Abstract
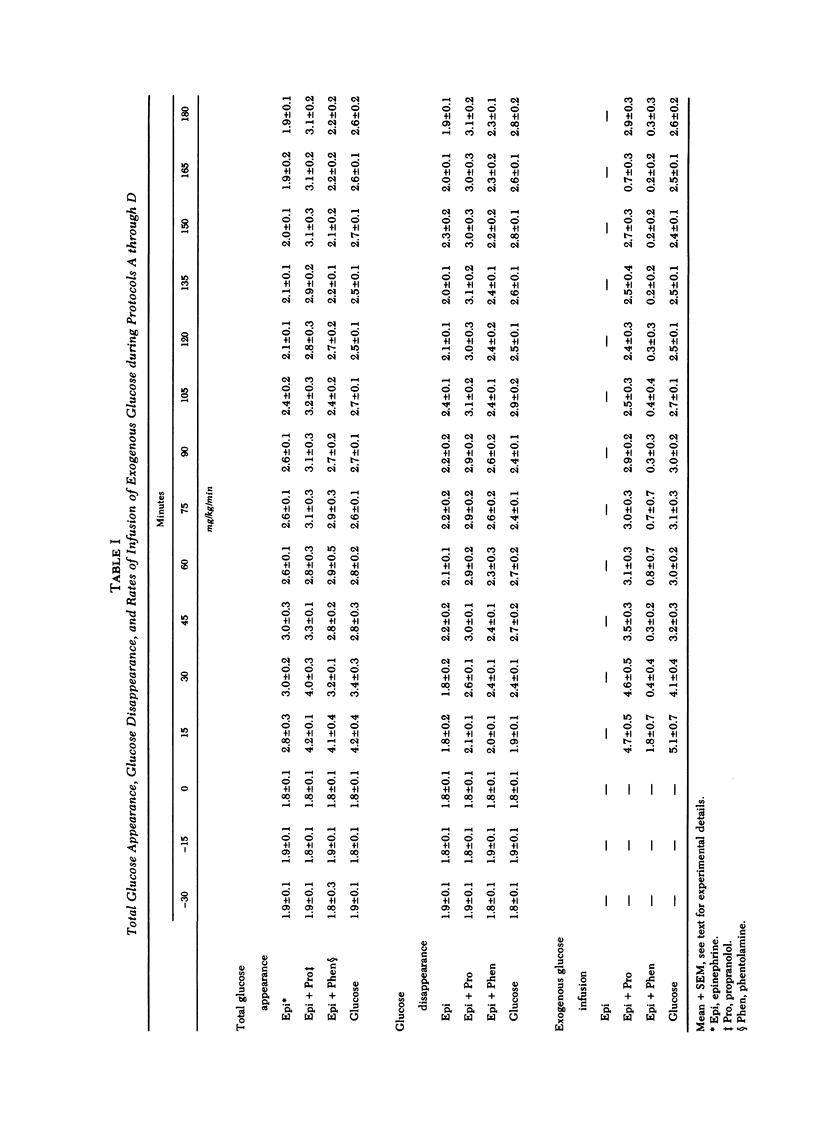
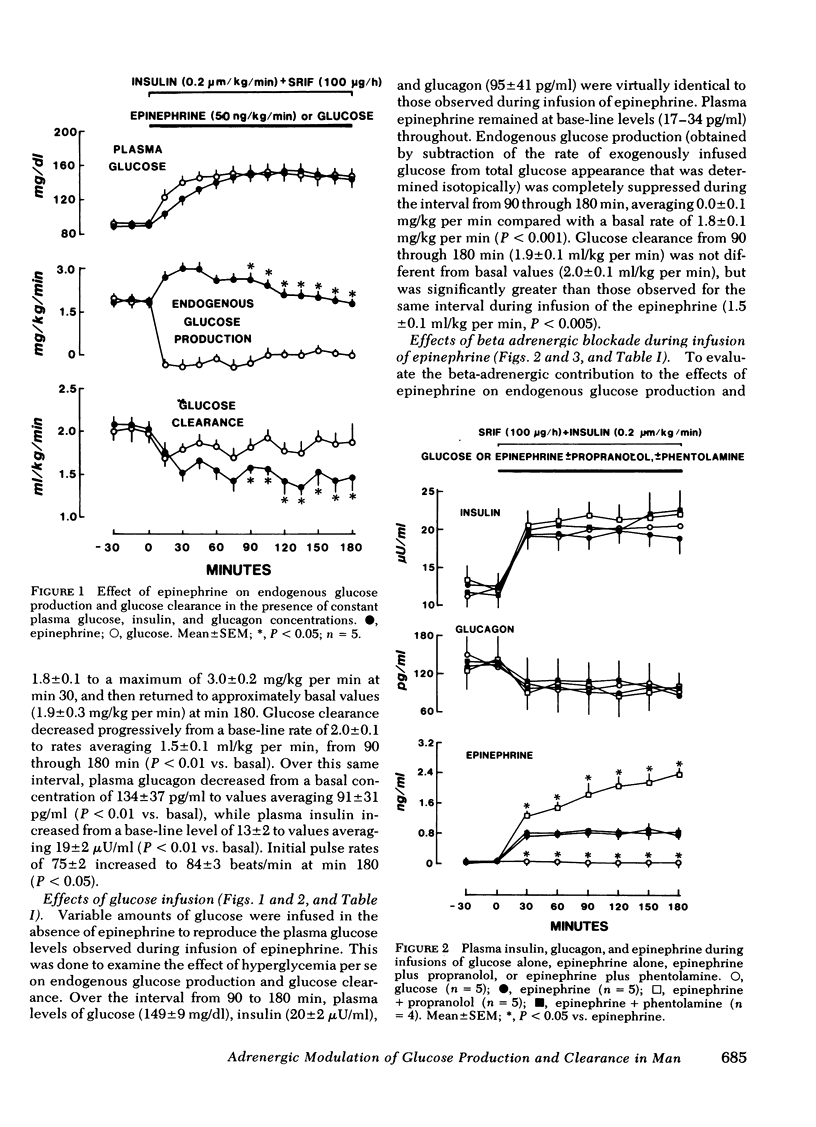
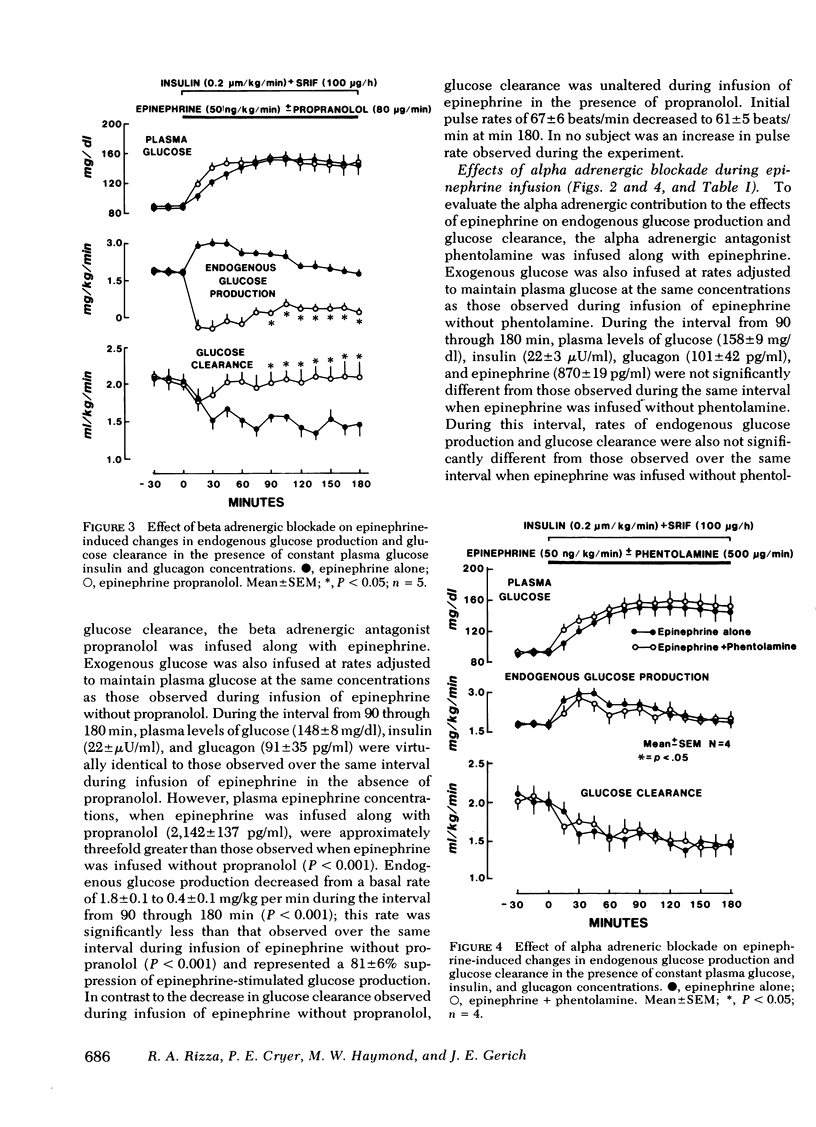
The present studies were undertaken to assess the adrenergic mechanisms by which epinephrine stimulates glucose production and suppresses glucose clearance in man: epinephrine (50 ng/kg per min) was infused for 180 min alone and during either alpha (phentolamine) or beta (propranolol)-adrenergic blockade in normal subjects under conditions in which plasma insulin, glucagon, and glucose were maintained at comparable levels by infusion of somatostatin (100 μg/h), insulin (0.2 mU/kg per min), and variable amounts of glucose. In additional experiments, to control for the effects of the hyperglycemia caused by epinephrine, variable amounts of glucose without epinephrine were infused along with somatostatin and insulin to produce hyperglycemia comparable with that observed during infusion of epinephrine. This glucose infusion suppressed glucose production from basal rates of 1.8±0.1 to 0.0±0.1 mg/kg per min (P < 0.01), but did not alter glucose clearance. During infusion of epinephrine, glucose production increased transiently from a basal rate of 1.8±0.1 to a maximum of 3.0±0.2 mg/kg per min (P < 0.01) at min 30, and returned to near basal rates at min 180 (1.9±0.1 mg/kg per min). Glucose clearance decreased from a basal rate of 2.0±0.1 to 1.5±0.2 ml/kg per min at the end of the epinephrine infusion (P < 0.01). Infusion of phentolamine did not alter these effects of epinephrine on glucose production and clearance. In contrast, infusion of propranolol completely prevented the suppression of glucose clearance by epinephrine, and inhibited the stimulation of glucose production by epinephrine by 80±6% (P < 0.001). These results indicate that, under conditions in which plasma glucose, insulin, and glucagon are maintained constant, epinephrine stimulates glucose production and inhibits glucose clearance in man predominantly by beta adrenergic mechanisms.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abramson E. A., Arky R. A. Role of beta-adrenergic receptors in counterregulation to insulin-induced hypoglycemia. Diabetes. 1968 Mar;17(3):141–146. doi: 10.2337/diab.17.3.141. [DOI] [PubMed] [Google Scholar]
