Abstract
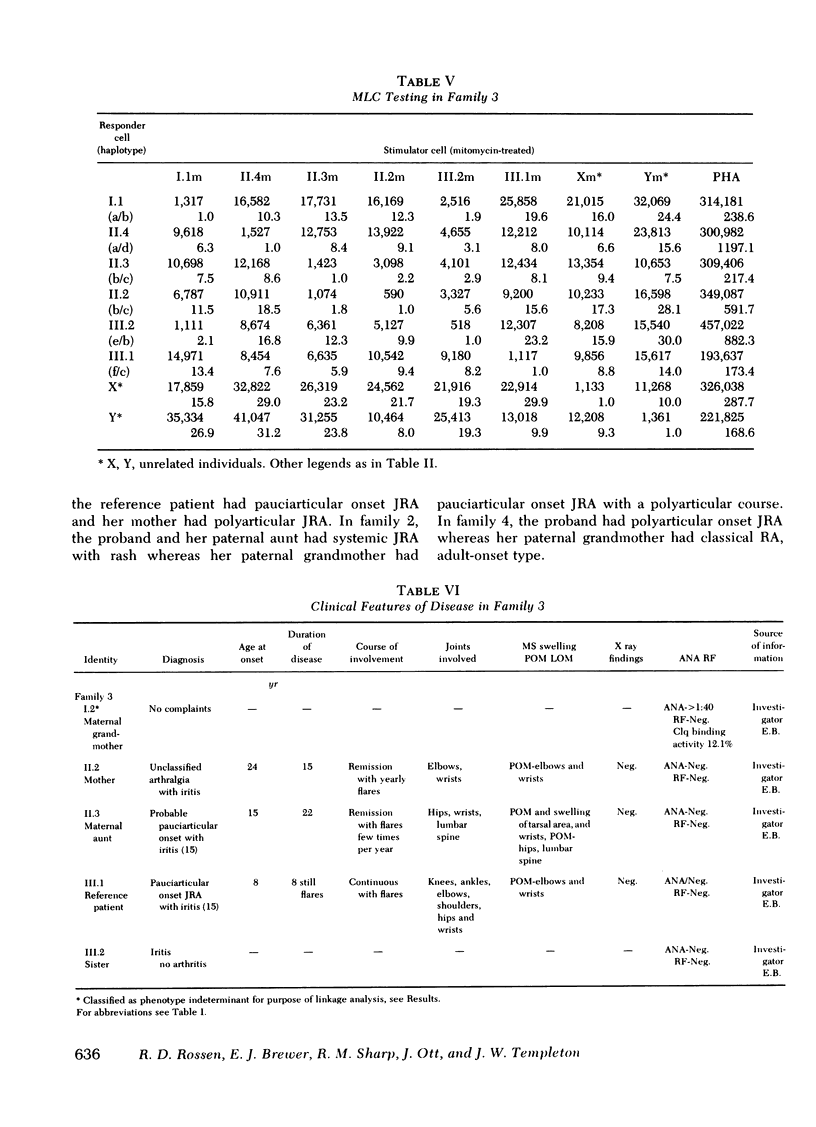
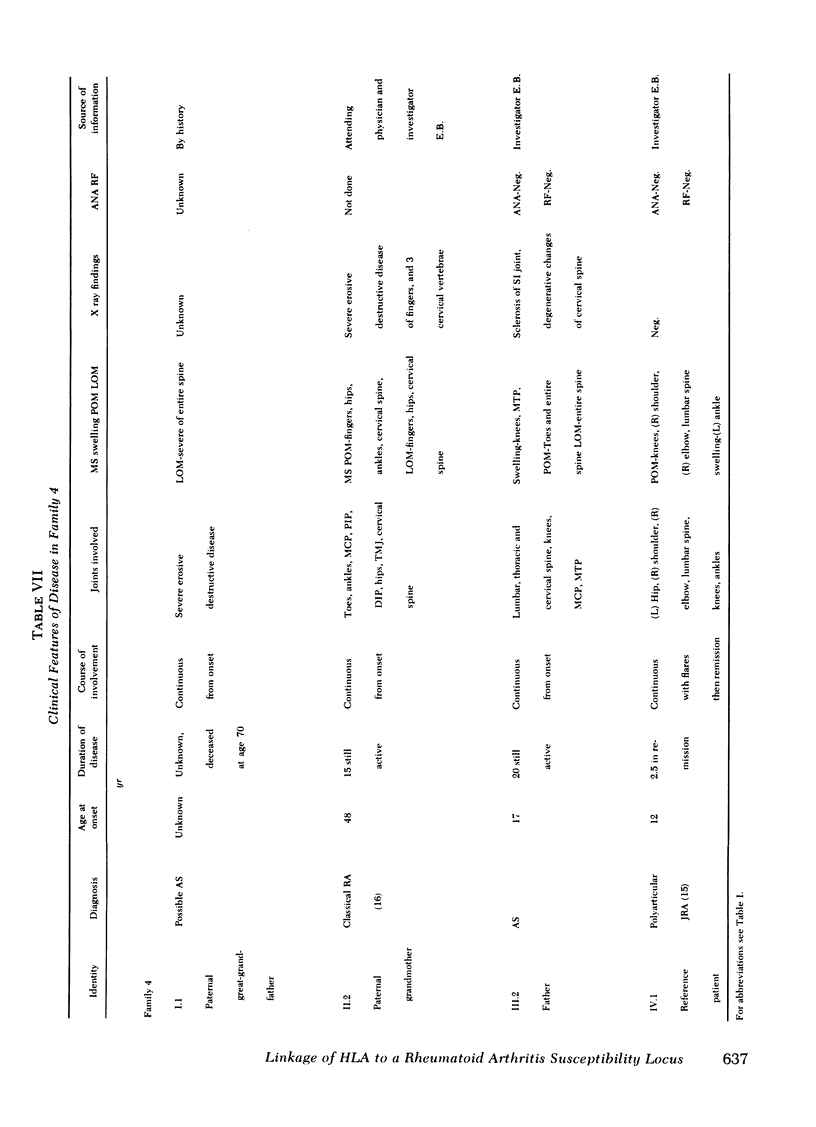
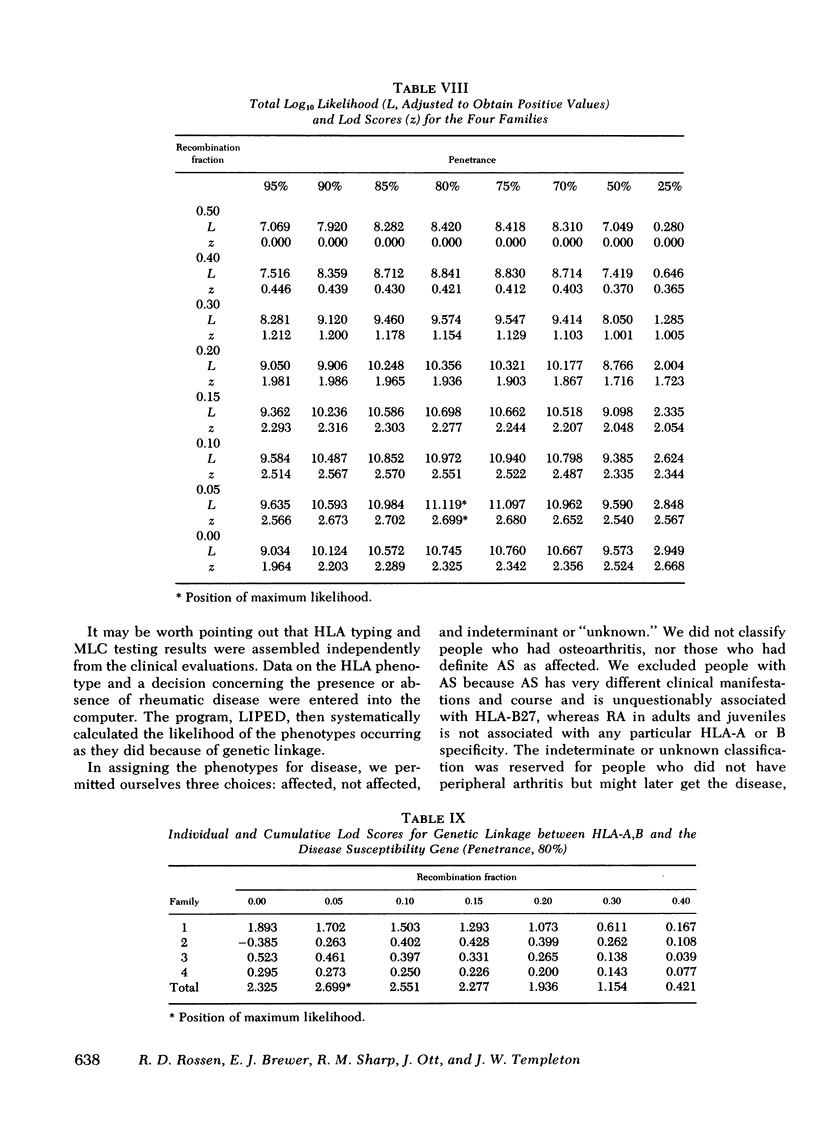
The occurrence of a chronic seronegative polyarthritis has been studied in four families in which the proband presented with some form of juvenile rheumatoid arthritis. In these families, histocompatibility testing suggested that susceptibility to arthritis was controlled by a dominant allele with variable penetrance and expressivity at the rheumatoid-like arthritis, first locus (RLA-1). The combined lod scores for the four families (2.70) indicated that the odds in favor of genetic linkage between the major histocompatibility complex and the postulated disease susceptibility gene, RLA-1, were 500:1. In one family, a recombinant event permitted localization of RLA-1 centromeric to HLA-D. Of major interest was the fact that there was significant pleomorphism in the clinical manifestations of arthritis in affected individuals. In some, symptoms first occurred in childhood and in others, in adult life. Even among those with childhood-onset arthritis, different types of juvenile rheumatoid arthritis were observed within the same family.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benacerraf B., McDevitt H. O. Histocompatibility-linked immune response genes. Science. 1972 Jan 21;175(4019):273–279. doi: 10.1126/science.175.4019.273. [DOI] [PubMed] [Google Scholar]
- Bianco N. E., Panush R. S., Stillman J. S., Schur P. H. Immunologic studies of juvenile rheumatoid arthritis. Arthritis Rheum. 1971 Nov-Dec;14(6):685–696. doi: 10.1002/art.1780140603. [DOI] [PubMed] [Google Scholar]
- Bowie E. J., Didisheim P., Thompson J. H., Jr, Owen C. A., Jr The spectrum of von Willebrand's disease. Thromb Diath Haemorrh. 1967 Aug 15;18(1-2):40–56. [PubMed] [Google Scholar]
