Abstract
Background
Benign breast disease and high breast density are prevalent, strong risk factors for breast cancer. Women with both risk factors may be at very high risk.
Methods
We included 42818 women participating in the Breast Cancer Surveillance Consortium who had no prior diagnosis of breast cancer and had undergone at least one benign breast biopsy and mammogram; 1359 women developed incident breast cancer in 6.1 years of follow-up (78.1% invasive, 21.9% ductal carcinoma in situ). We calculated hazard ratios (HRs) using Cox regression analysis. The referent group was women with nonproliferative changes and average density. All P values are two-sided.
Results
Benign breast disease and breast density were independently associated with breast cancer. The combination of atypical hyperplasia and very high density was uncommon (0.6% of biopsies) but was associated with the highest risk for breast cancer (HR = 5.34; 95% confidence interval [CI] = 3.52 to 8.09, P < .001). Proliferative disease without atypia (25.6% of biopsies) was associated with elevated risk that varied little across levels of density: average (HR = 1.37; 95% CI = 1.11 to 1.69, P = .003), high (HR = 2.02; 95% CI = 1.68 to 2.44, P < .001), or very high (HR = 2.05; 95% CI = 1.54 to 2.72, P < .001). Low breast density (4.5% of biopsies) was associated with low risk (HRs <1) for all benign pathology diagnoses.
Conclusions
Women with high breast density and proliferative benign breast disease are at very high risk for future breast cancer. Women with low breast density are at low risk, regardless of their benign pathologic diagnosis.
Atypical hyperplasia (1–3) and high breast density (4–6) are two of the strongest risk factors for breast cancer. If these two risk factors are independent, then the presence of both would identify a group of women at very high risk for breast cancer. These women may benefit from more-intensive approaches to screening for breast cancer or interventions to lower their risk for breast cancer.
A small prior study found a statistically significant interaction between benign breast disease and breast density, such that women with high breast density and atypical hyperplasia were at lower than expected risk (7). This is in contrast to other recent, large studies showing that breast density in combination with other risk factors is associated with increased risk of breast cancer. For example, women with high density and postmenopausal hormone therapy use are at higher risk of breast cancer than postmenopausal non–hormone therapy users with high breast density (8). Similarly, women with a first-degree relative with breast cancer and high breast density are at higher risk of breast cancer than those without a family history of breast cancer who have high breast density (9). In contrast, women with atypical hyperplasia are at increased breast cancer risk, but presence of family history does not statistically significantly modify the risk (2). The presence or absence of a statistically significant interaction between benign breast disease and breast density has important implications for improving risk assessment models of breast cancer.
We used data from the large, prospective Breast Cancer Surveillance Consortium (BCSC) to test the hypothesis that benign breast disease and breast density are independent risk factors for breast cancer and to obtain reliable estimates for the risks associated with combinations of these two factors. This is the first large study with sufficient power to examine these two strong, prevalent risk factors in US women evaluated using modern clinical practices for mammography and breast biopsies.
Methods
Study Population
The National Cancer Institute (NCI)–funded BCSC (http://breastscreening.cancer.gov) (10), is a community-based, geo graphically diverse cohort study that broadly represents the population of women presenting for screening mammography in the United States (11). We included the five registries that collect data on benign breast disease (North Carolina, New Hampshire, New Mexico, Vermont, Washington). Our sample consisted of 42818 women aged 30 years and older who had at least one biopsy with a benign diagnosis on pathology and had a mammographic measurement of breast density. All mammograms and biopsies took place between 1994 and 2009. We excluded all women who had a diagnosis of invasive breast cancer or ductal carcinoma in situ (DCIS) prior to their first eligible biopsy and women with cancers diagnosed in the first 6 months of follow-up to exclude cancers diagnosed on the basis of the initial biopsy. In addition, we excluded women with lobular carcinoma in situ (LCIS) on biopsy because the small number of women and subsequent breast cancers (n = 263 and 21, respectively) would not allow for analysis of this subgroup. Classic LCIS is not managed by surgical excision but is considered to be a risk factor for developing invasive breast cancer similar to atypical lobular hyperplasia. Lobular neoplasia is a term used to encompass the spectrum from atypical lobular hyperplasia to LCIS. When we combined LCIS with atypical hyperplasia, the results were identical to those presented in the paper. Thus, the data and outcomes presented exclude LCIS from the final analysis.
Each registry obtains annual approval from its institutional review board for consenting processes or a waiver of consent, enrollment of participants, and ongoing data linkage for research purposes. All registries have received a Federal Certificate of Confidentiality that protects the identities of research participants.
