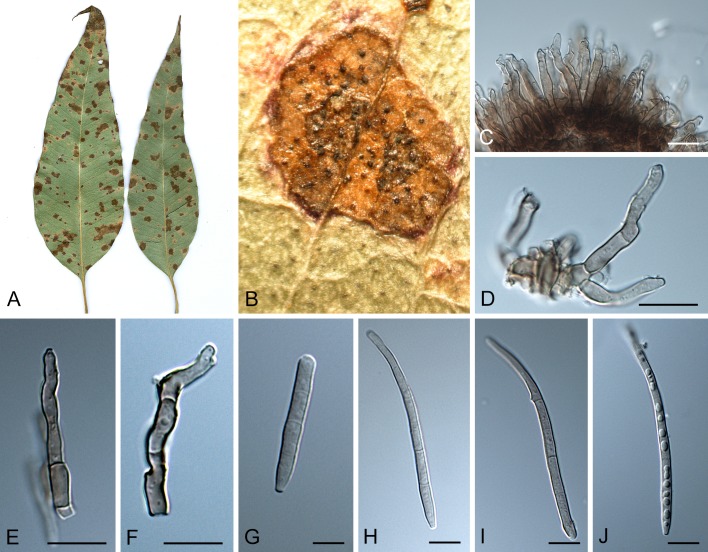
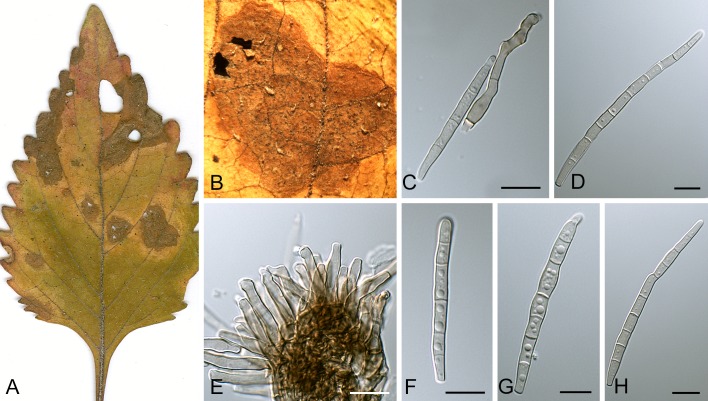
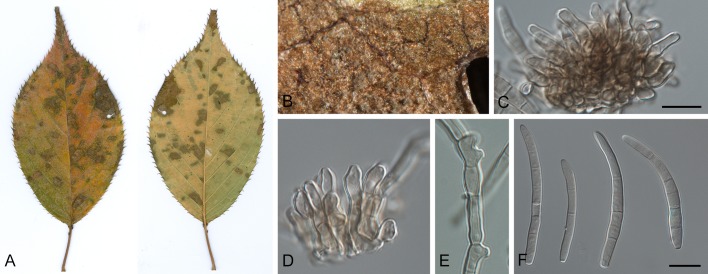
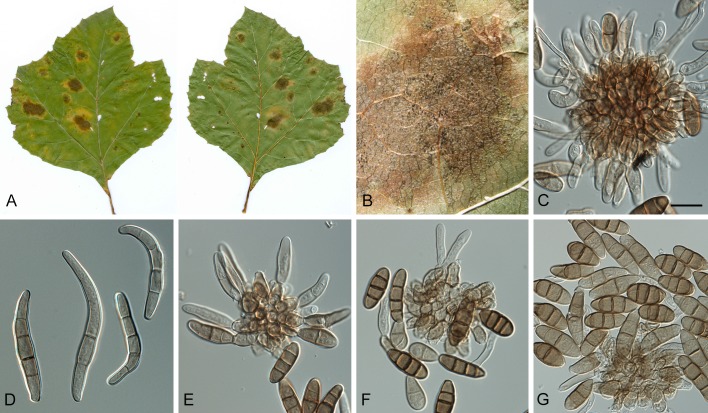
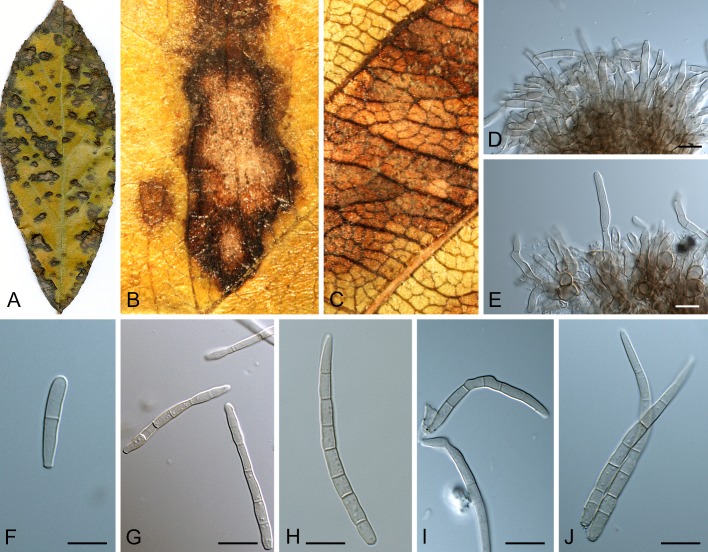
Leaf spot symptoms associated with various species from the Pseudocercospora complex. A. P. fatouae on Fatoua villosa. B. P. clematidis on Clematis apiicola. C. P. griseola on Phaseolus vulgaris. D. P. rhododendron-indici on Rhododendron indicum. E. P. pyracanthae on Pyracantha angustifolia. F. P. lonicericola on Lonicera japonica. G. Scolecostigmina mangiferae on Mangifera indica. H. P. fraxinites on Fraxinus rhynchophylla. I. Pseudocercosporella potentillae on Potentilla kleiniana. J. Pseudocercospora udagawana on Hovenia dulcis.
The classic monograph of the hyphomycete genus Cercospora (Chupp 1954) considered morphological features, including the structure of conidiomata as well as conidial pigmentation, septation, wall thickness, length, width, and shape as valuable features to define species within the genus. Chupp’s circumscription of Cercospora was rather broadly defined, and the genus was later shown to be extremely heterogenous (Deighton 1976). Deighton (1976) distinguished different groups within Cercospora based on characters such as superficial mycelium (and the texture thereof), conidial scar type, conidiophore and conidium pigmentation, septation, and conidial catenulation. These additional features resulted in many Cercospora species being transferred to several alternative genera such as Cercosporella, Mycocentrospora, Mycovellosiella, Phaeoramularia, Paracercospora, Passalora, Pseudocercospora, Ramularia, Stenella and Stigmina (Deighton 1971, 1976, 1979, 1987, Braun 1995, 1998). A subsequent morphological treatment of names published in Cercospora (Crous & Braun 2003) provided some rationalisation, with the following concepts proposed for the taxonomic treatment of cercosporoid fungi: structure of conidiogenous loci (scars) and hila, as either unthickened (or almost so, but slightly darkened or refractive) or unthickened; presence or absence of pigmentation in conidiophores and conidia.
Pseudocercospora was originally introduced by Spegazzini (1910) based on the type species Pseudocercospora vitis, a foliar pathogen of grapevines. The majority of Pseudocercospora species known to date are regarded as pathogens on a wide variety of plants, predominantly in tropical and sub-tropical environments where they cause leaf spots, blights, fruit spot and fruit rot (Chupp 1954, Deighton 1976, von Arx 1983, Pons & Sutton 1988). Some important plant pathogens include the species associated with Sigatoka disease on banana (Arzanlou et al. 2007, 2008, 2010, Churchill 2010), angular leaf spot of bean (Crous et al. 2006), husk spot of macadamia (Beilharz et al. 2003), Cercospora leaf spot of olive (Ávila et al. 2005), cactus (Ayala-Escobar et al. 2005), avocado (Deighton 1976), and eucalypts (Braun & Dick 2002). The importance of these diseases is also reflected in quarantine regulations, e.g. for Pseudocercospora angolensis the cause of fruit and leaf spot disease on citrus (Pretorius et al. 2003) (Fig. 2), and P. pini-densiflorae the cause of brown needle blight of pine (Evans 1984, Crous et al. 1990).
Fig. 2.
Pseudocercospora species of quarantine importance. A. P. fijiensis on Musa (Black Leaf Streak or Black Sigatoka) (Photo G.H.J. Kema). B, C. P. angolensis on Citrus (Phaeoramularia Fruit and Leaf Spot).
Pseudocercospora was established to accommodate synnematal analogues of Cercospora, as well as species that produce pigmented conidiogenous structures and conidia with neither thickened nor darkened conidial hila (Deighton 1976, Braun 1995) (Fig. 3). It was proposed that Pseudocercospora be divided into several genera (Deighton 1976) based on morphological differences, a view later supported by several authors (Pons & Sutton 1988, Braun 1995, Crous & Braun 1996). Since the first study applied DNA phylogenetic analyisis to species in the Mycosphaerella complex (Stewart et al. 1999), Pseudocercospora has been shown to be heterogenous, accommodating hundreds of species (Crous et al. 2000, 2001, Crous & Braun 2003).
Fig. 3.
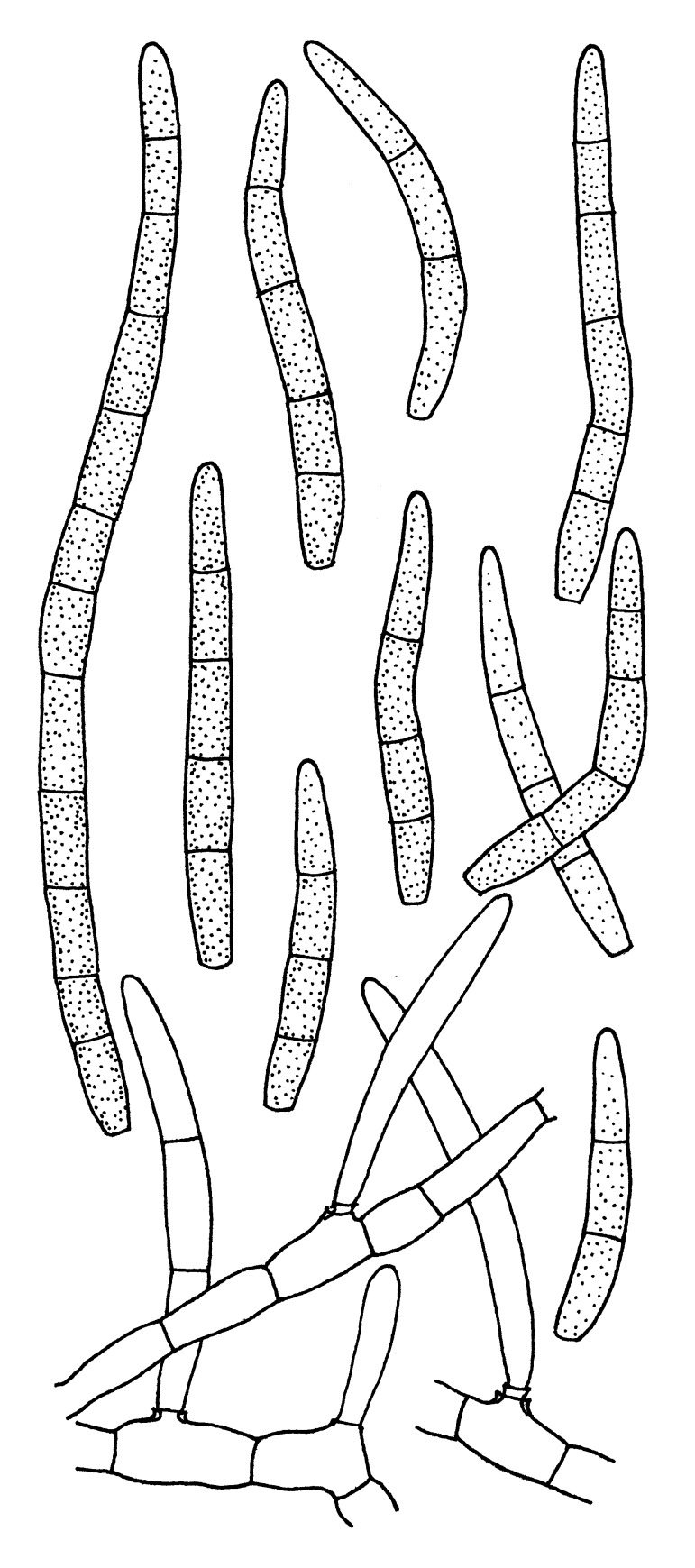
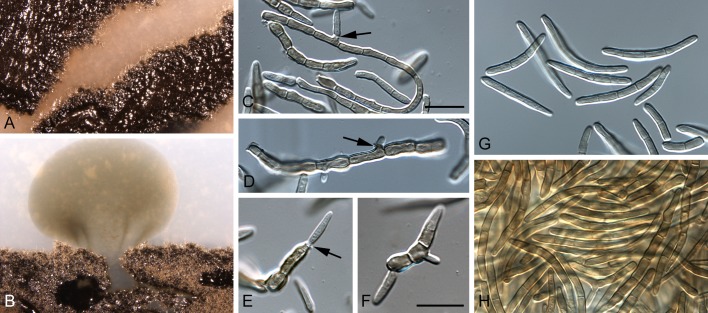
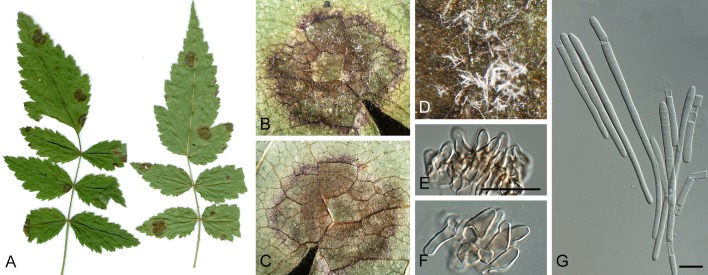
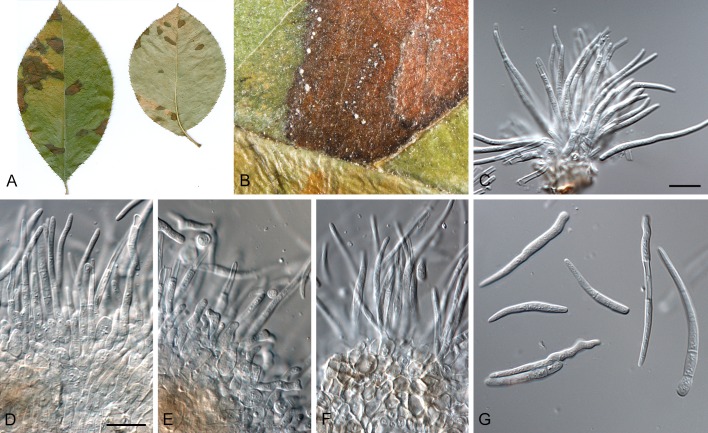
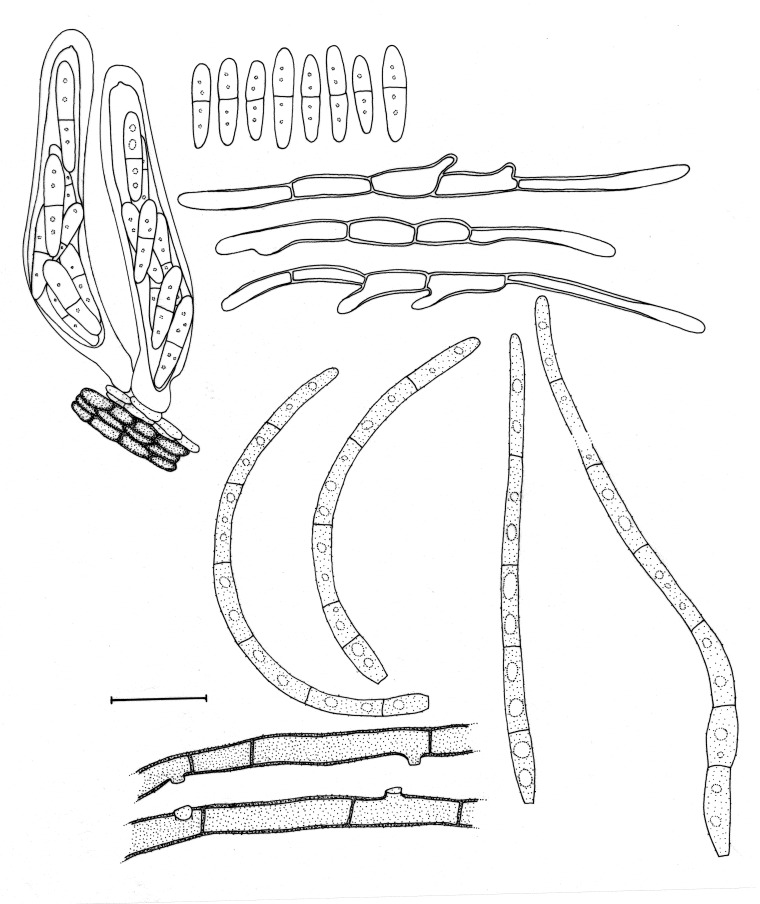
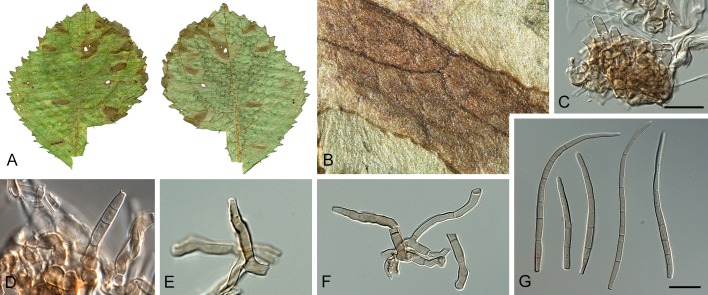
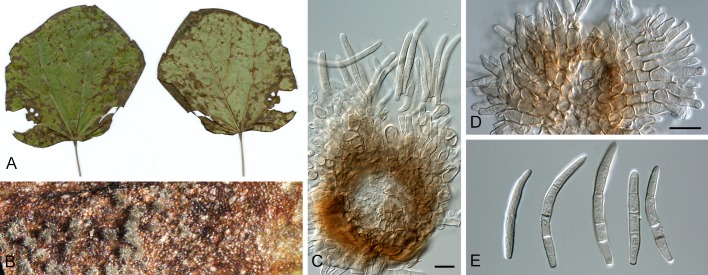
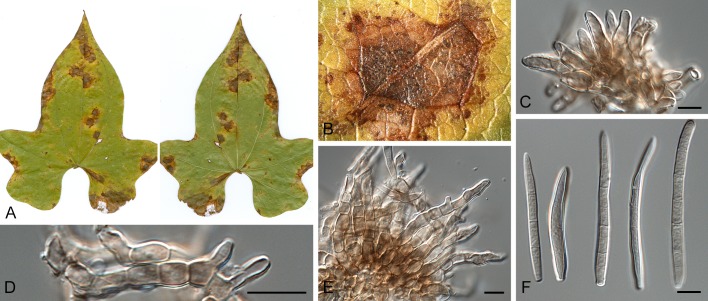
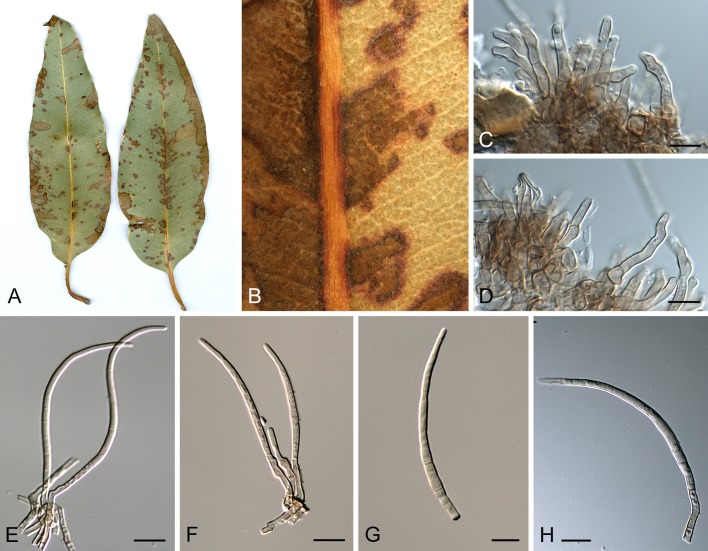
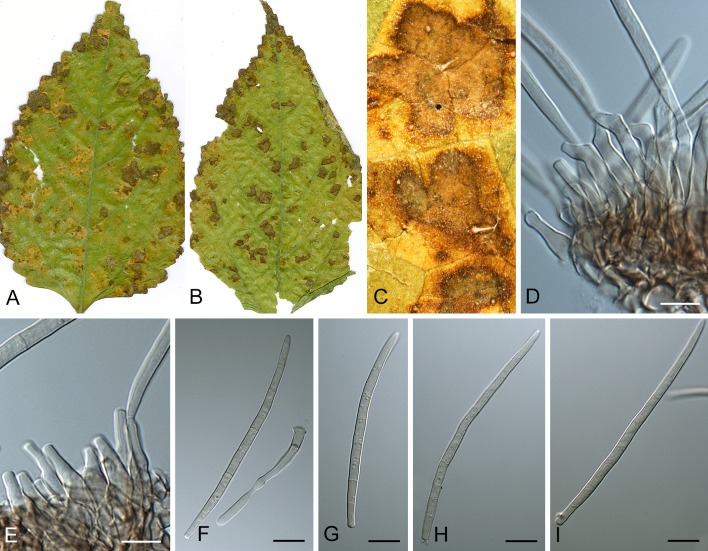
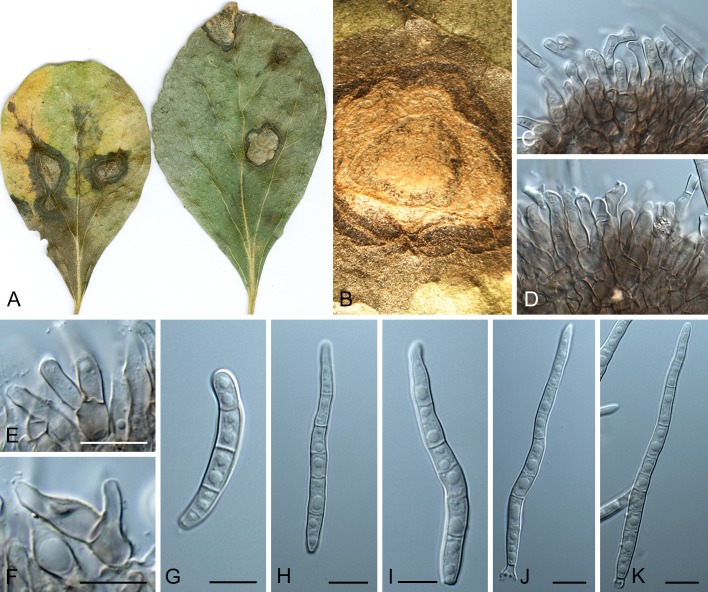
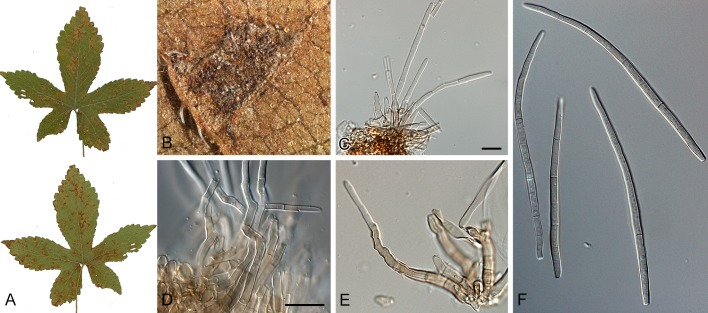
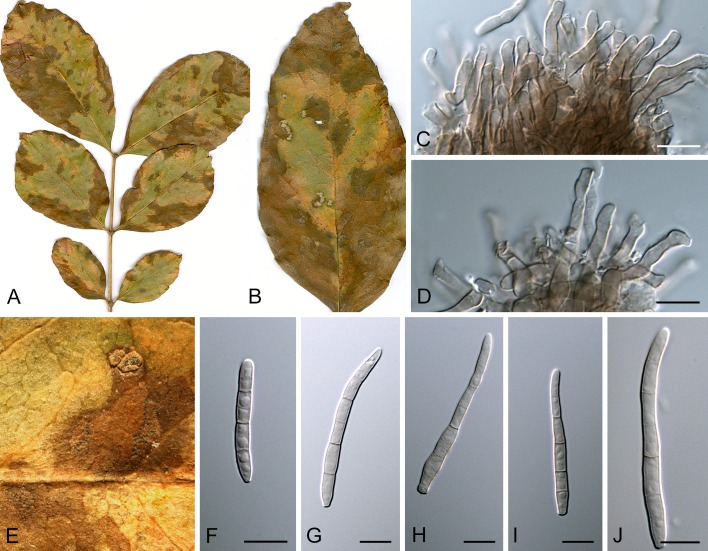
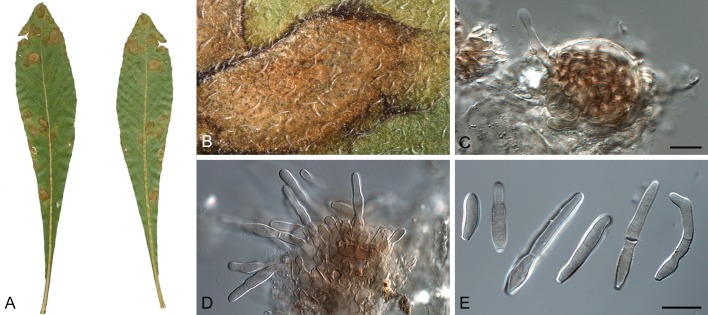
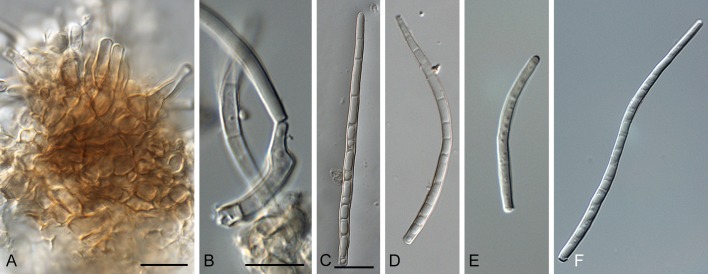
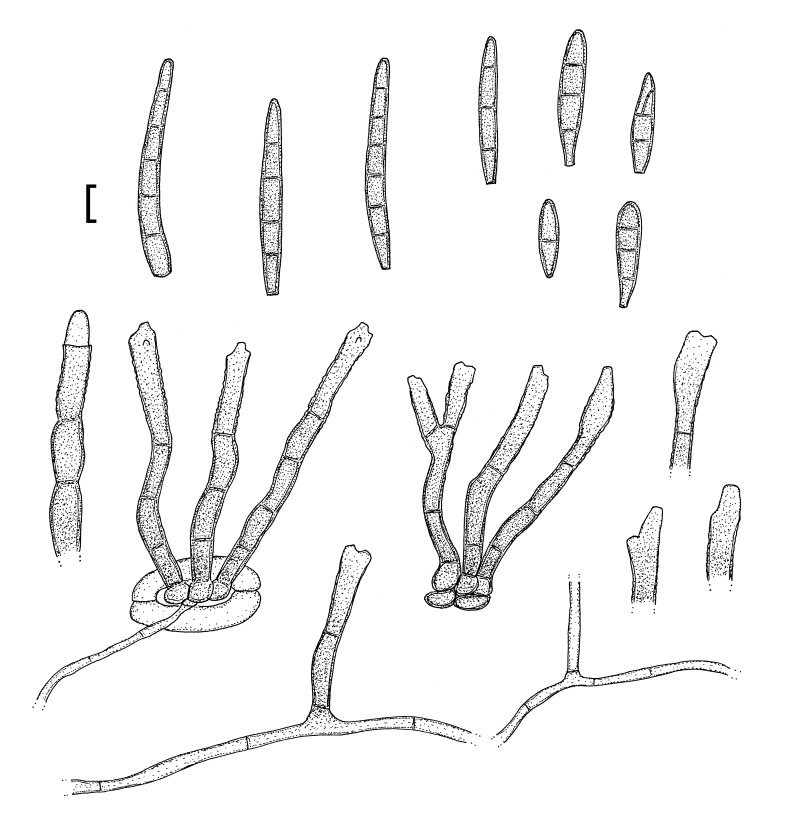
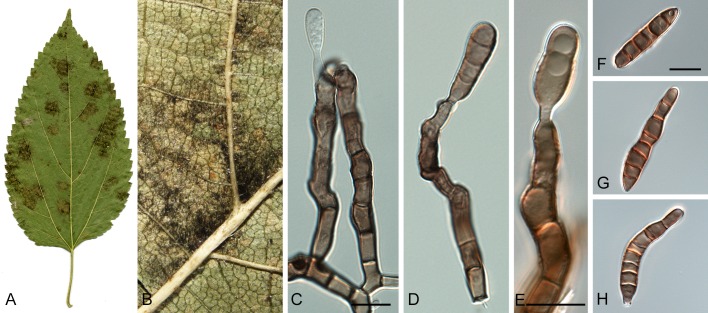
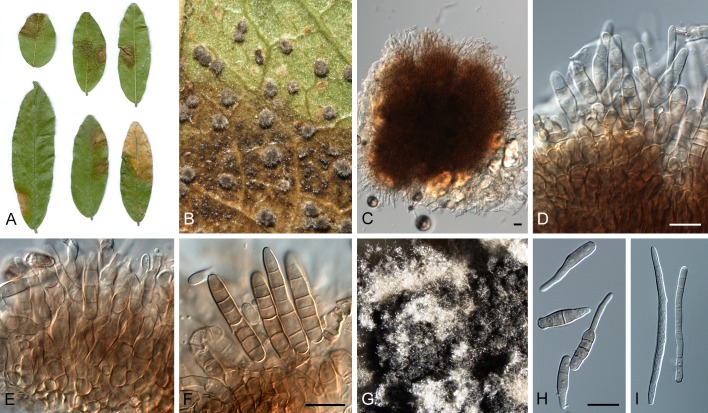
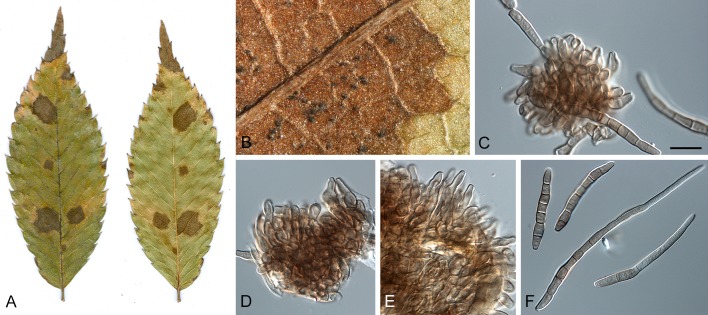
Morphological structures of Pseudocercospora spp. A. Synnematous conidiophore. B. Densely aggregated fascicle of conidiophores with well-developed brown stroma. C, D. Loosely branched fascicles of conidiophores with moderate (C) and poorly (D) developed brown stroma. E. Fascicle reduced to conidiogenous cells. F. Conidiophore fascicles arising from stomata. G, H. Solitary conidiogenous cells on superficial hyphae. I. Geniculate conidiophore (arrow) with truncate apical locus. J, K. Conidiophores branched below (arrows). L. Conidiogenous cells with percurrent proliferations (arrows). M, N. Conidiophores with sympodial proliferation. O. Conidiophores with conidiogenous cells (note minutely thickened scars, arrows). P. Subcylindrical conidium with subacute apex and truncate base. Q. Conidia with constrictions at septa. R. Conidium with guttules. S. Cylindrical conidium with obtuse apex, and truncate base. T. Undulate conidia. U. Curved conidium. Aseptate to 1-septate conidia. V. 1-septate conidia. W, X. Obclavate conidia with obconical base. Y. Obclavate conidium with short obconical base. Z. Dark brown, muriformly euseptate conidia (thick-walled, not distoseptate).
There are very few morphological features that are informative at the generic level within the Pseudocercospora complex. Deighton (1983) found it difficult to distinguish Cercoseptoria from Pseudocercospora on the basis of conidial shape, with conidia in the former genus acicular and those in the latter obclavate to cylindrical. In delimiting Pseudocercospora as an anamorph of Mycosphaerella, von Arx (1983) considered Pseudocercospora together in a group of related genera characterised by hyaline or subhyaline conidiogenous structures and unthickened, truncate, flat and broad conidiogenous loci. Later, Braun (1992) and Crous et al. (2000) argued that the arrangement of the conidiophores did not distinguish between sections within Pseudocercospora due to transitions from solitary to fasciculate to subsynnematal conidiophores. Crous et al. (2001) also regarded the slight thickening of conidial scars as a taxonomically uninformative generic character.
DNA sequence data for various gene regions have in recent years provided substantial information to support the generic circumscription of Pseudocercospora. Several studies have employed DNA sequence data from the Internal Transcribed Spacer (ITS) region of the rDNA operon for Pseudocercospora species from various hosts. Crous et al. (2000) examined isolates of Pseudocercospora from Eucalyptus and found that they could be separated into two clades within Mycosphaerella. Another clade of Pseudocercospora species occurred on banana, indicating that Pseudocercospora could be polyphyletic within the Mycosphaerella complex. Further evidence supporting this view emerged in subsequent studies that included many Pseudocercospora isolates (Crous et al. 2001). These phylogenetic studies have shown that several other genera are congeneric with Pseudocercospora and thus Cercostigmina, Paracercospora, Phaeoisariopsis and Pseudophaeoramularia were reduced to synonymy with Pseudocercospora (Stewart et al. 1999, Crous et al. 2001, Braun & Hill 2002, Crous et al. 2006). Based on these studies, the necessity arose to conserve Pseudocercospora over Stigmina, which represented an older generic name (Braun & Crous 2006).
Extensive DNA-based phylogenetic research has in recent years been conducted on Mycosphaerella and many of its anamorphic genera. These studies have not provided substantial resolution of Pseudocercospora. The aims of this study were to define phylogenetic lineages (reflecting genera) within what is perceived to be Pseudocercospora. An additional aim was to use the molecular data to infer host range and thus to consider the importance of host specificity in this important genus.
MATERIALS AND METHODS
Isolates
Direct isolations were made from fascicles of conidiophores on leaves. Some leaves were incubated in moist chambers for up to 1 wk to enhance sporulation before single conidial colonies were established on 2 % malt extract agar (MEA) (Crous 2002). Leaf spots bearing ascomata were soaked in water for approximately 2 h, after which they were attached to the inner surface of Petri dish lids over plates containing MEA. Ascospore germination patterns were examined after 24 h, and single ascospore and conidial cultures established as described previously (Crous et al. 1991, Crous 1998). Colonies were sub-cultured onto synthetic nutrient-poor agar (SNA), potato-dextrose agar (PDA), oatmeal agar (OA), and MEA (Crous et al. 2009d), and incubated at 25 °C under continuous near-ultraviolet light to promote sporulation. Isolates were also sourced from the culture collections of the CBS-KNAW Fungal Biodiversity Centre (CBS), the working collection of Pedro Crous (CPC), Chiharu Nakashima (CNS) and the culture collection of the laboratory of plant pathology, Mie University, Japan (MUCC), and the mycological herbarium of Mie University (MUMH). Furthermore, isolates representing fungal species from genera allied to Pseudocercospora, e.g. Cercospora, Cercostigmina, Cyphellophora, Davidiella, Dissoconium, Miuraea, Mycocentrospora, Passalora, Phaeoisariopsis, Phleospora, Septoria, Strelitziana, Stigmina, Teratosphaeria, Thedgonia, Trochophora, and Xenostigmina, were included in this study (Table 1).
Table 1.
Pseudocercospora and pseudocercospora-like isolates included in the morphological and/or phylogenetic analyses.
