Abstract
Neuroendocrine (NE) prostate tumors and neuroendocrine differentiation (NED) in prostatic adenocarcinomas have been associated with poor prognosis. In this study, we used the TRAMP mouse model that develops NE prostate tumors to identify key factors that can lead to NED. We have previously reported that NE tumors express the forkhead transcription factor, Foxa2, Mash1 (mouse achaete scute homolog-1), as well as Synaptophysin. In TRAMP, the prostatic intraepithelial neoplasia (PIN) first expresses Foxa2 and Synaptophysin, which then progresses to NE cancer. In order to determine if Foxa2 is dispensable for development or maintenance of NE cancer, a conditional knock-out of Foxa2 in TRAMP mice was generated by breeding mice with two floxed alleles of Foxa2 and one copy of Nkx3.1-Cre. Nkx3.1-Cre/Foxa2loxP/loxP mice showed loss of Foxa2 expression in embryonic prostatic buds. No expression of Foxa2 was seen in the adult prostate in either conditional null or control mice. Foxa2 is universally expressed in all wild type TRAMP NE tumors, but Mash1 expression is seen only in a few samples in a few cells. With the loss of Foxa2 in the NE tumors of the TRAMP/Nkx3.1-Cre/Foxa2loxP/loxP mice, the expression of the pro-neuronal gene Mash1 is upregulated. NE tumors from both the TRAMP control and Foxa2-deficient TRAMP prostate express Synaptophysin and SV40 Large T-antigen, and both show a loss of androgen receptor expression in NE cells. These studies suggest that the TRAMP NE tumors can form in the absence of Foxa2 by an up regulation of Mash1.
Keywords: prostate, TRAMP, Foxa2, Mash1, neuroendocrine
INTRODUCTION
Neuroendocrine (NE) prostate cancer (also called small cell carcinoma) is rare and occurs in <5% of the prostate cancer patients. However, increases in neuroendocrine differentiation (NED) of adenocarcinoma and NE secretory products are closely correlated with tumor progression [1,2]. Focal NED and expression of neuropeptides are observed in the majority of clinically localized PCa, closely correlated with tumor metastases, and the failure of androgen ablation therapy [3–6]. Recent work has demonstrated that NE cells promote adenocarcinoma to continue to grow and metastasize at castrate levels of androgens [7,8] by activating the NF-κB pathway within the adenocarcinoma [9].
We are interested in understanding the molecular mechanisms that lead to NED of prostate tumors. NED occurs in the LPB-Tag 12T-10 transgenic mouse prostate by a sequential activation of the same transcription factors as are involved in the endocrine differentiation of pancreatic beta cells [10]. This pathway starts with the expression of Foxa2 and Mash1 in prostatic intraepithelial neoplasia (PIN). The PIN also expresses NE markers such as Chromogranin A and Synaptophysin and eventually progresses to NE cancer [10]. Foxa2 is expressed in prostate of NE mouse models (LPB-Tag 12T-10, CR2-T-Ag, and TRAMP) as well as in human (NE) prostate tumors [11,12]. Previous studies have shown that transgenic mouse model for small cell lung carcinomas also express Foxa2 [13]. Since Foxa2 is required for endocrine differentiation of the pancreas [14,15] and Foxa2 expression is routinely detected in NE prostate cancer cells, we have investigated whether there is an important role of Foxa2 in NED of prostate tumors.
Since TRAMP mice develop large poorly differentiated NE prostate tumors, we have conditionally ablated Foxa2 to determine if Foxa2 is required for progression to NE cancer. We employed the Cre recombinase driven by the Nkx3.1 gene promoter, a prostate epithelial cell specific promoter expressed early in the embryonic development of the prostate and active in adult prostate epithelial cells. We found that the ablation of Foxa2 in the embryonic caused no obvious change later in prostate development. Further, the removal of Foxa2 did not prevent the formation of prostate NE tumors in TRAMP. However, the ablation of Foxa2 in the epithelium results in an increase in the expression of Mash1, which is detected only in a limited number of TRAMP cells. To test if Mash1 was sufficient to cause NED, a non-NE mouse prostate cell line was transfected with a Mash1 expression vector. The Mash1 over-expressing prostate epithelial cells when recombined with rat urogenital mesenchyme (UGM) and grafted under the kidney capsule of nude mice, give rise to sheets of cells which do not express Mash1 suggesting that cells are intolerant to high levels of Mash1 and cannot grow in vivo. It has been reported that the bitransgenic mice expressing the SV40 Large T-antigen and Mash1 in the lung epithelial cells develop small cell lung carcinomas [16]. These results suggest that there are two different cell types in the NE tumors, one that express Foxa2 and the other that express Mash1. It is possible that either Foxa2 or Mash1 alone is sufficient to maintain the NE phenotype of the cells, and both transcription factors serve as early markers of prostatic NED.
