Abstract
A synthesis of 11-methoxy mitragynine pseudoindoxyl, a new member of the mitragynine class of opioid agonists, from a derivative of the Geissman-Waiss lactone is described. An internal attack of an electron-rich aromatic ring on an electrophilic nitrilium ion and a late-stage construction of the functionalized piperidine ring by the method of reductive cyclization are the pivotal transformations; both ring annulations proceed in a highly diastereoselective fashion. The construction of substituted indoxyl frameworks by the interrupted Ugi method offers an attractive alternative to the strategy of oxidatively rearranging indoles.
Introduction
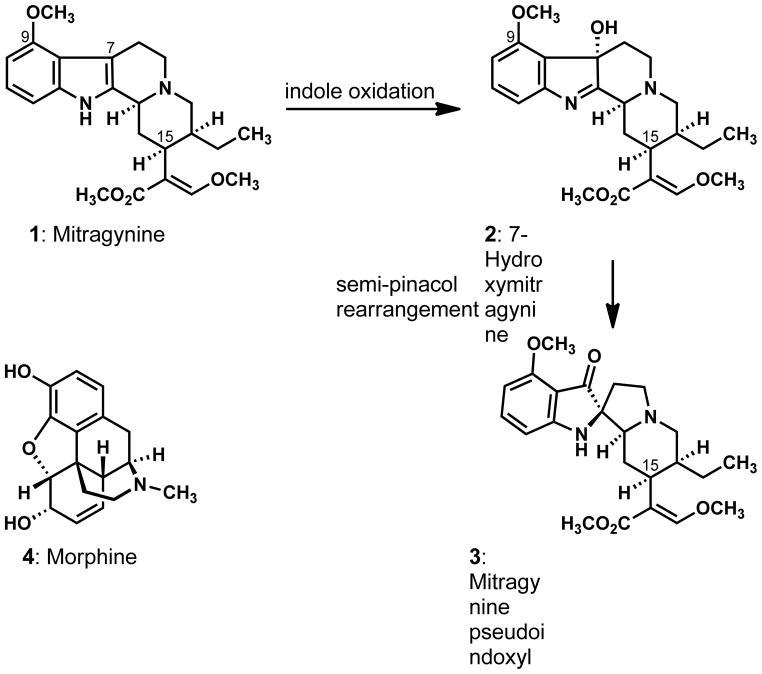
Mitragyna speciosa Korth, also known as the Kratom tree, is native to South East Asia. Extracts from the leaves of this Rubiaceae tree are known as “kratom”, and their stimulatory and powerful pain-relieving effects have been known for centuries in Thailand.1 Historically, kratom leaves were used as a substitute for opium, and, more than 100 years ago, they earned distinction as a remedy for preventing or ameliorating the effects of opium withdrawal. The mature leaves of Mitragyna speciosa, which were described by Pieter Korthals as resembling the shape of a bishop’s mitre, contain the indole alkaloid mitragynine (1) as a major constituent, as well as smaller amounts of several other alkaloids including 7-hydroxymitragynine (2).2 An oxidation of the indole nucleus of mitragynine (1) produces compound 2, which can undergo a base-induced rearrangement to the isomeric metabolite mitragynine pseudoindoxyl (3).3–5 Compounds 1–3 display potent opioid agonistic activities in guinea pig ileum and mouse vas deferens assays.6 7-Hydroxymitragynine (2) and mitragynine pseudoindoxyl (3) are more active as opioid agonists than the parent indole mitragynine (1), and all three compounds are more potent than the well-known opiate morphine (4).6c, 1c The significance of kratom to the traditional medicine of Thailand and Malaysia, the unique molecular structures of compounds 1–3, which are distinct from the structures of the naturally occurring opiates, and the pharmacological investigations that have been reported to date justify continued efforts to expand the therapeutic potential and the structural diversity of the mitragynine family of natural products.
