SUMMARY
Although plasma membranes benefit cells by regulating the flux of materials to and from the environment, these membranes cost energy to maintain. Because smaller cells provide relatively more membrane area for transport, ectotherms that develop in warm environments should consist of small cells despite the energetic cost. Effects of constant temperatures on cell size qualitatively match this prediction, but effects of thermal fluctuations on cell size are unknown. Thermal fluctuations could favour either small or large cells; small cells facilitate transport during peaks in metabolic demand whereas large cells minimize the resources needed for homeoviscous adaptation. To explore this problem, we examined effects of thermal fluctuations during development on the size of epidermal cells in the wings of Drosophila melanogaster. Flies derived from a temperate population were raised at two mean temperatures (18 and 25°C), with either no variation or a daily variation of ±4°C. Flies developed faster at a mean temperature of 25°C. Thermal fluctuations sped development, but only at 18°C. An increase in the mean and variance of temperature caused flies to develop smaller cells and wings. Thermal fluctuations reduced the size of males at 18°C and the size of females at 25°C. The thorax, the wings and the cells decreased with an increase in the mean and in the variance of temperature, but the response of cells was the strongest. Based on this pattern, we hypothesize that development of the greater area of membranes under thermal fluctuations provides a metabolic advantage that outweighs the greater energetic cost of remodelling membranes.
KEY WORDS: body size, cell size, Drosophila melanogaster, homeoviscous adaptation, oxygen permeability, phenotypic plasticity, plasma membrane, thermal adaptation
INTRODUCTION
When one considers the diversity of life, cell size rarely comes to mind as a variable trait that impacts the fitness of multicellular organisms. Indeed, foundations of some ecological theories were built on the assumption that all organisms have cells of the same size (West et al., 1997; reviewed in Kozlowski and Konarzewski, 2004). Yet, cell size responds to artificial selection (Trotta et al., 2007) and varies among species (Kozlowski et al., 2010), populations (Goodman and Heah, 2010) and even life stages (Davison, 1955). We have good reasons to suspect that natural selection contributes to this variation in cell size. A given change in the volume of a cell causes a smaller change in the area of its surface. This disproportional scaling determines the costs and benefits of cell size (Szarski, 1983; Woods, 1999; Kozlowski et al., 2003; Atkinson et al., 2006). On the one hand, a substantial portion of a cell's energy goes to maintain ionic gradients across cell membranes (Rolfe and Brown, 1997), which enable cellular communication and trans-membrane transport. In fact, cell size correlates with resting metabolic rate in some animals (Davison, 1955; Starostová et al., 2009). All else being equal, the energetic cost of supporting cell membranes should favour large cells, which possess little surface area relative to cytoplasmic volume [frugal strategy in Szarski's (Szarski, 1983) cell size model]. On the other hand, because cell membranes regulate the exchange of chemicals between cells and their environment, the relatively large surface area of small cells should enhance metabolic performance. Therefore, when organisms must quickly process large quantities of resources, natural selection should favour genotypes that develop small cells despite the elevated energetic cost [wasteful strategy in Szarski's (Szarski, 1983) cell size model].
Researchers have used this framework to predict the responses of cells to constant temperatures during development. Smaller cells are predicted to develop in warmer environments, where metabolic capacity and demands for resources are high, and oxygen supplies are low (Woods, 1999; Atkinson et al., 2006). Indeed, smaller cells have been observed at higher developmental temperatures in a wide range of ectotherms, including protists (Butler and Rogerson, 1996; Atkinson et al., 2003), rotifers (Stelzer, 2002), planarians (Romero and Baguna, 1991), nematodes (Van Voorhies, 1996), fish (Van Voorhies, 1996), lizards (Goodman and Heah, 2010) and flies (Partridge et al., 1994; Blanckenhorn and Llaurens, 2005). Moreover, Drosophila melanogaster evolved smaller cells at a higher temperature under controlled conditions (Partridge et al., 1994). These data support the idea that optimal cell size depends on the thermal environment, but tell us little about the responses of organisms to natural environments that fluctuate in temperature.
We use the theoretical framework described above to evaluate the plasticity of cell size in fluctuating thermal environments. The balance of opposing selective pressures should determine the optimal cell size in environments where temperatures fluctuate. As the temperature changes, cells rebuild their membranes to maintain the fluidity that enables normal functions, a process referred to as homeoviscous adaptation (Hazel, 1995). When the temperature drops, the distribution of fatty acids in phospholipids shifts from saturated to unsaturated. This process requires dietary fatty acids as substrates, molecular oxygen for processes governed by desaturases, and ATP to fuel enzymatic activity (Stanley-Samuelson et al., 1988; Shanklin and Cahoon, 1998; Martin-Creuzburg et al., 2012). Because other biochemical reactions compete for these resources, homeoviscous adaptation imposes some cost that likely depends on the area of cell membranes. Thus, an organism composed of large cells, which sum to less cell membrane area, would save resources at fluctuating temperatures. Alternatively, cells that spend some time at high temperatures require sufficient resources to meet extreme metabolic demands (Pörtner, 2002; Angilletta, 2009). Small cells would provide the relatively large surface areas and short diffusion distances needed to transport resources (Szarski, 1983; Woods, 1999; Kozlowski et al., 2003). Depending on the relative importance of these processes, selection could favour genotypes that either increase or decrease cell sizes when thermal environments fluctuate.
