Abstract
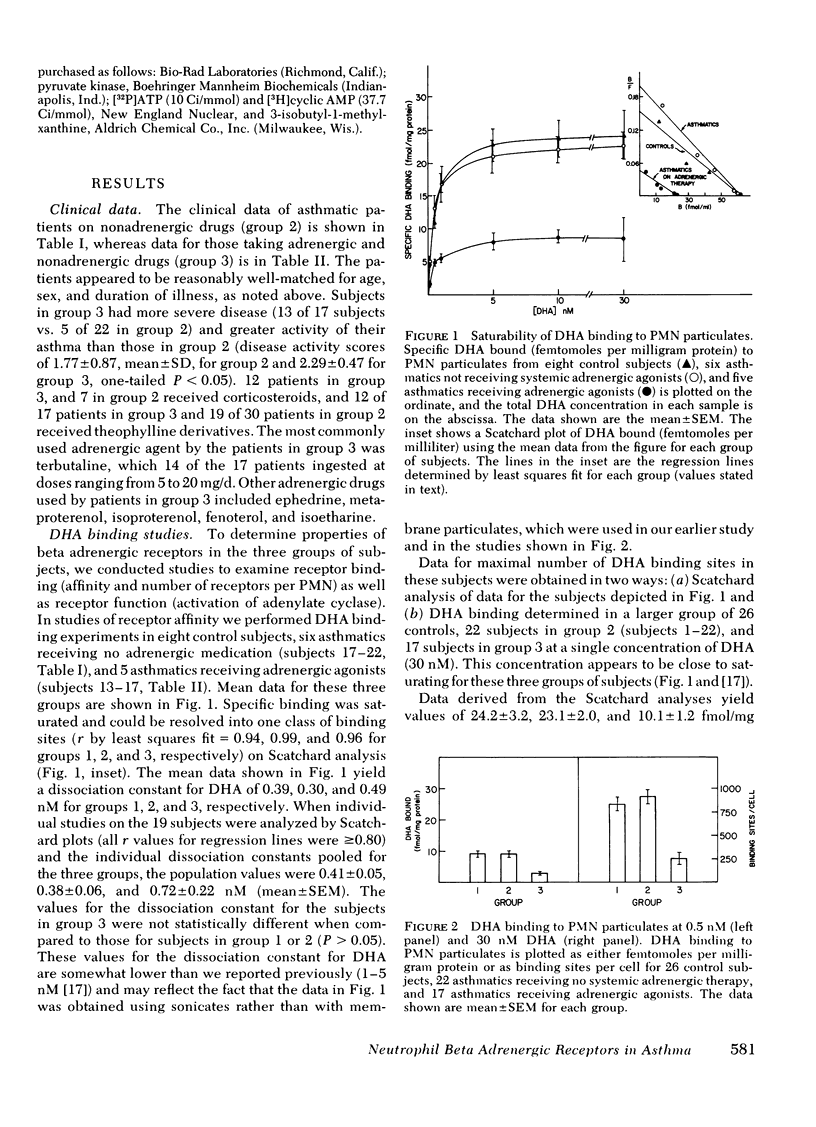
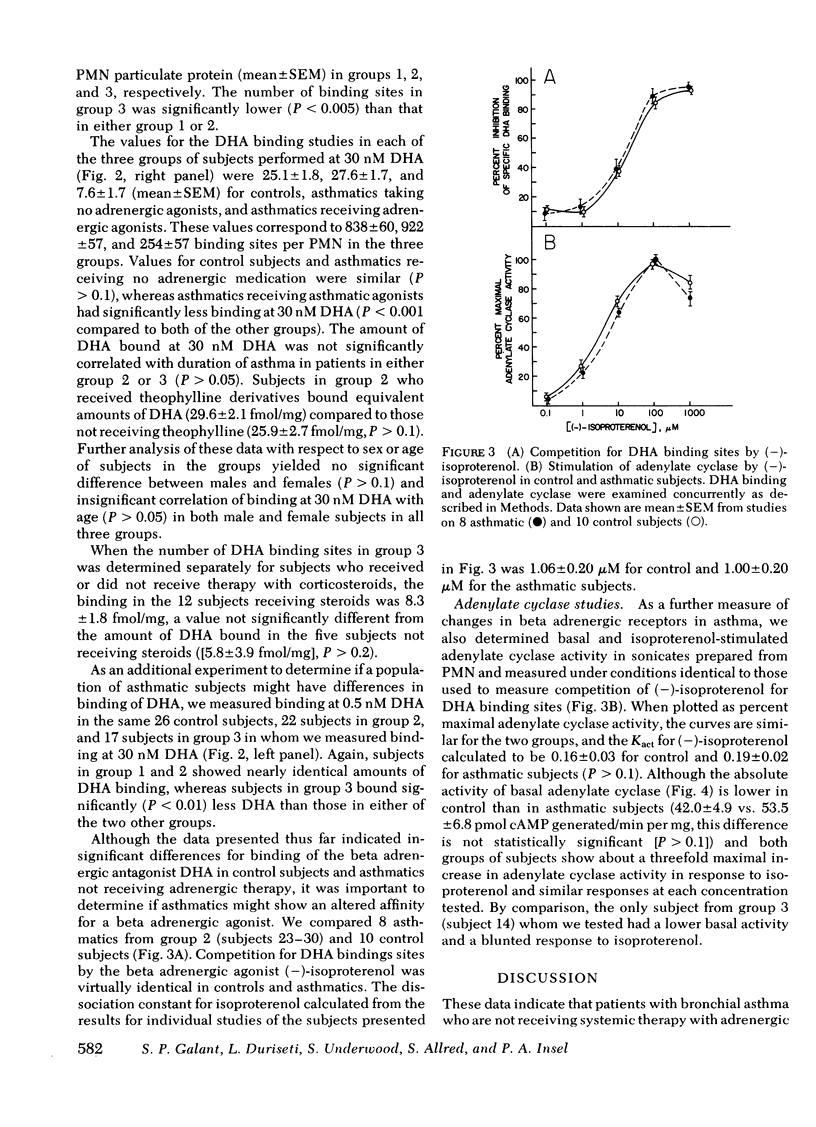
We have tested the beta adrenergic receptor theory of bronchial asthma by determining the number and affinity of binding sites of the beta adrenergic radioligand [3H]dihydroalprenolol (DHA) and the activity of adenylate cyclase in broken cell preparations of polymorphonuclear leukocytes (PMN). We studied 31 control subjects (group 1), 30 asthmatics receiving no systemic adrenergic medication (group 2), and 17 asthmatics receiving adrenergic agonists systemically (group 3). Control subjects and asthmatics taking no adrenergic drugs bound similar amounts of DHA at 0.5 nM and 30 nM DHA and had about 900 binding sites per PMN. In contrast, asthmatics receiving adrenergic agonists had a >70% decrease in their number of DHA binding sites per PMN (254±57). In a subset of our three groups of subjects (eight from