Abstract
We report a case of transfusion-associated bacteremia caused by Psychrobacter arenosus. This psychrotolerant bacterium was previously isolated in 2004 from coastal sea ice and sediments in the Sea of Japan, but not from humans. P. arenosus should be considered a psychrotolerant bacterial species that can cause transfusion-transmitted bacterial infections.
Keywords: Psychrobacter arenosus, bacteria, bacteremia, transfusion-transmitted infection, blood transfusion, France
Bacteria are the leading cause of transfusion-transmitted infections (1). Contamination occurs more frequently in platelet concentrates than in erythrocyte units, especially because of different storage conditions (20°C–24°C for platelet concentrates vs. 1°C–6°C for erythrocyte units). However, several bacterial species are able to grow at 4°C (1–3). We report a case of transfusion-transmitted bacterial infection caused by Psychrobacter arenosus, an environmental psychrotolerant and halotolerant bacterium.
The Patient
In October 2009, a 58-year-old man was admitted to Grenoble University Hospital (Grenoble, France) for a blood transfusion because of severe anemia. Idiopathic medullary aplasia had been diagnosed in 1997, and he had had grade 3 myelofibrosis since 2006. He had been receiving palliative care since November 2007, and received transfusions of erythrocyte units every 3 weeks. On October 27, 2009, he received 3 erythrocyte units (at 8:30 am, 10:30 am, and 12:15 pm). While receiving the third unit, he became febrile (temperature of 38°C that rapidly increased to 40°C) and had chills and headache. The transfusion was stopped and the patient transferred to the Department of Internal Medicine. At examination, there was no hypotension, jaundice, or red urine.
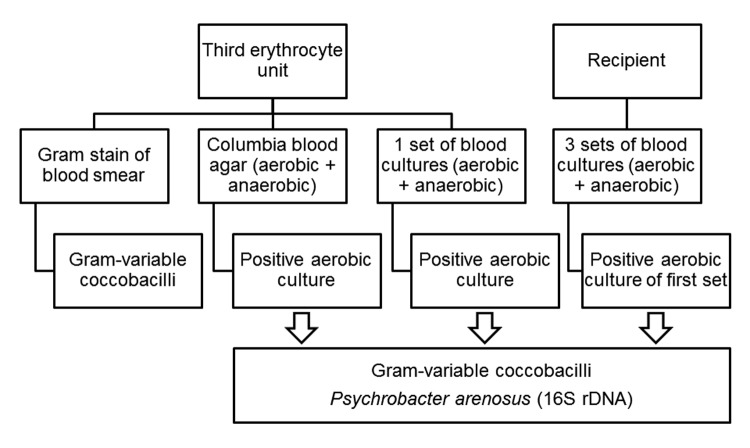
Standard laboratory testing showed no ABO incompatibility, hemoglobinemia, hemoglobinuria, and coagulation disorders. According to recommendations of the Agence Nationale de Sécurité du Médicament (Saint-Denis, France), 3 sets of aerobic and anaerobic blood cultures (Bactec; Becton Dickinson, Pont de Clay, France) for the recipient (1 immediately and 2 others 4 hours later) and the remaining part of the third erythrocyte unit were sent to the bacteriology laboratory for culture. Gram staining of a blood smear prepared from the third erythrocyte unit showed a large number (≈106 CFU/mL) of gram-variable coccobacilli.
Samples were placed on Columbia blood agar (bioMérieux, Marcy L’Etoile, France) and incubated at 37°C in anaerobic or 5% CO2–enriched atmospheres. Sample inoculated into blood culture bottles were incubated at 37°C under aerobic and anaerobic conditions (Figure). The aerobic blood culture bottle of the first sample obtained from the recipient and aerobic cultures of the third erythrocyte unit enabled isolation of the same gram-variable coccobacilli after incubation for 48 hours (Figure). Colonies obtained on Columbia blood agar were monomorphic, small, and gray, and had positive results for oxidase and catalase tests. Phenotypic traits of the bacterial strains isolated from the blood of the patient and the erythrocyte unit were similar, but identification using the Vitek2 Gram negative card and API 20E, API 20NE, and ID 32 GN Kits (bioMérieux) was not successful.
Figure.