To explore the impact of thermal fluctuations during development on cell size, we raised isofemale lines of D. melanogaster (Meigen) under constant and fluctuating temperatures and compared the sizes of epidermal cells in their wings. The genotypes used in this experiment were derived from a temperate population that experiences strong diel and seasonal fluctuations in temperature; such fluctuations induce changes in membrane composition of D. melanogaster (Overgaard et al., 2006; Cooper et al., 2012). The sizes of cells in wings have been used frequently as a proxy for the sizes of other cells throughout the body (reviewed by Arendt, 2007). In these cases, one assumes that the mean size of wing cells correlates with the mean sizes of other types of cells. In species of Drosophila, this assumption has gained empirical support (Stevenson et al., 1995). More importantly, the sizes of cells in different organs of D. melanogaster were observed to respond similarly to developmental conditions. Cells in both the wings and two other organs were smaller at higher temperatures (Azevedo et al., 2002), and a concerted reduction of cell sizes in wings and the abdomen occurred during hypoxia (Heinrich et al., 2011). Thus, sizes of epidermal cells and their responses to temperature should provide a proxy of these traits in other tissues of flies.
MATERIALS AND METHODS
Experimental design
In June of 2008, we used banana-bait traps to collect females of D. melanogaster at three sites in Terre Haute, IN, USA. Thirteen isofemale lines were formed by placing inseminated females into freshly yeasted vials containing moist instant medium (Formula 424, Carolina Biological Supply, Burlington, NC, USA). These vials were maintained at 21°C with a 12 h:12 h light:dark cycle. In the following generation, emerging males were examined to verify that the species was D. melanogaster. To form replicates within isofemale lines, four pairs of virgin males and virgin females from each line were transferred to new vials containing the same medium. After mating, females were transferred to new vials and were allowed to lay eggs for 24 h. After this period, each female was transferred to another new vial for a period of 24 h, and this process was repeated until we had four vials of eggs from each female.
At each transfer, the vial of eggs from each isofemale line was placed into one of four thermal treatments: (1) a constant environment of 25°C, (2) a fluctuating environment with a mean of 25°C (21 and 29°C during scotophase and photophase, respectively), (3) a constant environment of 18°C and (4) a fluctuating environment with a mean of 18°C (14 and 22°C during scotophase and photophase, respectively). Lines were allocated to these thermal treatments using a Latin square design (Bradley, 1958). The treatments were maintained by programmable incubators (model 818, Precision Scientific, Chicago, IL, USA). To ensure an adequate level of humidity, we placed containers of water in each incubator. The accuracy of each thermal treatment was verified to the nearest 0.5°C using data loggers (iButton Thermochron, Dallas Semiconductors, Dallas, TX, USA); mean temperatures were nearly identical between the fluctuating treatments (18.2 and 25.1°C) and constant treatments (18.1 and 25.2°C). The light cycle of each thermal treatment was 12 h:12 h light:dark.
Measuring sizes of thorax, wings and cells
We measured development time and morphological traits of flies in each thermal treatment. Developmental time was estimated as the number of days between oviposition and the first sign of emergence. The length of the thorax and the dimensions of the left wing were used as proxies for body size and wing size, respectively. The mean size of epidermal cells in the wing was a convenient estimate of cell size.
For measurements of morphology, we sampled up to four males and four females from each vial at 7 days after eclosion. After anaesthesia with CO2 and chloroform, flies were placed on their sides under a dissecting microscope (model M28, Leica Microsystems, Buffalo Grove, IL, USA). An ocular micrometer was used to measure thorax length to the nearest 0.025 mm. Following Partridge et al. (Partridge et al., 1994), we measured the distance between the base of the most anterior humeral bristle to the posterior tip of the scutellum. The left wing of each fly was removed with surgical micro-scissors. Each wing was then flattened with a drop of xylene on a microscopic slide and mounted with Permount medium (Fisher Scientific, Fair Lawn, NJ, USA). The dorsal surface of each wing was digitized under a microscope (model DC5-163, National Optical, San Antonio, TX, USA). The images were used to calculate the dimensions of the wing and the density of trichomes. As each epidermal cell supports a single trichome, the density of trichomes reflects the mean size of epidermal cells.
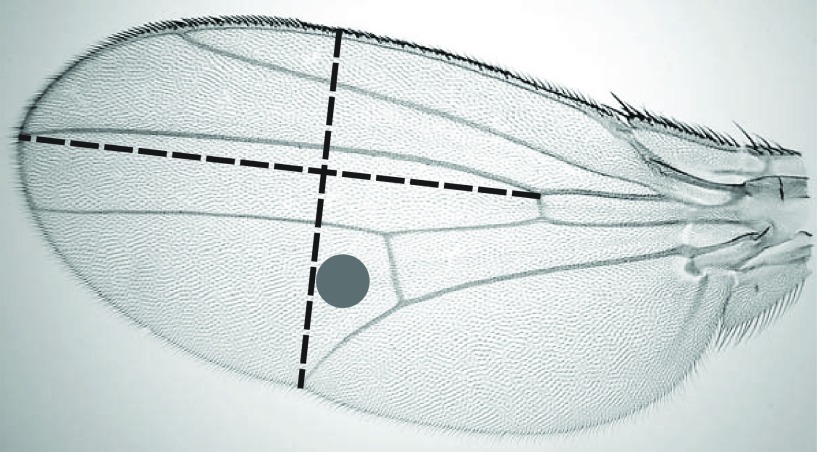
Following Gilchrist et al. (Gilchrist et al., 2001), we used imaging software (ImageJ, National Institutes of Health, Bethesda, MD, USA) to measure two dimensions of each wing (Fig. 1): (1) the width of the wing, from the intersection of vein V and the trailing edge to the leading edge along a trajectory perpendicular to vein III, and (2) the length of the distal segment of vein III. A principal component analysis (PCA) was used to generate an index of wing size from these two dimensions. To calculate trichome density, we counted trichomes in a circle (0.01 mm2) between the distal segments of wing veins IV and V (Fig. 1). Following Dobzhansky (Dobzhansky, 1929), the reciprocal of trichome density was considered an estimate of the mean area of epidermal cells in the wing.
