Abstract
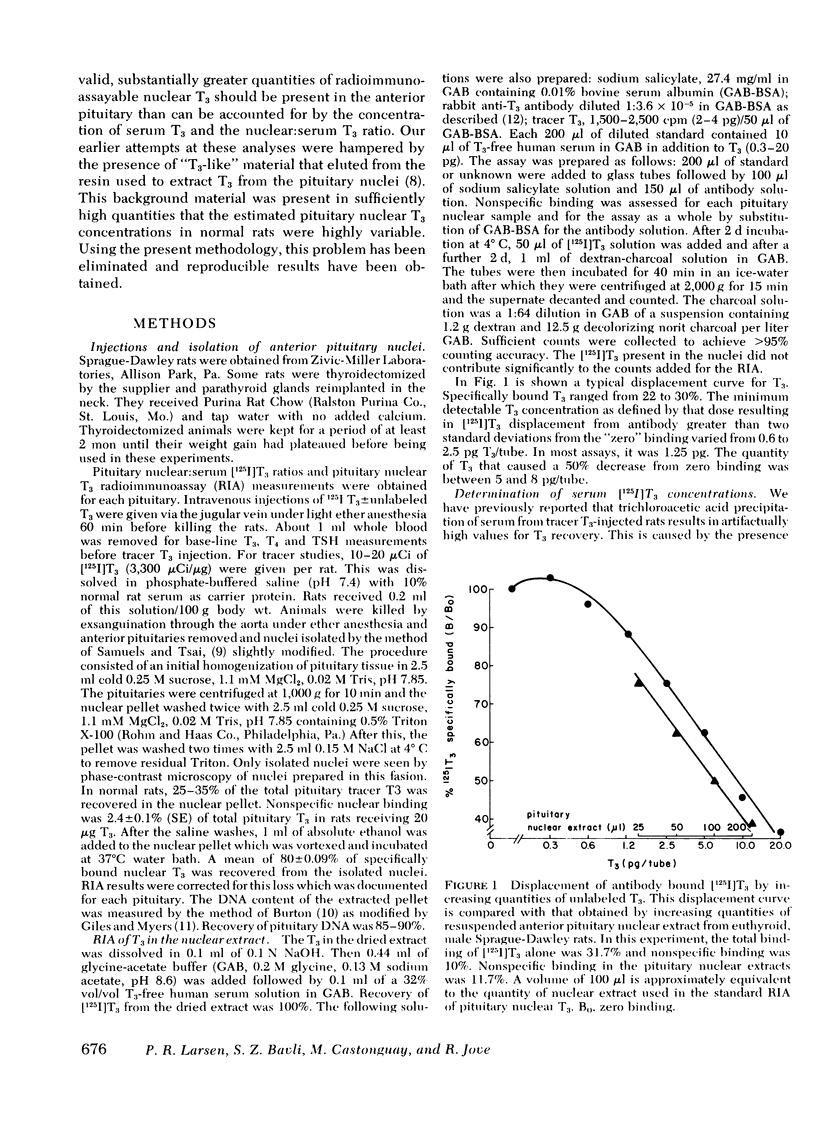
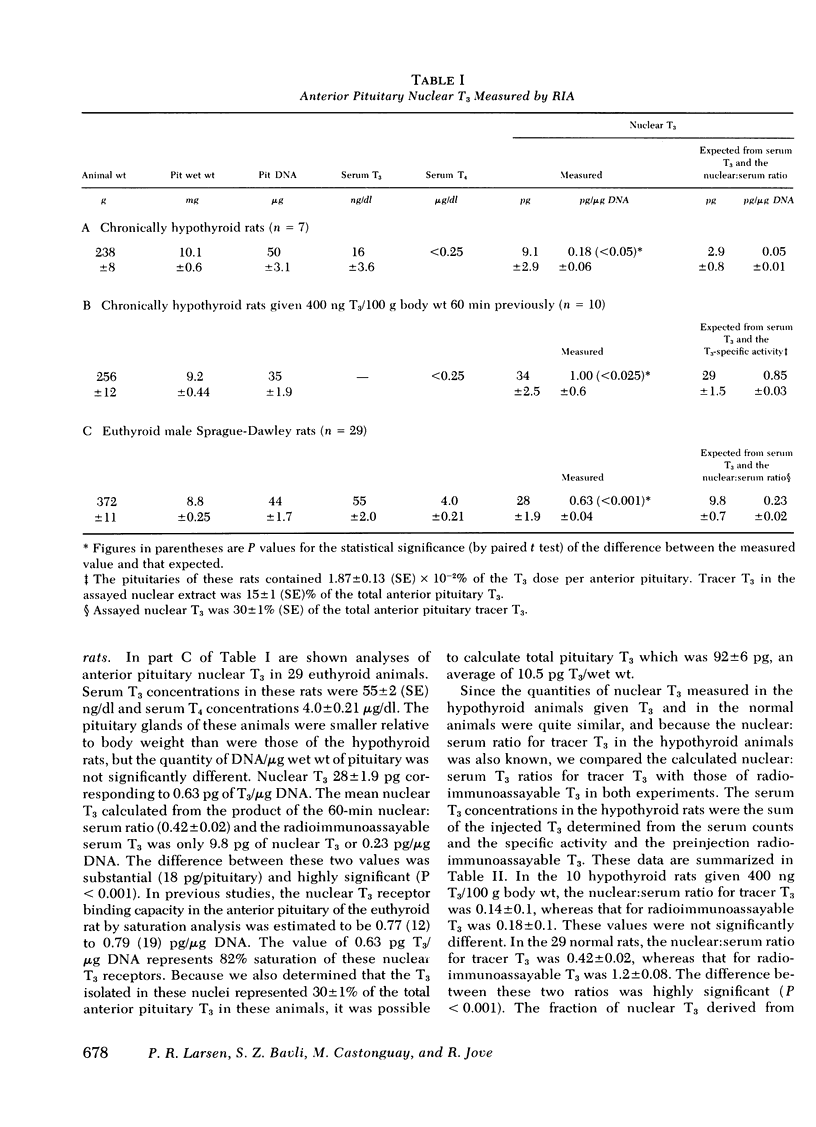
Previous tracer studies have suggested that 5′-monodeiodination of l-thyroxine (T4) in anterior pituitary may contribute a substantial portion of specifically bound nuclear 3,5,3′ l-triiodothyronine (T3) in this tissue in rats. To evaluate this possibility, a radioimmunoassay for nuclear T3 in individual anterior pituitaries was developed. Animals received [125I]T3 60 min before removal of the anterior pituitary and isolation of the nuclei by differential centrifugation. This allowed calculation of the nuclear:serum T3 ratio and comparison of expected with measured T3. T3 was extracted in ethanol, dried, and reconstituted in assay buffer. In untreated hypothyroid rats, anterior pituitary nuclear T3 was 0.18 ± 0.06 pg/μg DNA which was 0.13 pg/μg DNA greater than expected from the serum T3 concentration and the pituitary nuclear:serum [125I]T3 ratio. In 10 hypothyroid rats given a single bolus of 400 ng T3/100 g body wt., the nuclear T3 by radioimmunoassay was 1.0 ± 0.06 pg/μg DNA, whereas that expected from the T3 specific activity calculations was 0.85 pg/μg DNA (P < 0.025). Serum T4 concentrations in these rats were < 0.25 μg/dl but the nuclear T3 derived from as little as 0.2 μg/dl T4 could explain a large portion of these small discrepancies between observed and measured nuclear T3. In 29 normal rats, anterior pituitary nuclear T3 was 0.63±0.04 pg/μg DNA, whereas that expected from the serum T3 concentration (55±2 ng/dl) was 0.23±0.02 pg/μg DNA (P < 0.001). Total pituitary T3 based on this measurement was 92±6 pg. Because the maximal nuclear binding capacity for T3 in rat anterior pituitary is 0.77 pg/μg DNA, these results suggest there is 82% occupancy of these nuclear receptors. The requirement for normal serum concentrations of both T4 and T3 to achieve normal nuclear T3 saturation in anterior pituitary is in marked contrast to the situation in liver, kidney, and heart muscle which appear to require only a normal serum T3. As a consequence, the anterior pituitary can monitor both serum T4 and T3 and respond appropriately to changes in their concentrations.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheron R. G., Kaplan M. M., Larsen P. R. Physiological and pharmacological influences on thyroxine to 3,5,3'-triiodothyronine conversion and nuclear 3,5,3'-triiodothyronine binding in rat anterior pituitary. J Clin Invest. 1979 Nov;64(5):1402–1414. doi: 10.1172/JCI109598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulombe P., Schwartz H. L., Oppenheimer J. H. Relationship between the accumulation of pituitary growth hormone and nuclear occupancy by triiodothyronine in the rat. J Clin Invest. 1978 Nov;62(5):1020–1028. doi: 10.1172/JCI109206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frumess R. D., Larsen P. R. Correlation of serum triiodothyronine (T3) and thyroxine (T4) with biologic effects of thyroid hormone replacement in propylthiouracil-treated rats. Metabolism. 1975 Apr;24(4):547–554. doi: 10.1016/0026-0495(75)90079-7. [DOI] [PubMed] [Google Scholar]
