Abstract
Background
Obesity and diabetes mellitus are associated with an increased risk of pancreatic cancer. These associations may be secondary to consequences of peripheral insulin resistance, pancreatic β-cell dysfunction, or hyperglycemia itself. Hemoglobin A1c (HbA1c) is a measure of hyperglycemia, whereas plasma insulin and proinsulin are markers of peripheral insulin resistance, and the proinsulin to insulin ratio marks pancreatic β-cell dysfunction.
Methods
This was a prospective, nested case-control study of 449 case patients and 982 control subjects with prediagnostic blood samples and no diabetes history from five prospective US cohorts followed through 2008. Two or three control subjects were matched to each case patient by year of birth, cohort, smoking, and fasting status. Pancreatic cancer risk was assessed by prediagnostic HbA1c, insulin, proinsulin, and proinsulin to insulin ratio with multivariable-adjusted logistic regression. All P values were two-sided.
Results
The highest vs lowest quintiles of HbA1c, insulin, and proinsulin were associated with with an increased risk for pancreatic cancer (odds ratio [OR] = 1.79; 95% confidence interval [CI] = 1.17 to 2.72, P trend = .04 for HbA1c; OR = 1.57; 95% CI = 1.08 to 2.30; Ptrend = .002 for insulin; and OR = 2.22; 95% CI = 1.50 to 3.29; P trend < .001 for proinsulin). Proinsulin to insulin ratio was not associated with pancreatic cancer risk. Results were similar across studies (all P heterogeneity > .29). In cancers developing 10 or more years after blood collection, the associations with insulin and proinsulin became stronger (highest vs lowest quintile, OR = 2.77; 95% CI = 1.28 to 5.99 for insulin and OR = 3.60; 95% CI = 1.68 to 7.72 for proinsulin). In mutually adjusted models including HbA1c, insulin, and proinsulin, only proinsulin remained statistically significant ( highest vs lowest quintile, OR = 2.55; 95% CI = 1.54 to 4.21; Ptrend < .001).
Conclusions
Among participants from five large prospective cohorts, circulating markers of peripheral insulin resistance, rather than hyperglycemia or pancreatic β-cell dysfunction, were independently associated with pancreatic cancer risk.
Pancreatic cancer is the fourth-leading cause of cancer-related death in the United States (1). Among individuals who develop pancreatic adenocarcinoma, only 5% will survive five years after diagnosis, and most patients live for less than 12 months (2). A better understanding of predisposing factors is greatly needed to improve outcomes for patients with this highly lethal malignancy.
The risk of pancreatic cancer is increased among individuals with glucose intolerance, including those with excess weight (3–5), diabetes mellitus (6), and high serum glucose (7–9). Nevertheless, this increased risk with high glucose levels may be due to consequences of hyperinsulinemia, abnormal pancreatic β-cell function, hyperglycemia itself, or a combination of these (9–12). Furthermore, concern remains that observed glucose intolerance may be the result of subclinical cancer, rather than an etiologic factor (13).
Hemoglobin A1c (HbA1c) reflects an individual’s mean plasma glucose over approximately the preceding three months and therefore is a direct measure of chronic glucose exposure. The American Diabetes Association (14) and International Expert Committee (15) have advocated measurement of HbA1c as the preferred approach to the diagnosis and management of hyperglycemia and diabetes mellitus, rather than fasting or postload plasma glucose. The favorable features of HbA1c cited by these groups include stability after collection, low within-individual variability, and no preparation necessary prior to measurement, such as with an overnight fast or ingestion of an oral glucose load. However, measurement of HbA1c does not provide information on the relative contributions of peripheral insulin resistance and pancreatic β-cell dysfunction to hyperglycemia.
In secretory granules within pancreatic β-cells, proinsulin is cleaved to liberate insulin for secretion into circulation. A proportion of proinsulin molecules remain uncleaved when granules fuse with the β-cell membrane, such that proinsulin also enters the circulation. However, with insulin resistance and pancreatic β-cell dysfunction, circulating insulin, proinsulin, and the ratio of proinsulin to insulin are altered (16). Notably, production of insulin and proinsulin increase with increasing insulin resistance to compensate for impaired peripheral insulin signaling (10). In contrast, the proinsulin to insulin ratio is relatively stable in states of peripheral insulin resistance, but increases as pancreatic β-cell secretory capacity declines, due to impaired insulin processing by the β-cell mass (17–19). In sum, disproportionate elevation in circulating proinsulin compared to insulin acts as a marker of impaired β-cell function, while elevated circulating insulin and proinsulin mark peripheral insulin resistance.
To prospectively evaluate markers of glycemia, peripheral insulin resistance, and impaired β-cell function in relation to pancreatic cancer risk, we measured circulating prediagnostic HbA1c, insulin, proinsulin, and proinsulin to insulin ratio among male and female participants from five large, US cohort studies with plasma samples collected prior to cancer diagnosis. To address the issue of preclinical cancer leading to glucose intolerance, we performed analyses with exclusion of case patients diagnosed within two, five, or 10 years of blood collection.
Methods
Study Population
Our study population included patients with pancreatic cancer and control subjects from five prospective studies: Health Professionals Follow-up Study (HPFS), Nurses’ Health Study (NHS), Physicians’ Health Study (PHS), Women’s Health Initiative–Observational Study (WHI-OS), and Women’s Health Study (WHS). HPFS was initiated in 1986 when 51529 US men aged 40–75 years working in health professions completed a mailed questionnaire (20). NHS was initiated in 1976 when 121 700 female nurses aged 30–55 years completed a mailed questionnaire (21). Participants have responded to biennial questionnaires. PHS is a completed trial initiated in 1982 of aspirin and β-carotene among 22071 male physicians, aged 40–84 years. After trial completion, participants were followed as an observational cohort (22). WHI-OS consists of 93676 postmenopausal women, aged 50–79 years, enrolled 1994–1998 at 40 US clinical centers (23,24). Participants completed a baseline clinic visit and annual mailed questionnaires. WHS is a completed trial initiated in 1992 of aspirin and vitamin E among 39876 female health professionals aged ≥45 years. After trial closure, 88% of participants continued as an observational cohort (25). The current study was approved by the Human Research Committee at Brigham and Women’s Hospital, Boston, MA, and participants provided informed consent.
We included incident pancreatic adenocarcinoma cases diagnosed through 2008 with available plasma and no prior cancer except nonmelanoma skin cancer. Control subjects were alive without cancer at the case patient’s diagnosis and provided blood samples. We randomly selected two or three control subjects per case patient, matching by cohort (also matches by sex), year of birth (±5 years), smoking status (never, past, current, missing), and fasting status (<8 hours, ≥8 hours).