MATERIAL AND METHODS
Tissue Preparation and Processing
Mice were sacrificed by cervical dislocation after the inhalation of an anesthetic agent according to the policy of the Vanderbilt University Animal Care and Use Committee. The prostates were generally dissected into four different lobes (ventral, lateral, dorsal, and anterior lobe) under a dissecting microscope. When it was not possible to separate the lateral and dorsal lobes, the tissue was taken together as the dorsolateral lobe. Tissues were fixed in 10% buffered formalin and processed and embedded in paraffin using standard techniques. Paraffin-embedded tissues were cut at 5 μm, and sections were either stained with Haematoxylin and Eosin (H&E) or with relevant antibodies for immunohistochemical analysis.
Immunohistochemistry
Slides were deparaffinized by immersing in xylene twice for 10 min each and hydrated by immersing in a series of 100%, 95%, 70%, 50% ethanol, and one time in dH2O for 5 min each. Slides for histological analysis were stained with H&E by standard methods, with generally three to four sections reviewed per specimen. For Foxa2, Synaptophysin, T-antigen, AR, and Mash1 immunostaining, antigen retrieval was achieved by microwaving in antigen unmasking solution (Catalog # H-3300, Vector Laboratories, Inc., Burlingame, CA) for 30 min, and the slides were then equilibrated at room temperature for 1 hr. Endogenous peroxidase activity was blocked by peroxidase blocking reagent (Dako) 30 min followed by washing in PBS (pH 7.4). After rinsing with PBS, the slides were placed in blocking solution (goat, horse, or rabbit serum as appropriate) for 20 min to block non-specific binding of antibody to the tissue. Sections were incubated with primary antibody overnight at 4°C. The following primary antibodies were used (with the indicated dilutions in PBS): Foxa2, P19, goat antibody (Santa Cruz Biotechnology Inc., Santa Cruz, CA, 1:1,000); Synaptophysin (Cat# 611880, BD Biosciences Pharmingen, San Jose, CA, 1:500); T-antigen (SV40 T-Ag, monoclonal mouse IgG Calbiochem, San Diego, CA, 1:1,000); AR, N-20 (Santa Cruz Biotechnology Inc., 1:1,000); Mash1 (Cat #556604, BD Biosciences Pharmingen, 1:500). The respective secondary antibodies were used at a dilution of 1:200. Staining was visualized using VectastainABC kit (Vector Laboratories, Inc., Burlingame, CA) and 3,3′-diaminobenzidine tetrahydrochloride (Dako, Carpentaria, CA). Slides were counterstained with hematoxylin, dehydrated and cover slipped. For immunofluorescence staining, tissue sections were blocked with PBS containing 5% normal donkey serum (Vector Laboratories, Inc.) for 1 hr, and incubated overnight at 4°C with primary antibodies. After washing in PBS, sections were incubated with Alexa Fluor conjugated secondary antibody (Molecular Probes, Eugene, OR) for 1 hr (diluted 1:200 in blocking buffer). Sections were washed in PBS and coverslipped using mounting solution with DAPI (Vector Laboratories, Inc.).
Generation of Foxa2 Knock-Out TRAMP Mice
The TRAMP Foxa2-deficient mice were generated by breeding two alleles of floxed Foxa2 along with one copy of Nkx3.1-Cre in the TRAMP background. The Nkx3.1-Cre mice were in the C57Bl6 background. The Nkx3.1-Cre mice were generated by replacing one copy of Nkx3.1 allele with the Cre transgene by gene targeting. Foxa2loxP mice [17] were a mixed background of CD1 and C57Bl6. The breeding was a three step process.
Two copies of Foxa2loxP allele were bred into the TRAMP background resulting in TRAMP/Foxa2loxP/loxP mice.
Two copies of Foxa2loxP allele were bred into the Nkx3.1-Cre+/− background resulting in Nkx3.1-Cre/Foxa2loxP/loxP mice.
The animals obtained from the above two breeding were bred to each other and the next generation was screened for TRAMP/Nkx3.1-Cre+/−/Foxa2loxP/loxP mice.
