Abstract
BACKGROUND
There is debate about the value of assessing levels of C-reactive protein (CRP) and other biomarkers of inflammation for the prediction of first cardiovascular events.
METHODS
We analyzed data from 52 prospective studies that included 246,669 participants without a history of cardiovascular disease to investigate the value of adding CRP or fibrinogen levels to conventional risk factors for the prediction of cardiovascular risk. We calculated measures of discrimination and reclassification during follow-up and modeled the clinical implications of initiation of statin therapy after the assessment of CRP or fibrinogen.
RESULTS
The addition of information on high-density lipoprotein cholesterol to a prognostic model for cardiovascular disease that included age, sex, smoking status, blood pressure, history of diabetes, and total cholesterol level increased the C-index, a measure of risk discrimination, by 0.0050. The further addition to this model of information on CRP or fibrinogen increased the C-index by 0.0039 and 0.0027, respectively (P<0.001), and yielded a net reclassification improvement of 1.52% and 0.83%, respectively, for the predicted 10-year risk categories of “low” (<10%), “intermediate” (10% to <20%), and “high” (≥20%) (P<0.02 for both comparisons). We estimated that among 100,000 adults 40 years of age or older, 15,025 persons would initially be classified as being at intermediate risk for a cardiovascular event if conventional risk factors alone were used to calculate risk. Assuming that statin therapy would be initiated in accordance with Adult Treatment Panel III guidelines (i.e., for persons with a predicted risk of ≥20% and for those with certain other risk factors, such as diabetes, irrespective of their 10-year predicted risk), additional targeted assessment of CRP or fibrinogen levels in the 13,199 remaining participants at intermediate risk could help prevent approximately 30 additional cardiovascular events over the course of 10 years.
CONCLUSIONS
In a study of people without known cardiovascular disease, we estimated that under current treatment guidelines, assessment of the CRP or fibrinogen level in people at intermediate risk for a cardiovascular event could help prevent one additional event over a period of 10 years for every 400 to 500 people screened. (Funded by the British Heart Foundation and others.)
There is an evolving debate about the value of assessing levels of C-reactive protein (CRP) and other soluble biomarkers of inflammation for the prediction of first cardiovascular events. In 2003, the Centers for Disease Control and Prevention and the American Heart Association (AHA) concluded that CRP may be used at the discretion of a physician as part of a global assessment of cardiovascular risk.1 In 2007, the European Society of Cardiology described as “premature” the incorporation of CRP assessment into standard models for the prediction of cardiovascular risk.2 In 2009, the Canadian Cardiovascular Society recommended CRP assessment in patients at “intermediate risk,” which was defined as the predicted risk of a cardiovascular event over the subsequent 10 years of 10% to less than 20%.3 Also in 2009, the National Academy of Clinical Biochemistry Laboratory Medicine Practice Guidelines concluded that measurement of CRP levels might be useful in the stratification of patients at intermediate risk for a cardiovascular event, although the evidence for the usefulness of measures of fibrinogen and other biomarkers of inflammation was considered to be inconclusive.4 A report by the American College of Cardiology Foundation–AHA Task Force on Practice Guidelines in 2010 stated that assessment of CRP levels is reasonable for patients at intermediate risk.5 It is expected that further guidelines regarding these biomarkers will emerge, such as the updated guidelines on cholesterol (Adult Treatment Panel [ATP] IV) that are part of the integrated set of guidelines on cardiovascular risk reduction from the National Heart, Lung, and Blood Institute.6
We analyzed individual records of 246,669 people without a history of cardiovascular disease at baseline from 52 prospective cohort studies. Our aim was to quantify the improvement in the prediction of a first cardiovascular event when the assessment of circulating biomarkers of inflammation was added to the assessment of risk factors used in standard risk scores. Our principal focus was CRP and fibrinogen levels and our secondary focus was leukocyte count and albumin level — all of which are acute-phase reactants.
METHODS
STUDY OVERSIGHT
Details of the Emerging Risk Factors Collaboration have been described previously.7 The current study was designed and conducted by the independent coordinating center of the collaboration and was approved by the ethics review committee in Cambridgeshire, United Kingdom. The first three members and the last member of the writing committee vouch for the accuracy of the data and analyses and submitted the article for publication. GlaxoSmithKline, which provided an unrestricted educational grant to the coordinating center, played no role in the collection, analysis, or reporting of the data.
STUDY COHORTS
Prospective cohort studies were included in the current analysis if they met all the following criteria: participants who did not have a recorded baseline history of cardiovascular disease (i.e., myocardial infarction, angina, or stroke, which were defined in each study at the initial examination) were included; cause-specific deaths or vascular events (nonfatal myocardial infarction or stroke) or both, as assessed according to well-defined criteria, were recorded during follow-up; and data were recorded for more than 1 year of follow-up. In addition, studies were included only if data on baseline levels of CRP, fibrinogen, or both had been obtained and if complete information on age, sex, smoking status, history of diabetes, systolic blood pressure, and levels of total and high-density lipoprotein (HDL) cholesterol (i.e., “conventional risk factors” included in standard clinical risk scores8) had been collected.
Some data used in the reported analyses were available only in a subset of study participants. These included data on leukocyte count, levels of albumin and directly measured low-density lipo-protein (LDL) cholesterol, socioeconomic factors, and family history of cardiovascular disease. In registering fatal outcomes, all contributing studies used International Classification of Disease, revisions 8, 9, and 10, coding to at least three digits, and ascertainment was based on death certificates, with 46 studies also using medical records, autopsy findings, and other supplementary sources. All the studies used a definition of myocardial infarction that was based on World Health Organization (or similar) criteria and a definition of stroke that was based on the results of clinical examination and findings on brain imaging.