| Species | Culture accession numbers1 | Collector | Host | Family | Country |
GenBank accession numbers2 |
|||
|---|---|---|---|---|---|---|---|---|---|
| LSU | ITSy | EF-1α | ACT | ||||||
| Cercospora eucommiae | CPC 10047 | H.D. Shin | Eucommia ulmoides | Eucommiaceae | South Korea | GU253741 | GU269702 | GU384418 | GU320406 |
| CPC 10802; CBS 131932 | H.D. Shin | Eucommia ulmoides | Eucommiaceae | South Korea | GU214674 | GU269851/GU214674 | GU384563 | GU320555 | |
| CPC 11508; CBS 132026 | H.D. Shin | Eucommia ulmoides | Eucommiaceae | South Korea | GU253742 | GU269703 | GU384419 | GU320407 | |
| Cercospora sojina | CPC 12322; CBS 132018 | H.D. Shin | Glycine soja | Fabaceae | South Korea | GU253861 | GU214655 | JQ324984 | JQ325008 |
| Cyphellophora eucalypti | CBS 124764; CPC 13412 | P.W. Crous | Eucalyptus sp. | Myrtaceae | Australia | GQ303305 | GQ303274 | GU384510 | JQ325009 |
| Dissoconium dekkeri | CBS 110748; CPC 825; CMW 14906 | G. Kemp | Eucalyptus grandis | Myrtaceae | South Africa | GU214422 | AF173315 | JQ324985 | DQ147651 |
| Microcyclospora quercina | CPC 10712; CBS 130827 | G. Verkley | Quercus sp. | Fagaceae | Netherlands | GU214681 | GU269789 | GU384499 | GU320490 |
| Miuraea persicae | CPC 10069; CBS 132307 | H.D. Shin | Prunus persica | Rosaceae | South Korea | GU253859 | GU269843 | GU384556 | GU320546 |
| CPC 10828; CBS 131935 | H.D. Shin | Prunus armeniaca | Rosaceae | South Korea | JQ324939 | GU269844 | GU384557 | GU320547 | |
| “Mycosphaerella” laricina | CBS 326.52 | E. Müller | Larix decidua | Pinaceae | Switzerland | GU253693 | GU269643 | GU384361 | GU320353 |
| “Mycosphaerella” madeirae | CBS 112895; CPC 3745 | S. Denman | Eucalyptus globulus | Myrtaceae | Portugal | DQ204756 | AY725553 | DQ211672 | DQ147641 |
| “Mycosphaerella” marksii | CBS 110920; CPC 935; CMW 5150 | A.J. Carnegie | Eucalyptus botryoides | Myrtaceae | Australia | DQ246250/GU253694 | AF309588/GU269644 | DQ235134 | DQ147625 |
| Pallidocercospora acaciigena | CBS 112516; CPC 3838 | M.J. Wingfield | Acacia mangium | Fabaceae | Venezuela | GU214661/GU253697 | GU269648 | GU384366 | GU320356 |
| CBS 120740; CPC 13290 | B. Summerell | Eucalyptus sp. | Myrtaceae | Australia | GU253698 | EF394822/GU269649 | GU384367 | GU320357 | |
| Pallidocercospora crystallina | CBS 681.95; CBS 116158; CPC 802; CMW 3033 | M.J. Wingfield | Eucalyptus bicostata | Myrtaceae | South Africa | DQ204747 | AY490757 | DQ147636/DQ211662 | DQ147636 |
| Pallidocercospora heimii | CBS 110682; CPC 760; CMW 4942 | P.W. Crous | Eucalyptus sp. | Myrtaceae | Madagascar | DQ204751 | AF309606 | DQ211667 | DQ147638 |
| Pallidocercospora heimioides | CBS 111190; CPC 1312; CMW 3046 | M.J. Wingfield | Eucalyptus sp. | Myrtaceae | Indonesia | DQ204753 | AF309609 | DQ211669 | DQ147633 |
| Pallidocercospora irregulariramosa | CBS 114774; CBS 114777; CPC 1360; CMW 4943 | M.J. Wingfield | Eucalyptus saligna | Myrtaceae | South Africa | DQ204754 | AF309607 | DQ211670 | DQ147634 |
| Pallidocercospora konae | CBS 120748; CPC 13469 | W. Himaman | Eucalyptus camaldulensis | Myrtaceae | Thailand | GU253852 | EF394842 | GU384549 | GU320538 |
| Paracercospora egenula | CBS 485.81 | N. Ponnapa | Solanum melongena | Solanaceae | India | JQ324940 | GU269699 | GU384415 | GU320403 |
| CPC 12537; CBS 132030 | H.D. Shin | Solanum melongena | Solanaceae | South Korea | GU253738 | GU269698 | GU384414 | GU320402 | |
| MUCC 883 | T. Mikami | Solanum melongena | Solanaceae | Japan | GU253739 | GU269700 | GU384416 | GU320404 | |
| Passalora eucalypti | CBS 111318; CPC 1457 | P.W. Crous | Eucalyptus saligna | Myrtaceae | Brazil | GU253860 | GU269845 | GU384558 | GU320548 |
| Phaeomycocentrospora cantuariensis | CPC 10157 | H.D. Shin | Humulus scandens | Cannabaceae | South Korea | GU253712 | GU269664 | GU384381 | GU320370 |
| CPC 10762; CBS 131928 | H.D. Shin | Luffa cylindrica | Cucurbitaceae | South Korea | GU253713 | GU269665 | GU384382 | GU320371 | |
| CPC 11646; CBS 132013 | H.D. Shin | Acalypha australis | Euphorbiaceae | South Korea | GU253715 | GU269667 | GU384384 | GU320373 | |
| CPC 11694; CBS 132014 | H.D. Shin | Humulus scandens | Cannabaceae | South Korea | GU253716 | GU269668 | GU384385 | GU320374 | |
| Phloeospora ulmi | CBS 344.97 | W. Gams | Ulmus glabra | Ulmaceae | Austria | GU253841 | JQ324974 | JQ324986 | GU320528 |
| CBS 613.81 | H.A. Van der Aa | Ulmus sp. | Ulmaceae | Austria | GU253842 | GU269825 | JQ324987 | GU320529 | |
| Pseudocercospora abelmoschi | CPC 14478; CBS 132103 | H.D. Shin | Hibiscus syriacus | Malvaceae | South Korea | GU253696 | GU269647 | GU384365 | GU320355 |
| Pseudocercospora acericola | CBS 122279 | R. Kirschner | Acer albopurpurascens | Aceraceae | Taiwan | GU253699 | GU269650 | GU384368 | GU320358 |
| Pseudocercospora ampelopsis | CPC 11680; CBS 131583 | H.D. Shin | Ampelopsis brevipenduncula var. heterophylla | Vitaceae | South Korea | GU253846 | GU269830 | GU384542 | GU320534 |
| Pseudocercospora angolensis | CBS 112933; CPC 4118 | M.C. Pretorius | Citrus sp. | Rutaceae | Zimbabwe | GU214470 | AY260063/GU269836 | GU384548 | JQ325010 |
| CBS 149.53 | T. de Carvalho & O. Mendes | Citrus sinensis | Rutaceae | Angola | JQ324941 | JQ324975 | JQ324988 | JQ325011 | |
| Pseudocercospora araliae | CPC 10154 | H.D. Shin | Aralia elata | Araliaceae | South Korea | GU253701 | GU269652 | GU384370 | GU320360 |
| MUCC 873 | T. Kobayashi & C. Nakashima | Aralia elata | Araliaceae | Japan | GU253702 | GU269653 | GU384371 | GU320361 | |
| Pseudocercospora arecacearum | CBS 118406 | C.F. Hill | Rhopalostylis sapidis | Arecaceae | New Zealand | GU253704 | GU269655 | GU384373 | GU320363 |
| CBS 118792 | C.F. Hill | Howea forsteriana | Arecaceae | New Zealand | GU253703 | GU269654 | GU384372 | GU320362 | |
| Pseudocercospora assamensis | CBS 122467 | I. Buddenhagen | Musa cultivar | Musaceae | India | GU253705 | GU269656 | GU384374 | GU320364 |
| Pseudocercospora atromarginalis | CBS 114640 | C.F. Hill | Solanum sp. | Solanaceae | New Zealand | GU253706 | GU269658 | GU384376 | GU320365 |
| CPC 11372; CBS 132010 | H.D. Shin | Solanum nigrum | Solanaceae | South Korea | GU214671 | GU269657 | GU384375 | – | |
| Pseudocercospora balsaminae | CPC 10044; CBS 131882 | H.D. Shin | Impatiens textori | Balsaminaceae | South Korea | GU253708 | GU269660 | GU384379 | GU320367 |
| Pseudocercospora basiramifera | CBS 111072; CPC 1266 | M.J. Wingfield | Eucalyptus pellita | Myrtaceae | Thailand | GU253709 | GU269661 | DQ211677 | GU320368 |
| CBS 114757; CPC 1267 | M.J. Wingfield | Eucalyptus pellita | Myrtaceae | Thailand | GU253802 | GU269781 | GU384492 | GU320484 | |
| Pseudocercospora basitruncata | CBS 114664; CPC 1202 | M.J. Wingfield | Eucalyptus grandis | Myrtaceae | Colombia | GU253710/DQ204759 | DQ267600/GU269662 | DQ211675 | DQ147622 |
| Pseudocercospora callicarpae | MUCC 888 | T. Kobayashi | Callicarpa japonica | Verbenaceae | Japan | GU253711 | GU269663 | GU384380 | GU320369 |
| Pseudocercospora catalpigena | MUCC 743 | C. Nakashima & I. Araki | Catalpa ovata | Bignoniaceae | Japan | GU253731 | GU269690 | GU384406 | GU320395 |
| Pseudocercospora catappae | MUCC 809 | C. Nakashima & T. Akashi | Terminalia catappa | Combretaceae | Japan | GU253717 | GU269669 | GU384386 | GU320375 |
| Pseudocercospora cercidicola | MUCC 896 | T. Kobayashi & Y. Kobayashi | Cercis chinensis | Fabaceae | Japan | GU253719 | GU269671 | GU384388 | GU320377 |
| Pseudocercospora cercidis-chinensis | CPC 14481; CBS 132109 | H.D. Shin | Cercis chinensis | Fabaceae | South Korea | GU253718 | GU269670 | GU384387 | GU320376 |
| Pseudocercospora cf. cruenta | CBS 117232 | R. Kirschner | Phaseolus vulgaris | Fabaceae | Taiwan | GU253730 | GU269689 | GU384405 | GU320394 |
| Pseudocercospora cf. kaki | CPC 10636; CBS 131921 | H.D. Shin | Diospyros lotus | Ebenaceae | South Korea | GU214677 | GU269728 | GU384441 | GU320430 |
| Pseudocercospora chengtuensis | CPC 10696; CBS 131924 | H.D. Shin | Lycium chinense | Solanaceae | South Korea | JQ324942 | GU269673 | GU384390 | GU320379 |
| MUCC 828 | I. Araki & M. Harada | Lycium chinense | Solanaceae | Japan | JQ324943 | – | – | – | |
| Pseudocercospora chionanthi-retusi | CPC 14683; CBS 132110 | H.D. Shin | Chionanthus retusus | Oleaceae | South Korea | GU253721 | GU269674 | GU384391 | GU320380 |
| Pseudocercospora chrysanthemicola | CPC 10633; CBS 131888 | H.D. Shin | Chrysanthemum sp. | Asteraceae | South Korea | GU253722 | GU269675 | GU384392 | GU320381 |
| Pseudocercospora cladosporioides | CBS 117482; CPC 10913 | P.W. Crous | Olea europaea | Oleaceae | Tunisia | JQ324944 | GU269678 | GU384395 | GU320383 |
| “Pseudocercospora” colombiensis | CBS 110969; CPC 1106; CMW 4944 | M.J. Wingfield | Eucalyptus urophylla | Myrtaceae | Colombia | DQ204744 | AY752149 | DQ211660 | DQ147639 |
| Pseudocercospora contraria | CPC 14714; CBS 132108 | H.D. Shin | Dioscorea quinqueloba | Dioscoreaceae | South Korea | JQ324945 | GU269677 | GU384394 | GU320385 |
| Pseudocercospora coprosmae | CBS 114639 | C. F. Hill | Coprosma robusta | Rubiaceae | New Zealand | JQ324946 | GU269680 | GU384397 | GU320386 |
| Pseudocercospora cordiana | CBS 114685; CPC 2552 | P.W. Crous & R.L. Benchimol | Cordia goeldiana | Boraginaceae | Brazil | GU214472 | AF362054/GU269681 | GU384398 | GU320387 |
| Pseudocercospora coriariae | MUCC 840 | I. Araki & M. Harada | Coriaria japonica | Coriariaceae | Japan | GU253725 | GU269682 | GU384399 | GU320388 |
| Pseudocercospora cornicola | MUCC 909 | C. Nakashima & E. Imaizumi | Cornus alba var. sibirica | Cornaceae | Japan | GU253726 | GU269683 | GU384400 | GU320389 |
| Pseudocercospora corylopsidis | MUCC 874 | T. Kobayashi & C. Nakashima | Hamamelis japonica | Hamamelidaceae | Japan | GU253757 | GU269721 | GU384437 | GU320425 |
| MUCC 908 | C. Nakashima & E. Imaizumi | Corylopsis spicata | Hamamelidaceae | Japan | GU253727 | GU269684 | GU384401 | GU320390 | |
| Pseudocercospora cotoneastri | MUCC 876 | T. Kobayashi & C. Nakashima | Cotoneaster salicifolius | Rosaceae | Japan | GU253728 | GU269685 | GU384402 | GU320391 |
| Pseudocercospora crispans | CPC 14883; CBS 125999 | P.W.Crous | Eucalyptus sp. | Myrtaceae | South Africa | GU253825 | GU269807 | GU384518 | GU320510 |
| Pseudocercospora crocea | CPC 11668; CBS 126004 | H.D. Shin | Pilea hamaoi | Urticaceae | South Korea | JQ324947 | GU269792 | GU384502 | GU320493 |
| Pseudocercospora crousii | CBS 119487 | C.F. Hill | Eucalyptus sp. | Myrtaceae | New Zealand | GU253729 | GU269686 | GU384403 | GU320392 |
| Pseudocercospora cruenta | CPC 10846; CBS 132021 | H. Booker | Vigna sp. | Fabaceae | Trinidad | GU214673 | GU269688 | GU384404 | JQ325012 |
| Pseudocercospora cydoniae | CPC 10678; CBS 131923 | H.D. Shin | Chaenomeles speciosa | Rosaceae | South Korea | GU253732 | GU269691 | GU384407 | GU320396 |
| Pseudocercospora cymbidiicola | CBS 115132 | C.F. Hill | Cymbidium sp. | Orchidaceae | New Zealand | GU253733 | GU269692 | GU384408 | GU320397 |
| Pseudocercospora davidiicola | MUCC 296 | C. Nakashima & I. Araki | Davidia involucrata | Nyssaceae | Japan | GU253734 | GU269693 | GU384409 | GU320398 |
| Pseudocercospora dendrobii | MUCC 596 | C. Nakashima & K. Motohashi | Dendrobium sp. | Orchidaceae | Japan | GU253737 | GU269696 | GU384412 | GU320401 |
| Pseudocercospora destructiva | MUCC 870 | S. Uematsu & C. Nakashima | Euonymus japonicus | Celastraceae | Japan | GU253735 | GU269694 | GU384410 | GU320399 |
| Pseudocercospora dianellae | CBS 117746 | C.F. Hill | Dianella caerulae | Liliaceae | New Zealand | GU253736 | GU269695 | GU384411 | GU320400 |
| Pseudocercospora dodonaeae | CBS 114647 | C.F. Hill | Dodonaea viscosa | Sapindaceae | New Zealand | JQ324948 | GU269697 | GU384413 | JQ325013 |
| Pseudocercospora dovyalidis | CPC 13771; CBS 126002 | P.W. Crous | Dovyalis zeyheri | Flacourtiaceae | South Africa | GU253818 | GU269800 | GU384513 | GU320503 |
| Pseudocercospora elaeocarpi | MUCC 925 | C. Nakashima | Elaeocarpus sp. | Elaeocarpaceae | Japan | GU253740 | GU269701 | GU384417 | GU320405 |
| “Pseudocercospora” epispermogonia | CBS 110750; CPC 822 | G. Kemp | Eucalyptus grandis | Myrtaceae | South Africa | DQ204757 | DQ267596 | DQ211673 | DQ147629 |
| Pseudocercospora eucalyptorum | CBS 110777; CPC 16; CMW 5228 | P.W. Crous | Eucalyptus nitens | Myrtaceae | South Africa | DQ204762 | AF309598 | DQ211678 | DQ147614 |
| CBS 114242; CPC 10390; CMW 14908 | J.P. Mansilla | Eucalyptus globulus | Myrtaceae | Spain | GU214481 | AY725526 | DQ211681 | DQ147613/GU320465 | |
| CBS 116359; CPC 3751 | P.W. Crous | Eucalyptus sp. | Myrtaceae | Madeira | GU253829 | GU269812 | GU384524 | GU320514 | |
| CPC 10500; CBS 114243 | P.W. Crous | Eucalyptus nitens | Myrtaceae | New Zealand | JQ324949 | AY725527 | GU384474 | JQ325014 | |
| CPC 10507; CBS 116371 | P.W.Crous | Eucalyptus nitens | Myrtaceae | New Zealand | JQ324950 | GU269687 | JQ324989 | GU320393 | |
| CPC 10916 | P.W. Crous | Eucalyptus sp. | Myrtaceae | South Africa | GU253788 | GU269763 | GU384475 | GU320464 | |
| CPC 11713; CBS 132015 | P. Mansilla | Eucalyptus globulus | Myrtaceae | Spain | JQ324951 | GU269811 | GU384523 | JQ325015 | |
| CPC 12406; CBS 132029 | I. Smith | Eucalyptus globulus | Myrtaceae | Australia | GU253811 | GU269793 | GU384503 | GU320494 | |
| CPC 12568; CBS 132309 | C. Mohammed | Eucalyptus nitens | Myrtaceae | Australia | GU253814 | GU269796 | GU384506 | GU320497 | |
| CPC 12802; CBS 132032 | A. Phillips | Eucalyptus globulus | Myrtaceae | Portugal | GU253789 | JQ324976 | JQ324990 | GU320466 | |
| CPC 12957; CBS 132033 | B. Summerell | Eucalyptus deanei | Myrtaceae | Australia | GU253815 | GU269797 | JQ324991 | JQ325016 | |
| CPC 13455; CBS 132034 | P.W. Crous | Eucalyptus sp. | Myrtaceae | Portugal | GU253816 | GU269798 | GU384511 | GU320501 | |
| CPC 13769; CBS 132035 | P.W. Crous | Eucalyptus punctata | Myrtaceae | South Africa | GU253707 | GU269659 | GU384378 | GU320366 | |
| CPC 13816; CBS 132114 | S. Denman | Eucalyptus glaucescens | Myrtaceae | UK | GU253819 | GU269801 | JQ324992 | GU320504 | |
| CPC 13926; CBS 132105 | S. Denman | Eucalyptus sp. | Myrtaceae | USA | GU253820 | GU269802 | JQ324993 | GU320505 | |
| Pseudocercospora eupatoriella | CBS 113372 | M.J. Morris | Chromolaena odorata | Asteraceae | Jamaica | GU253743 | GU269704 | GU384420 | GU320408 |
| Pseudocercospora eustomatis | CBS 110822 | G. Dal Bello | Eustroma grandiflorum | Gentianaceae | Argentina | GU253744 | GU269705 | GU384421 | GU320409 |
| Pseudocercospora exosporioides | MUCC 893 | T. Kobayashi | Sequoia sempervirens | Taxodiaceae | Japan | GU253746 | GU269707 | GU384423 | GU320411 |
| Pseudocercospora fijiensis | CBS 120258; CIRAD 86 | J. Carlier | Musa sp. | Musaceae | Cameroon | JQ324952 | EU514248 | Genome3 | Genome3 |
| MUCC 792 | T. Kobayashi & C. Nakashima | Musa sp. | Musaceae | Japan | GU253776 | GU269748 | JQ324994 | GU320450 | |
| Pseudocercospora flavomarginata | CBS 118841; CMW 13586 | M.J. Wingfield | Eucalyptus camaldulensis | Myrtaceae | Thailand | DQ153306 | DQ155657 | DQ156548 | DQ166513 |
| CBS 124990; CPC 13492 | W. Himaman | Eucalyptus camaldulensis | Myrtaceae | Thailand | GU253817 | GU269799 | GU384512 | GU320502 | |
| CPC 14142; CBS 126001 | X. Zhou | Eucalyptus sp. | Myrtaceae | China | GU253822 | GU269804 | GU384515 | GU320507 | |
| Pseudocercospora fori | CBS 113285; CMW 9095 | G.C. Hunter | Eucalyptus grandis | Myrtaceae | South Africa | DQ204748 | AF468869 | DQ211664 | DQ147618 |
| CPC 14880; CBS 132113 | P.W. Crous | Eucalyptus sp. | Myrtaceae | South Africa | GU253824 | GU269806 | GU384517 | GU320509 | |
| Pseudocercospora fraxinites | CPC 10743; CBS 131927 | H.D. Shin | Fontanesia phillyraeoides | Oleaceae | South Korea | GU253720 | GU269672 | GU384389 | GU320378 |
| MUCC 891 | T. Kobayashi | Fraxinus excelsior | Oleaceae | Japan | GU253748 | GU269710 | GU384426 | GU320414 | |
| Pseudocercospora fukuokaensis | CPC 14689; CBS 132111 | H.D. Shin | Styrax japonicus | Styracaceae | South Korea | GU253750 | GU269713 | GU384429 | GU320417 |
| MUCC 887 | T. Kobayashi | Styrax japonicus | Styracaceae | Japan | GU253751 | GU269714 | GU384430 | GU320418 | |
| Pseudocercospora fuligena | CPC 12296; CBS 132017 | Z. Mersha | Lycopersicon sp. | Solanaceae | Thailand | JQ324953 | GU269711 | GU384427 | GU320415 |
| MUCC 533 | C. Nakashima | Lycopersicon esculentum | Solanaceae | Japan | GU253749 | GU269712 | GU384428 | GU320416 | |
| Pseudocercospora glauca | CPC 10062; CBS 131884 | H.D. Shin | Albizzia julibrissin | Fabaceae | South Korea | GU253752 | GU269715 | GU384431 | GU320419 |
| Pseudocercospora gracilis | CBS 243.94; CPC 730 | P.W. Crous | Eucalyptus urophylla | Myrtaceae | Indonesia | DQ204750 | DQ267582 | DQ211666 | DQ147616 |
| Pseudocercospora griseola f. griseola | CBS 119112; CPC 10460 | F.S. Ngulu & C. Mushi | Phaseolus vulgaris | Fabaceae | Tanzania | GU253753 | GU269717 | GU384433 | GU320421 |
| CBS 194.47 | – | Phaseolus vulgaris | Fabaceae | Portugal | JQ324954 | DQ289801 | JQ324995 | DQ289868 | |
| CBS 880.72 | H.A. van Kesteren | Phaseolus vulgaris | Fabaceae | Netherlands | GU214476 | GU269716 | GU384432 | GU320420 | |
| CPC 10462 | M.M. Liebenberg | Phaseolus vulgaris | Fabaceae | South Africa | GU253865 | GU269849 | GU384562 | GU320553 | |
| CPC 10480; CBS 131887 | M.M. Liebenberg | Phaseolus vulgaris | Fabaceae | South Africa | GU253864 | GU269848 | GU384561 | DQ289882 | |
| CPC 10779; CBS 131929 | H.D. Shin | Phaseolus vulgaris | Fabaceae | South Korea | GU253862 | GU269846 | GU384559 | DQ289885 | |
| CPC 12239 | G. Mahuku | Phaseolus vulgaris | Fabaceae | Colombia | GU253863 | GU269847 | GU384560 | DQ289887 | |
| Pseudocercospora guianensis | MUCC 855 | C. Nakashima & T. Akashi | Lantana camara | Verbenaceae | Japan | GU253755 | GU269719 | GU384435 | GU320423 |
| MUCC 879 | C. Nakashima | Lantana camara | Verbenaceae | Japan | GU253756 | GU269720 | GU384436 | GU320424 | |
| Pseudocercospora haiweiensis | CPC 14084; CBS 131584 | X. Zhou | Eucalyptus sp. | Myrtaceae | China | GU253821 | GU269803 | GU384514 | GU320506 |
| Pseudocercospora hakeae | CBS 112226; CPC 3145 | P.W. Crous & B. Summerell | Grevillea sp. | Proteaceae | Australia | GU253805 | GU269784 | GU384495 | JQ325017 |
| Pseudocercospora humuli | MUCC 742 | C. Nakashima & I. Araki | Humulus lupulus var. lupulus | Cannabaceae | Japan | GU253758 | GU269725 | GU384439 | GU320428 |
| Pseudocercospora humulicola | CPC 10049; CBS 131883 | H.D. Shin | Humulus scandens | Cannabaceae | South Korea | JQ324955 | GU269724 | JQ324996 | JQ325018 |
| CPC 11358; CBS 131585 | H.D. Shin | Humulus scandens | Cannabaceae | South Korea | JQ324956 | GU269723 | GU384438 | GU320427 | |
| Pseudocercospora indonesiana | CBS 122473 | I.W. Buddenhagen | Musa sp. | Musaceae | Sumatra | GU253765 | GU269735 | GU384448 | GU320437/EU514340 |
| CBS 122474 | I.W. Buddenhagen | Musa sp. | Musaceae | Indonesia | JQ324957 | EU514283 | JQ324997 | JQ325019 | |
| Pseudocercospora ixorae | CBS 118760 | R. Kirschner | Ixora sp. | Rubiaceae | Taiwan | GU253759 | GU269726 | GU384440 | GU320429 |
| Pseudocercospora jussiaeae | CPC 14625; CBS 132117 | H.D. Shin | Ludwigia prostrata | Onagraceae South | Korea | JQ324958 | JQ324977 | JQ324998 | JQ325020 |
| Pseudocercospora kaki | MUCC 900 | S. Uematsu & C. Nakashima | Diospyros kaki | Ebenaceae | Japan | GU253761 | GU269729 | GU384442 | GU320431 |
| Pseudocercospora kiggelariae | CPC 11853; CBS 132016 | W. Gams | Kiggelaria africana | Flacourtiaceae | South Africa | GU253762 | GU269730 | GU384443 | GU320432 |
| Pseudocercospora latens | MUCC 763 | C. Nakashima & T. Akashi | Lespedeza wilfordii | Fabaceae | Japan | GU253763 | GU269732 | GU384445 | GU320434 |
| Pseudocercospora leucadendri | CPC 1869 | S. Denman & P.W. Crous | Leucadendron sp. | Proteaceae | South Africa | GU214480 | GU269842 | GU384555 | GU320545 |
| Pseudocercospora libertiae | CBS 114643 | C.F. Hill | Libertia ixioides | Iridaceae | New Zealand | JQ324959 | GU269733 | GU384446 | GU320435 |
| Pseudocercospora lilacis | CPC 12767; CBS 132031 | C. Hodges | Ligustrum japonicum | Oleaceae | USA | GU253767 | GU269737 | GU384449 | GU320439 |
| Pseudocercospora longispora | CBS 122470 | D.R. Jones | Musa sp. | Musaceae | Malaysia | GU253764 | GU269734 | GU384447 | GU320436/EU514342 |
| Pseudocercospora lonicericola | MUCC 889 | T. Kobayashi | Lonicera gracilipes var. glabra | Caprifoliaceae | Japan | GU253766 | GU269736 | JQ324999 | GU320438 |
| Pseudocercospora luzardii | CPC 2556 | A.C. Alfenas | Hancornia speciosa | Apocynaceae | Brazil | GU214477 | AF362057/GU269738 | GU384450 | GU320440 |
| Pseudocercospora lyoniae | MUCC 910 | C. Nakashima & E. Imaizumi | Lyonia ovalifolia var. elliptica | Ericaceae | Japan | GU253768 | GU269739 | GU384451 | GU320441 |
| Pseudocercospora lythracearum | CPC 10707; CBS 131925 | H.D. Shin | Lagerstroemia indica | Lythraceae | South Korea | GU253769 | GU269740 | GU384452 | GU320442 |
| MUCC 890 | T. Kobayashi | Lagerstroemia indica | Lythraceae | Japan | GU253770 | GU269741 | GU384453 | GU320443 | |
| Pseudocercospora lythri | CPC 14588; CBS 132115 | H.D. Shin | Lythrum salicaria | Lythraceae | South Korea | GU253771 | GU269742 | GU384454 | GU320444 |
| MUCC 865 | I. Araki & M. Harada | Lythrum salicaria | Lythraceae | Japan | GU253772 | GU269743 | GU384455 | GU320445 | |
| Pseudocercospora macrospora | CBS 114696; CPC 2553 | P.W. Crous & R.L. Benchimol | Bertholletia excelsa | Lecythidaceae | Brazil | GU214478 | AF362055/GU269745 | GU384457 | GU320447 |
| Pseudocercospora mali | MUCC 886 | T. Kobayashi | Malus sieboldii | Rosaceae | Japan | GU253773 | GU269744 | GU384456 | GU320446 |
| Pseudocercospora marginalis | CPC 12497; CBS 131582 | H.D. Shin | Fraxinus rhynchophylla | Oleaceae | South Korea | GU253812 | GU269794 | GU384504 | GU320495 |
| Pseudocercospora melicyti | CBS 115023 | M. Fletcher | Melicytus macrophyllus | Violaceae | New Zealand | JQ324968 | GU269769 | GU384481 | GU320472 |
| Pseudocercospora metrosideri | CBS 118795 | C.F. Hill | Metrosideros collina | Myrtaceae | New Zealand | GU253774 | GU269746 | GU384458 | GU320448 |
| Pseudocercospora musae | CBS 116634 | J. Carlier | Musa sp. | Musaceae | Cuba | GU253775 | GU269747 | GU384459 | GU320449 |
| Pseudocercospora myrticola | MUCC 632 | C. Nakashima & K. Motohashi | Myrtus communis | Myrtaceae | Japan | GU253777 | GU269749 | GU384460 | GU320451 |
| Pseudocercospora nandinae | CBS 117745 | C.F. Hill | Nandina domestica | Berberidaceae | New Zealand | GU253778 | GU269750 | GU384461 | GU320452 |
| Pseudocercospora natalensis | CBS 111069; CPC 1263 | T. Coutinho | Eucalyptus nitens | Myrtaceae | South Africa | DQ267576 | DQ303077 | JQ325000 | DQ147620 |
| CBS 111071; CPC 1265 | T. Coutinho | Eucalyptus nitens | Myrtaceae | South Africa | GU253801 | GU269780 | GU384491 | GU320483 | |
| Pseudocercospora nephrolepidis | CBS 119121 | R. Kirschner | Nephrolepis auriculata | Oleandraceae | Taiwan | GU253779 | GU269751 | GU384462 | GU320453 |
| Pseudocercospora nogalesii | CBS 115022 | C.F. Hill | Chamaecytisus proliferus | Fabaceae | New Zealand | JQ324960 | GU269752 | GU384463 | GU320454 |
| Pseudocercospora norchiensis | CBS 114641 | C.F. Hill | Rubus sp. | Rosaceae | New Zealand | GU253794 | GU269772 | GU384484 | GU320475 |
| CBS 120738; CPC 13049 | W. Gams | Eucalyptus sp. | Myrtaceae | Italy | GU253780 | EF394859/GU269753 | GU384464 | GU320455 | |
| Pseudocercospora ocimi-basilici | CPC 10283 | M.E. Palm | Ocimum basilicum | Lamiaceae | Mexico | GU214678 | GU269754 | GU384465 | GU320456 |
| Pseudocercospora oenotherae | CPC 10290; CBS 131885 | H.D. Shin | Oenothera odorata | Onagraceae | South Korea | JQ324961 | GU269856 | GU384567 | GU320559 |
| CPC 10630; CBS 131920 | H.D. Shin | Oenothera odorata | Onagraceae | South Korea | GU253781 | GU269755 | GU384466 | GU320457 | |
| Pseudocercospora paederiae | CPC 10007 | H.D. Shin | Paederia foetida | Rubiaceae | South Korea | GU253783 | GU269757 | GU384468 | – |
| Pseudocercospora palleobrunnea | CBS 124771; CPC 13387 | P.W. Crous | Syzygium sp. | Myrtaceae | Australia | GQ303319 | GQ303288 | GU384509 | GU320500 |
| Pseudocercospora pallida | CPC 10776; CBS 131889 | H.D. Shin | Campsis grandiflora | Bignoniaceae | South Korea | GU214680 | GU269758 | GU384469 | GU320459 |
| Pseudocercospora pancratii | CBS 137.94 | R.F. Castaneda | – | – | Cuba | GU253784 | GU269759 | GU384470 | GU320460 |
| Pseudocercospora paraguayensis | CBS 111286; CPC 1459 | P.W. Crous | Eucalyptus nitens | Myrtaceae | Brazil | GU214479/DQ204764 | DQ267602 | DQ211680 | DQ147606 |
| CBS 111317; CPC 1458 | P.W. Crous | Eucalyptus nitens | Myrtaceae | Brazil | GQ852634 | JQ324978 | GU384522 | JQ325021 | |
| Pseudocercospora pini-densiflorae | MUCC 534 | Y. Tokushige | Pinus thunbergii | Pinaceae | Japan | GU253785 | GU269760 | GU384471 | GU320461 |
| Pseudocercospora plecthranthi | CPC 11462; CBS 131586 | H.D. Shin | Plectranthus sp. | Lamiaceae | South Korea | JQ324962 | GU269791 | GU384501 | GU320492 |
| Pseudocercospora pouzolziae | CBS 122280 | R. Kirschner | Gonostegia hirta | Urticaceae | Taiwan | GU253786 | GU269761 | GU384472 | GU320462 |
| Pseudocercospora profusa | CPC 10042 | H.D. Shin | Acalypha australis | Euphorbiaceae | South Korea | GU253808 | GU269787 | GU384497 | GU320488 |
| CPC 10055; CBS 132306 | H.D. Shin | Acalypha australis | Euphorbiaceae | South Korea | GU253787 | GU269762 | GU384473 | GU320463 | |
| Pseudocercospora proteae | CPC 15217; CBS 131587 | F. Roets | Protea mundii | Proteaceae | South Africa | GU253826 | GU269808 | GU384519 | GU320511 |
| Pseudocercospora prunicula | CPC 14511; CBS 132107 | H.D. Shin | Prunus × yedoensis | Rosaceae | South Korea | GU253723 | GU269676 | GU384393 | GU320382 |
| Pseudocercospora pseudostigmina-platani | CPC 11726; CBS 131588 | H.D. Shin | Platanus occidentalis | Platanaceae | South Korea | JQ324963 | GU269857 | GU384568 | GU320560 |
| Pseudocercospora puderi | MUCC 906 | S. Maruyama | Rosa sp. | Rosaceae | Japan | GU253790 | GU269764 | GU384476 | GU320467 |
| Pseudocercospora punctata | CPC 14734; CBS 132116 | P.W. Crous | Syzygium sp. | Myrtaceae | Madagascar | GU253791 | GU269765 | GU384477 | GU320468 |
| Pseudocercospora purpurea | CBS 114163; CPC 1664 | P.W. Crous | Persea americana | Lauraceae | Mexico | GU253804 | GU269783 | GU384494 | GU320486 |
| Pseudocercospora pyracanthae | MUCC 892 | T. Kobayashi & C. Nakashima | Pyracantha angustifolia | Rosaceae | Japan | GU253792 | GU269767 | GU384479 | GU320470 |
| Pseudocercospora pyracanthigena | CPC 10808; CBS 131589 | H.D. Shin | Pyracantha angustifolia | Rosaceae | South Korea | – | GU269766 | GU384478 | GU320469 |
| Pseudocercospora ranjita | CPC 11141; CBS 126005 | M.J. Wingfield | Gmelina sp. | Verbenaceae | Indonesia | GU253810 | GU269790 | GU384500 | GU320491 |
| Pseudocercospora ravenalicola | CBS 122468 | M. Arzanlou & W. Gams | Ravenala madagascariensis | Strelitziaceae | India | GU253828 | GU269810 | GU384521 | GU320513 |
| Pseudocercospora rhabdothamni | CBS 114872 | M. Fletcher | Rhabdothamnus solandri | Gesneriaceae | New Zealand | JQ324964 | GU269768 | GU384480 | GU320471 |
| Pseudocercospora rhamnellae | CPC 12500; CBS 131590 | H.D. Shin | Rhamnella frangulioides | Rhamnaceae | South Korea | GU253813 | GU269795 | GU384505 | GU320496 |
| Pseudocercospora rhapisicola | CBS 282.66 | K. Tubaki | Rhapis flabellifornis | Arecaceae | Japan | GU253793 | GU269770 | GU384482 | GU320473 |
| Pseudocercospora rhododendri-indici | CPC 10822; CBS 131591 | H.D. Shin | Rhododendron indicum | Ericaceae | South Korea | JQ324965 | GU269722 | – | GU320426 |
| Pseudocercospora rhoina | CPC 11464; CBS 131891 | H.D. Shin | Rhus chinensis | Anacardiaceae | South Korea | JQ324966 | GU269771 | GU384483 | GU320474 |
| Pseudocercospora robusta | CBS 111175; CPC 1269; CMW 5151 | M.J. Wingfield | Eucalyptus robur | Myrtaceae | Malaysia | DQ204767 | AY309597 | DQ211683 | DQ147617 |
| Pseudocercospora rubi | MUCC 875 | T. Kobayashi & C. Nakashima | Rubus allegheniensis | Rosaceae | Japan | GU253795 | GU269773 | GU384485 | GU320476 |
| Pseudocercospora rumohrae | CBS 117747 | C.F. Hill | Marattia salicina | Marattiaceae | New Zealand | GU253796 | GU269774 | GU384486 | GU320477 |
| Pseudocercospora sambucigena | CPC 10292; CBS 131886 | H.D. Shin | Sambucus williamsii | Caprifoliaceae | South Korea | GU253809 | GU269788 | GU384498 | GU320489 |
| CPC 14397; CBS 126000 | P.W. Crous | Sambucus nigra | Caprifoliaceae | Netherlands | GU253823 | GU269805 | GU384516 | GU320508 | |
| Pseudocercospora sawadae | CBS 115024 | C.F. Hill | Psidium guajava | Myrtaceae | New Zealand | JQ324967 | GU269775 | – | GU320478 |
| Pseudocercospora securinegae | CPC 10793; CBS 131930 | H.D. Shin | Flueggea suffruticosa | Euphorbiaceae | South Korea | GU253797 | GU269776 | GU384487 | GU320479 |
| Pseudocercospora snelliana | CPC 11654; CBS 131592 | H.D. Shin | Morus bombycis | Moraceae | South Korea | – | GU269731 | GU384444 | GU320433 |
| Pseudocercospora sordida | MUCC 913 | C. Nakashima & E. Imaizumi | Campsis radicans | Bignoniaceae | Japan | GU253798 | GU269777 | GU384488 | GU320480 |
| Pseudocercospora sp. | CBS 110993; CPC 1057 | M.J. Wingfield | Populus sp. | Salicaceae | South Africa | GU253800 | GU269779 | GU384490 | GU320482 |
| CBS 110998; CPC 1054 | M.J. Wingfield | Eucalyptus grandis | Myrtaceae | South Africa | GU253799 | GU269778 | GU384489 | GU320481 | |
| CBS 111373; CPC 1493 | M.J. Wingfield | Eucalyptus globulus | Myrtaceae | Uruguay | GU253803 | GU269782 | GU384493 | GU320485 | |
| CBS 112725; CPC 3961 | K.A. Seifert | Melilotus alba | Fabaceae | Canada | GU253806 | GU269785 | – | – | |
| CBS 113387 | A. den Breeyen | Lantana camara | Verbenaceae | Jamaica | GU253754 | GU269718 | GU384434 | GU320422 | |
| CPC 10058 | H.D. Shin | Potentilla kleiniana | Rosaceae | South Korea | – | JQ324979 | JQ325001 | JQ325022 | |
| CPC 10645; CBS 131922 | P.W. Crous | – | – | Brazil | GU253700 | GU269651 | GU384369 | GU320359 | |
| CPC 14711; CBS 132102 | H.D. Shin | Pyracantha angustifolia | Rosaceae | South Korea | – | JQ324980 | JQ325002 | JQ325023 | |
| CPC 15116; NC1 37A1a | J. Batzer | Malus sp. cv. Golden Delicious | Rosaceae | USA: North Carolina | JQ324969 | JQ324981 | JQ325003 | JQ325024 | |
| Pseudocercospora stahlii | CBS 117549 | R. Kirschner | Passiflora foetida | Passifloraceae | Taiwan | GU253830 | GU269813 | GU384525 | GU320515 |
| Pseudocercospora stephanandrae | MUCC 914 | C. Nakashima & E. Imaizumi | Stephanandra incisa | Rosaceae | Japan | GU253831 | GU269814 | GU384526 | GU320516 |
| Pseudocercospora subsessilis | CBS 136.94 | R.F. Castaneda | – | – | Cuba | GU253832 | GU269815 | GU384527 | GU320517 |
| Pseudocercospora subtorulosa | CBS 117230 | R. Kirschner | Melicope sp. | Rutaceae | Taiwan | GU253833 | GU269816 | GU384528 | GU320518 |
| Pseudocercospora subulata | CBS 118489; CPC 10849 | M. Dick | Eucalyptus botryoides | Myrtaceae | New Zealand | JQ324970 | DQ303090 | JQ325004 | GU320519 |
| Pseudocercospora tereticornis | CBS 124996; CPC 12960 | A.J. Carnegie | Eucalyptus nitens | Myrtaceae | Australia | GQ852647 | JQ324982 | GU384377 | JQ325025 |
| CPC 13299; CBS 125214 | P.W. Crous | Eucalyptus tereticornis | Myrtaceae | Australia | GQ852649 | GQ852770 | GU384508 | GU320499 | |
| “Pseudocercospora” thailandica | CBS 116367; CPC 10547 | K. Pongpanich | Acacia mangium | Fabaceae | Thailand | GU253837 | – | DQ835102/GU384533 | GU320523/AY752217 |
| CPC 10548; CBS 116367 | K. Pongpanich | Acacia mangium | Fabaceae | Thailand | GU253853 | AY752157 | AY840477 | GU320539 | |
| Pseudocercospora theae | CBS 128.30 | M. Curzi | Camelia sinensis | Theaceae | Italy | GU253838 | GU269821 | GU384534 | GU320524 |
| “Pseudocercospora” tibouchinigena | CBS 116462 | C.F. Hill | Tibouchina sp. | Melastomataceae | New Zealand | GU253839 | GU269822 | GU384535 | GU320525 |
| Pseudocercospora timorensis | MUCC 819 | C. Nakashima & T. Akashi | Ipomoea indica | Convolvulaceae | Japan | GU253840 | GU269823 | GU384536 | GU320526 |
| Pseudocercospora udagawana | CPC 10799; CBS 131931 | H.D. Shin | Hovenia dulcis | Rhamnaceae | South Korea | – | GU269824 | GU384537 | GU320527 |
| Pseudocercospora variicolor | MUCC 746 | C. Nakashima & I. Araki | Paeonia lactiflora var. trichocarpa | Paeoniaceae | Japan | GU253843 | GU269826 | GU384538 | GU320530 |
| Pseudocercospora viburnigena | CPC 15249; CBS 125998 | M.L. Crous | Viburnum davidii | Caprifoliaceae | Netherlands | GU253827 | GU269809 | GU384520 | GU320512 |
| Pseudocercospora viticicola | MUCC 777 | C. Nakashima | Vitex trifolia | Verbenaceae | Japan | GU253845 | GU269828 | GU384540 | GU320532 |
| Pseudocercospora vitis | CPC 11595; CBS 132012 | H.D. Shin | Vitis vinifera | Vitaceae | South Korea | GU214483 | DQ289829/GU269829 | GU384541 | GU320533 |
| CPC 14661; CBS 132112 | H.D. Shin | Vitis vinifera | Vitaceae | South Korea | GU253844 | GU269827 | GU384539 | GU320531 | |
| Pseudocercospora weigelae | MUCC 899 | T. Kobayashi & Y. Kobayashi | Weigela coraeensis | Caprifoliaceae | Japan | GU253847 | GU269831 | GU384543 | GU320535 |
| Pseudocercospora xanthocercidis | CPC 11665; CBS 131593 | A.R. Wood | Xanthocercis zambesiaca | Fabaceae | South Africa | JQ324971 | JQ324983 | JQ325005 | JQ325026 |
| Pseudocercospora xanthoxyli | CPC 10065 | H.D. Shin | Xanthoxylum ailanthoides | Rutaceae | South Korea | GU253848 | GU269832 | GU384544 | GU320536 |
| Pseudocercospora zelkovae | CPC 14484; CBS 132106 | H.D. Shin | Zelkova serrata | Ulmaceae | South Korea | GU253849 | GU269833 | GU384545 | JQ325027 |
| CPC 14717; CBS 132118 | H.D. Shin | Zelkova serrata | Ulmaceae | South Korea | GU253850 | GU269834 | GU384546 | JQ325028 | |
| MUCC 872 | T. Kobayashi & C. Nakashima | Zelkova serrata | Ulmaceae | Japan | GU253851 | GU269835 | GU384547 | GU320537 | |
| Pseudocercosporella arcuata | CPC 10050 | H.D. Shin | Rubus oldhamii | Rosaceae | South Korea | GU214685 | GU269850 | JQ325006 | GU320554 |
| Pseudocercosporella capsellae | CPC 14773; CBS 131896 | H.D. Shin | Raphanus sativus | Brassicaceae | South Korea | GU253714 | GU269666 | GU384383 | GU320372 |
| Pseudocercosporella chaenomelis | CPC 14795; CBS 131897 | H.D. Shin | Chaenomeles speciosa | Rosaceae | South Korea | GU253834 | GU269817 | GU384530 | GU320520 |
| MUCC 1510; CBS 132131 | C. Nakashima | Chaenomeles sinensis | Rosaceae | Japan | – | JQ793663 | – | JQ793664 | |
| Pseudocercosporella fraxini | CPC 11509 | H.D. Shin | Fraxinus rhynchophylla | Oleaceae | South Korea | GU214682 | GU269709 | GU384425 | GU320413 |
| Pseudocercosporella koreana | CPC 11414 | H.D. Shin | Vicia amurensis | Fabaceae | South Korea | GU214683 | GU269852 | GU384564 | GU320556 |
| Pseudocercosporella oxalidis | CBS 118758 | R. Kirschner | Oxalis debilis | Oxalidaceae | Taiwan | GU253782 | GU269756 | GU384467 | GU320458 |
| Pseudocercosporella sp. | CPC 10864; CBS 131890 | H.D. Shin | Trigonotis peduncularis | Boraginaceae | South Korea | JQ324972 | GU269858 | GU384569 | JQ325029 |
| Pseudocercosporella zelkovae | CPC 11592; CBS 132011 | H.D. Shin | Zelkova serrata | Ulmaceae | South Korea | GU214482 | GU269853 | – | GU320557 |
| Scolecostigmina mangiferae | CBS 125467; CPC 17351 | P.W. Crous | Mangifera indica | Anacardiaceae | Australia | GU253877 | GU269870 | GU384578 | GU320566 |
| CPC 17352; CBS 125467 | P.W. Crous | Mangifera indica | Anacardiaceae | Australia | GU253878 | GU269871 | GU384579 | GU320567 | |
| Septoria cerastii | CPC 12343; CBS 132028 | H.D. Shin | Cerastium holosteoides var. hallasanense | Caryophyllaceae | South Korea | GU253869 | GU269859 | GU384570 | JQ325030 |
| Septoria chelidonii | CPC 12337; CBS 132027 | H.D. Shin | Chelidonium majus var. asiaticum | Papaveraceae | South Korea | GU253870 | GU269860 | GU384571 | GU320561 |
| Septoria crepidis | CPC 12539; CBS 131895 | H.D. Shin | Crepis japonica | Asteraceae | South Korea | GU253871 | GU269861 | GU384572 | GU320562 |
| Septoria dysentericae | CPC 12328; CBS 131892 | H.D. Shin | Inula britannica var. chinensis | Asteraceae | South Korea | GU253866 | GU269854 | GU384565 | GU320558 |
| Septoria erigerontis | CPC 12340; CBS 131893 | H.D. Shin | Erigeron annuus | Asteraceae | South Korea | GU253872 | GU269862 | GU384573 | JQ325031 |
| Septoria eucalyptorum | CPC 11282; CBS 118505 | W. Gams | Eucalyptus sp. | Myrtaceae | India | GU253873 | GU269863 | GU384574 | GU320563 |
| Septoria justiciae | CPC 12509; CBS 131894 | H.D. Shin | Justicia procumbens | Acanthaceae | South Korea | GU253874 | GU269864 | GU384575 | GU320564 |
| Septoria quercicola | CBS 663.94 | H.A. van der Aa | Quercus robur | Fagaceae | Netherlands | GU253867 | GU269855 | GU384566 | JQ325032 |
| Septoria rubi | CPC 12331; CBS 132022 | H.D. Shin | Rubus crataegifolius | Rosaceae | South Korea | GU253875 | GU269865 | GU384576 | – |
| Stigmina platani | CBS 336.33 | R.M. Nattrass | Platanus orientalis | Platanaceae | India | GU253868 | – | JQ325007 | – |
| Strelitziana australiensis | CBS 124778; CPC 13421 | P.W. Crous | Eucalyptus sp. | Myrtaceae | Australia | GQ303326 | GQ303295 | GU384362 | – |
| CPC 13556; CBS 132310 | P.W. Crous | Eucalyptus sp. | Myrtaceae | Australia | GU253695 | GU269645 | GU384363 | GU320354 | |
| Teratosphaeria alcornii | CBS 313.76; CPC 3632 | J.L. Alcorn | Eucalyptus tessellaris | Myrtaceae | Australia | GU253876 | GU269866 | GU384577 | GU320565 |
| Teratosphaeria dimorpha | CPC 14132; CBS 124051 | B.A. Summerell | Eucalyptus caesia | Myrtaceae | Australia | FJ493215 | FJ023537 | – | – |
| Teratosphaeria stellenboschiana | CBS 124989; CPC 13767 | P.W. Crous | Eucalyptus punctata | Myrtaceae | South Africa | GQ852715 | GQ852823 | – | – |
| Thedgonia ligustrina | CPC 10019 | H.D. Shin | Ligustrum ovalifolium | Oleaceae | South Korea | GU253854 | GU269837 | GU384550 | GU320540 |
| CPC 10530; CBS 132130 | P.W. Crous | Ligustrum sp. | Oleaceae | Netherlands | GU253855 | GU269838 | GU384551 | GU320541 | |
| CPC 10861; CBS 132025 | H.D. Shin | Ligustrum ovalifolium | Oleaceae | South Korea | GU253856 | GU269839 | GU384552 | GU320542 | |
| Trochophora fasciculata | CPC 10282 | H.D. Shin | Daphniphyllum macropodum | Daphniphyllaceae | South Korea | FJ839668 | FJ839632 | – | – |
| Trochophora simplex | CBS 124744 | H.D. Shin | Daphniphyllum macropodum | Daphniphyllaceae | South Korea | GU253880 | GU269872 | GU384580 | GU320568 |
| MUCC 952 | C. Nakashima & I. Araki | Daphniphyllum teijsmannii | Daphniphyllaceae | Japan | GU253879 | – | – | – | |
| Xenostigmina zilleri | CBS 115685 | K.A. Seifert | Acer sp. | Aceraceae | Canada | GU253857 | GU269840 | GU384553 | GU320543 |
| CBS 115686 | K.A. Seifert | Acer sp. | Aceraceae | Canada | FJ839676/GU253858 | GU269841 | GU384554 | GU320544 | |
| Zasmidium nabiacense | CBS 125010; CPC 12748 | A.J. Carnegie | Eucalyptus sp. | Myrtaceae | Australia | GQ852734 | GQ852841 | GU384507 | GU320498 |
CBS: CBS-KNAW Fungal Biodiversity Centre, Utrecht, The Netherlands; CIRAD: Centre de Coopération Internationale en Recherche Agronomique pour le Développement, UMR-BGPI, Montpellier, France; CMW: Culture Collection of the Forestry and Agricultural Biotechnology Institute (FABI) of the University of Pretoria, Pretoria, South Africa; CPC: Culture collection of Pedro Crous, housed at CBS; MUCC: Culture Collection, Laboratory of Plant Pathology, Mie University, Tsu, Mie Prefecture, Japan.