Fig. 1.
Two dimensions of wings were measured and they were described with a principal component analysis. The density of trichomes in the circle (0.01 mm2) was used to estimate the mean area of cells in each wing blade.
Statistical modelling
We used general linear models to estimate the effects of mean temperature (18°C versus 25°C), thermal variation (present versus absent) and sex (male versus female) on developmental time, thorax length, wing size and cell size. Line and family (nested within line) were included as random factors when they improved the fit of the model. For the analysis of wing size (score for PC1), thorax length was included as a continuous factor. For the analysis of cell size, wing size was included as a continuous factor. Initially, we modelled all main effects and interactions. Then, we removed terms from the model, starting with the highest order term, until we arrived at the model with the lowest Akaike's information criterion (AIC) value (Burnham and Anderson, 2002). Following the procedure described by Zuur and colleagues (Zuur et al., 2009), we fitted models using the nlme library (Pinheiro et al., 2011) of the R Statistical Package (R Development Core Team, 2011).
RESULTS
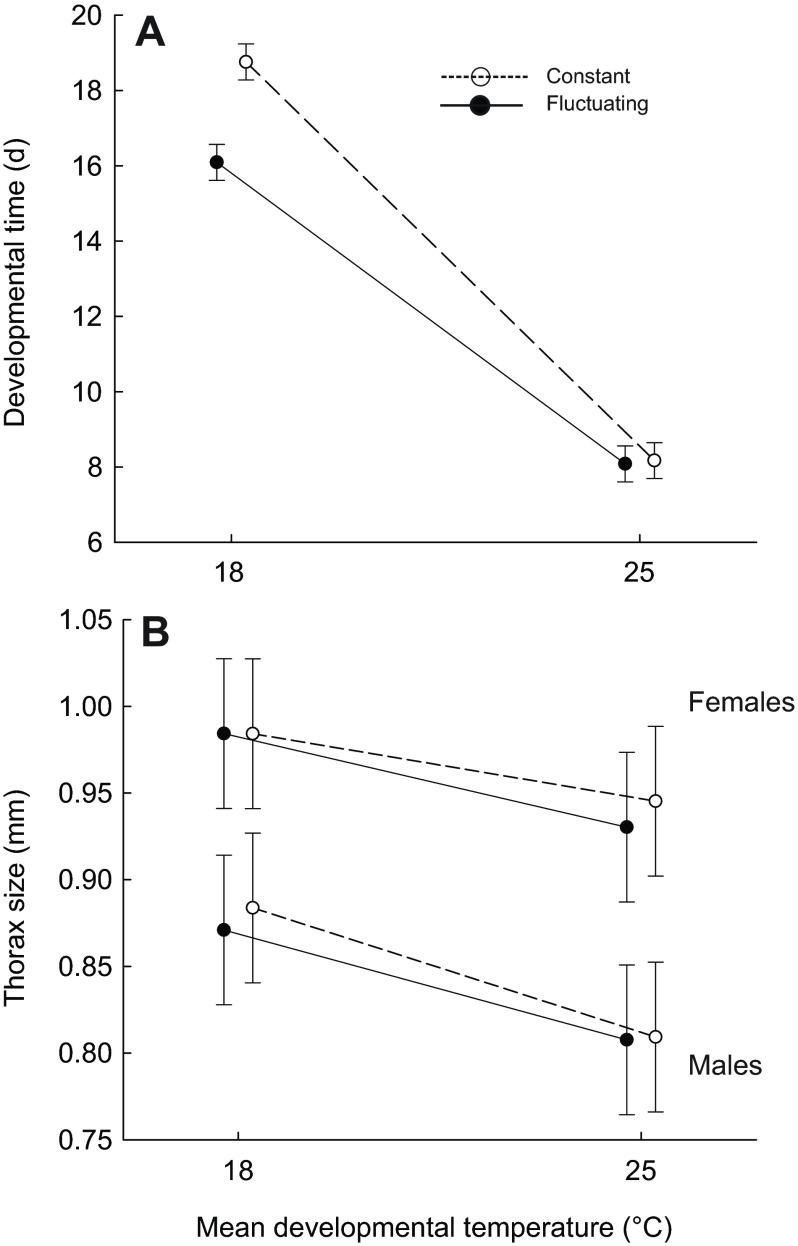
As in previous studies (e.g. Partridge et al., 1994), both developmental time and body size were affected by temperature. An increase in the mean or variance of temperature accelerated development (Table 1). The effect of thermal fluctuations was more pronounced at low mean temperature (Fig. 2A). The mean and variance of temperature interacted to determine thorax sizes of males and females (Table 2, Fig. 2B). A higher mean temperature during development yielded flies with smaller thoraxes. In constant environments, the thermal dependence of thorax size was more pronounced for males than it was for females. However, in thermally fluctuating environments, thorax sizes of males and females decreased by a similar magnitude with increasing mean temperature. Thermal fluctuations caused a decrease in thorax size, but this response was much smaller in magnitude than was the response to an increase in mean temperature; moreover, this response was observed only among males that developed at a mean of 18°C and among females that developed at a mean of 25°C (Fig. 2B).
Table 1.