- Altszuler N., Barkai A., Bjerknes C., Gottlieb B., Steele R. Glucose turnover values in the dog obtained with various species of labeled glucose. Am J Physiol. 1975 Dec;229(6):1662–1667. doi: 10.1152/ajplegacy.1975.229.6.1662. [DOI] [PubMed] [Google Scholar]
- Altszuler N., Steele R., Rathgeb I., De Bodo R. C. Glucose metabolism and plasma insulin level during epinephrine infusion in the dog. Am J Physiol. 1967 Mar;212(3):677–682. doi: 10.1152/ajplegacy.1967.212.3.677. [DOI] [PubMed] [Google Scholar]
- Bloomgarden Z. T., Liljenquist J. E., Cherrington A. D., Rabinowitz D. Persistent stimulatory effect of glucagon on glucose production despite downregulation. J Clin Endocrinol Metab. 1978 Nov;47(5):1152–1155. doi: 10.1210/jcem-47-5-1152. [DOI] [PubMed] [Google Scholar]
- Bomboy J. D., Jr, Lewis S. B., Lacy W. W., Sinclair-Smith B. C., Liljenquist J. E. Transient stimulatory effect of sustained hyperglucagonemia on splanchnic glucose production in normal and diabetic man. Diabetes. 1977 Mar;26(3):177–174. doi: 10.2337/diab.26.3.177. [DOI] [PubMed] [Google Scholar]
- Burns T. W., Mohs J. M., Langley P. E., Yawn R., Chase G. R. Regulation of human lipolysis. In vivo observations on the role of adrenergic receptors. J Clin Invest. 1974 Jan;53(1):338–341. doi: 10.1172/JCI107556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen N. J., Alberti K. G., Brandsborg O. Plasma catecholamines and blood substrate concentrations: studies in insulin induced hypoglycaemia and after adrenaline infusions. Eur J Clin Invest. 1975 Sep 12;5(5):415–423. doi: 10.1111/j.1365-2362.1975.tb00473.x. [DOI] [PubMed] [Google Scholar]