LSU: partial 28S nrRNA gene; ITS: internal transcribed spacer regions 1 & 2 including 5.8S nrRNA gene; EF-1α: partial translation elongation factor 1-alpha gene; ACT: partial actin gene.
Sequence for this locus obtained from: http://genome.jgi-psf.org/Mycfi1/Mycfi1.home.html
DNA isolation
Mycelium from actively growing fungal cultures was scraped from the surface of MEA or PDA plates using a sterile scalpel blade. Harvested mycelium was ground to a fine powder using liquid nitrogen and DNA was isolated using the CTAB extraction protocol as outlined by Crous et al. (2009d) or the UltraClean™ Microbial DNA Isolation Kit (MoBio Laboratories, Inc., Solana Beach, CA, USA) following the manufacturers’ protocols. Isolated DNA was visualised by electrophoresis in 1 % agarose gels (w/v) stained with ethidium bromide and viewed under near ultra-violet light. DNA concentrations were determined by measuring electrophoresed DNA samples against a HyperLadder™ I molecular marker (BIOLINE) or alternatively by a NanoDrop quantification as outlined by the manufacturer.
PCR amplification
DNA isolated from fungal isolates was used as template for further Polymerase Chain Reaction (PCR) amplifications. Four nuclear gene regions were targeted for PCR amplification and subsequent sequencing. These regions included the Internal Transcribed Spacer regions ITS-1, ITS-2 and the 5.8S nrRNA gene regions (ITS), the first 900 bp of the Large Subunit (28S, LSU) (domains D1-D3) of the rDNA operon and partial gene regions of the translation elongation factor 1-alpha (EF-1α) and the actin (ACT) genes.
The ITS region was amplified using primers ITS-1 or ITS-5 and ITS-4 (White et al. 1990) while primers used for amplification of the LSU region were LR0R (Rehner & Samuels 1994) or LSU1Fd (Crous et al. 2009b) and LR5 or LR7 (Vilgalys & Hester 1990). Primers employed for the amplification of EF-1α included EF1-728F and EF1-986R (Carbone & Kohn 1999) or EF-2 (O’Donnell et al. 1998) while ACT-512F and ACT-783R (Carbone & Kohn 1999) were used to amplify a portion of the ACT gene. All PCR reaction mixtures and conditions followed those outlined by Hunter et al. (2006b). Following PCR amplification, amplicons were visualized on 1.5 % agarose gels stained with ethidium bromide and viewed under ultra-violet light and sizes of amplicons were determined against a HyperLadder™ I molecular marker (BIOLINE). The PCR amplicons for the four loci were subsequently diluted 1 to 10 times in preparation for further DNA sequencing reactions.
DNA sequencing and phylogenetic inference
PCR amplicons of the four gene regions targeted in this study served as templates for DNA sequencing reactions with the BigDye® Terminator Cycle Sequencing Kit v. 3.1 (Applied Biosystems Life Technologies, Carlsbad, CA, USA) following the protocol of the manufacturer. DNA sequencing reactions used the same primers as those for the PCR reactions. However, additional internal primers LR3R (http://www.biology.duke.edu/fungi/mycolab/primers.htm), LR16 (Moncalvo et al. 1993) and LR5 were used to sequence the LSU in order to obtain reliable sequences spanning the entire D1-D3 region. DNA sequencing amplicons were purified through Sephadex® G-50 Superfine columns (Sigma Aldrich, St. Louis, MO) in MultiScreen HV plates (Millipore, Billerica, MA). Purified sequence reactions were run on an ABI Prism 3730xl DNA Sequencer (Life Technologies, Carlsbad, CA, USA).
Generated DNA sequence electropherograms were analysed using MEGA (Molecular Evolutionary Genetics Analysis) v. 4.0 (Tamura et al. 2007), 4Peaks v. 1.7.2 (http://www.mekentosj.com/) and SeqMan v. 8.0.2. from the DNASTAR Lasergene® software package. Consensus sequences were generated and imported into MEGA for initial alignment and the construction of sequence datasets. DNA sequences representing isolates of closely allied genera, for which material could not be obtained were downloaded from the NCBI GenBank nucleotide database (www.ncbi.nlm.nih.gov) and added to the DNA sequence datasets generated in this study. Sequence datasets for the four genomic loci were aligned in MAFFT (“Multiple alignment program for amino acids or nucleotide sequences”) v. 6.0 (Katoh & Toh 2006, Katoh et al. 2005; http://mafft.cbrc.jp/alignment/server/index.html) using the Auto alignment strategy with the 200PAM/ K=2 scoring matrix and a gap opening penalty of 1.53 with an offset value of 0.0. Resulting sequence alignments were manually evaluated and adjusted in MEGA, MacClade v.4.08 (Maddison & Maddison 2000) or Sequence Alignment Editor v. 2.0a11 (Rambaut 2002).
A phylogenetic re-construction was conducted for the aligned LSU data set to determine generic relationships using MrBayes v. 3.1.2 (Ronquist & Huelsenbeck 2003). Subsequently, a species level phylogeny was derived from the combined ITS, ACT and EF-1α alignment of Pseudocercospora s. str. sequences using PAUP v. 4.0b10 (Swofford 2003). For the LSU alignment, MrModeltest v. 2.2 (Nylander 2004) was used to determine the best nucleotide substitution model settings for MrBayes. Based on the results of the MrModeltest, a phylogenetic analysis was performed with MrBayes v. 3.1.2 applying a general time-reversible (GTR) substitution model with inverse gamma rates and dirichlet base frequencies and a heating parameter set at 0.3. The Markov Chain Monte Carlo (MCMC) analysis of 4 chains started in parallel from a random tree topology and had 8 000 000 generations. Trees were saved each 1 000 generations, resulting in 8 001 saved trees in each of the two tree files. Burn-in was set at 2 000 000 generations after which the likelihood values were stationary. For parsimony analysis of the combined ITS, ACT and EF-1α alignment, alignment gaps were treated as a fifth character state and all characters were unordered and of equal weight. Maximum parsimony analysis was performed in PAUP using the heuristic search option with 100 random taxon additions and tree bisection and reconnection (TBR) as the branch-swapping algorithm. Branches of zero length were collapsed and all multiple, equally most parsimonious trees were saved. The robustness of the trees was evaluated by 1 000 bootstrap replicates (Hillis & Bull 1993). Tree length (TL), consistency index (CI), retention index (RI) and rescaled consistency index (RC) were calculated and the resulting trees were printed with Geneious v. 5.5.4 (Drummond et al. 2011). Sequences derived in this study were deposited in GenBank (Table 1), the alignments in TreeBASE (www.treebase.org/treebase/index.html), and taxonomic novelties in MycoBank (www.MycoBank.org; Crous et al. 2004b).
Taxonomy
All taxonomic descriptions were based on structures on herbarium material. Diseased leaf tissue was viewed under a Nikon® SMZ1500 stereoscopic zoom microscope and relevant morphological structures were lifted from lesions with a sterile dissecting needle and mounted on glass slides in clear lactic acid. For measurements, 30-50 replicates of all relevant morphological features were made at ×1 000 magnification using a Carl Zeiss® Axioskop 2 plus light microscope. High-resolution photographic images of diseased material, leaf lesions and microscopic fungal structures were captured with a Nikon® digital sight DS-fi1 high definition colour camera mounted on the light microscope or a Nikon® digital sight DS-5M camera mounted on a stereoscopic zoom microscope. Images of morphological structures were captured, and measurements taken, using the Nikon® software NIS-Elements v. 2.34 while Adobe Photoshop was used for the final editing of acquired images and photographic preparations. Novel Pseudocercospora taxa were plated onto MEA and incubated at 24 °C for 2-4 wk in the dark in duplicate. The mycological colour charts of Rayner (1970) were used to define colours of the fungal colonies.
RESULTS
DNA sequencing and phylogenetic analyses
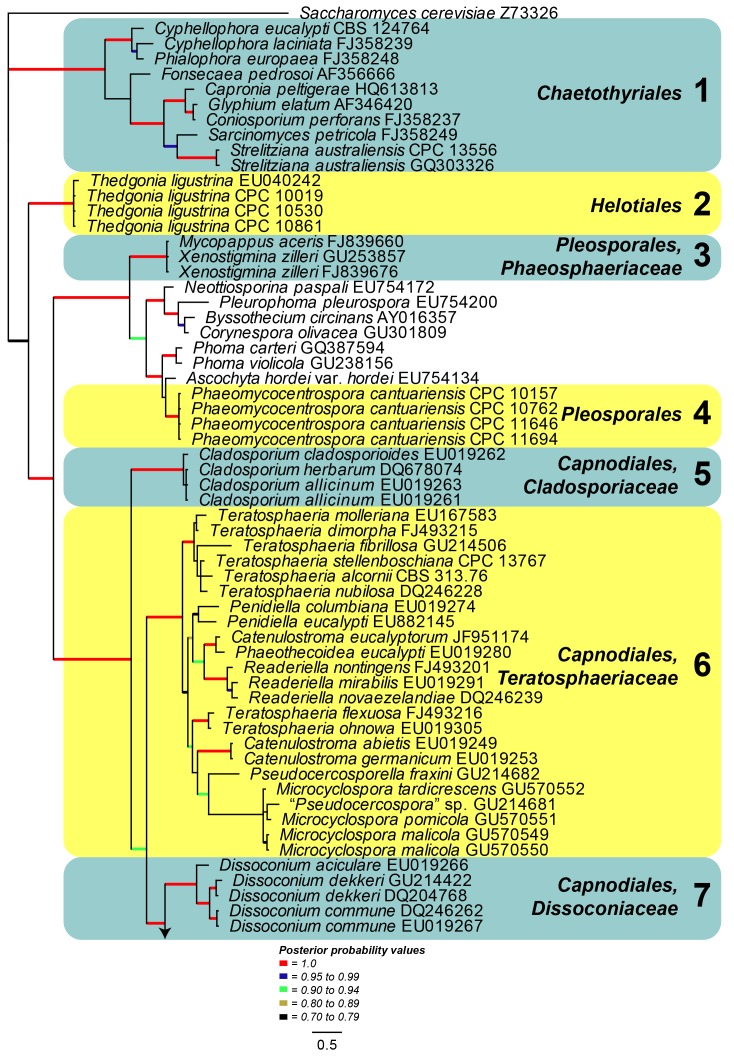
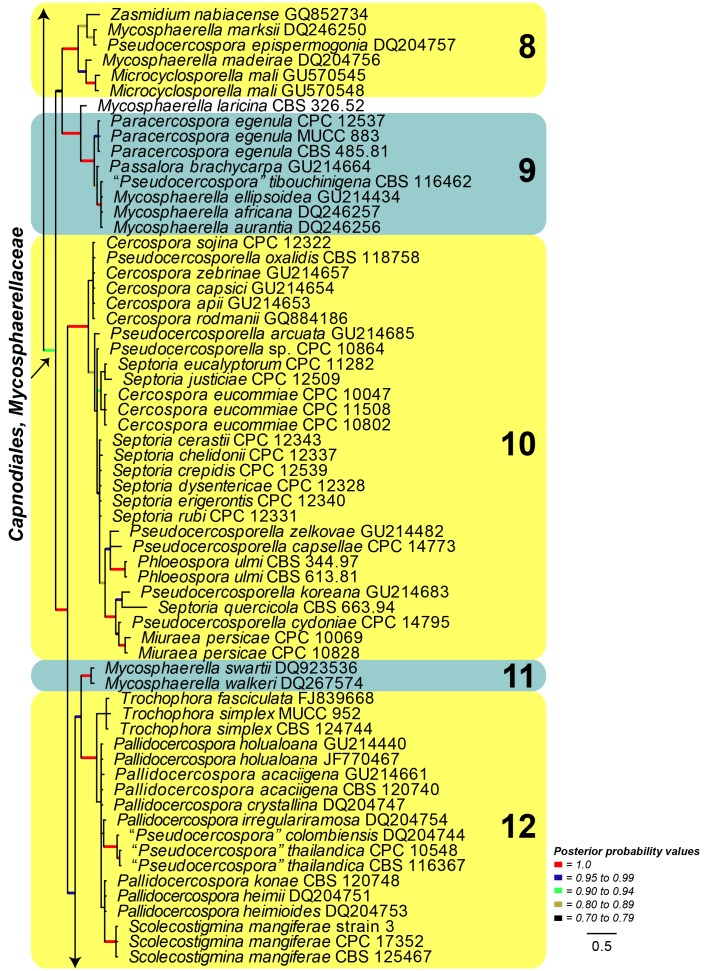
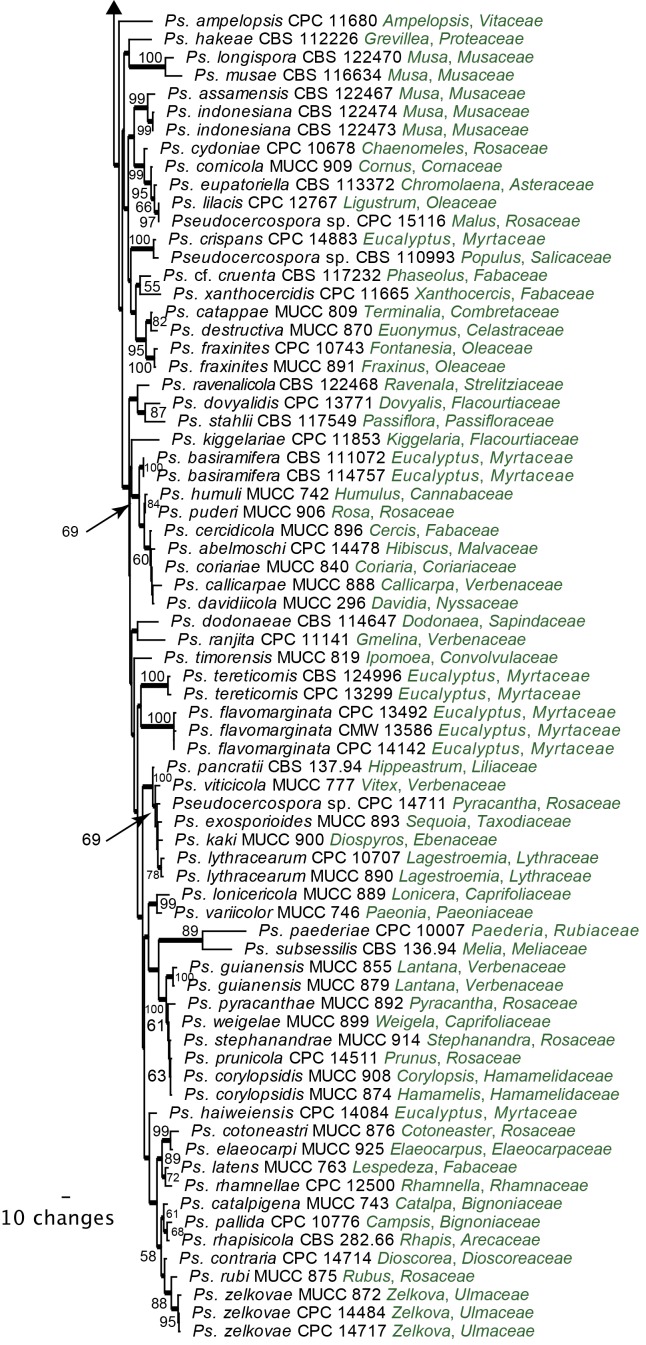
Large Subunit (LSU) phylogeny: The final aligned LSU dataset contained 316 ingroup taxa with a total of 1305 characters and Saccharomyces cerevisiae (GenBank Accession: Z73326) served as the outgroup taxon. From this alignment 827 characters were used for the Bayesian analysis; the consensus trees and posterior probabilities were calculated (Fig. 4) from the 12 002 trees left after discarding those used for burn-in. The resulting LSU phylogeny resolved several clades (Clades 1-14) grouping species of Pseudocercospora and allied genera (Fig. 4). Clade 1 (Posterior Probability (PP) value of 1.0) including Cyphellophora and Strelitziana represented by one of the two basal lineages. Thedgonia ligustrina (100 %) represented the second basal clade (PP = 1.0). In the Pleosporales, Clade 3 included Xenostigmina zilleri (PP = 1.0) and Clade 4 Pseudocercospora cantuariensis (PP = 1.0), the latter being described below as Phaeomycocentrospora cantuariensis. Clade 5 contained Cladosporium species belonging to the teleomorph genus Davidiella (PP = 1.0). Clade 6 (PP = 1.0) represented species belonging to Teratosphaeria and including the recently established genus Microcyclospora. Clade 7 (PP = 1.0) accommodated species of Dissoconium. Clade 8 (PP = 1.0) including species representing Mycosphaerella, Pseudocercospora and Zasmidium, as well as the recently established genus Microcyclosporella. Clade 9 (PP = 1.0) included Pseudocercospora tibouchinigena, Pseudocercospora egenula described below as Paracercospora egenula and the Mycosphaerella ellipsoidea complex. Clade 10 (PP = 1.0) accommodated species of other genera namely Pseudocercosporella, Mycosphaerella ulmi (Phleospora), Muiraea, Cercospora and Septoria. Clade 11 (PP = 1.0) included Mycosphaerella species with Sonderhenia anamorphs. Clade 12 (PP = 1.0) is sister to Clade 11 and included species representing taxa of Mycosphaerella and their associated pseudocercospora-like anamorphs, appeared to represent a novel genus. Other genera in this clade included Scolecostigmina and Trochophora. The isolates representing Trochophora are accommodated at a basal position in this clade with no PP support. The three isolates of Scolecostigmina mangiferae resided in a well-supported sub-clade (PP = 1.0) close to isolates regarded as part of the Mycosphaerella heimii complex (P. acaciigena, M. irregulariramosa, M. colombiensis, P. thailandica, M. heimii, M. heimioides, M. konae), described below in Pallidocercospora. Clade 13 (PP = 1.0) accommodated Passalora eucalypti. The remainder of the phylogeny encompassed Clade 14 (PP = 1.0), representing Pseudocercospora s. str., and accommodated the majority of Pseudocercospora species from many different hosts. The type species of Pseudocercospora, P. vitis was included in this clade. Interestingly, P. vitis was basal in this clade with the majority of Pseudocercospora species radiating out from the basal Pseudocercospora isolates. The LSU phylogeny provided a well-supported sub-clade (PP = 1.0) representing the second half of the sensu stricto clade (Clade 14). Several isolates representing species from genera morphologically allied to Pseudocercospora were also grouped in Clade 14. These included Stigmina platani, Cercostigmina protearum var. leucadendri (as Pseudocercospora leucadendri, see below), Cercostigmina protearum var. hakeae (as Pseudocercospora hakea, see below), Phaeoisariopsis griseola f. griseola (as Pseudocercospora griseola f. griseola, see Crous et al. 2006) and Pseudophaeoramularia angolensis (as Pseudocercospora angolensis, see below), which supports previous proposals to include these genera in Pseudocercospora s. str.
Fig. 4.
Consensus phylogram (50 % majority rule) of 12 002 trees resulting from a Bayesian analysis of the LSU sequence alignment using MrBayes v. 3.1.2. Bayesian posterior probabilities are indicated with colour-coded branches (see legend) and the scale bar represents the expected changes per site. Important clades are indicated in coloured blocks and numbered. The tree was rooted to Saccharomyces cerevisiae (GenBank Z73326).
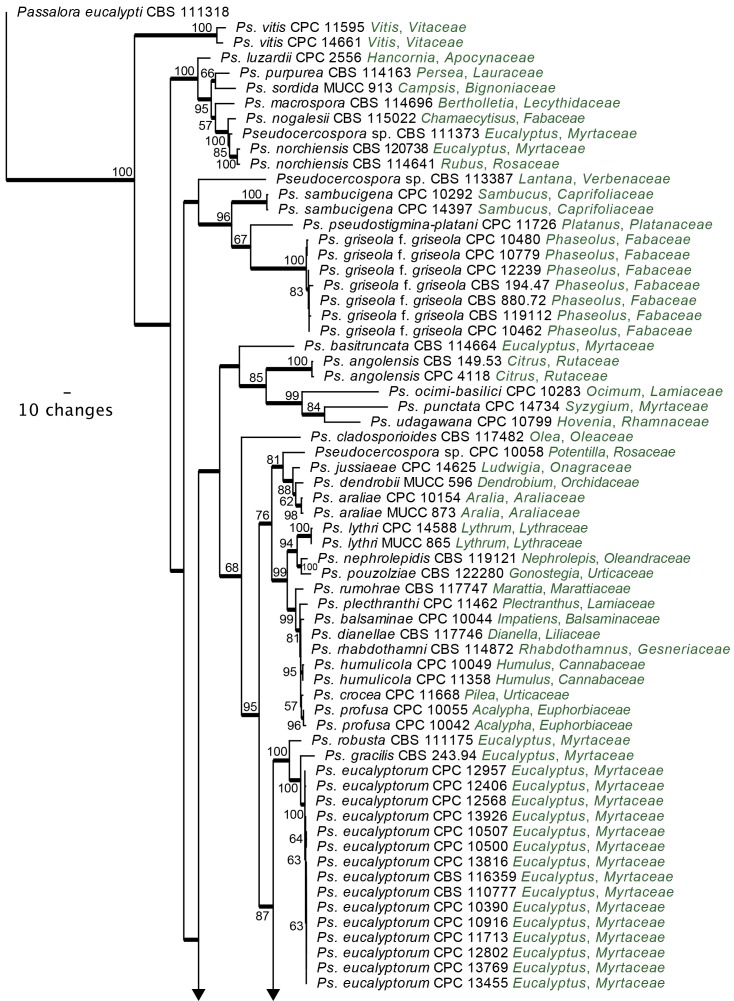
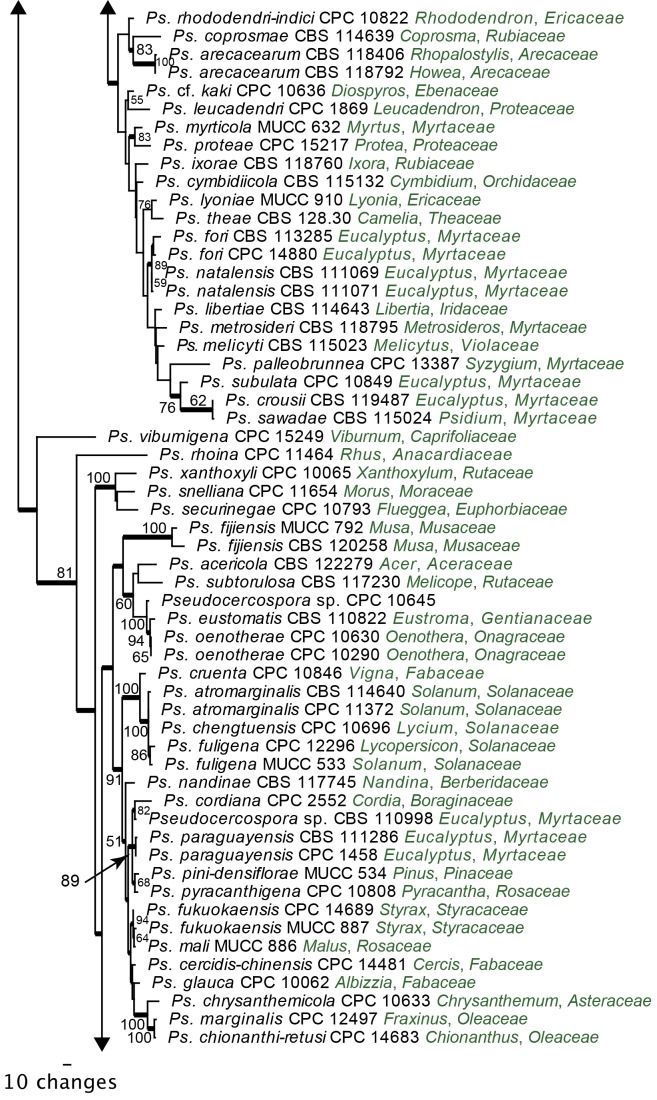
Pseudocercospora s. str. phylogeny: A further analysis was conducted on Clade 14 (Fig. 4), representing Pseudocercospora s. str. For this analysis, DNA sequence data from the ITS, ACT and EF-1α gene regions were combined in the parsimony analysis. For this dataset, there was a total of 194 taxa, each representing 1 029 characters. Passalora eucalypti (CBS 111318) served as the outgroup taxon for this analysis. From the combined alignment of 1 029 characters, 414 were constant, 124 were variable and 491 characters were parsimony uninformative. Only the first 1 000 equally most parsimonious trees were saved, the first of which is shown (Fig. 5) (TL = 4315, CI = 0.312, RI = 0.819, RC = 0.256).
Fig. 5.
The first of 1 000 equally most parsimonious trees obtained from a heuristic search with 100 random taxon additions of the combined ITS, ACT and EF-1α sequence alignment using PAUP v. 4.0b10. The scale bar shows 10 changes, and bootstrap support values from 1 000 replicates are shown at the nodes. Thickened lines indicate those branches present in the strict consensus tree and the tree was rooted to Passalora eucalypti strain CBS 111318 (GenBank GU269845, GU320548 and GU384558, respectively).
The phylogeny resulting from the combined sequence data was more structured towards the terminal nodes than the LSU phylogeny. Similar to the LSU phylogeny, a split was observed within Pseudocercospora s. str., with at least two main clades being evident. Although present in the strict consensus tree, this split was not well-supported in the phylogeny. Deeper nodes of the backbone were poorly supported. There were high levels of support for several of the smaller sub-clades in this tree, which are discussed in the Taxonomy section below.
Taxonomy
Isolates representing 146 species of Pseudocercospora were subjected to DNA analysis and morphological comparison. Phylogenetic analyses based on the LSU gene resolved a total of 14 clades in the Pseudocercospora complex.
Clade 1 represented Strelitziana (pseudocercospora-like but with a separating cell between conidia and conidiogenous cells) and Cyphellophora (pseudocercospora-like but phialides with flaring collarettes, situated directly on hyphae). Thedgonia ligustrina (pseudocercosporella-like, but conidia in chains) represented Clade 2. Clade 3 included several isolates of Pseudocercospora cantuariensis, which represents a novel genus, distinguished from Pseudocercospora based on its broad conidial hila and scars, as well hyaline mycelium, and the presence of hyphopodia-like structures. Xenostigmina zilleri, characterised as being stigmina-like, but also having sympodial proliferation of the conidiogenous cells, clustered in Clade 4, which was basal to Cladosporium (Cladosporiaceae; Clade 5). Clade 6 represented several members of Teratosphaeriaceae, known to have a wide range of anamorphs, including Microcyclospora. Clade 7 represented species of Dissoconium (Dissoconiaceae), distinct due to their dimorphic conidia that are actively discharged. Clade 8 remains unresolved, and was represented by disjunct elements appearing Zasmidium- and pseudocercospora-like in morphology, including Microcyclosporella. Clade 9 was represented by several Mycosphaerella species such as M. laricina (anamorph Pseudocercospora sp.), and Paracercospora egenula. Paracercospora was separated from Pseudocercospora based on a combination of characters, including pale olivaceous conidia, and a minute thickening along the rim of its conidial hila and scars. Clade 10 included a diverse assemblage of genera. Two genera that differ mainly based on their conidiomatal structure, Pseudocercosporella and Septoria, clustered in this clade. Miuraea, a genus intermediate between Cercospora and Pseudocercospora, also resided within this clade. Clade 11 was represented by two coelomycetous species of Sonderhenia that clustered basal to Clade 12. The latter included a new genus with pseudocercospora-like anamorphs, mostly distinguished from Pseudocercospora s. str. by having species with smooth, pale brown conidia, and the frequent production of red crystals in agar (previously referred to in literature as the Mycosphaerella heimii complex). Scolecostigmina (based on S. mangiferae), which is characterised by verruculose conidia and percurrently proliferating conidiogenous cells, clustered alongside to Trochophora, characterised by brown sickle-shaped conidia with three thick, dark septa. Passalora eucalypti formed a separate lineage in Clade 13 that was adjacent to Pseudocercospora s. str. in Clade 14. This clade included the type species, P. vitis that is basal in this cluster. Although there was structure within the clade, we regard it as representing a single genus, including Stigmina platani, the type of Stigmina, Phaeoisariopsis (P. griseola), and Pseudophaeoramularia (P. angolensis). Several isolates identified from different countries as representing the same species based on host, disease symptoms and general morphology, clustered apart from one another. These collections were found to represent novel cryptic species.
Treatment of species
Several novel taxa were identified in this study on the basis of phylogenetic analyses of the various gene regions together with morphological examination of the specimens and isolates. Recognised clades, as well as novel species and genera, are described and discussed below. Where descriptions of known taxa are freely available online in MycoBank or journals, they are not repeated here, other than their generic circumscriptions.
Clade 1: Strelitziana and Cyphellophora
Strelitziana M. Arzanlou & Crous, Fungal Planet No. 8: 2006.
Conidiophores erect, solitary, arising from aerial and submerged mycelium, subcylindrical, straight to geniculate-sinuous, pale brown. Conidiogenous cells terminal, integrated, rejuvenating percurrently, proliferating apically via several short, conspicuous denticles; conidiogenesis holoblastic with rhexolytic conidial secession. Conidia solitary, pale brown, smooth, long obclavate, multi-euseptate; microcyclic conidiation present in culture.
Type species: Strelitziana africana M. Arzanlou & Crous, Fungal Planet No. 8. 2006.
Notes: The genus Strelitziana presently accommodates four species that are primarily distinguished based on their conidial dimensions. These include S. africana, S. australiensis, S. eucalypti and S. mali (Arzanlou & Crous 2006, Cheewangkoon et al. 2009, Zhang et al. 2009, Crous et al. 2010).
Cyphellophora G.A. de Vries, Mycopathol. Mycol. Appl. 16: 47. 1962.
Colonies (on OA) with moderate to rapid growth, velvety to lanose, in various shades of grey; reverse black. Fertile hyphae pale brown, sometimes with constrictions at the septa. Conidiogenous cells phialidic, intercalary, sometimes on short side branches, each with a short, lateral or terminal collarette. Conidia sickle-shaped, brown, smooth-walled, transversely septate, adhering in small bundles (from de Vries 1962).
Type species: Cyphellophora laciniata G.A. de Vries, Mycopathol. Mycol. Appl. 16: 47. 1962.
Notes: The genus Cyphellophora, which is based on C. laciniata (isolated from human skin; De Vries et al. 1986), appears to be heterogeneous (Decock et al. 2003, Crous et al. 2007a, 2009a, Cheewangkoon et al. 2009) and requires further study.
Clade 2: Thedgonia
Thedgonia B. Sutton, Trans. Brit. Mycol. Soc. 61: 426. 1973.
Foliicolous, phytopathogenic, causing discrete leaf spots. Conidiomata fasciculate, punctiform. Mycelium internal, hyphae subhyaline, septate, branched, forming substomatal stromata, hyaline to pale brown. Conidiophores fasciculate, arising from stromata, simple, rarely branched, subcylindrical, straight to geniculate-sinuous, continuous to septate, smooth, hyaline to pale yellowish green. Conidiogenous cells integrated, terminal, occasionally conidiophores reduced to conidiogenous cells, holoblastic-thalloblastic, sympodial, conidiogenous loci more or less planate, unthickened, non-pigmented. Conidia in disarticulating chains, rarely in branched chains, subcylindrical to obclavate, with one to several transverse eusepta, hyaline or almost so, apex rounded to truncate, base truncate, hila flat, unthickened, hyaline (Crous et al. 2009a).
Type species: Thedgonia ligustrina B. Sutton, Trans. Brit. Mycol. Soc. 61: 426. 1973.
Thedgonia ligustrina (Boerema) B. Sutton, Trans. Brit. Mycol. Soc. 61: 428. 1973.
Basionym: Cercospora ligustrina Boerema, Tijdschr. Plantenziekten 68: 117. 1962.
≡ Cercoseptoria ligustrina (Boerema) Arx, Genera of Fungi Sporulating in Pure Culture, ed. 3: 306, Lehre 1981.
Specimens examined: Asia, on Ligustrum sp., H. Evans, CPC 4296 = W2072, CPC 4297 = W 2073, CPC 4298 = W 1877. Netherlands, Eefde, on Ligustrum ovalifolium, 23 Mar. 1959, G.H. Boerema, holotype L, ex-type culture CBS 148.59; Bilthoven, on L. ovalifolium, 2003, P.W. Crous, CPC 10530 = CBS 124332, CPC 10532, 10533. South Korea, Namyangju, on L. ovalifolium, 9 Oct. 2002, leg. H.D. Shin, isol. P.W. Crous, CBS H-20204, CPC 10019, 10861-10863; Suwon, on L. obtusifolium, 2 Oct. 2007, leg. H.D. Shin, isol. P.W. Crous, CBS H-20207, CPC 14754-14756.
Notes: Contrary to the earlier hypothesis that Thedgonia belonged to the Mycosphaerellaceae (Kaiser & Crous 1998), Crous et al. (2009a) showed that it resides in Helotiales. Consequently, thedgonia-like anamorphs that occur in the Mycosphaerellaceae must be accommodated elsewhere.
Clade 3: Xenostigmina
Xenostigmina Crous, Mycol. Mem. 21: 154. 1998.
Foliicolous, phytopathogenic, causing discrete leaf spots. Mycelium internal, consisting of hyaline to pale brown, septate, branched, smooth hyphae. Conidiomata sporodochial, brown to black. Conidiophores densely aggregated, arising from the upper cells of a pale brown stroma, finely verruculose, hyaline to pale brown, multiseptate, subcylindrical, straight to variously curved, branched. Conidiogenous cells terminal and intercalary, hyaline to pale brown, finely verruculose, doliiform to subcylindrical, tapering to flat tipped loci, mono- to polyblastic, proliferating sympodially and percurrent; loci not thickened or conspicuous. Conidia solitary, pale to medium brown, with pale brown apical and basal regions, finely verruculose, mostly straight, ellipsoidal, apex subobtuse, frequently extending into a beak; base truncate at dehiscence, inner part extending later to form a short, subobtuse basal appendage; septation muriform; basal marginal frill present (Crous et al. 2009a).
Type species: Xenostigmina zilleri (A. Funk) Crous, Mycol. Mem. 21: 155. 1998.
Specimens examined: Canada, British Columbia, 15 km east of Sardis, on living leaves of Acer macrophyllum, 22 Oct. 1985, A. Funk & C.E. Dorworth, holotype DAVFP 23272; British Columbia, on living leaves of Acer sp., 2002, leg. K.A. Seifert, isol. P.W. Crous, CBS 115686 =CPC 4010, CBS 115685 =CPC 4011; Victoria BC, 48°30’25.63”N, 123°30’46.99”W, 115 m, fallen leaves of A. macrophyllum, 6 Sep. 2007, leg. B. Callan, isol. P.W. Crous, epitype designated here CBS H-20208, cultures ex-epitype CPC 14376 = CBS 124108, CPC 14377, 14378 (Xenostigmina zilleri), CPC 14379 = CBS 124109, CPC 14380, 14381 (Mycopappus aceris).
Notes: Xenostigmina with its Mycopappus synanamorph is distinct from Stigmina s. str., which is a synonym of Pseudocercospora s. str. (Crous et al. 2006, Braun & Crous 2006, 2007). The genus Xenostigmina (Crous 1998) appears related to Seifertia (Seifert et al. 2007) in the Dothideomycetes (Crous et al. 2009b).
Clade 4: Phaeomycocentrospora
Phaeomycocentrospora Crous, H.D. Shin & U. Braun, gen. nov. MycoBank MB564813.
Etymology: Name reflects the pale brown appearance of conidia and the superficial similarity to Mycocentrospora.
Foliicolous, phytopathogenic, causing discrete leaf spots. Mycelium internal and external, consisting of hyaline, septate, branched, smooth, 3-5 μm diam hyphae; hyphopodium-like structures present. Caespituli amphigenous. Conidiophores in loose fascicles, arising from a poorly developed stroma, or from superficial hyphae emerging from stomata, or erumpent through the cuticle; erect on superficial hyphae, olivaceous-brown, straight to slightly curved, unbranched, not geniculate, obconically truncate at the apex; conidiogenous cells integrated, terminal or conidiophores reduced to conidiogenous cells, mono- to polyblastic, proliferating sympodially, transversely septate; conidiogenous loci broad, more or less planate, neither thickened nor darkened. Conidia solitary, filiform to cylindrical, straight to moderately curved, subhyaline to pale olivaceous, transversely euseptate, usually not constricted at septa, tapering somewhat towards an obtuse apex, truncate at the base; hilum unthickened, not darkened, broad.
Type species: Phaeomycocentrospora cantuariensis (E.S. Salmon & Wormald) Crous, H.D. Shin & U. Braun, comb. nov.
Notes: Phaeomycocentrospora is similar to Pseudocercospora in that its conidia and conidiophores appear to be pigmented and its conidiogenous loci are unthickened and not darkened. It is distinct from Pseudocercospora in that its mycelium is hyaline, hyphopodia-like structures are present, and conidia are hyaline with a pale brown inner wall layer, giving the impression of pigmented conidia. This fungus also has extremely broad conidial loci and scars that are untypical of Pseudocercospora. Chupp (1954) commented that Cercospora cantuariensis represented an unusual species that should be transferred to a genus of its own. Based on its unique phylogenetic placement (Fig. 4) and morphology, Phaeomycocentrospora gen. nov. is established for this taxon. Deighton (1971, 1972) assigned this species to Mycocentrospora, but the type species M. acerina is phylogenetically distinct from other genera morphologically similar to it and differs in having conidia with filiform appendages and often with strongly swollen intercalary cells.
Phaeomycocentrospora cantuariensis (E.S. Salmon & Wormald) Crous, H.D. Shin & U. Braun, comb. nov. MycoBank MB564814. Fig. 6.
Fig. 6.
Phaeomycocentrospora cantuariensis (CPC 11691-11693). A. Leaf spots on upper and lower leaf surface. B, C. Sporulation of leaf surface. D-I. Conidiophores and conidiogenous cells. J-M. Conidia. Scale bars = 10 μm.
Basionym: Cercospora cantuariensis E.S. Salmon & Wormald, J. Bot. (London) 61: 134. 1923.
≡ Centrospora cantuariensis (E.S. Salmon & Wormald) Deighton, Mycol. Pap. 124: 8. 1971.
≡ Mycocentrospora cantuariensis (E.S. Salmon & Wormald) Deighton, Taxon 21: 716. 1972.
≡ Pseudocercospora cantuariensis (E.S. Salmon & Wormald) U. Braun, Mycotaxon 48: 281. 1993.
