Abstract
Selective attention mechanisms route behaviorally relevant information through large-scale cortical networks. While evidence suggests that populations of cortical neurons synchronize their activity to preferentially transmit information about attentional priorities, it is unclear how cortical synchrony across a network is accomplished. Based on its anatomical connectivity with the cortex, we hypothesized that the pulvinar, a thalamic nucleus, regulates cortical synchrony. We mapped pulvino-cortical networks within the visual system using diffusion tensor imaging and simultaneously recorded spikes and field potentials from these interconnected network sites in monkeys performing a visuo-spatial attention task. The pulvinar synchronized activity between interconnected cortical areas according to attentional allocation, suggesting not only a critical role for the thalamus in attentional selection, but more generally in regulating information transmission across visual cortex.
The limited capacity of the visual system does not permit simultaneous processing of all information from our cluttered environment in detail. Selective attention helps overcome this limitation by preferentially routing behaviorally relevant information across the visual system. Simultaneous neural recordings from two cortical areas suggest that this selective routing depends on the degree of synchrony between neuronal groups in each cortical area (1-4). However, it is unclear how different cortical areas synchronize their activity. While direct interaction between two cortical areas may give rise to their synchrony, an alternative possibility is that a third area, connected to both of them, mediates cortical synchronization.
Higher-order thalamic nuclei, like the pulvinar, predominantly receive input from the cortex rather than the periphery and their output strongly influences cortical activity in in vitro experiments (5). Because directly-connected cortical areas are also indirectly connected via the pulvinar (Fig. S1), the pulvinar is ideally positioned to synchronize activity across visual cortex (6-8). However, little is known about the functional role of these cortico-pulvino-cortical loops. Selective attention modulates the response magnitude of macaque pulvinar neurons (9, 10), and both humans and macaques with pulvinar lesions commonly present with attentional deficits (11, 12). We therefore hypothesized that the pulvinar increases synchrony between sequential processing stages across visual cortex during selective attention.
Information transmitted along the ventral visual cortical pathway is sequentially processed in interconnected areas V4 and TEO. We simultaneously recorded neural activity in the pulvinar, V4 and TEO during 51 recording sessions (13). Spike trains and local field potentials (LFPs) were recorded in each area from neurons with overlapping receptive fields (RFs). Monkeys performed a variant of the Eriksen flanker task, in which a spatial cue signals the location of a subsequent target flanked by distracter stimuli (> 80% accuracy overall; Fig. 1A). Because directly-connected cortical areas like V4 and TEO only connect with restricted, but overlapping zones in the pulvinar (8, 14), we used diffusion tensor imaging (DTI) to ensure electrodes targeted interconnected pulvino-cortical sites.
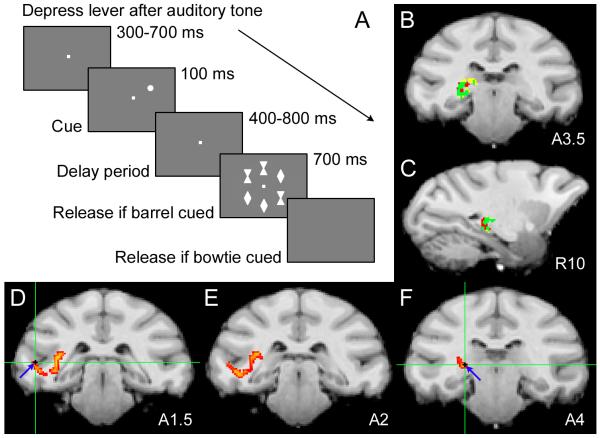
Fig. 1.
DTI-defined pulvino-cortical network probed with spatial attention task. (A) We simultaneously recorded from the pulvinar, V4 and TEO of monkeys performing a flanker task. Monkeys maintained fixation throughout trials, while we manipulated their locus of attention. The monkeys’ attention was drawn to the location of a cue, which randomly appeared at one of six locations. The cue signaled the location of the target in the subsequent array of six stimuli. To receive juice reward, monkeys immediately released the lever after onset of a barrel-shaped target or after disappearance of the stimulus array for a bowtie-shaped target. (B) Coronal and (C) sagittal slices containing pulvinar voxels with high probability of connection with V4 (yellow), TEO (red) or both (green). (D-F) Sequential coronal slices showing probable paths (yellow-red) between electrodes tips (blue arrows; green cross-hairs) in TEO (D) and pulvinar (F) for one session.