Case patients were identified by self-report or death follow-up. Deaths were ascertained from next of kin, postal service, or National Death Index; this method captures greater than 98% of deaths (26). Medical records were reviewed by physicians blinded to exposure data. The initial dataset included 478 case patients and 1075 control subjects. We excluded 122 participants who reported a diabetes history at blood collection (29 case patients, 38 control subjects, and 55 control subjects matched to excluded case patients). For insulin, the assay failed for 2 case patients and 1 control subject. For proinsulin, the assay failed for 1 case patient and 3 control subjects. Stored red cells were available for HbA1c measurement in 428 case patients and 927 control subjects. Covariates were obtained from baseline questionnaires in PHS, WHI, and WHS and questionnaires prior to blood draw in HPFS and NHS, as described previously (27).
Plasma Samples
Blood samples were collected from 18225 men in HPFS from 1993 to 1995, 32826 women in NHS from 1989 to 1990, 14916 men in PHS from 1982 to 1984, 93676 women in WHI from 1994 to 1998, and 28345 women in WHS from 1992 to 1995. Details on blood collection procedures have been described previously (HPFS (28), NHS (29), PHS (30), WHI (31), WHS (32)).
HbA1c was assayed by the laboratory of Dr Nader Rifai (Children’s Hospital, Boston, MA) using reagents from Roche Diagnostics (Indianapolis, IN). Plasma insulin and proinsulin were assayed by the laboratory of Dr Michael Pollak (McGill University, Montreal, Canada). To calculate the proinsulin to insulin ratio, we multiplied insulin measurements by 6.945 to convert units to pM (1 µIU/mL = 6.945 pM) and then divided proinsulin by insulin. Plasma insulin was measured in two batches. The first batch included 140 case patients and 243 control subjects, using reagents from Diagnostic Systems Laboratory (Webster, TX), as previously described (33). The second batch included 307 case patients and 734 control subjects, using reagents from Millipore Corporation (Billerica, MA). Plasma proinsulin was measured in a single batch, using reagents from Millipore Corporation. Intact insulin assays were non-cross-reactive with proinsulin. The total proinsulin assay had 100% cross-reactivity with intact proinsulin and des-31,32 proinsulin, 81% cross-reactivity to des-64,65 proinsulin, and no cross-reactivity with insulin. Blinded, randomly inserted samples from quality control plasma pools had mean intra-assay coefficients of variance of 2.0% for HbA1c, 5.4% for insulin, and 3.1% for proinsulin. For measurements below the limit of assay detection (less than 2 µIU/mL for insulin and less than 2 pM for proinsulin), participants were assigned 1 µIU/mL for insulin and 1 pM for proinsulin.
Statistical Analysis
To compare baseline characteristics, we used χ2 test for categorical variables, paired t test for continuous variables, and nonparametric Wilcoxon signed-rank test for plasma markers, as they were not normally distributed. Partial Spearman correlation coefficients adjusted for batch, fasting status, and cohort were calculated for plasma markers and covariates. Quintiles of plasma insulin and proinsulin to insulin ratio were defined by batch (1/2) and fasting status (≥8 hours/<8 hours), based on levels in control subjects. Quintiles of plasma proinsulin were defined by fasting status (≥8 hours/<8 hours) based on levels in control subjects, as proinsulin was measured in a single batch. Quintiles of HbA1c were defined among all control subjects together, as fasting status is not relevant to HbA1c measurement (15).
In primary analyses, we pooled participant-level data from quintile groups and computed odds ratios (ORs) to estimate relative risks and 95% confidence intervals [CIs] using unconditional logistic regression. We used unconditional regression because one of two control subjects was newly selected for 76 case patients from WHI. We also performed conditional logistic regression and meta-analysis of quintile groupings using a random effects model (34), to confirm results with different analytic approaches. Tests for trend were calculated by entering pooled quintile-specific medians for the analyte as a continuous variable in regression models. We also evaluated a “global” trend test by including the log-transformed analyte as a continuous variable in regression models.
In our base model, we adjusted for matching factors. We evaluated covariates for confounding by adding each covariate in turn to the base model. No covariate changed the risk estimate by greater than 10%, including race, body mass index (BMI), multivitamin use, physical activity, alcohol, and total calories. As no strong confounders were identified, we included race and BMI in our multivariable-adjusted models, as in prior analyses (27,33,35).
To evaluate whether subclinical malignancy influenced our results, we performed analyses with exclusion of case patients diagnosed within two, five, or 10 years of blood collection and their matched control subjects. We assessed heterogeneity across the five cohorts using Cochran’s Q statistic (36), which was 0.29, 0.38, 0.67, and 0.93 for HbA1c, insulin, proinsulin, and proinsulin to insulin ratio, respectively, for comparison of extreme quintiles. We also conducted a meta-analysis of individual study data, in which we calculated odds ratios for each cohort and then pooled the cohort-specific odds ratios to compute a summary risk estimate (34). Given limited case patient and control subject numbers in individual cohorts, we evaluated collapsed quintiles 2–5 vs quintile 1 (referent) for HbA1c, and quintile 5 vs collapsed quintiles 1–4 (referent) for insulin, proinsulin, and proinsulin to insulin ratio.
In exploratory analyses, we evaluated associations of circulating markers with pancreatic cancer risk stratified by other predisposing factors, using the condensed quintiles. We assessed statistical interaction by entering main effect terms and a cross-product term of the binary analyte and stratification variable into the model, evaluating likelihood ratio tests. We also used receiver operating characteristic (ROC) curve analysis to calculate the area under the curve, known as the concordance statistic, for our base model and after addition of the circulating markers (37). All statistical analyses were performed with the SAS 9.2 statistical package (SAS Institute). A P value of less than .05 was considered statistically significant. All P values are two-sided.
Results
Characteristics of 449 pancreatic cancer case patients and 982 matched control subjects are shown in Table 1 and by cohort in Supplementary Table 1 (available online). Median levels of HbA1c, insulin, and proinsulin were higher in case patients than in control subjects, whereas the plasma proinsulin to insulin ratio was similar. Partial Spearman correlation coefficients among control subjects for BMI and circulating analytes were 0.14 for HbA1c, 0.36 for insulin, 0.43 for proinsulin, and –0.09 for proinsulin to insulin ratio (Supplementary Table 2, available online).
Table 1.