- Brewer E. J., Jr, Bass J., Baum J., Cassidy J. T., Fink C., Jacobs J., Hanson V., Levinson J. E., Schaller J., Stillman J. S. Current proposed revision of JRA Criteria. JRA Criteria Subcommittee of the Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Section of The Arthritis Foundation. Arthritis Rheum. 1977 Mar;20(2 Suppl):195–199. [PubMed] [Google Scholar]
- Calin A., Fries J. F. Striking prevalence of ankylosing spondylitis in "healthy" w27 positive males and females. N Engl J Med. 1975 Oct 23;293(17):835–839. doi: 10.1056/NEJM197510232931701. [DOI] [PubMed] [Google Scholar]
- Cleland L. G., Bell D. A., Willans M., Saurino B. C. Familial lupus. Family studies of HLA and serologic findings. Arthritis Rheum. 1978 Mar;21(2):183–191. doi: 10.1002/art.1780210202. [DOI] [PubMed] [Google Scholar]
- Espinoza L. R., Vasey F. B., Oh J. H., Wilkinson R., Osterland C. K. Association between HLA-BW38 and peripheral psoriatic arthritis. Arthritis Rheum. 1978 Jan-Feb;21(1):72–75. doi: 10.1002/art.1780210112. [DOI] [PubMed] [Google Scholar]
- Gershwin M. E., Opelz G., Terasaki P. I., Castles J. J., Gorman T. A. Frequency of HLA-Dw3 in juvenile rheumatoid arthritis. Tissue Antigens. 1977 Oct;10(4):330–336. doi: 10.1111/j.1399-0039.1977.tb00765.x. [DOI] [PubMed] [Google Scholar]
- Ghose T., Woodbury J. F., Hansell M. M. Interaction in vitro between synovial cells and autologous lymphocytes and sera from arthritis patients. J Clin Pathol. 1975 Jul;28(7):550–558. doi: 10.1136/jcp.28.7.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson D. J., Carpenter C. M., Stillman J. S., Schur P. H. Re-examination of histocompatibility antigens found in patients with juvenile rheumatoid arthritis. N Engl J Med. 1975 Sep 25;293(13):636–638. doi: 10.1056/NEJM197509252931305. [DOI] [PubMed] [Google Scholar]
- Glass D., Soter N. A., Gibson D., Carpenter C. B., Schur P. H. Association between HLA and cutaneous necrotizing venulitis. Arthritis Rheum. 1976 Sep-Oct;19(5):945–949. doi: 10.1002/art.1780190519. [DOI] [PubMed] [Google Scholar]