Flow diagram showing samples collected from the blood donor unit (third erythrocyte unit) and a 58-year-old man (transfusion recipient) and results for isolation and identification of Psychrobacter arenosus, France.
Molecular identification was performed by 16S rRNA gene amplification and sequencing with fD1 and rP2 primers (4), and DNA sequence analysis was performed by using BLAST (www.ncbi.nlm.nih.gov) and leBIBI (http://umr5558-sud-str1.univ-lyon1.fr/lebibi/lebibi.cgi) software. DNA sequences obtained were identical (Genbank accession no. JX416703) and showed 99.7% homology with the P. arenosus 16SrDNA sequence previously reported by Romanenko et al. (5) (Genbank accession no. AJ609273). Consistent with this identification, subcultures of the isolated strain obtained on tryptic soy agar plates incubated at 4°C, 25°C, and 37°C showed opaque, circular, convex, cream-colored colonies; no subcultures were obtained on Drigalski medium. Phenotypic characteristics of this strain and the strain isolated by Romanenko at al. (5) are summarized in the Table. To determine the source of the P. arenosus contamination, environmental samples were collected at sites in which erythrocyte units were prepared and stored, but culture results were negative.
Antimicrobial drug susceptibility was determined by using an agar disk diffusion method, and results were interpreted by using MIC breakpoints recommended for other oxidative gram-negative bacilli by the Comité de l’Antibiogramme de la Société Française de Microbiologie (Paris, France) (6). The isolate was resistant to lincomycin and susceptible to amoxicillin, amoxicillin/clavulanate, ticarcillin/clavulanate, piperacillin, piperacillin/tazobactam, cefalotin, cefotaxime, ceftazidime, cefpirome, cefepime, imipenem, gentamicin, tobramycin, netilmicin, amikacin, erythromycin, pristinamycin, polymyxin B, trimethoprim/sulfamethoxazole, nalidixic acid, ofloxacin, ciprofloxacin, and fosfomycin.
The patient initially received intravenous ticarcillin/clavulanate (5 g/200 mg, 3×/d) and vancomycin (1g, 2×/d). When the antibiogram was available, treatment was switched to oral administration of amoxicillin/clavulanate (1 g/125 mg, 3×/d) and ofloxacin (200 mg, twice a day) for 12 days, which resulted in rapid recovery.
Conclusions
Psychrobacter species are nonmotile, nonpigmented, aerobic, gram-negative coccobacilli, although Gram staining results are often variable (7). These bacteria are psychrotolerant and halotolerant environmental microorganisms (7). They have been isolated from many sources, including sea water, ornithogenic soil, air contaminants, fish, poultry, milk, cheese, and irradiated food (7). P. arenosus was isolated in 2004 from coastal sea ice and sediments in the Sea of Japan (5).
Psychrobacter species are considered rare opportunistic human pathogens (8) and have been isolated from specimens obtained from human blood, cerebrospinal fluid, brain tissue, urine, ears, eyes, vulvae, wounds, and other cutaneous sources (8,9). P. phenylpyruvicus (formerly Moraxella phenylpyruvica) has been associated with bacteremia, endocarditis, septic arthritis, foot abscess, and surgical wound infection (9–11). P. immobilis has caused fatal infections in a patient who had AIDS (12), nosocomial ocular infection (13), and meningitidis in a 2-day-old infant (14). However, recently the taxonomy of Psychrobacter species has been revised, and most human isolates other than P. phenylpyruvicus belong to the newly characterized species P. faecalis and P. pulmonis (8). Also, a novel species, P. sanguinis, has been isolated from human blood samples (15). Thus, the spectrum of human infections associated with the different species of the genus Psychrobacter could change rapidly.
We report a case of human moderate septic transfusion reaction caused by P. arenosus. The clinical and laboratory findings did not support an acute hemolytic transfusion reaction. Gram staining of a direct smear prepared from the erythrocyte unit showed a high bacterial inoculum, strongly suggesting multiplication of bacteria in this unit before transfusion. P. arenosus was isolated from a contaminated erythrocyte unit and blood of the patient obtained after the transfusion was stopped. The patient recovered rapidly after receiving appropriate antimicrobial drug therapy. These findings confirm that the transfusion reaction was attributable to P. arenosus contamination of the erythrocyte unit. However, the isolated strain was not identified until 16S rRNA gene amplification and sequencing were performed.