Inferential statistics for the most likely general linear model of developmental time in Drosophila melanogaster, as determined by Akaike's information criterion
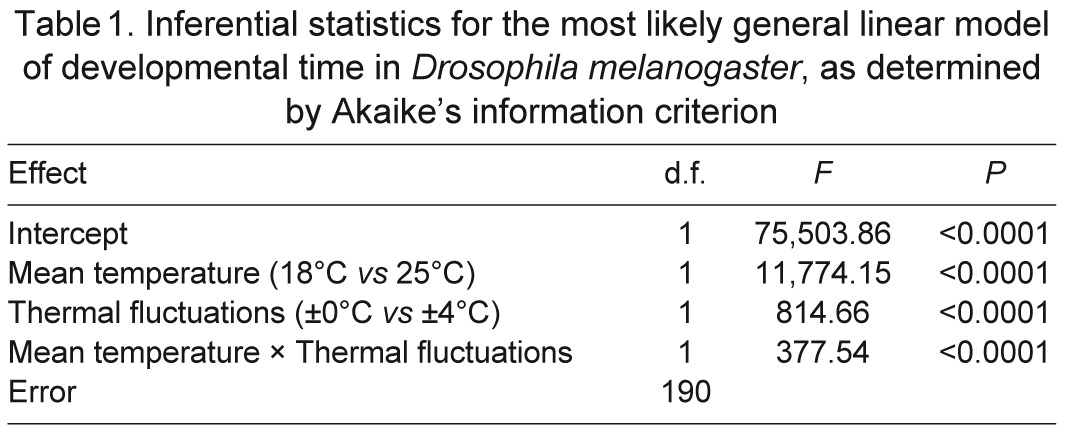
Fig. 2.
Generally, flies in warmer environments developed faster. When the mean temperature was low, fluctuations in temperature also sped development (A). Although females were generally larger than males, both sexes developed smaller bodies in warmer environments (B). Data are means ± s.d. estimated from the most likely statistical model.
Table 2.
Inferential statistics for the most likely general linear mixed model of thorax length in Drosophila melanogaster, as determined by Akaike's information criterion
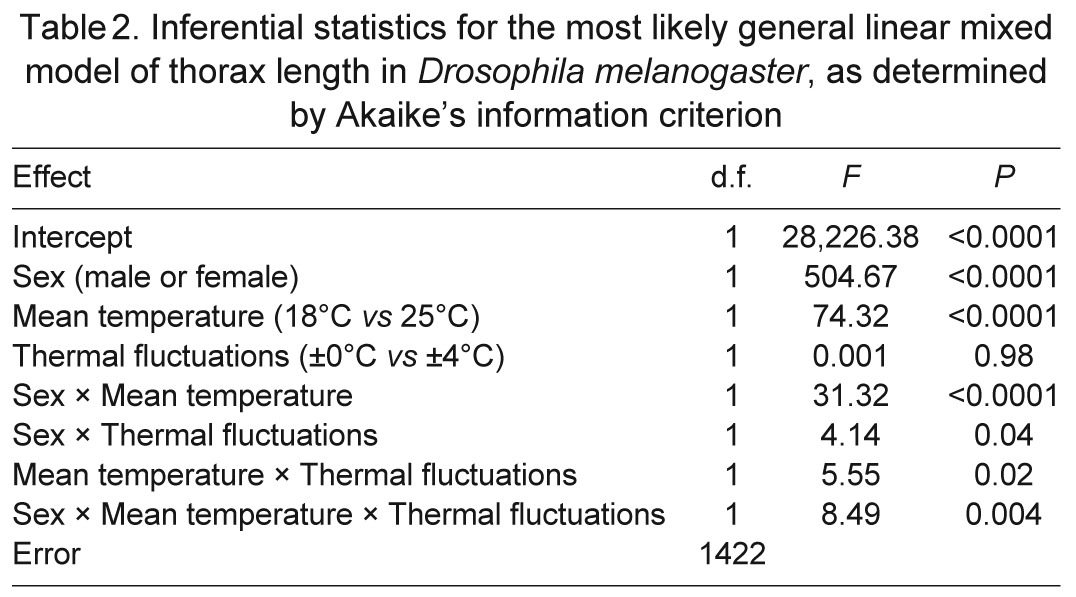
Wing size, independent of thorax size, also depended on sex and developmental temperatures (Table 3, Fig. 3). The first principal component of wing dimensions described 98% of the variation. The two dimensions contributed equally to this principal component, loading at a value of 0.99. Thus, greater scores for this principal component reflected larger wings. The score for this principal component generally increased with increasing thorax size, but females developed larger wings than males if both sexes were compared at the same thorax size. Disproportionately larger wings were also produced when mean temperature or thermal variance was low. As with thorax size, wing size decreased more with increasing mean temperature than it did with increasing thermal variance. The scaling of wing size with thorax size was steeper for flies that developed at 18°C than for flies that developed at 25°C. This interaction was particularly pronounced in males (Fig. 3).
Table 3.