Leaf spots amphigenous, scattered, often confluent, subcircular to irregular, 1-5 mm diam, becoming up to 10 mm diam when confluent, greyish to white, centre reddish brown with yellowish brown zone on upper surface; greyish brown to grey on lower surface. Caespituli amphigenous, but predominantly hypophyllous. Mycelium internal and external; internal hyphae hyaline, septate, branched, smooth, 3-4 μm diam; external hyphae plagiotropous, branched, septate, smooth, hyaline, 3-5 μm diam. Conidiophores in loose fascicles, arising from a poorly developed stroma, or from superficial hyphae emerging from stomata, or erumpent through the cuticle; erect on superficial hyphae, olivaceous-brown, straight to slightly curved, unbranched, not geniculate, obconically truncate at the apex, proliferating sympodially, 0-3-septate, 30-140 × 7-20 μm. Conidiogenous cells terminal, unbranched, pale brown, smooth, tapering to flat-tipped apical loci, with scars neither thickened nor darkened, 4-7 μm diam; at times proliferating percurrently, with 1-3 percurrent proliferations at the apex, 12-45 × 5-8 μm. Conidia solitary, filiform to cylindrical, straight to moderately curved, subhyaline to pale olivaceous, smooth, 3-15(-21)-septate, usually not constricted at septa, tapering somewhat towards obtuse apex, truncate at the base, or long obconically subtruncate, (100-)140-200(-500) × (5-)7-12(-20) μm; hilum unthickened, not darkened, 4-7 μm diam; conidia appear to have an inner wall layer that is pale brown when studied in culture (adapted from Shin & Kim 2001).
Specimens examined: South Korea, Hoengseong, on Humulus scandens (= H. japonicus), 4 Sep. 2005, H.D. Shin, CBS H-20830; Suwon, Acalypha australis, 5 Nov. 2004, H.D. Shin, cultures CPC 11691-11693; Suwon, H. scandens, 5 Nov. 2004, H.D. Shin, CBS H-20831, cultures CPC 11694-11696; Hoengseong, on H. scandens, 11 Oct. 2004, H.D. Shin, CBS H-20832, cultures CPC 11646, 11647; Wonju, on H. scandens, 18 Oct. 2002, H.D. Shin, CBS H-20833, cultures 10157, 10158; Namyangju, on Luffa aegyptica (= L. cylindrica), 22 Oct. 2003, H.D. Shin, CBS H-20834, cultures CPC 10762-10766.
Clade 5: Cladosporium (Cladosporiaceae)
Cladosporium Link, Ges. Naturf. Freunde Berlin Mag. Neuesten Entdeck. Gesammten Naturk. 7: 37. 1816.
Teleomorph: Davidiella Crous & U. Braun, Mycol. Progr. 2: 8. 2003.
Saprobic or phytopathogenic. Ascomata pseudothecial, black to red-brown, globose, inconspicuous and immersed beneath stomata to superficial, situated on a reduced stroma, with 1(-3) short, periphysate ostiolar necks; periphysoids frequently growing down into cavity; wall consisting of 3-6 layers of textura angularis. Asci fasciculate, short-stalked or not, bitunicate, subsessile, obovoid to broadly ellipsoid or subcylindrical, straight to slightly curved, 8-spored. Pseudoparaphyses frequently present in mature ascomata, hyaline, septate, subcylindrical. Ascospores bi- to multiseriate, hyaline, obovoid to ellipsoid-fusiform, with irregular luminar inclusions, mostly thick-walled, straight to slightly curved; frequently becoming brown and verruculose in asci; at times covered in mucoid sheath (from Schubert et al. 2007). Mycelium superficial, loosely branched, septate, sometimes constricted at septa, hyaline, subhyaline to pale brown, smooth or almost so to verruculose or irregularly rough-walled, sometimes appearing irregular in outline due to small swellings and constrictions, walls unthickened to somewhat thickened. Conidiophores both macro- and micronematous, arising laterally from plagiotropous hyphae or terminally from ascending hyphae. Macronematous conidiophores erect, straight to flexuous, somewhat geniculate-sinuous, nodulose or not, unbranched or occasionally branched, pluriseptate, pale to medium brown, older ones almost dark brown, walls thickened, sometimes even two-layered. Conidiogenous cells integrated, terminal or intercalary, mono- to usually polyblastic, nodulose to nodose or not, proliferation sympodial, with several conidiogenous loci, mostly situated on small lateral shoulders, more or less protuberant, characteristically coronate (SEM), i.e. with a convex central dome surrounded by a low to distinctly raised rim, appearing to be thickened and somewhat darkened-refractive. Micronematous conidiophores hardly distinguishable from hyphae, sometimes only as short lateral outgrowth with a single apical scar, short, conical to almost filiform or narrowly cylindrical, pluriseptate, usually short, subhyaline to pale brown, almost smooth to minutely verruculose or irregularly rough-walled, 0-3-septate. Conidiogenous cells integrated, terminal or conidiophores reduced to conidiogenous cells, narrowly cylindrical or filiform, with a single or two loci. Conidia solitary (in heterosporium-like species) to usually catenate, in unbranched or loosely branched chains, straight to slightly curved; small terminal conidia without distal hilum, obovoid to ellipsoid to subcylindrical, aseptate, subhyaline to pale brown; intercalary conidia with a single or sometimes up to three distal hila, limoniform, ellipsoid to subcylindrical, 0-1-septate; secondary ramoconidia with up to four distal hila, ellipsoid to cylindrical-oblong, 0-1(-2)-septate, pale greyish brown or brown to medium brown, smooth to minutely verruculose to verrucose, walls slightly to distinctly thickened, apex obtuse or slightly truncate, towards the base sometimes distinctly attenuated with hila situated on short stalk-like prolongations, hila slightly to distinctly protuberant, coronate structure as in conidiogenous loci, somewhat thickened and darkened-refractive; microcyclic conidiogenesis occurring; primary ramoconidia similar to secondary ramoconidia, except base truncate, uniform with conidiogenous cell, and more subcylindrical in shape (adapted from Schubert et al. 2007).
Type species: Cladosporium herbarum (Pers.: Fr.) Link, Ges. Naturf. Freunde Berlin Mag. Neuesten Entdeck. Gesammten Naturk. 7: 37. 1816.
Notes: Cladosporium is well-defined by having Davidiella teleomorphs and conidiophores that give rise to conidial chains with unique coronate scars (David 1997, Braun et al. 2003a, Schubert et al. 2007, Bensch et al. 2010, 2012), which easily distinguish it from a range of other morphologically similar genera (Crous et al. 2007a, b; Braun & Crous, in Seifert et al. 2011).
Clade 6: Teratosphaeriaceae
Teratosphaeria Syd. & P. Syd., Ann. Mycol. 10: 39. 1912.
Phytopathogenic, commonly associated with leaf spots, but also on fruit, or causing cankers on stems. Ascomata pseudothecial, superficial to immersed, frequently situated in a stroma of brown pseudoparenchymatal cells, globose, unilocular, papillate, ostiolate, canal periphysate, with periphysoids frequently present; wall consisting of several layers of brown textura angularis; inner layer of flattened, hyaline cells. Pseudoparaphyses frequently present, subcylindrical, branched, septate, anastomosing. Asci fasciculate, 8-spored, bitunicate, frequently with multi-layered endotunica. Ascospores ellipsoid-fusoid to obovoid, 1-septate, hyaline, but becoming pale brown and verruculose, frequently covered in mucoid sheath (from Crous et al. 2007a).
Type species: Teratosphaeria fibrillosa Syd. & P. Syd., Ann. Mycol. 10: 40. 1912.
Notes: Teratosphaeria accommodates a group of plant pathogenic fungi that can cause serious leaf spot, blotch and canker diseases of a range of hosts (Crous 2009, Crous et al. 2007a, 2009b, Hunter et al. 2009, 2011). The Teratosphaeriaceae remains to be clearly resolved, and several different genera are presently recognised in the family. Some are plant-associated such as Batcheloromyces, Baudoinea, Capnobotryella, Catenulostroma, Davisoniella, Devriesia, Hortea, Penidiella, Phaeothecoidea, Pseudotaeniolina, Readeriella, Staninwardia, and Stenella s. str. (Crous et al. 2007a, 2009a, 2011b), and others including Cystocoleus, Racodium, Friedmanniomyces, Elasticomyces, Recurvomyces (Selbmann et al. 2008) and Xanthoriicola (Ruibal et al. 2011) are lichenicolous or rock inhabiting.
Microcyclospora Jana Frank, Schroers & Crous, Persoonia 24: 99. 2010.
Epiphytic and endophytic, occurring on leaves and fruit. Mycelium consisting of branched, septate, pale brown, smooth, 2-3 μm wide hyphae. Conidiophores reduced to conidiogenous cells, integrated in hyphae, giving rise to peg-like lateral protuberances, 1 μm wide, 1-2 μm tall, mono- to polyblastic. Conidia scolecosporous, cylindrical, straight to variously curved, flexuous, apex obtuse, base truncate, 1-multi-septate, somewhat constricted at septa, smooth, pale brown, guttulate, aggregated in mucoid masses; hila not thickened or darkened; microcyclic conidiation observed in culture.
Type species: Microcyclospora pomicola Jana Frank, B. Oertel, Schroers & Crous, Persoonia 24: 100. 2010.
Notes: Microcyclospora was recently introduced in Teratosphaeriaceae for three taxa associated with sooty blotch of apple (Frank et al. 2010). The species described here resembles others presently known in Microcyclospora by having pigmented structures and undergoing microcyclic condiation. Other than having distinct conidial dimensions, it differs from other genera in that its conidiogenous cells are annellidic (not mono- to polyblastic), and its conidia are darker brown and verruculose to warty, not pale brown and smooth.
Microcyclospora quercina Crous & Verkley, sp. nov. MycoBank MB564815. Figs 7, 8.
Fig. 7.
Microcyclospora quercina (CPC 10712). Line drawing showing conidiogenous cells and conidia formed in culture. Scale bar = 10 μm.
Fig. 8.
Microcyclospora quercina (CPC 10712). A, B. Colony on oatmeal and potato-dextrose agar, respectively. C-E. Conidiogenous cells giving rise to conidia (arrows). F. Microcyclic conidiation. G, H. Conidia. Scale bars = 10 μm.
Etymology: Name reflects its host, Quercus.
Foliicolous, endophytic. Mycelium consisting of branched, septate, brown, 1.5-3 μm diam hyphae, guttulate, smooth to verruculose or warty, with or without mucoid sheath. Conidiophores reduced to conidiogenous cells. Conidiogenous cells lateral on hyphae, brown, solitary, not aggregated, 1.5-2 μm diam, with 1-4 percurrent proliferations and flaring collarettes. Conidia solitary, subcylindrical (rarely obclavate), gently curved, apex obtuse (rarely subobtuse), base truncate or long obconically truncate, with slight basal taper to hilum that is 2 μm diam, unthickened, nor darkened, frequently with small marginal frill, brown, guttulate to granular, smooth, appearing warty or roughened due to external mucoid layer which is sometimes present, transversely (1-)3-4(-11)-euseptate, becoming constricted at septa with age, (12-)30-45(-70) × (2-) 2.5-3 μm; microcyclic conidiation commonly observed.
Culture characteristics: Colonies after 2 wk in the dark up to 15 mm diam, with sparse aerial mycelium, folded surface and uneven to somewhat feathery, lobate margins, exuding copious amounts of slime on PDA, but less so on MEA and OA; colonies olivaceous-black on all media.
Specimen examined: Netherlands, endophytic in leaves of Quercus robur, Sep. 2003, G.J.M. Verkley, holotype CBS H-20835, culture ex-type CPC 10712 = CBS 130827.
Clade 7: Dissoconium (Dissoconiaceae)
Dissoconium de Hoog, Oorschot & Hijwegen, Proc. K. Ned. Akad. Wet., Ser. C, Biol. Med. Sci. 86(2): 198. 1983.
Hyperparasitic, but also reported to be phytopathogenic. Ascomata pseudothecial, immersed, globose, unilocular, papillate, ostiolate, canal periphysate; wall consisting of 3-4 layers of brown textura angularis; inner layer of flattened, hyaline cells. Pseudoparaphyses absent. Asci fasciculate, 8-spored, bitunicate. Ascospores ellipsoid-fusoid, 1-septate, hyaline, with or without mucoid sheath. Mycelium internal and external, consisting of branched, septate, smooth, hyaline to pale brown hyphae. Conidiophores separate, arising from hyphae, subcylindrical, subulate or lageniform to cylindrical, tapering to a bluntly rounded or truncate apex, straight to once geniculate, smooth, medium brown, 0-multi-septate; conidiogenous cells polyblastic, with terminal and lateral conidiogenous loci, visible as slightly thickened, darkened scars on a rachis. Conidia solitary, pale olivaceous-brown, smooth, ellipsoid to obclavate or globose, 0-1-septate; hila somewhat darkened. Secondary conidia present or absent; developing adjacent to primary conidia, pale olivaceous to subhyaline, aseptate, pyriform; conidium discharge active or passive (from Crous et al. 2009b).
Type species: Dissoconium aciculare de Hoog, Oorschot & Hijwegen, Proc. K. Ned. Akad. Wet., Ser. C, Biol. Med. Sci. 86(2): 198. 1983.
Notes: Dissoconium has mycosphaerella-like teleomorphs (Crous 1998, Crous et al. 2004c) and was recently shown to represent a distinct family, Dissoconiaceae (Crous et al. 2009b). Species are different from other taxa in Capnodiales in that they form primary and secondary conidia that are actively discharged and anastomose on the agar surface shortly after germination (De Hoog et al. 1991).
Clade 8: Microcyclosporella and zasmidium-like
Microcyclosporella Jana Frank, Schroers & Crous, Persoonia 24: 101. 2010.
Epiphytic on leaves and fruit. Mycelium consisting of pale brown, smooth to finely verruculose, branched, septate, 2-3.5 μm wide hyphae, at times covered in a mucoid layer, with integrated, lateral, truncate conidiogenous loci. Conidiophores mostly reduced to conidiogenous cells. Conidiogenous cells integrated, intercalary on hyphae, rarely terminal, cylindrical to doliiform, pale brown, but hyaline if occurring in yeast-like sectors of colonies, smooth, mono- or polyblastic, proliferating sympodially; loci inconspicuous, truncate, unthickened, not darkened, pale brown to hyaline. Conidia hyaline, smooth, subcylindrical to narrowly obclavate or narrowly fusoid with acutely rounded apex and obconically truncate base, guttulate, transversely 0-6-septate; microcyclic conidiation common.
Type species: Microcyclosporella mali Jana Frank, Schroers & Crous, Persoonia 24: 101. 2010.
Notes: Microcyclosporella was treated as part of the Pseudocercosporella generic complex (Batzer et al. 2005), but has since been shown to be polyphyletic within Mycosphaerellaceae (Crous 2009, Crous et al. 2003, 2009b, c, Frank et al. 2010). The clade accommodating Microcyclosporella contains many disjunct elements that vary in morphology from Microcyclosporella s. str. (hyaline structures) to pigmented structures, namely zasmidium-like (verrucuclose conidia) to pseudocercospora-like (smooth conidia) (see Crous et al. 2009b). We suspect that these groups may eventually be recognised as distinct genera, but more taxa need to be examined to resolve this issue.
Clade 9: Paracercospora and pseudocercospora-like
Paracercospora Deighton, Mycol. Pap. 144: 47. 1979.
Foliicolous, phytopathogenic, causing leaf spots. Mycelium internal, hyaline to pale olivaceous. Stromata absent to poorly developed. Conidiophores fasciculate, smooth, subhyaline to pale olivaceous. Conidiogenous cells integrated, terminal, mono- to usually polyblastic, proliferating sympodially; conidiogenous loci moderately conspicuous, with narrow thickening along the rim. Conidia solitary, subcylindrical to obclavate-cylindrical, smooth, subhyaline to pale olivaceous, with a narrow thickening along the rim of the hilum.
Type species: Paracercospora egenula (Syd.) Deighton, Mycol. Pap. 144: 48. 1979.
Specimens examined: Japan, Shimane, on leaves of Solanum melongena, 5 Aug. 1998, T. Mikami, CNS-415, cultures MUCC 883, MAFF 237766. South Korea, Hongcheon, on leaves of S. melongena, 26 Oct. 2005, H.D. Shin, CBS H-20836, culture CPC 12537.
Notes: Stewart et al. (1999) conducted the first phylogenetic analysis of the Mycosphaerellaceae and concluded that the marginal thickening that occurs along the rims of conidial scars and hila, originally thought to be the main character to distinguish Paracercospora from Pseudocercospora, was not taxonomically significant and suggested that Paracercospora be reduced to synonymy with Pseudocercospora. The current study provides new evidence that Paracercospora is not a synonym of Pseudocercospora, but no consistent morphological characters that distinguish it from Pseudocercospora s. str. have been identified. Conidia of Paracercospora egenula are subhyaline to pale olivaceous with minimal marginal thickening of the conidiogenous loci (Fig. 9). Conidial scars and hila of Ps. fijiensis (Arzanlou et al. 2008) and Ps. basiramifera (Crous 1998) are marginally thickened. Both of the latter species, which belong to Pseudocercospora s. str., have pale to medium brown conidia. At present Paracercospora may be defined by a combination of the minimal marginal thickening of the conidiogenous loci and its subhyaline conidia.
Fig. 9.
Paracercospora egenula (CPC 12537). A. Leaf spots on upper and lower leaf surface. B. Close-up of lesion. C-F. Fascicles with conidiogenous cells. G. Conidia. Scale bars = 10 μm.
The taxonomic placement of Paracercospora is complicated by two other taxa that resolve in the clade together with it. These are Passalora brachycarpa (pale olivaceous, catenate conidia, and prominent, thickened, darkened scars; also visible when sporulating in culture), and Pseudocercospora tibouchinigena described below, which has subhyaline conidia, and unthickened hila and scars. This indicates that it is neither a species of Pseudocercospora s. str. (subhyaline conidia), nor Paracercospora (lacking any form of scar thickening). As a temporary solution, the species on Tibouchina is described in Pseudocercospora, although taxa in this subclade may eventually be shown to represent a distinct genus.
Pseudocercospora tibouchinigena Crous & U. Braun, sp. nov. MycoBank MB564816. Fig. 10.
Fig. 10.
Pseudocercospora tibouchinigena (CBS 116462). A. Leaf spots on upper and lower leaf surface. B, C. Close-up of lesions. D-G. Fascicles with conidiogenous cells. H. Conidia. Scale bar = 10 μm.
Etymology: Name is derived from Tibouchina, the host on which it was collected.
Leaf spots amphigenous, angular to irregular, 1-3 mm diam, up to 10 mm long, medium brown, with raised, dark brown border. Mycelium internal, hyaline, smooth, consisting of septate, branched, smooth, 1.5-2 μm diam hyphae. Caespituli fasciculate, predominantly hypophyllous, hyaline to pale olivaceous on leaves, up to 60 μm wide and 40 μm high. Conidiophores aggregated in dense fascicles, arising from the upper cells of a hyaline to subhyaline stroma, up to 50 μm wide and 20 μm high; conidiophores subcylindrical to ampulliform, 0-3-septate, straight to variously curved or geniculate-sinuous, unbranched, 15-25 × 3-5 μm. Conidiogenous cells terminal, unbranched, hyaline, smooth, tapering to flat-tipped apical loci, proliferating sympodially, 5-10 × 2.5-3.5 μm. Conidia solitary, subhyaline, smooth, guttulate or not, subcylindrical or narrowly obclavate, apex subobtuse, base obconically truncate, straight to variously curved, 3-10-septate, (15-)30-40(-60) × (1.5-)2-2.5(-3) μm; hila unthickened, not darkened nor refractive, 1-1.5 μm diam; prominent microcyclic conidiation observed in vivo.
Culture characteristics: Colonies after 1 mo at 24 °C in the dark on MEA; erumpent, spreading, with moderate aerial mycelium, and smooth, lobate margins. Surface pale olivaceous-grey; reverse olivaceous-grey. Colonies reaching 30 mm diam.
Specimen examined: New Zealand, Auckland, Princes Street, Auckland University Campus, on leaves of Tibouchina sp. (Melastomataceae), 9 Aug. 2004, C.F. Hill 1061, holotype HAL 2359F, culture ex-type CBS 116462.
Notes: Pseudocercospora tibouchinigena was initially reported from New Zealand as P. tibouchina (Braun et al. 2006), which is hitherto known only from Brazil. It differs from P. tibouchinae in that the latter species has narrowly subcylindrical conidia that are larger, 40-120 × 2-3 μm (Viégas 1945), than those of P. tibouchinigena. The subhyaline conidia of P. tibouchinigena are not typical of Pseudocercospora s. str., but for the present, we choose to name it in Pseudocercospora until the clade in which it resides has been more fully resolved (Fig. 5).
Clade 10: Cercospora, Miuraea, Phloeospora, Pseudocercosporella, Septoria, Xenocercospora
Cercospora Fresen., in Fuckel, Hedwigia 1(15): 133. 1863 and in Fuckel, Fungi Rhen. Exs., Fasc. II, No. 117. 1863.
Mostly phytopathogenic producing conspicuous lesions, but also including saprobes. Mycelium internal, rarely also external; hyphae colourless or almost so to pigmented, branched, septate, smooth to faintly rough-walled. Stromata lacking to well-developed, subhyaline to usually pigmented, substomatal to intraepidermal. Conidiophores mononematous, macronematous, solitary to fasciculate, arising from internal hyphae or stromata, emerging through stomata or erumpent, very rarely arising from superficial hyphae, erect, continuous to pluriseptate, subhyaline to pigmented, smooth to faintly rough-walled, thin- to moderately thick-walled. Conidiogenous cells integrated, terminal or intercalary or conidiophores reduced to conidiogenous cells, monoblastic, determinate to usually polyblastic, sympodial, rarely with a few enteroblastically percurrent rejuvenations which are not connected with conidiogenesis; conidiogenous loci (scars) conspicuous, thickened and darkened, planate. Conidia solitary, very rarely catenate, scolecosporous, obclavate, cylindrical-filiform, acicular, hyaline or subhyaline (with a pale greenish tinge), mostly pluriseptate, euseptate, rarely with 0-1 or few septa, smooth or almost so, hila thickened and darkened, planate (from Crous & Braun 2003).
Type species: Cercospora penicillata (Ces.) Fresen., Beiträge zur Mykologie 3: 93. 1863. [= C. depazeoides (Desm.) Sacc.].
Cercospora sojina Hara, Nogyo Sekai, Tokyo 9: 28. 1915. Fig. 11.
Fig. 11.
Cercospora sojina (CPC 12322). A. Leaf spots on upper and lower leaf surface. B. Close-up of lesion. C-G. Fascicles with conidiophores and conidiogenous cells. H. Conidia. Scale bars = 10 μm.
≡ Passalora sojina (Hara) H.D. Shin & U. Braun, Mycotaxon 58: 163. 1996.
Specimen examined: South Korea, Hongcheon, on Glycine soja (= G. max subsp. soja), 20 Jul. 2004, H.D. Shin, CBS H-20837, culture CPC 12322.
Notes: Despite sparingly septate and broadly obclavate-cylindrical conidia that tend to be subhyaline, this species is better accommodated in Cercospora than Passalora (Shin & Braun 1996) based on phylogenetic analysis.
Cercospora eucommiae Crous, U. Braun & H.D. Shin, sp. nov. MycoBank MB564817. Fig. 12.
Fig. 12.
Cercospora eucommiae (CPC 10047). A. Leaf spots on upper and lower leaf surface. B. Close-up of lesion. C-G. Fascicles with conidiophores and conidiogenous cells. H, I. Conidia. Scale bars = 10 μm.
Etymology: Name derived from Eucommia, the host on which it occurs.
Leaf spots amphigenous, irregular to subcircular, 2-5 mm diam; surface grey-brown to brown with diffuse border; reverse olivaceous-brown with diffuse border. Mycelium internal, hyaline, consisting of septate, branched, smooth, 2-3 μm diam hyphae. Caespituli fasciculate, pale brown, amphigenous, up to 40 μm diam and 50 μm high (conidial mass white on leaf surface). Conidiophores aggregated in loose fascicles arising from the upper cells of a weakly developed brown stroma, up to 30 μm diam and 20 μm high, conidiophores pale brown, smooth, 1-3-septate, subcylindrical, straight to variously curved, unbranched, 20-50 × 4-5 μm. Conidiogenous cells terminal, unbranched, pale brown, smooth, tapering to flat-tipped apical loci that are thickened, somewhat darkened, slightly refractive, 2 μm diam, 15-25 × 4-5 μm, proliferating sympodially at the apex. Conidia solitary, or in unbranched short chains, hyaline to pale olivaceous (with age), smooth, guttulate, obclavate, apex obtuse to subobtuse or clavate, base obconically subtruncate, straight to mildly curved, 3-8-septate, (35-)60-75(-80) × (4-)5-6(-8) μm; hila thickened along the rim, but not darkened or planate, 1.5-2 μm diam.
Culture characteristics: Colonies after 2 wk at 24 °C in the dark on MEA; erumpent, spreading, with sparse aerial mycelium, and smooth, lobate margins. Surface folded, dark mouse-grey with patches of dirty white; reverse fuscous black becoming greyish sepia at margin. Colonies reaching 12 mm diam.
Specimens examined: South Korea, Chuncheon, on Eucommia ulmoides, 7 Oct. 2003, H.D. Shin, holotype CBS H-20839, cultures ex-type CPC 10802 = CBS 131932, CPC 10803, 10804; Chuncheon, on E. ulmoides, 11 Oct. 2002, H.D. Shin, CBS H-20838, culture CPC 10047.
Notes: In the Korean material C. eucommiae occurred in mixed infections with a Pseudocercospora species (conidia 22-160 × 4-7 μm) that resembles P. eucommiae (conidia 15-75 × 2-4 um), which is known from this host in China (Guo & Hsieh 1995). The description of C. eucommiae reveals the genus Cercospora to be paraphyletic. Morphologically C. eucommiae is distinct from other species in Cercospora in that the conidial hila and conidiogenous scars are different (thickened along the rim, not darkened and planate), and conidia also tend to occur in unbranched chains, which is not typical of Cercospora. Interestingly, it does not cluster with C. eremochloae, which also forms conidia in chains (Crous et al. 2011a). Although this species is not part of Cercospora s. str., we name it in this genus until further taxa are collected and studied to resolve the status of this subclade in relation to Cercospora s. str.
Miuraea Hara, Byochugai-Hoten (Manual of Pests and Diseases): 779. 1948.
Synonyms: See Braun (1995).
Foliicolous, phytopathogenic, causing leaf spots. Mycelium internal and external, consisting of septate, branched, hyaline to subhyaline hyphae. Conidiophores semi-macronematous, mononematous, reduced to a single conidiogenous cell, integrated on hyphae, with small lateral peg-like protuberances; conidiogenesis holoblastic, monoblastic, determinate, occasionally polyblastic, proliferation sympodial or percurrent; conidiogenous loci more or less truncate, inconspicuous, unthickened, not darkened. Conidia solitary, ellipsoid-ovoid, subcylindrical-vermiform, obclavate, subclavate, somewhat asymmetrical, euseptate, transversely pluriseptate to muriformly septate, hyaline to faintly pigmented, thin-walled; hila truncate to somewhat convex, unthickened, not darkened (adapted from Braun 1995).
Type species: Miuraea degenerans (Syd. & P. Syd.) Hara, Byochugai-Hoten (Manual of Pests and Diseases): 260. 1948.
Notes: Morphologically Miuraea is intermediate between Pseudocercospora and Pseudocercosporella, which explains its phylogenetic position in this clade (Fig. 4). It differs from Pseudocercosporella in having superficial mycelium, and very broad, muriformly septate conidia.
Miuraea persicae (Sacc.) Hara, Byochugai-Hoten (Manual of pests and diseases): 224. 1948. Fig. 13.
Fig. 13.
Miuraea persicae (CPC 10069). A. Leaf spots on upper and lower leaf surface. B, C. Close-up of fruiting (rather inconspicuous). D, E. Fascicles with conidiophores and conidiogenous cells. F. Conidia (note septation). Scale bars = 10 μm.
Basionym: Cercospora persicae Sacc., Hedwigia 15: 119. 1876.
Teleomorph: “Mycosphaerella” pruni-persicae Deighton, Trans. Brit. Mycol. Soc. 50: 328. 1967.
Specimens examined: South Korea, Chuncheon, Prunus persica, 11 Oct. 2002, H.D. Shin, CBS H-20841, culture CPC 10069; Chuncheon, 7 Oct. 2003, P. armeniaca, H.D. Shin, CBS H-20840, CPC 10828-10830.
Phloeospora Wallr., Flora Cryptogamica Germaniae 2: 176. 1833.
Phytopathogenic, commonly associated with leaf spots, occurring on leaves and fruit. Mycelium immersed, consisting of hyaline, septate, branched hyphae. Conidiomata acervular, subepidermal, erumpent; wall of thin-walled textura angularis, opening by means of an irregular split. Conidiophores reduced to conidiogenous cells. Conidiogenous cells hyaline, smooth, cylindrical, discrete, indeterminate, proliferating via percurrent proliferations, or sympodially, formed from the upper cells of the acervulus. Conidia solitary, hyaline, smooth, septate, cylindrical, apex subobtuse to obtuse, base truncate, straight to curved.
Type species: Phloeospora ulmi (Fr.) Wallr., Flora Cryptogamica Germaniae 2: 177. 1833.
Specimens examined: Austria, Ulmus sp., H.A. van der Aa, CBS 613.81; Ulmus glabra, G. Verkley, CBS 344.97. Netherlands, Ulmus sp., H.A. van der Aa, CBS 101564.
Notes: Phloeospora is distinguished from Septoria by the production of conidia in acervuli, whereas conidiomata in the latter genus are pycnidial. Both genera are known to be polyphyletic (Verkley & Priest 2000, Quaedvlieg et al. 2011) and require further revision.
Pseudocercosporella Deighton, Mycol. Pap. 133: 38. 1973.
Foliicolous, phytopathogenic, causing discrete leaf spots. Mycelium mostly consistently internal, in some species with internal as well as external hyphae, hyaline to pale brown, septate, branched, smooth or almost so; stromata lacking or weakly to well-developed, substomatal to intraepidermal, usually colourless. Conidiophores solitary to fasciculate, emerging through stomata or erumpent through the cuticle, arising from inner hyphae or from stromata, sometimes formed as lateral branches of superficial hyphae, or aggregated in crustose to subglobose sporodochia; conidiophores simple, rarely branched, straight and subcylindrical to geniculate-sinuous, hyaline, occasionally faintly pigmented at the base, rarely throughout, one-celled or septate. Conidiogenous cells integrated, terminal, or reduced to conidiogenous cells, mono- to polyblastic, sympodial; conidiogenous loci inconspicuous, unthickened, neither darkened nor conspicuously refractive. Conidia formed singly, rarely in simple or branched chains, subcylindrical, filiform, somewhat obclavate, 1-multi-euseptate, hyaline, thin-walled, mostly smooth, apex obtuse to subacute, base subtruncate, hilum unthickened, neither darkened, nor refractive (adapted from Braun 1995).
Type species: Pseudocercosporella ipomoeae Deighton, Mycol. Pap. 133: 39. 1973. [= P. bakeri (Syd. & P. Syd.) Deighton, Mycol. Pap. 133: 41. 1973].
Note: Pseudocercosporella is polyphyletic (see Frank et al. 2010, Crous et al. 2011b) and new taxonomically useful morphological features will need to be determined to delineate all the genera presently accommodated in this clade.
Pseudocercosporella arcuata S.K. Singh, P.N. Singh & Bhalla, Mycol. Res. 101: 542. 1997. Fig. 14.
Fig. 14.
Pseudocercosporella arcuata (CPC 10050). A. Leaf spots on upper and lower leaf surface. B-D. Close-up of lesions. E, F. Fascicles with conidiophores and conidiogenous cells. G. Conidia. Scale bars = 10 μm.
Specimen examined: South Korea, Chuncheon, on Rubus oldhamii (≡ R. pungens var. oldhamii), 11 Oct. 2002, H.D. Shin, CBS H-20842, culture CPC 10050.
Pseudocercosporella capsellae (Ellis & Everh.) Deighton, Mycol. Pap. 133: 42. 1973.
Basionym: Cylindrosporium capsellae Ellis & Everh., J. Mycol. 3(11): 130. 1887.
Additional synonyms in Braun (1995).
Teleomorph: “Mycosphaerella” capsellae A.J. Ingman & Sivan., Mycol. Res. 95: 1339. 1991.
Specimen examined: South Korea, Namyangju, Raphanus sativus, 22 Oct. 2007, H.D. Shin, CBS H-20843, cultures CPC 14773 = CBS 131896.
Pseudocercosporella chaenomelis (Y. Suto) C. Nakash., Crous, U. Braun & H.D. Shin, comb. nov. MycoBank MB564818. Fig. 15.
Fig. 15.
Pseudocercosporella chaenomelis (CPC 14795). A. Leaf spots on upper and lower leaf surface. B. Close-up of leaf spot with white fruiting (rather inconspicuous). C-F. Fascicles with conidiophores and conidiogenous cells. G. Conidia. Scale bars = 10 μm.
Basionym: Cercosporella chaenomelis Y. Suto, Mycoscience 40: 513. 1999.
= Mycosphaerella chaenomelis Y. Suto, Mycoscience 40: 513. 1999.
Leaf spots amphigenous, irregular to angular, 5-20 mm diam, brown, delimited by leaf veins. Mycelium internal, hyaline, consisting of septate, branched, smooth, 1.5-2 μm diam hyphae. Caespituli fasciculate to sporodochial, white, predominantly epiphyllous, up to 200 μm diam and 120 μm high. Conidiophores aggregated in dense fascicles, arising from the upper cells of a hyaline stroma, up to 180 μm diam and 100 μm high; conidiophores hyaline, smooth, subcylindrical to ampulliform, straight to variously curved, unbranched, reduced to conidiogenous cells, 5-12 × 3-4 μm, proliferating sympodially at the apex. Conidia solitary, hyaline, smooth, guttulate to granular, subcylindrical to obclavate, apex subobtuse, base obconically truncate, straight to variously curved, 1-4-septate, (10-)30-38(-50) × (2-)2.5-3(-4) μm; hila unthickened, not darkened nor refractive, 1.5-2 μm diam; undergoing microcyclic conidiation on the host. Description based on CPC 14795.
Culture characteristics: Colonies after 1 mo at 24 °C in the dark on MEA; Colonies erumpent, spreading, with aerial mycelium sparse to absent, margins smooth, lobate. Surface irregularly folded, with a prominent network of ridges; folds appearing cinnamon, with surrounding areas and border brown-vinaceous; reverse sepia to chestnut, reaching up to 35 mm diam.
Specimens examined: Japan, Shimane Pref., Matsue, on leaves of Chaenomeles sinensis, Y. Suto, 6 Nov. 1983, holotype SFH-917, in Herbarium of SPFRC; Mie Pref., Tsu, on leaves of C. sinensis, C. Nakashima, 29 Oct. 2011, epitype designated here TFM: FPH-8101, culture ex-epitype MUCC 1510 = CBS 132131. South Korea, Kimhae, C. speciosa (= C. lagenaria), 14 Nov. 2007, H.D. Shin, CBS H-20844, culture CPC 14795 = CBS 131897.
Notes: Suto (1999) established the connection between Pseudocercosporella chaenomelis (as Cercosporella) and Mycosphaerella chaenomelis, which is the cause of a serious leaf spot disease referred to as frosty mildew on Chaenomeles sinensis in Japan. The fungus was found to overwinter by means of ascomata on fallen leaves, which provided the primary inoculum for new infections (April to June). Since the disease was previously known in Japan to be caused by a species of Cercosporella, Suto (1999) chose the latter genus to accommodate the anamorph. The hyaline conidia with unthickened conidial hila indicate that the fungus is better placed in Pseudocercosporella, and hence a new combination is proposed. Based on DNA sequence data from the ITS and ACT gene regions, strains from Japan and Korea appear identical (unpubl. data).
Pseudocercosporella chaenomelis occurs in mixed infections with Pseudocercospora cydoniae. Pseudocercosporella chaenomelis is morphologically comparable only with Ps. gei, known on Geum spp. in North America and the Far East of Russia (Braun 1995). The latter species differs in having smaller stromata (20-45 μm diam) and much longer filiform-acicular conidia, 20-120 × 1-3(-4) μm (Braun 1995). Pseudocercosporella crataegi on Crataegus spp. in North America is distinct, forming superficial hyphae with solitary conidiophores, and its much smaller stromata and much longer conidia, and Ps. potentillae on Potentilla sp. in Russia also differs by having very long conidia (Braun 1995).
Pseudocercosporella koreana Crous, U. Braun & H.D. Shin, sp. nov. MycoBank MB564819. Fig. 16.
Fig. 16.
Pseudocercosporella koreana (CPC 11414). A. Leaf spots on upper and lower leaf surface. B. Close-up of leaf spot with white fruiting. C. Substomatal stroma. D-H. Fascicles with conidiophores and conidiogenous cells. I. Conidia. Scale bars = 10 μm.
Etymology: Name derived from the country where it was collected.
Leaf spots amphigenous, indistinct, irregular, chlorotic, up to 6 mm diam. Mycelium internal, hyaline, consisting of septate, branched, smooth, 1.5-2.5 μm diam hyphae. Caespituli fasciculate, white, amphigenous, up to 60 μm diam and 90 μm high. Conidiophores aggregated in dense fascicles, on the upper cells of a pale brown to hyaline, usually substomatal stroma, up to 45 μm diam and 20 μm high; conidiophores hyaline or pale brown at the base, smooth, 0-2-septate, but frequently reduced to conidiogenous cells, subcylindrical, straight to variously curved or geniculate-sinuous, unbranched or branched below, 15-25 × 4-5 μm, proliferating sympodially at the apex. Conidia solitary, hyaline, smooth, prominently guttulate, narrowly obclavate, apex obtuse to subobtuse, base obconically subtruncate, straight to variously curved, 3-13-septate, (40-)60-80(-130) × (2.5-)3(-4) μm; hila unthickened, neither darkened nor refractive, 2 μm diam.
Culture characteristics: Colonies after 2 wk at 24 °C in the dark on MEA; surface folded with a prominent network of ridges, erumpent, spreading, with sparse aerial mycelium, and smooth, lobate margins. Surface olivaceous-grey to iron-grey; reverse iron-grey to greenish black. Colonies reaching 6 mm diam.
Specimen examined: South Korea, Hoengseong, on Vicia amurensis, 4 Aug. 2004, H.D. Shin, holotype CBS H-20845, isotype HAL 1850 F, culture ex-holotype CPC 11414.
Notes: Braun (1995) listed several species of Pseudocercosporella on Fabaceae. None of these occur on Vicia, and only one, Ps. tephrosiae (on Tephrosia, Africa), has conidia of similar length (40-110 × 3-4.5 μm), although they are wider, subcylindrical-acicular, and have 3-6 septa.
Pseudocercosporella oxalidis (Goh & W.H. Hsieh) U. Braun, Nova Hedwigia 55: 218. 1992.
Basionym: Pseudocercospora oxalidis Goh & W.H. Hsieh, Bot. Bull. Acad. Sinica 30: 127. 1989.
Specimen examined: Taiwan, Taipei, Wulai, on living leaves of Oxalis debilis (= O. corymbosa), R. Kirschner, 2258, 22 Feb. 2005, culture CBS 118758.
Septoria Sacc., Syll. Fung. 3: 474. 1884.
Synonyms: See Sutton (1980).
Phytopathogenic and endophytic, occurring on leaves, fruit and stems, causing discrete lesions. Conidiomata pycnidial, immersed, separate or aggregated, globose, papillate or not, brown, with a thin wall of brown textura angularis. Ostiole single, circular, central, sometimes papillate. Conidiophores reduced to conidiogenous cells. Conidiogenous cells hyaline, smooth, ampulliform, doliiform or lageniform to short cylindrical, holoblastic, determinate or indeterminate, proliferating sympodially and/or percurrently; conidiogenous loci unthickened. Conidia solitary, hyaline, multiseptate, guttulate or not, thin-walled, filiform, smooth, continuous or constricted at the septa; hila unthickened.
Type species: Septoria cytisi Desm. Ann. Sci. Nat., Bot., Sér. 3, 8: 24. 1847.
Note: Septoria is polyphyletic (Quaedvlieg et al. 2011).
Clade 11: Sonderhenia
Sonderhenia H.J. Swart & J. Walker, Trans. Brit. Mycol. Soc. 90: 640. 1988.
Foliicolous, phytopathogenic, causing discrete leaf spots. Leaf spots amphigenous, round to confluent and irregular, surrounded by a purple border when young, which becomes dark red to brown and raised with age. Ascomata pseudothecial, amphigenous, on one side of each lesion, often 1-3, intermingled with conidiomata, immersed, black, punctiform, globose to subglobose; apical ostiole substomatal; wall olive-brown, of 3-4 layers of textura angularis, subhymenium of 1-2 layers of colorless cells. Asci fasciculate, bitunicate, subsessile, 8-spored, ovoid to obclavate, straight to incurved. Ascospores 2-3-seriate, hyaline, guttulate, straight or slightly curved, fusiform, 1-septate, widest just above median septum, slightly constricted at septum. Conidiomata pycnidial, amphigenous, subepidermal with central non-projecting ostiole, scattered, black, globose; wall of 2-3 layers of brown cells. Conidiogenous cells minute, olivaceous, proliferating enteroblastically and percurrently, lining the inner pycnidial wall layer. Conidia ellipsoid to cylindrical or ovoid, straight or bent, brown, 3-distoseptate, not constricted, verruculose, apex obtuse, base truncate with marginal frill (adapted from Crous 1998).
Type species: Sonderhenia eucalyptorum H.J. Swart & J. Walker, Trans. Brit. Mycol. Soc. 90: 640. 1988.
Notes: Sonderhenia includes taxa with mycosphaerella-like teleomorphs and pycnidial anamorphs that form brown, transversely distoseptate conidia on brown, percurrently proliferating conidiogenous cells. Only two species, S. eucalypticola and S. eucalyptorum are known.
Clade 12: Pallidocercospora, Scolecostigmina, Trochophora and pseudocercospora-like
Pallidocercospora Crous, gen. nov. MycoBank MB564820. Fig. 17.
Fig. 17.
Pallidocercospora heimii (CPC 1395). Asci, ascospores, germinating ascospores (after 24 h on malt extract agar), hyphae with conidiogenous loci, and conidia. Scale bar = 10 μm.
Etymology: The name reflects the pale brown cercospora-like conidia in this genus.