A guiding concept for synthesis
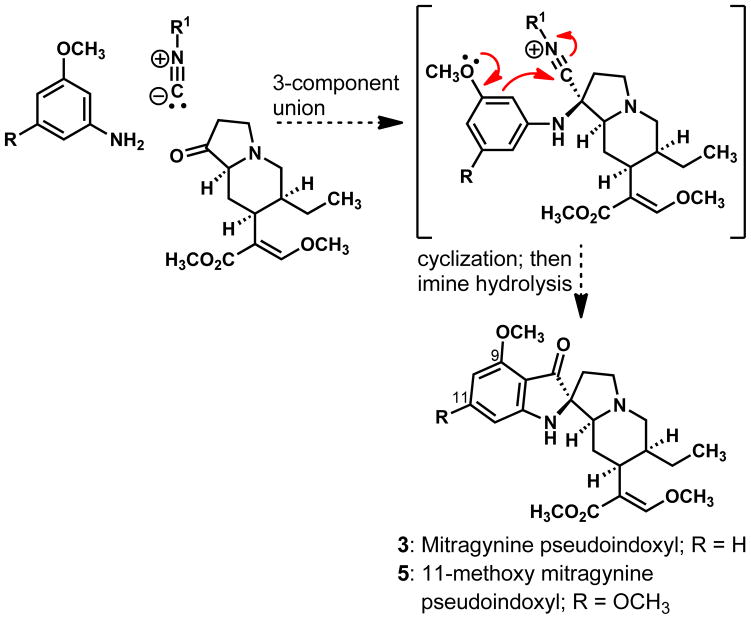
Mitragynine (1) and its derivatives 2 and 3 are believed to arise in nature from the Battersby-Arigoni biosynthetic route to indole alkaloids by analogy to the derivation of corynantheidine from strictosidine.7 The only structural feature that differentiates mitragynine (1) from corynantheidine is the methoxyl group at C-9, and it has been reported that this single methoxyl group is essential for the opioid agonistic activity of compound 1;6c this group is presumed to be vital to the analgesic activities of 7-hydroxymitragynine (2) and mitragynine pseudoindoxyl (3) as well. Our laboratory was mindful of the biologic imperative of the C-9 methoxyl group and drawn to the idea that its donor properties would reinforce the directing effect of the aniline nitrogen and facilitate the ring annulation outlined in Scheme 1, a cornerstone idea in our planned synthesis of mitragynine pseudoindoxyl (3) and related compounds. This appealing concept for constructing the spiro-fused indoxyl system would offer an alternative to the method of oxidatively rearranging indoles to indoxyls, which had served the previously reported semi-synthesis3a and total synthesis5 of mitragynine pseudoindoxyl (3) from mitragynine (1).
Scheme 1.
A concept for a synthesis of the indoxyl ring system.
Inspired by the structure of compound 3, our laboratory developed a simple and useful method for synthesizing substituted indoxyl and 3-amino indole motifs;8 the method combines mechanistic elements of both the Ugi and Houben-Hoesch reactions and features intramolecular additions of arenes to electrophilic nitrilium ions in a three-component coupling of an aniline, an isocyanide, and an aldehyde or ketone. This ‘interrupted Ugi method’ for heterocycle synthesis produces a new carbon-nitrogen bond and two carbon-carbon bonds and is especially effective when the aromatic ring of the aniline component bears at least one π-donor atom in addition to the nitrogen atom that enables the initial condensation with the carbonyl component. Our studies of internal trappings of nitrilium ions by pendent, electron-rich aromatic rings culminated in a highly diastereoselective synthesis of 11-methoxy mitragynine pseudoindoxyl (5), a new member of the mitragynine family. The manner in which this synthesis was achieved is the subject of this report.9
Results and discussion
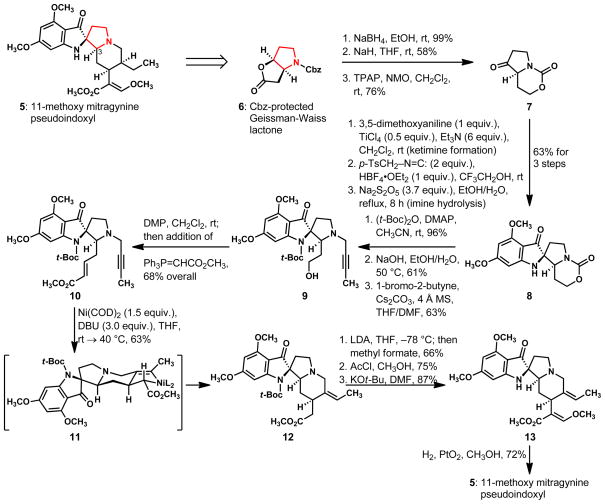
Our experiences with this chemical problem led us to favor a design wherein the substituted pyrrolidine ring at the heart of the structure (see highlighted ring in 5, Scheme 2) would become the foundation for the synthesis. Compound 6, with its serviceable pyrrolidine frame, C–O bond, and nitrogen-bearing stereocenter, emerged as an especially attractive starting material for the effort; this substance is the Cbz-protected derivative of the Geissman-Waiss lactone10 and available by an effective, three-step synthesis.11 The flexibility that this compound bestows to synthesis planning allowed our laboratory to investigate the feasibility of both “late-stage” and “early-stage” annulations of the spiro-fused indoxyl frame of 11-methoxy mitragynine pseudoindoxyl (5). As matters transpired, the latter strategy featuring an early-stage introduction of the indoxyl structure proved superior.12 This design came to fruition by the successful execution of the structural transformations shown in Scheme 2 and commenced with the three-step conversion of compound 6 to bicyclic ketone 7; in this sequence, the reductive cleavage of the lactone ring of 6 was followed sequentially by the formation of the cyclic carbamate via an internal attack of a primary alkoxide ion on the carbonyl of the N-Cbz group and a Ley-Griffith oxidation13 of the remaining secondary alcohol to the desired ketone 7.