Measurement of Risk Factors
Patient information was obtained primarily from self-report at the time of the mammogram. This included age, race, ethnicity, family history of breast cancer, history of prior breast biopsies, parity, age at first live birth, menopausal status, height, and weight. We calculated body mass index as the weight in kilograms divided by the square of the height in meters. Ethnicity was coded using the expanded race/ethnicity definition currently used in Surveillance Epidemiology and End Results (SEER) program and US Vital Statistics (non-Hispanic white, non-Hispanic black, Asian/Pacific Islander, Hispanic, other).
Benign Breast Disease
Community pathologists at each site classified breast biopsy results using their local practice. We grouped each diagnosis from the pathology reports into one of three categories: nonproliferative, proliferative without atypia, and proliferative with atypia using the taxonomy proposed by Dupont and Page and Page et al (12–14). Nonproliferative diagnoses included fibroadenomas, calcifications, fibrocystic changes, nonsclerosing adenosis, lipomas, and fat necrosis. Proliferative diagnoses without atypia included usual ductal hyperplasia, complex fibroadenomas, sclerosing adenosis, and papillomas or papillomatosis. Finally, proliferative diagnoses with atypia included atypical ductal hyperplasia and atypical lobular hyperplasia. If there was more than one diagnosis on a single biopsy or multiple biopsies were performed within a six-month window, we chose the biopsy with the highest grade (atypical hyperplasia > proliferative without atypia > nonproliferative) to represent the biopsy for that time period.
Mammographic Breast Density
Community radiologists at each site classified breast density on screening mammograms as part of routine clinical practice using the four American College of Radiology Breast Imaging Reporting and Data System (BI-RADS) density categories (15): almost entirely fat (low density); scattered fibroglandular densities (average density); heterogeneously dense (high density); extremely dense (very high density). The BI-RADS 2 category (average density) was used as the reference group for breast density because it is a large group not at increased risk of breast cancer.
For each eligible biopsy with benign breast disease, we preferentially used the most recent screening mammogram up to five years prior to the date of the biopsy for the breast density measurement (82.5% of mammograms). If no screening mammogram was available, then we used the most recent diagnostic mammogram within five years prior to the biopsy. The density measure occurred within the two years prior to the biopsy for 89.7% of women. If no density measure was available within five years prior to the biopsy then any measure within six months after the biopsy was used (0.8% of records).
Ascertainment of Breast Cancer Cases
Breast cancer outcomes (1062 invasive cancer and 297 ductal carcinoma in situ) were obtained at each site through linkage with the regional population-based SEER program, state tumor registries, and pathology databases.
Vital Status
Vital status was obtained through linkage to SEER registries, state tumor registries, and the individual state Vital Statistics.
Statistical Analysis
We used partly conditional Cox regression to estimate the hazard ratios for incident breast cancer in order to incorporate biopsies occurring after the initial biopsy (16). We used a robust sandwich estimator for repeated measures survival data to account for multiple observations per woman (17). Women entered the model six months after the index biopsy and were censored at the time of death or the end of follow-up. All models were adjusted for age, race/ethnicity, and study site. The proportional hazards assumption was assessed using log-log plots and a test based on scaled Schoenfeld residuals for each predictor variable. All predictors met the proportional hazards assumption. The interaction between benign breast disease and breast density was assessed on a multiplicative scale by using the Wald test.
The following sensitivity analyses were performed: We limited the outcome to invasive breast cancer by censoring women at the diagnosis of DCIS; we used only the earliest eligible biopsy for women; we censored observations after 10 years of follow-up; we restricted the analysis to observations with breast density measured on a screening exam prior to the biopsy; we excluded observations with a prior breast procedure; we added the remaining variables from Table 1 to the Cox regression; and we restricted the analysis to postmenopausal women and adjusted for current hormone therapy and body mass index. There were no important changes in the hazard associated with benign breast disease or breast density in any of these sensitivity analyses. A P value of less than .05 was considered statistically significant. All statistical tests were two-sided.
Table 1.
