Abstract
Summary
Methicillin-resistant Staphylococcus aureus (MRSA) emerged via acquisition of a mobile element, staphylococcal cassette chromosome mec (SCCmec). Integration and excision of SCCmec is mediated by an unusual site-specific recombination system. Most variants of SCCmec encode two recombinases, CcrA and CcrB, that belong to the large serine family. Since CcrA and CcrB are always found together, we sought to address their specific roles. We show here that CcrA and CcrB can carry out both excisive and integrative recombination in E. coli in the absence of any host-specific or SCCmec-encoded cofactors. CcrA and CcrB are promiscuous in their substrate choice: they act on many non-canonical pairs of recombination sites in addition to the canonical ones, which may explain tandem insertions into the SCCmec attachment site. Moreover, CcrB is always required, but CcrA is only required if one of the four half sites is present. Recombinational activity correlates with DNA-binding: CcrA recognizes only that half site, which overlaps a conserved coding frame on the host chromosome. Therefore, we propose that CcrA serves as a specificity factor that emerged through modular evolution to enable recognition of a bacterial recombination site that is not an inverted repeat.
Keywords: methicillin-resistance, MRSA, site-specific recombination, mobile elements
Introduction
Staphylococcus aureus causes a variety of human and animal diseases ranging from minor skin and soft tissue infections to severe bacteremia and fatal necrotizing pneumonia. Many of the genes that make particular S. aureus strains dangerous, such as virulence factors and antibiotic resistance determinants, are carried on mobile genetic elements (Novick et al., 2001). SCCmec is one such element. It is inserted at a unique site in the staphylococcal chromosome by Ccr (cassette chromosome recombinase) proteins that are encoded by the element (Ito et al., 1999; Katayama et al., 2000). SCCmec varies in size (21 – 67 kb) and the number of encoded genes (Hanssen and Ericson Sollid, 2006), but most importantly it carries the mecA gene that encodes a low-affinity penicillin-binding protein 2′ (PBP2′), that is necessary for the methicillin resistant phenotype of “MRSA” strains of S. aureus (Hartman and Tomasz, 1981). MRSA has become a serious public health problem worldwide and understanding the molecular mechanism behind SCCmec mobility may lay the groundwork for identifying ways to stop the rapid spread of methicillin resistance.
The Ccr proteins that integrate and excise the SCCmec element belong to the “large serine” family of site-specific DNA recombinases, named after their large C-terminal domain and their active site nucleophile (serine) (Katayama et al., 2000; Smith et al., 2010). This subfamily includes many bacteriophage integrases such as those of phiC31, Bxb1, TP901-1, R4, A118 and phiRv1(Christiansen et al., 1996; Matsuura et al., 1996; Thorpe and Smith, 1998; Ghosh et al., 2003; Bibb et al., 2005; Mandali et al., 2013), as well as the transposases TndX and TnpX from Tn5397 and Tn4451 (Wang and Mullany, 2000; Lyras et al., 2004). The chemical reactions of recombination are carried out by a catalytic domain that is conserved among all serine recombinases and generally found at the N-terminus. After formation of a tetramer that synapses the two DNA substrates, the active serine residues attack at positions 2 bp apart on each duplex, displacing the 3′ hydroxyls to create double strand breaks in which each 5′ end is covalently linked to a recombinase subunit. The strand exchange mechanism is widely accepted to occur via a 180° rotation of two subunits within the tetramer with respect to the other two. The rotation aligns the broken ends for religation in a recombinant configuration (through the reverse of the cleavage reaction) (Grindley et al., 2006). Although all well-characterized serine recombinases function as homotetramers, most types of SCCmec encode two large serine recombinases, termed CcrA and CcrB. Overexpression of these in MRSA strains triggers excision of a cognate SCCmec element as a circle (Noto and Archer, 2006), and CcrB has been shown to bind DNA in vitro (Wang and Archer, 2010; Wang et al, 2012), but little else is known about their function.
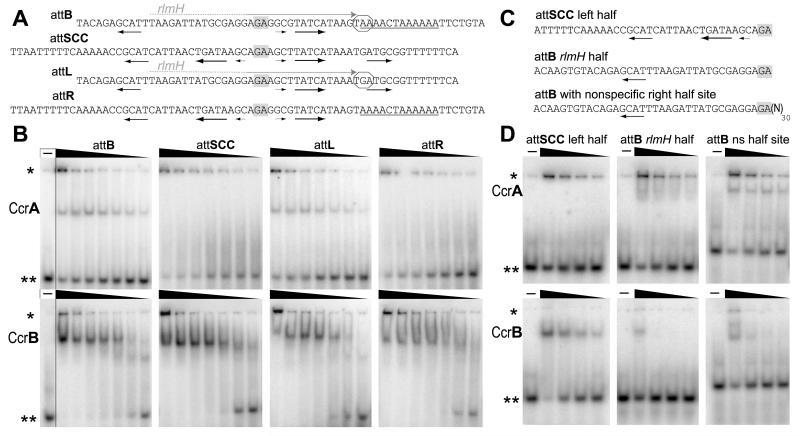
The recombination target sites for the Ccr proteins are degenerate inverted repeats. SCCmec is always found at a unique location in the staphylococcal genome, within the 3′ end of a highly conserved gene, rlmH, previously called orfX, encoding a 23S rRNA pseudouridine methyltransferase (Purta et al, 2008; Ero et al, 2010). RlmH structures are known from S. aureus and other bacteria (Badger et al, 2005; Benach et al, PDB ID 1NS5; Boundy et al, 2012). The 3′ end of the gene encodes part of the proposed binding site for pseudouridine (Purta et al, 2008). The integration of SCCmec occurs via Ccr-mediated recombination between this unique sequence (designated attB) and a specific site in the circularized SCCmec element (designated attSCC), and it regenerates the C-terminus of RlmH (Ito et al., 1999; Katayama et al., 2000). When SCCmec is inserted, a new pair of sites is generated, referred to as attL and attR, flanking the element’s left (rlmH-proximal) and right end, respectively. During SCCmec excision the reverse happens-attR and attL sites are recombined to regenerate the original sites, attB and attSCC (Fig.1). Three of the four half-sites that constitute these att sites have similar sequences, but the one that completely overlaps the rlmH coding region (half of attB and attL) is quite different.
Fig. 1.
SCCmec cassette transposition. Simplified cartoon of an SCCmec element. SCCmec, which is assumed to exist in a circular form before integration, contains the mecA gene that is responsible for methicillin resistance and the ccrA ccrB operon that encodes the recombinases that integrate and excise the element. The Ccr recombinases recognize unique sequences: one on the staphylococcal chromosome (attB), which overlaps the 3′ end of the rlmH (formerly orfX) gene and the other on the SCCmec element (attSCC). Binding of Ccr recombinases to attB and attSCC brings the two sites together which triggers DNA cleavage and exchange of the half sites, presumably via a 180° rotation. Religation of the DNA leads to stable incorporation of SCCmec into the staphylococcal genome and generation of hybrid sites termed attL and attR. During excision the attL and attR sites are recombined leading to removal of SCCmec from the staphylococcal chromosome and regeneration of the circular form of the element. Note that we are using the left/right convention of the IWG-SCC (2009), based on the orientation of the integrated SCCmec element relative to the bacterial origin of replication.