Foliicolous, phytopathogenic, causing discrete leaf spots. Ascomata single, black, immersed, globose, glabrous; wall of 3-4 layers of medium brown textura angularis. Asci fasciculate, bitunicate, aparaphysate, subsessile, 8-spored, ellipsoid to obclavate or cylindrical, straight or curved, numerous. Ascospores 2-multi-seriate, oblique, overlapping, straight ellipsoidal to obovoid, colourless, smooth, 1-septate. Mycelium predominantly immersed, consisting of olivaceous-brown hyphae, smooth, branched, septate, 2-4 μm diam. Conidiophores in vivo fasciculate, or occurring singly on superficial mycelium as lateral projections, unbranched or branched, septate, cylindrical, straight to geniculate-sinuous, olivaceous-brown. Conidiogenous cells integrated, terminal, cylindrical, straight to geniculate-sinuous, olivaceous-brown, proliferating sympodially or percurrently; conidiogenous loci unthickened, not darker than the surrounding conidiogenous cell. Conidia solitary, straight to irregularly curved, guttulate, pale olivaceous to olivaceous-brown, subcylindrical to narrowly obclavate, multiseptate; hila neither thickened nor darkened.
Type species: Pallidocercospora heimii (Crous) Crous, comb. nov.
Notes: Species of Pallidocercospora have pale olivaceous, smooth conidia (generally referred to as the Mycosphaerella heimii complex; Crous et al. 2004c), and form red crystals when cultivated in agar (on WA, SNA, PDA, MEA), which distinguishes them from Pseudocercospora. Pseudocercospora has several synonyms (see Seifert et al. 2011). Cercoseptoria with its mostly acicular conidia, was correctly treated as synonym of Pseudocercospora by Deighton (1976). Other synonyms include Ancylospora Sawada (based on A. costi), now treated as P. costina; Cercocladospora G.P. Agarwal & S.M. Singh (based on C. adinae, nom. non rite publ.), now treated as P. adinicola; and Helicominia L.S. Olive (based on H. caperonia), now P. caperoniae, and Pantospora Cif. (based on P. guazumae) (see Ellis 1971, Deighton 1976), the muriformly septate conidia of the latter are similar to those of Pseudocercospora pseudostigmina-platani, though Pantospora has been shown to be a genus in its own right (Minnis et al. 2011).
Pallidocercospora acaciigena (Crous & M.J. Wingf.) Crous & M.J. Wingf., comb. nov. MycoBank MB564821.
Basionym: Pseudocercospora acaciigena Crous & M.J. Wingf., Stud. Mycol. 50: 464. 2004.
Teleomorph: “Mycosphaerella” acaciigena Crous & M.J. Wingf., Stud. Mycol. 50: 463. 2004.
Specimen examined: Venezuela, Acarigua, on leaves of Acacia mangium, May 2000, M.J. Wingfield, CBS H-9873, holotype of M. acaciigena and P. acaciigena; cultures ex-type CBS 115432, 112515, 112516 = CPC 3836-3838.
Pallidocercospora crystallina (Crous & M.J. Wingf.) Crous & M.J. Wingf., comb. nov. MycoBank MB564822.
Basionym: Pseudocercospora crystallina Crous & M.J. Wingf., Mycologia 88: 451. 1996.
Teleomorph: “Mycosphaerella” crystallina Crous & M.J. Wingf., Mycologia 88: 451. 1996.
Specimens examined: South Africa, Kwazula-Natal Province,Umvoti, on leaves of Eucalyptus bicostata, Oct. 1994, M.J. Wingfield (holotypes PREM 51922, teleomorph; PREM 51923, anamorph, cultures ex-type CPC 800-802); Kwazula-Natal Province, leaf litter of E. grandis × camaldulensis, Jun. 1995, M.J. Wingfield (PREM 51937, cultures CPC 1178-1180).
Pallidocercospora heimii (Crous) Crous, comb. nov. MycoBank MB564823. Fig. 17.
Basionym: Pseudocercospora heimii Crous, S. African For. J. 172: 4. 1995.
Teleomorph: “Mycosphaerella” heimii Crous, S. African For. J. 172: 2. 1995.
≡ “Mycosphaerella” heimii Bouriquet, Encycl. Mycol. 12: 418. 1946, nom. nud.
Specimens examined: Madagascar, Moramanga, on leaves of Eucalyptus sp., Apr. 1994, P.W. Crous, PREM 51749, holotype of teleomorph; PREM 51748, holotype of anamorph, cultures ex-type CPC 760-761 = CBS 110682.
Pallidocercospora heimioides (Crous & M.J. Wingf.) Crous & M.J. Wingf., comb. nov. MycoBank MB564824.
Basionym: Pseudocercospora heimioides Crous & M.J. Wingf., Can. J. Bot. 75: 787. 1997.
Teleomorph: “Mycosphaerella” heimioides Crous & M.J. Wingf., Can. J. Bot. 75: 787. 1997.
Specimens examined: Indonesia, N. Sumatra, Lake Toba area, leaves of Eucalyptus sp., Mar. 1996, M.J. Wingfield, holotype of teleomorph PREM 54966; holotype of anamorph PREM 54967; cultures ex-type CPC 1311, 1312 = CBS 111190).
Pallidocercospora holualoana (Crous, Joanne E. Taylor & M.E. Palm) Crous, comb. nov. MycoBank MB564825.
Basionym: “Mycosphaerella” holualoana Crous, Joanne E. Taylor & M.E. Palm, Mycotaxon 78: 458. 2001.
Specimen examined: USA, Hawaii, Kona district, Holualoa, on a living leaf of Leucospermum sp., P.W. Crous & M.E. Palm, 17 Nov. 1998, holotype PREM 56926, cultures ex-type CPC 2126-2128).
Pallidocercospora irregulariramosa (Crous & M.J. Wingf.) Crous & M.J. Wingf., comb. nov. MycoBank MB564826.
Basionym: Pseudocercospora irregulariramosa Crous & M.J. Wingf., Can. J. Bot. 75: 785. 1997.
Teleomorph: “Mycosphaerella” irregulariramosa Crous & M.J. Wingf., Can. J. Bot. 75: 785. 1997.
Specimens examined: South Africa, Northern Province, Tzaneen, on leaves of Eucalyptus saligna, Mar. 1996, M.J. Wingfield, holotype of teleomorph PREM 54964; holotype of anamorph PREM 54965; cultures ex-type CPC 1360 = CBS 114777).
Pallidocercospora konae (Crous, Joanne E. Taylor & M.E. Palm) Crous, comb. nov. MycoBank MB564827.
Basionym: “Mycosphaerella” konae Crous, Joanne E. Taylor & M.E. Palm, Mycotaxon 78: 459. 2001.
Specimen examined: USA, Hawaii, Kona district, Holualoa, on a living leaf on Leucadendron cv. Safari Sunset, 17 Nov. 1998, P.W. Crous & M.E. Palm, holotype PREM 56921; ex-type cultures CPC 2123-2125.
Scolecostigmina U. Braun, N. Z. J. Bot. 37: 323. 1999. Fig. 18.
Fig. 18.
Scolecostigmina mangiferae (CBS 125467). A. Leaf spots on upper and lower leaf surface. B. Close-up of leaf spot with fruiting. C-E. Fascicles with conidiophores and conidiogenous cells (note rough percurrent proliferations). F. Conidia. Scale bars = 10 μm.
Foliicolous, phytopathogenic, associated with leaf spots. Mycelium immersed, consisting of septate, branched, pigmented hyphae. Sporodochia immersed to erumpent; stromata subglobose to applanate, composed of brown, angular to subglobose cells. Conidiophores numerous, densely aggregated, arising from stroma, subcylindrical or somewhat tapered towards the apex, occasionally ampulliform, continuous or septate, pigmented, wall somewhat thickened, usually verruculose; conidiogenous cells integrated, terminal or at times conidiophores reduced to conidiogenous cells, holoblastic, proliferating percurrently via conspicuous annellations. Conidia solitary, scolecosporous, usually subcylindrical-obclavate, transversely pluriseptate, occasionally with few longitudinal or oblique septa, euseptate, rarely with few intermixed distosepta, thick-walled, pigmented, dark, smooth to verrucose, apex obtuse to subacute, base truncate or obconically truncate; secession schizolytic (adapted from Braun et al. 1999).
Type species: Scolecostigmina mangiferae (Koord.) U. Braun & Mouch., N. Z. J. Bot. 37: 323. 1999.
Specimen examined: Australia, Queensland, Mareeba, S16°58’75.5” E145°20’60.8”, leaves of Mangifera indica, 10 Aug. 2009, P.W. Crous & R.G. Shivas, CBS H-20846, culture CPC 17352, 17351 = CBS 125467.
Trochophora R.T. Moore, Mycologia 47: 90. 1955. Fig. 19.
Fig. 19.
Trochophora simplex (CBS 124744). A. Leaf spots on upper and lower leaf surface. B, C. Close-up of leaf spot with fruiting. D-G. Fascicles with conidiophores and conidiogenous cells. H, I. Conidia. Scale bars = 10 μm.
Foliicolous, but pathogenicity unproven. Colonies hypophyllous, medium to dark brown, consisting of numerous synnemata. Stroma absent, but with a superficial network of hyphae linking the various synnemata. Conidiophores synnematous, mostly unbranched and straight, or with 1-2 short branches, straight or curved, cylindrical, individual conidiophores tightly aggregated, but separating near the apex, pale to medium brown, smooth. Conidiogenous cells polyblastic, integrated, terminal, determinate to sympodial, with visible unthickened scar, clavate. Conidia solitary, terminal or lateral on conidiogenous cells, prominently curved to helicoid, pale to medium brown, smooth, transversely euseptate with a darkened, thickened band at the septa (adapted from Crous et al. 2009a).
Type species: Trochophora simplex (Petch) R.T. Moore, Mycologia, 47: 90. 1955.
Specimens examined: Japan, Shimane, on Daphniphyllum teijsmannii, 26 April 2008, C. Nakashima & I. Araki, MUMH 11134, culture MUCC 952. South Korea, Jeju, Halla arboretum, on D. macropodum, 29 Oct. 2005, H.D. Shin, CBS H-20847, culture CBS 124744.
Notes: Other pseudocercospora-like species found in this clade are P. colombiensis (foliar pathogen of Eucalyptus; Crous 1998), and P. thailandica (foliar pathogen of Acacia; Crous et al. 2004d), both also having mycosphaerella-like teleomorphs. Morphologically, these taxa appear typical members of Pseudocercospora s. str. so it would be difficult to identify these as different from Pseudocercospora without the aid of DNA sequence comparisons.
Clade 13: Passalora-like
Notes: This clade is represented by Passalora eucalypti, which was originally described as a leaf spot pathogen of Eucalyptus saligna in Brazil (Crous 1998, Crous & Braun 2003). Recently, a second species was found to belong to this clade, namely Passalora leptophlebiae, which was described from Eucalyptus leptophlebia leaves collected in Brazil (Crous et al. 2011a). Both species are charaterised by fasciculate conidiophores and catenate, pale brown conidia, with thickened, darkened and refractive scars and hila.
Clade 14: Pseudocercospora s. str.
Pseudocercospora Speg., Anales Mus. Nac. Hist. Nat. Buenos Aires, Ser. 3, 20: 437. 1910.
Foliicolous, chiefly phytopathogenic, but also endophytic; commonly associated with leaf spots, but also occurring on fruit. Mycelium internal and external, consisting of smooth, septate, subhyaline to brown, branched hyphae. Stroma absent to well-developed. Conidiophores in vivo arranged in loose to dense fascicles, sometimes forming distinct synnemata or sporodochia, emerging through stomata or erumpent through the cuticle, often arising from substomatal or subcuticular to intraepidermal stromata, or occurring singly on superficial hyphae, short to long, septate or continuous, i.e. conidiophores may be reduced to conidiogenous cells, simple to branched and straight to geniculate-sinuous, pale to dark brown, smooth to finely verruculose. Conidiogenous cells integrated, terminal, occasionally intercalary, polyblastic, sympodial, or monoblastic, proliferating percurrently via inconspicuous or darkened, irregular annellations, at times denticulate, pale to dark brown; scars inconspicous, or only thickened along the rim, or flat, and slightly thickened and darkened, but never pronounced. Conidia solitary, rarely in simple chains, subhyaline, olivaceous, pale to dark brown, usually scolecosporous, i.e. obclavate-cylindrical, filiform, acicular, and transversely plurieuseptate, occasionally also with oblique to longitudinal septa, conidia rarely amero- to phragmosporous, short subcylindrical or ellipsoidal-ovoid, aseptate or only with few septa, apex subacute to obtuse, base obconically truncate to truncate, or bluntly rounded, with or without a minute marginal frill, straight to curved, rarely sigmoid, smooth to finely verruculose; hila usually unthickened, not darkened, at most somewhat refractive, occasionally slightly thickened along the rim, or rarely flat, and slightly thickened and darkened, but never pronounced.
Type species: P. vitis (Lév.) Speg., Anales Mus. Nac. Hist. Nat. Buenos Aires, Ser. 3, 20: 438. 1910.
Specimens examined: South Korea, Namyangju, on Vitis vinifera, 30 Sep. 2004, H.D. Shin, CBS H-20848, CPC 11595 = CBS 132012; V. vinifera, 1 Oct. 2007, H.D. Shin, CPC 14661 = CBS 132112.
Pseudocercospora abelmoschi (Ellis & Everh.) Deighton, Mycol. Pap. 140: 138. 1976. Fig. 20.
Fig. 20.
Pseudocercospora abelmoschi (CPC 14478). A. Leaf spots on upper and lower leaf surface. B. Close-up of leaf spot with fruiting. C-G. Hyphae giving rise to conidiogenous cells and conidia. H. Conidia. Scale bars = 10 μm.
Basionym: Cercospora abelmoschi Ellis & Everh., J. Inst. Jamaica 1: 347. 1893.
= Cercospora hibisci Tracy & Earle, Bull. Torrey Bot. Club 22: 179. 1895.
= Cercospora hibisci-manihotis Henn., Hedwigia 43: 146. 1904.
Specimen examined: South Korea, Suwon, on Hibiscus syriacus, 2 Oct. 2007, H.D. Shin, CBS H-20849, CPC 14478 = CBS 132103.
Pseudocercospora ampelopsis Crous, U. Braun & H.D. Shin, sp. nov. MycoBank MB564828. Fig. 21.
Fig. 21.
Pseudocercospora ampelopsis (CPC 11680). A. Leaf spots on upper and lower leaf surface. B. Close-up of leaf spot with fruiting. C. Conidiophores and conidiogenous cells. D. Conidia. Scale bar = 10 μm.
Etymology: Name derived from the host Ampelopsis, from which it was collected.
Leaf spots amphigenous, irregular to subcircular, 2-8 mm diam, dark brown on upper surface, dull brownish green on lower surface. Mycelium internal and external, pale brown to brown, consisting of septate, branched, smooth, 1.5-4 μm diam hyphae, anastomosing on surface. Caespituli fasciculate, brown, amphigenous, emerging through stomata (but stromata lacking). Conidiophores aggregated in loose fascicles, or solitary, arising from superficial mycelium, medium to dark brown, smooth to finely verruculose, 3-6-septate, subcylindrical, straight to variously curved, unbranched, 20-80 × (2.5-)3-5(-6) μm. Conidiogenous cells terminal, unbranched, brown, finely verruculose, tapering to flat-tipped apical loci, proliferating sympodially, 10-15 × 4-5 μm. Conidia solitary, dark brown, finely verruculose, guttulate, obclavate-cylindrical, apex obtuse, base obconically subtruncate, straight to gently curved, 3-12-septate, (35-)40-90(-110) × 3-5(-6) μm; hila unthickened, neither darkened nor refractive, 2 μm diam.
Culture characteristics: Colonies after 2 wk at 24 °C in the dark on MEA; surface folded, erumpent, spreading, with sparse aerial mycelium, and smooth, lobate margins. Surface olivaceous-grey; reverse iron-grey. Colonies reaching 7 mm diam.
Specimen examined: South Korea, Hongcheon, on Ampelopsis glandulosa var. heterophylla, 24 Oct. 2004, H.D. Shin, holotype CBS H-20850, isotype HAL 1866 F, culture ex-type CPC 11680 = CBS 131583.
Notes: Pseudocercospora brachypus, which also occurs on Ampelopsis, has much shorter and narrower conidia, 25-60 × 2-3.5 μm (Guo & Hsieh 1995). Pseudocercospora ampelopsis is morphologically close to P. riachuelii var. horiana on Ampelocissus, Cissus and Parthenocissus species (Crous & Braun 2003). The two are similar in that conidiophores are solitary and form in fascicles and arise from superficial hyphae, and conidia of the two taxa are similar in size. Pseudocercospora ampelopsis differs in having much longer pluriseptate conidiophores whereas those of P. riachuelii var. horiana are much shorter and 0-1-septate.
Pseudocercospora angolensis (T. Carvalho & O. Mendes) Crous & U. Braun, Sydowia 55: 301. 2003.
Basionym: Cercospora angolensis T. Carvalho & O. Mendes, Bol. Soc. Brot. 27: 201. 1953.
≡ Phaeoramularia angolensis (T. Carvalho & O. Mendes) P.M. Kirk, Mycopathologia 94: 177. 1986.
≡ Pseudophaeoramularia angolensis (T. Carvalho & O. Mendes) U. Braun, Cryptog. Mycol. 20: 171. 1999.
Specimens examined: Angola, Mozambique Province, on leaves of Citrus × aurantium (= × sinensis), Dec. 1951, Carvalho & O. Mendes, BPI 432660, BPI 442839 (paratypes), BPI 442837 (holotype), IMI 56597 (isotype). Camaroon, Yaoundé, on leaves of C. × aurantium, 17 Mar. 1978, E. Milla, IMI 252792. Ethiopia, on leaves of Citrus sp., IMI 361170. Kenya, on leaves of C. × aurantium, 15 Nov. 1991, A. Seif W3753, IMI 351626. Uganda, on leaves of C. × aurantium, 14 Jun. 1991, W.T.H. Peregrine, IMI 384297. West Africa, intercepted at San Pedro, California, USA, on leaves of Citrus sp., 2 Oct. 1953, L.A. Hart, BPI 432661, BPI 432659. Zambia, on leaves of Citrus sp., 18 Jun. 1973, R.H. Raemakers 7837, IMI 176562; Chilanga, on leaves of C. × aurantium, 28 Sep. 1983, D.M. Naik, IMI 280618; Chilanga, on leaves of Citrus sp., 18 Jul. 1975, B.K. Patel, IMI 196889; Lusaka, on leaves of Citrus sp., 17 June 1977, I. Javaid, IMI 214501. Zimbabwe, Bindura, on leaves of Citrus sp., 13 Aug. 1979, A. Rothwell, IMI 240682; on leaves of Citrus sp., Sep. 2000, M.C. Pretorius, epitype designated here CBS H-20851, culture ex-epitype CPC 4112-4118, 4111 = CBS 112933.
Pseudocercospora araliae (Henn.) Deighton, Mycol. Pap. 140: 19. 1976. Fig. 22.
Fig. 22.
Pseudocercospora araliae (CPC 10154). A. Leaf spots on upper and lower leaf surface. B. Close-up of leaf spot with fruiting. C-E. Fascicles with conidiophores and conidiogenous cells. F. Conidia. Scale bars = 10 μm.
Basionym: Cercospora araliae Henn., Bot. Jahrb. Syst. 31: 742. 1902; also 37: 165. 1906.
≡ Cercosporiopsis araliae (Henn.) Miura, Fl. Manchuria & E. Mongolia, 27, 3: 533. 1928.
= Cercospora atromaculans auct., non Ellis & Everh.
Specimens examined: Japan, Tosa, Ushioe-yama, on Aralia elata var. glabrescens, Aug. 1901, T. Yoshinaga, holotype B 700015014; A. elata, T. Kobayashi & C. Nakashima, epitype designated here TFM: FPH-8094, ex-epitype cultures MUCC 873, MAFF 238192. South Korea, Jeju, Halla Arboretum, on A. elata, 14 Sep. 2002, H.D. Shin, CBS H-20852, culture CPC 10154; Wonju, on A. elata, 21 Sep. 2003, H.D. Shin, CBS H-20853, cultures CPC 10782-10784.
Pseudocercospora atromarginalis (G.F. Atk.) Deighton, Mycol. Pap. 140: 139. 1976. Fig. 23.
Fig. 23.
Pseudocercospora atromarginalis (CPC 11372-11374). A. Leaf spots on upper and lower leaf surface. B. Close-up of leaf spot with fruiting. C, D. Fascicles with conidiophores and conidiogenous cells. E. Conidia. Scale bar = 10 μm.
Basionym: Cercospora atromarginalis G.F. Atk. (atramarginalis), J. Elisha Mitchell Sci. Soc. 8: 59. 1892.
= Cercospora rigospora G.F. Atk., J. Elisha Michell Sci. Soc. 8: 65. 1892.
= Cercospora tosensis Henn., Bot. Jahrb. Syst. 34: 605. 1905.
= Cercospora nigri Tharp, Mycologia 9: 112. 1917.
= Cercospora solani-biflori Sawada, Formosan Agric. Rev. 39: 701. 1942, nom. inval.
Specimens examined: Japan, Prov. Tosa, Aki-machi, on Solanum nigrum, Oct. 1903, Yoshinaga No. 43, (holotype of C. tosensis, B 700015016). South Korea, Namyangju, on S. nigrum, 27 Jul. 2004, H.D. Shin, CBS H-20854, CPC 11372-11374. New Zealand, Auckland, Jan. 2004, C.F. Hill 970, CBS 114640.
Notes: Pseudocercospora atromarginalis was described from Solanum collected in Auburn Alabama, USA. Material studied here from New Zealand and Korea represents the same species, which might be authentic for the name. Fresh material from Solanum in the USA, and a detailed study of the synonyms listed by Chupp (1954) would resolve this issue. An isolate identified as P. chengtuensis (on Lycium, Solanaceae) appears identical to Pseudocercospora atromarginalis.
Pseudocercospora balsaminae (Syd.) Deighton, Mycol. Pap. 140: 139. 1976. Fig. 24.
Fig. 24.
Pseudocercospora balsaminae (CPC 10044). A. Leaf spots on upper and lower leaf surface. B. Close-up of leaf spot with fruiting. C-F. Fascicles and solitary conidiophores with conidiogenous cells. G. Conidia. Scale bars = 10 μm.
Basionym: Cercoseptoria balsaminae Syd., Ann. Mycol. 33: 69. 1935.
Specimens examined: South Korea, Chuncheon, on Impatiens textorii, 11 Oct. 2002, H.D. Shin, CBS H-20856, CPC 10044 = CBS 131882; Dongducheon, on I. textorii, 11 Oct. 2004, H.D. Shin, CBS H-20855, CPC 10699-10701.
Pseudocercospora callicarpae (Cooke) Y.L. Guo & W.X. Zhao, Acta Mycol. Sin. 8: 118. 1989.
Basionym: Cercospora callicarpae Cooke, Grevillea 6: 140. 1878.
= ? Cercospora callicarpicola Naito, Mem. Coll. Agric. Kyoto Imp. Univ. 47: 49. 1940.
Specimen examined: Japan, Ibaraki, on Callicarpa japonica, 11 Sep. 1998, T. Kobayashi, MUCC 888, MAFF 237784, CNS-442.
Pseudocercospora catalpigena U. Braun & Crous, Mycol. Progr. 2: 198. 2003.
Specimen examined: Japan, Wakayama, on Catalpa ovata, 30 Oct. 2007, C. Nakashima & I. Araki, MUMH 10868, culture MUCC 743.
Pseudocercospora catappae (Henn.) X.J. Liu & Y.L. Guo, Mycosystema 2: 230. 1989.
Basionym: Cercospora catappae Henn., Bot. Jahrb. Syst. 34: 56. 1905.
= Pseudocercospora catappae Goh & W.H. Hsieh, in Hsieh & Goh, Cercospora and similar fungi from Taiwan: 57. 1990, homonym of P. catappae (Henn.) X.J. Liu & Y.L. Guo, 1989.
= Ramularia catappae Racib., Paras. Algen u. Pilze Javas II, Batavia: 41. 1900.
= Cercospora terminaliae Sawada (terminariae), Taiwan Agric. Rev. 38: 701. 1942, nom. illeg., homonym of C. terminaliae Syd. 1929.
Specimens examined: Tanzania, Zanzibar, Dar-es-Salam, on Terminalia catappa, 26 Oct. 1901, Stuhlmann holotype B 700015015. Japan, Okinawa, on T. catappa, 17 Nov. 2007, C. Nakashima & T. Akashi, MUMH 10913, culture MUCC 809.
Pseudocercospora cercidicola Crous, U. Braun & C. Nakash., sp. nov. MycoBank MB564829. Fig. 25.
Fig. 25.
Pseudocercospora cercidicola (CBS H-20895). A. Leaf spots on upper and lower leaf surface. B. Close-up of leaf spot with fruiting. C, D. Fascicles with conidiophores and conidiogenous cells. E, F. Conidiophores on superficial hyphae. G. Conidia. Scale bars = 10 μm.
Etymology: Name reflects the host Cercis, from which it was collected.
Leaf spots amphigenous, irregular to angular, 1-5 mm diam, confined by leaf veins, brown on upper surface, with raised, dark brown border, on lower surface medium brown, with indistinct borders. Mycelium internal, consisting of pale brown, smooth, septate, branched, 2-3 μm diam hyphae. Caespituli fasciculate to sporodochial, amphigenous, but predominantly epiphyllous, grey-brown on leaves, up to 130 μm wide and 150 μm high. Conidiophores aggregated in dense fascicles arising from the upper cells of a brown stroma up to 80 μm wide and 60 μm high; conidiophores brown, finely verruculose, 2-6-septate, subcylindrical, straight to variously curved, unbranched or branched above, 20-50 × 3-5 μm. Conidiogenous cells terminal or lateral, unbranched, medium brown, finely verruculose, tapering to flat-tipped apical loci, proliferating sympodially, 10-20 × 2-3 μm. Conidia solitary, medium brown, smooth, guttulate, subcylindrical to narrowly obclavate, apex subobtuse, base long obconically subtruncate, straight to variously curved, (0-)3-6-septate, (27-)30-50(-60) × (2.5-)3(-3.5) μm; hila neither thickened, nor darkened-refractive, 1.5-2 μm diam.
Culture characteristics: Colonies on MEA 10-15 mm after 2 wk at 20 °C in the dark, restricted, with margin mildly lobed, felty, pale olivaceous or greyish olivaceous, surrounded by greyish margin; reverse olivaceous.
Specimens examined: Japan, Ibaraki, on Cercis chinensis, 10 Sep. 1998, T. & Y. Kobayashi, holotype CBS H-20895, culture ex-type MUCC 896, MAFF 237791 = CBS 132041; Tokyo, Koishikawa Botanical Garden, on Cercis chinensis, 10 Nov. 2007, I. Araki & M. Harada, MUMH 11108, culture MUCC 937; Japan, Kanagawa, on Cercis chinensis, May 1992, K.Kishi, culture MAFF 237128.
Notes: Asian collections of cercosporoid fungi on Cercis chinensis were considered as representative of Cercospora chionea by Chupp (1954). The latter species was shown to be a member of Passalora by Braun (1993). Shin & Braun (2000) introduced a new species of Pseudocercospora for the taxon occurring on Cercis in Asia, namely P. cercidis-chinensis, based on material collected in Korea. Phylogenetic data obtained in the present study (Fig. 5) show that the Japanese collections are distinct. As the name Cercospora cercidis Nishikado is illegitimate, a new name, P. cercidicola is introduced for the species occurring on Cercis in Japan. Pseudocercospora cercidicola is morphologically very close to P. cercidis-chinensis but superficial hyphae with solitary conidiophores are not formed and the conidia are shorter.
Pseudocercospora cercidis-chinensis H.D. Shin & U. Braun, Mycotaxon 74: 109. 2000. Fig. 26.
Fig. 26.
Pseudocercospora cercidis-chinensis (CPC 14481). A. Leaf spots on upper and lower leaf surface. B. Close-up of leaf spot with fruiting. C, D. Fascicles with conidiophores and conidiogenous cells. E. Conidia. Scale bars = 10 μm.
Specimens examined: South Korea, Kyeongju, on Cercis chinensis, 26 Aug. 1998, H.D. Shin, holotype KUS-F 14914, isotype HAL; Suwon, C. chinensis, 2 Oct. 2007, H.D. Shin, epitype designated here CBS H-20857, culture ex-epitype CPC 14481 = CBS 132109.
Note: See P. cercidicola.
Pseudocercospora chengtuensis (F.L. Tai) Deighton, Mycol. Pap. 140: 141. 1976. Fig. 27.
Fig. 27.
Pseudocercospora chengtuensis (CPC 10696-10698). A. Leaf spots on upper and lower leaf surface. B. Close-up of leaf spot with fruiting. C-G. Fascicles with conidiophores and conidiogenous cells. H. Conidia. Scale bars = 10 μm.
Basionym: Cercospora chengtuensis F.L. Tai, Lloydia 11: 40. 1948.
Specimens examined: China, Szechuan, Chengtu, Lycium chinense, Lee Ling No. 126, 1943, holotype (not seen). South Korea, Dongducheon, Lycium chinense, 28 Sep. 2003, H.D. Shin, CBS H-20858, culture CPC 10696-10698.
Notes: The isolate identified here as P. chengtuensis appears to be identical to P. atromarginalis (also on Solanaceae) based on phylogenetic analysis and the two are morphologically similar. Study of of additional collections of both are needed to determine whether they are synonymous or distinct species.
Pseudocercospora chionanthi-retusi Goh & W.H. Hsieh, in Hsieh & Goh, Cercospora and similar fungi from Taiwan: 249. 1990. Fig. 28.
Fig. 28.
Pseudocercospora chionanthi-retusi (CPC 14683). A. Leaf spots on upper and lower leaf surface. B. Close-up of leaf spot with fruiting. C-H. Fascicles and solitary conidiophores with conidiogenous cells. I. Conidia. Scale bars = 10 μm.
-
= Cercospora chionanthi-retusi Togashi & Katsuki, Sci. Rep. Yokohama Nat. Univ. Sect. II, 1: 1. 1952.
≡ Pseudocercospora chionanthi-retusi (Togashi & Katsuki) Nishijima, C. Nakash. & Tak. Kobay., Mycoscience 40: 270. 1999, nom. illeg., homonym of P. chionanthi-retusi Goh & Hsieh, 1990.
= Pseudocercospora chionanthicola C. Nakash. & Tak. Kobay., Mycoscience 43: 98. 2002.
Specimen examined: South Korea, Osan, on Chionanthus retusus, 30 Oct. 2007, H.D. Shin, CBS H-20859, culture CPC 14683 = CBS 132110.
Pseudocercospora chrysanthemicola (J.M. Yen) Deighton, Mycol. Pap. 140: 141. 1976.
Basionym: Cercospora chrysanthemicola J.M. Yen, Rev. Mycol. 29: 216. 1964.
Specimen examined: South Korea, Seoul, on Chrysanthemum sp., 6 Sep. 2003, H.D. Shin, CPC 10633.
Pseudocercospora contraria (Syd. & P. Syd.) Deighton, Mycol. Pap. 140: 30. 1976. Fig. 29.
Fig. 29.
Pseudocercospora contraria (CPC 14714). A. Leaf spots on upper and lower leaf surface. B. Close-up of leaf spot with fruiting. C-E. Fascicles with conidiophores, and solitary loci on hyphae. F. Conidia. Scale bars = 10 μm.
Basionym: Cercospora contraria Syd. & P. Syd., Ann. Mus. Congo, Bot., Ser. V, 3: 21. 1909.
= Cercospora wildemanii Syd. & P. Syd., Ann. Mus. Congo, Bot., Ser. V, 3: 21. 1909.
= Mycosphaerella contraria Hansf., Proc. Linn. Soc. London 153: 22. 1941.
Specimen examined: South Korea, Bukjeju, Jeolmul recreation forest, on Dioscorea quinqueloba, 2. Nov. 2007, H.D. Shin, CBS H-20861, CPC 14714 = CBS 132108.
Notes: This fungus was first reported from Korea by Shin & Kim (2001). Conidial measurements (16-75 × 2.5-4.5 μm) are smaller than those of the type collected in the Democratic Republic of the Congo (20-120 × 5-8 μm, Chupp 1954), and the Korean material may eventually be shown to represent a distinct species.
Pseudocercospora coriariae (Chupp) X.J. Liu & Y.L. Guo, Mycosystema 2: 232. 1989.
Basionym: Cercospora coriariae Chupp, J. Dept. Agric. Puerto Rico 14: 285. 1930.
= Cercospora coriariae F.L. Tai, Lloydia 11: 43. 1948, nom. illeg., homonym of C. coriariae Chupp, 1930.
Specimen examined: Japan, Tokyo, on Coriaria japonica, 10 Nov. 2007, I. Araki & M. Harada, MUMH 10942, culture MUCC 840.
Pseudocercospora cornicola (Tracy & Earle) Y.L. Guo & X.J. Liu, Mycosystema 2: 232. 1989.
Basionym: Cercospora cornicola Tracy & Earle, Bull. Torrey Bot. Club 23: 205. 1896.
Specimen examined: Japan, Tokyo, Cornus alba var. sibirica, 7 Nov. 1998, C. Nakashima & E. Imaizumi, CNS-494, culture MUCC 909, MAFF 237773.
Pseudocercospora corylopsidis (Togashi & Katsuki) C. Nakash. & Tak. Kobay., Mycoscience 40: 270. 1999.
≡ Cercospora corylopsidis Togashi & Katsuki, Bot. Mag. (Tokyo) 65: 20. 1952.
= Cercospora hamamelidis auct.; sensu Togashi & Katsuki, Bot Mag. (Tokyo) 65: 21. 1952, non (Peck) Ellis & Everh.
Specimens examined: Japan, Kagoshima, on Corylopsis pauciflora, 26 Oct. 1949, S. Katsuki, holotype YNU, Isotype TNS-F-243824; Ibaraki, Tsukuba Botanical Garden, on C. pauciflora, Oct. 1996, T. Kobayashi; Ibaraki, on C. pauciflora, 9 Nov. 1998, T. Kobayashi; Tokyo, Todori, on C. pauciflora, 12 Oct. 1979, M. Kusunoki, TFM:FPH-6152; Tokyo, Jindai Bot. Park, on C. spicata, 7 Nov. 1998, C. Nakashima & E. Imaizumi, epitype designated here TFM: FPH-8095, ex-epitype cultures MUCC 908, MAFF 237795; Saitama, isolated from C. pauciflora, Nov. 1995, MUCC1249, MAFF 237302; Kagoshima, 26 Oct. 1949, on Hamamelis japonica, S. Katsuki, SK2077; Shizuoka, 2 Nov. 1996, on H. japonica, T. Koboyashi & C. Nakashima, CNS-114, cultures MAFF 237632, MUCC 874.
Notes: Isolate MUCC 874, which was isolated from Hamamelis japonica (Hamamelidaceae), appears to be phylogenetically identical to P. corylopsidis. Based on morphology, there is little difference between these specimens other than the presence or absence of external mycelium.
Togashi & Katsuki (1952) reported a fungus on Hamamelis japonica as Cercospora hamamelidis (Peck) Ellis & Everh. based on a specimen collected in Kagoshima (SK2077). Recently, C. hamamelidis was transferred to the genus Passalora (Crous & Braun 2003). The Japanese specimens of C. hamamelidis are morphologically and phylogenetically identical to Pseudocercospora corylopsidis. We conclude that the fungus on Corylopsis and Hamamelis in Japan represents P. corylopsidis. In addition, a species of Pseudocercospora collected in Tokyo (TFM:FPH-4348, isolate MAFF 410032) was recognised as a distinct taxon on Corylopsis plants, based on its longer and narrower conidia, and DNA phylogeny.
Pseudocercospora cotoneastri (Katsuki & Tak. Kobay.) Deighton, Trans. Brit. Mycol. Soc. 88: 389. 1987.
Basionym: Cercospora cotoneastri Katsuki & Tak. Kobay. (as “cotoneasteris”), Trans. Mycol. Soc. Japan 17: 276. 1976.
Specimens examined: Japan, Tokyo, Asakawa Experimental Forest Station, on Cotoneaster dammeri, 13 Aug. 1974, T. Kobayashi, holotype TFM:FPH-4185, ex-holotype culture MAFF 410089; Tokyo, Tokyo Agric. Exp. Stn., on C. franchetii, 27 Sep. 1978, T. Kobayashi, TFM:FPH-4924; Tokyo, Jindai Bot. Park, on C. horizontalis, 4 Sep. 1975, H. Horie, TFM: FPH-4417; Tokyo, on C. horizontalis, 23 Oct. 1975, K. Sasaki, TFM:FPH-4798; Tokyo, culture isolated from Cotoneater sp., 1977, H. Horie, culture MAFF 305633; Fukuoka, Kitakyushu, on C. horizontalis,4 Oct. 1975, S. Ogawa (TFM:FPH-4401); Shizuoka, Hamamatsu, on C. salicifolius, 1 Nov. 1996, T. Kobayashi & C. Nakashima, CNS-126, culture MUCC 876, MAFF 237629.
Note: Three isolates including the ex-holotype, MAFF 410089, 305633 and 237629, were identical based on ACT gene sequence data (data not shown).
Pseudocercospora crispans G.C. Hunter & Crous, sp. nov. MycoBank MB564830. Fig. 30.
Fig. 30.
Pseudocercospora crispans (CPC 14883). A. Leaf spots on upper and lower leaf surface. B. Close-up of leaf spot with fruiting. C-F. Fascicles with conidiophores and conidiogenous cells. G, H. Conidia. Scale bars = 10 μm.
Etymology: Name reflects the characteristic curling or undulate nature of the conidia produced by this fungus.
Leaf spots amphigenous, angular to irregular, predominantly occurring next to or close to the mid-rib, 2-15 mm diam, pale brown on the upper side of the leaf, and pale to darker brown on the bottom side of the lesion, surrounded by a raised, dark brown border with a diffuse red pigment emanating away from the border; single, discrete lesions may coalesce to form larger lesions. Mycelium smooth, septate, guttulate, thick-walled, branched, internal and external, pale brown, 2-4 mm wide. Caespituli amphigenous, sparsely scattered over lesion, floccose, whitish. Stromata hypophyllous, brown, well-developed, immersed, globular to irregular, 40-120 mm diam. Conidiophores brown at the base, becoming paler toward apex, arising from cells of brown stroma; arranged in loose fascicles, smooth, thick-walled, guttulate, unbranched, straight to curved, 0-4-septate, straight to geniculate-sinuous, (14-)17-31(-42) × (2-)3-4(-5) μm. Conidiogenous cells terminal, unbranched, smooth, guttulate, pale brown, straight to geniculate to geniculate-sinuous, proliferating sympodially and percurrently, tapering toward apex; apex obtuse to truncate, (8-)9-15(-19) × (2-)3(-4) μm. Conidia solitary, smooth, guttulate, curved to undulate, pale brown, 3-9-septate, apex acute to subacute, base truncate, (40-)65-96(-102) × (2-)3(-4) μm; hila unthickened, not darkened.
Culture characteristics: Colonies on MEA reaching 54 mm diam after 30 d at 24 °C. Colonies circular, flat to slightly convex, with a feathery margin and profuse aerial mycelium; lavender-grey to glaucous-grey (surface) and olivaceous-grey (reverse).
Specimen examined: South Africa, Western Cape Province, Knysna, on leaves of Eucalyptus sp., Jan. 2008, P.W. Crous, holotype CBS H-20392, culture ex-type CPC 14883 = CBS 125999.
Notes: Pseudocercospora crispans is phylogenetically distinct from other taxa described from Eucalyptus (Crous et al. 1989, Crous & Alfenas 1995, Crous & Wingfield 1997, Crous 1998, Braun & Dick 2002, Hunter et al. 2006a), and can be distinguished morphologically by its prominently curled conidia.
Pseudocercospora crocea Crous, U. Braun, G.C. Hunter & H.D. Shin, sp. nov. MycoBank MB564831. Fig. 31.
Fig. 31.
Pseudocercospora crocea (CPC 11668). A, B. Leaf spots on upper and lower leaf surface. C. Close-up of leaf spot with fruiting. D, E. Fascicles with conidiophores and conidiogenous cells. F-I. Conidia. Scale bars = 10 μm.
Etymology: Name reflects the typical diffuse yellow border surrounding leaf lesions caused by this fungus.
Leaf spots distinct, scattered and at the leaf margin, pale brown to brown, circular to irregular, 2-5 mm diam, indefinite border, with a pale yellow diffuse halo. Mycelium, internal and external, subhyaline, septate, branched, smooth, 2-5 mm wide. Caespituli amphigenous, grey, scattered over the lesion surface, arachnoid. Stromata well-developed, 40-100 mm diam, subimmersed, globular, dark brown. Conidiophores fasciculate, brown, becoming paler toward the apex, 0-1-septate, smooth, unbranched, straight to curved, apex truncate to subtruncate, 0-1-septate, (14-)17-24(-32) × (3-)4(-5) μm. Conidiogenous cells terminal, unbranched, pale brown, smooth to slightly verruculose, proliferating percurrently, (9-) 13-18(-21) × (3-)4(-5) μm. Conidia solitary, 4-10-septate, straight to curved, sparsely guttulate, narrowly obclavate, apex subobtuse, base obconically truncate to long obconically truncate, smooth, subhyaline, (67-)79-94(-104) × (3-)4(-5)μm, hila unthickened not darkened.
Culture characteristics: Colonies on MEA reaching 53 mm diam after 30 d at 24 °C. Colonies circular with feathery margin, flat to slightly convex, some folding occurs, with a darker radial ring toward the colony margin, aerial mycelium medium; iron-grey to olivaceous-grey (surface) and iron-grey (reverse).
Specimen examined: South Korea, Suwon, on leaves of Pilea hamaoi (≡ P. pumila var. hamaoi), 5 Nov. 2004, H.D. Shin, holotype CBS H-20387, isotype HAL 1860 F, cultures ex-type CPC 11668 = CBS 126004.
Notes: Singh et al. (1996) provide an account of the Pseudocercospora spp. present on members of Urticaceae. Of these, P. crocea is most similar to P. pileae as it also has a well-developed stroma. Pseudocercospora pileae is distinct from P. crocea, which lacks stromata and has conidiophores that are consistently solitary, arising from superficial hyphae.
Pseudocercospora cydoniae (Ellis & Everh.) Y.L. Guo & X.J. Liu, Mycosystema 5: 103. 1992. Fig. 32.
Fig. 32.
Pseudocercospora cydoniae (CPC 10678). A. Leaf spots on upper and lower leaf surface. B. Close-up of leaf spot with fruiting. C. Fascicle with conidiophores and conidiogenous cells. D, E. Conidiogenous cells. F. Conidia. Scale bars = 10 μm.
Basionym: Cercospora cydoniae Ellis & Everh., J. Mycol. 8: 72. 1902.
≡ Cercosporina cydoniae (Ellis & Everh.) Sacc., Syll. Fung. 25: 915. 1931.