We performed probabilistic tractography on DTI data for each monkey, to map probable connections between the pulvinar, V4 and TEO. We identified pulvinar zones connected with V4 (yellow) and TEO (red), and delineated the region of overlap (green) through which the V4- pulvinar-TEO pathway likely traverses (Fig. 1, B and C). V4 and TEO predominantly connected to ventral pulvinar, and there was substantial overlap between V4 and TEO projection zones in the pulvinar, with the TEO projection zone extending more caudally, consistent with previous anatomical tracer work (8, 14). However, probabilistic tractography data had the advantage of delineating projection zones specific to individual monkeys, which cannot be precisely ascribed based on published tracer data. Guided by our structural connectivity maps, we positioned electrodes in the appropriate projection zones, and verified their location therein by taking structural scans of each electrode track (Fig. S7). We performed additional tractography analyses between voxels containing electrode tips, to show probable paths running directly between recording sites (Fig. 1, D-F).
If the pulvinar plays an important role in selective attention, then pulvinar neurons should signal where the monkey attends in our flanker task. Figure 2, A and B, show the population activity of pulvinar neurons aligned to the cue and target onsets, respectively. Pulvinar neurons responded robustly to the cue in their RF. While the monkey maintained attention at the RF location, there remained a small, but significant increase in pulvinar activity across the delay period (t test, p < 0.05). When a stimulus later appeared in the RF, pulvinar neurons showed a significantly greater response when the monkey attended to the RF location rather than outside the RF (in opposite visual hemifield; t test, p < 0.05). These results are consistent with previously reported attention-enhanced pulvinar responses to visual stimuli (10) and additionally show that the attentional locus is represented by pulvinar spike rate throughout the delay period when no stimuli are present.
Fig. 2.
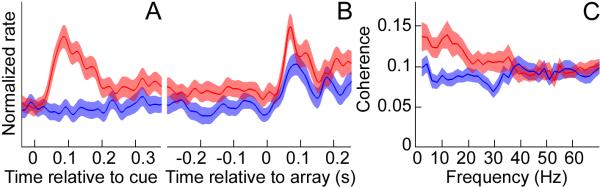
Attention modulated pulvinar spike rate and spike timing. Population activity (±SE) aligned to (A) cue and (B) target onset. Mean of 51 pulvinar cells. In (B), the preferred stimulus (barrel/bowtie) appeared at the RF, flanked by congruent distracters. (C) Population average of the transformed spike-field coherence in the pulvinar, calculated in the 300 ms window prior to target onset. Red, attention at RF; blue, attention away from RF.
We further tested whether attention influenced pulvinar spike timing, specifically the synchrony between pulvinar neurons, by calculating the degree of synchrony between spike times and the LFP, or spike-field coherence. For spectral analyses, we largely focused on the delay period after the cue-evoked response until the array onset, because not only did the monkey maintain spatial attention during this interval, but the data in each session generally satisfied methodological assumptions of stationarity as well. Figure S2, A and B, show a typical session in which pulvinar spike-field coherence increased immediately after the cue appeared in the RF, predominantly in the alpha-frequency range. While the monkey attended to the RF location, the spike-field coherence remained significantly elevated throughout the delay period until target presentation (t test, p < 0.05). When the cue appeared outside the RF (Fig. S2, C and D), drawing the monkey’s attention away from the RF, there was much weaker spike-field coherence. At the population level, there was significantly greater spike-field coherence in the 8-15 Hz range (alpha band) during the delay period until target presentation when the monkey attended to the RF location rather than outside the RF (Holm’s controlled t tests, p < 0.05; Fig. 2C). Because synchronized thalamic output provides increased drive to the cortex in anesthetized animals (15, 16), increased synchrony of pulvinar neurons may be an effective means to influence visual cortex during selective attention.