- Cryer P. E., Santiago J. V., Shah S. Measurement of norepinephrine and epinephrine in small volumes of human plasma by a single isotope derivative method: response to the upright posture. J Clin Endocrinol Metab. 1974 Dec;39(6):1025–1029. doi: 10.1210/jcem-39-6-1025. [DOI] [PubMed] [Google Scholar]
- DEBODO R. C., STEELE R., ALTSZULER N., DUNN A., BISHOP J. S. ON THE HORMONAL REGULATION OF CARBOHYDRATE METABOLISM; STUDIES WITH C14 GLUCOSE. Recent Prog Horm Res. 1963;19:445–488. [PubMed] [Google Scholar]
- Exton J. H., Mallette L. E., Jefferson L. S., Wong E. H., Friedmann N., Miller T. B., Jr, Park C. R. The hormonal control of hepatic gluconeogenesis. Recent Prog Horm Res. 1970;26:411–461. doi: 10.1016/b978-0-12-571126-5.50014-5. [DOI] [PubMed] [Google Scholar]
- Exton J. H., Robison G. A., Sutherland E. W., Park C. R. Studies on the role of adenosine 3',5'-monophosphate in the hepatic actions of glucagon and catecholamines. J Biol Chem. 1971 Oct 25;246(20):6166–6177. [PubMed] [Google Scholar]
- Felig P., Wahren J., Hendler R. Influence of physiologic hyperglucagonemia on basal and insulin-inhibited splanchnic glucose output in normal man. J Clin Invest. 1976 Sep;58(3):761–765. doi: 10.1172/JCI108523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerich J. E., Karam J. H., Forsham P. H. Stimulation of glucagon secretion by epinephrine in man. J Clin Endocrinol Metab. 1973 Sep;37(3):479–481. doi: 10.1210/jcem-37-3-479. [DOI] [PubMed] [Google Scholar]
- Herbert V., Lau K. S., Gottlieb C. W., Bleicher S. J. Coated charcoal immunoassay of insulin. J Clin Endocrinol Metab. 1965 Oct;25(10):1375–1384. doi: 10.1210/jcem-25-10-1375. [DOI] [PubMed] [Google Scholar]
- Himms-Hagen J. Sympathetic regulation of metabolism. Pharmacol Rev. 1967 Sep;19(3):367–461. [PubMed] [Google Scholar]
- Hornbrook K. R. Adrenergic receptors for metabolic responses in the liver. Fed Proc. 1970 Jul-Aug;29(4):1381–1385. [PubMed] [Google Scholar]
- McGuire E. A., Helderman J. H., Tobin J. D., Andres R., Berman M. Effects of arterial versus venous sampling on analysis of glucose kinetics in man. J Appl Physiol. 1976 Oct;41(4):565–573. doi: 10.1152/jappl.1976.41.4.565. [DOI] [PubMed] [Google Scholar]