≡ Pseudocercospora cydoniae (Ellis & Everh.) U. Braun & H.D. Shin, Mycotaxon 49: 356. 1993.
Specimens examined: South Korea, Seoul, on Chaenomeles speciosa (= C. lagenaria), 17 Sep. 2003, H.D. Shin, cultures CPC 10678 = CBS 131923; Jeonju, C. sinensis, 15 Oct. 2003, H.D. Shin, CBS H-20863.
Pseudocercospora dovyalidis (Chupp & Doidge) Deighton, Mycol. Pap. 140: 143. 1976. Fig. 33.
Fig. 33.
Pseudocercospora dovyalidis (CPC 13771-13773). A. Leaf spots on upper and lower leaf surface. B. Close-up of leaf spot with fruiting. C, D. Fascicles with conidiophores and conidiogenous cells. E, F. Conidiogenous cells. G-K. Conidia. Scale bars = 10 μm.
Basionym: Cercospora dovyalidis Chupp & Doidge, Bothalia 4: 885. 1948.
≡ Pseudocercosporella dovyalidis (Chupp & Doidge) B. Sutton, Mycol. Pap. 138: 99. 1975.
Leaf spots amphigenous, distinct, 1-3 lesions per leaf, scattered over the leaf, 3-10 mm diam, pale brown surrounded by a dark brown to black border. Mycelium internal, consisting of pale brown, septate, smooth, 2-6 μm diam hyphae. Caespituli hypophyllous, evenly distributed over the leaf spot, floccose to punctiform, olivaceous to black. Stromata well-developed, subimmersed to erumpent, globular, dark brown, 40-100 mm diam. Conidiophores fasciculate, emerging from the upper cells of stromata, brown, becoming paler toward the apex, smooth, 0-2-septate, straight to variously curved, guttulate, apex rounded, conidiophores rarely branched below, (12-)13-22(-34) × (3-)3-5(-6) μm. Conidiogenous cells terminal, pale brown, smooth, guttulate, proliferating percurrently, (4-)6-12(-15) × (2-) 3-4(-5) μm. Conidia solitary, pale brown or subhyaline, smooth, distinctively guttulate, 1-10-septate, thick-walled, straight to curved, broadly filliform to cylindrical, apex rounded to subacute, base long obconically truncate, (20-)30-70(-84) × (3-)3-5(-6) μm; hila neither thickened nor darkened.
Culture characteristics: Colonies on MEA reaching 32 mm diam after 30 d at 24 °C. Colonies circular with a smooth margin, either flat with excessive folding into the media or convex, aerial mycelium moderate, margin of colony darker than colony interior; greenish glaucous to olivaceous-grey (surface) and olivaceous-grey (reverse).
Specimens examined: South Africa, Gauteng, Pretoria, Groenkloof, on Dovyalis zeyheri, 18 Feb. 1914, E.M. Doidge, holotype PREM 7398; Gauteng, Walter Susulu Botanical Garden, on leaves of D. zeyheri, 2 Mar. 2007, P.W. Crous, epitype designated here CBS H-20389, culture ex-type CPC 13771 = CBS 126002.
Pseudocercospora eucalyptorum Crous, M.J. Wingf., Marasas & B. Sutton, Mycol. Res. 93: 394. 1989.
= Pseudocercospora pseudoeucalyptorum Crous, Stud. Mycol. 50: 210. 2004.
Specimens examined: South Africa, Western Cape Province, Stellenbosch, Stellenbosch Mountain, on leaves of E. nitens, 21 Dec. 1987, P.W. Crous, holotype of P. eucalyptorum PREM 49112, cultures ex type CPC16 = CBS 110777. Spain, Pontevedra, Lourizán, Areeiro, on leaves of E. globulus, 2003, J.P. Mansilla, holotype of P. pseudoeucalyptorum CBS H-9893, culture ex-type CPC 10390 = CBS 114242.
Note: Pseudocercospora pseudoeucalyptorum is reduced to synonymy with P. eucalyptorum on the basis of the phylogeny obtained here and similarity in pigmentation (Crous et al. 2004c).
Pseudocercospora exosporioides (Bubák) B. Sutton & Hodges, Mycologia 82: 320. 1990.
Basionym: Cercospora exosporioides Bubák, Ann. Mycol. 13: 33. 1915.
Specimen examined: Japan, Ibaraki, on Sequoia sempervirens, 11 Sep. 1998, T. Kobayashi, CNS-448, cultures MUCC 893, MAFF 237788.
Pseudocercospora flavomarginata G.C. Hunter, Crous & M.J. Wingf., Fungal Diversity 22: 80. 2006. Fig. 34.
Fig. 34.
Pseudocercospora flavomarginata (CPC 14142). A. Leaf spots on upper and lower leaf surface. B. Close-up of leaf spot with fruiting. C. Fascicle with conidiophores and conidiogenous cells. D-F. Conidiogenous cells. G-J. Conidia. Scale bars = 10 μm.
Specimens examined: Thailand, Chang Gao Province near Pratchinburi, on leaves of Eucalyptus camaldulensis, 2004, M.J. Wingfield, holotype PREM 58952, cultures ex-type CBS 118841, 118823, 118824; Chachoengsao Province, on leaves of E. camaldulensis, 2001, W. Himaman, CBS H-20388, culture CPC 13492-13494. China, on leaves of Eucalyptus sp., 2003, X. Zhou, CBS H-20390, culture ex-type CPC 14142 = CBS 126001.
Notes: Pseudocercospora flavomarginata was described as the causal agent of a prominent leaf spot disease of E. camaldulensis in Thailand (Hunter et al. 2006a). Based on this study it appears that it is present also on this host in China.
Pseudocercospora fukuokaensis (Chupp) X.J. Liu & Y.L. Guo, Mycosystema 5: 103. 1992. Fig. 35.
Fig. 35.
Pseudocercospora fukuokaensis (CPC 14689). A. Leaf spots on upper and lower leaf surface. B. Close-up of leaf spot with fruiting. C, D. Fascicles with conidiophores and conidiogenous cells. E. Conidia. Scale bars = 10 μm.
Basionym: Cercospora fukuokaensis Chupp, Sci. Rep. Yokahama Natl. Univ., Sect. II, Biol. Sci. 1: 2. 1952.
Specimens examined: Japan, Fukuoka, Futsukaichi-machi, on Styrax japonicus, 5 Sep. 1951, S. Katsuki, holotype TNS-F243813; Ibaraki, on S. japonicus, 11 Sep. 1998, T. Kobayashi & C. Nakashima, epitype designated here TFM: FPH-8096, ex-epitype cultures MUCC 887, MAFF 237768; Ibaraki, Ibaraki Nat. Mus., on S. japonicus, 10 Sep. 1998, T. & Y. Kobayashi; Fukuoka, Fukuoka For. Exp. Stn., on S. japonicus, 30 Jul. 1975, S. Ogawa (TFM: FPH-4356); Kaogshima, Tanegashima Is., on S. japonicus, 18 Oct. 1997, T. Kobayashi & C. Nakashima (culture: MAFF238203); Kagoshima, Tokunoshima Is., on S. japonicus, 8 Nov. 1993, T. Kobayashi & T. Hosoya (Culture: MAFF236995); Okinawa, Kunigami, on S. japonicus, 18 Nov. 1999, T. Kobayashi & C. Nakashima; Fukuoka, Fukuoka For. Exp. Stn., on S. obassia, 14 Sep. 1978, S. Ogawa (TFM: FPH -4941); Fukuoka, on S. grandiflora (= S. japonicus var. kotoensis), Oct. 2001, T. Kobayashi (MAFF 238480); Yamaguchi, on S. japonicus, Dec. 1996, T. Kobayashi (MAFF 237634); Saitama, on S. japonicus, Sep. 2002, T. Kobayashi & Y.Ono (MAFF 239411). South Korea, Osan, S. japonicus, 30 Oct. 2007, H.D. Shin, culture CPC 14689 = CBS 132111.
Notes: DNA sequence data for different isolates from Styrax japonica collected in Japan are identical, and distinct from the strain collected in Korea, suggesting that the Korean material represents a different taxon.
Pseudocercospora fuligena (Roldan) Deighton, Mycol. Pap. 140: 144. 1976.
Basionym: Cercospora fuligena Roldan, Philipp. J. Sci. 66: 8. 1938.
Holotype: Philippines, Luzon, Laguna, College of Agriculture Campus, on Solanum lycopersicum (≡ Lycopersicon esculentum), E.F. Roldan No 32, holotype (not seen).
Specimens examined: Thailand, on Solanum lycopersicum (variety FMMT260), 28 Aug. 2005, Z. Mersha, CBS H-20864, culture CPC 12296 = CBS 132017. Japan, Mie, on Lycopersicon esculentum, 6 Feb. 2007, C. Nakashima, MUCC 533.
Notes: DNA sequence data (ITS and EF-1α) for 40 Japanese isolates revealed variation in only one position (data not shown) and the culture from Thailand is very similar genetically. The collections of P. fuligena treated in this study are also morphologically similar to the description of the holotype specimen, which was collected in the Philippines. Chupp (1954) did not see the holotype, nor did Deighton (1976) refer to it. Fresh collections from the type location are needed to resolve this apparent species complex.
Pseudocercospora glauca (Syd.) Y.L. Guo & X.J. Liu, Acta Mycol. Sin. 11: 132. 1992. Fig. 36.
Fig. 36.
Pseudocercospora glauca (CPC 10062). A. Leaf spots on upper and lower leaf surface. B. Close-up of leaf spot with fruiting. C. Fascicle with conidiophores and conidiogenous cells. D. Conidiophores. E, F. Conidia. Scale bars = 10 μm.
Basionym: Cercospora glauca Syd., Ann. Mycol. 27: 432. 1929.
Specimen examined: South Korea, Wando, Wando arboretum, on Albizzia julibrissin, 9 Nov. 2002, H.D. Shin, CBS H-20865, culture CPC 10062 = CBS 131884.
Pseudocercospora guianensis (F. Stevens & Solheim) Deighton, Mycol. Pap. 140: 145. 1976.
Basionym: Cercospora guianensis F. Stevens & Solheim, Mycologia 23: 375. 1931.
Specimen examined: Japan, Tateyama, Chiba, on Lantana camara, 4 June 1997, C. Nakashima CNS-162, cultures MUCC 879, MAFF 238239.
Pseudocercospora haiweiensis Crous & X. Zhou, sp. nov. MycoBank MB564832. Fig. 37.
Fig. 37.
Pseudocercospora haiweiensis (CPC 14084). A. Leaf spots on upper and lower leaf surface. B. Close-up of leaf spot with fruiting. C. Fascicle with conidiophores and conidiogenous cells. D-F. Conidiophores. G. Conidia. Scale bars = 10 μm.
Etymology: Name is derived from Hai Wei, China, where this fungus was collected.
Leaf spots amphigenous, irregular to subcircular or angular, 2-4 mm diam, brown, with raised border, and at times with a red-purple margin. Mycelium internal, subhyaline, consisting of septate, branched, smooth, 2-3 μm diam hyphae. Caespituli fasciculate to sporodochial, amphigenous, breaking through epidermis, appearing almost acervular, grey-brown on leaves, up to 90 μm wide and 50 μm high. Conidiophores aggregated in dense fascicles arising from the upper cells of a brown stroma up to 60 μm wide and 30 μm high; conidiophores brown, smooth to finely verruculose, 0-2-septate, subcylindrical, straight to variously curved or geniculate-sinuous, unbranched, 10-25 × 3-4 μm. Conidiogenous cells terminal, unbranched, brown, subcylindrical, smooth to finely verruculose, tapering to flat-tipped apical loci, proliferating sympodially, rarely percurrently near apex, 10-15 × 2.5-3.5 μm. Conidia solitary, brown, finely verruculose, guttulate, subcylindrical, apex obtuse, base obconically subtruncate to truncate, straight to gently curved, 3(-5)-septate, (25-)30-40(-45) × 3(-4) μm; hila unthickened, neither darkened nor refractive, 1.5 μm wide.
Culture characteristics: Colonies after 2 wk at 24 °C in the dark on MEA; surface folded, erumpent, spreading, with moderate aerial mycelium, and smooth, lobate margins. Surface olivaceous-grey with patches of pale olivaceous-grey; reverse olivaceous-grey. Colonies reaching 12 mm diam.
Specimen examined: China, Hai Wei, on leaves of Eucalyptus sp. (APP 21), 3 June 2007, X. Zhou, holotype CBS H-20866, culture ex-type CPC 14084 = CBS 131584.
Notes: A combination of relatively short conidia (1-3-septate, 25-45 × 3-4 μm) that are subcylindrical in shape, the absence of superficial mycelium, and dense fascicles with well-developed stromata, distinguish this new species on Eucalyptus from other taxa known from this host (Crous 1998, Braun & Dick 2002).
Pseudocercospora hakeae (U. Braun & Crous) U. Braun & Crous, comb. et stat. nov. MycoBank MB564833.
Basionym: Cercostigmina protearum var. hakeae U. Braun & Crous, Sydowia 46: 206. 1994.
≡ Pseudocercospora protearum var. hakeae (U. Braun & Crous) U. Braun & Crous, Mycol. Progr. 1: 22. 2002.
Specimens examined: South Africa, Northern Province, Louis Trichardt, Hangklip Forest Station, on leaves of Hakea salicifolia (= H. saligna), Apr. 1988, C. Roux, holotype PREM 51117. Australia, New South Wales, Mount Annan Botanic Gardens, on leaves of Grevillea sp., Aug. 1999, P.W. Crous & B. Summerell, JT 926, DAR 74861, CPC 2968; Mount Tomah Botanic Gardens, on leaves of Grevillea sp., Aug. 1999, P.W. Crous & B. Summerell, JT 873, DAR 74862, CPC 3145 = CBS 112226.
Note: No culture from Hakea is presently available, and thus the position of this taxon on Hakea and Grevillea has yet to be confirmed based on DNA sequence comparisons.
Pseudocercospora humuli (Hori) Y.L. Guo & X.J. Liu, Acta Mycol. Sin., Suppl. 1: 345. (1986) 1987.
Basionym: Cercospora humuli Hori, in S. Takimoto, Trans. Agric. Assoc. Chosen 13(12): 34. 1918.
≡ Cercospora humuli Hori, in Salmon & Wormald. J. Bot. (London) 61: 135. 1923.
-
= Cercospora humuli-japonici Sawada, Taiwan Agric. Rev. 38: 697. 1942, nom. inval.
≡ Pseudocercospora humuli-japonici Sawada ex Goh & W.H. Hsieh, in Hsieh & Goh, Cercospora and similar fungi from Taiwan: 239. 1990.
Specimens examined: Japan, Tokyo, Nishigahara, on Humulus scandens, 28 Sep. 1915, S. Hori, holotype NIAES herbarium C-487; Wakayama, on H. lupulus var. lupulus, 30 Oct. 2007, C. Nakashima & I. Araki, epitype designated here TFM: FPH-8097, ex-epitype culture MUCC 742.
Pseudocercospora humulicola Crous, U. Braun & H.D. Shin, sp. nov. MycoBank MB564834. Fig. 38.
Fig. 38.
Pseudocercospora humulicola (CPC 11358). A. Leaf spots on upper and lower leaf surface. B. Close-up of leaf spot with fruiting. C-E. Fascicles with conidiophores and conidiogenous cells. F. Conidia. Scale bars = 10 μm.
Etymology: Name derived from Humulus, the plant on which it was collected.
Leaf spots amphigenous, irregular to angular, 0.5-1.5 mm diam, brown, with raised border and wide chlorotic halo. Mycelium internal, subhyaline, consisting of septate, branched, smooth, 2-3 μm diam hyphae. Caespituli fasciculate to sporodochial, amphigenous, predominantly epiphyllous, pale brown on leaves, up to 90 μm wide and 200 μm high. Conidiophores aggregated in dense fascicles arising from the upper cells of a brown stroma up to 80 μm wide and 30 μm high; conidiophores pale brown, smooth, 2-5-septate, subcylindrical, straight to variously curved or geniculate-sinuous, unbranched, 40-90 × 3-4 μm. Conidiogenous cells terminal, unbranched, subhyaline to pale brown, subcylindrical, smooth, tapering to flat-tipped apical conidiogenous loci, 2 μm diam, proliferating sympodially, 10-30 × 3-4 μm. Conidia solitary, subhyaline, smooth, finely granular, subcylindrical, apex obtuse, base truncate, straight to gently curved, 3-12-septate, (70-)80-95(-120) × 2.5(-3) μm; hila unthickened, neither darkened nor refractive, 2-3 μm wide.
Culture characteristics: Colonies after 2 wk at 24 °C in the dark on MEA; surface folded, erumpent, spreading, with sparse aerial mycelium, and smooth, lobate margins. Surface pale olivaceous-grey; reverse olivaceous-grey. Colonies reaching 10 mm diam.
Specimens examined: South Korea, Hongchon, on leaves of Humulus scandens, 9 Jul. 2004, H.D. Shin, holotype CBS H-20867, culture ex-type CPC 11358 = CBS 131585; Chuncheon, on H. scandens, 11 Oct. 2002, H.D. Shin, CBS H-20868, culture CPC 10049 = CBS 131883; Cheongju, on H. scandens, 4 June 2004, H.D. Shin, CBS H-20869, culture CPC 10002.
Notes: Pseudocercospora humulicola is very similar to P. humuli, originally described from Japan, but it is distinct based on DNA sequence comparisons. In P. humuli conidia are obclavate-cylindrical, 35-120 × 2.5-4 μm (Chupp 1954), while conidia of P. humulicola are subcylindrical, and on average longer than 80 μm. Furthermore, P. humuli has shorter conidiophores (10-55 μm long, 0-2-septate) than those of P. humulicola, which are 2-5-septate, and 40-90 μm long.
Pseudocercospora jussiaeae (G.F. Atk.) Deighton, Mycol. Pap. 140: 146. 1976. Fig. 39.
Fig. 39.
Pseudocercospora jussiaeae (CPC 14625). A. Leaf spots on upper and lower leaf surface. B. Close-up of leaf spot with fruiting. C, D. Fascicles with conidiophores and conidiogenous cells. E. Conidia. Scale bars = 10 μm.
Basionym: Cercospora jussiaeae G.F. Atk., J. Elisha Mitchell Sci. Soc. 8: 50. 1892.
= Cercospora ludwigiae G.F Atk., J. Elisha Mitchell Sci. Soc. 8: 58. 1892.
Specimen examined: South Korea, Hongcheon, on Ludwigia prostrata, 9 Oct. 2007, H.D. Shin, KUS-F22981, CBS H-20870, culture CPC 14625 = CBS 132117.
Pseudocercospora kaki Goh & W.H. Hsieh, in Hsieh & Goh, Cercospora and similar fungi from Taiwan: 109. 1990. Fig. 40.
Fig. 40.
Pseudocercospora kaki (CPC 10837-10839). A. Leaf spots on upper and lower leaf surface. B. Close-up of leaf spot with fruiting. C, D. Fascicles with conidiophores and conidiogenous cells. E, F. Conidiogenous cells. G. Conidia. Scale bars = 10 μm.
Specimens examined: Japan, Toyama, Kureha, on Diospyros kaki, 25 Sep. 1998, T. Kobayashi & E. Imaizumi, CNS-472, culture MAFF 238214; Chiba, on D. kaki, 18 Sep. 1998, S. Uematsu & C. Nakashima, CNS-464, cultures MUCC 900, MAFF 238238; Chiba, on D. kaki, Nov. 1993, T. Kobayashi, cultures MAFF 237013. South Korea, Gongju, on D. lotus, 28 Oct. 2003, H.D. Shin, CBS H-20871, cultures CPC 10837-10839.
Additional isolates examined (representing a different lineage): Japan, Kagoshima, Oshima Is., on D. kaki, 11 Nov. 1993, T. Kobayashi, CNS-993, culture MAFF 236999; Chiba, on D. kaki, Oct. 1991, T. Kobayashi, culture MAFF 235880.
Notes: The type specimen of this species is from Taiwan but the type was not cultured or sequenced. It may be synonymous with Cercospora kaki, which is based on material from the USA. The Japanese material studied here is different from the Korean material based on DNA sequence data. Actin sequences generated for additional Japanese isolates resolved two different lineages, one of which may be attributed to Cercospora kakivora, but this can only be resolved once fresh collections from Taiwan and the USA have been obtained.
Pseudocercospora kiggelariae (Syd.) Crous & U. Braun, Sydowia 46: 215. 1994.
Basionym: Cercospora kiggelariae Syd., Ann. Mycol. 22: 434. 1924.
Holotype: South Africa, Western Cape Province, Stellenbosch, on leaves of Kiggelaria africana, May 1924, C.K. Brain No 1449 (not preserved).
Specimens examined: South Africa, Gauteng, Walter Susulu Botanical Garden, on leaves of K. africana, Jan. 2005, W. Gams, neotype designated here CBS H-20872, cultures ex-neotype CPC 11853 = CBS 132016; Western Cape Province, Hermanus, Fernkloof Botanical Garden, S34°23’52.1” E19°15’58.5”, K. africana, 2 May 2010, P.W. Crous, CBS H-20873, CPC 18286, 18287.
Pseudocercospora latens (Ellis & Everh.) Y.L. Guo & X.J. Liu, Mycosystema 2: 236. 1989.
Basionym: Cercospora latens Ellis & Everh., J. Mycol. 4: 3. 1888.
≡ Pseudocercospora latens (Ellis & Everh.) U. Braun, Trudy Bot. Inst. im. V.L. Komarova 20: 67. 1997, comb. superfl.
Specimen examined: Japan, Okinawa, on Lespedeza wilfordii (= L. thunbergii subsp. formosa), 18 Nov. 2007, C. Nakashima & T. Akashi, MUMH 10815, culture MUCC 763.
Pseudocercospora leucadendri (Cooke) U. Braun & Crous, comb. et stat. nov. MycoBank MB564835.
Basionym: Cercospora protearum var. leucadendri Cooke, Grevillea 12: 39. 1883.
≡ Stigmina protearum var. leucadendri (Cooke) M.B. Ellis, Mycol. Pap. 131: 7. 1972.
≡ Cercostigmina protearum var. leucadendri (Cooke) U. Braun & Crous, in Crous & Braun, Sydowia 46: 206. 1994.
≡ Pseudocercospora protearum var. leucadendri (Cooke) U. Braun & Crous, Mycol. Progr. 1: 22. 2002.
= Passalora protearum Kalchbr. & Cooke, Grevillea 19: 6. 1890.
Specimen examined: South Africa, Western Cape Province, Stellenbosch, Devon Valley, Protea Heights, on Leucadendron sp., 3 Apr. 1998, S. Denman & P.W. Crous, specimen JT-178, culture CPC 1869 (no longer viable).
Note: Pseudocercospora protearum has three varieties on Proteaceae, viz. protearum, leucadendri and hakeae (Braun & Hill 2002), that should be recognised as distinct species (Crous et al. 2004a) as shown here (Fig. 5).
Pseudocercospora lonicericola (W. Yamam.) Deighton, Mycol. Pap. 140: 146. 1976.
Basionym: Cercospora lonicericola W. Yamam. J. Soc. Trop. Agric. 6: 604. 1934.
Holotype: Taiwan, Taihoku, on Lonicera japonica var. sempervillosa, 3 Nov. 1933, W. Yamamoto (holotype could not be located, and is probably lost).
Specimens examined: Japan, Tokyo, Jindai Bot. Park, on L. japonica, 21 Oct. 1976, T. Kobayashi, TFM: FPH-4479; Chiba, Matsudo, on L. japonica, 14 Sep. 1951, E. Kurosawa, SK -2207; Fukuoka, Yame, on L. japonica, 29 Nov. 1949, S. Katsuki, SK -2206; Kagoshima, Yaku Is., on L. japonica, 29 Dec. 1952, S. Katsuki, SK -392; Ibaraki, L. gracilipes var. glabra, 11 Sep. 1998, T. Kobayashi, neotype designated here TFM: FPH-8098, ex-neotype cultures MUCC 889, MAFF 237785.
Pseudocercospora lyoniae (Katsuki & Tak. Kobay.) Deighton, Trans. Brit. Mycol. Soc. 88: 389. 1987.
Basionym: Cercospora lyoniae Katsuki & Tak. Kobay., Trans. Mycol. Soc. Japan 16: 3. 1975.
Specimens examined: Japan, Tokyo, Asakawa Experimental Forest, Government Forest Experimental Station, on Lyonia ovalifolia var. elliptica, 21 Sep. 1973, H. Horie, holotype TFM: FPH-3999; Tokyo, Jindai Bot. Garden, on L. ovalifolia var. elliptical, 25 Sep. 1974, T. Kobayashi, TFM: FPH -4202; Tokyo, Jindai Bot. Garden, on L. ovalifolia var. elliptica, 7 Nov. 1998, C. Nakashima & E. Imaizumi, epitype designated here TFM: FPH-8100, ex-epitype cultures MUCC 910, MAFF 237775.
Pseudocercospora lythracearum (Heald & F.A. Wolf) X.J. Liu & Y.L. Guo, Acta Mycol. Sin. 11: 294. 1992. Fig. 41.
Fig. 41.
Pseudocercospora lythracearum (CPC 10707). A. Leaf spots on upper and lower leaf surface. B. Close-up of leaf spot with fruiting. C, D. Fascicles with conidiophores and conidiogenous cells. E. Conidiophore with conidiogenous cells. F. Conidia. Scale bars = 10 μm.
Basionym: Cercospora lythracearum Heald & F.A. Wolf, Mycologia 3: 18. 1911.
≡ Cercosporina lythracearum (Heald & F.A. Wolf) Sacc., Syll. Fung. 25: 909. 1931.
= Cercospora lagerstroemiae Syd. & P. Syd., Ann. Mycol. 12: 203. 1914.
-
= Cercospora lagerstroemiae-subcostatae Sawada, Taiwan Agric. Res. Inst. Rept. 51: 129. 1931.
≡ Pseudocercospora lagerstroemiae-subcostatae (Sawada) Goh & W.H. Hsieh, in Hsieh & Goh, Cercospora and similar fungi from Taiwan: 212. 1990.
= Cercospora lagerstroemiicola Sawada, Taiwan Agric. Res. Inst. Rept. 85: 112. 1943, nom. inval.
Specimens examined: Japan, Ibaraki, on Lagerstroemia indica, 11 Sep. 1998, T. Kobayashi, CNS-444, cultures MUCC 890, MAFF 237786; Kanagawa, isolated from L. subcostata, collection date unknown, T. Kobayashi, MAFF 410017; Ibaraki, isolated from L. subcostata, Oct. 1994, T. Nishijima, MAFF 237185; Chiba, isolated from L. subcostata, Oct. 1993, T. Kobayashi, MAFF 236964. South Korea, Jinju, L. indica, 15 Oct. 2003, H.D. Shin, CBS H-20874, KUS-F 19899, culture CPC 10707 = CBS 131925.
Notes: The material collected from Korea is genetically similar to that from Japan (Fig. 5). However, fresh collections from the USA are required to determine if the Asian material is the same as that from the USA. The synonyms cited by Chupp (1954) could represent different species.
Pseudocercospora lythri H.D. Shin & U. Braun, Mycotaxon 74: 111. 2000. Fig. 42.
Fig. 42.
Pseudocercospora lythri (CPC 14588). A. Leaf spots on upper and lower leaf surface. B. Close-up of leaf spot with fruiting. C, D. Fascicles with conidiophores and conidiogenous cells. E. Conidia. Scale bars = 10 μm.
Specimens examined: Japan, Tokyo, on Lythrum salicaria (incl. L. anceps) 10 Nov. 2007, I. Araki & M. Harada, MUMH 11104, culture MUCC865. South Korea, Chuncheon, on L. salicaria, 21 Sep. 1991, H.D. Shin, holotype KUS-F 11109; Yangku, on L. salicaria, 28 Sep. 2007, H.D. Shin, epitype designated here CBS H-20875, culture ex-epitype CPC 14588 = CBS 132115.
Pseudocercospora marginalis G.C. Hunter, Crous, U. Braun & H.D. Shin, sp. nov. MycoBank MB564836. Fig. 43.
Fig. 43.
Pseudocercospora marginalis (CPC 12497). A, B. Leaf spots on upper and lower leaf surface. C, D. Fascicles with conidiophores and conidiogenous cells. E. Close-up of leaf spot with fruiting. F-J. Conidia. Scale bars = 10 μm.
Etymology: Margo, marginalis, referring to border or margin; indicating leaf spots that extend along the leaf margin.
Leaf spots distinct, 2-5 mm diam, also predominantly forming larger blotches extending along the length of the leaf margin, brown, irregular; border indefinite. Mycelium internal and external, septate, smooth, subhyaline, branched, 2-4 μm wide. Caespituli epiphyllous, aggregated along leaf veins, floccose, olivaceous, emerging from stomata. Stromata well-developed, subimmersed to erumpent, globular to elongated, brown, 20-75 μm diam. Conidiophores fasciculate, pale brown to brown, straight to curved to undulate, cylindrical, unbranched, apex rounded to subtruncate, smooth, finely guttulate, 0-4-septate, (15-)18-31(-41) × (3-)4(-5) μm. Conidiogenous cells terminal, unbranched, smooth, finely guttulate, pale brown, straight to curved, cylindrical, apex rounded to subtruncate, proliferating sympodially or percurrently, (5-)8-11(-14) × 3(-4) μm. Conidia solitary, smooth, cylindrical to narrowly obclavate, guttulate, thick-walled, straight to curved, pale brown to pale olivaceous, apex rounded to obtuse, base obconic to long obconically truncate, 1-7-septate, (19-)30-48(-58) × (3-)4(-5) μm; hila neither thickened nor darkened.
Culture characteristics: Colonies after 2 wk at 24 °C in the dark on MEA; erumpent, spreading, with moderate aerial mycelium, and smooth, even margins. Surface pale olivaceous-grey; reverse olivaceous-grey. Colonies reaching 10 mm diam.
Specimen examined: South Korea, Jeju, Halla arboretum, on leaves of Fraxinus rhynchophylla (≡ F. chinensis subsp. rhynchophylla), 29 Oct. 2005, H.D. Shin, holotype CBS H-20397, culture ex-type CPC 12497 = CBS 131582, CPC 12498, 12499.
Specimens examined of P. fraxinites: South Korea, Jinju, on Fontanesia phillyreoides, 15 Oct. 2003, H.D. Shin, CBS H-20876, cultures CPC 10743-10745. Japan, Ibaraki, on Fraxinus excelsior, 11 Sep. 1998, T. Kobayashi, CNS-445, cultures MUCC 891, MAFF 237787.
Notes: Although similar to P. fraxinites (conidia 20-60 × 1.5-3 μm; Chupp 1954) (Fig. 44), conidia of P. marginalis are wider and cluster apart from isolates of P. fraxinites on Fontanesia from Korea (CPC 10743-10745) and Fraxinus from Japan (MUCC 891). Pseudocercospora fraxinites was originally described from Fraxinus in the USA. Morphological and molecular characterisation of new collections and cultures from this host in the USA are needed to clarify the limits of P. fraxinites and P. marginalis.
Fig. 44.
Pseudocercospora fraxinites (CPC 10743-10745). A. Leaf spots on upper and lower leaf surface. B. Close-up of leaf spot with fruiting. C-E. Fascicles with conidiophores and conidiogenous cells. F. Conidiogenous cells. G. Conidia. Scale bars = 10 μm.
Pseudocercospora melicyti U. Braun & C.F. Hill, Australas. Pl. Pathol. 33: 489. 2004.
Specimen examined: New Zealand, Auckland, Waiatarua, on Melicytus macrophyllus, 13 Mar. 2003, C.F. Hill, holotype HAL 1787 F (isotype PDD 77567), culture ex-type ICMP 14984 = CBS 115023.
Pseudocercospora myrticola (Speg.) Deighton, Mycol. Pap. 140: 148. 1976.
Basionym: Cercospora myrticola Speg., Anales Soc. Ci. Argent. 16: 167. 1883.
= Cercospora myrti Erikss., Bidrag Känn. om vara odlade Vaxters s jukdomar, Stockholm 8: 79. 1885 and Rev. Mycol. 8: 60. 1886.
= Cercospora saccardoana Scalia, Atti Accad. Gioenia Sci. Nat. Catania, Ser. 4, 14: 35. 1901.
= Cercospora amadelpha Syd., Ann. Mycol. 30: 89. 1932.
= Fusariella cladosporioides P. Karst., Hedwigia 30: 248. 1891.
Specimen examined: Japan, Kagoshima, on Myrtus communis, 29 May 2007, C. Nakashima & K. Motohashi, MUMH 10572, culture MUCC 632.
Pseudocercospora ocimi-basilici Crous, M.E. Palm & U. Braun, sp. nov. MycoBank MB564837. Fig. 45.
Fig. 45.
Pseudocercospora ocimi-basilici (CPC 10283-10285). A. Leaf spots on upper and lower leaf surface. B. Close-up of leaf spot with fruiting. C-E. Fascicles with conidiophores and conidiogenous cells. F. Conidia. Scale bars = 10 μm.
Etymology: Name derived from Ocimum basilicum, the host from which it was collected.
Leaf spots amphigenous, subcircular, circular or somewhat irregular, 2-10 mm diam, greyish green, dull grey to dark brown, border indistinct, at times raised. Mycelium internal, pale brown, consisting of septate, branched, smooth, 2-3 μm diam hyphae. Caespituli fasciculate to sporodochial, brown, predominantly hypophyllous, up to 90 μm diam and 70 μm high. Conidiophores aggregated in mostly dense, small to large, sometimes almost sporodochial fascicles, emerging through stomata or erumpent through the cuticle, arising from the upper cells of a brown, substomatal to mostly intraepidermal stroma, 10-80 μm; conidiophores pale to medium brown or olivaceous-brown, smooth, thin-walled, 0-2-septate, subcylindrical or attenuated towards the tip, straight to moderately geniculate-sinuous, unbranched or branched above, 5-35 × 2-5 μm. Conidiogenous cells integrated, terminal or conidiophores reduced to conidiogenous cells, pale olivaceous-brown, smooth, tapering to flat-tipped apical loci, 1-2 μm wide, proliferating sympodially, 5-20 × 2-4 μm. Conidia solitary, subhyaline to pale olivaceous-brown, smooth, guttulate, shape and size variable, small conidia short obclavate-cylindrical to fusiform, longer conidia narrowly obclavate-filiform, sometimes acicular, apex subacute to subobtuse, base short to long obconically truncate to truncate in acicular conidia, straight to curved, 3-12-septate, (25-)30-120 (-130) × (2-)2.5-5(-5.5) μm; hila unthickened, neither darkened nor refractive, 1.5-2.5 μm diam.
Specimens examined: Fiji (intercepted at the Auckland International Airport, on basil foliage imported from Fiji), on Ocimum basilicum, 24 Feb. 2002, C.F. Hill 529, HAL. Mexico, on O. basilicum, Dec. 2001, without collector (cultured as MEP 1515), BPI 841445; (intercepted at Los Angeles), 2 Nov. 2002, L.C. Lastra 1395 A, BPI 747831; 6 Dec. 2002, M.E. Palm, holotype CBS H-20877, culture ex-type CPC 10283-10285 (unfortunately no longer viable). New Zealand, Auckland, Botanical Garden, on O. basilicum, 9 Mar. 2002, C.F. Hill 546, HAL. Vanuatu, Efate, Vanuatu Tropical Products, on O. basilicum, 25 Oct. 1996, E. McKenzie, PDD 66438; Rainbow Garden, on O. basilicum, 22 Oct. 1996, E. McKenzie, PDD 66537.
Notes: Braun et al. (2003b) examined Pseudocercospora collections on Ocimum basilicum from Fiji, New Zealand, and Vanuatu and identified those collections as P. ocimicola, in spite of some morphological differences observed. Pseudocercospora ocimicola differs from collections on Ocimum basilicum, herein described as P. ocimi-basilici, in having shorter conidia (about 25-80 μm long), conidiophores in small, loose fascicles as well as solitary conidiophores arising from superficial hyphae, and lacking or almost lacking stromata.
The description of Cercospora ocimicola provided by Chupp (1954) covers type material of this species as well as material on O. basilicum. Based on type material and additional collections, C. ocimicola is redescribed as P. ocimicola in the current study (see below).
Pseudocercospora ocimicola (Petr. & Cif.) Deighton, Mycol. Pap. 140: 149. 1976.
Basionym: Cercospora ocimicola Petr. & Cif., Ann. Mycol. 30: 324. 1932.
= C. hyptidicola (as “hypticola”) Chupp & A.S. Mull., Bol. Soc. Venez. Ci. Nat. 8: 47. 1942, nom. inval.
Leaf spots lacking or almost so to indistinct or angular-irregular, yellowish ochraceous, olivaceous to brownish, centre finally sometimes paler, dingy greyish brown to grey, 1-10 mm diam., margin indefinite. Mycelium internal and external, superficial, hyphae emerging through stomata, sparingly branched, septate, subhyaline to olivaceous-brown, 1-3 μm wide, thin-walled, smooth. Stromata lacking or small, mostly substomatal, occasionally intraepidermal, 10-30 μm diam. Caespituli amphigenous, usually not very conspicuous, olivaceous-brown, finely punctiform to subeffuse. Conidiophores in small, loose to moderately large and denser fascicles, arising from stromata or internal hyphae, through stomata or erumpent through the cuticle, or conidiophores solitary, arising from superficial hyphae, lateral or occasionally terminal, straight and subcylindrical to conical or usually geniculate-sinuous, unbranched or occasionally branched, pale olivaceous to olivaceous-brown, 0-3-septate, thin-walled, smooth, 5-50 × (2-)3-5 μm. Conidiogenous cells integrated, terminal or conidiophores reduced to conidiogenous cells, 5-20 × 2-4 μm, proliferating sympodially, with a single or several inconspicus to flat-tipped conidiogenous loci, 1-2 μm wide. Conidia solitary, subhyaline to pale olivaceous or olivaceous-brown, thin-walled, smooth, obclavate-subcylindrical, apex obtuse to subacute, base truncate to obconically truncate, 1-8-septate, (15-)25-75(-85) × 2-4 μm, hila unthickened, neither darkened nor refractive, 1-2 μm diam.
Specimens examined: Brazil, State of Ceará, Pentecoste County, on Ocimum sp., 2 Mar. 2001, F. Freire, HAL; State of Ceará, Cascavel County, Preaoca, on Marsypianthes chamaedrys; 12 June 1999, F. Freire, HAL. Cuba, Habana, Santiago de las Vegas, on Ocimum gratissimum, 6 Sep. 1988, R.F. Castañeda [C88/316], HAL; Habana, Santiago de las Vegas, on O. sanctum, 28 Dec. 1987, R.F. Castañeda [C87/382], HAL. Dominican Republic, Santiago, Valle del Cibao, Prov. Santiago, Hato del Yonque, on O. campechianum (= O. micranthum), 26 Nov. 1930, E.L. Ekman, Cif., Mycofl. Doming. Exs. 359, lectotype designated here BPI 845245 and isolectotype BPI 438987. India, Midnapur, Daspur, on O. sanctum, 3 Dec. 1967, M. Mandal, BPI 438988. Venezuela, Les Tincheras, Edo Carabobo, on Hyptis sp., 24 Feb. 1940, M.F. Barrus & A.S. Muller, type of Cercospora hyptidicola, CUP-VZ 3863; La Cuchilla, Río Claro, Lara, on Hyptis suaveolens, June 2007, R. Urtiaga, HAL.
Notes: Chupp (1954) reduced C. hyptidicola, described from Venezuela on Hyptis sp., to synonymy with C. lycopodis, and Crous & Braun (2003) followed this treatment. Braun & Urtiaga (2008) examined type material of this species and an additional new collection from Venezuela and considered C. hyptidicola a synonym of C. ocimicola since the two species are morphologically indistinguishable. Both also occur on two closely related plants, Hyptis and Ocimum, in the Lamiaceae subfam. Ocimoideae. Pseudocercospora collections on Marsypianthes (subfam. Ocimoideae) in Brazil, is morphologically also indistinguishable from collections on Ocimum spp. and was assigned to P. ocimicola by Braun & Freire (2002).
Pseudocercospora oenotherae (Ellis & Everh.) Y.L. Guo & X.J. Liu, Acta Mycol. Sin. 11: 297. 1992. Fig. 46.
Fig. 46.
Pseudocercospora oenotherae (CPC 10290, 10041). A. Leaf spots on upper and lower leaf surface. B. Close-up of leaf spot with fruiting. C, D. Fascicles with conidiophores and conidiogenous cells. E. Conidia. Scale bars = 10 μm.
Basionym: Cercospora oenotherae Ellis & Everh., Proc. Acad. Nat. Sci. Philadelphia 46: 380. 1894.
Specimens examined: South Korea, Seoul, Oenothera odorata, 6 Sep. 2003, H.D. Shin, KUS-F 19606, CPC 10630 = CBS 131920; O. odorata, 2 Oct. 2002, H.D. Shin, CBS H-20878, cultures CPC 10290 = CBS 131885, CPC 10041.
Pseudocercospora paederiae Goh & W.H. Hsieh, Cercospora and similar fungi from Taiwan: 291. 1990. Fig. 47.
Fig. 47.
Pseudocercospora paederiae (CPC 10007). A. Leaf spots on upper and lower leaf surface. B. Close-up of leaf spot with fruiting. C. Broken base of detached fascicle. D-G. Synnematal fascicles with conidiophores and conidiogenous cells. H. Conidia. Scale bars = 10 μm.
Leaf spots amphigenous, irregular to subcircular, 3-7 mm diam, pale brown in centre, with raised, dark brown border, at times with concentric zones delimited by dark borders. Mycelium internal, occasionally in addition with a few external hyphae emerging through stomata, pale to medium brown, consisting of septate, branched, smooth to finely verruculose, 3-4 μm diam hyphae. Caespituli predominantly hypophyllous, synnematous, dark brown on leaves, 25-50 μm wide and 100-200 μm high. Conidiophores aggregated in dense synnemata arising from the upper cells of a brown substomatal stroma 20-40 μm diam; individual conidiophores subhyaline to olivaceous-brown, smooth, multiseptate, subcylindrical-filiform, straight to gently curved, unbranched, 80-200 × 3-5 μm. Conidiogenous cells terminal, unbranched, brown, subcylindrical to clavate, smooth, tapering to flat-tipped apical loci, neither thickened nor darkened, proliferating sympodially, or rarely percurrently near apex, 20-35 × 2-5 μm. Conidia solitary, subhyaline, greenish yellow to pale brown, smooth to finely verruculose, guttulate, obclavate, short conidia sometimes cylindrical or fusiform, apex obtuse to subobtuse, base obconically truncate, straight to curved, 1-10-septate, (20-)40-60(-70) × 3-7 μm; hila not thickened nor darkened or refractive, 1-2 μm diam.