We next aimed to establish that selective attention increased synchrony between cortical areas (1-3). We calculated the coherence between V4 and TEO LFPs, which measures the synchrony between oscillatory processes in the two areas, as a function of oscillation frequency. Attention generally increased coherence between V4 and TEO LFPs in two frequency bands. There was significantly increased mean coherence in the 8-15 Hz range as well as a smaller, but significant increase in the 30-60 Hz range (gamma band) across the population (Holm’s controlled t tests, p < 0.05) during the delay period until target presentation (Fig. 3A).
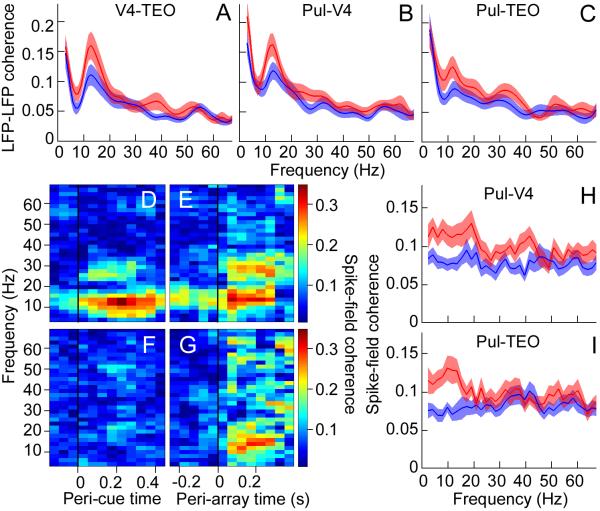
Fig. 3.
Attention modulated neural synchrony in pulvino-cortical networks. Population average of the transformed coherence between LFPs in (A) V4 and TEO, (B) pulvinar and V4, and (C) pulvinar and TEO, calculated in the 200 ms window before target onset. (D-G) Coherence (color-coded) between pulvinar spikes and V4 LFP for one session, calculated in successive 300 ms windows with 50 ms step size. (D) Cue and (E) target at RF. (F) Cue and (G) target away from RF. Same stimuli presented in (E) and (G). Window immediately before black line in (E) and (G) represents the coherence 0-300 ms before target onset. Population average of the transformed coherence between (H) pulvinar spikes and V4 LFP, and (I) pulvinar spikes and TEO LFP, calculated in the 300 ms window before target onset. Red, attention at RF; blue, attention away from RF.
Because low frequency oscillations modulate higher frequency oscillations (18-20), we tested whether attention increased cross-frequency coupling between alpha and gamma oscillations within V4 and TEO. To measure cross-frequency coupling, we calculated the synchronization index between cortical alpha oscillations and the gamma power envelope. Across the population, there was a significantly greater synchronization index for V4 and TEO during the delay period when attention was directed to the RF location rather than outside the RF (sign tests, p < 0.05; Fig. S3, A and B), suggesting that alpha oscillations contributed to the attention effect on gamma frequencies.
If the pulvinar interacts with the cortex during attentional processing, then attention should also modulate pulvino-cortical synchrony. Across the population, there was significantly greater alpha-band coherence between the pulvinar LFP and V4 LFP (Fig. 3B) as well as between the pulvinar LFP and TEO LFP (Fig. 3C) during the delay period until target presentation, when the monkey attended to the RF location rather than outside the RF (Holm’s controlled t tests, p < 0.05). This result is consistent with previous reports of synchrony between the cat lateral posterior-pulvinar complex and visual cortex (21, 22). Pulvinar spike trains also synchronized with cortical LFPs. Figure 3, D and E, respectively show attention effects aligned to cue and target onsets for a typical session. Attention significantly increased coherence between pulvinar spikes and V4 LFP after the cue appeared in the RF until target presentation, predominantly in the 8-15 Hz range (t test, p < 0.05). There was much weaker coherence after the cue had drawn attention away from the RF (Fig. 3, F and G). We obtained similar attention effects on the coherence between pulvinar spikes and TEO LFP (Fig. S4). Across the population, spatial attention significantly increased the coherence between pulvinar spikes and V4 LFP (Fig. 3H) as well as between pulvinar spikes and TEO LFP (Fig. 3I) predominantly in the 8-15 Hz range, throughout the delay period until target presentation (Holm’s controlled t tests, p < 0.05). These findings support the idea that the pulvinar is part of the brain’s attention network (9, 12, 23-25) and that it uses the alpha band as a fundamental operating mode.