Baseline characteristics of nested pancreatic cancer case patients and matched control subjects*
| Characteristic | Case patients | Control subjects | P† |
|---|---|---|---|
| No. of subjects | 449 | 982 | |
| Age, y | 63.1 (8.4) | 62.5 (8.5) | — |
| Female sex, % | 71.5 | 70.7 | — |
| Race/ethnicity, % | <.001 | ||
| White | 88.6 | 92.6 | |
| Black | 3.3 | 1.5 | |
| Other | 3.4 | 5.2 | |
| Missing | 4.7 | 0.7 | |
| Height, inches | 66.1 (3.5) | 66.2 (3.7) | .81 |
| Body mass index, kg/m2 | 26.4 (5.1) | 25.8 (4.4) | .03 |
| Physical activity, MET-h/wk | 17. 9 (22.4) | 20.0 (25.9) | .15 |
| Tobacco use, % | — | ||
| Never | 40.8 | 42.6 | |
| Past | 45.6 | 44.4 | |
| Current | 12.9 | 12.2 | |
| Missing | 0.7 | 0.8 | |
| Regular multivitamin use, % | 43.9 | 40.2 | .19 |
| Total calories, kcal/d‡ | 1664 (664) | 1716 (608) | .18 |
| Alcohol (≥1 drink/d), % | 25.4 | 22.4 | .22 |
| Fasting samples (≥8h) | 76.4 | 76.9 | — |
| Hemoglobin A1c, % (median) | 5.11 | 5.09 | .03 |
| Plasma markers | |||
| Median fasting levels | |||
| Insulin, µIU/mL | 5.71 | 4.63 | <.001 |
| Proinsulin, pM | 8.07 | 7.09 | <.001 |
| Proinsulin to insulin ratio, pM/pM | 0.24 | 0.24 | .85 |
| Median nonfasting levels | |||
| Insulin, µIU/mL | 9.08 | 6.32 | .07 |
| Proinsulin, pM | 17.2 | 13.7 | .006 |
| Proinsulin to insulin ratio, pM/pM | 0.35 | 0.31 | .46 |
* Continuous variables presented as mean (SD).
HPFS = Health Professionals Follow-up Study; MET-h/wk = metabolic equivalent task-hours per week; NHS = Nurses’ Health Study; PHS = Physicians’ Health Study; WHI = Women’s Health Initiative; WHS = Women’s Health Study.
† Case patients and control subjects matched on year of birth, cohort (HPFS, NHS, PHS, WHI, WHS; which also matches for sex), smoking status (never, past, current), and fasting status (<8 hours, ≥ 8 hours). P values calculated by χ2 test for categorical variables, paired t test for continuous variables, and nonparametric Wilcoxon signed-rank test for plasma markers, as they were not normally distributed. All P values were two-sided.
‡ Data not available for PHS participants.
Multivariable-adjusted global trend tests calculated by including the log-transformed circulating markers in pooled logistic regression models were .004 for HbA1c, less than .001 for insulin, and less than .001 for proinsulin (Table 2). Compared to the bottom quintile of HbA1c, those in the top quintile had an OR of 1.79 (95% CI = 1.17 to 2.72). Conditional regression provided similar results, with an OR of 1.83 (95% CI = 1.17 to 2.86), comparing extreme quintiles. Compared to the bottom quintile of plasma insulin, those in the top quintile had an OR of 1.57 (95% CI = 1.08 to 2.30). Conditional regression and meta-analysis of the quintile groupings provided similar results, with ORs of 1.43 (95% CI = 0.98 to 2.09) and 1.57 (95% CI = 0.91 to 2.71), respectively, comparing extreme quintiles, although the lower 95% confidence intervals crossed one. Compared to the bottom quintile of proinsulin, those in the top quintile had an OR of 2.22 (95% CI = 1.50 to 3.29). Conditional regression and meta-analysis of the quintile groupings demonstrated similar results with ORs of 2.21 (95% CI = 1.48 to 3.29) and 2.39 (95% CI = 1.25 to 4.59), respectively, comparing extreme quintiles.
Table 2.
Odds of pancreatic cancer by quintile of hemoglobin A1c, plasma insulin, plasma proinsulin, and plasma proinsulin to insulin ratio*
| Risk marker | Quintile of circulating marker | P trend† | ||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| Hemoglobin A1c | ||||||
| Median, % | 4.77 | 4.95 | 5.09 | 5.24 | 5.50 | |
| No. of cases/controls | 61/185 | 92/184 | 101/185 | 74/188 | 100/185 | |
| Base model, OR (95% CI)‡ | 1.0 | 1.61 (1.09 to 2.38) | 1.83 (1.24 to 2.69) | 1.36 (0.90 to 2.06) | 1.85 (1.23 to 2.77) | .02 |
| Adjusted model, OR (95% CI)§ | 1.0 | 1.59 (1.07 to 2.36) | 1.82 (1.22 to 2.70) | 1.36 (0.89 to 2.07) | 1.79 (1.17 to 2.72) | .04 |
| Insulin | ||||||
| Median, µIU/mL | 1.0 | 3.33 | 4.69 | 9.11 | 17.2 | |
| No. of cases/controls | 89/211 | 75/192 | 72/191 | 88/193 | 123/190 | |
| Base model, OR (95% CI)‡ | 1.0 | 0.98 (0.68 to 1.41) | 0.94 (0.64 to 1.37) | 1.14 (0.79 to 1.65) | 1.61 (1.14 to 2.29) | <.001 |
| Adjusted model, OR (95% CI)§ | 1.0 | 0.92 (0.63 to 1.35) | 0.94 (0.64 to 1.38) | 1.13 (0.77 to 1.66) | 1.57 (1.08 to 2.30) | .002 |
| Proinsulin | ||||||
| Median, pM | 3.69 | 5.55 | 7.35 | 10.5 | 22.0 | |
| No. of cases/controls | 67/195 | 79/196 | 80/195 | 78/196 | 144/195 | |
| Base model, OR (95% CI)‡ | 1.0 | 1.18 (0.80 to 1.74) | 1.21 (0.82 to 1.78) | 1.17 (0.79 to 1.73) | 2.18 (1.52 to 3.13) | <.001 |
| Adjusted model, OR (95% CI)§ | 1.0 | 1.18 (0.80 to 1.74) | 1.23 (0.83 to 1.82) | 1.20 (0.80 to 1.80) | 2.22 (1.50 to 3.29) | <.001 |
| Proinsulin to insulin ratio | ||||||
| Median, pM/pM | 0.12 | 0.20 | 0.27 | 0.39 | 0.72 | |
| No. of cases/controls | 78/193 | 82/195 | 85/195 | 118/195 | 83/194 | |
| Base model, OR (95% CI)‡ | 1.0 | 1.02 (0.71 to 1.48) | 1.05 (0.73 to 1.52) | 1.45 (1.02 to 2.07) | 1.02 (0.70 to 1.48) | .76 |
| Adjusted model, OR (95% CI)§ | 1.0 | 1.01 (0.69 to 1.48) | 1.05 (0.72 to 1.53) | 1.39 (0.97 to 2.00) | 1.03 (0.70 to 1.51) | .71 |
* CI = confidence interval; HPFS = Health Professionals Follow-up Study; NHS = Nurses’ Health Study; OR = odds ratio; PHS = Physicians’ Health Study; WHI = Women’s Health Initiative; WHS = Women’s Health Study.