Baseline characteristics of women with at least one benign breast biopsy
| Characteristic | No breast cancer, no. (n = 41459) | Breast cancer, no. (n = 1359) |
P* |
|---|---|---|---|
| Pathology from benign biopsy | <.001 | ||
| Nonproliferative | 29138 (70.3%) | 792 (58.3%) | |
| Proliferative disease without atypia | 10483 (25.3%) | 431 (31.7%) | |
| Atypical hyperplasia | 1838 (4.4%) | 136 (10.0%) | |
| Breast density† | <.001 | ||
| Almost entirely fat | 1920 (4.6%) | 39 (2.9%) | |
| Scattered fibroglandular densities | 15892 (38.3%) | 436 (32.1%) | |
| Heterogeneously dense | 18424 (44.4%) | 667 (49.1%) | |
| Extremely dense | 5223 (12.6%) | 217 (16.0%) | |
| Age at biopsy, y | <.001 | ||
| 30–39 | 4928 (11.9%) | 58 (4.3%) | |
| 40–49 | 14987 (36.1%) | 413 (30.4%) | |
| 50–59 | 11340 (27.4%) | 382 (28.1%) | |
| 60–69 | 5942 (14.3%) | 291 (21.4%) | |
| 70–79 | 3361 (8.1%) | 169 (12.4%) | |
| ≥80 | 901 (2.2%) | 46 (3.4%) | |
| Race or ethnicity | .008 | ||
| White, non-Hispanic | 32319 (78.0%) | 1098 (80.8%) | |
| Black, non-Hispanic | 2749 (6.6%) | 78 (5.7%) | |
| Asian | 571 (1.4%) | 19 (1.4%) | |
| Hispanic | 2230 (5.4%) | 59 (4.3%) | |
| Other/mixed | 847 (2.0%) | 11 (0.8%) | |
| Unknown/not reported | 2743 (6.6%) | 94 (6.9%) | |
| Family history of breast cancer in first-degree relative | 5879 (16.9%) | 272 (24.3%) | <.001 |
| Postmenopausal | 18134 (52.2%) | 780 (66.7%) | <.001 |
| Current hormone therapy use | 7920 (20.8%) | 355 (29.3%) | <.001 |
| Nulliparous | 3576 (14.3%) | 143 (15.3%) | .40 |
| Age at first live birth ≥30 y (if parous) | 2596 (12.9%) | 103 (13.8%) | .52 |
| Body mass index, kg/m2 | .66 | ||
| <18.5 | 479 (1.9%) | 14 (1.6%) | |
| 18.5 to <25 | 11144 (44.3%) | 387 (43.8%) | |
| 25 to <30 | 7065 (28.1%) | 244 (27.6%) | |
| 30 to <35 | 3826 (15.2%) | 150 (17.0%) | |
| ≥35 | 2619 (10.4%) | 89 (10.1%) |
* Two-sided P value using the χ2 test.
† Using the Breast Imaging Reporting and Data System (BI-RADS) density categories: 1 = almost entirely fat (low density); 2 = scattered fibroglandular densities (average density); 3 = heterogeneously dense (high density); 4 = extremely dense (very high density).
Results
Women in the BCSC with benign breast disease had a median age of 52.2 years (Table 1), and the majority of the women were white (74.0%). During a median follow-up of 6.1 years, 1359 of the women developed breast cancer. As expected, older age, non-Hispanic white race/ethnicity, a family history of breast cancer, postmenopausal status, hormone therapy use, proliferative disease on breast biopsy, and high breast density were all associated with the development of breast cancer (Table 1).
The majority of the biopsies showed nonproliferative lesions (69.7%). These included benign calcifications, fibroadenomas, and a mix of other benign findings (Table 2). Approximately 25.6% of the biopsies showed proliferative lesions without atypia and another 4.7% showed atypical hyperplasia including both atypical ductal and lobular hyperplasia (Table 2).
Table 2.
Distribution of the subtypes of benign breast diagnoses
| Diagnosis | No. | % |
|---|---|---|
| Nonproliferative* | 32526 | 69.7% |
| Fibroadenoma | 8172 | 17.5% |
| Calcification | 9897 | 21.2% |
| Phyllodes tumor† | 108 | 0.2% |
| Other benign | 29256 | 62.7% |
| Proliferative disease without atypia | 11942 | 25.6% |
| Ductal hyperplasia‡ | 11942 | 25.6% |
| Atypical hyperplasia | 2179 | 4.7% |
| Atypical ductal hyperplasia | 1376 | 2.9% |
| Atypical lobular hyperplasia | 304 | 0.7% |
| Atypical ductal and lobular hyperplasia | 63 | 0.1% |
| Atypical hyperplasia, not otherwise specified | 436 | 0.9% |
| Total | 46647 | 100.0% |
* Women having biopsies with multiple subtypes are counted more than once in the table.
† Phyllodes tumor (coded historically as cystosarcoma phyllodes) may be malignant but is typically benign and has been included in the nonproliferative category; it has no association with epithelial carcinoma.
‡ Ductal hyperplasia includes usual ductal hyperplasia, complex fibroadenomas, sclerosing adenosis, and papillomas or papillomatosis. The clinical sites map these diagnoses to ductal hyperplasia prior to submitting data to the coordinating center, so no breakdown is available for presentation.
Table 3 describes the distribution of benign breast disease within breast density categories. The two most commonly observed combinations were nonproliferative changes and heterogeneously dense breasts (30.6% of biopsies) or scattered fibroglandular dense breasts (26.9% of biopsies). Proliferative disease without atypia was relatively common among women with average breast density (9.1%) and high breast density (12.1%). The combination of atypical hyperplasia and very high breast density was rarely observed (0.6%). Low breast density was uncommon in this sample of women with a history of benign breast disease (4.5%). Proliferative disease with or without atypia was rare in women with low breast density (0.9% of biopsies).