STATISTICAL ANALYSIS
For all analyses, values for CRP and leukocyte count were loge-transformed. The main outcome was a first cardiovascular event, defined as a myocardial infarction or fatal coronary heart disease or any stroke. Cox proportional-hazards modeling allowed for the determination of separate baseline hazards according to study and sex, but common coefficients (log hazard ratios) were estimated across studies (see the Supplementary Appendix, available with the full text of this article at NEJM .org). We censored outcomes for patients who died from noncardiovascular causes. The proportional-hazards assumption was satisfied.
Prognostic models were compared with the use of measures of discrimination (i.e., the C-index and the D measure, both of which quantify the degree to which a model can predict the order of disease events)9 and risk reclassification10 (see the Supplementary Appendix). We extended our previous methods9 to include a two-stage approach that allowed for the examination of between-study heterogeneity through calculation of the C-index, the D measure, and changes therein within each individual study before pooling results. Studies were weighted according to the numbers of cardiovascular outcomes contributed. Between-study heterogeneity in the risk-discrimination measures and their changes was quantified with the use of the I2 statistic.11 We used chi-square tests to determine differences in changes in discrimination measures across subgroups. We constructed reclassification tables with data from studies in which both fatal and nonfatal cardiovascular outcomes had been recorded to examine the movement of participants among three predicted 10-year cardiovascular risk categories (“low,” <10%; “intermediate,” 10% to <20%; and “high,” ≥20%) when CRP or fibrinogen values were added to a model that included conventional risk factors.5 For clinical modeling, we assumed that statin treatment reduces cardiovascular risk by 20%.12–14 Analyses were performed with the use of Stata software, version 11.0. All P values are two-sided.
RESULTS
CHARACTERISTICS OF THE STUDY PARTICIPANTS
A total of 52 studies involving 246,669 participants were included in the analysis. Overall, the mean (±SD) age at baseline was 61±9 years. In aggregate, 56% of the participants were men; 57% were from Western Europe, and 34% were from North America. Information on CRP levels was available for 166,596 participants from 38 prospective cohorts (Table S1 in the Supplementary Appendix), among whom there were 13,568 first cardiovascular events (8816 coronary heart disease events and 4752 strokes) during 1.6 million person-years at risk (median number of years to first outcome, 6.7). Information on fibrinogen levels was available for 185,892 participants from 40 cohorts (Table S2 in the Supplementary Appendix), among whom there were 12,021 first cardiovascular events (7475 coronary heart disease events and 4546 strokes) during 1.7 million person-years at risk (median number of years to first outcome, 5.9). Information on both CRP and fibrinogen levels was available for 95,733 participants. Table 1 shows the baseline characteristics of participants who were included in the CRP and fibrinogen analyses, as well as adjusted hazard ratios for first cardiovascular events.
Table 1.
Baseline Characteristics and Hazard Ratios for First-Onset Cardiovascular Disease.*
| Characteristic | Participants with Assessment of C-Reactive Protein (N = 166,596) | Hazard Ratio (95% CI)† | Participants with Assessment of Fibrinogen (N = 185,892) | Hazard Ratio (95% CI)† |
|---|---|---|---|---|
| Mean age at time of survey — yr | 59.7±8.6 | 1.90 (1.86–1.94) | 59.3±8.4 | 1.81 (1.77–1.85) |
| Male sex — no. (%) | 82,077 (49) | NA‡ | 100,530 (54) | NA‡ |
| Current smoker — no. (%) | 35,779 (21) | 1.64 (1.58–1.71) | 46,799 (25) | 1.71 (1.64–1.78) |
| Systolic blood pressure — mm Hg | 136±19 | 1.26 (1.24–1.28) | 137±18 | 1.30 (1.28–1.32) |
| History of diabetes — no. (%) | 10,802 (6) | 1.74 (1.64–1.85) | 11,287 (6) | 1.89 (1.79–2.00) |
| Total cholesterol — mmol/liter | 5.86±1.06 | 1.17 (1.15–1.19) | 5.80±1.08 | 1.19 (1.17–1.21) |
| HDL cholesterol — mmol/liter | 1.32±0.38 | 0.86 (0.84–0.88) | 1.32±0.39 | 0.85 (0.83–0.87) |
| Loge CRP — mg/liter | 0.59±1.09 | 1.20 (1.18–1.22) | — | — |
| Fibrinogen — g/liter | — | — | 3.15±0.74 | 1.15 (1.13–1.17) |
Plus–minus values are means ±SD. Data on C-reactive protein are from 38 studies in which 13,568 participants had a first-ever cardiovascular disease outcome during follow-up, and data on fibrinogen are from 40 studies in which 12,021 participants had a first-ever cardiovascular disease outcome during follow-up. Dashes indicate that summary statistics are not applicable for either CRP or fibrinogen because the models used were based on two separate sets of data to maximize the information on each marker. CRP denotes C-reactive protein.
The hazard ratios were calculated per 1-SD increment in the measured level or as compared with the relevant reference category. Where appropriate, hazard ratios were adjusted for age, sex, smoking status, systolic blood pressure, diabetes status, and levels of total cholesterol, high-density lipoprotein (HDL) cholesterol, and C-reactive protein or fibrinogen.