- Griffiths M. M., Smith C. B., Pepper B. J. Susceptibility of rheumatoid and nonrheumatoid synovial cells to antibody-dependent cell-mediated cytotoxicity. Arthritis Rheum. 1978 Jan-Feb;21(1):97–105. doi: 10.1002/art.1780210116. [DOI] [PubMed] [Google Scholar]
- Hartzman R. J., Segall M., Bach M. L., Bach F. H. Histocompatibility matching. VI. Miniaturization of the mixed leukocyte culture test: a preliminary report. Transplantation. 1971 Mar;11(3):268–273. doi: 10.1097/00007890-197103000-00005. [DOI] [PubMed] [Google Scholar]
- Hiller C., Bischoff M., Schmidt A., Bender K. Analysis of the HLA-ABC linkage disequilibrium: decreasing strength of gametic association with increasing map distance. Hum Genet. 1978 Apr 24;41(3):301–312. doi: 10.1007/BF00284764. [DOI] [PubMed] [Google Scholar]
- Jaraquemada D., Pachoula-Papasteriadis C., Festenstein H., Sachs J. A., Roitt I. M., Corbett M., Ansell B. HLA-D and DR determinants in rheumatoid arthritis. Transplant Proc. 1979 Jun;11(2):1306–1306. [PubMed] [Google Scholar]
- Khan M. A., Kushner I., Ballou S. P., Braun W. E. Familial rheumatoid arthritis and HLA-DRw4. Lancet. 1979 Apr 28;1(8122):921–922. doi: 10.1016/s0140-6736(79)91395-3. [DOI] [PubMed] [Google Scholar]
- Marcusson J., Elman A., Möller E., Thyresson N. Psoriasis, sacro-iliitis and peripheral arthritis occurring in patients with the same HLA haplotype. A preliminary family report and a hypothetical explanation of the interaction between MHS products. Tissue Antigens. 1976 Aug;8(2):131–138. [PubMed] [Google Scholar]
- McMichael A. J., Sasazuki T., McDevitt H. O., Payne R. O. Increased frequency of HLA-Cw3 and HLA-Dw4 in rheumatoid arthritis. Arthritis Rheum. 1977 Jun;20(5):1037–1042. doi: 10.1002/art.1780200501. [DOI] [PubMed] [Google Scholar]
- Miller J. J., 3rd, Olds-Arroyo L., Akasaka T. Antiglobulins in juvenile rheumatoid arthritis. Arthritis Rheum. 1977 Mar;20(2):729–735. doi: 10.1002/art.1780200214. [DOI] [PubMed] [Google Scholar]