- Gordon A., Spira O. Triiodothyronine binding in rat anterior pituitary, posterior pituitary, median eminence and brain. Endocrinology. 1975 Jun;96(6):1357–1365. doi: 10.1210/endo-96-6-1357. [DOI] [PubMed] [Google Scholar]
- Larsen P. R., Dick T. E., Markovitz B. P., Kaplan M. M., Gard T. G. Inhibition of intrapituitary thyroxine to 3.5.3'-triiodothyronine conversion prevents the acute suppression of thyrotropin release by thyroxine in hypothyroid rats. J Clin Invest. 1979 Jul;64(1):117–128. doi: 10.1172/JCI109430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen P. R. Direct immunoassay of triiodothyronine in human serum. J Clin Invest. 1972 Aug;51(8):1939–1949. doi: 10.1172/JCI107000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen P. R., Dockalova J., Sipula D., Wu F. M. Immunoassay of thyroxine in unextracted human serum. J Clin Endocrinol Metab. 1973 Aug;37(2):177–182. doi: 10.1210/jcem-37-2-177. [DOI] [PubMed] [Google Scholar]
- Larsen P. R., Frumess R. D. Comparison of the biological effects of thyroxine and triiodothyronine in the rat. Endocrinology. 1977 Apr;100(4):980–988. doi: 10.1210/endo-100-4-980. [DOI] [PubMed] [Google Scholar]
- MATTHEWS C. M. The theory of tracer experiments with 131I-labelled plasma proteins. Phys Med Biol. 1957 Jul;2(1):36–53. doi: 10.1088/0031-9155/2/1/305. [DOI] [PubMed] [Google Scholar]
- Obregon M. J., Pascual A., de Escobar G. M., Escobar del Rey F. Pituitary and plasma thyrotropin, thyroxine, and triiodothyronine after hyperthyroidism. Endocrinology. 1979 May;104(5):1467–1473. doi: 10.1210/endo-104-5-1467. [DOI] [PubMed] [Google Scholar]
- Oppenheimer J. H., Schwartz H. L., Koerner D., Surks M. I. Limited binding capacity sites for L-triiodothyronine in rat liver nuclei. Nuclear-cytoplasmic interrelation, binding constants, and cross-reactivity with L-thyroxine. J Clin Invest. 1974 Mar;53(3):768–777. doi: 10.1172/JCI107615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheimer J. H., Schwartz H. L., Surks M. I. Tissue differences in the concentration of triiodothyronine nuclear binding sites in the rat: liver, kidney, pituitary, heart, brain, spleen, and testis. Endocrinology. 1974 Sep;95(3):897–903. doi: 10.1210/endo-95-3-897. [DOI] [PubMed] [Google Scholar]
- PEARSON J. D., VEALL N., VETTER H. A practical method for plasma albumin turnover studies. Strahlentherapie. 1958;107(SONDERBD):290–297. [PubMed] [Google Scholar]