RESULTS
Foxa2 Expression Is Absent in the Prostatic Buds of Nkx3.1-Cre/Foxa2loxP/loxP Mice
The TRAMP transgenic mice were bred with Nkx3.1-Cre mice and Foxa2loxP/loxP to generate TRAMP/Nkx3.1-Cre/Foxa2loxP/loxP, TRAMP/Foxa2loxP/loxP, TRAMP/Nkx3.1-Cre, Nkx3.1-Cre/Foxa2loxP/loxP, Foxa2loxP/loxP, and Nkx3.1-Cre mice. Prostates from the six lines were examined to control for the loss of one copy of the Nkx3.1 gene (the Cre was placed into the Nkx3.1 locus by homologous recombination) and to determine if the loss of Foxa2 early in development resulted in a prostate phenotype that could influence the development of the TRAMP tumor. Figure 1A shows that Foxa1 is expressed in all the epithelial cells including the prostatic buds, as previously reported [18]. Figure 1B shows that the loss of one copy of Nkx3.1 does not affect Foxa1 expression and the presence of two Foxa2loxP alleles does not affect Foxa1 expression (Fig. 1C), Loss of Foxa2 (Nkx3.1-Cre/Foxa2loxP/loxP) had no affect on Foxa1 expression (Fig. 1D). In control mice, Foxa2 expression is detected only in the epithelial cells at the tips of growing prostatic buds (Fig. 1E) and Foxa2 expression is not affected by loss of one copy of Nkx3.1 due to knock-in of Cre (Fig. 1F), or floxing of Foxa2 alleles (Fig. 1G). Figure 1H shows that Foxa2 expression is lost at the tips of growing buds by Nkx3.1-Cre mediated conditional deletion. Some scattered cells still express Foxa2, suggesting that the Nkx3.1-Cre is not expressed in these epithelial cells.
Fig. 1.
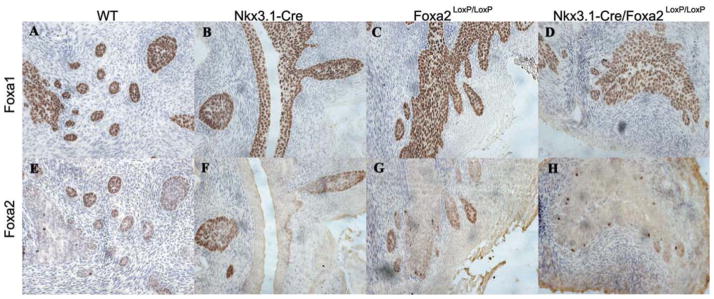
Foxa2 expression is lost at the prostatic tips at E19.5 UGS from Nkx3.1-Cre+/−/Foxa2loxP/loxP. Foxa1 is expressed in the urogenital epithelial (UGE) cells in C57Blk6 wild type mice (A), Nkx3.1-Cre+/− (B), Nkx3.1-Crewt/wt/Foxa2loxP/loxP (C), Nkx3.1-Cre+/−/Foxa2loxP/loxP (D).Foxa2 is expressed in the UGE cells of C57Blk6 wild type mice (E),Nkx3.1-Cre+/− (F),Nkx3.1-Crewt/wt/Foxa2loxP/loxP (G).Foxa2 expression is deleted in epithelial cells from the E19.5 UGS of Nkx3.1-Cre+/−/Foxa2loxP/loxP (H).
Loss of Foxa2 During Early Budding Does not Affect the Normal Prostate Development
In the normal prostate, Foxa2 expression is observed only at the tips of growing prostatic buds from E18 to E21; thus we investigated if the loss of Foxa2 can cause defects in normal prostate development, architecture, and differentiation. The four prostatic lobes (anterior, dorsal, lateral, and ventral) were dissected from five Nkx3.1-Cre/Foxa2loxP/loxP and five Foxa2loxP/loxP mice at 2, 6, and 12 months of age. H&E staining of the lobes at all ages showed no structural changes. Figure 2 shows the H&E staining on the anterior (A), dorsal (B), lateral (C), ventral (D) lobes of six months old Foxa2loxP/loxP mice (controls) and anterior (E), dorsal (F), lateral (G) and ventral (H) lobes from six months old Nkx3.1-Cre/Foxa2loxP/loxP mice. Immunohistochemistry was performed using the androgen receptor and Foxa1 as epithelial cell markers, and p63 as a basal cell marker. The architecture and ductal branching of the prostate was unaffected by the loss of Foxa2.
Fig. 2.
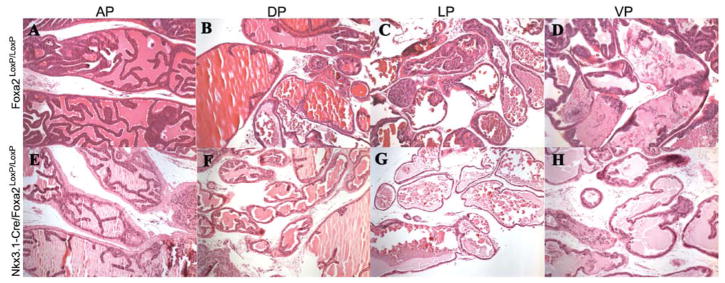
Haemotoxylin and Eosin staining of the prostatic lobes does not show any histological defects in prostates of the Foxa2-deficient mice. H and E on anterior (A), dorsal (B), lateral (C), and ventral (D) prostatic lobes of Foxa2loxP/loxP mice. H and E on the anterior (E), dorsal (F),lateral(G),and ventral (H) prostatic lobes of Nkx3.1-Cre/Foxa2loxP/loxP.