The directionality of the reaction (e.g., integration vs. excision) is regulated by accessory factors in many other well-studied serine recombinase systems. Members of the resolvase/invertase subfamily of serine recombinases employ DNA bending proteins and/or additional recombinase subunits to construct a regulatory complex of defined DNA topology that controls proper alignment of recombination sites and reaction outcome. For these systems, the two recombination crossover sites themselves can be identical, perfectly symmetric inverted repeats. The “large serine recombinase” subfamily that Ccr proteins belong to includes bacteriophage integrases and DNA transposases. Their crossover sites (e.g., attP and attB) often differ significantly in sequence (Smith and Thorpe, 2002). The bacteriophage integrases will typically carry out integration reactions on relatively short, linear attB and attP sites, with no additional proteins required. However, an additional phage-encoded directionality factor is required for the excision reaction (Ghosh et al., 2006; Smith et al., 2010; Khaleel et al., 2011). In contrast, the large serine transposase TnpX will catalyze both reactions without additional factors (Lyras et al., 2004). Whether or not Ccr-mediated recombination requires accessory factors has not been previously addressed.
Although ccr genes have been extensively sequenced from numerous MRSA isolates, there is very little biochemical data on the proteins themselves. A vast genotyping analysis revealed substantial sequence differences between ccr genes (IWG-SCC, 2009). To date, three phylogenetically distinct Ccr proteins have been identified in S. aureus: CcrA, CcrB, and CcrC with DNA similarities below 50%. CcrA and CcrB are part of a two-gene operon and they are always present together, whereas some cassette types carry only CcrC. The majority of SCCmec types carry ccrA and ccrB genes and because of their significant nucleotide variations, from 50% to 85%, they have been further classified into four allotypes. On the other hand, all ccrC genes identified to date have shown high nucleotide identity (≥87%) and are assigned to only one allotype, CcrC1 (IWG-SCC, 2009). When overexpressed, CcrA and CcrB from one cassette type have been reported to trigger excision of not only that type but also certain other ones, whereas CcrC can only act on the type of SCCmec element that encodes it (Ito et al., 2004; Noto and Archer, 2006). This observation is quite intriguing considering the sequence variations of CcrA and CcrB between different SCCmec types. We therefore sought to investigate the roles of CcrA and CcrB in SCCmec recombination, to try to explain why they are always present together and what the specific function of each of them is in the recombination process. Our study is one of the first attempts to elucidate the molecular mechanisms of CcrA and CcrB activity and how they facilitate the introduction of methicillin resistance into new S. aureus genetic backgrounds.
Results
Specific DNA binding of CcrA & CcrB
To investigate DNA binding properties of CcrA and CcrB we performed a series of EMSA assays. The DNA probes were double stranded radiolabelled synthetic oligonucleotides containing the attachment sites: attSCC, attB, attL, attR, or indicated half sites. The MRSA strain used for cloning of the Ccr proteins and prediction of the attachment sites’ sequences was S. aureus 923 (USA300), which containsSCCmec type IV. The proteins were overexpressed in E. coli and purified to near-homogeneity (Fig. S1). A C-terminal His6 tag was added for ease of purification, but initial in vivo experiments showed no difference in activity between these tagged constructs and ones that included the natural stop codon before tag, implying that the tag did not significantly affect activity. All binding reactions were performed in the presence of excess unlabeled nonspecific DNA competitor to assess site-specific binding. Substrate lengths were based on a careful examination of SCCmec att site sequences and on the literature of the large serine recombinases. The ~60 bp sites used include all the sequences conserved among half sites. They are also longer than the characterized att sites for other large serine recombinases: e.g. minimal sizes of attB and attP were defined for phiC31 to be 34 bp and 39 bp, for Bxb1 38 bp and 48 bp, and for R4 50 bp and 49 bp, respectively (Groth et al., 2000; Ghosh et al., 2003; Miura et al., 2011). Binding of CcrB to 320 bp attL and 387 bp attR PCR products was also assayed, and the results were not significantly different from those described below.
CcrA binds specifically to two of the four attachment sites. It recognizes attB and attL sites with nanomolar affinity forming a single DNA-protein complex, whereas it does not form a shifted band with the two other sites (attSCC and attR), even at high protein concentrations (although some aggregated material becomes trapped in the wells) (Fig. 2B, top panel). This is the first report of CcrA binding selectively to specific att sites. A previous study (Wang and Archer, 2010) did not detect any DNA binding activity for CcrA. We speculate that the difference is due to the tag used in protein purification – we used a small His6 tag, whereas Wang and Archer used GST, which can dimerize (Tudyka and Skerra, 1997; Vinckier et al., 2011). If CcrA dimerizes with different geometry than GST does, such a tag could trigger non-native oligomerization. GST might also enhance the solubility of misfolded proteins, reducing the overall activity of the purified material (Wissmueller et al., 2011).
Fig. 2.
Specific binding of CcrA and CcrB proteins to their cognate attachment sites. A. Sequences of attL, attR, attB, attSCC sites used in EMSA assay. The top strand of each duplex is shown. GA bases shown in grey boxes represent the central dinucleotide where DNA cleavage and strand exchange presumably occur during recombination. The rlmH coding frame and stop codon (octagon) are indicated, and the A-tract that was deleted in later experiments is underlined. Short inverted repeats are indicated by horizontal arrows. The position of the third motif from central dinucleotide differs by one nucleotide between attB and attSCC and between the two halves of attSCC (which are otherwise very similar). B. CcrA and CcrB titrations with indicated att sites. Proteins concentrations: 400, 200, 100, 50, 25, 12, 6 (in nM). CcrA binds attB and attL, but does not bind attSCC and attR, while CcrB binds all four. The first lanes marked as ( - ) indicate that no protein was added showing the mobility of free DNA. C. Substrates used to test binding to half sites (the top strand of each duplex is shown). D. CcrA recognizes the unique attB rlmH half site shared between attB and attL, which fully overlaps the rlmH gene, whereas it does not bind attSCC half site (conc: 700, 350, 175, 87 nM). Extra non-specific (ns) DNA is necessary for stable complex formation between CcrA and attB rlmH half site. In contrast to CcrA, CcrB binds the attSCC half site more tightly than the attB rlmH half (conc: 500, 250, 125, 63 nM). All DNA-binding reactions were analyzed on 5% (w/v) polyacrylamide non-denaturing gels and contained radiolabeled specific DNA and excess of unlabeled DNA competitor. (*) marks wells, (**) free DNA.
CcrB binds with roughly similar affinities (Kd apparent = ~10-20 nM) to all four attachment sites. Two bands of different mobility are seen, with the slower-migrating one predominating at higher protein concentrations (Fig. 2B, bottom panel). Large serine recombinases are known to dimerize in solution (Smith et al., 2010), which agrees with our observation for CcrB (Fig. S2). Therefore we assume that the slower-migrating complex is a CcrB dimer bound to one att site and the faster-migrating complex is a CcrB monomer bound to one att site. Comparison of binding to half- and full attSCC sites carried on similar length DNA segments supports this conclusion (Fig. S3B). However, other stoichiometries remain a formal possibility. Previous studies also reported specific binding of CcrB to all four att sites, although with lower affinity. The quantitative differences probably reflect different protein purification protocols (Wang and Archer, 2010; Wang et al. 2012). The first of those studies also reported that DNA binding by GST-CcrB was inhibited by GST-CcrA, but we found that titrating CcrA into CcrB binding reactions did not inhibit binding (Fig. S3C).
Intrigued by the specificity of CcrA for only two attachment sites, attB and attL, we explored the binding of the Ccr proteins to individual half sites. The attB and attL sites share one half-site in common (dark blue in Fig. 1; the left half of each in Fig. 2A). This unique half site is the half of the bacterial insertion site that lies entirely within the conserved rlmH reading frame, and is designated here “attB rlmH half” (note that rlmH also overlaps part of the other half of attB). It shares the lowest sequence identity with the other three half sites, which are very similar to one another. We compared binding of CcrA to this unique half site to binding to a more canonical half site (the left half of attSCC, as shown in Fig. 2D, top panel). The DNA probes were truncated to comprise only the half site in question. CcrA does not bind the half site from attSCC, confirming our EMSA result with the full attachment sites. As we expected, CcrA interacts with attB rlmH half, however, the resulting complex appears to be unstable: a smear instead of a sharp band is observed. We therefore tested a longer DNA fragment containing the sequence of attB rlmH half on one side of the central dinucleotide, and random-sequence DNA of the same length on the other. This fragment formed a discrete complex, indicating that CcrA can selectively recognize the attB rlmH half site, but needs additional non-specific contacts to form a stable complex.