† Test for trend calculated by entering quintile-specific median values for circulating marker as a continuous variable in logistic regression models. All P values were two-sided.
‡ Adjusted for matching factors: prospective cohort (HPFS, NHS, PHS, WHI, WHS; which also adjusted for sex), smoking status (never, past, current, missing), fasting time (<4, 4–8, 8–12, ≥12 hours, missing) and age at blood collection (continuous)
§ Adjusted for prospective cohort (HPFS, NHS, PHS, WHI, WHS; which also adjusted for sex), smoking status (never, past, current <25 cigarettes/day, current ≥25 cigarettes/day, missing), fasting time (<4, 4–8, 8–12, ≥12 hours, missing) age at blood collection (continuous), race (white, black, other, missing) and body mass index (<18.5, 18.5–24.9, 25–29.9, ≥30kg/m2).
For plasma proinsulin to insulin ratio, the multivariable-adjusted global test for trend was .66 (Table 2). Compared to the bottom quintile, those in the top quintile had an OR of 1.03 (95% CI = 0.70 to 1.51). Similar non–statistically significant results were noted for conditional regression and meta-analysis of the quintile groupings.
The association between insulin, proinsulin, and pancreatic cancer risk became more pronounced after limiting inclusion to cases (and their matched controls) with greater time between blood collection and pancreatic cancer diagnosis (Table 3), but were not statistically significant for HbA1c and the proinsulin to insulin ratio. Among case patients who developed cancer 10 or more years after blood collection and their matched control subjects, the fifth compared to first quintile had an OR of 2.77 (95% CI = 1.28 to 5.99; Ptrend = .004) for insulin and 3.60 (95% CI = 1.68 to 7.72; Ptrend < .001) for proinsulin.
Table 3.
Odds of pancreatic cancer by quintile of plasma markers and time between blood collection and diagnosis*
| Years between plasma collection and cancer diagnosis† | No. of cases/controls | OR (95% CI) for quintile of plasma marker‡ | P trend§ | ||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |||
| Insulin | |||||||
| ≥2 y | 405/889 | 1.0 | 1.13 (0.75 to 1.69) | 1.20 (0.79 to 1.82) | 1.51 (1.00 to 2.28) | 2.00 (1.32 to 3.00) | <.001 |
| ≥5 y | 296/655 | 1.0 | 1.15 (0.72 to 1.85) | 1.13 (0.69 to 1.83) | 1.24 (0.75 to 2.03) | 1.93 (1.19 to 3.15) | .003 |
| ≥10 y | 129/295 | 1.0 | 1.33 (0.62 to 2.85) | 1.41 (0.65 to 3.06) | 2.51 (1.19 to 5.30) | 2.77 (1.28 to 5.99) | .004 |
| Proinsulin | |||||||
| ≥2 y | 406/890 | 1.0 | 1.21 (0.80 to 1.83) | 1.24 (0.81 to 1.89) | 1.36 (0.88 to 2.08) | 2.28 (1.49 to 3.48) | <.001 |
| ≥5 y | 296/656 | 1.0 | 1.16 (0.72 to 1.89) | 1.16 (0.71 to 1.89) | 1.02 (0.61 to 1.71) | 2.29 (1.41 to 3.72) | <.001 |
| ≥10 y | 130/298 | 1.0 | 1.50 (0.70 to 3.19) | 1.54 (0.72 to 3.30) | 1.26 (0.55 to 2.88) | 3.60 (1.68 to 7.72) | <.001 |
* CI = confidence interval; HPFS = Health Professionals Follow-up Study; NHS = Nurses’ Health Study; OR = odds ratio; PHS = Physicians’ Health Study; WHI = Women’s Health Initiative; WHS = Women’s Health Study.
† For the pancreatic cancer cases in the ≥2 years, ≥5 years, and ≥10 years groups, the median time between blood collection and pancreatic cancer diagnosis was 7.6 years, 9.3 years, and 13.5 years, respectively.
‡ Adjusted for prospective cohort (HPFS, NHS, PHS, WHI, WHS; which also adjusted for sex), smoking status (never, past, current <25 cigarettes/day, current ≥25 cigarettes/day, missing), fasting time (<4, 4–8, 8–12, ≥12 hours, missing) age at blood collection (continuous), race (white, black, other, missing) and body mass index (<18.5, 18.5–24.9, 25–29.9, ≥30kg/m2).
§ Test for trend calculated by entering quintile-specific median values for plasma marker as a continuous variable in logistic regression models. All P values were two-sided.
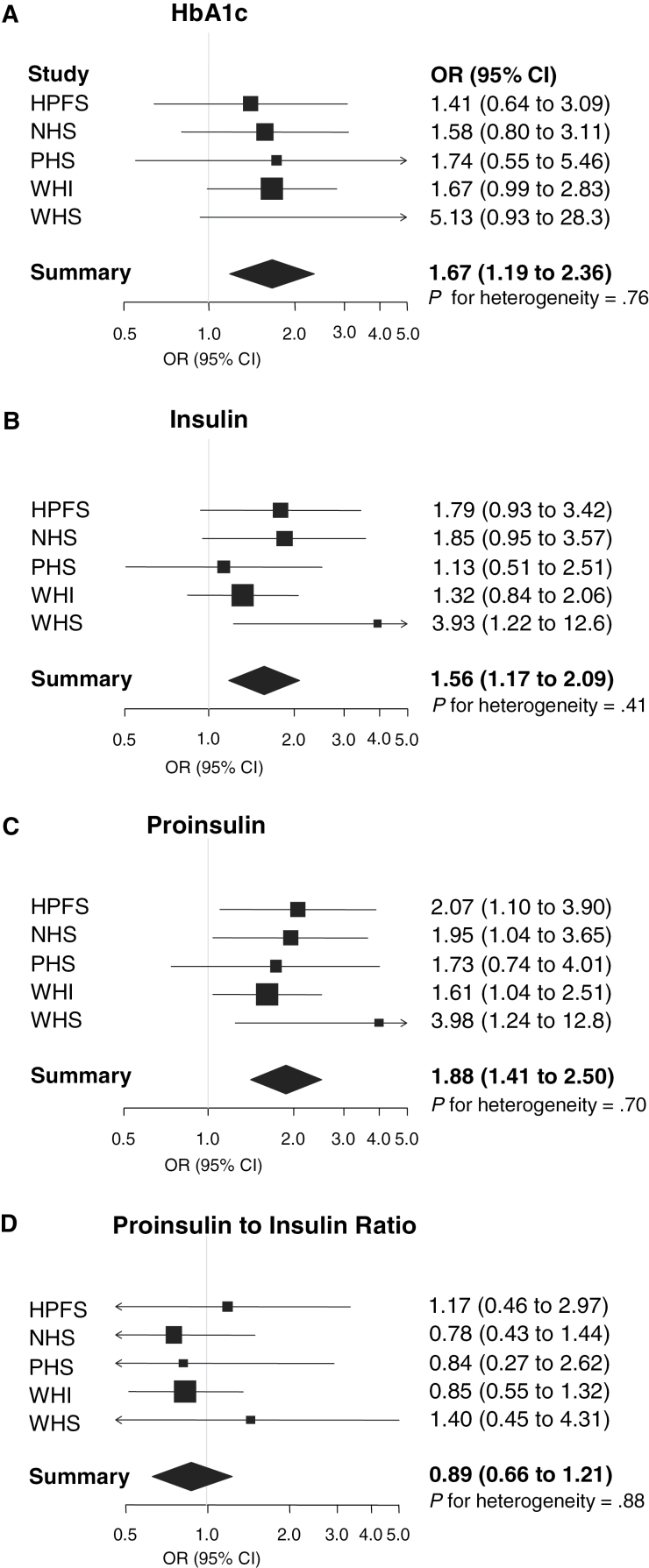
For the four markers, results were similar across the five individual cohorts (Figure 1). No statistically significant interactions of plasma insulin, proinsulin (Table 4), HbA1c, or proinsulin to insulin ratio with pancreatic cancer risk were seen by sex, smoking status, BMI, physical activity, or fasting status.