Loss of Foxa2 Results in Increased Mash1 Expression in the TRAMP Neuroendocrine Prostate Tumors
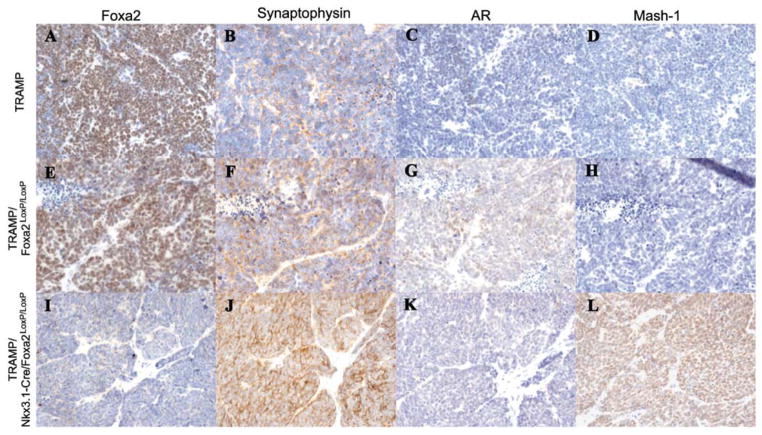
Previous studies have shown that Foxa2 is expressed in the NE PIN lesions from the TRAMP mice [11]. The advanced NE tumors from the TRAMP mice show high levels of expression of Foxa2 (Fig. 3A), Synaptophysin (Fig. 3B), and a loss of androgen receptor expression (Fig. 3C). The TRAMP tumors are largely negative for Mash1 (Fig. 3D), with occasional individual positive cells. Mash1 is reported as a pro-endocrine gene previously that causes lung adenocarcinoma in the SV40 Large T-antigen model to differentiate into small cell carcinomas (NE cancer). In the presence of two Foxa2loxP alleles in the TRAMP background we see the expression of Foxa2, as expected (Fig. 3E), Synaptophysin (Fig. 3F), the loss of androgen receptor (Fig. 3G), and absence of Mash1 (Fig. 3H). NE prostate tumors still develop in the TRAMP/Nkx3.1-Cre/Foxa2loxP/loxP and they show a loss of Foxa2 expression (Fig. 3I). The NE prostate tumors from the Foxa2-deficient TRAMP mice still express Synaptophysin (Fig. 3J) and do not express androgen receptor (Fig. 3K). This indicates that loss of Foxa2 alone is not sufficient to prevent the formation of NE tumors. However, with the loss of Foxa2 expression, an increase in Mash1 expression was detected in NE cancer (Fig. 3L).
Fig. 3.
Ablation of Foxa2 in the TRAMP tumors results in increase in Mash-1 expression. Foxa2 is expressed in the neuroendocrine prostate tumors from the TRAMP mice (A), TRAMP/Foxa2floxP/floxP (E). Foxa2 expression is lost in the NE tumors from TRAMP/NKX3.1-Cre+/−/Foxa2floxP/floxP (I). Synaptophysin is expressed by the NE prostate tumors from TRAMP mice (B), TRAMP/Foxa2floxP/floxP (F), and TRAMP/NKX3.1-Cre+/−/Foxa2floxP/floxP (J). Androgen receptor expression is lost in the tumors from wild type TRAMP mice (C), TRAMP/Foxa2floxP/floxP (G), and TRAMP/NKX3.1-Cre+/−/Foxa2floxP/floxP (K). Mash-1 is not expressed in the NE tumors of TRAMP (D), TRAMP/Foxa2floxP/floxP (H). Mash-1 expression is switched on in the NE prostate tumors of the TRAMP/Nkx3.1-Cre+/−/Foxa2loxP/loxP(L).