Hazard ratios according to sex are not available (NA) because these models were stratified by sex.
INCREMENTAL VALUE IN RISK PREDICTION
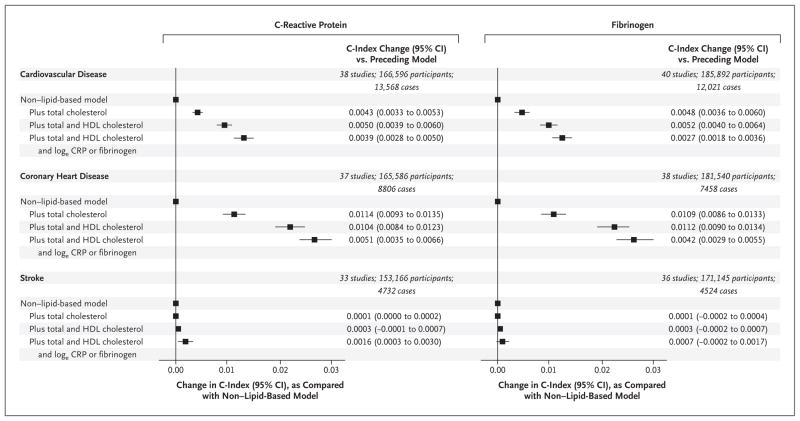
The addition of HDL cholesterol to a prognostic model for first cardiovascular events that included data on age, sex, smoking status, blood pressure, history of diabetes, and total cholesterol increased the C-index by 0.0050 (Fig. 1, and Table S3 in the Supplementary Appendix). The further addition of information on a biomarker of inflammation to this model increased the C-index by 0.0039 with CRP levels and by 0.0027 with fibrinogen levels (Fig. 1, and Table S4 and Fig. S1 and S2 in the Supplementary Appendix), yielding a net reclassification improvement of 1.52% and 0.83%, respectively, for the predicted 10-year risk categories of low (<10%), intermediate (10% to <20%), and high (≥20%) (P<0.02 for both comparisons) (Tables S5 and S6 in the Supplementary Appendix). The corresponding values for the integrated discrimination index were 0.0036 and 0.0027.
Figure 1. Changes in the C-Index after the Addition of Information on Lipid Markers and C-Reactive Protein or Fibrinogen to a Non–Lipid-Based Model.
The nonlipid model denotes a risk score that includes information on age, systolic blood pressure, smoking status, and diabetes status, stratified according to sex. CI denotes confidence interval, CRP C-reactive protein, and HDL high-density lipoprotein.
In analyses that limited the outcome to coronary heart disease, the combined predictive value of total and HDL cholesterol was greater than that of either CRP or fibrinogen (Fig. 1). In contrast, in analyses of stroke, neither the biomarkers of inflammation nor the measures of cholesterol increased the C-index substantially over the C-index for the model that did not include measures of lipids.
In analyses limited to participants with data on both CRP and fibrinogen, the change in the C-index when both markers were used was broadly similar to the change when either marker was used alone (Table 2). The effects of adding information on CRP or fibrinogen were similar in analyses that included measures of body-mass index, family history of cardiovascular disease, or both as risk factors in the standard prediction model (Table S7 in the Supplementary Appendix); in analyses that omitted extreme CRP or fibrinogen values; and in analyses that omitted participants known to be taking medications at study entry that lowered lipid levels or blood pressure (Fig. S3 in the Supplementary Appendix). Total cholesterol levels were also similarly effective in predicting cardiovascular events in participants known to be taking statins and in those not taking statins (P = 0.74 for the interaction).
Table 2.
Changes in the C-Index after the Addition of Information on Biomarkers of Inflammation to a Model Including Conventional Risk Factors.*
| Data Source and Risk Factor | Change in C-Index after Addition of Biomarker (95% CI) | P Value for Comparison with Model Including Conventional Risk Factors | P Value for Comparison with Reference |
|---|---|---|---|
| 25 Studies with 95,733 participants, 6609 with CVD | |||
| Conventional risk factors plus loge CRP | 0.0035 (0.0018–0.0051) | <0.001 | Reference |
| Conventional risk factors plus fibrinogen | 0.0022 (0.0010–0.0035) | <0.001 | 0.13 |
| Conventional risk factors plus loge CRP and fibrinogen | 0.0040 (0.0023–0.0057) | <0.001 | 0.10† |
| 10 Studies with 32,160 participants, 3498 with CVD | |||
| Conventional risk factors plus loge CRP | 0.0031 (0.0010–0.0053) | 0.004 | Reference |
| Conventional risk factors plus loge leukocyte count | 0.0028 (0.0011–0.0045) | 0.002 | 0.78 |
| 17 Studies with 61,002 participants, 8646 with CVD | |||
| Conventional risk factors plus loge CRP | 0.0038 (0.0023–0.0053) | <0.001 | Reference |
| Conventional risk factors plus albumin | 0.0022 (0.0014–0.0030) | <0.001 | 0.05 |
| 15 Studies with 46,699 participants, 5227 with CVD | |||
| Conventional risk factors plus fibrinogen | 0.0037 (0.0020–0.0053) | <0.001 | Reference |
| Conventional risk factors plus loge leukocyte count | 0.0036 (0.0022–0.0051) | <0.001 | 0.97 |
| 16 Studies with 62,502 participants, 6476 with CVD | |||
| Conventional risk factors plus fibrinogen | 0.0034 (0.0020–0.0048) | <0.001 | Reference |
| Conventional risk factors plus albumin | 0.0032 (0.0019–0.0044) | <0.001 | 0.79 |
Conventional risk factors include age, systolic blood pressure, smoking status, diabetes status, and levels of total and HDL cholesterol. “Reference” refers to the comparator model. CRP denotes C-reactive protein, and CVD cardiovascular disease.
