To the Editor: Campylobacter spp. infection is the leading cause of bacterial enteritis worldwide. The epidemiology of Campylobacter infection in developing countries differs substantially from that in industrialized countries. In many studies from the United States and other industrialized countries, Campylobacter spp. are among the most common bacterial causes of diarrhea (1), with an incidence of ≈10% in persons with diarrhea. Reports from developing countries also suggest that C. jejuni and C. coli have been isolated mostly from patients with diarrheal illness (2). We investigated for the prevalence of Campylobacter infection in patients hospitalized with diarrhea at the Infectious Disease Hospital in Kolkata, India, and their resistance patterns to different antimicrobial drugs.
During January 2008–December 2010, we screened 3,186 fecal samples on brain–heart infusion agar with 5% defibrinated sheep blood and antimicrobial drugs (bacitracin, cycloheximide, colistin sulfate, cephazoline sodium, novobiocin) and incubated under microaerophillic environment (5% O2, 10% CO2, and 85% N2) at 37°C for 48 h. Each isolate was tested by Gram staining, cytochrome oxidase, and hippurate hydrolysis for presumptive identification and species-specific PCR (3) to identify 5 species from Campylobacter genus. The overall isolation rate of Campylobacter spp. was ≈7% (222/3,186). Sole infection with Campylobacter spp. accounted for only 40% of cases; others were mixed infection. C. jejuni was the predominant species (78%), with C. coli, C. fetus, C. lari, and C. upsaliensis isolated less frequently. Campylobacter infection prevailed throughout the year, with no seasonality. The C. jejuni isolation rate was significantly higher (10.0%; p<0.001) for children <5 years of age who had diarrhea than for persons in other age groups (3.7%). Although we used the culture method, which is the standard for screening fecal samples, some molecular methods, such as PCR and real-time PCR, are now used for screening Campylobacter spp. from fecal samples on the Indian subcontinent (4,5). The results from molecular methods are showing more infection with Campylobacter spp. and high mixed infection cases and suggest the usefulness of molecular methods in combination with cultures.
Macrolides and fluoroquinolones generally are the first- and second-line choices, respectively, of antimicrobial drugs for treating Campylobacter enteritis. Since the late 1980s, resistance to these drugs has complicated treatment. In India, resistance of Campylobacter spp. to several antimicrobial drugs has been reported since the early 1990s (6). Fluoroquinolone resistance was not reported in 1994 but reached 79% during 2001–2006 (2). Likewise, ciprofloxacin resistance in Campylobacter spp. increased markedly in Dhaka, Bangladesh, during 2005–2008 (7) and in Karachi, Pakistan, during 1992–2002 (8). We tested 142 C. jejuni isolates for antimicrobial susceptibility by disk diffusion method on Muller-Hinton agar with 5% sheep blood and incubated them at 37°C in microaerophillic environment for 48 h before obtaining results. All tested strains were resistant to trimethoprim–sulfamethoxazole, and most (97%) were resistant to quinolone (nalidixic acid) and fluoroquinolones (norfloxacin, ciprofloxacin, and ofloxacin) (Figure). Approximately 26.1% and 17.6% of the isolates were resistant to ampicillin and tetracycline, respectively. Susceptibility to erythromycin, azithromycin, gentamicin, furazolidone, and chloramphenicol was very high (>97%) in most isolates. Multidrug resistance was frequent among many of the isolates: ampicillin, nalidixic acid, norfloxacin, ciprofloxacin, ofloxacin, and trimethoprim–sulfamethoxazole (19%); tetracycline, nalidixic acid, norfloxacin, ciprofloxacin, ofloxacin, and trimethoprim–sulfamethoxazole (10.2%); and tetracycline, ampicillin, nalidixic acid, norfloxacin, ciprofloxacin, ofloxacin, and trimethoprim–sulfamethoxazole (6.8%). These results indicate that macrolides may be useful for treating campylobacteriosis in this region.
Figure.
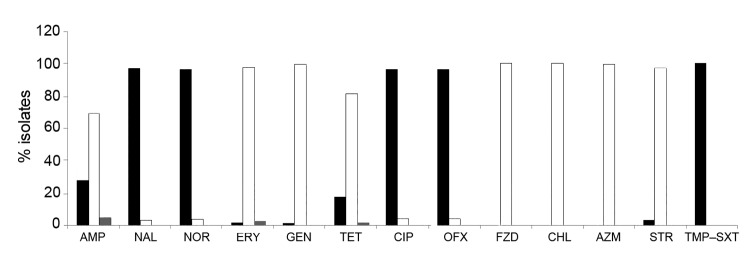
Antimicrobial drug susceptibility profile of 142 Campylobacter jejuni isolates, Kolkata, India, 2008–2010. Black bars, resistant; gray bars, intermediate resistance; white bars, susceptible. AMP, ampicillin; NAL, nalidixic acid; NOR, norfloxacin; ERY, erythromycin; GEN, gentamicin; TET, tetracycline; CIP, ciprofloxacin; OFX, ofloxacin; FZD, furazolidone; CHL, chloramphenicol; AZM, azithromycin; STR, streptomycin; TMP–SXT, trimethoprim–sulfamethoxazole.