Table 3.
The frequency and prevalence of benign breast diagnoses and breast density categories
| Pathology from benign biopsy | BI-RADS breast density, No. (%)* | Total | |||
|---|---|---|---|---|---|
| Almost entirely fat | Scattered fibroglandular densities | Heterogeneously dense | Extremely dense | ||
| Nonproliferative | 1629 | 12527 | 14260 | 4110 | 32526 |
| (3.5%) | (26.9%) | (30.6%) | (8.8%) | (69.7%) | |
| Proliferative disease without atypia | 388 | 4247 | 5626 | 1681 | 11942 |
| (0.8%) | (9.1%) | (12.1%) | (3.6%) | (25.6%) | |
| Atypical hyperplasia | 65 | 768 | 1079 | 267 | 2179 |
| (0.1%) | (1.6%) | (2.3%) | (0.6%) | (4.7%) | |
| Total | 2082 | 17542 | 20965 | 6058 | 46647 |
| (4.5%) | (37.6%) | (44.9%) | (13.0%) | (100.0%) | |
* Using the Breast Imaging Reporting and Data System (BI-RADS) density categories: 1 = almost entirely fat (low density); 2 = scattered fibroglandular densities (average density); 3 = heterogeneously dense (high density); 4 = extremely dense (very high density).
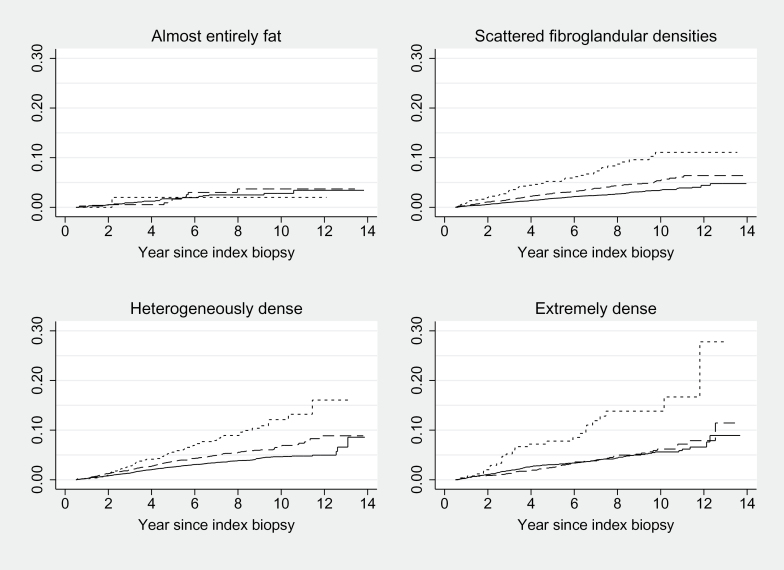
Proliferative lesions were strongly associated with breast cancer after adjustment for age, race/ethnicity, and registry (hazard ratio [HR] = 1.31; 95% confidence interval [CI] = 1.16 to 1.48 for hyperplasia; HR = 2.45; 95% CI = 2.03 to 2.95 for atypical hyperplasia; P < .001 for both). In stratified analyses, the association of benign breast diagnoses with breast cancer was similar within the three highest categories of breast density, but hyperplasia and atypical hyperplasia were not associated with an increased risk for breast cancer in women with low breast density (Figure 1). In a post hoc analysis, the P value for interaction with low breast density was not statistically significant (P = .36).
Figure 1.
Cumulative hazard of breast cancer for benign breast disease within breast density strata. The unit of analysis is benign biopsy. Observations were entered into the analysis at six months after the index biopsy. The solid line represents nonproliferative disease, the dashed line represents proliferative disease without atypia, and the dotted line represents proliferative disease with atypia. The interaction was not statistically significant (P = .28) using the two-sided Wald test.
Table 4 presents the hazard ratios associated with each combination of benign breast diagnosis and breast density using women with nonproliferative biopsy results and average breast density as the reference group. As seen in Figure 1, women with low breast density were at low risk for future breast cancer even if they had proliferative disease without atypia or atypical hyperplasia (all HRs < 1.0). The large group of women with proliferative disease without atypia was associated with a statistically significantly elevated risk for breast cancer in all of the three higher categories of breast density; average (HR = 1.37; 95% CI = 1.11 to 1.69, P = .003), high (HR = 2.02; 95% CI = 1.68 to 2.44, P < .001), or very high (HR = 2.05; 95% CI = 1.54 to 2.72, P < .001). Women with atypical hyperplasia and very high breast density were at highest risk for future breast cancer (HR = 5.34; 95% CI = 3.52 to 8.09, P < .001). The P value for interaction between benign breast disease and breast density was not statistically significant (P = .28).
Table 4.