In contrast to CcrA, CcrB binds more tightly to the three similar half sites than to the attB rlmH half site (Fig. 2D, bottom panel and Fig. S3B). Only at high protein concentrations (0.5 μM) was a complex formed with the truncated probe containing the attB rlmH half site sequence or the longer probe with extra non-specific DNA. On the other hand, the same shifting of the other three half-sites was seen even at lowest CcrB concentration (63 nM). These results agree qualitatively with the results of Wang et al. (2012), who found that CcrB bound specifically to a 14 bp segment found entirely within the conserved right half of attB, but not to the rlmH half.
In vivo recombination activity of CcrA and CcrB in a non-host expression system
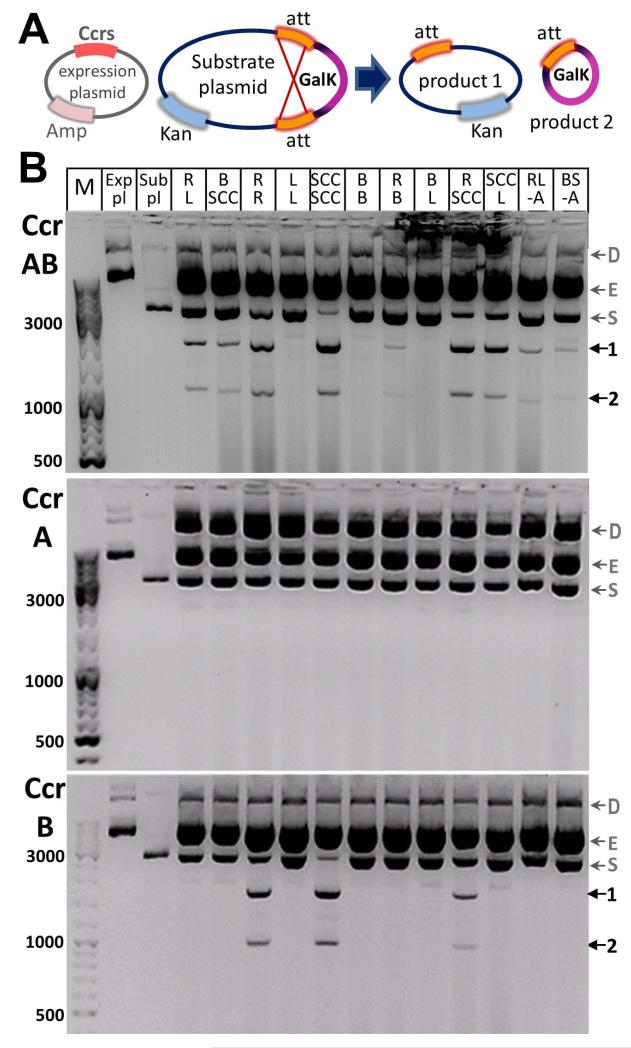
Previous studies reported CcrA and CcrB recombinational activity only in the context of the natural host S. aureus and thus could not exclude additional host-specific or SCCmec-encoded factors that may be needed for excision or integration. To more precisely examine the Ccr recombination system and to determine if any additional factors are required, we designed an in vivo assay in E. coli, based on one used previously for other serine recombinases (Rowland et al., 2009) (Fig. 3A). This assay involves transformation of the cells with two plasmids: the substrate plasmid containing a reporter gene galKflanked by att sites in a direct orientation (as defined by their asymmetric central dinucleotide), and the recombinase-encoding plasmid. Recombination can be easily monitored by the deletion of galK gene from the substrate plasmid which causes a change in the colony color. If the recombination is efficient (galK deletion), white colonies can be seen on MacConkey indicator plates, while red colonies are seen if recombination is slow or absent.
Fig. 3.
In vivo CcrA and CcrB recombinational activity in a non-host environment. A. Schematic diagram of plasmids used in the assay and expected recombination products. SCCmec attachment sites (att) flank the reporter gene galK that is excised upon Ccr-mediated recombination. B. Agarose gel analysis of parental and recombinant products after plasmid DNA extraction from E. coli cells. Top gel: Ccr proteins were co-expressed; middle gel: CcrA was expressed alone; bottom gel: CcrB was expressed alone. R, L, B, SCC indicate att sites present on the substrate plasmid. All possible combinations of att pairs were tested for Ccr recombinational activity plus two additional substrates with the A-tract deleted from the attR and attB sites. Recombination products of the expected sizes are marked by arrows 1 and 2. Bands of plasmids dimers (D), expression plasmids (E), substrate plasmids (S) are indicated with gray arrows. Controls include E. coli cells transformed with expression plasmid only (“Exp pl” lanes) and substrate plasmid only (“Sub pl” lanes). Molecular size markers (“M” lanes).
To assess the specific functions of CcrA and CcrB in the recombination reaction, E. coli cells were transformed with plasmids encoding CcrA, CcrB, or the two proteins together. Next, a substrate plasmid was introduced containing a pair of att sites: either attB and attSCC or attL and attR to test for integrative and excisive recombination, respectively. The cells expressing both CcrA and CcrB showed some recombinational activity and a few individual white colonies were visible on the indicator plates, whereas no white colonies could be observed when CcrA and CcrB were expressed separately. Since a change in the colony color is dependent in this assay on galK deletion from a multi-copy plasmid, a low level of recombination may not be evident. Therefore, recombination was monitored by extracting total plasmid DNA and analyzing it on agarose gels. The recombination products from substrate plasmids containing attB and attSCC or attL and attR were only present in cells expressing CcrA and CcrB together (Fig. 3B; leftmost five lanes). No products were seen when the substrate plasmid contained non-cognate att sites (those for Sin recombinase; data not shown). To confirm that the products seen were from site-specific recombination, the two lower bands were excised, the DNA was purified, and the sequences of the junctions were verified. When CcrA or CcrB were expressed individually, no recombination product was detected. This result indicates that both recombinases are necessary for excision and integration of the SCCmec element and spread of the methicillin resistance, consistent with previous observations (Katayama et al., 2000; Ito et al., 2001; Jansen et al., 2006). This result also reveals that no staphylococcal or SCCmec cassette-specific factors other than CcrA and CcrB are strictly required for integration or excision of the element. However, it does not completely rule out the possibility that recombination might be more robust in the presence of an as-yet-undiscovered cofactor, or that the system uses a host cofactor that is sufficiently conserved between E. coli and S. aureus. Furthermore, the data show that the isolated att sites are sufficient for the Ccr recombination reaction, and accessory DNA sites are not necessary, consistent with data from other large serine recombinases such as phiC31, Bxb1, R4, A118, phiRv1, and TnpX (Thorpe and Smith, 1998; Ghosh et al., 2003; Lyras et al., 2004; Bibb et al., 2005; Miura et al., 2011; Mandali et al., 2013). It should be noted that our results disagree with a previous report in which additional DNA sequences flanking attB were required for recombination in a plasmid fusion assay. (Wang et al., 2012). However, there is no precedent for large serine recombinases requiring accessory sequence elements. Many tyrosine recombinases with similar functions (e.g., phage λ and SaPI integrases (Grindley et al., 2006; Ubeda et al., 2003; Novick et al., 2010) do require additional DNA sequences, but we are unaware of any examples where those sequences are found in the host’s genome rather than the mobile element’s.