- Porte D., Jr A receptor mechanism for the inhibition of insulin release by epinephrine in man. J Clin Invest. 1967 Jan;46(1):86–94. doi: 10.1172/JCI105514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randle P. J., Newsholme E. A., Garland P. B. Regulation of glucose uptake by muscle. 8. Effects of fatty acids, ketone bodies and pyruvate, and of alloxan-diabetes and starvation, on the uptake and metabolic fate of glucose in rat heart and diaphragm muscles. Biochem J. 1964 Dec;93(3):652–665. doi: 10.1042/bj0930652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizza R. A., Gerich J. E. Persistent effect of sustained hyperglucagonemia on glucose production in man. J Clin Endocrinol Metab. 1979 Feb;48(2):352–355. doi: 10.1210/jcem-48-2-352. [DOI] [PubMed] [Google Scholar]
- Rizza R., Haymond M., Cryer P., Gerich J. Differential effects of epinephrine on glucose production and disposal in man. Am J Physiol. 1979 Oct;237(4):E356–E362. doi: 10.1152/ajpendo.1979.237.4.E356. [DOI] [PubMed] [Google Scholar]
- Rizza R., Verdonk C., Miles J., Service F. J., Gerich J. Effect of intermittent endogenous hyperglucagonemia on glucose homeostasis in normal and diabetic man. J Clin Invest. 1979 Jun;63(6):1119–1123. doi: 10.1172/JCI109404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEELE R., WALL J. S., DE BODO R. C., ALTSZULER N. Measurement of size and turnover rate of body glucose pool by the isotope dilution method. Am J Physiol. 1956 Sep;187(1):15–24. doi: 10.1152/ajplegacy.1956.187.1.15. [DOI] [PubMed] [Google Scholar]
- Sloan I. G., Sawh P. C., Bihler I. Influence of adrenalin on sugar transport in soleus, a red skeletal muscle. Mol Cell Endocrinol. 1978 Feb-Mar;10(1):3–12. doi: 10.1016/0303-7207(78)90054-0. [DOI] [PubMed] [Google Scholar]
- WALAAS O., WALAAS E. Effect of epinephrine on rat diaphragm. J Biol Chem. 1950 Dec;187(2):769–776. [PubMed] [Google Scholar]