group 1, six from group 2, and five from group 3) we measured DHA binding at several DHA concentrations and found similar values (0.4-0.7 nM) for the dissociation constant of DHA among these subjects.
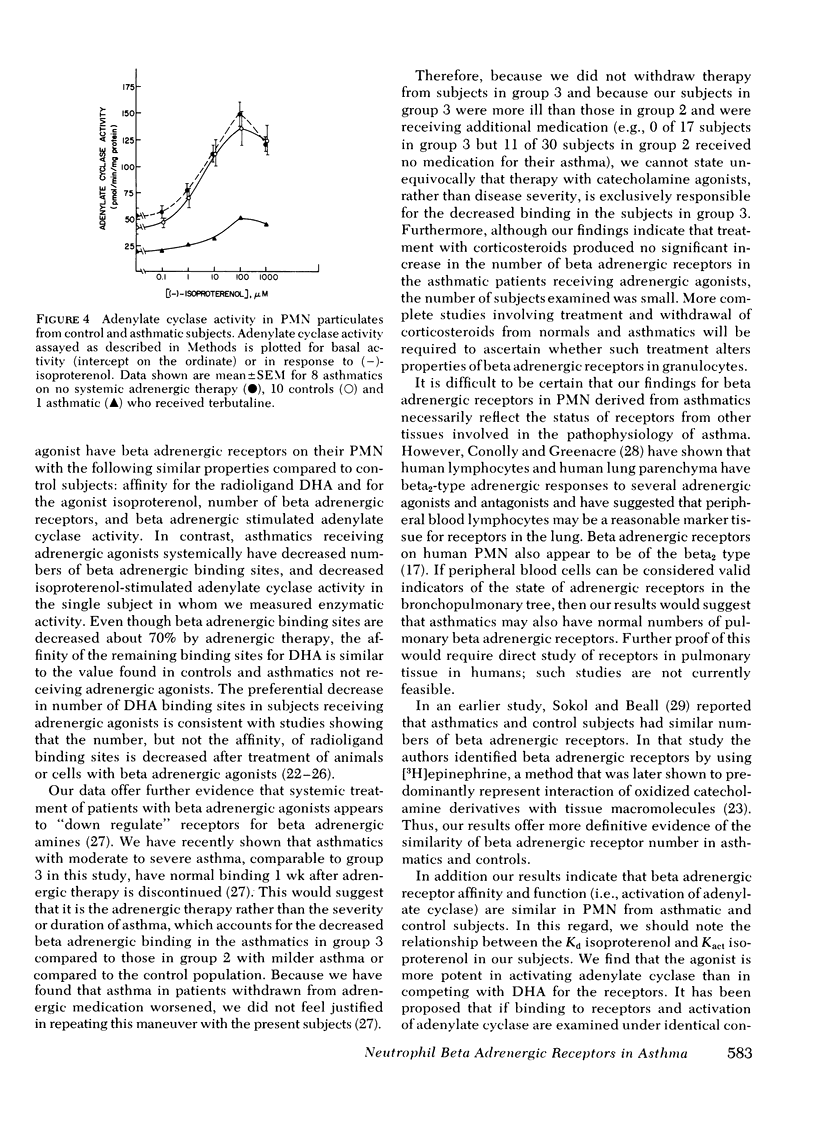
In further studies we examined the interaction of the agonist (−)-isoproterenol with beta adrenergic receptors in 8 normal subjects and 10 asthmatics not receiving adrenergic medication. We tested the ability of isoproterenol to compete for DHA binding sites and to stimulate adenylate cyclase in sonicates prepared from PMN and examined under identical conditions. The dissociation constants for the competition of isoproterenol for DHA binding sites in normal and asthmatic subjects were virtually identical (∼1.0 μM). In addition, the (activation constant) values for stimulation of adenylate cyclase were similar (0.16-0.19 μM) in the two groups of subjects.