We next explored the in vivo activity of CcrA and CcrB using the same assay to determine preferred recombination substrates. We constructed 8 more substrate plasmids containing all possible combinations of att sites flanking the galK gene. Since CcrA binds attB and attL in vitro, we speculated that it might be proficient in recombining those two attachment sites. However, recombination products were not detected for any combination of sites when CcrA was expressed alone (Fig. 3B, middle gel). This result suggests that CcrA may have no or very low recombinational activity on its own and requires CcrB for function. Interestingly, CcrB when expressed alone efficiently recombined substrate plasmids containing two copies of attSCC (“SCC SCC” lane), two copies of attR (“R R” lane), and a combination of those two sites (“SCC R” lane) (Fig. 3B, bottom gel). These are the sites that do not contain the unique attB rlmH half site sequence. Of the well characterized large serine recombinases, only A118 integrase has been reported to recombine two copies of the mobile element attachment site (called attP, which for bacteriophages is the equivalent of attSCC), and it did so much less efficiently than it recombined attP with attB (Mandali et al., 2013). The combination of attP × attP has been called a non-permissive site pair (Thorpe et al., 2000; Ghosh et al., 2005; Wang et al., 2006), whereas for CcrB it seems to be a preferred substrate for recombination. The mechanism of site selection by CcrB must therefore be different than in the other known large serine recombinases.
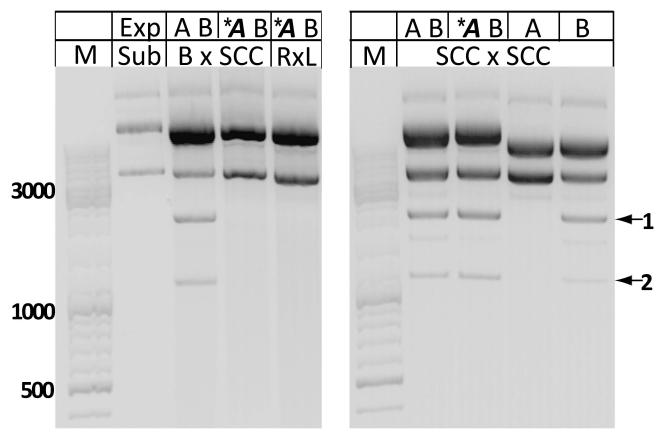
We also tested all possible combinations of the four att sites as recombination substrates for CcrA and CcrB expressed together. Intriguingly, most combinations of attachment sites gave recombination products of the expected size (Fig. 3B, top gel): only the attB × attB, attL × attL, attB × attL pairs were not recombined. As discussed below, the ability to recombine attSCC with attL or attR suggests a mechanism for independent insertions of multiple SCCmec cassettes into the bacterial chromosome. This result also indicates that CcrA cooperates with CcrB to recognize a broader array of recombination substrates. Interaction between CcrA and CcrB has been reported previously (Wang and Archer, 2010). This pattern of recombination agrees with our in vitro DNA binding data, which show that CcrA selectively binds the attB rlmH half site from the bacterial insertion site and CcrB prefers the other half sites. To test whether CcrA participates in catalysis or merely recognizes the unique half site sequence, we repeated the assays with a mutant CcrA lacking the conserved active site serine (S11A). In contrast to the WT pair, the CcrA(S11A)CcrB pair failed to recombine attB × attSCC and attL × attR substrate plasmids, implying that CcrA fully participates in the recombination reaction (Fig. 4). As a control, we also showed that CcrA(S11A) neither aids nor interferes with CcrB-mediated attSCC × attSCC recombination.
Fig. 4.
The catalytic activity of CcrA is required for integrative and excisive recombination. The activities of WT CcrA and CcrA with the catalytic serine (S11) mutated to alanine (*A) were compared (S11 aligns with the nucleophilic serines of other serine recombinases.) The left gel shows that the catalytic activity of CcrA is required for integrative and excisive recombination. The right gel shows that the CcrA S11A does not inhibit attSCC x attSCC recombination by CcrB.
Next we wanted to assess whether the relative orientation of the att sites is important. Note that we are defining the orientation in the case of these degenerate inverted repeats by the direction of the central GA dinucleotide. One might expect that the att sites in the substrates above could be recombined in either of two relative local alignments: one that would delete the intervening galK gene and one that would invert it. However, studies on other serine recombinases have shown that if the central dinucleotide is mismatched after DNA cleavage and a 180° rotation, a second 180° rotation occurs and the broken ends are religated to their original partners (McIlwraith et al., 1997). Because the central GA of our att sites is asymmetric, synapsis of the two att sites in one relative orientation will lead to recombinant products, whereas in the other orientation it will not. The substrates described above were expected to produce recombinants only when the sites were aligned such that the overall result would be deletion of galK. To test the importance of site orientation in the CcrAB system, the attSCC site in the attSCC × attB substrate plasmid was cloned in the opposite direction so that the galK gene was flanked by inversely oriented instead of directly oriented sites. As expected, co-expressed CcrA and CcrB inverted rather than deleted the galK gene in this substrate (Fig. S4).
Lastly, motivated by previous reports on MRSA strains deficient in CcrAB-mediated SCCmec excision (Noto and Archer, 2006), we attempted to determine whether sequence differences play a role in the inability to excise the cassette. We compared left and right attachment sites of SCCmec elements from various excision competent and deficient MRSA isolates. One of the biggest variations we noticed was that strains deficient in SCCmec excision such as MW2 and J28, were missing an A-tract in their right attachment sites located 14 bp away from the central dinucleotide. Therefore, we deleted this sequence from the attR site in our substrate plasmid and tested for excisive recombination by co-expressing CcrA and CcrB. Recombination products were still detected for this substrate (Fig. 3B, “RL –A” lane) (although the exact efficiency is hard to quantify), suggesting that this difference in sequence between excision competent and deficient MRSA strains may not be the primary reason for loss of SCCmec mobility. We also deleted the same A-tract from the attB site and found it did not prevent integrative recombination when CcrA and CcrB were present together (Fig. 3B, “BS –A” lane). As with excisive and integrative recombination of WT att sites, no products were detected when CcrA and CcrB were expressed separately.
Discussion
CcrA and CcrB represent a unique system within the large serine recombinase family where two recombinases are required for efficient integration and excision of DNA. Understanding the molecular mechanism of their action is of great importance since they mobilize the methicillin resistance gene causing spread of MRSA. In this study, we show for the first time that no other staphylococcal proteins, except for CcrA and CcrB, are necessary for the recombination reaction, although we cannot rule out the possibility that some E. coli –encoded protein substitutes for a missing cofactor. The two recombinases, when co-expressed, supported efficient recombination in a heterologous gram-negative E. coli host, and are likely to be functional in other model organisms. No mobile element-encoded directionality factor is required for their action, unlike the well-studied large serine integrases phiC31, Bxb1, and phiRv1 where an additional phage-encoded protein controls the directionality of recombination (Bibb and Hatfull, 2002; Ghosh et al., 2006; Khaleel et al., 2011). In this respect, the Ccr proteins more closely resemble the large serine transposases TnpX or TndX, which can also carry out both excision and integration without cofactors (Wang and Mullany, 2000; Lyras et al., 2004). Our data also demonstrated that CcrA and CcrB recognize and act on short specific sequences of length no more than 58-72 bp, which indicates that no accessory binding sites are required. Recombination of short DNA substrates follows the mode of action of other large serine recombinases, where attachment sites were reported to be approximately 50 bp long (Smith et al., 2010).