- Morris R., Metzger A. L., Bluestone R., Terasaki P. I. HL-A W27--a clue to the diagnosis and pathogenesis of Reiter's syndrome. N Engl J Med. 1974 Mar 7;290(10):554–556. doi: 10.1056/NEJM197403072901008. [DOI] [PubMed] [Google Scholar]
- Möller E., Olhagen B. Studies on the major histocompatibility system in patients with ankylosing spondylitis. Tissue Antigens. 1975 Oct;6(4):237–246. doi: 10.1111/j.1399-0039.1975.tb00638.x. [DOI] [PubMed] [Google Scholar]
- Nydegger U. E., Zubler R. H., Gabay R., Joliat G., Karagevrekis C. H., Lambert P. H., Miescher P. A. Circulating complement breakdown products in patients with rheumatoid arthritis. Correlation between plasma C3d, circulating immune complexes, and clinical activity. J Clin Invest. 1977 May;59(5):862–868. doi: 10.1172/JCI108708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyulassy S., Buc M., Sasinka M., Pavlovic M., Slugen I., Hirschová V., Kaiserová M., Menkyna R., Stefanovic J. The HLA system in glomerulonephritis. Clin Immunol Immunopathol. 1977 May;7(3):319–323. doi: 10.1016/0090-1229(77)90064-2. [DOI] [PubMed] [Google Scholar]
- Ott J. A simple scheme for the analysis of HLA linkages in pedigrees. Ann Hum Genet. 1978 Oct;42(2):255–257. doi: 10.1111/j.1469-1809.1978.tb00657.x. [DOI] [PubMed] [Google Scholar]
- Ott J. Estimation of the recombination fraction in human pedigrees: efficient computation of the likelihood for human linkage studies. Am J Hum Genet. 1974 Sep;26(5):588–597. [PMC free article] [PubMed] [Google Scholar]
- Panayi G. S., Wooley P. H., Batchelor J. R. HLA-DRw4 and rheumatoid arthritis. Lancet. 1979 Mar 31;1(8118):730–730. doi: 10.1016/s0140-6736(79)91186-3. [DOI] [PubMed] [Google Scholar]
- Pasternack A., Tiilikainen A. HLA-B27 in rheumatoid arthritis and amyloidosis. Tissue Antigens. 1977 Feb;9(2):80–89. doi: 10.1111/j.1399-0039.1977.tb01086.x. [DOI] [PubMed] [Google Scholar]