Scheme 2.
A synthesis of 11-methoxy mitragynine pseudoindoxyl (5) from a derivative of the Geissman-Waiss lactone (6). Cbz = benzyloxycarbonyl; TPAP = tetrapropylammonium perruthenate; NMO = N-methylmorpholine-N-oxide; DMAP = 4-dimethylaminopyridine; DMP = Dess-Martin periodinane; COD = cyclooctadiene; DBU = 1,8-diazabicyclo[5.4.0]undec-7-ene; LDA = lithium diisopropylamide; Ac = C(O)CH3; THF = tetrahydrofuran; DMF = N,N-dimethylformamide; MS = molecular sieves.
The concave nature of bicycle 7 was anticipated to favor a diastereoselective outcome in the pivotal attack of an isocyanide on an iminium ion derived from the keto group. Our intent was to advance 7 to the tetracyclic indoxyl 8 by a sequence involving an in situ imine formation with a concomitant, face-selective attack of an isocyanide on a reactive iminium ion, and a subsequent imine hydrolysis. While this direct channel for synthesis is possible, we found that higher overall yields of 8 are obtained if 7 is first condensed with 3,5-dimethoxyaniline under the conditions shown, followed by exposure of the resulting, crude secondary ketimine to the combined action of fluoroboric acid diethyl ether complex and tosylmethyl isocyanide. In the wake of this process, which afforded a new carbon-nitrogen and two carbon-carbon bonds, the intermediate, spiro-fused tosylmethyl imine was heated at 95 °C for 8 hours in a mixture of water and ethanol containing sodium metabisulfite14 to accomplish the desired hydrolysis. By this sequence of reactions, compound 8 was produced as a single diastereoisomer and isolated in an overall yield of 63%. In the second stage of the construction, fluoroboric acid diethyl ether complex is a sufficiently strong acid catalyst with a non-nucleophilic conjugate base that does not compete with the π-electron-rich aromatic ring in the attack on the putative nitrilium ion. Incidentally, 3-methoxyaniline will participate in this type of ring annulation as well, however, the more electron-rich and symmetrical 3,5-dimethoxyaniline affords the desired indoxyl as a single regioisomer and in higher yield; 3-methoxyaniline preferentially affords an indoxyl regioisomer with a para relationship between the methoxyl and the indoxyl keto groups.
In response to the problem of constructing the functionalized piperidine ring, we envisioned a strategy in which unsaturated side chains on adjacent atoms would join in the course of a metal-mediated, reductive cyclization. In the planning phase, some of the published examples of low-valent nickel-induced reductive cyclizations from the Montgomery laboratory15 were especially supportive of this general idea. To investigate the feasibility of a Montgomery cyclization in our synthesis, we selected compound 10 as a subgoal for the effort and achieved its synthesis in four steps from compound 8. Thus, a high-yielding protection of the indole nitrogen in 8 was followed by a selective, hydrolytic cleavage of the cyclic carbamate under basic conditions. The latter transformation enabled a subsequent N-alkylation reaction with 1-bromo-2-butyne and a final, one-flask oxidative homologation to ester 10. We were delighted to find that the combined action of bis(cyclooctadiene)nickel(0) and 1,8-diazabicycloundec-7-ene (DBU) on compound 10 in tetrahydrofuran at 40 °C causes a highly diastereoselective, reductive cyclization to the desired tetracycle 12.15, 16 This metal-templated reaction presumably passes through the metalacyclic intermediate 11 and produces the desired piperidine ring, as well as the geometrically defined ethylidene function. A simple protonation of the carbon-nickel bonds during workup completes the construction of 12, which was isolated as a single diastereoisomer in 63% yield.17
While efforts to directly form the vinylogous carbonate moiety found in the mitragynine class by condensing compound 12 with trimethyl orthoformate were unsuccessful, we achieved the conversion of 12 to 13 via the same three-step sequence of reactions that had served Takayama’s pioneering sythesis of mitragynine (1)5 and the more recent syntheses of this alkaloid by the groups of Cook18 and Ma.19 The second step in this sequence produces a dimethyl acetal function β to the methoxycarbonyl group and causes removal of the t-Boc protecting group under conditions that liberate HCl. After a simple base-mediated, E1cb elimination of methoxide ion to give compound 13, the exocyclic alkene was hydrogenated in the presence of Adam’s catalyst. The latter transformation proceeded in a completely diastereoface-selective fashion and afforded 11-methoxy mitragynine pseudoindoxyl (5) in 72% yield.