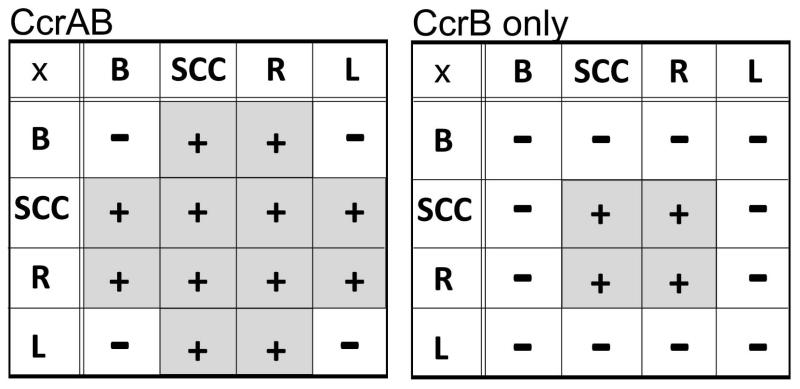
The CcrA and CcrB pair differs from other large serine recombinases in their ability to recombine most of the non-canonical pairs of attachment sites. Such promiscuous recombination is rather uncommon in the large serine recombinase family except for a few examples of serine transposases with variable target sites (Wang et al., 2006). The best-studied members of this group, the phage serine integrases mentioned above, were shown to selectively synapse only the biologically appropriate partners – attP (equivalent of attSCC) and attB in the absence of any additional factors (Smith et al., 2004a; Ghosh et al., 2005; Zhang et al., 2010). In this study, we report that CcrA and CcrB can recombine all of the 10 possible site pairs except for three: attB × attB, attL × attL, attB × attL (Fig. 5). This recombination pattern implies that Ccr proteins are selective for any pair of attachment sites that contains no more than one copy of the attB rlmH half site.
Fig. 5.
Recombination pattern of CcrA and CcrB when expressed together and when CcrB is expressed alone. CcrAB efficiently recombine any pairing of att sites except BxB, BxL, LxL pairs (left table). CcrB alone recognizes as recombination substrates only SCCxSCC, SCCxR, and RxR pairs (right table). Grey box with (+) indicate efficient recombination reaction between the sites within the lines, whereas (−) signifies no detected recombination.
Such promiscuity in recombining att sites may allow the SCCmec cassette to rapidly acquire new genes. Tandem arrangements of different SCC elements have been reported in epidemic strains of MRSA. For instance, the virulent, community-acquired USA300 strain used here has an ACME (Arginine Catabolic Mobile Element) element integrated into attR (Diep et al., 2006; Zhang et al., 2009; Zong and Lü, 2010), while a different element was found there in other strains (Jansen et al., 2006). Both of these additional elements could be mobilized by CcrAB. These findings suggest that other mobile elements with some sequence similarity to the SCCmec att sites can potentially be inserted into the staphylococcal chromosome at attL or attR via Ccr-mediated recombination. Acquisition of genetic material by SCCmec may provide an opportunity for the host bacteria to acquire new toxins and resistance factors. The recombinational promiscuity of CcrA and CcrB may help explain the rapid evolution and high genetic plasticity of the SCCmec island, and may explain why this extraordinarily successful family of mobile elements is found in sizes ranging from 21 kb to 67 kb.
We are unaware of any other serine recombinases that are expressed together and act in concert as CcrA and CcrB do. Our data suggest that CcrA may allow for modular selection of new target sites in the bacterial chromosome. Integrating into the 3′ end of a conserved ribosomal synthesis gene gives SCCmec a reliable landing spot on the staphylococcal chromosome. However, site-specific recombination reactions generally require that one recombinase subunit bind on each side of the cleavage site, which is most easily accommodated if the crossover site contains an inverted repeat. Many bacteriophage integrases that belong to the otherwise unrelated tyrosine recombinase family target the natural inverted repeats found in tRNA genes (Reiter et al., 1989; Williams, 2002). Serine integrases, although less well-characterized, seem to employ a different tactic. For example, Bxb1 and phiC31 integrases target small fortuitous inverted repeats found within protein coding regions (Combes et al., 2002; Kim et al., 2003). SCCmec seems to have solved this problem by using CcrB to recognize most half-sites, and CcrA for the half site that fully overlaps the rlmH gene (here termed “attB rlmH half”). This conclusion is supported by our DNA binding data, and by the fact that CcrB is active without CcrA as long as the attB rlmH half site is not present (Fig. 5). Excision of SCCmec was reported in the presence of CcrB only, however, a five-fold longer induction time for CcrB expression was necessary to detect any excision product and an alternate attL site was used (Wang and Archer, 2010) (note: we use the left/right convention of the IWG-SCC (2009), whereas Wang and Archer, 2010 used the opposite convention).
There is some precedent for site-specific recombinases acting as heterotetramers; in the tyrosine recombinase family, E. coli XerC and XerD form a heterotetramer that resolves dimeric replicons (Blakely et al., 1993). In that case, the logic of using two recombinases with different sequence specificity appears to be in regulating the direction of recombination. In contrast, CcrAB is not an obligate heterotetramer, since CcrB can act independently on some pairs of sites. CcrA appears to be an alternate subunit that helps target recombination to the appropriate host site. Our results are consistent with two scenarios for integrative recombination: one in which attB is bound by a homodimer of CcrA, and one in which it is bound by a CcrAB heterodimer. In either case, attSCC must be bound by a homodimer of CcrB, which implies that after subunit exchange, the product attL would be bound (and religated) by a CcrAB heterodimer. Heterodimers must therefore be functional. Why then was no recombination seen when both substrates contained the CcrA-binding rlmH half site (e.g. attB × attL)? It may be that CcrA is defective in synaptic interactions, but more experiments will be needed to address this question. It remains to be seen how the subset of SCCmec types that use only CcrC for integration and excision solve this issue.
In summary, we find that CcrA and CcrB comprise an unusual site-specific DNA recombination system. These two enzymes cooperate to catalyze excision and integration of the SCCmec elements that encode them, with CcrA specifically recognizing one half of the crossover site on the host chromosome. We propose that this unique use of two serine recombinases facilitates recognition of a site on the host chromosome that is not an inverted repeat. Unlike most large serine recombinases, CcrAB will recombine most pairs of att sites. Non-canonical pairs of sites that do not include the attB rlmH half site can be recombined by CcrB alone. We also show that the CcrAB pair is more like the large serine transposases than the large serine integrases in that it does not appear to require a recombination directionality factor for excisive recombination. Finally, by demonstrating activity in an E. coli host, we have ruled out the need for any other staphylococcal-specific or element-encoded cofactors in CcrAB-mediated recombination.
Experimental procedures
Plasmid construction and DNA substrates
Oligonucleotide primers are listed in Supplementary material, Table S1. To generate protein overexpression plasmids, PCR amplified CcrA and CcrB genes from US300 MRSA strain (923) were inserted into the cloning site of pET101/D-TOPO vector (Invitrogen) following the manufacturer’s protocol. CcrA and ccrB genes were first cloned separately for individual expression and contained their natural stop codons (immediately preceding the vector-encoded tag) which were next removed by QuikChange Site-Directed mutagenesis (Agilent Technologies, Inc). In these constructs, the natural start codons of CcrA and CcrB are downstream of the strong T7 promoter and T7 ribosome binding site of pET101. CcrAB co-expression plasmids were made by subcloning CcrB (with its natural stop codon) into the BglII and XbaI restriction sites of the pET101/D-TOPO vector containing CcrA. The two genes are under the same T7 promoter, but each has its own T7 ribosome binding site. Two versions, one with and one without a His6 tag on CcrA, produced the same experimental results. More details on expression plasmid design are available on request. The substrate plasmids containing att sites for the in vivo recombination assay were all generated from the pMS183 plasmid backbone (Rowland et al., 2009). They carry the pSC101 origin and the Kan(r) marker. The fragments containing att sites were produced by annealing complementary oligonucleotides with the same sequences as those used for the electrophoretic mobility shift assays (listed below), except they had additional 5′ overhangs for cloning into restriction sites (NcoI – XbaI and BsrGI – BglII) flanking the reporter galK gene. Standard digestion and ligation procedures with appropriate restriction enzymes and T4 ligase, respectively, were used to incorporate the attB + attSCC and attL + attR pairs of sites into the pMS183 vector generating the first set of substrate plasmids. All the other substrate plasmids with different pairs of att sites were made via QuikChange Site-Directed mutagenesis kit (Agilent Technologies, Inc) using the first set of substrate plasmids as templates. The att-galK-att segments of all newly-generated vectors were sequenced to ensure that no unintended mutations had been introduced. The sequences of all plasmids are available on request.