Thus, these data suggest that asthma per se is not associated with alteration in either the number or affinity of beta adrenergic receptors in PMN. Our findings indicate that previous reports of abnormal beta adrenergic receptor function in asthmatic patients may in part be explained by prior treatment of such patients with adrenergic agonists. Because the asthmatics who received adrenergic agonists in our study tended to be more ill and to receive additional medication compared to subjects in group 2, we cannot rule out unequivocally that severe asthma may be associated with decreased binding to beta adrenergic receptors. Nevertheless, we conclude that beta adrenergic receptors on PMN from asthmatics are relatively normal unless such patients are treated with adrenergic agonists.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alston W. C., Patel K. R., Kerr J. W. Response of leucocyte adenyl cyclase to isoprenaline and effect of alpha-blocking drugs in extrinsic bronchial asthma. Br Med J. 1974 Jan 19;1(5898):90–93. doi: 10.1136/bmj.1.5898.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks S. M., McGowan K., Bernstein I. L., Altenau P., Peagler J. Relationship between numbers of beta adrenergic receptors in lymphocytes and disease severity in asthma. J Allergy Clin Immunol. 1979 Jun;63(6):401–406. doi: 10.1016/0091-6749(79)90213-6. [DOI] [PubMed] [Google Scholar]
- Busse W. W. Decreased granulocyte response to isoproterenol in asthma during upper respiratory infections. Am Rev Respir Dis. 1977 May;115(5):783–791. doi: 10.1164/arrd.1977.115.5.783. [DOI] [PubMed] [Google Scholar]
- Busse W. W., Sosman J. Decreased H2 histamine response of granulocytes of asthmatic patients. J Clin Invest. 1977 Jun;59(6):1080–1087. doi: 10.1172/JCI108731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Cheng Y., Prusoff W. H. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol. 1973 Dec 1;22(23):3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- Conolly M. E., Greenacre J. K. The beta-adrenoceptor of the human lymphocyte and human lung parenchyma. Br J Pharmacol. 1977 Jan;59(1):17–23. doi: 10.1111/j.1476-5381.1977.tb06971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conolly M. E., Greenacre J. K. The lymphocyte beta-adrenoceptor in normal subjects and patients with bronchial asthma: the effect of different forms of treatment on receptor function. J Clin Invest. 1976 Dec;58(6):1307–1316. doi: 10.1172/JCI108586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galant S. P., Duriseti L., Underwood S., Insel P. A. Decreased beta-adrenergic receptors on polymorphonuclear leukocytes after adrenergic therapy. N Engl J Med. 1978 Oct 26;299(17):933–936. doi: 10.1056/NEJM197810262991707. [DOI] [PubMed] [Google Scholar]
- Galant S. P., Underwood S., Duriseti L., Insel P. A. Characterization of high-affinity beta2-adrenergic receptor binding of (-)-[3H]-dihydroalprenolol to human polymorphonuclear cell particulates. J Lab Clin Med. 1978 Oct;92(4):613–618. [PubMed] [Google Scholar]
- Ignarro L. J. Nonphagocytic release of neutral protease and beta-glucuronidase from human neutrophils. Regulation by autonomic neurohormones and cyclic nucleotides. Arthritis Rheum. 1974 Jan-Feb;17(1):25–36. doi: 10.1002/art.1780170106. [DOI] [PubMed] [Google Scholar]
- Insel P. A., Stoolman L. M. Radioligand binding to beta adrenergic receptors of intact cultured S49 cells. Mol Pharmacol. 1978 Jul;14(4):549–561. [PubMed] [Google Scholar]