The resistance patterns are influenced by various factors, possibly including pressure exerted by use of antimicrobial drugs. Various reports have stated that introduction of fluoroquinolones for use in veterinary practice has been associated with a dramatic rise in Campylobacter strains showing resistance to these drugs (9). Increasing antimicrobial drug resistance limits the number of therapeutic options, which makes empirical treatment more difficult. Therefore, constant monitoring of Campylobacter susceptibility to antimicrobial agents is essential. We could not detect any allele of plasmid-mediated quinolone resistance genes (qnr) among C. jejuni isolates and the different class of mobile genetic elements that generally carry the antimicrobial resistance gene cassettes. However, we found that most of the C. jejuni isolates had a mutation in the quinolone-resistance determining region of gyrA (Thr-86 to Ile), which led the isolates to become resistant for quinolone and fluoroquinolones.
Recent microbiome analysis of the gut of a malnourished child residing in an urban slum in Kolkata showed 35 times more Campylobacter bacteria than in healthy child in the same setting (10). This finding suggests that intestinal inflammation may directly influence malabsorption of nutrients. Hence, it is essential to examine the effect of Campylobacter infection in the developing world in the context of many recent developments in the human gut microbiome.
Acknowledgments
This work was supported in part by the Indian Council of Medical Research, Government of India, and by the Japan Initiative for Global Research Network on Infectious Diseases Ministry of Education, Culture, Sports, Science and Technology of Japan.
Footnotes
Suggested citation for this article: Mukherjee P, Ramamurthy T, Bhattacharya MK, Rajendran K, Mukhopadhyay AK. Campylobacter jejuni in hospitalized patients with diarrhea, Kolkata, India [letter]. Emerg Infect Dis [Internet]. 2013 Jul [date cited]. http://dx.doi.org/10.3201/eid1907.121278
References
- 1.Centers for Disease Control and Prevention. Vital signs: incidence and trends of infection with pathogens transmitted commonly through food—Foodborne Diseases Active Surveillance Network, 10 U.S. sites, 1996–2010. MMWR Morb Mortal Wkly Rep. 2011;60:749–55 . [PubMed] [Google Scholar]
- 2.Jain D, Sinha S, Prasad KN, Pandey CM. Campylobacter species and drug resistance in a north Indian rural community. Trans R Soc Trop Med Hyg. 2005;99:207–14 . 10.1016/j.trstmh.2004.09.006 [DOI] [PubMed] [Google Scholar]
- 3.Asakura M, Samosornsuk W, Taguchic M, Kobayashic K, Misawad N, Kusumoto M, et al. Comparative analysis of cytolethal distending toxin (cdt) genes among Campylobacter jejuni, C. coli and C. fetus strains. Microb Pathog. 2007;42:174–83 . 10.1016/j.micpath.2007.01.005 [DOI] [PubMed] [Google Scholar]
- 4.Rajendran P, Babji S, George AT, Rajan DP, Kang G, Ajjampur SS. Detection and species identification of Campylobacter in stool samples of children and animals from Vellore, south India. Indian J Med Microbiol. 2012;30:85–8 . 10.4103/0255-0857.93049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sinha A, Sengupta S, Guin S, Dutta S, Ghosh S, Mukherjee P, et al. Culture-independent real-time PCR reveals extensive polymicrobial infections in hospitalized diarrhoea cases in Kolkata, India. Clin Microbiol Infect. 2013;19:173–80 . 10.1111/j.1469-0691.2011.03746.x [DOI] [PubMed] [Google Scholar]
- 6.Prasad KN, Mathur SK, Dhole TN, Ayyagari A. Antimicrobial susceptibility and plasmid analysis of Campylobacter jejuni isolated from diarrhoeal patients and healthy chickens in northern India. J Diarrhoeal Dis Res. 1994;12:270–3 . [PubMed] [Google Scholar]
- 7.Ahmed D, Hoque A, Elahi MS, Endtz HP, Hossain MA. Bacterial aetiology of diarrhoeal diseases and antimicrobial resistance in Dhaka, Bangladesh, 2005–2008. Epidemiol Infect. 2012;140:1678–84 . 10.1017/S0950268811002135 [DOI] [PubMed] [Google Scholar]
- 8.Ibrahim NG, Zafar A, Hasan R. Evaluation of frequency of isolation and trends in antibiotic resistance among Campylobacter isolates over 11 year period. J Pak Med Assoc. 2004;54:291–4 . [PubMed] [Google Scholar]
- 9.Phillips I, Casewell M, Cox T, De Groot B, Friis C, Jones R, et al. Does the use of antibiotics in food animals pose a risk to human health? A critical review of published data. J Antimicrob Chemother. 2004;53:28–52 . 10.1093/jac/dkg483 [DOI] [PubMed] [Google Scholar]
- 10.Gupta SS, Mohammed MH, Ghosh TS, Kanungo S, Nair GB, Mande SS. Metagenome of the gut of a malnourished child. Gut Pathog. 2011;3:7. [DOI] [PMC free article] [PubMed]