Breast cancer risk associated with benign breast disease cross-classified with breast density
| Benign breast disease | BI-RADS breast density, HR (95% CI)* | |||
|---|---|---|---|---|
| Almost entirely fat | Scattered fibroglandular densities | Heterogeneously dense | Extremely dense | |
| Nonproliferative | 0.85 (0.56 to 1.28), | 1.0 (reference) | 1.51 (1.28 to 1.78), | 2.15 (1.73 to 2.68), |
| P = .44 | P < .001 | P < .001 | ||
| Proliferative without atypia | 0.67 (0.30 to 1.52), | 1.37 (1.11 to 1.69), | 2.02 (1.68 to 2.44), | 2.05 (1.54 to 2.72), |
| P = .34 | P = .003 | P < .001 | P < .001 | |
| Atypical hyperplasia | 0.68 (0.09 to 4.90), | 2.57 (1.85 to 3.58), | 3.37 (2.58 to 4.40), | 5.34 (3.52 to 8.09), |
| P = .70 | P < .001 | P < .001 | P < .001 | |
* Using the Breast Imaging Reporting and Data System (BI-RADS) density categories: 1 = almost entirely fat (low density); 2 = scattered fibroglandular densities (average density); 3 = heterogeneously dense (high density); 4 = extremely dense (very high density). The hazard ratios are relative to women with nonproliferative breast pathology and scattered fibroglandular densities and are adjusted for age, race/ethnicity, and registry. The P value for interaction between benign breast disease and breast density = 0.28, based on a two-sided Wald test.
Discussion
In this analysis of the BCSC cohort, we examined the independent contribution of benign breast diagnoses and breast density to breast cancer risk in 42818 women with at least one benign breast biopsy; over 6.1 years of follow-up, 1359 breast cancers developed. This represents the largest study to date of benign breast biopsies and breast density and the only study using data collected after 1990, thus reflecting contemporary mammography and pathologic evaluation of breast lesions. We found that benign breast disease and high breast density independently predict incident breast cancer. Women found on breast biopsy to have atypical hyperplasia and very high breast density had the highest risk for breast cancer. Notably, women with the more common proliferative forms of benign breast disease without atypia were at statistically significantly increased risk for breast cancer in all but the lowest category of breast density, that is, average density, high density, and very high density categories. Women with low breast density, whose breast tissue is almost entirely fat, were at low risk for future breast cancer regardless of the histology of their breast biopsy. However, the number of women with low breast density and proliferative disease was small and the test for interaction did not achieve statistical significance, indicating that this may represent a chance finding.
It is known that simply having a history of a breast biopsy increases a woman’s risk for future breast cancer. Previous breast biopsy is incorporated as a risk factor in the majority of risk assessment models for breast cancer including the Gail model (18), the revision of the Gail model that incorporates breast density (19), our own BCSC model (9), and the Tyrer-Cuzick model (20). However, some models do not include the histopathological diagnosis of the biopsy in the risk calculation (9,21), and those that do only modify a woman’s predicted risk if her histopathological diagnosis is atypical hyperplasia (18–20). In our study, proliferative lesions without atypia were identified in one-quarter of all breast biopsy results and the increased risk for breast cancer associated with these diagnoses, particularly in the presence of high breast density, is not included in current risk assessment models. These findings suggest that breast cancer risk assessment models have the potential to be improved by incorporating the full range of biopsy results into the risk calculations.
Our results could be used to better tailor prevention to individual patients. For example, tamoxifen is recommended for women with atypical ductal hyperplasia (ADH) according to the American Society of Clinical Oncology (22) and the US Preventive Services Task Force (23). This recommendation is based on results from the Breast Cancer Prevention Trial (24) showing that women with ADH reduced their risk of breast cancer by 86% when taking tamoxifen. Our results suggest that tamoxifen may not be appropriate for all women with ADH. Women with ADH and low breast density are not at increased risk of breast cancer compared to women with nonproliferative lesions and average breast density; women with ADH and average breast density have a modest increased risk of breast cancer. However, women with ADH and high or very high breast density are at high risk of breast cancer and would likely benefit the most from tamoxifen. Given that women have been reluctant to take tamoxifen for prevention because of medication side effects and the poor discrimination of risk prediction models (25–28), results from our study may assist women and their providers when deciding on tamoxifen therapy for prevention.