P = 0.003 for the comparison of the addition of loge CRP to a model with fibrinogen.
Improvements observed in the C-index when leukocyte count or albumin level was added to the analysis did not differ significantly from those observed with the addition of CRP or fibrinogen (Table 2), although concomitant information on CRP, fibrinogen, and at least one other biomarker of inflammation was available for only about one third of the participants. Results qualitatively similar to those described above were observed in analyses that used the D measure (Table S8 in the Supplementary Appendix). In analyses in which one risk factor at a time was omitted from a model containing conventional risk factors plus measures of CRP or fibrinogen, the results when values for CRP or fibrinogen were omitted were broadly similar to those when values for total or HDL cholesterol were omitted (Fig. S4 in the Supplementary Appendix).
EFFECT OF ASSESSMENT IN CLINICALLY RELEVANT SUBGROUPS
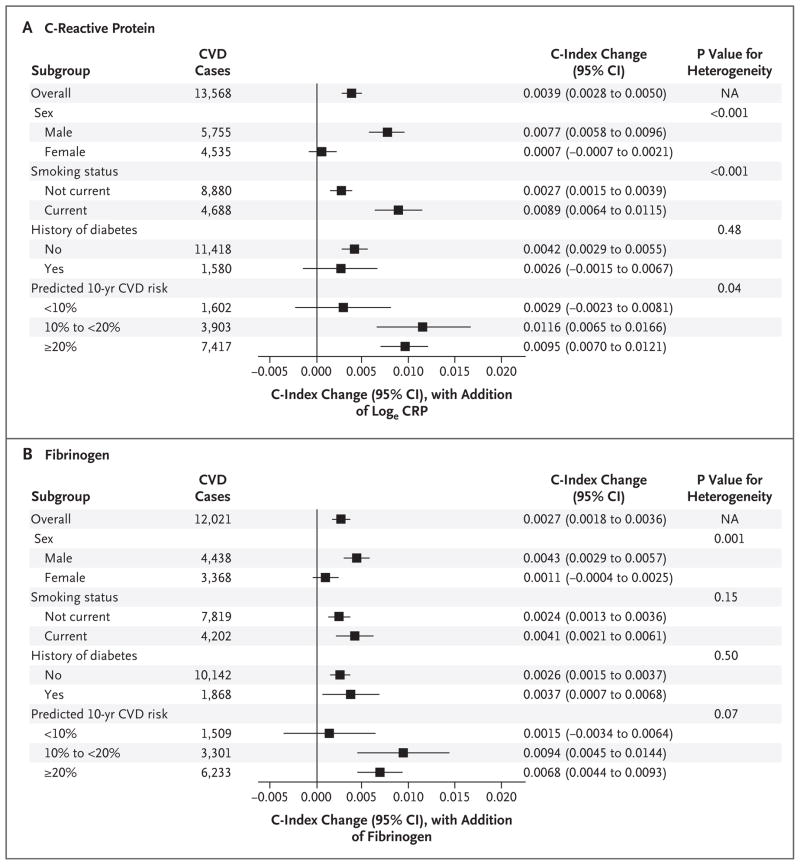
The use of information on CRP and fibrinogen improved cardiovascular risk discrimination in men but not in women (P≤0.001 for the interaction). CRP also had greater predictive value in current smokers than in nonsmokers (P<0.001 for the interaction) (Fig. 2). There were no significant differences in cardiovascular risk discrimination in other clinically relevant subgroups (Fig. 2, and Fig. S3 in the Supplementary Appendix). The differences in the improvement of cardiovascular risk discrimination according to sex with the use of information on CRP and fibrinogen levels persisted in further analyses in which participants were stratified according to smoking status (or in which smokers were omitted) and in analyses in which women who were known to be receiving hormonal treatment at baseline were excluded, although information on such treatments was incomplete.
Figure 2. Changes in the C-Index with the Addition of Information on C-Reactive Protein or Fibrinogen to a Model with Conventional Risk Factors, According to Subgroup.
P values are for differences in the C-index changes across subgroups. Error bars indicate 95% confidence intervals. For each comparison, only studies with information on all subgroup levels were used (hence, subgroup total numbers do not always equal overall total numbers). Since not all the contributing studies had complete information across all subgroup levels, comparisons across subgroups (e.g., men vs. smokers) may not be reliable owing to between-study differences. “Predicted 10-year CVD risk” refers to the predicted 10-year risk of cardiovascular disease events calculated on the basis of the 2008 version of the Framingham risk score.
To further explore sex-specific findings, we assessed reclassification using data only from 15 studies that involved both men and women with at least 10 years of follow-up (19,467 men with 2784 first cardiovascular events and 25,157 women with 2323 first cardiovascular events). In these studies, the net improvement in reclassification with measurement of CRP among men was 1.24% (95% confidence interval [CI], −0.20 to 2.69; P = 0.09), and the net improvement among women was 0.36% (95% CI, −0.70 to 1.42; P=0.51). The effect modification according to sex was not observed with measures of HDL cholesterol or systolic blood pressure (Fig. S5 in the Supplementary Appendix). Similarly, there was no evidence of effect modification according to age when analyses of CRP or fibrinogen were conducted only with data from studies that included participants in each of three age groups (40 to <60 years, 60 to <70 years, and ≥70 years; P = 0.62) (Fig. S6 in the Supplementary Appendix).