Conclusions
This synthesis has afforded a new member of the mitragynine class, and we are now in a position to evaluate its analgesic properties. Since mitragynine pseudoindoxyl (3) is significantly more active as an opioid agonist than the parent mitragynine (1), new compounds with modified aromatic rings are of immediate interest as opioid agonists and potential analgesics. In a sense, this work is the culmination of our development of a new, convergent, and non-oxidative method for synthesizing structurally complex indoxyl motifs.8, 9 However, we now have an opportunity to leverage the strategy and chemical reactions described herein in syntheses of additional new members of the mitragynine class of opioid agonists.
Supplementary Material
Figure 1.
The molecular structures of mitragynine (1), 7-hydroxymitragynine (2), mitragynine pseudoindoxyl (3), and morphine (4).
Acknowledgments
We gratefully acknowledge financial support from the National Institute of General Medical Sciences (GM065483) to E.J.S., a NIH postdoctoral fellowship (F32GM078910) to J.S.S., Jr., and the Horst Witzel Fellowship in Organic Chemistry from Cephalon, Inc. to J.K. We thank Drs. István Pelczer and John Eng (both of Princeton University) for spectroscopic assistance.
Footnotes
Electronic Supplementary Information (ESI) available: [details of any supplementary information available should be included here]. See DOI: 10.1039/b000000x/
References
- 1.(a) Jansen KLR, Prast CJ. J Ethnopharmacol. 1988;23:115–119. doi: 10.1016/0378-8741(88)90121-3. [DOI] [PubMed] [Google Scholar]; (b) Jansen KLR, Prast CJ. J Psychoact Drugs. 1988;20(4):455–457. doi: 10.1080/02791072.1988.10472519. [DOI] [PubMed] [Google Scholar]; (c) Rosenbaum CD, Carreiro SP, Babu KM. J Med Toxicol. 2012;8:15–32. doi: 10.1007/s13181-011-0202-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.(a) Ponglux D, Wongseripipatana S, Takayama H, Kikuchi M, Kurihara M, Kitajima M, Aimi N, Sakai S. Planta Medica. 1994;60:580–581. doi: 10.1055/s-2006-959578. [DOI] [PubMed] [Google Scholar]; (b) Kikura-Hanajiri R, Kawamura M, Maruyama T, Kitajima M, Takayama H, Goda Y. Forensic Toxicol. 2009;27:67–74. [Google Scholar]
- 3.Microbial transformation of mitragynine by the fungus Helminthosporum sp. produced mitragynine pseudoindoxyl, see: Zarembo JE, Douglas B, Valenta J, Weisbach JA. J Pharm Sci. 1974;63(9):1407–1415. doi: 10.1002/jps.2600630916.For an earlier example of an indole → indoxyl transformation by oxidative rearrangement, see: Finch N, Taylor WI, Ulshafer PR. Experientia. 1963;XIX(6):296. doi: 10.1007/BF02150414.
- 4.Takayama H, Kurihara M, Subhadhirasakul S, Kitajima M, Aimi N, Sakai S-i. Heterocycles. 1996;42(1):87–92. [Google Scholar]
- 5.For the first total synthesis of mitragynine, which also described the mitragynine → mitragynine pseudoindoxyl oxidative rearrangement, see: Takayama H, Aimi N, Sakai S-i. Yakugaku Zasshi. 2000;120(10):959–967. doi: 10.1248/yakushi1947.120.10_959.The oxidative rearrangement was achieved in three steps with an overall yield of 21%.