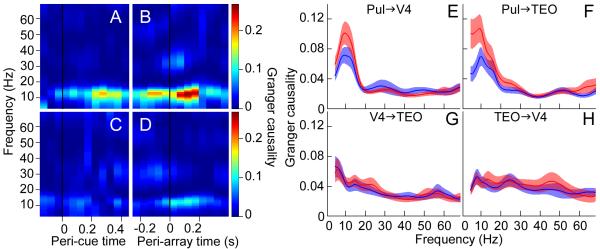
To determine the direction of pulvino-cortical interactions, we calculated the conditional Granger causality in the frequency domain, for the connections between the pulvinar, V4 and TEO. Conditional Granger causality measures the influence that one area (e.g., pulvinar) has on a second area (e.g., TEO) accounting for the influence of other areas (e.g., V4). This allowed us to dissect contributions from each connection, and thus test our overall hypothesis that the pulvinar modulates cortical synchrony according to attentional demands. The pulvinar influenced oscillatory activity in both V4 and TEO when the monkey attended to the RF location. Figure 4, A and B, show pulvinar influence on alpha activity in V4 significantly increased (p < 0.05) after the cue and continued until target presentation. There was much weaker pulvinar influence on V4 when the cue had drawn attention away from the RF (Fig. 4, C and D). Across the population, pulvinar influence on V4 (Fig. 4E; Holm’s controlled t tests, p < 0.05) and on TEO (Fig. 4F; Holm’s controlled t tests, p < 0.05) in the alpha-frequency range during the delay period was significantly greater with attention at the RF location versus outside the RF. Importantly, pulvinar influence on alpha activity in both V4 and TEO correlated with the attentional modulation of synchrony between V4 and TEO in the same frequency range (Fig. 3A), suggesting that the pulvinar regulated alpha synchrony between cortical areas according to attention allocation.
Fig. 4.
Pulvinar causally influenced cortical synchrony. (A-D) Conditional Granger causality (color-coded) from the pulvinar to V4 (accounting for TEO) for one session, calculated in successive 200 ms windows with 50 ms step size. (A) Cue and (B) target at RF. (C) Cue and (D) target away from RF. Same stimuli presented in (B) and (D). Window immediately before black line in (B) and (D) represents the Granger causality 0-200 ms before target onset. Population average of the conditional Granger causality for (E) pulvinar influence on V4, (F) pulvinar influence on TEO, (G) V4 influence on TEO, and (H) TEO influence on V4, calculated in the 200 ms window before target onset. Red, attend in; Blue, attend out.
In contrast to pulvino-cortical influences, direct cortico-cortical influences during the delay period were weak. Figure 4, G and H, show the population conditional Granger causality spectra for V4′s influence on TEO and TEO’s influence on V4, respectively. Spatial attention did not significantly change the weak influence of V4 on TEO, nor the weak influence of TEO on V4, during the delay period (t tests, p > 0.05). However, there was evidence consistent with strong cortico-cortical influences during visual stimulation (Fig. S5). These results suggest that maintenance of attention in the absence of visual stimulation depended on pulvino-cortical interactions (supplementary online text) rather than direct cortico-cortical interactions.
Our results show that the pulvinar modulates the synchrony between cortical areas according to the locus of attention. The pulvinar predominantly influenced cortical alpha oscillations, consistent with another thalamic nucleus, the lateral geniculate nucleus, driving occipital alpha rhythms (26). Evidence suggests that the rhythmic excitability of alpha oscillations gates visual events, with the phase of alpha oscillations critical for transmission of visual information (17, 27, 28). Thus, the pulvinar, by synchronizing distributed patches of cortical alpha activity, can selectively facilitate transmission of information about attentional priorities across cortex. Because pulvinar-controlled alpha activity modulated gamma activity in cortex through cross-frequency coupling, pulvinar influence on cortical synchrony extends to frequencies higher than alpha.