One prior study examined the combined effect of breast density and benign breast disease on risk for incident breast cancer in a case-control study nested in the Breast Cancer Detection Demonstration Project (BCDDP) (7). In that study, breast density and benign breast disease were both associated with breast cancer. However, women with both high breast density and atypical hyperplasia were at statistically significantly lower risk for breast cancer than both the group of women with low breast density and atypical hyperplasia and the group of women with high breast density and either nonproliferative or proliferative disease without atypia (P interaction = .002). There are several important differences between the two studies: 1) The BCDDP data collection took place between 1973 and 1980 with follow-up through 1989, whereas the mammograms and biopsies for our BCSC analysis occurred between 1994 and 2009 and are more representative of current practice. 2) The BCDDP study of benign breast disease and breast density also had limited statistical power because it included only 347 women with benign breast disease who developed cancer and 410 age-matched control subjects. In the BCDDP there were only 13 women with both high breast density and atypical hyperplasia, compared to 267 in our BCSC analysis. Thus, the confidence interval around the risk estimate for women in the BCDDP was wide. 3) The BCDDP used a different referent group than our study, women with nonproliferative histopathology and a percentage breast density less than 50%, and adjusted for additional covariates including family history, alcohol consumption, nulliparity, years of education, weight, menopause status, age of menopause, and postmenopausal hormone use. We performed sensitivity analyses to mirror this approach using the BCSC data, but they did not change our findings.
When comparing the relative hazards reported in this paper to those of benign breast disease reported in prior publications, it is important to keep in mind that all women included in this analysis were required to have undergone at least one breast biopsy. This was done to avoid introducing any bias due to a propensity to biopsy based on breast density. Because having a biopsy itself is a risk factor for breast cancer, the relative risk for women with high density and proliferative disease on biopsy will be even higher than that reported in this paper when the referent group is women who have never had a breast biopsy. The distribution of benign breast disease observed in the BCSC is almost identical to that of the large prior cohort study reported by the Mayo Clinic (2). When women with nonproliferative findings are used as the referent group, the relative risk estimates for proliferative lesions in the Mayo Clinic study are very similar to those in our study.
A second study from the Mayo Clinic investigated the relationship between lobular involution on breast biopsy and breast density (29,30). They hypothesized that lobular involution might explain some of the relationship between breast density and breast cancer risk because lobular involution increases with age and is associated with the replacement of epithelial tissue with adipose tissue (fat). Although they did find some association of the degree of lobular involution with breast density, both were independent risk factors for breast cancer (29,30). As in our analysis, the Mayo Clinic study demonstrates that clinically relevant changes in breast histology are associated with breast cancer risk independent of breast density.
There are several potential limitations to our study. Community radiologists reported breast density as part of routine clinic practice. Thus, the results are likely less precise than they would be if performed at a central facility by one trained reader (31,32). There was also a transition from film mammography to digital mammography during the study period, but we have demonstrated in a prior article that this has not affected the qualitative BI-RADS measure of breast density (33). Similarly, community pathologists read and reported the histopathological findings of the breast biopsies as part of routine clinical care. We did not perform any central review of pathology diagnoses or mammographic density assessment, nor did we perform any training to encourage standardization in the interpretation of the biopsies or mammograms. Several studies have documented poor agreement between pathologists for some histologic diagnoses (34–39). The decreased precision from lack of standardization would tend to bias the results toward the null. Thus, our associations may underestimate or overestimate the true strength of the associations between breast density, benign breast disease, and breast cancer because of misclassification of breast density and histologic findings. Finally, the small number of women with LCIS on biopsy precluded meaningful evaluation of the contribution of LCIS to risk within breast density subgroups.
In summary, we found that BI-RADS breast density and benign breast disease were independent risk factors for breast cancer associated with a stepwise increase in risk with increasing density and increasing proliferation. Women with high breast density and proliferative lesions with atypia were at highest risk for future breast cancer. The nearly 16% of women with proliferative lesions without atypia in the upper two categories of breast density had twice the risk of women with non-proliferative breast diagnoses. Women with low breast density were at low risk even if their biopsy results indicated the presence of proliferative lesions with or without atypia. The potential benefits and harms of screening mammography (40,41) are directly influenced by the level of breast cancer risk. Women at higher risk will have greater absolute benefit and fewer harms and women at low risk will have lower absolute benefit and greater harms. To maximize the potential benefit and minimize the potential harms of primary and secondary preventions for breast cancer, facilities and screening programs are starting to implement risk-based screening programs (42,43). Women and providers may use our results when discussing their breast cancer risk and the potential benefits and harms of interventions and choices for primary and secondary breast cancer prevention.
Funding
This work was supported by a National Cancer Institute–funded Program Project (P01CA154292), the Breast Cancer Surveillance Consortium (U01CA63740, U01CA86076, U01CA86082, U01CA63736, U01CA70013, U01CA69976, U01CA63731, U01CA70040, HHSN261201100031C), and the R01 CA140286.
Supplementary Material
The collection of cancer and vital status data used in this study was supported in part by several state public health departments and cancer registries throughout the United States. For a full description of these sources, please see http://www.breastscreening.cancer.gov/work/acknowledgement.html. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health. We thank the participating women, mammography facilities, and radiologists for the data they have provided for this study. A list of the BCSC investigators and procedures for requesting BCSC data for research purposes are provided at http://breastscreening.cancer.gov/.