Mash1 Is not Expressed in the Embryonic Prostates of Foxa2-Deficient Mice
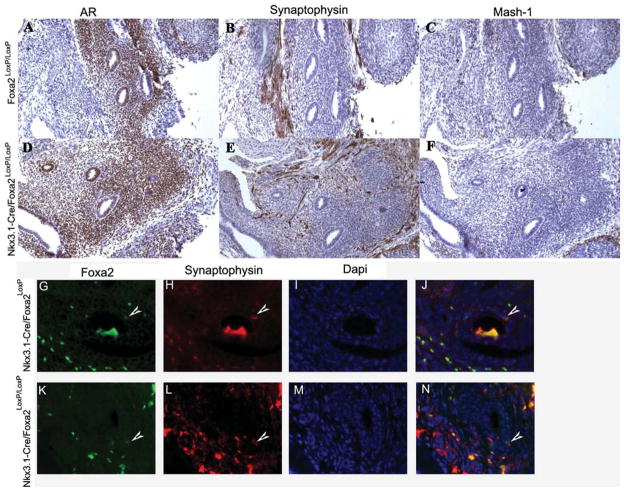
In order to determine if Mash1 expression was indirectly transcriptionally activated due to loss of Foxa2, we analyzed the expression of Mash1 in early prostate buds from both the Foxa2-deficient and control mice. Figure 4A and D shows androgen receptor expression in the urogenital sinus from Foxa2loxP/loxP and Nkx3.1-Cre+/−/Foxa2loxP/loxP E19.5 embryos, respectively, where, as expected, both the urogenital epithelium of the prostate ducts and the stroma express the AR. Synaptophysin expression is not detected in the UGS from both the Foxa2 floxed (Fig. 4B) and Foxa2-deficient (Fig. 4E) embryos. Rare Foxa2 and Synaptophysin double positive cells were detected in 3-week-old prostate from both control (G–J) and Nkx3.1-Cre+/−/Foxa2loxP/loxP (K–N) mice. Mash1 is not expressed in the UGS from Foxa2loxP/loxP mice (Fig. 4C). Loss of Foxa2 in the prostatic buds does not induce Mash1 expression in the UGS from Nkx3.1-Cre+/−/Foxa2loxP/loxP mice (F). These results suggest that Mash1 is not expressed in the embryonic prostate of Foxa2-deficient mice, and that expression of Mash1 in the adult prostate of the Foxa2-deficient TRAMP is related to the NE phenotype.
Fig. 4.
Ablation of Foxa2 does not result in Mash-1 expression in the E19.5 UGS. AR is expressed in the epithelial and stromal cells from UGS of Foxa2loxP/loxP (A) and Nkx3.1-Cre+/−/Foxa2loxP/loxP (D) mice. Synaptophysin expression is not detected in the UGS of Foxa2loxP/loxP (B) and Nkx3.1-Cre+/−/Foxa2loxP/loxP (E) mice. Mash-1 is not expressed in UGS of either Foxa2loxP/loxP (C) or Nkx3.1-Cre+/−/Foxa2loxP/loxP (F)mice. Foxa2 and Synaptophysin are co-expressed in 3-week-old prostate of control (G^J) and Nkx3.1-Cre+/−/Foxa2loxP/loxP (K^N) mice.
DISCUSSION
The androgen receptor is key to both normal prostate function and prostate cancer biology. The transcriptional activity of the AR is regulated by its interaction with other tissue specific transcription factors. The Foxa1 and Foxa2 transcription factors play fundamental roles in AR action, prostate development, and NE differentiation [11,19,20]. The expression of both Foxa1 and Foxa2 is detected at E18 in epithelial cells that express either basal and/or luminal markers. By E21, Foxa2 is expressed in a large number of cells at the tips of growing prostatic buds and is still expressed in the prostate until neonatal day 1, but is only expressed in the normal adult prostate in rare NE cells [21]. Previous studies have shown that Foxa1 interacts with AR and regulates several AR target genes in the prostate [19]. Loss of Foxa1 results in a loss of complete differentiation of prostate epithelium, with accumulation of cells co-expressing both secretory epithelial cells markers (Cytokeratin 8) and basal cell markers (p63) [20]. Similar coregulation of target genes by Foxa1/a2 and AR also was observed in liver. Here AR-mediated promotion of hepatocarcinogenesis was lost in Foxa1/2-deficient mice, indicating an involvement of Foxa1/2 in sexual dimorphism of hepatocellular carcinoma [22]. Although, Foxa2 expression in prostate buds is highly conserved between embryonic mouse and human prostate [21], the loss of Foxa2 did not result in any obvious detectable morphological or histological phenotype.
We have previously shown that Foxa2 is expressed in rodent and human androgen-independent NE prostate cancer [11]. Because Foxa2 is a critical factor that starts a cascade in endocrine differentiation of the pancreas [23], we reasoned a similar pathway may occur in prostate NE cells. Using the 12T-10 transgenic mouse model that develops NE prostate cancer, we found that NED in prostate cancer follows that same pathway [10]. In this study, we employed the TRAMP NE prostate cancer mouse model to study the role of Foxa2 in NED. TRAMP tumors also express Foxa2, and loss of Foxa2 in these tumors could not prevent the formation of NE cancers. However, the loss of Foxa2 is accompanied by expression of the pro-endocrine gene Mash1.