Figure 1.
Forest plot of odds ratios (ORs) for pancreatic cancer in five prospective cohorts for hemoglobin A1c (HbA1c) (A), insulin (B), proinsulin (C), and proinsulin to insulin ratio (D). The solid squares and horizontal lines correspond to the cohort-specific multivariable-adjusted odds ratios and 95% confidence intervals (CIs). The area of the solid square reflects the cohort-specific weight (inverse of the variance). The diamond represents the pooled multivariable-adjusted odds ratios and 95% confidence intervals. The vertical line indicates an odds ratio of 1.0. Odds ratios adjusted for smoking status (never, past, current <25 cigarettes/day, current ≥25 cigarettes/day, missing), fasting time (<4, 4–8, 8–12, ≥12 hours, missing) age at blood collection (continuous), race (white, nonwhite), and body mass index (<25, 25–29.9, ≥30kg/m2). HPFS = Health Professionals Follow-up Study; NHS = Nurses’ Health Study; PHS = Physicians’ Health Study; WHI = Women’s Health Initiative; WHS = Women’s Health Study.
Table 4.
Odds of pancreatic cancer according to plasma markers stratified by potential risk modifiers*
| Covariates | Insulin | Proinsulin | ||||||
|---|---|---|---|---|---|---|---|---|
| No. of cases/ controls | OR (95% CI) for quintiles† | P interaction‡ | No. of cases/ controls | OR (95% CI) for quintiles† | P interaction‡ | |||
| 1–4 | 5 | 1–4 | 5 | |||||
| Sex | .89 | .73 | ||||||
| Female | 319/689 | 1.0 | 1.59 (1.12 to 2.25) | 320/691 | 1.0 | 1.80 (1.29 to 2.52) | ||
| Male | 128/288 | 1.0 | 1.59 (0.96 to 2.65) | 128/286 | 1.0 | 2.21 (1.32 to 3.69) | ||
| Smoking status | .77 | .23 | ||||||
| Never | 182/417 | 1.0 | 1.48 (0.95 to 2.29) | 183/417 | 1.0 | 2.30 (1.49 to 3.56) | ||
| Ever | 262/553 | 1.0 | 1.66 (1.14 to 2.42) | 262/552 | 1.0 | 1.73 (1.19 to 2.50) | ||
| Body mass index | .34 | .46 | ||||||
| <25kg/m2 | 204/473 | 1.0 | 1.85 (1.13 to 3.03) | 205/473 | 1.0 | 2.19 (1.30 to 3.71) | ||
| ≥25kg/m2 | 243/504 | 1.0 | 1.46 (1.04 to 2.05) | 243/504 | 1.0 | 1.83 (1.32 to 2.54) | ||
| Physical activity§ | .85 | .59 | ||||||
| <12.6 MET-h/wk | 242/485 | 1.0 | 1.69 (1.16 to 2.48) | 242/488 | 1.0 | 1.85 (1.27 to 2.68) | ||
| ≥12.6 MET-h/wk | 205/492 | 1.0 | 1.47 (0.96 to 2.26) | 206/489 | 1.0 | 1.98 (1.29 to 3.05) | ||
| Fasting time | .42 | .59 | ||||||
| <8 h | 106/227 | 1.0 | 1.32 (0.74 to 2.36) | 106/227 | 1.0 | 2.40 (1.39 to 4.17) | ||
| ≥8 h | 341/750 | 1.0 | 1.59 (1.16 to 2.19) | 342/750 | 1.0 | 1.72 (1.25 to 2.36) | ||
* CI = confidence interval; HPFS = Health Professionals Follow-up Study; MET-h/wk = metabolic equivalent task-hours per week; NHS = Nurses’ Health Study; OR = odds ratio; PHS = Physicians’ Health Study; WHI = Women’s Health Initiative; WHS = Women’s Health Study.
† Adjusted for prospective cohort (HPFS, NHS, PHS, WHI, WHS; which also adjusted for sex), smoking status (never, past, current <25 cigarettes/day, current ≥25 cigarettes/day, missing), fasting time (<4, 4–8, 8–12, ≥12 hours, missing) age at blood collection (continuous), race (white, black, other, missing) and body mass index (<18.5, 18.5–24.9, 25–29.9, ≥30kg/m2).
‡ P values for interaction were calculated by including a cross-product term of the binary analyte and stratification variable in the regression model and evaluating likelihood ratio tests. All P values were two-sided.
§ Stratified at the median of physical activity among control subjects.
Finally, we conducted a multivariable model with mutual adjustment for HbA1c, insulin, and proinsulin to assess the independent effects of these markers of hyperglycemia and insulin resistance. Following mutual adjustment, the association of pancreatic cancer risk with proinsulin remained strong, whereas the associations with HbA1c and insulin were no longer statistically significant (Table 5). Comparing extreme quintiles in the mutually adjusted multivariate model, the OR was 1.30 (95% CI = 0.84 to 2.02; Ptrend = .66) for HbA1c, 0.93 (95% CI = 0.58 to 1.48; Ptrend = .69) for insulin, and 2.55 (95% CI = 1.54 to 4.21; Ptrend < .001) for proinsulin. ROC curve analysis also suggested that the discriminatory ability of our circulating markers was predominantly due to the addition of plasma proinsulin to our base model (Supplementary Table 3, available online).
Table 5.