Protein purification
C-terminally His6-tagged CcrA and CcrB proteins were individually expressed in Rosetta (DE3)pLysS cells (Novagen). A short linker, a V5 tag, and a His6 tag were added to each protein’s C-terminus. The exact sequence of the added segment is as follows (with the tags in italics): K G E L N S K L E G K P I P N P L L G L D S T R T G H H H H H H. The cells were grown in Luria-Bertani medium with 100 μg ml−1 ampicillin at 37°C until they reached OD600 ≈0.6 and then were induced with 0.5 mM isopropyl-β-D-thiogalactopyranoside (IPTG). After induction, cells containing the plasmid expressing CcrA were grown over night (16 h) at 15°C, whereas cells containing the plasmid expressing CcrB were grown for 3 h at 37°C. Cells were harvested by centrifugation at 10,733×g in an F10S rotor for 7 min. Cell pellets were resuspended in buffer A (42.5 mM Na2HPO4, 7.5 mM NaH2PO4, 1 mM DTT, 5% glycerol, pH 7.5) containing protease inhibitor cocktails (Complete Mini, Roche Diagnostics GmbH). Due to the lower solubility of CcrA the buffers used for its purification had additional 1M NaCl and 1M urea. Lysozyme was added to 200 μg ml−1, and the mixture was incubated on ice for 10 min, sonicated, then centrifuged at 38,759×g in an SS-34 rotor at 4°C for 1 h. Ccr proteins remained in the pellet, which was subsequently resuspended and centrifuged again in buffer A. The pellets were next resuspended in buffer B (buffer A with 1% Triton-X), sonicated for a second time and centrifuged. The supernatant was incubated with a slurry of nickel beads (1 ml of the slurry pre-equilibrated in buffer B per 1 L of cell culture) and softly shaken at 4°C for 1 h. The nickel beads were washed twice with buffer A, after which the proteins were eluted with buffer C (buffer A plus 0.5 M imidazole). After overnight dialysis into buffer A, proteins were loaded onto a His-Trap™ HP (GE Healthcare) nickel column, and eluted with buffer C. Fractions containing full-length Ccr proteins were combined and dialyzed into 25 mM Tris (pH 8), 0.5 mM EDTA, 1 M NaCl, 1 mM DTT, 20% glycerol and stored at − 80°C until needed. When expressed on a large scale (2-6 L culture volume) the proteins underwent additional steps of purification: CcrA was loaded a second time onto a His-Trap™ HP (GE Healthcare) nickel column, whereas CcrB was further purified on HiPrep™ 16/10 Heparin FF (GE Healthcare) and MonoS™ 5/50 GL (GE Healthcare) columns according to the manufacturer’s protocols.
Electrophoretic mobility shift assay
The DNA substrates were oligonucleotides containing predicted Ccr attachment sites or their half sites ordered from Integrated DNA Technologies (IDT). The sequences of the top strands were as follows: attL58:5′TACAGAGCATTTAAGATTATGCGAGGAGAAGCTTATCATAAATGATGCGGTTTTTTCA; attR72:5′TTAATTTTTCAAAAACCGCATCATTAACTGATAAGCAGAGGCGTATCATAAGTAAAACTAAAAAATTCTGTA; attB62:5′TACAGAGCATTTAAGATTATGCGAGGAGAGGCGTATCATAAGTAAAACTAAAAAATTCTGTA; attSCC68:5′TTATTTTTTCAAAAACCGCATCATTAACTGATAAGCAGAAGCTTATCATAAATGATGCGGTTTTTTCA; SCChalfsite36:5′ATTTTTCAAAAACCGCATCATTAACTGATAAGCAGA; attBrlmHhalfsite36:5′ACAAGTGTACAGAGCATTTAAGATTATGCGAGGAGA; attBnshalfsite62:5′TACAGAGCATTTAAGATTATGCGAGGAGATGGCTGAGAACACCCCTTGTGAGGTACTTCATG, where the subscript indicates number of bases. Each of the listed top strands at 1 μM concentration were 5′- end 32P-phosphorylated using T4 kinase, then 10× of unlabeled top and bottom strands were added and annealed. The attachment sites at 1 nM were incubated with Ccr proteins in a binding buffer containing 20 nM Tris-HCl, pH 8.0, 50 mM NaCl, 1 mM DTT, 5% glycerol, and 2ng μl−1 of sonicated salmon sperm DNA as a competitor DNA. The concentrations of the proteins are indicated in the figures legends. The total volume of the samples was 20 μl. After 20 min at room temperature glycerol was added to a final concentration of 20% and the samples were loaded onto 5% nondenaturing polyacrylamide gel. To separate protein-DNA complexes from free DNA, the gels were run in 0.5 × TBE buffer for 2.5-3h at 4°C and 140 V. Bands were visualized by phosphor-imaging.
In vivo recombination assay
Recombination reactions were performed in E. coli strain DS941 (recF−, galK−, F−; this strain is deficient in homologous recombination). Chemically competent cells were first transformed with a pET101/D-TOPO-based plasmid expressing Ccr proteins: either CcrA, CcrB, or both. Expression is supported by the strong T7-derived ribosome binding site of pET101 (Dubendorff and Studier, 1991) and presumably by read-through transcription from an upstream E. coli promoter (since T7 RNA polymerase is absent in DS941). Similar pET-derived constructs have previously been invaluable for in vivo expression and mutagenesis screens with diverse serine recombinases (Burke et al, 2004; Rowland et al, 2009). In a second transformation, the substrate plasmid was introduced carrying the galK gene flanked by different combinations of att sites. The transformants were either plated on MacConkey agar (Difco Ltd) indicator plates (containing 1% D(+) galactose, 50 μg ml−1 kanamycin, 100 μg ml−1 ampicillin) if recombination was to be monitored by colony color. Otherwise, transformants were suspended in a liquid Luria-Bertani media (supplemented with 50 μg ml−1 kanamycin and 100 μg ml−1 ampicillin) and were grown over night at 37°C. To analyze infrequent recombination events, plasmid DNA was isolated from the over-night liquid cultures using Qiagen miniprep kits, and the size of the extracted DNA was analyzed by agarose gel electrophoresis. Additional restriction enzyme digestion was performed on plasmid DNA samples from cells transformed with substrate plasmid containing att sites in inverted orientation (Fig. S4). Similar recombination efficiencies were seen when the experiments were repeated in a RecA− host (BLR). The in vivo recombination assays were initially carried out with two Ccr-expression constructs (retaining natural stop codons and His6-tag fused genes). Since the results were very similar, we present our results for tagged constructs only (for direct comparison to the in vitro DNA binding results with the same constructs).
Supplementary Material
Acknowledgments
This work was funded by NIH grant R21 AI087559 (to P.A.R., S. B.-V. and R.S.D).