Pulvinar control of cortical processing challenges the common conceptualizing of cognitive functions as being restricted to cortex. Pulvino-cortical influences dominated during the delay period, suggesting that internal processes such as maintenance of attention in expectation of visual stimuli and short-term memory rely heavily on pulvino-cortical interactions. Pulvinar regulation of alpha activity is consistent with the important role ascribed to alpha oscillations in these internal processes (17, 29).
The prevailing view that information about our visual environment is transmitted through a network of cortical areas for detailed processing needs to be revised by considering extensive pulvino-cortical loops that regulate the information transmitted between each cortical stage of visual processing. Because of common cellular mechanisms and thalamo-cortical connectivity principles across sensorimotor domains, a general function of higher-order thalamic nuclei may be regulation of cortical synchrony to selectively route information across cortex.
Supplementary Material
Acknowledgments
We thank C. D. Brody and C. J. Honey for useful comments on the manuscript; and M. Ding for Granger causality routines. Supported by NIH (R21 EY021078) and NSF (BCS-1025149).
Footnotes
This manuscript has been accepted for publication in Science. This version has not undergone final editing. Please refer to the complete version of record at http://www.sciencemag.org/. The manuscript may not be reproduced or used in any manner that does not fall within the fair use provisions of the Copyright Act without the prior, written permission of AAAS.
Science’s embargo policy applies. The embargo time for Science is 2 p.m. U.S. Eastern Time, Thursday. That means no news coverage of the paper should appear anywhere before 2 p.m. U.S. E.T. on the Thursday immediately before the paper’s publication.
References and Notes
- 1.Buschman TJ, Miller EK. Top-down versus bottom-up control of attention in the prefrontal and posterior parietal cortices. Science. 2007;315:1860–1862. doi: 10.1126/science.1138071. [DOI] [PubMed] [Google Scholar]
- 2.Gregoriou GG, Gotts SJ, Zhou H, Desimone R. High-frequency, long-range coupling between prefrontal and visual cortex during attention. Science. 2009;324:1207–1210. doi: 10.1126/science.1171402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saalmann YB, Pigarev IN, Vidyasagar TR. Neural mechanisms of visual attention: how top-down feedback highlights relevant locations. Science. 2007;316:1612–1615. doi: 10.1126/science.1139140. [DOI] [PubMed] [Google Scholar]
- 4.Womelsdorf T, et al. Modulation of neuronal interactions through neuronal synchronization. Science. 2007;316:1609–1612. doi: 10.1126/science.1139597. [DOI] [PubMed] [Google Scholar]
- 5.Theyel BB, Llano DA, Sherman SM. The corticothalamocortical circuit drives higher-order cortex in the mouse. Nat. Neurosci. 2010;13:84–88. doi: 10.1038/nn.2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jones EG. The thalamic matrix and thalamocortical synchrony. Trends Neurosci. 2001;24:595–601. doi: 10.1016/s0166-2236(00)01922-6. [DOI] [PubMed] [Google Scholar]
- 7.Saalmann YB, Kastner S. Cognitive and perceptual functions of the visual thalamus. Neuron. 2011;71:209–223. doi: 10.1016/j.neuron.2011.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shipp S. The functional logic of cortico-pulvinar connections. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2003;358:1605–1624. doi: 10.1098/rstb.2002.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bender DB, Youakim M. Effect of attentive fixation in macaque thalamus and cortex. J. Neurophysiol. 2001;85:219–234. doi: 10.1152/jn.2001.85.1.219. [DOI] [PubMed] [Google Scholar]
- 10.Petersen SE, Robinson DL, Keys W. Pulvinar nuclei of the behaving rhesus monkey: visual responses and their modulation. J. Neurophysiol. 1985;54:867–886. doi: 10.1152/jn.1985.54.4.867. [DOI] [PubMed] [Google Scholar]
- 11.Petersen SE, Robinson DL, Morris JD. Contributions of the pulvinar to visual spatial attention. Neuropsychologia. 1987;25:97–105. doi: 10.1016/0028-3932(87)90046-7. [DOI] [PubMed] [Google Scholar]
- 12.Snow JC, Allen HA, Rafal RD, Humphreys GW. Impaired attentional selection following lesions to human pulvinar: evidence for homology between human and monkey. Proc. Natl. Acad. Sci. U. S. A. 2009;106:4054–4059. doi: 10.1073/pnas.0810086106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Materials and Methods are available as supplementary material on Science Online.