We found differences in biochemical characteristics between this P. arenosus strain and the strain isolated by Romanenko et al. (5) (Table). P. arenosus is able to grow at 4°C–37°C (5) and thus could multiply in the erythrocyte unit stored at 4°C for 1 month before transfusion. As in most cases of transfusion-transmitted bacterial infections, source of contamination of the erythrocyte unit was not identified. P. arenosus could not be detected in environmental samples collected at sites in which the erythrocyte unit was prepared and stored. As for other gram-negative bacteria, transient bacteremia in an asymptomatic blood donor could be the source of the erythrocyte unit contamination (1,3), but exogenous contamination at the time of blood collection or preparation of units occurs more frequently (3).
Table. Characteristics of Psychrobacter arenosus isolated in this study (France) and a strain isolated in Russia* .
| Characteristic | Isolate from this study | Isolate from Russia† |
|---|---|---|
| Growth at 5°C | + | + |
| Growth at 22°C | + | + |
| Growth at 37°C |
+ |
+ |
| Nitrate reduction | – | – |
| Urease | – | – |
| Arginine dihydrolase | – | – |
| β-galactosidase | – | – |
| Esculin hydrolysis | – | – |
| Gelatinase | – | – |
| Indole production |
– |
– |
| Metabolic assay result | ||
| l-arabinose | – | + |
| Malate | + | + |
| Citrate | + | + |
| Caprate | – | – |
| Acetate | + | + |
| Propionate | + | + |
| 3-hydroxybutyrate | + | – |
| Lactate | + | – |
| Itaconic acid | + | – |
| l-proline | + | – |
| l-alanine | + | – |
| l histidine | + | – |
| l-serine | + | UNK |
| Valeric acid | + | UNK |
| Adipic acid | – | UNK |
| 3-hydroxybenzoate | – | UNK |
| 4-hydroxybenzoate | – | UNK |
| l-fucose | – | UNK |
| Gluconate | – | UNK |
| 2-ketogluconate | – | UNK |
| n-acetylglucosamine | – | UNK |
| d-glucose | – | UNK |
| Glycogen | – | UNK |
| Inositol | – | UNK |
| Malonate | – | UNK |
| d-maltose | – | UNK |
| d-mannitol | – | UNK |
| d-melibiose | – | UNK |
| d-mannose | – | UNK |
| Phenylacetate | – | UNK |
| l-rhamnose | – | UNK |
| d-ribose | – | UNK |
| d-saccharose | – | UNK |
| Salicin | – | UNK |
| d-sorbitol | – | UNK |
| Suberic acid | – | UNK |
*+, positive; –, negative; UNK, unknown. †Romanenko et al. (5).
Psychrobacter spp. strains are highly susceptible to antimicrobial drugs; only 1 strain of P. phenylpyruvicus was reported to be resistant to penicillin and aztreonam, 2 strains of P. immobilis resistant to penicillin (10,13,14), and 1 strain of P. immobilis resistant to gentamicin, tobramycin, ampicillin, and lincomycin (12). Most human infections have been treated with a third-generation cephalosporin, leading to rapid recovery (10,11,14). One patient who had AIDS died from septic shock, despite appropriate treatment (12).
In conclusion, P. arenosus should be considered a psychrotolerant bacterial species responsible for transfusion-transmitted bacterial infections, similar to Yersinia enterocolitica, Listeria monocytogenes, and psychrophilic Pseudomonas spp. (1,2). However, phenotypic identification of P. arenosus is problematic and might require amplification and sequencing of its 16S rRNA gene.
Acknowledgment
We thank Jeanne-Noëlle Del Bano for technical assistance in 16S rRNA gene amplification and sequencing.
Biography
Dr Caspar is an intern in clinical microbiology in the bacteriology laboratory at Grenoble University Hospital, France. His research interest is medical bacteriology.