References
- 1. Carter CL, Corle DK, Micozzi MS, et al. A prospective study of the development of breast cancer in 16,692 women with benign breast disease. Am J Epidemiol. 1988;128(3):467–477 [DOI] [PubMed] [Google Scholar]
- 2. Hartmann LC, Sellers TA, Frost MH, et al. Benign breast disease and the risk of breast cancer. N Engl J Med. 2005;353(3):229–237 [DOI] [PubMed] [Google Scholar]
- 3. London SJ, Connolly JL, Schnitt SJ, et al. A prospective study of benign breast disease and the risk of breast cancer. JAMA. 1992;267(7):941–944 [PubMed] [Google Scholar]
- 4. Boyd NF, Guo H, Martin LJ, et al. Mammographic density and the risk and detection of breast cancer. N Engl J Med. 2007;356(3):227–236 [DOI] [PubMed] [Google Scholar]
- 5. Cummings SR, Tice JA, Bauer S, et al. Prevention of breast cancer in postmenopausal women: approaches to estimating and reducing risk. J Natl Cancer Inst. 2009;101(6):384–398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ziv E, Shepherd J, Smith-Bindman R, et al. Mammographic breast density and family history of breast cancer. J Natl Cancer Inst. 2003;95(7):556–568 [DOI] [PubMed] [Google Scholar]
- 7. Byrne C, Schairer C, Brinton LA, et al. Effects of mammographic density and benign breast disease on breast cancer risk (United States). Cancer Causes Control. 2001;12(2):103–110 [DOI] [PubMed] [Google Scholar]
- 8. Kerlikowske K, Cook AJ, Buist DS, et al. Breast cancer risk by breast density, menopause, and postmenopausal hormone therapy use. J Clin Oncol. 2010;28(24):3830–3837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tice JA, Cummings SR, Smith-Bindman R, et al. Using clinical factors and mammographic breast density to estimate breast cancer risk: development and validation of a new predictive model. Ann Intern Med. 2008;148(5):337–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ballard-Barbash R, Taplin SH, Yankaskas BC, et al. Breast Cancer Surveillance Consortium: a national mammography screening and outcomes database. Am J Roentgenol. 1997;169(4):1001–1008 [DOI] [PubMed] [Google Scholar]
- 11. Sickles EA, Miglioretti DL, Ballard-Barbash R, et al. Performance benchmarks for diagnostic mammography. Radiology. 2005;235(3):775–790 [DOI] [PubMed] [Google Scholar]
- 12. Dupont WD, Page DL. Risk factors for breast cancer in women with proliferative breast disease. N Engl J Med. 1985;312(3):146–151 [DOI] [PubMed] [Google Scholar]
- 13. Page DL, Dupont WD, Rogers LW, et al. Atypical hyperplastic lesions of the female breast. A long-term follow-up study. Cancer. 1985;55(11):2698–2708 [DOI] [PubMed] [Google Scholar]
- 14. Page DL, Schuyler PA, Dupont WD, et al. Atypical lobular hyperplasia as a unilateral predictor of breast cancer risk: a retrospective cohort study. Lancet. 2003;361(9352):125–129 [DOI] [PubMed] [Google Scholar]
- 15. American College of Radiology Breast Imaging Reporting and Data System (BI-RADS) 4th ed Reston, VA: American College of Radiology; 2003. [Google Scholar]
- 16. Zheng Y, Heagerty PJ. Partly conditional survival models for longitudinal data. Biometrics. 2005;61(2):379–391 [DOI] [PubMed] [Google Scholar]
- 17. Lee EO, Wei L, Amato D. Cox-type regression analysis for large numbers of small groups of correlated failure time observations. Dordrecht, the Netherlands: Kluwer Academic Publishers; 1992. [Google Scholar]
- 18. Gail MH, Brinton LA, Byar DP, et al. Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. J Natl Cancer Inst. 1989;81(24):1879–1886 [DOI] [PubMed] [Google Scholar]
- 19. Chen J, Pee D, Ayyagari R, et al. Projecting absolute invasive breast cancer risk in white women with a model that includes mammographic density. J Natl Cancer Inst. 2006;98(17):1215–1226 [DOI] [PubMed] [Google Scholar]
- 20. Tyrer J, Duffy SW, Cuzick J. A breast cancer prediction model incorporating familial and personal risk factors. Stat Med. 2004;23(7):1111–1130 [DOI] [PubMed] [Google Scholar]
- 21. Barlow WE, White E, Ballard-Barbash R, et al. Prospective breast cancer risk prediction model for women undergoing screening mammography. J Natl Cancer Inst. 2006;98(17):1204–1214 [DOI] [PubMed] [Google Scholar]
- 22. Visvanathan K, Chlebowski RT, Hurley P, et al. American Society of Clinical Oncology clinical practice guideline update on the use of pharmacologic interventions including tamoxifen, raloxifene, and aromatase inhibition for breast cancer risk reduction. J Clin Oncol. 2009;27(19)3235–3258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chemoprevention of breast cancer: recommendations and rationale. Ann Intern. Med. 2002;137(1):56–58 [DOI] [PubMed] [Google Scholar]