Inferential statistics for the most likely general linear mixed model of wing size in Drosophila melanogaster developed at two average temperatures, as determined by Akaike's information criterion
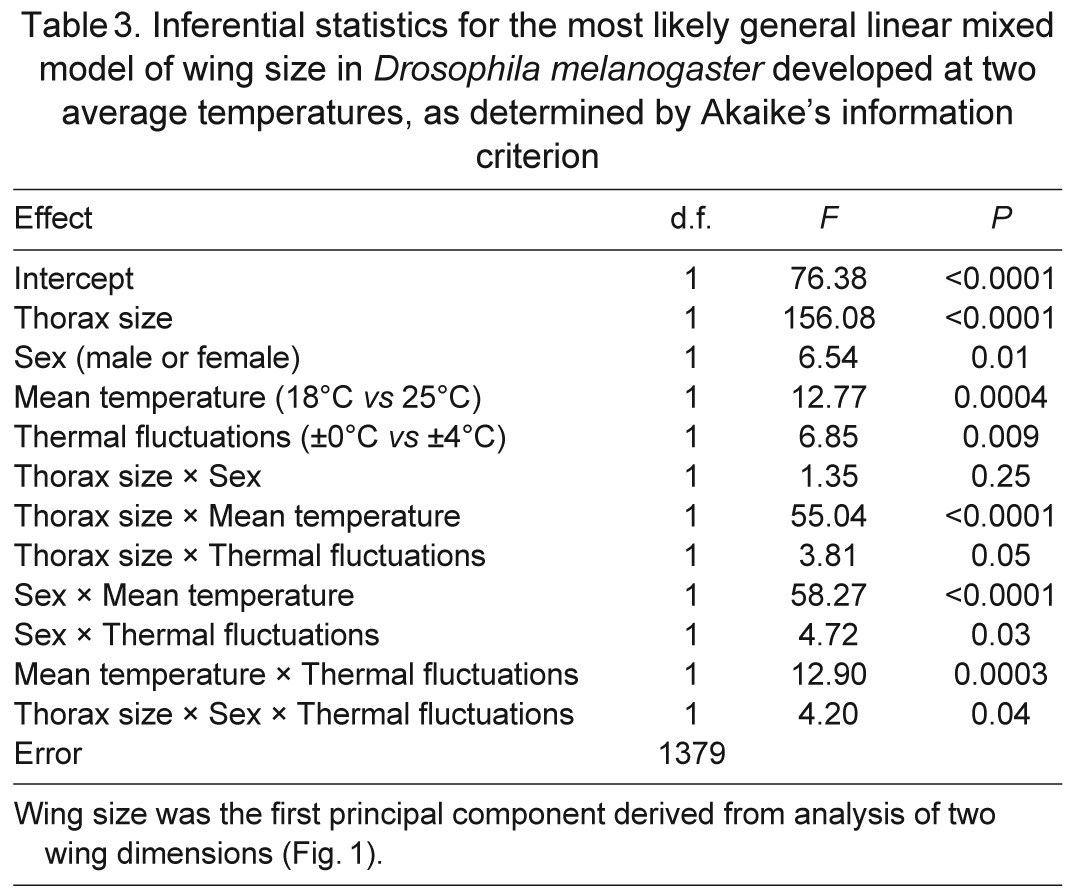
Fig. 3.
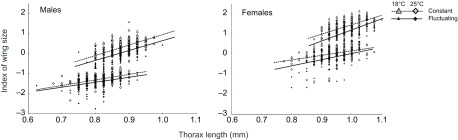
Flies at a low, constant temperature developed the largest wings for a given body size, whereas flies in a warm, thermally fluctuating environment developed the smallest wings. Wing size was indexed as the score for the first principal component of two wing dimensions (Fig. 1). Lines display the relationships estimated from the most likely statistical model.
The sizes of cells within wings were also affected by thermal conditions during development (Table 4, Fig. 4). Cell size scaled positively with wing size in both males and females, but the scaling was steeper for males. Females had larger cells than did males for a given wing size. Although large wings consisted of large cells, flies that developed at high or fluctuating temperatures had disproportionally smaller cells for their wing size. The effect of thermal fluctuations on cell size was particularly visible for females, but was still weaker in magnitude than the effect of mean temperature.
Table 4.
Inferential statistics for the most likely general linear mixed model of cell size in Drosophila melanogaster, as determined by Akaike's information criterion
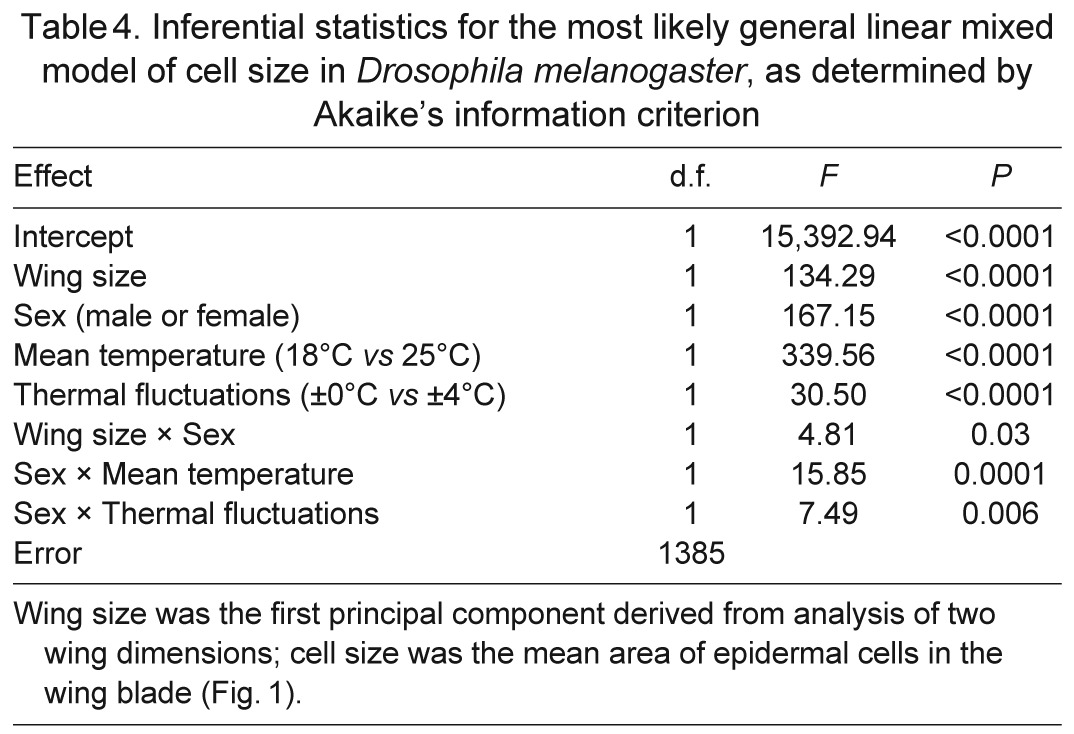
Fig. 4.