- 6.(a) Watanabe K, Yano S, Horie S, Yamamoto LT. Life Sci. 1997;60(12):933–942. doi: 10.1016/s0024-3205(97)00023-4. [DOI] [PubMed] [Google Scholar]; (b) Yamamoto LT, Horie S, Takayama H, Aimi N, Sakai S-i, Yano S, Shan J, Pang PKT, Ponglux D, Watanabe K. Gen Pharmacol. 1999;33:73–81. doi: 10.1016/s0306-3623(98)00265-1. [DOI] [PubMed] [Google Scholar]; (c) Takayama H, Ishikawa H, Kurihara M, Kitajima M, Aimi N, Ponglux D, Koyama F, Matsumoto K, Moriyama T, Yamamoto LT, Watanabe K, Murayama T, Horie S. J Med Chem. 2002;45:1949–1956. doi: 10.1021/jm010576e. [DOI] [PubMed] [Google Scholar]; (d) Matsumoto K, Horie S, Ishikawa H, Takayama H, Aimi N, Ponglux D, Watanabe K. Life Sci. 2004;74:2143–2155. doi: 10.1016/j.lfs.2003.09.054. [DOI] [PubMed] [Google Scholar]; (e) Matsumoto K, Horie S, Takayama H, Ishikawa H, Aimi N, Ponglux D, Murayama T, Watanabe K. Life Sci. 2005;78:2–7. doi: 10.1016/j.lfs.2004.10.086. [DOI] [PubMed] [Google Scholar]
- 7.(a) Battersby AR, Byrne JC, Kapil RS, Martin JA, Payne TG. Chem Commun. 1968:951–953. [Google Scholar]; (b) Nagakura N, Rüffer M, Zenk MH. J Chem Soc, Perkin Trans I. 1979:2308–2312. [Google Scholar]
- 8.(a) Deyrup JA, Vestling MM, Hagan WV, Yun HY. Tetrahedron. 1969;25:1467–1478. [Google Scholar]; (b) Schneekloth JS, Jr, Kim J, Sorensen EJ. Tetrahedron. 2009;65:3096–3101. doi: 10.1016/j.tet.2008.08.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.This manuscript is based on the Ph. D. thesis of Jimin Kim, Princeton University, September 2010.
- 10.Geissman T, Waiss AC., Jr J Org Chem. 1962;27:139–142. [Google Scholar]
- 11.(a) de Faria AR, Matos CRR, Correia CRD. Tetrahedron Lett. 1993;34:27–30. [Google Scholar]; (b) de Faria AR, Salvador EL, Correia CRD. J Org Chem. 2002;67:3651–3661. doi: 10.1021/jo016189u. [DOI] [PubMed] [Google Scholar]; (c) Takahata H, Banba Y, Momose T. Tetrahedron: Asymmetry. 1991;2:445–448. [Google Scholar]
- 12.Efforts to annulate the spiro-fused indoxyl system onto advanced, piperidine-containing intermediates were frustrated by low yields. Discussions are found in reference 9.
- 13.Griffith WP, Ley SV, Whitcombe GP, White AD. J Chem Soc, Chem Commun. 1987:1625–1627. [Google Scholar]
- 14.Rozenberg V, Danilova T, Sergeeva E, Vorontsov E, Starikova Z, Lysenko K, Belokon Y. Eur J Org Chem. 2000:3295–3303. [Google Scholar]
- 15.(a) Fornicola RS, Subburaj K, Montgomery J. Org Lett. 2002;4(4):615–617. doi: 10.1021/ol017213t. [DOI] [PubMed] [Google Scholar]; (b) Montgomery J. Angew Chem Int Ed. 2004;43:3890–3908. doi: 10.1002/anie.200300634. [DOI] [PubMed] [Google Scholar]
- 16.For a recent synthesis of (−)-corynantheidine featuring a nickel-catalyzed carboxylative cyclization of an enyne, see: Mizuno T, Oonishi Y, Takimoto M, Sato Y. Eur J Org Chem. 2011:2606–2609.
- 17.While the putative metalacycle 11 is shown as an η3 carbon-bound intermediate, an η1 oxygen-bound intermediate or an η3 enolate could be involved, see: Amarasinghe KKD, Chowdhury SK, Heeg MJ, Montgomery J. Organometallics. 2001;20:370–372.Ogoshi S, Nishimura A, Ohashi M. Org Lett. 2010;12:3450–3452. doi: 10.1021/ol101264r.
- 18.(a) Ma J, Yin W, Zhou H, Cook JM. Org Lett. 2007;9(18):3491–3494. doi: 10.1021/ol071220l. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Ma J, Yin W, Zhou H, Liao X, Cook JM. J Org Chem. 2009;74:264–273. doi: 10.1021/jo801839t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun X, Ma D. Chem Asian J. 2011;6:2158–2165. doi: 10.1002/asia.201100219. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.