ESTIMATE OF THE POTENTIAL FOR DISEASE PREVENTION
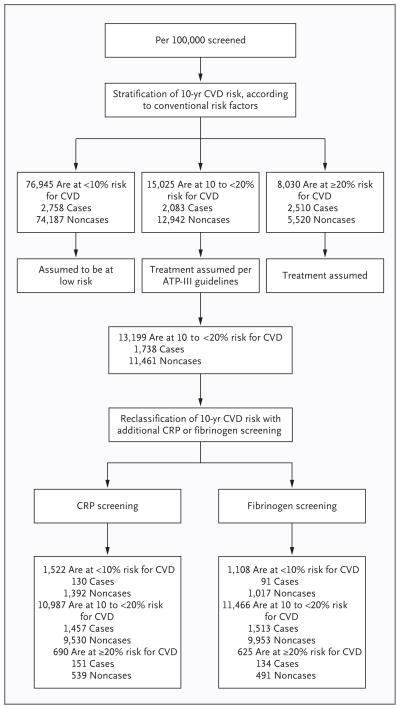
We modeled targeted CRP or fibrinogen assessment in people whose risk of a cardiovascular event was 10% to less than 20% over the subsequent 10 years. In a population of 100,000 adults 40 years of age or older, with an age profile similar to that in the European standard population and with age-specific and sex-specific incidences of cardiovascular events that are assumed to be the same as those observed in the current study (see the Supplementary Appendix), 15,025 persons would initially be classified as having a predicted risk of cardiovascular disease of 10% to less than 20% over a period of 10 years when the risk is calculated with conventional risk factors alone (Fig. 3). Assuming that allocation to statin treatment would be conducted according to the ATP-III guidelines15 (i.e., persons with a predicted risk of ≥20% and those with risk factors, such as diabetes, irrespective of their predicted 10-year risk), 13,199 participants at intermediate risk would currently not be eligible for statin treatment. Additional assessment of CRP in these 13,199 participants would reclassify 690 participants (5.2%) to a predicted risk of 20% or more, of whom approximately 151 would be expected to have a cardiovascular event within 10 years; correspondingly, additional assessment of fibrinogen in the same participants would reclassify 625 (4.7%) to a predicted risk of 20% or more, of whom approximately 134 would be expected to have a cardiovascular event within 10 years. Assuming that persons reclassified as being at a predicted risk of 20% or more would begin statin therapy, in accordance with the ATP-III criteria, such targeted assessment of CRP could help to prevent about 30 (i.e., 0.20×151) additional cardiovascular events over a period of 10 years; the corresponding assessment of fibrinogen could help to prevent about 27 (i.e., 0.20×134) additional cardiovascular events over a 10-year period.
Figure 3. Modeling of Reclassification per 100,000 Persons Initially Screened for Conventional Risk Factors Only and Then Screened for C-Reactive Protein or Fibrinogen.
Reclassification was based on observed data from participants with complete information on conventional risk factors and CRP levels (72,574 participants in 22 studies) or fibrinogen levels (83,061 participants in 27 studies). Adult Treatment Panel (ATP) III guidelines recommend treatment in only 1826 of these people (e.g., persons with diabetes) in the intermediate risk group, leaving 13,199 people in this group currently ineligible for treatment with statins. Details of the modeling are available in the Supplementary Appendix. CVD denotes cardiovascular disease.
In other words, targeted assessment of CRP in people at intermediate risk for a cardiovascular event could help to prevent one additional event over the course of 10 years for every 440 people so screened (i.e., 13,199 ÷ 30), as a result of about 23 additional people starting statin therapy (i.e., 5.2%×440). The corresponding number needed to screen with targeted fibrinogen assessment would be about 490 (i.e., 13,199 ÷ 27). Alternatively, assuming that only people with a predicted risk of 20% or more were started on statin therapy, as recommended in Canadian3 and U.K.16 guidelines, the assessment of CRP could help prevent one extra cardiovascular event for approximately 360 people at intermediate risk so screened (Fig. S7 in the Supplementary Appendix). If subsequent analyses confirm that such improvements are limited to men, one extra cardiovascular event could be prevented for approximately every 390 men at intermediate risk in whom CRP levels were assessed (the corresponding number for targeting women would be one event prevented for every 740 women with CRP assessment).
DISCUSSION
In an analysis comprising individual participant data from 52 prospective cohort studies, we evaluated the effects of adding information on CRP or fibrinogen to the standard risk factors used to predict the risk of a first cardiovascular event. We then modeled a scenario in which CRP or fibrinogen was assessed in people considered to be at intermediate risk (a predicted risk of 10% to <20% over a period of 10 years) after initial screening with the use of conventional risk factors alone. If such measurement of a biomarker of inflammation were to be coupled with initiation of statin therapy in accordance with the recommendations of the ATP-III guidelines,15 our data suggest that one extra cardiovascular disease outcome would be prevented over a period of 10 years for approximately every 440 people in whom CRP levels were assessed or approximately every 490 people in whom fibrinogen levels were assessed, as a result of the initiation of statin therapy in about 23 additional people. Analyses in which both CRP and fibrinogen levels were included showed results that were broadly similar to those in which either biomarker alone was used.