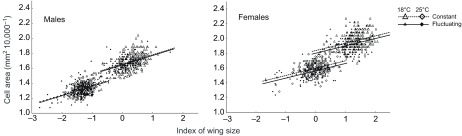
Flies at a low, constant temperature developed the largest cells for a given wing size, whereas flies in a warm, thermally fluctuating environment developed the smallest cells. Wing size was indexed as the score for the first principal component of two wing dimensions; cell area was calculated from the density of trichomes on each wing blade (Fig. 1). Lines display the relationships estimated from the most likely statistical model.
DISCUSSION
Either an increase in the mean or variance of temperature caused flies to develop smaller epidermal cells in their wings, suggesting that flies that experience higher temperatures produce organs from smaller cells. In a previous experiment, flies consistently produced smaller cells in wings and in two other organs when developing at a higher constant temperature (Azevedo et al., 2002). A correlated thermal plasticity of cell sizes in different cell types occurred also in dung flies (Blanckenhorn and Llaurens, 2005) and planarians (Romero and Baguna, 1991). In our experiment, small flies produced small wings composed of small cells. Because higher temperatures caused smaller body sizes, the thermal plasticity of cell size was partly coupled with the thermal plasticity of body size. Thermal conditions also directly affected cell size. Either a higher mean or variance of temperature caused flies to develop small wings relative to thorax size and small cells relative to wing size. Consequently, flies exposed to a higher maximal temperature developed the smallest epidermal cells relative to their body sizes. Thus, our results suggest that the thorax, the wings and the cells respond similarly to temperature, but the response of cells is the strongest of these responses. Although thermal effects on cell size have been observed previously in various ectotherms (Partridge et al., 1994; Butler and Rogerson, 1996; Blanckenhorn and Llaurens, 2005; Goodman and Heah, 2010), these effects were not dissected to separate the independent response to temperature from the response related to body and organ sizes.
The observation that flies produced smaller cells in thermally fluctuating environments accords with the hypothesis that the area of cell membranes, and thus cell size, should depend on metabolic demands. Given the nonlinear relationship between temperature and performance, ectotherms that experience fluctuating temperatures can perform equal to, better than or worse than conspecifics from constant environments with the same mean temperature (Ruel and Ayres, 1999; Angilletta, 2009; Bozinovic et al., 2011). Consistent with this idea, our flies developed faster in the thermally fluctuating environment when the mean temperature was 18°C but at similar rates when the mean temperature was 25°C. In a previous study (Bozinovic et al., 2011), thermal fluctuations sped population growth of D. melanogaster at a mean of 17°C but slowed population growth at a mean of 24°C. The greater performance of flies at fluctuating temperatures requires resource delivery to meet metabolic demand. Small cells might have enabled fast acquisition of resources during brief exposures to peak temperatures. Such conditions open ‘windows of opportunity’ for cells to grow and divide, which could ultimately enhance the fitness of the organism.
Cell membranes serve as points of exchange between the cytoplasm and its surroundings. Thus, the larger area of exchange and the shorter distance of diffusion associated with smaller cells could speed transport and enhance performance (Szarski, 1983; Woods, 1999; Kozlowski et al., 2003). Still other factors could favour larger surface areas of cell membranes at higher temperatures. For example, oxygen permeates more efficiently through the hydrocarbon phase of a membrane than through the aqueous phase of cytoplasm; thus, cell membranes form pathways, rather than barriers, along which oxygen can penetrate tissue (Subczynski et al., 1989). Because smaller cells provide a greater density of cell membranes, tissues should become perfused with more oxygen, speeding its delivery to mitochondria. Consistent with this idea, flies that developed in a hypoxic environment produced small cells in two types of tissues (Heinrich et al., 2011). Furthermore, experimental evolution of D. melanogaster in hypoxic conditions led to small cells that consumed oxygen faster than did large cells (Zhou et al., 2007). Importantly, the superior diffusion of oxygen through the hydrocarbon phase of membranes is more pronounced at high temperatures, suggesting that small cells especially benefit a warm organism (Subczynski et al., 1989).
Our findings should help to refine the theory of optimal cell size and metabolic scaling and direct researchers toward new hypotheses. Because flies that experience temporal changes in body temperature develop smaller cells, two scenarios remain possible: either membrane remodelling during thermal change requires little energy, or the metabolic advantages of small cells outweigh the energetic savings of large cells. We favour the latter hypothesis for two reasons. First, flies rapidly remodel their membranes during thermal change (Hazel, 1995; Overgaard et al., 2006). Although no one knows the specific cost of membrane remodelling during thermal change, phospholipid metabolism can require substantial amounts of ATP (Purdon et al., 2002). Second, females in our experiment shrunk their cells at fluctuating temperatures more than did males, both in absolute terms and in relative terms (3.2% versus 3.1% at 18°C, and 3.5% versus 1.9% at 25°C). This pattern makes sense when one considers that the balance between the cost of remodelling and the potential to acquire resources depends on the state of a cell. Females produced larger cells with a relatively small area of membrane compared with males, which should make them more prone to limitation imposed by a trans-membrane transport during expositions to higher temperatures. Therefore, females should gain a greater metabolic advantage from shrinking their cells than do males. At the same time, females would not gain a significant energetic advantage from enlarging their cells, given their already small area of membranes. At least one other study has generated patterns that accord with this hypothesis; bryozoans developed smaller cells at a higher temperature in a tissue that acquired oxygen through passive diffusion, but did not do so in a tissue that received active ventilation (Atkinson et al., 2006).