Footnotes
Supporting information
Additional supporting information may be found in the online version of this article
References
- Adams V, Lucet IS, Lyras D, Rood JI. DNA binding properties of TnpX indicate that different synapses are formed in the excision and integration of the Tn4451 family. Mol Microbiol. 2004;53:1195–1207. doi: 10.1111/j.1365-2958.2004.04198.x. [DOI] [PubMed] [Google Scholar]
- Badger J, Sauder JM, Adams JM, Antonysamy S, Bain K, Bergseid MG, et al. Structural analysis of a set of proteins resulting from a bacterial genomics project. Proteins. 2005;60:787–796. doi: 10.1002/prot.20541. [DOI] [PubMed] [Google Scholar]
- Benach J, Shen J, Rost B, Xiao R, Acton T, Montelione G, Hunt JF. Structure of YBEA from E. coli. PDB ID 1NS5. [Google Scholar]
- Bibb LA, Hancox MI, Hatfull GF. Integration and excision by the large serine recombinase phiRv1 integrase. Mol Microbiol. 2005;55:1896–1910. doi: 10.1111/j.1365-2958.2005.04517.x. [DOI] [PubMed] [Google Scholar]
- Bibb LA, Hatfull GF. Integration and excision of the Mycobacterium tuberculosis prophage-like element, phiRv1. Mol Microbiol. 2002;45:1515–1526. doi: 10.1046/j.1365-2958.2002.03130.x. [DOI] [PubMed] [Google Scholar]
- Blakely G, May G, McCulloch R, Arciszewska LK, Burke M, Lovett ST, Sherratt DJ. Two related recombinases are required for site-specific recombination at dif and cer in E. coli K12. Cell. 1993;75:351–361. doi: 10.1016/0092-8674(93)80076-q. [DOI] [PubMed] [Google Scholar]
- Boundy S, Safo MK, Wang L, Musayev FN, O’Farrell HC, Rife JP, Archer GL. Characterization of the Staphylococcus aureus rRNA Methyltransferase Encoded by orfX, the Gene Containing the Staphylococcus aureus SCCmec Insertion Site. J Biol Chem. 2013;288:132–40. doi: 10.1074/jbc.M112.385138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke ME, Arnold PH, He J, Wenwieser SV, Rowland SJ, Boocock MR, Stark WM. Activating mutations of Tn3 resolvase marking interfaces important in recombination catalysis and its regulation. Mol Microbiol. 2004;51:937–48. doi: 10.1046/j.1365-2958.2003.03831.x. [DOI] [PubMed] [Google Scholar]
- Christiansen B, Brøndsted L, Vogensen FK, Hammer K. A resolvase-like protein is required for the site-specific integration of the temperate lactococcal bacteriophage TP901-1. J Bacteriol. 1996;178:5164–5173. doi: 10.1128/jb.178.17.5164-5173.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combes P, Till R, Bee S, Smith MC. The streptomyces genome contains multiple pseudo-attB sites for the phiC31-encoded site-specific recombination system. J Bacteriol. 2002;184:5746–5752. doi: 10.1128/JB.184.20.5746-5752.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diep BA, Gill SR, Chang RF, Phan TH, Chen JH, Davidson MG, Lin F, Lin J, Carleton HA, Mongodin, E , et al. Complete genome sequence of USA300, an epidemic clone of community-acquired meticillin-resistant Staphylococcus aureus. Lancet. 2006;367:731–739. doi: 10.1016/S0140-6736(06)68231-7. [DOI] [PubMed] [Google Scholar]
- Dubendorff JW, Studier FW. Controlling basal expression in an inducible T7 expression system by blocking the target T7 promoter with lac repressor. J Mol Biol. 1991;219:45–59. doi: 10.1016/0022-2836(91)90856-2. [DOI] [PubMed] [Google Scholar]
- Ero R, Leppik M, Liiv A, Remme J. Specificity and kinetics of 23S rRNA modification enzymes RlmH and RluD. RNA. 2010;16:2075–2084. doi: 10.1261/rna.2234310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh P, Kim AI, Hatfull GF. The orientation of mycobacteriophage Bxb1 integration is solely dependent on the central dinucleotide of attP and attB. Mol Cell. 2003;12:1101–1111. doi: 10.1016/s1097-2765(03)00444-1. [DOI] [PubMed] [Google Scholar]
- Ghosh P, Pannunzio NR, Hatfull GF. Synapsis in phage Bxb1 integration: selection mechanism for the correct pair of recombination sites. J Mol Biol. 2005;349:331–48. doi: 10.1016/j.jmb.2005.03.043. [DOI] [PubMed] [Google Scholar]
- Ghosh P, Wasil LR, Hatfull GF. Control of phage Bxb1 excision by a novel recombination directionality factor. PLoS Biol. 2006;4:e186. doi: 10.1371/journal.pbio.0040186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grindley NDF, Whiteson KL, Rice PA. Mechanisms of site-specific recombination. Annu Rev Biochem. 2006;75:567–605. doi: 10.1146/annurev.biochem.73.011303.073908. [DOI] [PubMed] [Google Scholar]
- Groth AC, Olivares EC, Thyagarajan B, Calos MP. A phage integrase directs efficient site-specific integration in human cells. Proc Natl Acad Sci USA. 2000;97:5995–6000. doi: 10.1073/pnas.090527097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanssen AM, Ericson Sollid JU. SCCmec in staphylococci: genes on the move. FEMS Immunol. Med Microbiol. 2006;46:8–20. doi: 10.1111/j.1574-695X.2005.00009.x. [DOI] [PubMed] [Google Scholar]
- Hartman B, Tomasz A. Altered penicillin-binding proteins in methicillin-resistant strains of Staphylococcus aureus. Antimicrob Agents Chemother. 1981;19:726–735. doi: 10.1128/aac.19.5.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito T, Katayama Y, Asada K, Mori N, Tsutsumimoto K, Tiensasitorn C, Hiramatsu K. Structural Comparison of Three Types of Staphylococcal Cassette Chromosome mec Integrated in the Chromosome in Methicillin-Resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2001;45:1323–1336. doi: 10.1128/AAC.45.5.1323-1336.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito T, Katayama Y, Hiramatsu K. Cloning and nucleotide sequence determination of the entire mec DNA of pre-methicillin-resistant Staphylococcus aureus N315. Antimicrob Agents Chemother. 1999;43:1449–1458. doi: 10.1128/aac.43.6.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito T, Ma XX, Takeuchi F, Okuma K, Yuzawa H, Hiramatsu K. Novel type V staphylococcal cassette chromosome mec driven by a novel cassette chromosome recombinase, ccrC. Antimicrob Agents Chemother. 2004;48:2637–2651. doi: 10.1128/AAC.48.7.2637-2651.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen WTM, Beitsma MM, Koeman CJ, van Wamel WJB, Verhoef J, Fluit AC. Novel mobile variants of staphylococcal cassette chromosome mec in Staphylococcus aureus. Antimicrob Agents Chemother. 2006;50:2072–2078. doi: 10.1128/AAC.01539-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katayama Y, Ito T, Hiramatsu K. A new class of genetic element, staphylococcus cassette chromosome mec, encodes methicillin resistance in Staphylococcus aureus. Antimicrob Agents Chemother. 2000;44:1549–1555. doi: 10.1128/aac.44.6.1549-1555.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khaleel T, Younger E, McEwan AR, Varghese AS, Smith MCM. A phage protein that binds φC31 integrase to switch its directionality. Mol Microbiol. 2011;80:1450–1463. doi: 10.1111/j.1365-2958.2011.07696.x. [DOI] [PubMed] [Google Scholar]
- Kim AI, Ghosh P, Aaron MA, Bibb LA, Jain S, Hatfull GF. Mycobacteriophage Bxb1 integrates into the Mycobacterium smegmatis groEL1 gene. Mol Microbiol. 2003;50:463–473. doi: 10.1046/j.1365-2958.2003.03723.x. [DOI] [PubMed] [Google Scholar]