Previous work has shown that co-expression of Mash1 with SV 40 Large T-antigen can result in lung NE tumors [16]. These data together with ours suggest that the cells, along with the Large T-antigen, need either Foxa2 or Mash1 to progress to the NE phenotype. After the loss of Foxa2, the epithelial cells in the TRAMP model activate the expression of Mash1 and still progress to NE cancers. In our previous studies, we have shown that the 12T-10 NE prostate tumors express Ngn3 and Nkx2.2, transcription factors also expressed by pancreatic endocrine cells [10]. In the TRAMP model, neither Ngn3 nor Nkx2.2 are detected in the TRAMP tumors as well as TRAMP tumors without Foxa2 expression. The 12T-10 expresses a deletion construct of the SV40 early region that only allows the expression of the Large T-antigen but not the small t-antigen. However, the TRAMP tumor expresses the early region of the SV40 virus [24]. Thus, one possible explanation for the difference in Ngn3 and Nkx2.2 expression could be that the presence of both SV40 large and small t-antigen in TRAMP mouse model, where the small t-antigen might influence some pathways that inhibit the expression of these genes while still allowing NED to occur.
Although the expression of Foxa2 has so far been detected in all rodent and human NE tumors, its loss is not sufficient to prevent NE cancer. This suggests that Foxa2 could be a good marker for the NE phenotype, but that disease progression can take place independent of Foxa2 expression. Our recent studies show that activation of Wnt/β-Catenin signaling in the prostate induces the expression of Foxa2 [25,26], and prostates that express both SV40 Large T-antigen and stabilized β-Catenin (mimicking active Wnt signaling) display NED [26]. Further, since Wnt-signaling is active in the embryonic prostate buds [27,28], the expression of Foxa2 in these buds may serve as marker of active Wnt signaling.
A recent study has shown that Foxa2 synergistically cooperates with hypoxia-inducible factor HIF1a to regulate the expression of a network of transcription factors and promotes NE tumor progression in TRAMP mouse model [29]. A reduction of HIF1a level (achieved by ablating the ubiquitin ligase Siah2) significantly decreases NE tumor initiation and metastasis in the TRAMP mice. The lack of phenotypic difference in TRAMP/Foxa2-deficient mice compared with TRAMP mice in our study suggests that the tumors adopt some other ways, such as the induction of Mash1, to maintain NE differentiation. It is possible that a similar cooperation between Mash1 and HIF1 exists and this cooperation facilitates the NE tumor development in the TRAMP/Foxa2-deficient mice.
In summary, the results of this study show that loss of Foxa2 in TRAMP mice was insufficient to prevent the development of the small cell carcinomas/NE tumors. The increased expression of Mash1 upon loss of Foxa2 combined with previous studies that show Mash1 and T-antigen expression can cause NE lung cancer [16] suggests that Mash1 can substitute for Foxa2 during progression to small cell carcinoma. This could be investigated by generating Mash1 and Foxa2 combined conditional gene ablation in the TRAMP background. Loss of NE tumors in the compound mutants would indicate that Mash1 expression compensated for the loss of Foxa2 in these studies.
Acknowledgments
This work was supported by P01 CA154293 to M.M.S. and 5R01 DK055748-12, 4R01 CA076142-13, and R01-AG23490, P01 DK49210 to K.H.K., and the Joe C. Davis Foundation to R.J.M.
Grant sponsor: NIH; Grant numbers: 5R01 DK055748-12; 4R01 CA076142-13; R01-AG23490; P01 CA154293; P01 DK49210; Grant sponsor: Joe C. Davis Foundation.