To fully understand the impact of thermal fluctuations on cell size, we need to answer several questions. First, do cells of all tissues grow or shrink in concert, as we have assumed here? Although some evidence supports our assumption (Stevenson et al., 1995; Azevedo et al., 2002; Kozlowski et al., 2010), future research should address plastic responses and evolutionary changes of cell size in diverse tissues. Second, how much does the area of cell membranes impact the capacity of cells to acquire resources, especially oxygen? Third, how much energy does a cell spend to remodel a given area of membrane? The answers to these questions will enable biologists to develop quantitative models of optimal cell size. Hypotheses related to the fitness consequences of membrane maintenance and efficient resource delivery can be directly tested to explicitly determine the selective advantage of different cellular strategies among environments. For example, genotypes that differ in the degree of membrane plasticity and in cell size can be competed to determine whether thermal fluctuations favour large cells with a greater capacity for remodelling membranes. Genotypes that differ in rates of membrane transport and in cell size could be competed to determine whether resource pulses favour small cells with greater rates of transport. These types of experiments will be needed to understand how the plasticity of cell size evolves to enhance the ability to acquire resources while reducing the costs of membrane maintenance in changing thermal environments.
ACKNOWLEDGEMENTS
We thank Jeff Tharp and Angela Hatfield for helping with the experiment and Diana Hews for access to her laboratory facilities. This manuscript was improved by comments from Jan Kozlowski and members of the Montooth laboratory at Indiana University. We thank two anonymous reviewers for their constructive suggestions.
FOOTNOTES
COMPETING INTERESTS
No competing interests declared.
FUNDING
M.C. was supported by fellowships from the Fulbright and Kosciuszko Foundations. B.S.C. is supported by the Indiana University Genetics, Cellular and Molecular Sciences Training Grant [T32-GM007757] funded by the National Institutes of Health. The research was supported by the Polish Ministry of Science and High Education [N N304 373238] and the Jagiellonian University [DS/WBINS/INOS/757/2011]. Deposited in PMC for release after 12 months.
REFERENCES
- Angilletta M. J. (2009). Thermal Adaptation: a Theoretical and Empirical Synthesis. Oxford: Oxford University Press; [Google Scholar]
- Arendt J. (2007). Ecological correlates of body size in relation to cell size and cell number: patterns in flies, fish, fruits and foliage. Biol. Rev. Camb. Philos. Soc. 82, 241-256 [DOI] [PubMed] [Google Scholar]
- Atkinson D., Ciotti B. J., Montagnes D. J. S. (2003). Protists decrease in size linearly with temperature: ca. 2.5% °C−1. Proc. Biol. Sci. 270, 2605-2611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson D., Morley S. A., Hughes R. N. (2006). From cells to colonies: at what levels of body organization does the ‘temperature–size rule’ apply? Evol. Dev. 8, 202-214 [DOI] [PubMed] [Google Scholar]
- Azevedo R. B. R., French V., Partridge L. (2002). Temperature modulates epidermal cell size in Drosophila melanogaster. J. Insect Physiol. 48, 231-237 [DOI] [PubMed] [Google Scholar]
- Blanckenhorn W. U., Llaurens V. (2005). Effects of temperature on cell size and number in the yellow dung fly Scathophaga stercoraria. J. Therm. Biol. 30, 213-219 [Google Scholar]
- Bozinovic F., Bastías D. A., Boher F. E., Clavijo-Baquet S., Estay S. A., Angilletta M. J., Jr (2011). The mean and variance of environmental temperature interact to determine physiological tolerance and fitness. Physiol. Biochem. Zool. 84, 543-552 [DOI] [PubMed] [Google Scholar]
- Bradley J. V. (1958). Complete counterbalancing of immediate sequential effects in a Latin square design. J. Am. Stat. Assoc. 53, 525-528 [Google Scholar]
- Burnham K. P., Anderson D. R. (2002). Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach. New York, NY: Springer; [Google Scholar]
- Butler H., Rogerson A. (1996). Growth potential, production efficiency and annual production of marine benthic naked amoebae (Gymnamoebae) inhabiting sediments of the Clyde Sea area, Scotland. Aquat. Microb. Ecol. 10, 123-129 [Google Scholar]
- Cooper B. S., Hammad L. A., Fisher N. P., Karty J. A., Montooth K. L. (2012). In a variable thermal environment selection favors greater plasticity of cell membranes in Drosophila melanogaster. Evolution 66, 1976-1984 [DOI] [PubMed] [Google Scholar]
- Davison J. (1955). Body weight, cell surface and metabolic rate in anuran Amphibia. Biol. Bull. 109, 407-419 [Google Scholar]
- Dobzhansky T. (1929). The influence of the quantity and quality of chromosomal material on the size of the cells in Drosophila melanogaster. Wilhelm Roux Arch. Entwickl. Mech. Org. 115, 363-379 [DOI] [PubMed] [Google Scholar]
- Gilchrist G. W., Huey R. B., Serra L. (2001). Rapid evolution of wing size clines in Drosophila subobscura. Genetica 112-113, 273-286 [PubMed] [Google Scholar]
- Goodman R. M., Heah T. P. (2010). Temperature-induced plasticity at cellular and organismal levels in the lizard Anolis carolinensis. Integr. Zool. 5, 208-217 [DOI] [PubMed] [Google Scholar]
- Hazel J. R. (1995). Thermal adaptation in biological membranes: is homeoviscous adaptation the explanation? Annu. Rev. Physiol. 57, 19-42 [DOI] [PubMed] [Google Scholar]