Odds of pancreatic cancer by quintile of hemoglobin A1c, plasma insulin, and plasma proinsulin in mutually adjusted logistic regression model*
| Risk marker | Quintile of circulating marker OR (95% CI)† | P trend‡ | ||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| Hemoglobin A1c | 1.0 | 1.48 (1.00 to 2.21) | 1.61 (1.08 to 2.41) | 1.17 (0.76 to 1.80) | 1.30 (0.84 to 2.02) | .66 |
| Insulin | 1.0 | 0.90 (0.61 to 1.34) | 0.67 (0.43 to 1.03) | 0.73 (0.47 to 1.14) | 0.93 (0.58 to 1.48) | .69 |
| Proinsulin | 1.0 | 1.21 (0.80 to 1.83) | 1.47 (0.95 to 2.27) | 1.43 (0.90 to 2.29) | 2.55 (1.54 to 4.21) | <.001 |
* CI = confidence interval; HbA1c = hemoglobin A1c; HPFS = Health Professionals Follow-up Study; NHS = Nurses’ Health Study; OR = odds ratio; PHS = Physicians’ Health Study; WHI = Women’s Health Initiative; WHS = Women’s Health Study.
† Adjusted for prospective cohort (HPFS, NHS, PHS, WHI, WHS; which also adjusted for sex), smoking status (never, past, current <25 cigarettes/day, current ≥25 cigarettes/day, missing), fasting time (<4, 4–8, 8–12, ≥12 hours, missing) age at blood collection (continuous), race (white, black, other, missing) and body mass index (<18.5, 18.5–24.9, 25–29.9, ≥30kg/m2).
‡ Test for trend calculated by entering quintile-specific median values for circulating marker as a continuous variable in logistic regression models. All P values were two-sided.
Discussion
In this pooled, nested case-control study from five large, prospective US cohorts, we noted statistically significant positive associations between risk of pancreatic cancer and prediagnostic circulating markers of hyperglycemia (HbA1c) and peripheral insulin resistance (plasma insulin and proinsulin). In contrast, the plasma proinsulin to insulin ratio, a marker of impaired β-cell function, was not associated with pancreatic cancer risk in our cohort participants. When evaluated in joint models, high levels of plasma proinsulin (a marker of peripheral insulin resistance) were associated with a nearly 2.5-fold increase in risk for pancreatic cancer, whereas HbA1c (a marker of hyperglycemia) was no longer associated with risk. Notably, the associations of plasma insulin and proinsulin with pancreatic cancer risk became progressively stronger after excluding patients diagnosed with pancreatic cancer within 5 or 10 years of their blood collection. Therefore, the likelihood is low that preclinical cancer led to alterations in peripheral insulin resistance, thereby explaining the observed associations.
Glucose intolerance has emerged as an important predisposing factor for pancreatic adenocarcinoma. Prospective studies have demonstrated an increased risk of pancreatic cancer with increasing BMI (3–5), a predisposing factor for hyperglycemia (10). Although controversy remains regarding causation vs consequence (38), diabetes mellitus has also been associated with pancreatic cancer risk in prospective studies (6). Furthermore, prediagnostic fasting serum glucose and postload plasma glucose have been linked with the subsequent risk of pancreatic cancer (7–9). Nevertheless, a number of mechanisms might underlie the higher risk of pancreatic cancer among individuals with impaired glucose processing, including the consequences of peripheral insulin resistance, impaired pancreatic β-cell function, and/or hyperglycemia itself (9–12).
Prospective studies have reported associations between pancreatic cancer risk and prediagnostic circulating levels of insulin and C-peptide, supporting the hypothesis that peripheral insulin resistance confers an increased risk for this malignancy (33,39). In addition, a recent study with more limited follow-up time noted an elevated risk of pancreatic cancer with higher HbA1c values (9), highlighting the possible role of chronic hyperglycemia in promoting pancreatic tumorigenesis. In the current study with long median follow-up (12.2–25.3 years) and large case numbers with prediagnostic blood samples, we simultaneously measured circulating HbA1c, insulin, proinsulin, and proinsulin to insulin ratio to investigate hyperglycemia, insulin resistance, and impaired β-cell function in relation to pancreatic cancer risk.
HbA1c is a clinically relevant measure of chronic glucose exposure (15). Circulating levels of insulin and proinsulin increase in the setting of peripheral insulin resistance, as higher insulin secretion is necessary to overcome reduced peripheral insulin signaling (10,40,41). However, the proinsulin to insulin ratio is relatively stable in subjects with peripheral insulin resistance, as insulin and proinsulin levels rise largely in tandem. In contrast, β-cell dysfunction leads to impaired processing of proinsulin, with greater proinsulin in secretory granules and therefore a higher ratio of proinsulin to insulin in the circulation (17–19). Therefore, the observed positive associations with insulin and proinsulin, but not the ratio, implicate peripheral insulin resistance rather than impaired β-cell function in the etiology of pancreatic adenocarcinoma. Furthermore, we observed that HbA1c was no longer statistically significantly associated with pancreatic cancer after adjustment for proinsulin, suggesting the importance of peripheral insulin resistance, rather than hyperglycemia, to pancreatic tumorigenesis. Peripheral insulin resistance is associated with alterations in multiple metabolic pathways, and additional research is necessary to better understand which pathways may underlie an increased risk of pancreatic cancer. Of note, proinsulin itself may impact cancer risk, as it has activity at the insulin receptor, with effects on cell proliferation (42,43), or may mark other systemic metabolic alterations not directly referable to states of insulin resistance.
The current study has a number of important strengths. Its prospective design and long follow-up time greatly reduced the likelihood of bias due to reverse causation and allowed a thorough evaluation of possible influences of preclinical disease. We excluded cases and controls with a history of diabetes at blood collection, minimizing the effects of clinical diabetes on insulin secretion and risk. Assays were performed in a single laboratory and blinded to identifiers of case patient and control subject status with excellent coefficients of variance for quality control samples. Our sample size was large for a prospective plasma study of pancreatic cancer, and cases were rigorously coded with confirmation by review of patient records. Covariate data were also rigorously collected, allowing for control of confounding and evaluation of effect modification.
Limitations of the current study included having a single measurement of circulating markers. However, studies with similar design have demonstrated associations between these markers and disease risk, suggesting that a single measurement is a reasonable proxy for longer-term exposure (15,39, 44–46). We did not have other markers of insulin resistance and β-cell dysfunction, such as homeostatic model assessment (HOMA)–IR or HOMA-B. However, multiple studies have demonstrated our measured markers to be valid proxies of insulin resistance and pancreatic β-cell dysfunction (16–19,47,48). We cannot rule out that our findings may be influenced in part by residual confounding. This may be more likely in analyses involving several cohorts, as not all covariates are collected identically. Nonetheless, the studies included in this analysis provided detailed information on known or suspected risk factors for pancreatic cancer and we evaluated multiple possible confounding covariates without observing meaningful changes in our risk estimates. Finally, our participants were predominantly individuals of European descent and further studies in other populations are warranted.