Specimen examined: South Korea, Pocheon, National Arboretum, Paederia foetida (= P. scandens), 23 Oct. 2002, H.D. Shin, CBS H-20879, culture CPC 10007 (unfortunately no longer viable).
Notes: A brown leaf spot on P. scandens was reported from the Keryong Mountain in Chungnam district, South Korea, including the southern districts, Chonnam, Kyeongnam, and Jeju Island by Lee et al. (2001). The associated fungus was identified as Pseudocercospora paederiae. Characteristics of the Korean material are consistent with the original description of P. paederiae (from Taiwan), except for longer conidiophores and shorter conidia that are up to 10-septate. All characterstics overlap, and the Korean collections are tentatively assigned to P. paederiae. New collections from Taiwan, together with cultures and sequence data are necessary to reassess Pseudocercospora on Paederia scandens in Asia.
Pseudocercospora pallida (Ellis & Everh.) H.D. Shin & U. Braun, Mycotaxon 74: 114. 2000. Fig. 48.
Fig. 48.

Pseudocercospora pallida (CPC 10776-10778). A. Leaf spots on upper and lower leaf surface. B. Close-up of leaf spot with fruiting. C, D. Fascicles with conidiophores and conidiogenous cells. E. Conidia. Scale bars = 10 μm.
Basionym: Cercospora pallida Ellis & Everh., J. Mycol. 3: 21. 1887.
≡ Cercospora langloisii Sacc., Syll. Fung. 10: 647. 1892, nom. superfl.
= Cercospora duplicata Ellis & Everh., J. Mycol. 5: 70. 1889.
= Cercospora capreolata Ellis & Everh., J. Mycol. 8: 70. 1902.
Specimen examined: South Korea, Suwon, on Campsis grandiflora, 14 Oct. 2003, H.D. Shin, KUS-F 19888, CBS H-20880, CPC 10776 = CBS 131889.
Pseudocercospora paraguayensis (Kobayashi) Crous, Mycotaxon 57: 270. 1996.
Basionym: Cercospora paraguayensis Kobayashi, Trans. Mycol. Soc. Japan 25: 263. 1984.
Specimen examined: Brazil, São Paulo, Susano clonal orchard, leaves of Eucalyptus nitens, Jun. 1996, P.W. Crous, CPC 1458 = CBS 111317.
Pseudocercospora pini-densiflorae (Hori & Nambu) Deighton, Trans. Brit. Mycol. Soc. 88: 390. 1987.
Basionym: Cercospora pini-densiflorae Hori & Nambu, J. Pl. Protect. (Tokyo) 4: 353. 1917.
≡ Cercoseptoria pini-densiflorae (Hori & Nambu) Deighton, Mycol. Pap. 140: 167. 1976.
Teleomorph: “Mycosphaerella” gibsonii H.C. Evans, Mycol. Pap. 153: 61. 1984.
Specimens examined: Japan, C-511, NIAES herbarium; Shizuoka, Kanaya, on P. densiflora, 6 Mar. 1976, K. Kasai, TFM: FPH-4544; Kumamoto, isolated from P. thunbergii, 24 April 1964, Y. Tokushige, MUCC 534.
Pseudocercospora plectranthi G.C. Hunter, Crous, U. Braun & H.D. Shin, sp. nov. MycoBank MB564839. Fig. 49.
Fig. 49.
Pseudocercospora plectranthi (CPC 11462). A. Leaf spots on lower leaf surface. B. Close-up of leaf spot with fruiting. E. Fascicle with conidiophores and conidiogenous cells. C, D, F-H. Conidia. Scale bars = 10 μm.
Etymology: Name derived from the host genus Plectranthus, from which it was collected.
Leaf spots distinct, scattered over leaf surface and along leaf border, amphigenous, subcircular to irregular, 2-12 mm diam, brown to pale brown. Mycelium internal and external, pale brown to hyaline, branched, smooth, 1.5-4 mm diam. Caespituli amphigenous, predominantly epiphyllous, black, distributed evenly over the leaf spot, punctiform. Stromata almost absent, weakly developed, subimmersed, globular, olivaceous-brown, 20-70 μm diam. Conidiophores fasciculate, brown to pale brown, straight to curved, smooth, unbranched, apex rounded to truncate, 0-2-septate, (18-)22-35(-45) × (3-)4(-5) μm. Conidiogenous cells integrated, terminal, unbranched, brown to pale brown, smooth, proliferating sympodially, (9-)14-21(-25) × (2-)3-4(-5) μm. Conidia solitary, pale brown to subhyaline, guttulate, 2-10-septate, slightly constricted at septa, filiform, apex obtuse to subobtuse, base obconic to long obconic, (41-)62-98(-112) × (3-)4(-5) μm, hila unthickened, not darkened.
Culture characteristics: Colonies after 2 wk at 24 °C in the dark on MEA; erumpent, spreading, with moderate aerial mycelium, and smooth, lobate margins. Surface pale olivaceous-grey; reverse iron-grey. Colonies reaching 8 mm diam.
Specimen examined: South Korea, Jeonju, on leaves of Plectranthus sp., 1 July 2004, H.D. Shin, holotype CBS H-20396, cultures ex-type CPC 11462 = CBS 131586, CPC 11463.
Notes: No species of Pseudocercospora are presently known from Plectranthus and allied genera, and as P. plectranthi does not correspond to any sequences available in GenBank at present, it is described as a new species. Numerous Pseudocercospora species have been described from hosts in the Lamiaceae, e.g. P. anisomelicola, P. colebrookiae, P. colebrookiicola, P. lamiacearum, P. leucadis, P. lycopodis, P. ocimicola, P. perillulae, P. pogostemonis, P. salvia, and P. scutellariae, but all of them are morphologically easily distinguishable from P. plectranthi by having different conidial shapes (mostly obclavate-cylindrical), smaller or no stromata or abundant superficial mycelium with solitary conidiophores. Pseudocercospora salvia has filiform conidia similar to those of P. plectranthi but in the former they are narrower (Hsieh & Goh 1990) and conidiophores are not fasciculate.
Pseudocercospora profusa (Syd. & P. Syd.) Deighton, Trans. Brit. Mycol. Soc. 88: 388. 1987. Fig. 50.
Fig. 50.
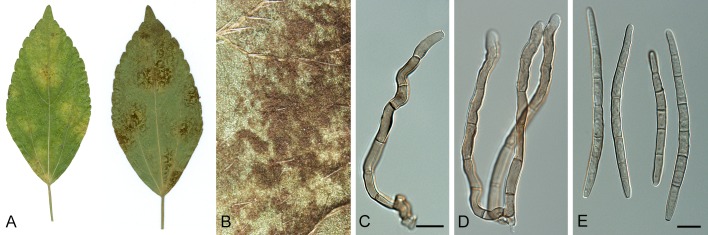
Pseudocercospora profusa (CPC 10055). A. Leaf spots on upper and lower leaf surface. B. Close-up of leaf spot with fruiting. C, D. Conidiophores and conidiogenous cells. E. Conidia. Scale bars = 10 μm.
Basionym: Cercospora profusa Syd. & P. Syd., Ann. Mycol. 7(2): 175. 1909.
≡ Cercosporiopsis profusa (Syd. & P. Syd.) Miura, in: M. Miura, Flora of Manchuria and East Mongolia. Part III. Cryptogams, fungi 3: 530. 1928.
Specimens examined: South Korea, Seoul, Acalypha australis, 17 Sep. 2003, H.D. Shin, CBS H-20882, culture CPC 10713-10715; Wonju, A. australis, 18 Oct. 2002, H.D. Shin, CBS H-20881, culture CPC 10055.
Pseudocercospora proteae Crous, sp. nov. MycoBank MB564840. Fig. 51.
Fig. 51.
Pseudocercospora proteae (CPC 15217). A. Fascicle with conidiophores and conidiogenous cells. B. Conidiogenous cell giving rise to a conidium. C-F. Conidia. Scale bars = 10 μm.
Etymology: Name derived from Protea, the host genus from which it was collected.
Leaf spots absent, with sporulation on adaxial leaf surface, prominent among leaf hairs. Mycelium internal and external, pale brown, consisting of septate, branched, smooth, 1.5-2 μm diam hyphae. Caespituli fasciculate, brown, hypophyllous, up to 120 μm diam and 40 μm high. Conidiophores aggregated in dense fascicles, arising from the upper cells of a brown stroma, up to 100 μm diam and 20 μm high; conidiophores pale brown to brown, smooth, 0-2-septate, subcylindrical to somewhat doliiform at the base, straight to geniculate-sinuous, unbranched or branched above, 15-40 × 3-6 μm. Conidiogenous cells terminal, unbranched, pale brown to brown, smooth, proliferating sympodially near apex, with flat-tipped loci, 10-15 × 2.5-5 μm. Conidia solitary, pale brown, smooth, guttulate, subcylindrical, straight to curved, apex obtuse, base truncate, (3-)8-12-septate, (35-)70-85(-100) × 3(-3.5) μm; hila unthickened, neither darkened nor refractive, 2.5-3 μm diam.
Culture characteristics: Colonies after 2 wk at 24 °C in the dark on MEA; erumpent, spreading, with sparse aerial mycelium, and smooth, even margins. Surface olivaceous-grey; reverse iron-grey. Colonies reaching 10 mm diam.
Specimen examined: South Africa, Western Cape Province, Stellenbosch, Assegaaibos, on leaves of Protea mundii, 16 Apr. 2008, F. Roets, holotype CBS H-20883, culture ex-type CPC 15216 = CBS 131587, CPC 15218, 15217.
Notes: The long, multi-septate, subcylindrical conidia of P. proteae are distinct from those of P. stromatosa (25-40 × 2.5-3 μm), and from the shorter, verruculose conidia of P. protearum (Taylor & Crous 2000, Crous et al. 2004a).
Pseudocercospora prunicola (Ellis & Everh.) U. Braun, in: Braun & Mel’nik, Trudy Bot. Inst. Im. V.L. Komarova 20: 82. 1997. Fig. 52.
Fig. 52.
Pseudocercospora prunicola (CPC 14511). A. Leaf spots on upper and lower leaf surface. B. Close-up of leaf spot with fruiting. C, D. Fascicles with conidiophores with conidiogenous cells. E. Hypha with conidiogenous loci. F. Conidia. Scale bars = 10 μm.
Basionym: Cercospora prunicola Ellis & Everh., J. Mycol. 3: 17. 1887.
≡ Cercoseptoria prunicola (Ellis & Everh.) J.M. Yen, Bull. Trimest. Soc. Mycol. France 97: 92. 1981.
-
= Cercospora pruni-yedoensis Sawada, Rep. Gov. Agric. Res. Inst. Taiwan 85: 120. 1943, nom. inval.
≡ Pseudocercospora pruni-yedoensis Goh & W.H. Hsieh, in Hsieh & Goh, Cercospora and similar genera from Taiwan: 282. 1990.
-
= Cercospora pruni-persicae J.M. Yen, Bull. Trimest. Soc. Mycol. France 94: 61. 1978 and Rev. Mycol. 42: 59. 1978.
≡ Cercoseptoria pruni-persicae (J.M. Yen) J. M. Yen, Bull. Trimest. Soc. Mycol. France 97: 92. 1981.
Misapplied name: Pseudocercospora circumscissa (Sacc.) Y.L. Guo & X.J. Liu, Mycosystema 2: 231. 1989.
Descriptions: Hsieh & Goh (1990: 282-283, as Pseudocercospora pruni-yedoensis), Braun & Mel’nik (1997: 82-83).
Illustrations: Hsieh & Goh (1990: 283, fig. 216, as Pseudocercospora pruni-yedoensis), Braun & Mel’nik (1997: 121, fig. 48).
Specimens examined: South Korea, Suwon, on Prunus yedoensis (≡ Cerasus yedoensis), 2 Oct. 2007, H.D. Shin, CBS H-20860, CPC 14511 = CBS 132107. Taiwan, Taipei, on Prunus yedoensis, 30 Nov. 1930, K. Sawada, holotype of Pseudocercospora pruni-yedoensis, NTU-PPE. USA, Louisiana, Point a la Hache, Langlois 542, holotype of Cercospora prunicola, NY (also Ellis & Everh., North American Fungi 1771, NY, isotype).
Notes: Braun & Mel’nik (1997) discussed the intricate taxonomy of Passalora and Pseudocercospora on species of Prunus s. lat. in detail and demonstrated, based on type material and other collections, that two distinct species are involved. Cercospora circumscissa is a true Passalora with somewhat thickened and darkened conidiogenous loci and hila. Its placement in Passalora s. str. has recently been confirmed based on molecular data (unpubl.). Superficial mycelium with solitary conidiophores is lacking, and the conidia are mostly somewhat rough-walled. Passalora circumscissa is also known from Asia, e.g. China, Iran and Japan. Some Chinese collections deposited at HMAS have been examined and proved to be true Passalora circumscissa (e.g. on Prunus mandshurica × Armeniaca mandshurica, Yanji, Jilin, HMAS 55845). Other collections belong to Pseudocercospora prunicola (e.g. on Prunus yedoensis, Nanjing, Jiangsu, HMAS 06632, and Changshan, Hunan, HMAS 55847). The Chinese authors misapplied the name Pseudocercospora circumscissa. The published descriptions of “Pseudocercospora circumscissa” in Guo & Hsieh (1995) and Guo & Liu (1998) cover both species, namely Passalora circumscissa as well as Pseudocercospora prunicola, but the illustrations seem to be based on material of the true Pseudocercospora on Prunus. Pseudocercospora prunicola is morphologically easily distinguishable from Passalora circumscissa by its inconspicuous, unthickened, not darkened conidiogenous loci and hila, well-developed superficial hyphae with solitary conidiophores and smooth conidia. The position of P. prunicola within the Pseudocercospora clade has been confirmed on the basis of sequence data retrieved from the present Korean culture.
Pseudocercospora pseudostigmina-platani Crous, U. Braun & H.D. Shin, sp. nov. MycoBank MB564841. Fig. 53.
Fig. 53.
Pseudocercospora pseudostigmina-platani (CPC 11726). A. Leaf spots on upper and lower leaf surface. B. Close-up of leaf spot with fruiting. C, E, F. Fascicles with conidiophores and conidiogenous cells, giving rise to dimorphic conidia. D. Pseudocercospora conidia. G. Conidia of stigmina-like synanamorph. Scale bars = 10 μm.
Etymology: Name reflects its morphological similarity to the Pseudocercospora anamorph of Mycosphaerella stigmina-platani. Leaf spots amphigenous, irregular to subcircular, 5-10 mm diam, medium brown with a wide chlorotic margin. Mycelium predominantly internal, pale brown, consisting of septate, branched, smooth, 2-3 μm diam hyphae. Caespituli fasciculate to sporodochial, brown, predominantly hypophyllous, up to 60 μm diam and 30 μm high. Conidiophores aggregated in loose to dense fascicles, arising from the upper cells of a brown stroma, up to 50 μm diam and 20 μm high; conidiophores brown, verruculose, 0-1-septate, subcylindrical to somewhat doliiform, straight to slightly curved, unbranched, 10-20 × 7-10 μm. Conidiogenous cells terminal, unbranched, brown, verruculose, proliferating percurrently near apex, with 1-4 irregular proliferations, 8-20 × 5-8 μm. Conidia dimorphic: cercostigmina-like conidia fusoid-ellipsoidal to obclavate, straight to curved, apex obtuse, base obconically subtruncate, brown, verruculose, 3-5-septate, at times constricted at septa, (28-)30-35(-38) × (5-)7-8(-9) μm; stigmina-like conidia broadly ellipsoid, straight to curved, apex obtuse, base obconically subtruncate, brown, verruculose, 3-septate, at times constricted at septa, which can also be darkened, and wall can appear thick though not distoseptate sensu stricto, (17-) 21-25(-28) × (9-)10-12 μm; hila unthickened, neither darkened nor refractive, 3-3.5 μm diam.
Culture characteristics: Colonies after 2 wk at 24 °C in the dark on MEA; surface folded, erumpent, spreading, with sparse aerial mycelium, and smooth, lobate margins. Surface pale olivaceous-grey, with thin, olivaceous-grey margin; reverse iron-grey. Colonies reaching 7 mm diam.
Specimen examined: South Korea, Suwon, on leaves of Platanus occidentalis, 7 Nov. 2007, H.D. Shin, holotype CBS H-20884, culture ex-type CPC 11726 = CBS 131588.
Notes: Pseudocercospora pseudostigmina-platani resembles the Pseudocercospora/Stigmina synanamorphs of Mycosphaerella stigmina-platani on Platanus in the USA, although its conidia are larger in size. The stigmina-like anamorph has conidia that are 3-6-septate, (15-)23-30(-45) × (6-)8-9(-10) μm, and the Pseudocercospora conidia are 3-7-septate, (35-)45-60(-100) × (4-)4.5-6(-6.5) μm (Crous & Corlett 1998). Based on DNA sequence comparisons, the genus Stigmina was treated as synonym of Pseudocercospora (Crous et al. 2006). The two species occurring on Platanus both with Pseudocercospora/Stigmina synanamorphs treated here, further support this synonymy.
Pseudocercospora pyracanthae (Katsuki) C. Nakash. & Tak. Kobay., Ann. Phytopathol. Soc. Japan 63: 313. 1997.
Basionym: Cercospora pyracanthae Katsuki, Bull. Agric. Improv. Sect. Econ. Dept. Fukuoka Pref. 1: 19. 1949.
Specimens examined: Japan, Fukuoka, Kurume, on Pyracantha angustifolia, 6 Nov. 1947, S Katsuki, holotype TNS-F-243829; Chiba, Sanbu, October 1976, E. Ishizawa, TFM: FPH-4432; Okayama, Okayama, on P. angustifolia, 20 Nov. 1960, H. Tanaka, TFM: FPH-3247; P. angustifolia, T. Koboyashi & C. Nakashima, CNS-446, culture MUCC892; Ibaraki, on P. angusti, Nov. 1994, T. Nishijima, culture MAFF 237140; Kumamoto, on P. crenulata, 1973, T. Kobayashi, culture MAFF 410022.
Notes: DNA sequence data obtained for Japanese isolates of this species indicate at least two different taxa. Further research is required to select a specimen and isolate that is authentic for the name, while other collections probably represent a novel species.
Pseudocercospora pyracanthigena Crous, U. Braun & H.D. Shin, sp. nov. MycoBank MB564842. Fig. 54.
Fig. 54.
Pseudocercospora pyracanthigena (CPC 10808). A. Leaf spots on upper and lower leaf surface. B. Close-up of leaf spot with fruiting. C, D. Fascicles with conidiophores and conidiogenous cells. E. Conidiogenous cell giving rise to a conidium. F. Conidia. Scale bars = 10 μm.
Etymology: Name derived from the host plant Pyracantha, from which it was collected.
Leaf spots amphigenous, irregular to angular, up to 7 mm diam, brown, with inconspicuous border. Mycelium internal, hyaline to pale brown, consisting of septate, branched, smooth, 2-3 μm diam hyphae. Caespituli fasciculate to sporodochial, amphigenous, but predominantly epiphyllous, olivaceous on leaves, up to 150 μm wide and 60 μm high. Conidiophores aggregated in dense fascicles arising from the upper cells of a brown stroma up to 120 μm wide and 35 μm high; conidiophores medium brown, smooth, 0-1-septate, subcylindrical to ampulliform, straight, unbranched, mostly reduced to conidiogenous cells, tapering to flat-tipped apical loci, proliferating sympodially or percurrently near apex, 7-15 × 2-3 μm. Conidia solitary, brown, smooth, guttulate, subcylindrical to narrowly obclavate, apex subobtuse, base obconically subtruncate to truncate, straight to gently curved, 1-4-septate, (30-)35-40(-45) × (2.5-)3(-3.5) μm; hila unthickened, neither darkened nor refractive, 1.5 μm wide.
Culture characteristics: Colonies after 2 wk at 24 °C in the dark on MEA; surface folded, erumpent, spreading, with sparse aerial mycelium, and smooth, lobate margins. Surface smoke-grey; reverse olivaceous-grey. Colonies reaching 15 mm diam.
Specimen examined: South Korea, Jeju, Halla arboretum, on leaves of Pyracantha angustifolia, 1 Nov. 2007, M.J. Park, holotype CBS H-20885, culture ex-type CPC 10808 = CBS 131589.
Notes: Pseudocercospora pyracanthigena is distinct from P. pyracanthae (conidia 25-65 × 2.4-4 μm, conidiophores 15-40 × 2.5-3 μm; Chupp 1954) in having shorter conidia and conidiophores. A second species has been recorded on Pyracantha angustifolia in Korea (CPC 14711-14713), for which a new name is required.
Pseudocercospora ranjita (S. Chowdhury) Deighton, Mycol. Pap. 140: 151. 1976. Fig. 55.
Fig. 55.
Pseudocercospora ranjita (CPC 11141). A. Leaf spots on upper leaf surface. B, C. Close-up of leaf spots with fruiting. D-F. Fascicles with conidiophores and conidiogenous cells. H. Branched conidiophore. G, I-K. Conidia. Scale bars = 10 μm.
Basionym: Cercospora ranjita S. Chowdhury, Lloydia 21: 155. 1958.
Leaf spots epiphyllous, distinct, scattered, white to pale brown, irregular, 1-4 mm diam, definite raised brown border, surrounded entirely or partly by brown to dark brown irregular halo. Mycelium internal and external, 2-5 mm wide, branched, smooth, septate, subhyaline to pale brown. Caespituli epiphyllous, few in number, distributed over the leaf spot, dark brown to black. Stromata well-developed, intraepidermal to subimmersed, brown, globular to irregular, 40-90 μm diam. Conidiophores fasciculate, arising from the upper cells of stromata, pale brown, straight to curved, unbranched and branched, 1-4-septate, irregular in width, apex truncate, (20-)27-38(-42) × (3-)3.5-4.5(-5) μm. Conidiogenous cells terminal, unbranched, pale brown, smooth to finely verrucose, proliferating percurrently, (8-)9-15(-19) × 3(-4) μm. Conidia solitary, cylindrical to obclavate, 2-9-septate, subhyaline to pale brown, smooth, apex rounded to subobtuse, base obconically to long obconically truncate, (26-)44-67(-84) × (3-)4-5(-6) μm; hila unthickened nor darkened.
Culture characteristics: Colonies on MEA reaching 27 mm diam after 30 d at 24 °C on MEA. Colonies circular with a smooth margin, that is darker than the colony centre, slight folding; aerial mycelium moderate; greyish blue to olivaceous-grey (surface) and iron-grey (reverse).
Specimen examined: Indonesia, Northern Sumatra, on leaves of Gmelina sp., Mar. 2004, M.J. Wingfield, CBS H-20386, culture CPC 11141 = CBS 126005.
Note: The present collection closely matches the morphological description of the type specimen, which was collected from India (Chowdhury 1958).
Pseudocercospora ravenalicola G.C. Hunter & Crous, sp. nov. MycoBank MB564843. Fig. 56.
Fig. 56.
Pseudocercospora ravenalicola (CBS 122468). A. Leaf spots on upper leaf surface. B, C. Close-up of leaf spots. D-G. Fascicles with conidiophores and conidiogenous cells. H-L. Conidia. Scale bars = 10 μm.
Etymology: Name derived from the plant host Ravenala, from which this fungus was isolated.
Leaf spots amphigenous, distinct, brown to pale, predominantly at leaf margin, but smaller spots are scattered over the whole leaf, elongated to irregular; border definite, raised, with dark brown to black border. Caespituli amphigenous, sparsely scattered over the leaf spot and aggregated toward the lesion margin, flocculose, pale to pale olivaceous. Stromata erumpent to superficial, globular, pale to dark brown, 30-80 μm diam. Conidiophores fasciculate, arising from the stromata, brown, becoming paler toward the apex, smooth, 0-3-septate, straight to curved, apex subtruncate to rounded, predominantly unbranched, sometimes branched below, (14-)17-25(-32) × (3-)4-5(-6) μm. Conidiogenous cells terminal, pale brown, smooth, straight to geniculate, tapering to a truncate to blunt apex, proliferating sympodially and percurrently, (7-)13(-15) × (3-)3.5(-4) μm. Conidia solitary, cylindrical, straight to curved, smooth, subhyaline to pale brown, 1-6-septate, infrequently constricted at the septa, apex obtuse to narrowly rounded, base obconically truncate to long obconically truncate, (16-)25-47(-60) × (3-)4(-5) μm; hila unthickened, nor darkened.
Culture characteristics: Colonies after 1 mo at 24 °C in the dark on MEA; erumpent, spreading, with moderate aerial mycelium, and smooth, lobate margins. Surface smoke-grey in centre, pale olivaceous-grey in outer region; reverse olivaceous-grey. Colonies reaching 35 mm diam.
Specimen examined: India, Chandigarh, on leaves of Ravenala madagascariensis, 2 Mar. 2004, W. Gams, holotype CBS H-20394, culture ex-type CBS 122468.
Note: Pseudocercospora ravenalicola represents the first species of Pseudocercospora known from this host and the Strelitziaceae.
Pseudocercospora rhabdothamni U. Braun & C.F. Hill, Australas. Plant Pathol. 33: 489. 2004.
Specimen examined: New Zealand, Auckland, University Campus, Princes Street, on Rhabdothamnus solanderi, 9 Nov. 2003, C.F. Hill, holotype HAL 1790 F, isotype PDD 80279, culture ex-isotype CBS 114872, ICMP 15289.
Note: Two strains have been deposited in CBS under the name Ps. rhabdothamni.
Pseudocercospora rhamnellae G.C. Hunter, H.D. Shin, U. Braun & Crous, sp. nov. MycoBank MB564844. Fig. 57.
Fig. 57.
Pseudocercospora rhamnellae (CPC 12500-12502). A. Leaf spots on upper leaf surface. B, C. Close-up of leaf spots with fruiting. D, E. Fascicles with conidiophores and conidiogenous cells. F-J. Conidia. Scale bars = 10 μm.
Etymology: Name derived from the plant host Rhamnella, from which this fungus was isolated.
Leaf spots distinct, amphigenous, subcircular to irregular, pale to dark brown, dark brown to black raised border with effuse spreading pale to dark brown halo, solitary or sometimes coalescing, 2-11 mm diam. Mycelium smooth, branched, internal and external, pale brown, septate 2-4 μm diam. Caespituli amphigenous, on adaxial surface single, scattered to slightly aggregated, pale to light brown, on abaxial surface significantly more dense, mostly aggregated over the lesions surface, light brown to light olive-green. Stromata medium to large, well-developed, superficial to intraepidermal, pale to dark brown, 30-85 μm diam. Conidiophores fasciculate, straight to curved, brown, becoming paler to the apex, unbranched, smooth to finely verruculose, subcylindrical, 0-1-septate, (10-)13-19(-23) × (2-)3-4(-5) μm. Conidiogenous cells terminal, unbranched, pale brown, smooth to slightly verruculose, proliferating sympodially or percurrently near apex, (3-)5-10(-15) × (2-)3-4(-5) μm. Conidia solitary, guttulate, straight to curved, apex obtusely rounded, base truncate, solitary, pale brown, thin-walled, smooth, subcylindrical to narrowly obclavate, 1-12-septate, (17-)33-57(-80) × (2-)3(-4) μm, hila neither thickened, nor darkened or refractive, 2-3 μm diam.
Culture characteristics: Colonies after 2 wk at 24 °C in the dark on MEA; surface folded, erumpent, spreading, with sparse aerial mycelium, and smooth, lobate margins. Surface olivaceous-grey with patches of pale olivaceous-grey; reverse iron-grey. Colonies reaching 10 mm diam.
Specimen examined: South Korea, Jeju, Halla arboretum, on leaves of Rhamnella franguloides, 29 Oct. 2005, H.D. Shin, holotype CBS H-20395, culture ex-type CPC 12500 = CBS 131590, CPC 12501, 12502.
Notes: No species of Pseudocercospora are presently known to occur on Rhamnella (Rhamnaceae). Pseudocercospora rhamnellae is distinct from P. rhamnaceicola (on Paliurus, Rhamnus and Zizyphus; conidia 18-85 × 1.5-2.5 μm, apex pointed, base obconically truncate, Hsieh & Goh 1990) by having wider conidia, which are subcylindrical-obclavate with an obtusely rounded apex and truncate base. The conidiophores are also shorter and wider. Further collections are needed to determine whether isolates from other hosts in the Rhamnaceae all represent P. rhamnaceicola.
Pseudocercospora rhododendri-indici Crous, U. Braun & H.D. Shin, sp. nov. MycoBank MB564845. Fig. 58.
Fig. 58.

Pseudocercospora rhododendri-indici (CPC 10822-10824). A. Leaf spots on upper and lower leaf surface. B. Close-up of leaf spot with fruiting. C, D. Fascicles with conidiophores and conidiogenous cells. E. Conidia. Scale bars = 10 μm.
Etymology: Name derived from the plant host Rhododendron indicum, from which it was collected.
Leaf spots amphigenous, subcircular to circular, 2-3 mm diam, medium brown with a raised, dark brown border. Mycelium internal, pale brown, consisting of septate, branched, smooth, 2-3 μm diam hyphae. Caespituli fasciculate to sporodochial, olivaceous-brown, predominantly epiphyllous, up to 100 μm diam and 80 μm high. Conidiophores aggregated in dense fascicles, arising from the upper cells of a brown stroma, up to 80 μm diam and 40 μm high; conidiophores pale brown, smooth, 0-2-septate, subcylindrical, straight to geniculate-sinuous, unbranched, 10-30 × 3-4 μm. Conidiogenous cells terminal, pale brown, smooth, tapering to flat-tipped apical loci, proliferating sympodially, 10-15 × 3-3.5 μm. Conidia solitary, pale brown, smooth, guttulate, subcylindrical, apex subobtuse, base truncate, straight to variously curved, 1-4-septate, (35-)40-55(-65) × (2-)3 μm; hila unthickened, neither darkened nor refractive, 2-3 μm diam.
Culture characteristics: Colonies after 2 wk at 24 °C in the dark on MEA; erumpent, spreading, with moderate aerial mycelium, and smooth, lobate margins. Surface olivaceous-grey in centre, pale olivaceous-grey in outer region; reverse iron-grey. Colonies reaching 14 mm diam.
Specimen examined: South Korea, Seoul, on Rhododendron indicum, 27 Oct. 2003, H.D. Shin, holotype CBS H-20886, cultures ex-type CPC 10822 = CBS 131591, CPC 10823, 10824.
Notes: Of the species occurring on Rhododendron, P. rhododendri-indici differs from P. handelii (conidia narrowly linear to obclavate, indistinctly multiseptate, 12-140 × 1.5-3 μm; Chupp 1954) by its subcylindrical, 1-4-septate conidia with truncate base and obtuse apex, and phylogentic position (Fig. 5). The description and illustration of P. handelii based on Chinese material (Guo & Hsieh 1995) agrees well with Chupp’s (1954) description. The identity of Korean collections on Rhododendron indicum described in Shin & Kim (2001), characterised by much longer acicular-filiform conidia with truncate base, is unclear. Pseudocercospora rhododendri-indici differs from P. rhododendricola (conidia 54-96 × 2-2.5 μm; Yen 1966) by its shorter conidia. Beside epiphyllous colonies, P. rhododendricola forms hypophyllous colonies composed of small, loose fascicles of conidiophores that emerge through stomata, together with superficial hyphae that give rise to solitary conidiophores. The hypophyllous fruiting was neither mentioned in the original description nor in Yen & Lim (1980). It was observed during the re-examination of type material (Singapore, Botanic Gardens, on Rhododendron sp., 13 Apr. 1965, S.H. Yen No. 112, holotype PC).
Pseudocercospora rhoina (Cooke & Ellis) Deighton, Mycol. Pap. 140: 152. 1976. Fig. 59.
Fig. 59.
Pseudocercospora rhoina (CPC 11464-11465). A. Leaf spots on upper and lower leaf surface. B. Close-up of leaf spot with fruiting. C-E. Fascicles with conidiophores and conidiogenous cells. F. Conidia. Scale bars = 10 μm.
Basionym: Cercospora rhoina Cooke & Ellis, Grevillea 6: 89. 1878.
= Cercospora copallina Cooke, Grevillea 12: 31. 1883.
= Cercospora rhoina var. nigromaculans Peck, Rep. (Annual) New York State Mus. Nat. Hist. 42: 129. 1889.
Specimen examined: South Korea, Namhae, on Rhus chinensis, 30 Jun. 2004, H.D. Shin, CBS H-20887, KUS-F 20367, CPC 11464 = CBS 131891.
Pseudocercospora sambucigena U. Braun, Crous & K. Schub., Mycotaxon 92: 400. 2005. Fig. 60.
Fig. 60.
Pseudocercospora sambucigena (CPC 14397-14399). A, B. Leaf spots on upper and lower leaf surface. C, D. Close-up of leaf spots with fruiting. E, F. Fascicles with conidiophores and conidiogenous cells. G. Conidiogenous cells. H-L. Conidia. Scale bars = 10 μm.
Leaf spots distinct, scattered over leaf surface, amphigenous, upper surface pale brown to grey, with definite border that is raised and dark brown in colour; lower surface pale grey to pale brown, with distinctly raised, brown border, 2-10 mm diam. Mycelium smooth, internal and external, consisting of branched, subhyaline, 2-4 μm diam hyphae. Caespituli amphigenous, predominantly occurring on the abaxial lesion surface, evenly distributed over the lesion, punctiform, grey to dark brown. Stromata well-developed, subimmersed becoming erumpent, globular, dark brown, 45-100 mm diam. Conidiophores fasciculate, emerging from stomata, brown, becoming paler toward the apex, unbranched, straight to curved, cylindrical, uniform or irregular in width, rounded apex, indistinctly 0-3-septate, (25-)35-51(-60) × (4-)5(-7) μm. Conidiogenous cells terminal, unbranched, smooth, pale brown, proliferating sympodially and percurrently, conidiogenous loci (scars) unthickened to slightly thickened, but not darkened, (10-) 19-34(-46) × (3-)5 μm. Conidia solitary, pale olivaceous to pale brown, smooth, guttulate, apex obtuse, base long obconically truncate, shape variable from cylindrical to obclavate, 1-7-septate, (40-)68-117(-156) × (4-)5-6(-7) μm; hila unthickened to slightly thickened, but not darkened.
Culture characteristics: Colonies on MEA reaching 16 mm diam after 30 d in the dark at 24 °C. Colonies circular to subcircular, smooth to slightly irregular margin, prominently convex, moderate aerial mycelium; pale greenish grey to pale olivaceous-grey (surface) and olivaceous-black (reverse).
Specimens examined: Italy, Parma, on leaves of Sambucus nigra, G. Passerini, paratype B 70-6710. Netherlands, Milingerwaard on leaves of Sambucus nigra, 2007, P.W. Crous, epitype designated here CBS H-20391, cultures ex-epitype CPC 14397 = CBS 126000. USA, Pennsylvania, Dauphin Co., on leaves of Sambucus pubens, 21 Aug. 1921, O.E. Jennings, Acc. 6736, holotype NY.
Pseudocercospora securinegae (Togashi & Katsuki) Deighton, Mycol. Pap. 140: 152. 1976. Fig. 61.
Fig. 61.
Pseudocercospora securinegae (CPC 10793). A. Leaf spots on upper and lower leaf surface. B. Close-up of leaf spot with fruiting. C, D. Fascicles with conidiophores and conidiogenous cells. E. Conidia. Scale bars = 10 μm.
Basionym: Cercospora securinegae Togashi & Katsuki, Ann. Phytopathol. Soc. Japan 17: 7. 1952.
Specimen examined: South Korea, Yangpyong, on Flueggea suffruticosa (≡ Securinega suffruticosa), 30 Sep. 2003, H.D. Shin, CBS H-20888, culture CPC 10793 = CBS 131930.
Pseudocercospora snelliana (Reichert) U. Braun, H.D. Shin, C. Nakash. & Crous, comb. nov. MycoBank MB564846. Figs 62, 63.
Fig. 62.
Pseudocercospora snelliana (B 700014740, holotype). Sparse fascicles, and solitary conidiophores on superficial mycelium giving rise to muriformly septate, thick-walled conidia. Scale bar = 10 μm.
Fig. 63.
Pseudocercospora snelliana (CPC 11654-11656). A. Leaf spots on the lower leaf surface. B. Close-up of leaf spot with fruiting. C-E. Solitary conidiophores and conidiogenous cells. F-H. Conidia. Scale bars = 10 μm.
Basionym: Cercospora snelliana Reichert, Bot. Jahrb. Syst. 56: 724. 1921.
-
= Clasterosporium mori Syd. & P. Syd., Mem. Herb. Boiss. 4: 6. 1900.
≡ Sirosporium mori (Syd. & P. Syd.) M.B. Ellis, Mycol. Pap. 87: 7. 1963.
≡ Cercospora kusanoi Sawada, Rep. Dept. Agric. Gov. Res. Inst. Formosa 35: 109. 1928, nom. nov., non Cercospora mori Hara, 1918.
= Cercospora bremeri Petr., Sydowia 2: 312. 1948.
= Cercospora flexuosa Tanaka, unknown, nom. nud., non Tracy & Earle, 1895.
Leaf spots lacking or amphigenous, but inconspicuous on upper leaf surface, chlorotic, irregular, as small speckles, up to 8 mm diam, or effuse and much larger, forming large blotches or covering large portions of the hypophyllous surface with blackish colonies. Mycelium internal and external; internal hyphae pale olivaceous to pale brown, smooth, 3-4 μm diam, arising through stomata, giving rise to external mycelium that is pale yellowish green, olivaceous to brown, smooth, thin-walled, 1.5-5 μm diam. Conidiophores arising singly from superficial mycelium and in small, divergent fascicles from a few substomatal swollen hyphal cells, 2-8 μm diam., emerging through stomata, brown, smooth, becoming roughened towards apex, wall up to 1 μm thick, 1-12-septate, subcylindrical to often subclavate, i.e. width somewhat increasing towards the apex, straight to variously curved or geniculate-sinuous, unbranched or branched above, 15-100 × 3-6 μm. Conidiogenous cells terminal or lateral, unbranched, brown, becoming paler towards the tip, roughened, tapering towards flat-tipped loci, 2-3 μm diam, proliferating sympodially (lateral scars as illustrated by Ellis 1971 observed), or percurrently near apex, 10-30 × 4-7 μm. Conidia solitary, medium to dark olivaceous-brown or brown, small young conidia sometimes subhyaline to pale olivaceous, wall up to 1 μm thick, smooth or almost so to verruculose, guttulate, smaller conidia ellipsoid-ovoid, subcylindrical, larger conidia usually distinctly obclavate, apex obtuse, base obconically truncate, subtruncate or sometimes rounded, straight to gently curved, 1-10-septate (septa somewhat refractive, at times also 1(-2) oblique or vertical septa present), (15-)30-70(-80) × (3-)4-6(-7) μm; hila neither thickened, nor darkened or refractive, 1-1.5(-2) μm diam.
Culture characteristics: Colonies after 2 wk at 24 °C in the dark on MEA; erumpent, spreading, with sparse aerial mycelium, and smooth, lobate margins. Surface pale olivaceous-grey; reverse olivaceous-grey. Colonies reaching 7 mm diam.
Specimens examined: Egypt, Kahirahm, near Bahtim, on Morus alba, Nov. 1913, Snell, holotype B 700014740. South Korea, Hoengseong, on Morus bombycis, 11 Oct. 2004, H.D. Shin, CBS H-20889, HAL 1867 F, culture CPC 11654 = CBS 131592, CPC 11655, 11656.
Notes: Cercospora kusanoi is based on the same type specimen used by Sydow to describe Clasterosporium mori. Sawada (1928) considered this fungus a species of Cercospora. He introduced the name Cercospora kusanoi because the species epithet mori was occupied in Cercospora. The Korean material we studied closely resembles the description of the type, which was originally described on Morus alba from Japan (Sawada 1928). Pseudocercospora mori is also already occupied so type material of P. snelliana, the next available epithet, was re-examined. We determined it to be conspecific with C. kusanoi, so P. snelliana is introduced as a new combination.
Pseudocercospora stephanandrae (Tak. Kobay. & H. Horie) C. Nakash. & Tak. Kobay., Mycoscience 41: 27. 2000.
Basionym: Cercospora stephanandrae Tak. Kobay. & H. Horie, Trans. Mycol. Soc. Japan 20: 331. 1979.
Specimens examined: Japan, Tokyo, Jindai Bot. Park, on Stephanandra incisa, 21 Oct. 1976, T. Kobayashi & H. Horie TFM: FPH-4712; Tokyo, Jindai Botanical Park, Chofu-City, on S. incisa, 26 Oct. 1974, H. Horie, holotype TFM: FPH 4411; Tokyo, Jindai Bot. Park, on S. incisa, 7 Nov. 1998, C. Nakashima & E. Imaizumi, epitype designated here TFM: FPH-8099, ex-epitype cultures MUCC 914, MAFF 237799.
Pseudocercospora timorensis (Cooke) Deighton, Mycol. Pap. 140: 154. 1976.
Basionym: Cercospora timorensis Cooke, Grevillea 12: 38. 1883.
= Ramularia batatae Racib., Paras. Algen Pilze Javas, Batavia 1: 35. 1900.
= Cercospora batatae A. Zimmerm., Ber. Land.-Forstw. Deutsch Ostafrikas 2: 28. 1904.
= Cercospora batatae Henn., Bot. Jahrb. Syst. 38: 118. 1907, nom. illeg., homonym of C. batatae A. Zimmerm., 1904.
-
= Cercospora ipomoeae-purpureae J.M. Yen, Rev. Mycol. 30: 173. 1965.
≡ Pseudocercospora ipomoea-purpureae (J.M. Yen) J.M. Yen, in Yen & Lim, Gard. Bull., Singapore 33: 177. 1980.
Specimen examined: Japan, Okinawa, Ipomoea indica, 19 Nov. 2007, C. Nakashima & T. Akashi, MUMH 10923, culture MUCC 819.
Pseudocercospora udagawana (Katsuki) X.J. Liu & Y.L. Guo, Mycosystema 2: 238. 1989. Fig. 64.
Fig. 64.
Pseudocercospora udagawana (CPC 10799-10801). A. Leaf spots on upper and lower leaf surface. B. Close-up of leaf spot with fruiting. C, D. Fascicles with conidiophores and conidiogenous cells. E. Solitary conidiogenous cell on superficial hypha. F. Conidia. Scale bar = 10 μm.