- Kaliner M., Austen K. F. A sequence of biochemical events in the antigen-induced release of chemical mediators from sensitized human lung tissue. J Exp Med. 1973 Nov 1;138(5):1077–1094. doi: 10.1084/jem.138.5.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy M. S., Insel P. A. Inhibitors of microtubule assembly enhance beta-adrenergic and prostaglandin E1-stimulated cyclic AMP accumulation in S49 lymphoma cells. Mol Pharmacol. 1979 Jul;16(1):215–223. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Maguire M. E., Ross E. M., Gilman A. G. beta-Adrenergic receptor: ligand binding properties and the interaction with adenylyl cyclase. Adv Cyclic Nucleotide Res. 1977;8:1–83. [PubMed] [Google Scholar]
- McNeill R. S., Ingram C. G. Effect of propranolol on ventilatory function. Am J Cardiol. 1966 Sep;18(3):473–475. doi: 10.1016/0002-9149(66)90072-5. [DOI] [PubMed] [Google Scholar]
- Middleton E., Jr Autonomic imbalance in asthma with special reference to beta adrenergic blockade. Adv Intern Med. 1972;18:177–197. [PubMed] [Google Scholar]
- Morris H. G., Rusnak S. A., Barzens K. Leukocyte cyclic adenosine monophosphate in asthmatic children. Effects of adrenergic therapy. Clin Pharmacol Ther. 1977 Sep;22(3):352–357. doi: 10.1002/cpt1977223352. [DOI] [PubMed] [Google Scholar]
- Morris H. G., Rusnak S. A., Selner J. C., Barzens K., Barnes J. Adrenergic desensitization in leukocytes of normal and asthmatic subjects. J Cyclic Nucleotide Res. 1977 Dec;3(6):439–446. [PubMed] [Google Scholar]
- Niaudet P., Beaurain G., Bach M. A. Differences in effect of isoproterenol stimulation on levels of cyclic AMP in human B and T lymphocytes. Eur J Immunol. 1976 Nov;6(11):834–836. doi: 10.1002/eji.1830061117. [DOI] [PubMed] [Google Scholar]
- Ouellette J. J., Reed C. E. The effect of partial beta adrenergic blockade on the bronchial response of hay fever subjects to ragweed aerosol. J Allergy. 1967 Mar;39(3):160–166. doi: 10.1016/0021-8707(67)90033-0. [DOI] [PubMed] [Google Scholar]
- Parker C. W., Smith J. W. Alterations in cyclic adenosine monophosphate metabolism in human bronchial asthma. I. Leukocyte responsiveness to -adrenergic agents. J Clin Invest. 1973 Jan;52(1):48–59. doi: 10.1172/JCI107173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomon Y., Londos C., Rodbell M. A highly sensitive adenylate cyclase assay. Anal Biochem. 1974 Apr;58(2):541–548. doi: 10.1016/0003-2697(74)90222-x. [DOI] [PubMed] [Google Scholar]
- Shear M., Insel P. A., Melmon K. L., Coffino P. Agonist-specific refractoriness induced by isoproterenol. Studies with mutant cells. J Biol Chem. 1976 Dec 10;251(23):7572–7576. [PubMed] [Google Scholar]
- Sheppard J. R., Gormus R., Moldow C. F. Catecholamine hormone receptors are reduced on chronic lymphocytic leukaemic lymphocytes. Nature. 1977 Oct 20;269(5630):693–695. doi: 10.1038/269693a0. [DOI] [PubMed] [Google Scholar]
- Sokol W. N., Beall G. N. Leukocytic epinephrine receptors of normal and asthmatic individuals. J Allergy Clin Immunol. 1975 May;55(5):310–324. doi: 10.1016/0091-6749(75)90003-2. [DOI] [PubMed] [Google Scholar]
- Terasaki W. L., Brooker G. [125I]Iodohydroxybenzylpindolol binding sites on intact rat glioma cells. Evidence for beta-adrenergic receptors of high coupling efficiency. J Biol Chem. 1978 Aug 10;253(15):5418–5425. [PubMed] [Google Scholar]
- Tse J., Powell J. R., Baste C. A., Priest R. E., Kuo J. F. Isoproterenol-induced cardiac hypertrophy: modifications in characteristics of beta-adrenergic receptor, adenylate cyclase, and ventricular contraction. Endocrinology. 1979 Jul;105(1):246–255. doi: 10.1210/endo-105-1-246. [DOI] [PubMed] [Google Scholar]
- Zurier R. B., Weissmann G., Hoffstein S., Kammerman S., Tai H. H. Mechanisms of lysosomal enzyme release from human leukocytes. II. Effects of cAMP and cGMP, autonomic agonists, and agents which affect microtubule function. J Clin Invest. 1974 Jan;53(1):297–309. doi: 10.1172/JCI107550. [DOI] [PMC free article] [PubMed] [Google Scholar]