- Person D. A., Sharp J. T., Lidsky M. D. The cytotoxicity of leukocytes and lymphocytes from patients with rheumatoid arthritis for synovial cells. J Clin Invest. 1976 Sep;58(3):690–698. doi: 10.1172/JCI108515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROPES M. W., BENNETT G. A., COBB S., JACOX R., JESSAR R. A. 1958 Revision of diagnostic criteria for rheumatoid arthritis. Bull Rheum Dis. 1958 Dec;9(4):175–176. [PubMed] [Google Scholar]
- Rachelefsky G. S., Terasaki P. I., Katz R., Stiehm E. R. Increased prevalence of W27 in juvenile rheumatoid arthritis. N Engl J Med. 1974 Apr 18;290(16):892–893. doi: 10.1056/NEJM197404182901608. [DOI] [PubMed] [Google Scholar]
- Raum D., Glass D., Carpenter C. B., Alper C. A., Schur P. H. The chromosomal order of genes controlling the major histocompatibility complex, properdin factor B, and deficiency of the second component of complement. J Clin Invest. 1976 Nov;58(5):1240–1248. doi: 10.1172/JCI108578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roitt I. M., Corbett M., Festenstein H., Jaraquemada D., Papasteriadis C., Hay F. C., Nineham L. J. HLA-DRW4 and prognosis in rheumatoid arthritis. Lancet. 1978 May 6;1(8071):990–990. doi: 10.1016/s0140-6736(78)90280-5. [DOI] [PubMed] [Google Scholar]
- Rossen R. D., Brewer E. J., Person D. A., Templeton J. W., Lidsky M. D. Circulating immune complexes and antinuclear antibodies in juvenile rheumatoid arthritis. Arthritis Rheum. 1977 Nov-Dec;20(8):1485–1490. doi: 10.1002/art.1780200807. [DOI] [PubMed] [Google Scholar]
- Rudnicki R. D., Ruderman M., Scull E., Goldenberg A., Rothfield N. Clinical features and serologic abnormalities in juvenile rheumatoid arthritis. Arthritis Rheum. 1974 Nov-Dec;17(6):1007–1015. doi: 10.1002/art.1780170613. [DOI] [PubMed] [Google Scholar]
- Schlosstein L., Terasaki P. I., Bluestone R., Pearson C. M. High association of an HL-A antigen, W27, with ankylosing spondylitis. N Engl J Med. 1973 Apr 5;288(14):704–706. doi: 10.1056/NEJM197304052881403. [DOI] [PubMed] [Google Scholar]