Footnotes
Suggested citation for this article: Caspar Y, Recule C, Pouzol P, Lafeuillade B, Mallaret M-R, Maurin M, et al. Psychrobacter arenosus bacteremia after blood transfusion, France. Emerg Infect Dis [Internet]. 2013 Jul [date cited]. http://dx.doi.org/10.3201/eid1907.121599
References
- 1.Brecher ME, Hay SN. Bacterial contamination of blood components. Clin Microbiol Rev. 2005;18:195–204. 10.1128/CMR.18.1.195-204.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wagner SJ. Transfusion-transmitted bacterial infections: risks, sources and interventions. Vox Sang. 2004;86:157–63. 10.1111/j.0042-9007.2004.00410.x [DOI] [PubMed] [Google Scholar]
- 3.Bihl F, Castelli D, Marincola F, Dodd RY, Brander C. Transfusions-transmitted infections. J Transl Med. 2007;5:25. 10.1186/1479-5876-5-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weisburg WG, Barns SM, Pelletier DA, Lane DJ. 16S ribosomal DNA amplification for phylogenetic study. J Bacteriol. 1991;173:697–703 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Romanenko LA, Lysenko AM, Rohde M, Mikhailov VV, Stackebrandt E. Psychrobacter maritimus sp. nov. and Psychrobacter arenosus sp. nov., isolated from coastal sea ice and sediments of the Sea of Japan. Int J Syst Evol Microbiol. 2004;54:1741–5. 10.1099/ijs.0.63096-0 [DOI] [PubMed] [Google Scholar]
- 6.Société Française de Microbiologie. Comité de l’Antibiogramme de la Société Française de Microbiologie. Recommendations 2009. [cited 2013Mar 28]. http://www.sfm-microbiologie.org/UserFiles/file/CASFM/casfm_2009-1.pdf
- 7.Juni E. Psychrobacter. In: Brenner DJ, Krieg NR, StaleyJT, editors. Bergey’s manual of systematic bacteriology, 2nd ed. Vol. 2. New York: Springer; 2005. p. 437–41. [Google Scholar]
- 8.Deschaght P, Janssens M, Vaneechoutte M, Wauters G. Psychrobacter isolates of human origin, other than Psychrobacter phenylpyruvicus, are predominantly Psychrobacter faecalis and Psychrobacter pulmonis, with emended description of P. faecalis. Int J Syst Evol Microbiol. 2012;62:671–4. 10.1099/ijs.0.032631-0 [DOI] [PubMed] [Google Scholar]
- 9.Stepanović S, Vuković D, Bedora-Faure M, K’ouas G, Djukić S, Švabić-Vlahović M, et al. Surgical wound infection associated with Psychrobacter phenylpyruvicus-like organism. Diagn Microbiol Infect Dis. 2007;57:217–9. 10.1016/j.diagmicrobio.2006.08.002 [DOI] [PubMed] [Google Scholar]
- 10.Tripodi MF, Adinolfi LE, Rosario P, Ragone E, Utili R. First definite case of aortic valve endocarditis due to Moraxella phenylpyruvica. Eur J Clin Microbiol Infect Dis. 2002;21:480–2. 10.1007/s10096-002-0744-y [DOI] [PubMed] [Google Scholar]
- 11.Leung WK, Chow VC, Chan MC, Ling JM, Sung JJ. Psychrobacter bacteremia in cirrhotic patient after consumption of raw geoduck clam. J Infect. 2006;52:e169–71 . 10.1016/j.jinf.2005.08.031 [DOI] [PubMed] [Google Scholar]
- 12.Lozano F, Florez C, Recio FJ, Gamboa F, Gómez-Mateas JM, Martín E. Fatal Psychrobacter immobilis infection in a patient with AIDS. AIDS. 1994;8:1189–90 and. 10.1097/00002030-199408000-00027 [DOI] [PubMed] [Google Scholar]
- 13.Gini GA. Ocular infection caused by Psychrobacter immobilis acquired in the hospital. J Clin Microbiol. 1990;28:400–1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lloyd-Puryear M, Wallace D, Baldwin T, Hollis DG. Meningitidis caused by Psychrobacter immobilis in an infant. J Clin Microbiol. 1991;29:2041–2 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wirth SE, Ayala-del-Río HL, Cole JA, Kohlerschmidt DJ, Musser KA, Sepúlveda-Torres LC. Psychrobacter sanguinis sp. nov., recovered from four clinical specimens over a 4-year period. Int J Syst Evol Microbiol. 2012;62:49–54. 10.1099/ijs.0.029058-0 [DOI] [PubMed] [Google Scholar]