- Lyras D, Adams V, Lucet I, Rood JI. The large resolvase TnpX is the only transposon-encoded protein required for transposition of the Tn4451/3 family of integrative mobilizable elements. Mol Microbiol. 2004;51:1787–1800. doi: 10.1111/j.1365-2958.2003.03950.x. [DOI] [PubMed] [Google Scholar]
- Mandali S, Dhar G, Avliyakulov NK, Haykinson MJ, Johnson RC. The site-specific integration reaction of Listeria phage A118 integrase, a serine recombinase. Mob DNA. 2013;4:2. doi: 10.1186/1759-8753-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuura M, Noguchi T, Yamaguchi D, Aida T, Asayama M, Takahashi H, Shirai M. The sre gene (ORF469) encodes a site-specific recombinase responsible for integration of the R4 phage genome. J Bacteriol. 1996;178:3374–6. doi: 10.1128/jb.178.11.3374-3376.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIlwraith MJ, Boocock MR, Stark WM. Tn3 resolvase catalyses multiple recombination events without intermediate rejoining of DNA ends. J Mol Biol. 1997;266:108–121. doi: 10.1006/jmbi.1996.0765. [DOI] [PubMed] [Google Scholar]
- Miura T, Hosaka Y, Yan-Zhuo Y, Nishizawa T, Asayama M, Takahashi H, Shirai M. In vivo and in vitro characterization of site-specific recombination of actinophage R4 integrase. J Gen Appl Microbiol. 2011;57:45–57. doi: 10.2323/jgam.57.45. [DOI] [PubMed] [Google Scholar]
- Noto MJ, Archer GL. A subset of Staphylococcus aureus strains harboring staphylococcal cassette chromosome mec (SCCmec) type IV is deficient in CcrAB-mediated SCCmec excision. Antimicrob Agents Chemother. 2006;50:2782–2788. doi: 10.1128/AAC.00032-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick RP, Schlievert P, Ruzin A. Pathogenicity and resistance islands of staphylococci. Microbes Infect. 2001;3:585–594. doi: 10.1016/s1286-4579(01)01414-9. [DOI] [PubMed] [Google Scholar]
- Novick RP, Christie GE, Penadés JR. The phage-related chromosomal islands of Gram-positive bacteria. Nat Rev Microbiol. 2010;8:541–51. doi: 10.1038/nrmicro2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purta E, Kaminska KH, Kasprzak JM, Bujnicki JM, Douthwaite S. YbeA is the m3Psi methyltransferase RlmH that targets nucleotide 1915 in 23S rRNA. RNA. 2008;14:2234–2244. doi: 10.1261/rna.1198108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiter WD, Palm P, Yeats S. Transfer RNA genes frequently serve as integration sites for prokaryotic genetic elements. Nucleic Acids Res. 1989;17:1907–1914. doi: 10.1093/nar/17.5.1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland S-J, Boocock MR, McPherson AL, Mouw KW, Rice PA, Stark WM. Regulatory mutations in Sin recombinase support a structure-based model of the synaptosome. MolMicrobiol. 2009;74:282–298. doi: 10.1111/j.1365-2958.2009.06756.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MCA, Till R, Brady K, Soultanas P, Thorpe H, Smith MCM. Synapsis and DNA cleavage in φC31 integrase-mediated site-specific recombination. Nucl Acids Res. 2004;32:2607–2617. doi: 10.1093/nar/gkh538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MCM, Brown WRA, McEwan AR, Rowley PA. Site-specific recombination by phiC31 integrase and other large serine recombinases. Biochem Soc Trans. 2010;38:388–394. doi: 10.1042/BST0380388. [DOI] [PubMed] [Google Scholar]
- Smith MCM, Thorpe HM. Diversity in the serine recombinases. Mol Microbiol. 2002;44:299–307. doi: 10.1046/j.1365-2958.2002.02891.x. [DOI] [PubMed] [Google Scholar]
- Thorpe HM, Smith MC. In vitro site-specific integration of bacteriophage DNA catalyzed by a recombinase of the resolvase/invertase family. Proc Natl Acad Sci USA. 1998;95:5505–5510. doi: 10.1073/pnas.95.10.5505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorpe HM, Wilson SE, Smith MC. Control of directionality in the site-specific recombination system of the Streptomyces phage phiC31. Mol Microbiol. 2000;38:232–41. doi: 10.1046/j.1365-2958.2000.02142.x. [DOI] [PubMed] [Google Scholar]
- Tudyka T, Skerra A. Glutathione S-transferase can be used as a C-terminal, enzymatically active dimerization module for a recombinant protease inhibitor, and functionally secreted into the periplasm of Escherichia coli. Protein Sci. 1997;6:2180–7. doi: 10.1002/pro.5560061012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubeda C, Tormo MA, Cucarella C, Trotonda P, Foster TJ, Lasa I, Penadés JR. Sip, an integrase protein with excision, circularization and integration activities, defines a new family of mobile Staphylococcus aureus pathogenicity islands. Mol Microbiol. 2003;49:193–210. doi: 10.1046/j.1365-2958.2003.03577.x. [DOI] [PubMed] [Google Scholar]
- Vinckier NK, Chworos A, Parsons SM. Improved isolation of proteins tagged with glutathione S-transferase. Protein Expr Purif. 2011;75:161–4. doi: 10.1016/j.pep.2010.09.006. [DOI] [PubMed] [Google Scholar]
- Wang H, Mullany P. The large resolvase TndX is required and sufficient for integration and excision of derivatives of the novel conjugative transposon Tn5397. J Bacteriol. 2000;182:6577–6583. doi: 10.1128/jb.182.23.6577-6583.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Smith MCM, Mullany P. The conjugative transposon Tn5397 has a strong preference for integration into its Clostridium difficile target site. J Bacteriol. 2006;188:4871–4878. doi: 10.1128/JB.00210-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Archer GL. Roles of CcrA and CcrB in excision and integration of staphylococcal cassette chromosome mec, a Staphylococcus aureus genomic island. J Bacteriol. 2010;192:3204–3212. doi: 10.1128/JB.01520-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Safo M, Archer GL. Characterization of DNA sequences required for the CcrAB-mediated integration of staphylococcal cassette chromosome mec, a Staphylococcus aureus genomic island. J Bacteriol. 2012;194:486–98. doi: 10.1128/JB.05047-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams KP. Integration sites for genetic elements in prokaryotic tRNA and tmRNA genes: sublocation preference of integrase subfamilies. Nucleic Acids Res. 2002;30:866–875. doi: 10.1093/nar/30.4.866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wissmueller S, Font J, Liew CW, Cram E, Schroeder T, Turner J, Crossley M, Mackay JP, Matthews JM. Protein-protein interactions: analysis of a false positive GST pulldown result. Proteins. 2011;79:2365–71. doi: 10.1002/prot.23068. [DOI] [PubMed] [Google Scholar]
- Zhang K, McClure J-A, Elsayed S, Conly JM. Novel staphylococcal cassette chromosome mec type, tentatively designated type VIII, harboring class A mec and type 4 ccr gene complexes in a Canadian epidemic strain of methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2009;53:531–540. doi: 10.1128/AAC.01118-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zong Z, Lü X. Characterization of a new SCCmec element in Staphylococcus cohnii. PLoS ONE. 2010;5:e14016. doi: 10.1371/journal.pone.0014016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Working Group on the Classification of Staphylococcal Cassette Chromosome Elements (IWG-SCC) Classification of staphylococcal cassette chromosome mec (SCCmec): guidelines for reporting novel SCCmec elements. Antimicrob Agents Chemother. 2009;53:4961–4967. doi: 10.1128/AAC.00579-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.