Abbreviations
- PC
aprostate cancer
- AR
androgen receptor
- HGPIN
high grade prostatic intraepithelial neoplasia
- Tag
SV40 large T-antigen
- NE
neuroendocrine
- NED
neuroendocrine differentiation
Footnotes
Disclosure Statement: The authors have nothing to disclose. There is no conflict of interest. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.di Sant’Agnese PA. Neuroendocrine differentiation in prostatic carcinoma: An update. Prostate Suppl. 1998;8:74–79. [PubMed] [Google Scholar]
- 2.Abrahamsson PA. Neuroendocrine cells in tumour growth of the prostate. Endocr Relat Cancer. 1999;6:503–519. doi: 10.1677/erc.0.0060503. [DOI] [PubMed] [Google Scholar]
- 3.Ito T, Yamamoto S, Ohno Y, Namiki K, Aizawa T, Akiyama A, Tachibana M. Up-regulation of neuroendocrine differentiation in prostate cancer after androgen deprivation therapy, degree and androgen independence. Oncol Rep. 2001;8:1221–1224. doi: 10.3892/or.8.6.1221. [DOI] [PubMed] [Google Scholar]
- 4.Chuang CK, Wu TL, Tsao KC, Liao SK. Elevated serum chromogranin A precedes prostate-specific antigen elevation and predicts failure of androgen deprivation therapy in patients with advanced prostate cancer. J Formos Med Assoc. 2003;102:480–485. [PubMed] [Google Scholar]
- 5.Tarle M, Ahel MZ, Kovacic K. Acquired neuroendocrine-positivity during maximal androgen blockade in prostate cancer patients. Anticancer Res. 2002;22:2525–2529. [PubMed] [Google Scholar]
- 6.Best CJ, Gillespie JW, Yi Y, Chandramouli GVR, Perlmutter MA, Gathright Y, Erickson HS, Georgevich L, Tangrea MA, Duray PH, Gonzalez S, Velasco A, Linehan WM, Matusik RJ, Price D, Figg WD, Emmert-Buck MR, Chuaqui RF. Molecular alterations in primary prostate cancer after androgen ablation therapy. Clin Cancer Res. 2005;11:6823–6834. doi: 10.1158/1078-0432.CCR-05-0585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jin RJ, Wang Y, Masumori N, Ishii K, Tsukamoto T, Shappell SB, Hayward SW, Kasper S, Matusik RJ. NE-10 neuroendocrine cancer promotes the LNCaP xenograft growth in castrated mice. Cancer Res. 2004;64:5489–5495. doi: 10.1158/0008-5472.CAN-03-3117. [DOI] [PubMed] [Google Scholar]
- 8.Uchida K, Masumori N, Takahashi A, Itoh N, Kato K, Matusik RJ, Tsukamoto T. Murine androgen-independent neuroendocrine carcinoma promotes metastasis of human prostate cancer cell line LNCaP. Prostate. 2006;66:536–545. doi: 10.1002/pros.20369. [DOI] [PubMed] [Google Scholar]
- 9.Jin RJ, Lho Y, Connelly L, Wang Y-Q, Yu X, Saint Jean L, Case T, Ellwood-Yen K, Sawyers CL, Bhowmick NA, Blackwell TS, Yull FE, Matusik RJ. The nuclear factor kappa B pathway controls progression of prostate cancer to androgen independent growth. Cancer Res. 2008;68:6762–6769. doi: 10.1158/0008-5472.CAN-08-0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gupta A, Wang Y-Q, Browne C, Kim S, Case TC, Paul M, Matusik RJ. Neuroendocrine differentiation in the 12T-10 transgenic prostate mouse model mimics endocrine differentiation of pancreatic beta cells. Prostate. 2008;68:50–60. doi: 10.1002/pros.20650. [DOI] [PubMed] [Google Scholar]
- 11.Mirosevich J, Gao N, Gupta A, Shappell SB, Jove R, Matusik RJ. Expression and role of Foxa proteins in prostate cancer. Prostate. 2006;66:1013–1029. doi: 10.1002/pros.20299. [DOI] [PubMed] [Google Scholar]
- 12.Hu Y, Ippolito JE, Garabedian EM, Humphrey PA, Gordon JI. Molecular characterization of a metastatic neuroendocrine cell cancer arising in the prostates of transgenic mice. J Biol Chem. 2002;277:44462–44474. doi: 10.1074/jbc.M205784200. [DOI] [PubMed] [Google Scholar]
- 13.Khoor A, Stahlman MT, Johnson JM, Olson SJ, Whitsett JA. Forkhead box A2 transcription factor is expressed in all types of neuroendocrine lung tumors. Hum Pathol. 2004;35:560–564. doi: 10.1016/j.humpath.2004.01.017. [DOI] [PubMed] [Google Scholar]