In sum, in this large, nested, prospective case-control study, we demonstrate that after mutual adjustment, markers of peripheral insulin resistance, rather than hyperglycemia or pancreatic β-cell dysfunction, are associated with risk of pancreatic adenocarcinoma. These findings provide important mechanistic insight into the established associations between obesity, type II diabetes mellitus, and pancreatic cancer risk. Furthermore, these findings underscore the value of correcting insulin resistance as a strategy for preventing this highly lethal malignancy.
Funding
The NHS and HPFS are supported by the National Cancer Institute, National Institutes of Health (NIH) (grant numbers P01 CA87969, P01 CA55075, P50 CA127003, R01 CA124908). The PHS is supported by the NIH (grant numbers CA97193, CA34944, CA40360, HL26490, and HL34595). The WHI program is funded by the National Heart, Lung, and Blood Institute, NIH, US Department of Health and Human Services (contract numbers N01WH22110, 24152, 32100–2, 32105–6, 32108–9, 32111–13, 32115, 32118–32119, 32122, 42107–26, 42129–32, and 44221). The WHS is supported by the NIH (grant numbers CA047988, HL043851, and HL080467). BMW is supported by NCI K07 CA140790, an American Society of Clinical Oncology Career Development Award, a Howard Hughes Medical Institute Early Career Physician-Scientist Award, the Lustgarten Foundation, and Promises for Purple. The study sponsors had no role in the design of the study; the collection, analysis, and interpretation of the data; the writing of the manuscript; or the decision to submit the manuscript for publication.
Supplementary Material
The authors thank the participants and staff of the HPFS, NHS, PHS, WHI, and WHS for their valuable contributions as well as the following state cancer registries for their help: AL, AZ, AR, CA, CO, CT, DE, FL, GA, ID, IL, IN, IA, KY, LA, ME, MD, MA, MI, NE, NH, NJ, NY, NC, ND, OH, OK, OR, PA, RI, SC, TN, TX, VA, WA, WY. A full listing of WHI investigators can be found at http://www.whiscience.org/publications/WHI_investigators_shortlist.pdf.
References
- 1. Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013, CA Cancer J Clin. 2013;63(1):11–30 [DOI] [PubMed] [Google Scholar]
- 2. Hidalgo M. Pancreatic cancer. N Engl J Med. 2010;362(17):1605–1617 [DOI] [PubMed] [Google Scholar]
- 3. Michaud DS, Giovannucci E, Willett WC, Colditz GA, Stampfer MJ, Fuchs CS. Physical activity, obesity, height, and the risk of pancreatic cancer. JAMA. 2001;286(8):921–929 [DOI] [PubMed] [Google Scholar]
- 4. Arslan AA, Helzlsouer KJ, Kooperberg C, et al. Anthropometric measures, body mass index, and pancreatic cancer: a pooled analysis from the Pancreatic Cancer Cohort Consortium (PanScan). Arch Intern Med. 2010;170(9):791–802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Li D, Morris JS, Liu J, et al. Body mass index and risk, age of onset, and survival in patients with pancreatic cancer. JAMA. 2009;301(24):2553–2562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Huxley R, Ansary-Moghaddam A, Berrington de Gonzalez A, Barzi F, Woodward M. Type-II diabetes and pancreatic cancer: a meta-analysis of 36 studies. Br J Cancer. 2005;92(11):2076–2083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gapstur SM, Gann PH, Lowe W, Liu K, Colangelo L, Dyer A. Abnormal glucose metabolism and pancreatic cancer mortality. JAMA. 2000;283(19):2552–2558 [DOI] [PubMed] [Google Scholar]
- 8. Jee SH, Ohrr H, Sull JW, Yun JE, Ji M, Samet JM. Fasting serum glucose level and cancer risk in Korean men and women. JAMA. 2005;293(2):194–202 [DOI] [PubMed] [Google Scholar]
- 9. Grote VA, Rohrmann S, Nieters A, et al. Diabetes mellitus, glycated haemoglobin and C-peptide levels in relation to pancreatic cancer risk: a study within the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort. Diabetologia. 2011;54(12):3037–3046 [DOI] [PubMed] [Google Scholar]
- 10. Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444(7121):840–846 [DOI] [PubMed] [Google Scholar]
- 11. Chari ST, Zapiach M, Yadav D, Rizza RA. Beta-cell function and insulin resistance evaluated by HOMA in pancreatic cancer subjects with varying degrees of glucose intolerance. Pancreatology. 2005;5(2–3):229–233 [DOI] [PubMed] [Google Scholar]
- 12. Jiao L, Weinstein SJ, Albanes D, et al. Evidence that serum levels of the soluble receptor for advanced glycation end products are inversely associated with pancreatic cancer risk: a prospective study. Cancer Res. 2011;71(10):3582–3589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pannala R, Basu A, Petersen GM, Chari ST. New-onset diabetes: a potential clue to the early diagnosis of pancreatic cancer. Lancet Oncol. 2009;10(1):88–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Standards of medical care in diabetes--2011. Diabetes Care. 2011;34 (suppl 1):S11–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care. 2009;32(7):1327–1334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Uchizono Y, Alarcon C, Wicksteed BL, Marsh BJ, Rhodes CJ. The balance between proinsulin biosynthesis and insulin secretion: where can imbalance lead?. Diabetes Obes Metab. 2007;9(suppl 2):56–66 [DOI] [PubMed] [Google Scholar]
- 17. Roder ME, Porte D, Jr., Schwartz RS, Kahn SE. Disproportionately elevated proinsulin levels reflect the degree of impaired B cell secretory capacity in patients with noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab. 1998;83(2):604–608 [DOI] [PubMed] [Google Scholar]
- 18. Mykkanen L, Zaccaro DJ, Hales CN, Festa A, Haffner SM. The relation of proinsulin and insulin to insulin sensitivity and acute insulin response in subjects with newly diagnosed type II diabetes: the Insulin Resistance Atherosclerosis Study. Diabetologia. 1999;42(9):1060–1066 [DOI] [PubMed] [Google Scholar]
- 19. Wang PW, Abbasi F, Carantoni M, Chen YD, Azhar S, Reaven GM. Insulin resistance does not change the ratio of proinsulin to insulin in normal volunteers. J Clin Endocrinol Metab. 1997;82(10):3221–3224 [DOI] [PubMed] [Google Scholar]