Our main model assumed that the assessment of CRP or fibrinogen would provide similar predictions of the risk of cardiovascular events across population subgroups. However, a finding from an exploratory analysis suggested that these biomarkers improve risk discrimination only in men. This result could, at least to some extent, be due to the play of chance, since we studied the interaction of CRP and fibrinogen with a number of factors, and there is currently no good explanation for the finding. We therefore believe that this finding requires cautious interpretation. Nevertheless, if this finding were confirmed in subsequent analyses, targeting the assessment of CRP or fibrinogen only in men at intermediate risk should enhance screening efficiency.
Recently developed risk prediction scores have tended to combine the outcomes of coronary heart disease and stroke because these outcomes have similar risk factors and treatments; therefore, the primary outcome of our study was any first cardiovascular event (defined as fatal or nonfatal coronary heart disease or stroke). However, some risk scores relate solely to coronary heart disease17 and others relate solely to stroke.18 Our results confirm that the relative usefulness of risk factors in prediction can vary considerably depending on whether the outcome chosen includes all cardiovascular events or coronary heart disease events only. Furthermore, risk scores proposed solely for the prediction of stroke may involve additional risk factors that are not considered here, such as atrial fibrillation.18
The strengths and potential limitations of this study merit consideration. Our analysis involved populations from 16 countries. We found broadly concordant results among several measures of risk reclassification and discrimination. Although we used a conventional 10-year time frame and standard clinical risk categories, we acknowledge that such reclassification analyses are sensitive to the choices of landmark time and risk categories. We found that the differences among studies in levels of biomarkers of inflammation contributed relatively little to the heterogeneity in our results, which was due mostly to differing age ranges across cohorts. We had incomplete information on statin use, which may have influenced our estimates of the effect of individual risk factors or risk models on outcomes. Our models could have overestimated the potential benefits of statins because we assumed that all eligible people would receive them. Conversely, somewhat greater clinical effect than that estimated here would be projected if we had used less conservative modeling assumptions, such as more efficacious statin regimens,12,19 or longer time horizons, such as those in lifetime risk estimates. However, the findings from this study should aid in the modeling of such scenarios.
Several other issues warrant further investigation. The measurement of biomarkers of inflammation should be compared with the use of other emerging biomarkers (including imaging biomarkers)4,5,20–23 with regard to cost-effectiveness,24,25 practicability, and clinical benefit.26 Because prospective associations have previously been observed between the biomarkers studied here and the risk of several major nonvascular diseases as well as cardiovascular disease,27–29 further study is needed to determine the value of these biomarkers in screening concurrently for various chronic diseases.30
In conclusion, we analyzed individual records from 52 cohort studies to investigate the value of adding information on CRP or fibrinogen levels to conventional models for the prediction of cardiovascular risk among people without known cardiovascular disease. We estimated that under current treatment guidelines, after initial screening with conventional risk factors alone, the additional assessment of CRP or fibrinogen in people at intermediate risk for a cardiovascular event could help prevent one additional event over a period of 10 years for every 400 to 500 people so screened.
Acknowledgments
Supported by a grant from the British Heart Foundation (RG/08/014), the U.K. Medical Research Council, the U.K. National Institute of Health Research Cambridge Biomedical Research Centre, a grant from the British United Provident Association Foundation, an unrestricted educational grant from GlaxoSmithKline, and others through support of the cohorts contributing data to the Emerging Risk Factors Collaboration (the investigators of several of these studies have contributed to other studies; a list of these studies that cites relevant funding sources can be found at http://ceu.phpc.cam.ac.uk/research/erfc/studies).
APPENDIX
The members of the writing committee for this report are as follows: Stephen Kaptoge, Emanuele Di Angelantonio, Lisa Pennells, and Angela M. Wood — all at the University of Cambridge, Cambridge, U.K.; Ian R. White, Medical Research Council (MRC) Biostatistics Unit, Cambridge, U.K.; Pei Gao, Matthew Walker, Alexander Thompson, and Nadeem Sarwar — all at the University of Cambridge, Cambridge, U.K.; Muriel Caslake, University of Glasgow, Glasgow, U.K.; Adam S. Butterworth, University of Cambridge, Cambridge, U.K.; Philippe Amouyel, Institut Pasteur de Lille, France; Gerd Assmann, Assmann-Stiftung für Prävention, Germany; Stephan J.L. Bakker, University Medical Center Groningen and University of Groningen, Groningen, the Netherlands; Elizabeth L.M. Barr, Baker IDI Heart and Diabetes Institute, Australia; Elizabeth Barrett-Connor, University of California, San Diego; Emelia J. Benjamin, Boston University, Boston; Cecilia Björkelund, University of