- Stankaitiené D. J., Matulis A. A., Guobys H., Jusénaité J. P. Serum antiimmunoglobulins reactive with human and rabbit IgG in rheumatoid arthritis and other conditions. Arthritis Rheum. 1978 Jan-Feb;21(1):120–128. doi: 10.1002/art.1780210119. [DOI] [PubMed] [Google Scholar]
- Stastny P. Association of the B-cell alloantigen DRw4 with rheumatoid arthritis. N Engl J Med. 1978 Apr 20;298(16):869–871. doi: 10.1056/NEJM197804202981602. [DOI] [PubMed] [Google Scholar]
- Stastny P., Fink C. W. Different HLA-D associations in adult and juvenile rheumatoid arthritis. J Clin Invest. 1979 Jan;63(1):124–130. doi: 10.1172/JCI109265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stastny P., Fink C. W. HLA-Dw4 in adult and juvenile rheumatoid arthritis. Transplant Proc. 1977 Dec;9(4):1863–1866. [PubMed] [Google Scholar]
- Stastny P. Mixed lymphocyte cultures in rheumatoid arthritis. J Clin Invest. 1976 May;57(5):1148–1157. doi: 10.1172/JCI108382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strelkauskas A. J., Callery R. T., McDowell J., Borel Y., Schlossman S. F. Direct evidence for loss of human suppressor cells during active autoimmune disease. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5150–5154. doi: 10.1073/pnas.75.10.5150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strelkauskas A. J., Schauf V., Wilson B. S., Chess L., Schlossman S. F. Isolation and characterization of naturally occurring subclasses of human peripheral blood T cells with regulatory functions. J Immunol. 1978 Apr;120(4):1278–1282. [PubMed] [Google Scholar]
- Ting A., Mickey M. R., Terasaki P. I. B-lymphocyte alloantigens in Caucasians. J Exp Med. 1976 Apr 1;143(4):981–986. doi: 10.1084/jem.143.4.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twomey J. J., Laughter A. H., Steinberg A. D. A serum inhibitor of immune regulation in patients with systemic lupus erythematosus. J Clin Invest. 1978 Sep;62(3):713–715. doi: 10.1172/JCI109180. [DOI] [PMC free article] [PubMed] [Google Scholar]