- 20. Giovannucci E, Ascherio A, Rimm EB, Colditz GA, Stampfer MJ, Willett WC. Physical activity, obesity, and risk for colon cancer and adenoma in men. Ann Intern Med. 1995;122(5):327–334 [DOI] [PubMed] [Google Scholar]
- 21. Colditz GA, Hankinson SE. The Nurses’ Health Study: lifestyle and health among women. Nat Rev Cancer. 2005;5(5):388–396 [DOI] [PubMed] [Google Scholar]
- 22. Manson JE, Grobbee DE, Stampfer MJ, et al. Aspirin in the primary prevention of angina pectoris in a randomized trial of United States physicians. Am J Med. 1990;89(6):772–776 [DOI] [PubMed] [Google Scholar]
- 23. Langer RD, White E, Lewis CE, Kotchen JM, Hendrix SL, Trevisan M. The Women’s Health Initiative Observational Study: baseline characteristics of participants and reliability of baseline measures. Ann Epidemiol. 2003;13(9 suppl):S107–121 [DOI] [PubMed] [Google Scholar]
- 24. Hays J, Hunt JR, Hubbell FA, et al. The Women’s Health Initiative recruitment methods and results. Ann Epidemiol. 2003;13(9 suppl):S18–77 [DOI] [PubMed] [Google Scholar]
- 25. Cook NR, Lee IM, Gaziano JM, et al. Low-dose aspirin in the primary prevention of cancer: the Women’s Health Study: a randomized controlled trial. JAMA. 2005;294(1):47–55 [DOI] [PubMed] [Google Scholar]
- 26. Rich-Edwards JW, Corsano KA, Stampfer MJ. Test of the National Death Index and Equifax Nationwide Death Search. Am J Epidemiol. 1994;140(11):1016–1019 [DOI] [PubMed] [Google Scholar]
- 27. Wolpin BM, Ng K, Bao Y, et al. Plasma 25-hydroxyvitamin D and risk of pancreatic cancer. Cancer Epidemiol Biomarkers Prev. 2012;21(1):82–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wei EK, Giovannucci E, Fuchs CS, Willett WC, Mantzoros CS. Low plasma adiponectin levels and risk of colorectal cancer in men: a prospective study. J Natl Cancer Inst. 2005;97(22):1688–1694 [DOI] [PubMed] [Google Scholar]
- 29. Hankinson SE, Willett WC, Manson JE, et al. Alcohol, height, and adiposity in relation to estrogen and prolactin levels in postmenopausal women. J Natl Cancer Inst. 1995;87(17):1297–1302 [DOI] [PubMed] [Google Scholar]
- 30. Final report on the aspirin component of the ongoing Physicians’ Health Study Steering Committee of the Physicians’ Health Study Research Group. N Engl J Med. 1989;321(3):129–135 [DOI] [PubMed] [Google Scholar]
- 31. Anderson GL, Manson J, Wallace R, Lund B, Hall D, Davis S, et al. Implementation of the Women’s Health Initiative study design. Ann Epidemiol. 2003;13(9 suppl):S5–17 [DOI] [PubMed] [Google Scholar]
- 32. Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA. 2001;286(3):327–334 [DOI] [PubMed] [Google Scholar]
- 33. Michaud DS, Wolpin B, Giovannucci E, et al. Prediagnostic plasma C-peptide and pancreatic cancer risk in men and women. Cancer Epidemiol Biomarkers Prev. 2007;16(10):2101–2109 [DOI] [PubMed] [Google Scholar]
- 34. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188 [DOI] [PubMed] [Google Scholar]
- 35. Wolpin BM, Michaud DS, Giovannucci EL, et al. Circulating insulin-like growth factor axis and the risk of pancreatic cancer in four prospective cohorts. Br J Cancer. 2007;97(1):98–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cochran WG. The combination of estimates from different experiments. Biometrics. 1954;10 101–129 [Google Scholar]
- 37. Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982;143(1):29–36 [DOI] [PubMed] [Google Scholar]
- 38. Pannala R, Leibson CL, Rabe KG, et al. Temporal association of changes in fasting blood glucose and body mass index with diagnosis of pancreatic cancer. Am J Gastroenterol. 2009;104(9):2318–2325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Stolzenberg-Solomon RZ, Graubard BI, Chari S, et al. Insulin, glucose, insulin resistance, and pancreatic cancer in male smokers. JAMA. 2005;294(22):2872–2878 [DOI] [PubMed] [Google Scholar]
- 40. Pfutzner A, Kunt T, Hohberg C, et al. Fasting intact proinsulin is a highly specific predictor of insulin resistance in type 2 diabetes. Diabetes Care. 2004;27(3):682–687 [DOI] [PubMed] [Google Scholar]
- 41. Pfutzner A, Standl E, Hohberg C, et al. IRIS II study: intact proinsulin is confirmed as a highly specific indicator for insulin resistance in a large cross-sectional study design. Diabetes Technol Ther. 2005;7(3):478–486 [DOI] [PubMed] [Google Scholar]
- 42. Malaguarnera R, Sacco A, Voci C, Pandini G, Vigneri R, Belfiore A. Proinsulin binds with high affinity the insulin receptor isoform A and predominantly activates the mitogenic pathway. Endocrinology. 2012;153(5):2152–2163 [DOI] [PubMed] [Google Scholar]
- 43. Belfiore A, Frasca F, Pandini G, Sciacca L, Vigneri R. Insulin receptor isoforms and insulin receptor/insulin-like growth factor receptor hybrids in physiology and disease. Endocr Rev. 2009;30(6):586–623 [DOI] [PubMed] [Google Scholar]
- 44. Schulze MB, Solomon CG, Rifai N, et al. Hyperproinsulinaemia and risk of type 2 diabetes mellitus in women. Diabet Med. 2005;22(9):1178–1184 [DOI] [PubMed] [Google Scholar]
- 45. Zethelius B, Byberg L, Hales CN, Lithell H, Berne C. Proinsulin is an independent predictor of coronary heart disease: report from a 27-year follow-up study. Circulation. 2002;105(18):2153–2158 [DOI] [PubMed] [Google Scholar]
- 46. Alssema M, Dekker JM, Nijpels G, Stehouwer CD, Bouter LM, Heine RJ. Proinsulin concentration is an independent predictor of all-cause and cardiovascular mortality: an 11-year follow-up of the Hoorn Study. Diabetes Care. 2005;28(4):860–865 [DOI] [PubMed] [Google Scholar]
- 47. Laakso M. How good a marker is insulin level for insulin resistance?. Am J Epidemiol. 1993;137(9):959–965 [DOI] [PubMed] [Google Scholar]
- 48. Howard G, Bergman R, Wagenknecht LE, et al. Ability of alternative indices of insulin sensitivity to predict cardiovascular risk: comparison with the “minimal model.” Insulin Resistance Atherosclerosis Study (IRAS) Investigators. Ann Epidemiol. 1998;8(6):358–369 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.