Gothenburg, Gothenburg, Sweden; Hermann Brenner, German Cancer Research Center, Heidelberg, Germany; Eric Brunner, University College London, London; Robert Clarke, University of Oxford, Oxford, U.K.; Jackie A. Cooper, University College London, London; Peter Cremer, Klinikum der Universität München LMU, Munich, Germany; Mary Cushman, University of Vermont, Burlington; Gilles R. Dagenais, Institut Universitaire de Cardiologie et Pneumologie de Québec, Quebec, Canada; Ralph B. D’Agostino, Sr., Boston University, Boston; Rachel Dankner, Gertner Institute for Epidemiology and Health Policy Research, Ramat Gan, Israel; George Davey-Smith, University of Bristol, Bristol, U.K.; Dorly Deeg and Jacqueline M. Dekker — both at Vrije Universiteit Medical Center, Amsterdam; Gunnar Engström, Lund University, Lund, Sweden; Aaron R. Folsom, University of Minnesota, Minneapolis; F. Gerry R. Fowkes, University of Edinburgh, Edinburgh; John Gallacher, Cardiff University, Cardiff, U.K.; J. Michael Gaziano, Harvard Medical School, Boston; Simona Giampaoli, Istituto Superiore di Sanità, Rome; Richard F. Gillum, Centers for Disease Control and Prevention, Hyattsville, MD; Albert Hofman, Erasmus MC, Rotterdam, the Netherlands; Barbara V. Howard, Medstar Health Research Institute, Hyattsville, MD; Erik Ingelsson, Karolinska Institutet, Stockholm; Hiroyasu Iso, Osaka University, Osaka, Japan; Torben Jørgensen, Glostrup University Hospital, Glostrup, Denmark, and University of Copenhagen, Copenhagen; Stefan Kiechl, Medical University Innsbruck, Innsbruck, Austria; Akihiko Kitamura, Osaka Medical Center for Health Science and Promotion, Osaka, Japan; Yutaka Kiyohara, Kyushu University, Fukuoka City, Japan; Wolfgang Koenig, University of Ulm Medical Center, Ulm, Germany; Daan Kromhout, Wageningen University, Wageningen, the Netherlands; Lewis H. Kuller, University of Pittsburgh, Pittsburgh; Debbie A. Lawlor, University of Bristol, Bristol, U.K.; Tom W. Meade, London School of Hygiene and Tropical Medicine, London; Aulikki Nissinen, National Institute for Health and Welfare, Helsinki, Finland; Børge G. Nordestgaard, University of Copenhagen, Copenhagen; Altan Onat, Istanbul University, Istanbul, Turkey; Demosthenes B. Panagiotakos, Harokopio University, Athens; Bruce M. Psaty, University of Washington, Seattle; Beatriz Rodriguez, University of Hawaii, Honolulu; Annika Rosengren, Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden; Veikko Salomaa, National Institute for Health and Welfare, Helsinki, Finland; Jussi Kauhanen, University of Eastern Finland, Kuopio; Jukka T. Salonen, Metabolic Analytical Services, Helsinki; Jonathan A. Shaffer and Steven Shea — both at Columbia University Medical Center, New York; Ian Ford, University of Glasgow, Glasgow, U.K.; Coen D.A. Stehouwer, Maastricht University Medical Centre, Maastricht, the Netherlands; Timo E. Strandberg, University of Helsinki and University of Oulu, Oulun Yliopisto, Finland; Robert W. Tipping, Merck Research Laboratories, Philadelphia; Alberto Tosetto, San Bortolo Hospital, Vicenza, Italy; Sylvia Wassertheil-Smoller, Albert Einstein College of Medicine, New York; Patrik Wennberg, Umeå University, Umeå, Sweden; Rudi G. Westendorp, Leiden University Medical Center, Leiden, the Netherlands; Peter H. Whincup, St. George’s, University of London, London; Lars Wilhelmsen, University of Gothenburg, Gothenburg, Sweden; Mark Woodward, University of Sydney, Sydney; Gordon D.O. Lowe, University of Glasgow, Glasgow, U.K.; Nicholas J. Wareham, MRC Epidemiology Unit, Cambridge, U.K.; Kay-Tee Khaw, University of Cambridge, Cambridge, U.K.; Naveed Sattar and Chris J. Packard — both at University of Glasgow, Glasgow, U.K.; Vilmundur Gudnason, Icelandic Heart Association and University of Iceland, Kópavogur; Paul M. Ridker, Brigham and Women’s Hospital, Boston; Mark B. Pepys, University College London, London; and Simon G. Thompson and John Danesh — both at University of Cambridge, Cambridge, U.K.
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Pearson TA, Mensah GA, Alexander RW, et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107:499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- 2.Graham I, Atar D, Borch-Johnsen K, et al. European guidelines on cardiovascular disease prevention in clinical practice: executive summary. Eur Heart J. 2007;28:2375–414. doi: 10.1093/eurheartj/ehm316. [DOI] [PubMed] [Google Scholar]
- 3.Genest J, McPherson R, Frohlich J, et al. 2009 Canadian Cardiovascular Society/Canadian guidelines for the diagnosis and treatment of dyslipidemia and prevention of cardiovascular disease in the adult — 2009 recommendations. Can J Cardiol. 2009;25:567–79. doi: 10.1016/s0828-282x(09)70715-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Myers GL, Christenson RH, Cushman M, et al. National Academy of Clinical Biochemistry Laboratory Medicine Practice Guidelines: emerging biomarkers for primary prevention of cardiovascular disease. Clin Chem. 2009;55:378–84. doi: 10.1373/clinchem.2008.115899. [DOI] [PubMed] [Google Scholar]
- 5.Greenland P, Alpert JS, Beller GA, et al. 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2010;56(25):e50–e103. doi: 10.1016/j.jacc.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 6.National Heart, Lung, and Blood Institute. Detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel IV) ( http://www.nhlbi.nih.gov/guidelines/cholesterol/atp4/index.htm) [PubMed]
- 7.Emerging Risk Factors Collaboration. The Emerging Risk Factors Collaboration: analysis of individual data on lipid, inflammatory and other markers in over 1. 1 million participants in 104 prospective studies of cardiovascular diseases. Eur J Epidemiol. 2007;22:839–69. doi: 10.1007/s10654-007-9165-7. [DOI] [PubMed] [Google Scholar]