- Heinrich E. C., Farzin M., Klok C. J., Harrison J. F. (2011). The effect of developmental stage on the sensitivity of cell and body size to hypoxia in Drosophila melanogaster. J. Exp. Biol. 214, 1419-1427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozlowski J., Konarzewski M. (2004). Is West, Brown and Enquist's model of allometric scaling mathematically correct and biologically relevant? Funct. Ecol. 18, 283-289 [Google Scholar]
- Kozłowski J., Konarzewski M., Gawelczyk A. T. (2003). Cell size as a link between noncoding DNA and metabolic rate scaling. Proc. Natl. Acad. Sci. USA 100, 14080-14085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozlowski J., Czarnoleski M., François-Krassowska A., Maciak S., Pis T. (2010). Cell size is positively correlated between different tissues in passerine birds and amphibians, but not necessarily in mammals. Biol. Lett. 6, 792-796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Creuzburg D., Wacker A., Ziese C., Kainz M. J. (2012). Dietary lipid quality affects temperature-mediated reaction norms of a freshwater key herbivore. Oecologia 168, 901-912 [DOI] [PubMed] [Google Scholar]
- Overgaard J., Sørensen J. G., Petersen S. O., Loeschcke P., Holmstrup M. (2006). Reorganization of membrane lipids during fast and slow cold hardening in Drosophila melanogaster. Physiol. Entomol. 31, 328-335 [Google Scholar]
- Partridge L., Barrie B., Fowler K., French V. (1994). Evolution and development of body size and cell size in Drosophila melanogaster in response to temperature. Evolution 48, 1269-1276 [DOI] [PubMed] [Google Scholar]
- Pinheiro J., Bates D., DebRoy S., Sarkar D., the R Development Core Team (2011). nlme: Linear and Nonlinear Mixed Effects Models. R package version 3.1-98 [Google Scholar]
- Pörtner H. O. (2002). Climate variations and the physiological basis of temperature dependent biogeography: systemic to molecular hierarchy of thermal tolerance in animals. Comp. Biochem. Physiol. 132A, 739-761 [DOI] [PubMed] [Google Scholar]
- Purdon A. D., Rosenberger T. A., Shetty H. U., Rapoport S. I. (2002). Energy consumption by phospholipid metabolism in mammalian brain. Neurochem. Res. 27, 1641-1647 [DOI] [PubMed] [Google Scholar]
- R Development Core Team (2011). R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; [Google Scholar]
- Rolfe D. F. S., Brown G. C. (1997). Cellular energy utilization and molecular origin of standard metabolic rate in mammals. Physiol. Rev. 77, 731-758 [DOI] [PubMed] [Google Scholar]
- Romero R., Baguna J. (1991). Quantitative cellular analysis of growth and reproduction in fresh-water planarians (Turbellaria, Tricladida). I. A cellular description of the intact organism. Invertebr. Reprod. Dev. 19, 157-165 [Google Scholar]
- Ruel J. J., Ayres M. P. (1999). Jensen's inequality predicts effects of environmental variation. Trends Ecol. Evol. 14, 361-366 [DOI] [PubMed] [Google Scholar]
- Shanklin J., Cahoon E. B. (1998). Desaturation and related modifications of fatty acids. Annu. Rev. Plant Physiol. Plant Mol. Biol. 49, 611-641 [DOI] [PubMed] [Google Scholar]
- Stanley-Samuelson D. W., Jurenka R. A., Cripps C., Blomquist G. J., de Renobales M. (1988). Fatty acids in insects: composition, metabolism, and biological significance. Arch. Insect Biochem. Physiol. 9, l-33 [Google Scholar]
- Starostová Z., Kubicka L., Konarzewski M., Kozłowski J., Kratochvíl L. (2009). Cell size but not genome size affects scaling of metabolic rate in eyelid geckos. Am. Nat. 174, E100-E105 [DOI] [PubMed] [Google Scholar]
- Stelzer C. P. (2002). Phenotypic plasticity of body size at different temperatures in a planktonic rotifer: mechanisms and adaptive significance. Funct. Ecol. 16, 835-841 [Google Scholar]
- Stevenson R. D., Hill M. F., Bryant P. J. (1995). Organ and cell allometry in Hawaiian Drosophila: how to make a big fly. Proc. Biol. Sci. 259, 105-110 [DOI] [PubMed] [Google Scholar]
- Subczynski W. K., Hyde J. S., Kusumi A. (1989). Oxygen permeability of phosphatidylcholine–cholesterol membranes. Proc. Natl. Acad. Sci. USA 86, 4474-4478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szarski H. (1983). Cell size and the concept of wasteful and frugal evolutionary strategies. J. Theor. Biol. 105, 201-209 [DOI] [PubMed] [Google Scholar]
- Trotta V., Calboli F. C. F., Ziosi M., Cavicchi S. (2007). Fitness variation in response to artificial selection for reduced cell area, cell number and wing area in natural populations of Drosophila melanogaster. BMC Evol. Biol. 7 Suppl. 2, S10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Voorhies W. A. (1996). Bergmann size clines: a simple explanation for their occurrence in ectotherms. Evolution 50, 1259-1264 [DOI] [PubMed] [Google Scholar]
- West G. B., Brown J. H., Enquist B. J. (1997). A general model for the origin of allometric scaling laws in biology. Science 276, 122-126 [DOI] [PubMed] [Google Scholar]
- Woods H. A. (1999). Egg-mass size and cell size: effects of temperature on oxygen distribution. Am. Zool. 39, 244-252 [Google Scholar]
- Zhou D., Xue J., Chen J., Morcillo P., Lambert J. D., White K. P., Haddad G. G. (2007). Experimental selection for Drosophila survival in extremely low O2 environment. PLoS ONE 2, e490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuur A. F., Leno E. N., Walker N., Saveliev A. A., Smith G. M. (2009). Mixed Effects Models and Extensions in Ecology With R. New York, NY: Springer; [Google Scholar]