- 14.Gao N, Le Lay J, Qin W, Doliba N, Schug J, Fox AJ, Smirnova O, Matschinsky FM, Kaestner KH. Foxa1 and Foxa2 maintain the metabolic and secretory features of the mature beta-cell. Mol Endocrinol. 2010;24:1594–1604. doi: 10.1210/me.2009-0513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee CS, Sund NJ, Behr R, Herrera PL, Kaestner KH. Foxa2 is required for the differentiation of pancreatic alpha-cells. Dev Biol. 2005;278:484–495. doi: 10.1016/j.ydbio.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 16.Borges M, Linnoila RI, van de Velde HJ, Chen H, Nelkin BD, Mabry M, Baylin SB, Ball DW. An achaete-scute homologue essential for neuroendocrine differentiation in the lung. Nature. 1997;386:852–855. doi: 10.1038/386852a0. [DOI] [PubMed] [Google Scholar]
- 17.Sund NJ, Ang SL, Sackett SD, Shen W, Daigle N, Magnuson MA, Kaestner KH. Hepatocyte nuclear factor 3beta (Foxa2) is dispensable for maintaining the differentiated state of the adult hepatocyte. Mol Cell Biol. 2000;20:5175–5183. doi: 10.1128/mcb.20.14.5175-5183.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mirosevich J, Gao N, Matusik RJ. Expression of Foxa transcription factors in the developing and adult murine prostate. Prostate. 2005;62:339–352. doi: 10.1002/pros.20131. [DOI] [PubMed] [Google Scholar]
- 19.Gao N, Zhang J-F, Rao MA, Case TC, Mirosevich J, Wang Y, Jin R, Gupta A, Rennie PS, Matusik RJ. The role of hepatocyte nuclear factor-3{alpha} (Foxa1) and androgen receptor in transcriptional regulation of prostatic genes. Mol Endocrinol. 2003;17:1484–1507. doi: 10.1210/me.2003-0020. [DOI] [PubMed] [Google Scholar]
- 20.Gao N, Ishii K, Mirosevich J, Kuwajima S, Oppenheimer SR, Roberts RL, Jiang M, Yu X, Shappell SB, Caprioli RM, Stoffel M, Hayward SW, Matusik RJ. Forkhead box A1 regulates prostate ductal morphogenesis and promotes epithelial cell maturation. Development. 2005;132:3431–3443. doi: 10.1242/dev.01917. [DOI] [PubMed] [Google Scholar]
- 21.DeGraff DJ, Yu X, Sun Q, Mirosevich J, Jin RJ, Wang Y-Q, Gupta A, Nandana S, Case TC, Paul M, Huang H-Y, Shapiro E, Logan SK, Suzuki K, Orgebin-Crist MC, Matusik RJ. The role of Foxa proteins in the regulation of the androgen receptor activity. In: Tindall DJ, Mohler JL, editors. Androgen action in prostate cancer. New York: Springer Science and Business Media; 2009. pp. 587–615. [Google Scholar]
- 22.Li Z, Tuteja G, Schug J, Kaestner KH. Foxa1 and Foxa2 are essential for sexual dimorphism in liver cancer. Cell. 2012;148:72–83. doi: 10.1016/j.cell.2011.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bort R, Zaret K. Paths to the pancreas. Nat Genet. 2002;32:85–86. doi: 10.1038/ng0902-85. [DOI] [PubMed] [Google Scholar]
- 24.Greenberg NM, DeMayo FJ, Sheppard PC, Barrios R, Lebovitz M, Finegold M, Angelopoulou R, Dodd JG, Duckworth ML, Rosen JM, Matusik RJ. The rat probasin gene promoter directs hormonally and developmentally regulated expression of a heterologous gene specifically to the prostate in transgenic mice. Mol Endocrinol. 1994;8:230–239. doi: 10.1210/mend.8.2.8170479. [DOI] [PubMed] [Google Scholar]
- 25.Yu X, Wang Y-Q, Jiang M, Bierie BB, Hayward SW, Shen MM, Taketo MM, Wills M, Matusik RJ. Activated beta-catenin in mouse prostate causes HGPIN and continuous prostate growth after castration. Prostate. 2009;69:249–262. doi: 10.1002/pros.20877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu X, Wang Y, DeGraff DJ, Wills ML, Matusik RJ. Wnt/beta-Catenin activation promotes prostate tumor progression in a mouse model. Oncogene. 2011;30:1868–1879. doi: 10.1038/onc.2010.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mehta V, Abler LL, Keil KP, Schmitz CT, Joshi PS, Vezina CM. Atlas of Wnt and R-spondin gene expression in the developing male mouse lower urogenital tract. Dev Dyn. 2011;240:2548–2560. doi: 10.1002/dvdy.22741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu X, Daniels G, Shapiro E, Xu K, Huang H, Li Y, Logan S, Greco MA, Peng Y, Monaco ME, Melamed J, Lepor H, Grishina I, Lee P. LEF1 identifies androgen-independent epithelium in the developing prostate. Mol Endocrinol. 2011;25:1018–1026. doi: 10.1210/me.2010-0513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Qi J, Nakayama K, Cardiff RD, Borowsky AD, Kaul K, Wil-liams R, Krajewski S, Mercola D, Carpenter PM, Bowtell D, Ronai ZA. Siah2-dependent concerted activity of HIF and FoxA2 regulates formation of neuroendocrine phenotype and neuroendocrine prostate tumors. Cancer Cell. 2010;18:23–38. doi: 10.1016/j.ccr.2010.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]