- 8.D’Agostino RB, Sr, Vasan RS, Pencina MJ, et al. General cardiovascular risk profile for use in primary care: the Framing-ham Heart Study. Circulation. 2008;117:743–53. doi: 10.1161/CIRCULATIONAHA.107.699579. [DOI] [PubMed] [Google Scholar]
- 9.Fibrinogen Studies Collaboration. Measures to assess the prognostic ability of the stratified Cox proportional hazards model. Stat Med. 2009;28:389–411. doi: 10.1002/sim.3378. [DOI] [PubMed] [Google Scholar]
- 10.Pencina MJ, D’Agostino RB, Sr, D’Agostino RB, Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157–72. doi: 10.1002/sim.2929. [DOI] [PubMed] [Google Scholar]
- 11.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–58. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 12.Cholesterol Treatment Trialists’ (CTT) Collaborators. The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: meta-analysis of individual data from 27 randomised trials. Lancet. 2012;380:581–90. doi: 10.1016/S0140-6736(12)60367-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heart Protection Study Collaborative Group. C-reactive protein concentration and the vascular benefits of statin therapy: a prospective analysis of 20,536 patients in the Heart Protection Study. Lancet. 2011;377:469–76. doi: 10.1016/S0140-6736(10)62174-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ridker PM, MacFadyen J, Libby P, Glynn RJ. Relation of baseline high-sensitivity C-reactive protein level to cardiovascular outcomes with rosuvastatin in the Justification for Use of Statins in Prevention: An Intervention Trial Evaluating Rosuvastatin (JUPITER) Am J Cardiol. 2010;106:204–9. doi: 10.1016/j.amjcard.2010.03.018. [DOI] [PubMed] [Google Scholar]
- 15.Executive summary of the third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–97. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 16.Cooper A, O’Flynn N. Risk assessment and lipid modification for primary and secondary prevention of cardiovascular disease: summary of NICE guidance. BMJ. 2008;336:1246–8. doi: 10.1136/bmj.39554.624086.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wilson PWF, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–47. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 18.Wolf PA, D’Agostino RB, Belanger AJ, Kannel WB. Probability of stroke: a risk profile from the Framingham Study. Stroke. 1991;22:312–8. doi: 10.1161/01.str.22.3.312. [DOI] [PubMed] [Google Scholar]
- 19.Ridker PM, Danielson E, Fonseca FAH, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359:2195–207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- 20.Emerging Risk Factors Collaboration. Lipoprotein(a) concentration and the risk of coronary heart disease, stroke, and nonvascular mortality. JAMA. 2009;302:412–23. doi: 10.1001/jama.2009.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Idem. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet. 2010;375:2215–22. doi: 10.1016/S0140-6736(10)60484-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lp-PLA2 Studies Collaboration. . Lipo-protein-associated phospholipase A2 and risk of coronary disease, stroke, and mortality: collaborative analysis of 32 prospective studies. Lancet. 2010;375:1536–44. doi: 10.1016/S0140-6736(10)60319-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Di Angelantonio E, Chowdhury R, Sarwar N, et al. B-type natriuretic peptides and cardiovascular risk: systematic review and meta-analysis of 40 prospective studies. Circulation. 2009;120:2177–87. doi: 10.1161/CIRCULATIONAHA.109.884866. [DOI] [PubMed] [Google Scholar]
- 24.Blake GJ, Ridker PM, Kuntz KM. Potential cost-effectiveness of C-reactive protein screening followed by targeted statin therapy for the primary prevention of cardiovascular disease among patients without overt hyperlipidemia. Am J Med. 2003;114:485–94. doi: 10.1016/s0002-9343(03)00074-3. [DOI] [PubMed] [Google Scholar]
- 25.Lee KK, Cipriano LE, Owens DK, Go AS, Hlatky MA. Cost-effectiveness of using high-sensitivity C-reactive protein to identify intermediate- and low-cardiovascular-risk individuals for statin therapy. Circulation. 2010;122:1478–87. doi: 10.1161/CIRCULATIONAHA.110.947960. [DOI] [PubMed] [Google Scholar]
- 26.Hlatky MA, Greenland P, Arnett DK, et al. Criteria for evaluation of novel markers of cardiovascular risk: a scientific statement from the American Heart Association. Circulation. 2009;119:2408–16. doi: 10.1161/CIRCULATIONAHA.109.192278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Emerging Risk Factors Collaboration. C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: an individual participant meta-analysis. Lancet. 2010;375:132–40. doi: 10.1016/S0140-6736(09)61717-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fibrinogen Studies Collaboration. Plasma fibrinogen level and the risk of major cardiovascular diseases and non-vascular mortality: an individual participant meta-analysis. JAMA. 2005;294:1799–809. doi: 10.1001/jama.294.14.1799. [DOI] [PubMed] [Google Scholar]
- 29.Danesh J, Collins R, Appleby P, Peto R. Association of fibrinogen, C-reactive protein, albumin, or leukocyte count with coronary heart disease: meta-analyses of prospective studies. JAMA. 1998;279:1477–82. doi: 10.1001/jama.279.18.1477. [DOI] [PubMed] [Google Scholar]
- 30.Godsland IF, North BV, Johnston DG. Simple indices of inflammation as predictors of death from cancer or cardiovascular disease in a prospective cohort after two decades of follow-up. QJM. 2011;104:387–94. doi: 10.1093/qjmed/hcq213. [DOI] [PubMed] [Google Scholar]