- 24. Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 1998;90(18):1371–1388 [DOI] [PubMed] [Google Scholar]
- 25. Amir E, Freedman OC, Seruga B, et al. Assessing women at high risk of breast cancer: a review of risk assessment models. J Natl Cancer Inst. 2010;102(10):680–691 [DOI] [PubMed] [Google Scholar]
- 26. Gail MH, Mai PL. Comparing breast cancer risk assessment models. J Natl Cancer Inst. 2010;102(10):665–668 [DOI] [PubMed] [Google Scholar]
- 27. Waters EA, Cronin KA, Graubard BI, et al. Prevalence of tamoxifen use for breast cancer chemoprevention among U.S. women. Cancer Epidemiol Biomarkers Prev. 2010;19(2):443–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Waters EA, McNeel TS, Stevens WM, et al. Use of tamoxifen and raloxifene for breast cancer chemoprevention in 2010. Breast Cancer Res Treat. 2012;134(2):875–880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ghosh K, Hartmann LC, Reynolds C, et al. Association between mammographic density and age-related lobular involution of the breast. J Clin Oncol. 2010;28(13):2207–2212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ghosh K, Vachon CM, Pankratz VS, et al. Independent association of lobular involution and mammographic breast density with breast cancer risk. J Natl Cancer Inst. 2010;102(22):1716–1723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ciatto S, Houssami N, Apruzzese A, et al. Categorizing breast mammographic density: intra- and interobserver reproducibility of BI-RADS density categories. Breast. 2005;14(4):269–275 [DOI] [PubMed] [Google Scholar]
- 32. Kerlikowske K, Grady D, Barclay J, et al. Variability and accuracy in mammographic interpretation using the American College of Radiology Breast Imaging Reporting and Data System. J Natl Cancer Inst. 1998;90(23):1801–1809 [DOI] [PubMed] [Google Scholar]
- 33. Harvey JA, Gard CC, Miglioretti DL, et al. Reported mammographic density: film-screen versus digital acquisition. Radiology. 2012;266(3):752–758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bodian CA, Perzin KH, Lattes R, et al. Reproducibility and validity of pathologic classifications of benign breast disease and implications for clinical applications. Cancer. 1993;71(12):3908–3913 [DOI] [PubMed] [Google Scholar]
- 35. Jain RK, Mehta R, Dimitrov R, et al. Atypical ductal hyperplasia: interobserver and intraobserver variability. Mod Pathol. 2011;24(7):917–923 [DOI] [PubMed] [Google Scholar]
- 36. Masood S, Rosa M. Borderline breast lesions: diagnostic challenges and clinical implications. Adv Anat Pathol. 2011;18(3):190–198 [DOI] [PubMed] [Google Scholar]
- 37. Rosai J. Borderline epithelial lesions of the breast. Am J Surg Pathol. 1991;15(3):209–221 [DOI] [PubMed] [Google Scholar]
- 38. Sidawy MK, Stoler MH, Frable WJ, et al. Interobserver variability in the classification of proliferative breast lesions by fine-needle aspiration: results of the Papanicolaou Society of Cytopathology Study. Diagn Cytopathol. 1998;18(2):150–165 [DOI] [PubMed] [Google Scholar]
- 39. Sloane JP, Ellman R, Anderson TJ, et al. Consistency of histopathological reporting of breast lesions detected by screening: findings of the U.K. National External Quality Assessment (EQA) Scheme. U.K. National Coordinating Group for Breast Screening Pathology. Eur J Cancer. 1994;30A(10):1414–1419 [DOI] [PubMed] [Google Scholar]
- 40. Schousboe JT, Kerlikowske K, Loh A, et al. Personalizing mammography by breast density and other risk factors for breast cancer: analysis of health benefits and cost-effectiveness. Ann Intern Med. 2011;155(1):10–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. van Ravesteyn NT, Miglioretti DL, Stout NK, et al. Tipping the balance of benefits and harms to favor screening mammography starting at age 40 years: a comparative modeling study of risk. Ann Intern Med. 2012;156(9):609–617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Brinton JT, Barke LD, Freivogel ME, et al. Breast cancer risk assessment in 64,659 women at a single high-volume mammography clinic. Acad Radiol. 2012;19(1):95–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Evans DG, Warwick J, Astley SM, et al. Assessing individual breast cancer risk within the U.K. National Health Service breast screening program: a new paradigm for cancer prevention. Cancer Prev Res (Phila). 2012;5(7):943–951 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.