Basionym: Cercospora udagawana Katsuki, Ann. Phytopathol. Soc. Japan 20(2-3): 72. 1955.
Specimen examined: South Korea, Dongducheon, on Hovenia dulcis, 28 Sep. 2003, H.D. Shin, CBS H-20890, CPC 10799 = CBS 131931.
Pseudocercospora viburnigena U. Braun & Crous, Mycol. Progr. 1: 23. 2002. Fig. 65.
Fig. 65.
Pseudocercospora viburnigena (CPC 15249). A. Leaf spots on upper leaf surface. B, C. Close-up of leaf spots with fruiting. D, E. Fascicles with conidiophores and conidiogenous cells. F-H. Conidia. Scale bars = 10 μm.
Basionym: Cercospora tinea Sacc., Michelia 1(2): 268. 1878 (non P. tinea Y.L. Guo & W.H. Hsieh, 1994).
≡ Cercoseptoria tinea (Sacc.) Deighton, Mycol. Pap. 140: 167. 1976.
≡ Cercostigmina tinea (Sacc.) U. Braun, Cryptog. Bot. 4: 108. 1993.
Leaf spots distinct, scattered, amphigenous, 4-15 mm diam, lesions on abaxial surface dark to pale brown, subcircular to irregular, surrounded by a slightly raised dark brown border, lesions on adaxial surface dark to pale brown, surrounded by a dark brown border with a light red diffuse pigment extending outward from the border in older lesions. Mycelium internal and external, smooth, subhyaline, branched, 1.5-4 μm wide. Caespituli amphigenous, but predominantly hypophyllous, evenly distributed over the leaf spot, velvety, olivaceous. Stromata well-developed, subimmersed, globular, dark brown, 30-80 μm diam. Conidiophores fasciculate, smooth, 0-2-septate, emerging from the upper cells of the stroma, pale brown, straight to curved, irregular in width, apex subtruncate to rounded, (14-)17-24(-30) × (3-)4-5(-6) μm. Conidiogenous cells integrated, terminal, inconspicuously proliferating percurrently, cylindrical, straight, pale brown, at times slightly verruculose, (5-)9-15(-19) × (2-) 3(-4) μm. Conidia solitary, pale brown, smooth, guttulate, apex obtusely rounded, base narrowly truncate, narrowly ellipsoidal to acicular, curved or sigmoid, 5-11-septate, (68-)87-110(-120) × (2-)3-4(-5) μm, hila unthickened.
Culture characteristics: Colonies on MEA reaching 23 mm diam after 30 d at 24 °C in the dark. Colonies circular, convex, smooth margin that is distinctly darker than the rest of the colony, slight folding occurs toward the edge of the colony, moderate to profuse aerial mycelium; olivaceous-grey (surface) and greenish black (reverse).
Specimens examined: Italy, Padova, Viburnum tinus, Oct. 1877, Bizzozera, Sacc., Mycoth. Venet. 1252, syntype HAL. Netherlands, Bilthoven, Sweelincklaan 87, on leaves of Viburnum davidii, 26 May 2008, M.K. Crous, epitype designated here CBS H-20393, culture ex-epitype CPC 15249 = CBS 125998.
Note: The epitype closely matches the morphology of the holotype (Braun & Hill 2002), representing a species that is common on Viburnum in Europe.
Pseudocercospora viticicola (J.M. Yen & Lim) J.M. Yen, Gardens Bulletin, Singapore 33: 190. 1980.
Basionym: Cercospora viticicola J.M. Yen & Lim, Cah. Pacifique 17: 104. 1973.
-
= Cercospora viticis Ellis & Everh. (as “viteae”), J. Mycol. 3: 18. 1887, non Pseudocercospora viticis Goh & W.H. Hsieh, 1989.
≡ Pseudocercosporella viticis (Ellis & Everh.) B.K. Gupta & Kamal, Indian Phytopathol. 42: 388. 1989, nom. inval.
≡ Pseudocercospora viticicola U. Braun, Mycotaxon 48: 296. 1993, nom. illeg., homonym of P. viticicola (J.M. Yen & Lim) J.M. Yen, 1980.
-
= Cercospora viticis Sawada, Rep. Gov. Agric. Res. Inst. Taiwan 87: 90. 1944, nom. illeg., homonym of C. viticis Ellis & Everh., 1887.
≡ Pseudocercospora viticis Goh & W.H. Hsieh, Trans. Mycol. Soc. Republ. China 4: 11. 1989.
-
= Cercospora viticis-quinatae J.M. Yen, Bull. Trimestriel Soc. Mycol. France 93: 158. 1977.
≡ Pseudocercospora viticis-quinatae (J.M. Yen) J.M. Yen, Bull. Trimestriel Soc. Mycol. France 94: 388. (1978) 1979.
= Pseudocercospora viticigena J.M. Yen, A.K. Kar & B.K. Das, Mycotaxon 16: 68. 1982.
Specimens examined: Japan, Okinawa, Okinawa Is, on Vitex trifolia, 19 Nov. 2007, C. Nakashima, MUMH 10828, culture MUCC 777; Chiba, Matsudo, on V. agnus-castus, 7 Nov. 1987, M. Nagashima & T. Kobayashi, TFM: FPH-6912; Shizuoka, Kanzanji, on V. agnus-castus, 1 Nov. 1996, T. Kobayashi & C. Nakashima, CNS-101, culture MUCC 1069, MAFF 237866; Kuroki, Fukuoka, on V. cannabifolia (≡ V. negundo var. cannabifolia), 25 Sep. 1974, S. Ogawa, TFM: FPH-4193.
Pseudocercospora weigelae (Ellis & Everh.) Deighton, Trans. Brit. Mycol. Soc. 88: 389. 1987.
Basionym: Cercospora weigelae Ellis & Everh., Proc. Acad. Nat. Sci. Philadelphia 45: 170. 1893.
Specimen examined: Japan, Ibaraki, on Weigela coraeensis, 10 Sep. 1998, T. & Y. Kobayashi, CNS-455, culture MUCC 899, MAFF 237794.
Pseudocercospora xanthocercidis Crous, U. Braun & A. Wood, sp. nov. MycoBank MB564847. Fig. 66.
Fig. 66.
Pseudocercospora xanthocercidis (CPC 11665-11667). A. Leaf spots on upper and lower leaf surface. B. Close-up of leaf spot with fruiting. C-E. Fascicles with conidiophores and conidiogenous cells. F. Conidia. G. Colony on malt extract agar. H, I. Conidia formed in culture. Scale bars = 10 μm.
Etymology: Name derived from the plant host Xanthocercis, from which it was collected.
Leaf spots amphigenous, irregular to subcircular, 3-8 mm diam, pale to medium brown, with indistinct border. Mycelium internal, pale brown, consisting of septate, branched, smooth, 2-3 μm diam hyphae. Caespituli sporodochial, hypophyllous, also occurring on green leaf tissue, prominent, appearing like insect galls, olivaceous-brown on leaves, up to 400 μm wide and 300 μm high. Conidiophores aggregated in dense sporodochial fascicles arising from the upper cells of a brown stroma up to 300 μm wide and 250 μm high; conidiophores brown, finely verruculose, 1-2-septate, subcylindrical, straight to slightly curved, 20-30 × 5-7 μm. Conidiogenous cells terminal, unbranched, brown, subcylindrical, finely verruculose, proliferating percurrently near apex, with several irregular, rough proliferations, 7-12 × 5-6 μm. Conidia solitary, brown, finely verruculose, guttulate, narrowly obclavate, apex obtuse, base obconically subtruncate to truncate, straight to gently curved, 5-8-septate, (25-)28-36(-40) × (5-)6-7 μm; hila unthickened, neither darkened nor refractive, 3-4 μm diam, with minute marginal frill visible.
Culture characteristics: Colonies after 2 wk at 24 °C in the dark on MEA; surface irregular, folded, erumpent, spreading, with sparse aerial mycelium, and smooth, irregularly lobate margins. Surface olivaceous-grey, with patches of iron-grey; reverse iron-grey. Colonies reaching 5 mm diam.
Specimen examined: South Africa, Mpumalanga, Nelspruit, Lowveld National Botanical Garden, on Xanthocercis zambesiaca, 14 Sep. 2004, A. Wood, holotype HAL 1859 F, isotype CBS H-20891, culture ex-type CPC 11665 = CBS 131593, CPC 11666, 11667.
Notes: No other species of Pseudocercospora are known from this host. Pseudocercospora xanthocercidis differs from other Pseudocercospora species on legumes by its very large sporodochial conidiomata with percurrently proliferating conidiogenous cells and verruculose conidia with visible marginal frill at the base. There is no comparable species on legumes.
Pseudocercospora xanthoxyli (Cooke) Y.L. Guo & X.J. Liu, Mycosystema 4: 115. 1991. Fig. 67.
Fig. 67.
Pseudocercospora xanthoxyli (CPC 10009, 10064-10065). A. Leaf spots on upper and lower leaf surface. B. Close-up of leaf spot with fruiting. C-E. Fascicles with conidiophores and conidiogenous cells. F. Close-up of conidiogenous cells. G. Conidia. Scale bars = 10 μm.
Basionym: Cercospora xanthoxyli Cooke, Grevillea 12: 30. 1883.
-
= Cercospora fagaricola Sawada (fagariae), Rep. Gov. Agric. Res. Inst. Taiwan 85: 105. 1943, nom. inval.
≡ Pseudocercospora fagaricola Goh & W.H. Hsieh, in Hsieh & Goh, Cercospora and similar species from Taiwan: 294. 1990.
Specimen examined: South Korea, Wando, Wando Arboretum, on Xanthoxylum ailanthoides, 9 Nov. 2002, H.D. Shin, CBS H-20892, CPC 10009, 10064-10065.
Pseudocercospora zelkovae (Hori) X.J. Liu & Y.L. Guo, Acta Mycol. Sin. 12: 33. 1993. Fig. 68.
Fig. 68.
Pseudocercospora zelkovae (CPC 14484). A. Leaf spots on upper and lower leaf surface. B. Close-up of leaf spot with fruiting. C-E. Fascicles with conidiophores and conidiogenous cells. F. Conidia. Scale bar = 10 μm.
Basionym: Cercospora zelkowae Hori, Nambu N. Jour. Plant Protection 8: 492. 1921.
Holotype: Japan, Tokyo, Forest Experimental Station, on Zelkova serrata, Jun. 1920 (not preserved).
Specimens examined: Japan, Yamagata, Kamabuchi, on Z. serrata, 5 July 1956, K. Ito, neotype designated here TFM:FPH169, cultures ex-neotype MAFF 410008, MUCC 1398. South Korea, Suwon, on Z. serrata, 2 Oct. 2007, H.D. Shin, CBS H-20893, culture CPC 14484 = CBS 132106; Osan, on Z. serrata, 30 Oct. 2007, H.D. Shin, CBS H-20894, CPC 14717 = CBS 132118.
DISCUSSION
This study provides a broad framework and phylogeny for the genus Pseudocercospora. These fungi are very common and the foundation that has been set will form the basis for additional species to be described and for specific groups to be more thoroughly investigated. Although the results clarify several issues relating to the taxonomy of Pseudocercospora s. str., the study also highlights many remaining taxonomic questions relating to this complex. To resolve these issues many species will need to be recollected, cultured, and sequenced so that they can be placed into this phylogenetic backbone. This is especially true for species described in some of the obscure genera treated by Braun (1995) and Crous & Braun (2003), many of which (or their type species) are not currently known from culture, and thus DNA sequence comparisons and phylogenetic inference has not been possible.
Amongst the cercosporoid fungi, it appears possible and even probable that the approximately 1 500 names in Pseudocercospora represent the tip of the iceberg in terms of biodiversity. Indeed it seems likely that this could emerge as the largest genus of cercosporoid fungi known. A significant result of this study was the determination that names based on American or European type specimens could in most cases not be used when identifying identical diseases on the same hosts in Asia, Africa or South America. In this regard, it was surprising to find diversity even within a region such as Asia, where isolates from the same host and disease symptoms from Korea frequently differed from similar collections made in Japan. These important issues, which have significant ramifications pertaining to plant health and quarantine, will only be resolved when fresh collections from the American and European type locations have been made, thus allowing DNA sequence based comparisons. Furthermore, it emphasises the need to ensure that a DNA sequence has been provided for all novel taxa in this complex and that an authentic DNA barcode (Schoch et al. 2012) is available. The ITS gene region was found to be capable of differentiating only 25 of the 146 Pseudocercospora taxa (17 %) to species level in the present study. Where the ITS locus fails to provide acceptable resolution, it can be supplemented with sequences from the ACT or EF-1α gene regions (Fig. 5), though these loci still proved relatively conserved, and 57 taxa had less than 1% variation from their closest neighbours, suggesting that additional loci still have to be found to provide a more robust identification of Pseudocercospora species.
Focused studies on specific crops such as those on Eucalyptus (Crous 1998, Hunter et al. 2006b), Musa (Arzanlou et al. 2007, 2008, 2010), Chromolaena (Den Breeÿen et al. 2006) and Citrus (Pretorius et al. 2003) will undoubtedly confirm the already emerging view that many plant species are infected by a complex of Pseudocercospora spp. Some of these will clearly be specific to the host from which they were isolated, while others reflect chance occurrences or infections or broader host ranges (Crous & Groenewald 2005). In some instances, these chance infections may be caused by fungi that are major pathogens of other, completely unrelated hosts (Crous & Groenewald 2005, Arzanlou et al. 2008). Although the present study has succeeded in delineating Pseudocercospora within the Mycosphaerellaceae, and in the process has also delineated several other pseudocercospora-like genera, the question relating to host specificity still remains largely unanswered.
The taxa investigated during this study represent the largest collection of Pseudocercospora and pseudocercospora-like taxa ever subjected to DNA sequence analysis. Of these, the vast majority appear to be host-specific. Of the 146 taxa subjected to multi-gene analysis, only four were found to occur on more than one host. These include P. norchiensis (Myrtaceae and Rosaceae), P. fraxinites (Oleaceae), P. atromarginalis (Solanaceae) and P. corylopsidis (Hamamelidaceae). In the latter three examples, the same species was found on different host genera within the same plant family, but never on unrelated hosts. This result was somewhat surprising as we initially expected to find at least some examples where species are generalists and occur on many hosts which are unrelated such as those in the Cercospora apii complex (Groenewald et al. 2006, 2007). The occurrence of P. norchiensis (a foliar pathogen of Eucalyptus in Italy; Crous et al. 2007c) on Rubus in New Zealand (CBS 114641), was highly unexpected, and further collections on Rubus from New Zealand will have to be made to resolve if this was a mere chance occurrence (Crous & Groenewald 2005), or true indication of its host range.
In future studies of Pseudocercospora, additional taxa should be included in the analyses, and further loci screened to obtain a better separation of species. There is an urgent need to conduct inoculation tests to confirm inferences from taxonomic studies about host specificity in this important group of predominantly plant pathogenic fungi. For example, it remains to be shown whether isolates from different hosts with identical DNA barcodes and similar morphology have the ability to cross-infect hosts under natural conditions in the field. It appears that for the most part, F.C. Deighton was correct in his statement “If a sparrow flies to a cherry tree, it’s a cherry tree sparrow. If the same sparrow sits in an apple tree, it is an apple tree sparrow”.
Acknowledgments
We thank the technical staff, Arien van Iperen (cultures), Marjan Vermaas (photographic plates), and Mieke Starink-Willemse (DNA isolation, amplification and sequencing) for their invaluable assistance.
REFERENCES
- Arx JA von. (1983). Mycosphaerella and its anamorphs. Proceedings of the Koninklijke Nederlandse Akademie van Wetenschappen, Series C 86: 15–54 [Google Scholar]
- Arzanlou M, Abeln ECA, Kema GHJ, Waalwijk C, Carlier J, et al. (2007). Molecular diagnostics for the Sigatoka disease complex of banana. Phytopathology 97: 1112–1118 [DOI] [PubMed] [Google Scholar]
- Arzanlou M, Crous PW. (2006). Strelitziana africana. Fungal Planet No. 8 CBS, Utrecht, Netherlands: [Google Scholar]
- Arzanlou M, Crous PW, Zwiers L-H. (2010). Evolutionary dynamics of mating-type loci of Mycosphaerella spp. occurring on banana. Eukaryotic Cell 9: 164–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arzanlou M, Groenewald JZ, Fullerton RA, Abeln ECA, Carlier J, et al. (2008). Multiple gene genealogies and phenotypic characters differentiate several novel species of Mycosphaerella and related anamorphs on banana. Persoonia 20: 19–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ávila A, Groenewald JZ, Trapero A, Crous PW. (2005). Characterisation and epitypification of Pseudocercospora cladosporioides, the causal organism of Cercospora leaf spot of olives. Mycological Research 109: 881–888 [DOI] [PubMed] [Google Scholar]
- Ayala-Escobar V, Yañez-Morales MJ, Braun U, Groenewald JZ, Crous PW. (2005). Cercospora agavicola - a new foliar pathogen of Agave tequilana var. azul from Mexico. Mycotaxon 93: 115–121 [Google Scholar]
- Batzer JC, Gleason ML, Harrington TC, Tiffany LH. (2005). Expansion of the sooty blotch and flyspeck complex on apples based on analysis of ribosomal DNA gene sequences and morphology. Mycologia 97: 1268–1286 [DOI] [PubMed] [Google Scholar]
- Beilharz V, Mayers PE, Pascoe IG. (2003). Pseudocercospora macadamiae sp. nov., the cause of husk spot of macadamia. Australasian Plant Pathology 32: 279–282 [Google Scholar]
- Bensch K, Braun U, Groenewald JZ, Crous PW. (2012). The genus Cladosporium. Studies in Mycology 72: 1–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bensch K, Groenewald JZ, Dijksterhuis J, Starink-Willemse M, Andersen B, et al. (2010). Species and ecological diversity within the Cladosporium cladosporioides complex (Davidiellaceae, Capnodiales). Studies in Mycology 67: 1–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun U. (1992). Taxonomic notes on some species of the Cercospora-complex. Nova Hedwigia 55: 211–221 [Google Scholar]
- Braun U. (1993). Taxonomic notes on some species of the Cercospora complex (III). Mycotaxon 48: 275–298 [Google Scholar]
- Braun U. (1995). A monograph of Cercosporella, Ramularia and allied genera (Phytopathogenic Hyphomycetes). Vol. 1 IHW Verlag, Eching, Germany: [Google Scholar]
- Braun U. (1998). A monograph of Cercosporella, Ramularia and allied genera (phytopathogenic hyphomycetes). Vol. 2 Eching, Germany: IHW-Verlag; [Google Scholar]
- Braun U, Crous PW. (2006). (1732) Proposal to conserve the name Pseudocercospora against Stigmina and Phaeoisariopsis (Hyphomycetes). Taxon 55: 803 [Google Scholar]
- Braun U, Crous PW. (2007). The diversity of cercosporoid hyphomycetes - new species, combinations, names and morphological clarifications. Fungal Diversity 26: 55–72 [Google Scholar]
- Braun U, Crous PW, Dugan F, Groenewald JZ, Hoog GS de. (2003a). Phylogeny and taxonomy of Cladosporium-like hyphomycetes, including Davidiella gen. nov., the teleomorph of Cladosporium s. str. Mycological Progress 2: 3–18 [Google Scholar]
- Braun U, Dick MA. (2002). Leaf spot diseases of eucalypts in New Zealand caused by Pseudocercospora species. New Zealand Journal of Forestry Science 32: 221–234 [Google Scholar]
- Braun U, Freire FCO. (2002). Some cercosporoid hyphomycetes from Brazil - II. Cryptogamie Mycologie 23(4): 295–328 [Google Scholar]
- Braun U, Hill CF. (2002). Some new micromycetes from New Zealand. Mycological Progress 1: 19–30 [Google Scholar]
- Braun U, Hill F, Dick M. (2003b). New cercosporoid leaf spot diseases from New Zealand. Australasian Plant Pathology 32: 87–97 [Google Scholar]
- Braun U, Hill F, Schubert K. (2006). New species and new records of biotrophic micromycetes from Australia, Fiji, New Zealand and Thailand. Fungal Diversity 22: 13–35 [Google Scholar]
- Braun U, Mel’nik VA. (1997). Cercosporoid fungi from Russia and adjacent countries. Trudy Botaniceskogo Instituta imeni V. L. Komarova 20: 1–130 [Google Scholar]
- Braun U, Mouchacca J, McKenzie EHC. (1999). Cercosporoid hyphomycetes from New Caledonia and some other South Pacific islands. New Zealand Journal of Botany 37: 297–327 [Google Scholar]
- Braun U, Urtiaga R. (2008). New species and new records of cercosporoid hyphomycetes from Venezuela. Feddes Repertorium 119(5-6): 484–506 [Google Scholar]
- Carbone I, Kohn LM. (1999). A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia 91: 553–556 [Google Scholar]
- Cheewangkoon R, Crous PW, Hyde KD, Groenewald JZ, To-anan C. (2008). Species of Mycosphaerella and related anamorphs on Eucalyptus leaves from Thailand. Persoonia 21: 77–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheewangkoon R, Groenewald JZ, Summerell BA, Hyde KD, To-anun C, Crous PW. (2009). Myrtaceae, a cache of fungal biodiversity. Persoonia 23: 55–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury S. (1958). Notes on fungi from Assam, III. Lloydia 21: 152–156 [Google Scholar]
- Chupp C. (1954). A monograph of the fungus genus Cercospora. Published by the author, Ithaca, New York: [Google Scholar]
- Churchill ACL. (2010). Mycosphaerella fijiensis, the black leaf streak pathogen of banana: progress towards understanding pathogen biology and detection, disease development, and the challenges of control. Molecular Plant Pathology 12: 307–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW. (1998). Mycosphaerella spp. and their anamorphs associated with leaf spot diseases of Eucalyptus. Mycologia Memoir 21: 1–170 [Google Scholar]
- Crous PW. (2002). Taxonomy and pathology of Cylindrocladium (Calonectria) and allied genera. APS Press, Minnesota, St. Paul, USA: [Google Scholar]
- Crous PW. (2009). Taxonomy and phylogeny of the genus Mycosphaerella and its anamorphs. Fungal Diversity 38: 1–24 [Google Scholar]
- Crous PW, Alfenas AC. (1995). Mycosphaerella gracilis and other species of Mycosphaerella associated with leaf spots of Eucalyptus in Indonesia. Mycologia 87: 121–126 [Google Scholar]
- Crous PW, Aptroot A, Kang JC, Braun U, Wingfield MJ. (2000). The genus Mycosphaerella and its anamorphs. Studies in Mycology 45: 107–121 [Google Scholar]
- Crous PW, Braun U. (1996). Cercosporoid fungi from South Africa. Mycotaxon 57: 233–321 [Google Scholar]
- Crous PW, Braun U. (2003). Mycosphaerella and its anamorphs. 1. Names published in Cercospora and Passalora. CBS Biodiversity Series 1: 1–571 Centraalbureau voor Schimmelcultures, Utrecht, Netherlands: [Google Scholar]
- Crous PW, Braun U, Groenewald JZ. (2007a). Mycosphaerella is polyphyletic. Studies in Mycology 58: 1–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Braun U, Schubert K, Groenewald JZ. (2007b). Delimiting Cladosporium from morphologically similar genera. Studies in Mycology 58: 33–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Braun U, Wingfield MJ, Wood AR, Shin HD, et al. (2009a). Phylogeny and taxonomy of obscure genera of microfungi. Persoonia 22: 139–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Corlett M. (1998). Reassessment of Mycosphaerella spp. and their anamorphs occurring on Platanus. Canadian Journal of Botany 76: 1523–1532 [Google Scholar]
- Crous PW, Denman S, Taylor JE, Swart L, Palm ME. (2004a). Cultivation and diseases of Proteaceae: Leucadendron, Leucospermum and Protea. CBS Biodiversity Series 2: 1–228 Centraalbureau voor Schimmelcultures, Utrecht, Netherlands: [Google Scholar]
- Crous PW, Gams W, Stalpers JA, Robert V, Stegehuis G. (2004b). MycoBank: an online initiative to launch mycology into the 21st century. Studies in Mycology 50: 19–22 [Google Scholar]
- Crous PW, Groenewald JZ. (2005). Hosts, species and genotypes: opinions versus data. Australasian Plant Pathology 34: 463–470 [Google Scholar]
- Crous PW, Groenewald J Z, Mansilla JP, Hunter GC, Wingfield MJ. (2004c). Phylogenetic reassessment of Mycosphaerella spp. and their anamorphs occurring on Eucalyptus. Studies in Mycology 50: 195–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Groenewald JZ, Gams W. (2003). Eyespot of cereals revisited: ITS phylogeny reveals new species relationships. European Journal of Plant Pathology 109: 841–850 [Google Scholar]
- Crous PW, Groenewald JZ, Pongpanich K, Himaman W, Arzanlou M, Wingfield MJ. (2004d). Cryptic speciation and host specificity among Mycosphaerella spp. occurring on Australian Acacia species grown as exotics in the tropics. Studies in Mycology 50: 457–469 [Google Scholar]
- Crous PW, Groenewald JZ, Shin HD. (2010). Strelitziana albiziae. Fungal Planet No. 56. Persoonia 25: 132–133 [Google Scholar]
- Crous PW, Groenewald JZ, Shivas RG, Edwards J, Seifert KA, et al. (2011a). Fungal Planet Description Sheets: 69-91. Persoonia 26: 108–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Kang JC, Braun U. (2001). A phylogenetic redefinition of anamorph genera in Mycosphaerella based on ITS rDNA sequence and morphology. Mycologia 93: 1081–1101 [Google Scholar]
- Crous PW, Liebenberg MM, Braun U, Groenewald JZ. (2006). Re-evaluating the taxonomic status of Phaeoisariopsis griseola, the causal agent of angular leaf spot of bean. Studies in Mycology 55: 163–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Schoch CL, Hyde KD, Wood AR, Gueidan C, et al. (2009b). Phylogenetic lineages in the Capnodiales. Studies in Mycology 64: 17–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Summerell BA, Carnegie AJ, Mohammed C, Himaman W, Groenewald JZ. (2007c). Foliicolous Mycosphaerella spp. and their anamorphs on Corymbia and Eucalyptus. Fungal Diversity 26: 143–185 [Google Scholar]
- Crous PW, Summerell BA, Carnegie AJ, Wingfield MJ, Hunter GC, et al. (2009c). Unravelling Mycosphaerella: do you believe in genera? Persoonia 23: 99–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Tanaka K, Summerell BA, Groenewald JZ. (2011b). Additions to the Mycosphaerella complex. IMA Fungus 2: 49–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Verkley GJM, Groenewald JZ, Samson RA. (eds) (2009d). Fungal Biodiversity. CBS Laboratory Manual Series 1: 1–269 Centraalbureau voor Schimmelcultures, Utrecht, Netherlands: [Google Scholar]
- Crous PW, Wingfield MJ. (1997). New species of Mycosphaerella occurring on Eucalyptus leaves in Indonesia and Africa. Canadian Journal of Botany 75: 781–790 [Google Scholar]
- Crous PW, Wingfield MJ, Marasas WFO, Sutton BC. (1989). Pseudocercospora eucalyptorum sp. nov. on Eucalyptus leaves. Mycological Research 93: 394–398 [Google Scholar]
- Crous PW, Wingfield MJ, Park RF. (1991). Mycosphaerella nubilosa, a synonym of M. molleriana. Mycological Research 95: 628–632 [Google Scholar]
- Crous PW, Wingfield MJ, Swart WJ. (1990). Shoot and needle diseases of pines in South Africa. South African Forestry Journal 154: 60–66 [Google Scholar]
- David JC. (1997). A contribution to the systematics of Cladosporium. Revision of the fungi previously referred to Heterosporium. Mycological Papers 172: 1–157 [Google Scholar]
- Decock C, Delgado-Rodríguez G, Buchet S, Seng JM. (2003). A new species and three new combinations in Cyphellophora, with a note on the taxonomic affinities of the genus, and its relation to Kumbhamaya and Pseudomicrodochium. Antonie van Leeuwenhoek 84: 209–216 [DOI] [PubMed] [Google Scholar]
- Deighton FC. (1971). Studies on Cercospora and allied genera. III. Centrospora. Mycological Papers 124: 1–13 [Google Scholar]
- Deighton FC. (1972). Mycocentrospora, a new name for Centrospora Neerg. Taxon 21: 716–716 [Google Scholar]
- Deighton FC. (1976). Studies on Cercospora and allied genera. VI. Pseudocercospora Speg., Pantospora Cif. and Cercoseptoria Petr. Mycological Papers 140: 1–168 [Google Scholar]
- Deighton FC. (1979). Studies on Cercospora and allied genera. VII. New species and redispositions. Mycological Papers 144: 1–56 [Google Scholar]
- Deighton FC. (1983). Studies on Cercospora and allied genera. VIII. Further notes on Cercoseptoria and some new species and redispositions. Mycological Papers 151: 1–13 [Google Scholar]
- Deighton FC. (1987). New species of Pseudocercospora and Mycovellosiella, and new combinations into Pseudocercospora and Phaeoramularia. Transactions of the British Mycological Society 88: 365–391 [Google Scholar]
- Den Breeÿen A, Groenewald JZ, Verkley GJM, Crous PW. (2006). Morphological and molecular characterisation of Mycosphaerellaceae associated with the invasive weed, Chromolaena odorata. Fungal Diversity 23: 89–110 [Google Scholar]
- Drummond AJ, Ashton B, Buxton S, Cheung M, Cooper A, Heled J, Kearse M, Moir R, Stones-Havas S, Sturrock S, Thierer T, Wilson A. (2011). Geneious v5.5. Available from http://www.geneious.com
- Ellis MB. (1971). Dematiaceous Hyphomycetes. Commonwealth Mycological Institute, Kew, Surrey, UK: [Google Scholar]
- Evans HC. (1984). The genus Mycosphaerella and its anamorphs Cercoseptoria, Dothistroma and Lecanosticta on pines. Mycological Papers 153: 1–102 [Google Scholar]
- Frank J, Crous PW, Groenewald JZ, Oertel B, Hyde KD, et al. (2010). Microcyclospora and Microcyclosporella: novel genera accommodating epiphytic fungi causing sooty blotch on apple. Persoonia 24: 93–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groenewald M, Groenewald JZ, Braun U, Crous PW. (2006). Host range of Cercospora apii and C. beticola, and description of C. apiicola, a novel species from celery. Mycologia 98: 275–285 [DOI] [PubMed] [Google Scholar]
- Groenewald M, Groenewald JZ, Linde CC, Crous PW. (2007). Development of polymorphic microsatellite and single nucleotide polymorphism markers for Cercospora beticola (Mycosphaerellaceae). Molecular Ecology Notes 7: 890–892 [Google Scholar]
- Guo Y-L, Hsieh W-H. (1995). The genus Pseudocercospora in China. International Academic Publishers, Beijing, China: [Google Scholar]
- Guo YL, Liu XJ. (1989). Studies on the genus Pseudocercospora in China I. Mycosystema 2: 225–240 [Google Scholar]
- Hawksworth DL, Crous PW, Redhead SA, Reynolds DR, Samson RA, et al. (2011). The Amsterdam Declaration on Fungal Nomenclature. IMA Fungus 2: 105–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillis DM, Bull JJ. (1993). An empirical test of bootstrapping as a method for assessing confidence in phylogenetic analysis. Systematic Biology 42: 182–192 [Google Scholar]
- Hoog GS de, Hijwegen T, Batenburg van der Vegte WH. (1991). A new species of Dissoconium. Mycological Research 95: 679–682 [Google Scholar]
- Hsieh WH, Goh TK. (1990). Cercospora and similar fungi from Taiwan. Maw Chang Book. Co., Taipei: [Google Scholar]
- Hunter GC, Crous PW, Carnegie AJ, Burgess TI, Wingfield MJ. (2011). Mycosphaerella and Teratosphaeria diseases of Eucalyptus; easily confused and with serious consequences. Fungal Diversity 50: 145–166 [Google Scholar]
- Hunter GC, Crous PW, Carnegie AJ, Wingfield MJ. (2009). Teratosphaeria nubilosa, a serious leaf disease pathogen of Eucalyptus spp. in native and introduced areas. Molecular Plant Pathology 10: 1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter GC, Crous PW, Wingfield BD, Pongpanich K, Wingfield MJ. (2006a). Pseudocercospora flavomarginata sp. nov., from Eucalyptus leaves in Thailand. Fungal Diversity 22: 71–90 [Google Scholar]
- Hunter GC, Wingfield BD, Crous PW, Wingfield MJ. (2006b). A multi-gene phylogeny for species of Mycosphaerella occurring on Eucalyptus leaves. Studies in Mycology 55: 147–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser W, Crous PW. (1998). Mycosphaerella lupini sp. nov., a serious leaf spot disease of perennial lupin in Southcentral Idaho, USA. Mycologia 90: 726–731 [Google Scholar]
- Katoh K, Kuma K, Toh H, Miyata T. (2005). MAFFT version 5: improvement in accuracy of multiple sequence alignment. Nucleic Acids Research 33: 511–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh K, Toh H. (2007). PartTree: an algorithm to build an approximate tree from a large number of aligned sequences. Bioinformatics 23: 372–372 [DOI] [PubMed] [Google Scholar]
- Koike SK, Baameur A, Groenewald JZ, Crous PW. (2011). Cercosporoid leaf pathogens from whorled milkweed and spineless safflower in California. IMA Fungus 2: 7–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HB, Yu SH, Kim C-J. (2001). First report of leaf spot of Paederia scandens caused by Pseudocercospora paederiae in Korea. New Disease Reports 4: 7 [Google Scholar]
- Maddison DR, Maddison WP. (2000). MacClade 4. Analysis of phylogeny and character evolution. Sinauer Associates, Inc. [DOI] [PubMed] [Google Scholar]
- Minnis AM, Kennedy AH, Grenier DB, Rehner SA, Bischoff JF. (2011). Asperisporium and Pantospora (Mycosphaerellaceae): epitypifications and phylogenetic placement. Persoonia 27: 1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moncalvo J-M, Rehner SA, Vilgalys R. (1993). Systematics of Lyophyllum section Difformia based on evidence from culture studies and ribosomal DNA sequences. Mycologia 85: 788–794 [Google Scholar]
- Nylander JAA. (2004). MrModeltest v2.2. Program distributed by the author Evolutionary Biology Centre, Uppsala University; [Google Scholar]
- O’Donnell K, Kistler HC, Cigelnik E, Ploetz RC. (1998). Multiple evolutionary origins of the fungus causing Panama disease of banana: concordant evidence from nuclear and mitochondrial gene genealogies. Proceedings of the National Academy of Sciences of the United States of America 95: 2044–2049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pons N, Sutton BC. (1988). Cercospora and similar fungi on Yams (Dioscorea species). Mycological Papers 160: 1–78 [Google Scholar]
- Pretorius MC, Crous PW, Groenewald JZ, Braun U. (2003). Phylogeny of some cercosporoid fungi from Citrus. Sydowia 55: 286–305 [Google Scholar]
- Quaedvlieg W, Kema GHJ, Groenewald JZ, Verkley GJM, Seifbarghi S, Razavi M, Mirzadi Gohari A, Mehrabi R, Crous PW. (2011). Zymoseptoria gen. nov.: a new genus to accommodate Septoria-like species occurring on graminicolous hosts. Persoonia 26: 57–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rambaut A. (2002). Sequence Alignment Editor. Version 2.0. Department of Zoology, University of Oxford, Oxford: [Google Scholar]
- Rayner RW. (1970). A mycological colour chart. CMI and British Mycological Society, Kew, Surrey, England: [Google Scholar]
- Rehner SA, Samuels GJ. (1994). Taxonomy and phylogeny of Gliocladium analysed from nuclear large subunit ribosomal DNA sequences. Mycological Research 98: 625–634 [Google Scholar]
- Ronquist F, Huelsenbeck JP. (2003). MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19: 1572–1574 [DOI] [PubMed] [Google Scholar]
- Ruibal C, Millanes AM, Hawksworth DL. (2011). Molecular phylogenetic studies on the lichenicolous Xanthoriicola physciae reveal Antarctic rock-inhabiting fungi and Piedraia species among closest relatives in the Teratosphaeriaceae. IMA Fungus 2: 97–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawada K. (1928). Descriptive catalogue of the Formosan fungi IV. Report of the Department of Agriculture, Government Research Institute of Formosa 35: 1–162 [Google Scholar]
- Schoch CL, Seifert KA, Huhndorf S, Robert V, Spouge JL, Levesque CA, Chen W, et al. (2012). Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for Fungi. Proceedings of the National Academy of Sciences of the United States of America 109: 6241–6246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert K, Groenewald JZ, Braun U, Dijksterhuis J, Starink M, Hill CF, Zalar P, de Hoog GS, Crous PW. (2007). Biodiversity in the Cladosporium herbarum complex (Davidiellaceae, Capnodiales), with standardisation of methods for Cladosporium taxonomy and diagnostics. Studies in Mycology 58: 105–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert KA, Hughes SJ, Boulay H, Louis-Seize G. (2007). Taxonomy, nomenclature and phylogeny of three cladosporium-like hyphomycetes, Sorocybe resinae, Seifertia azaleae and the Hormoconis anamorph of Amorphotheca resinae. Studies in Mycology 58: 235–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert KA, Morgan-Jones G, Gams W, Kendrick B. (2011). The Genera of Hyphomycetes. CBS Biodiversity Series 9: 1–997 Centraalbureau voor Schimmelcultures, Utrecht, Netherlands: [Google Scholar]
- Selbmann L, Hoog GS de, Zucconi L, Isola D, Ruisi S, et al. (2008). Drought meets acid: three new genera in a dothidealean clade of extremotolerant fungi. Studies in Mycology 61: 1–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin HD, Braun U. (1996). Notes on Korean Cercosporae and allied genera (II). Mycotaxon 58: 157–166 [Google Scholar]
- Shin HD, Braun U. (2000). Notes on Korean Cercosporae and allied genera (III). Mycotaxon 74: 105–118 [Google Scholar]
- Shin HD, Kim JD. (2001). Cercospora and allied genera from Korea. Plant Pathogens of Korea 7: 1–302 National Institute of Agricultural Science and Technology; Suwon, Korea: [Google Scholar]
- Singh PN, Singh SK, Tripathi SC. (1996). New species of Pseudocercospora causing leaf spots of forest plants in Nepal. Mycological Research 100: 1129–1132 [Google Scholar]
- Spegazzini C. (1910). Mycetes Argentinenses (Series V). Anales del Museo Nacional de Historia Natural, Buenos Aires 20: 329–467 [Google Scholar]
- Stewart EL, Liu Z, Crous PW, Szabo L. (1999). Phylogenetic relationships among some cercosporoid anamorphs of Mycosphaerella based on rDNA sequence analysis. Mycological Research 103: 1491–1499 [Google Scholar]
- Suto Y. (1999). Mycosphaerella chaenomelis sp. nov.: the teleomorph of Cercosporella sp., the causal fungus of frosty mildew in Chaenomeles sinensis, and its role as the primary infection source. Mycoscience 40: 509–516 [Google Scholar]
- Sutton BC. (1980). The Coelomycetes. Fungi imperfecti with pycnidia, acervuli and stromata. Commonwealth Mycological Institute, Kew, Surrey, UK: [Google Scholar]
- Swofford DL. (2003). PAUP*. Phylogenetic Analysis Using Parsimony (*and Other Methods). Version 4. Sinauer Associates, Sunderland, Massachusetts: [Google Scholar]
- Tamura K, Dudley J, Nei M, Kumar S. (2007). MEGA 4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Molecular Biology and Evolution 24: 1596–1599 [DOI] [PubMed] [Google Scholar]
- Taylor JE, Crous PW. (2000). Fungi occurring on Proteaceae. New anamorphs for Teratosphaeria, Mycosphaerella and Lembosia, and other fungi associated with leaf spots and cankers of Proteaceous hosts. Mycological Research 104: 618–636 [Google Scholar]
- Togashi K, Katsuki S. (1952). New or noteworthy Cercosporae from Japan. Botanical Magazine Tokyo 65: 18–26 [Google Scholar]
- Verkley GJM, Crous PW, Groenewald JZ, Braun U, Aptroot A. (2004). Mycosphaerella punctiformis revisited: morphology, phylogeny, and epitypification of the type species of the genus Mycosphaerella (Dothideales, Ascomycota). Mycological Research 108: 1271–1282 [DOI] [PubMed] [Google Scholar]
- Verkley GJM, Priest MJ. (2000). Septoria and similar coelomycetous anamorphs of Mycosphaerella. Studies in Mycology 45: 123–128 [Google Scholar]
- Viégas AP. (1945). Alguns fungos do Brasil - Cercosporae. Boletim de Sociedade Brasileira de Agronomia 8: 1–160 [Google Scholar]
- Vilgalys R, Hester M. (1990). Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. Journal of Bacteriology 172: 4238–4246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vries GA de. (1962). Cyphellophora laciniata nov. gen., nov. sp. and Dactylium fusarioides Fragoso et Ciferri. Mycopathologia et Mycologia Applicata 16: 47–54 [Google Scholar]
- Vries GA de, Elders MC, Luykx MH. (1986). Description of Cyphellophora pluriseptata sp. nov. Antonie van Leeuwenhoek 52: 141–143 [DOI] [PubMed] [Google Scholar]
- White TJ, Bruns T, Lee S, Taylor J. (1990). Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: PCR Protocols: a guide to methods and applications (Innis MA, Gelfand DH, Sninsky JJ, White TJ, eds). Academic Press, San Diego, California: 315–322 [Google Scholar]
- Wingfield MJ, De Beer ZW, Slippers B, Wingfield BD, Groenewald JZ, Lombard L, Crous PW. (2012). One fungus, one name promotes progressive plant pathology. Molecular Plant Pathology DOI: 10.1111/J.1364-3703.2011.00768.X [DOI] [PMC free article] [PubMed]
- Yen JM. (1966). Etude sur les champignons parasites du Sud-Est asiatique IV. Troisieme note sur quelques nouvelles especes de Cercospora de Singapour. Revue Mycologique 31: 109–149 [Google Scholar]
- Yen JM, Lim G. (1980). Cercospora and allied genera of Singapore and the Malay Peninsula. Garden’s Bulletin Singapore 33: 151–263 [Google Scholar]
- Zhang Y, Schoch CL, Fournier J, Crous PW, Gruyter J de, et al. (2009). Multi-locus phylogeny of Pleosporales: a taxonomic, ecological and evolutionary re-evaluation. Studies in Mycology 64: 85–102 [DOI] [PMC free article] [PubMed] [Google Scholar]