- 14.Baleydier C, Morel A. Segregated thalamocortical pathways to inferior parietal and inferotemporal cortex in macaque monkey. Vis. Neurosci. 1992;8:391–405. doi: 10.1017/s0952523800004922. [DOI] [PubMed] [Google Scholar]
- 15.Alonso JM, Usrey WM, Reid RC. Precisely correlated firing in cells of the lateral geniculate nucleus. Nature. 1996;383:815–819. doi: 10.1038/383815a0. [DOI] [PubMed] [Google Scholar]
- 16.Bruno RM, Sakmann B. Cortex is driven by weak but synchronously active thalamocortical synapses. Science. 2006;312:1622–1627. doi: 10.1126/science.1124593. [DOI] [PubMed] [Google Scholar]
- 17.Palva S, Palva JM. Functional roles of alpha-band phase synchronization in local and large-scale cortical networks. Front. Psychol. 2011;2:204. doi: 10.3389/fpsyg.2011.00204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Canolty RT, Knight RT. The functional role of cross-frequency coupling. Trends Cogn. Sci. 2010;14:506–515. doi: 10.1016/j.tics.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jensen O, Colgin LL. Cross-frequency coupling between neuronal oscillations. Trends Cogn. Sci. 2007;11:267–269. doi: 10.1016/j.tics.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 20.Schroeder CE, Lakatos P. Low-frequency neuronal oscillations as instruments of sensory selection. Trends Neurosci. 2009;32:9–18. doi: 10.1016/j.tins.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Molotchnikoff S, Shumikhina S. The lateral posterior-pulvinar complex modulation of stimulus-dependent oscillations in the cat visual cortex. Vision Res. 1996;36:2037–2046. doi: 10.1016/0042-6989(95)00311-8. [DOI] [PubMed] [Google Scholar]
- 22.Wrobel A, Ghazaryan A, Bekisz M, Bogdan W, Kaminski J. Two streams of attention-dependent beta activity in the striate recipient zone of cat’s lateral posterior-pulvinar complex. J. Neurosci. 2007;27:2230–2240. doi: 10.1523/JNEUROSCI.4004-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Desimone R, Wessinger M, Thomas L, Schneider W. Attentional control of visual perception: cortical and subcortical mechanisms. Cold Spring Harb. Symp. Quant. Biol. 1990;55:963–971. doi: 10.1101/sqb.1990.055.01.090. [DOI] [PubMed] [Google Scholar]
- 24.LaBerge D, Buchsbaum MS. Positron emission tomographic measurements of pulvinar activity during an attention task. J. Neurosci. 1990;10:613–619. doi: 10.1523/JNEUROSCI.10-02-00613.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Robinson DL, Petersen SE. The pulvinar and visual salience. Trends Neurosci. 1992;15:127–132. doi: 10.1016/0166-2236(92)90354-b. [DOI] [PubMed] [Google Scholar]
- 26.Hughes SW, Crunelli V. Thalamic mechanisms of EEG alpha rhythms and their pathological implications. Neuroscientist. 2005;11:357–372. doi: 10.1177/1073858405277450. [DOI] [PubMed] [Google Scholar]
- 27.Busch NA, Dubois J, VanRullen R. The phase of ongoing EEG oscillations predicts visual perception. J. Neurosci. 2009;29:7869–7876. doi: 10.1523/JNEUROSCI.0113-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mathewson KE, Gratton G, Fabiani M, Beck DM, Ro T. To see or not to see: prestimulus alpha phase predicts visual awareness. J. Neurosci. 2009;29:2725–2732. doi: 10.1523/JNEUROSCI.3963-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.von Stein A, Chiang C, Konig P. Top-down processing mediated by interareal synchronization. Proc. Natl. Acad. Sci. U. S. A. 2000;97:14748–14753. doi: 10.1073/pnas.97.26.14748. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.