- Samuels H. H., Tsai J. S. Thyroid hormone action in cell culture: domonstration of nuclear receptors in intact cells and isolated nuclei. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3488–3492. doi: 10.1073/pnas.70.12.3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva J. E., Dick T. E., Larsen P. R. The contribution of local tissue thyroxine monodeiodination to the nuclear 3,5,3'-triiodothyronine in pituitary, liver, and kidney of euthyroid rats. Endocrinology. 1978 Oct;103(4):1196–1207. doi: 10.1210/endo-103-4-1196. [DOI] [PubMed] [Google Scholar]
- Silva J. E., Kaplan M. M., Cheron R. G., Dick T. E., Larsen P. R. Thyroxine to 3,5,3'-triiodothyronine conversion by rat anterior pituitary and liver. Metabolism. 1978 Nov;27(11):1601–1607. doi: 10.1016/0026-0495(78)90282-2. [DOI] [PubMed] [Google Scholar]
- Silva J. E., Larsen P. R. Contributions of plasma triiodothyronine and local thyroxine monodeiodination to triiodothyronine to nuclear triiodothyronine receptor saturation in pituitary, liver, and kidney of hypothyroid rats. Further evidence relating saturation of pituitary nuclear triiodothyronine receptors and the acute inhibition of thyroid-stimulating hormone release. J Clin Invest. 1978 May;61(5):1247–1259. doi: 10.1172/JCI109041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva J. E., Larsen P. R. Peripheral metabolism of homologous thyrotropin in euthyroid and hypothyroid rats: acute effects of thyrotropin-releasing hormone, triiodothyronine, and thyroxine. Endocrinology. 1978 Jun;102(6):1783–1796. doi: 10.1210/endo-102-6-1783. [DOI] [PubMed] [Google Scholar]
- Silva J. E., Larsen P. R. Pituitary nuclear 3,5,3'-triiodothyronine and thyrotropin secretion: an explanation for the effect of thyroxine. Science. 1977 Nov 11;198(4317):617–620. doi: 10.1126/science.199941. [DOI] [PubMed] [Google Scholar]
- Surks M. I., Oppenheimer J. H. Concentration of L-thyroxine and L-triiodothyronine specifically bound to nuclear receptors in rat liver and kidney. Quantitative evidence favoring a major role of T3 in thyroid hormone action. J Clin Invest. 1977 Sep;60(3):555–562. doi: 10.1172/JCI108807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeke J., Orskov H. Synthesis of 125I monolabelled 3, 5, 3'-triiodothyronine and thyroxine of maximum specific activity for radioimmunoassay. Scand J Clin Lab Invest. 1973 Dec;32(4):357–360. doi: 10.3109/00365517309084359. [DOI] [PubMed] [Google Scholar]
- Zimmerman C. J., Izumi M., Larsen P. R. Isolation of labeled triiodothyronine from serum using affinity chromatography: application to the extimation of the peripheral T4 to T3 conversion in rats. Metabolism. 1978 Mar;27(3):303–313. doi: 10.1016/0026-0495(78)90110-5. [DOI] [PubMed] [Google Scholar]



