Abstract
Cultural neuroscience issues from the apparently incompatible combination of neuroscience and cultural psychology. A brief literature sampling suggests, instead, several preliminary topics that demonstrate proof of possibilities: cultural differences in both lower-level processes (e.g. perception, number representation) and higher-order processes (e.g. inferring others’ emotions, contemplating the self) are beginning to shed new light on both culture and cognition. Candidates for future cultural neuroscience research include cultural variations in the default (resting) network, which may be social; regulation and inhibition of feelings, thoughts, and actions; prejudice and dehumanization; and neural signatures of fundamental warmth and competence judgments.
Keywords: culture, emotion, neuroscience, perception, self, social cognition
Introduction
Cultural psychology and neuroscience might seem to inhabit opposite ends of the scientific spectrum. Recently, however, the emerging field of cultural neuroscience has sought to combine the theories and methods of these two disciplines (Fiske, 2009; Han & Northoff, 2008). At first blush, these theories and methods may seem incompatible— with cultural psychology characterized by ethnographic holism and neuroscience characterized by biological reductionism. A closer look, however, reveals that these two approaches to understanding people, instead, closely inter- relate. Culture is, after all, stored in people’s brains. More- over, human brains are biologically prepared to acquire culture: The ability to coordinate thoughts and behaviors within social groups has aided primate and hominid survival (A. P. Fiske, 2002). Because of this, the human brain is uniquely evolved to acquire basic cultural capacities, such as language (Chomsky, 1965) and morality (Mikhail, 2007). Without the requisite neurobiological capabilities, culture could not function, and the parameters of the human brain have, in this sense, shaped the progression of culture since our evolutionary beginnings. As such, culture’s bio- logical underpinnings help elucidate the formation, acquisition, and preservation of culture.
The present article reviews recent research in cultural neuroscience, first examining investigations of basic cognitive processes (e.g. perception), then moving toward higher-order processes (e.g. social coordination). (For another review, organized around five approaches to cultural psychology, see Zhou & Cacioppo, 2010). While the research progress thus far is impressive, much work yet remains. This article therefore concludes by identifying several candidates for future research in cultural neuroscience.
Recent research
Perception
The neural substrates of human perception might seem more or less universal; after all, people in all cultures face the same basic perceptual challenges (e.g. tactile discrimination, object recognition). However, recent neuroimaging research has revealed a set of (perhaps surprising) cultural differences in the neural mechanisms subserving various perceptual domains, including object processing, color discrimination, and taste. Behavioral studies widely suggest that East Asians and Westerners apply different ‘perceptual styles’ to the task of decoding visual scenes. Specifically, Westerners tend to focus on objects (in an analytical, context-free manner), whereas East Asians tend to focus more on contexts, relationships, and backgrounds (Chua, Boland, & Nisbett, 2005; Nisbett & Miyamoto, 2005). In a functional magnetic resonance imaging (fMRI) study examining the neural basis for this difference (Gutchess, Welsh, Boduroglu, & Park, 2006), Chinese and American participants judged various pictures of objects, backgrounds, and object–background combinations. Consistent with prior behavioral studies suggesting greater object-focused processing among Westerners, American participants (relative to Chinese participants) demonstrated stronger and more distributed neural activations during object processing. Specifically, Americans more often recruited the middle temporal gyrus (implicated in semantic knowledge retrieval during object perception; Martin, Wiggs, Ungerleider, & Haxby, 1996), right superior temporal / supramarginal gyrus (important for the encoding of spatial information; Aguirre & D’Esposito, 1997; Ungerleider, 1995), and superior parietal lobule (which tracks successful encoding of object locations; Sommer, Rose, Weiller, & Büchel, 2005). Few cross-cultural brain differences were associated with back- ground processing (and those few differences tended to be only marginally significant). Thus, whereas behavioral studies might seem to suggest that greater attention to context among East Asians drives cultural differences in perceptual styles, these neuroimaging data suggest that these differences primarily result from additional object processing among Westerners.
Other neural investigations support this conclusion. One such study (Goh et al., 2007) measured fMRI response adaptation (i.e. reduction of neural response following repeated presentation of the same stimulus). In this experiment, participants from Singapore and the USA both showed adaptation in the parahippocampal gyrus (linked to background processing) and lateral occipital cortex (linked to object processing) (see Fig. 1 panel 1 for these and other regions discussed in this section). Notably however, the adaptation observed in the object processing region was more pronounced in Americans than in Singaporeans, again suggesting more object-focused processing (but equivalent background processing) in Americans versus East Asians. Consistent with the idea that such differences might arise from years of cultural immersion, and with prior demonstrations that the extent of neural differences between two groups correlates with the number of years during which those groups have had divergent experiences (Maguire et al., 2000), greater object processing in Americans versus Singaporeans emerged only for older adults, and not for younger participants.
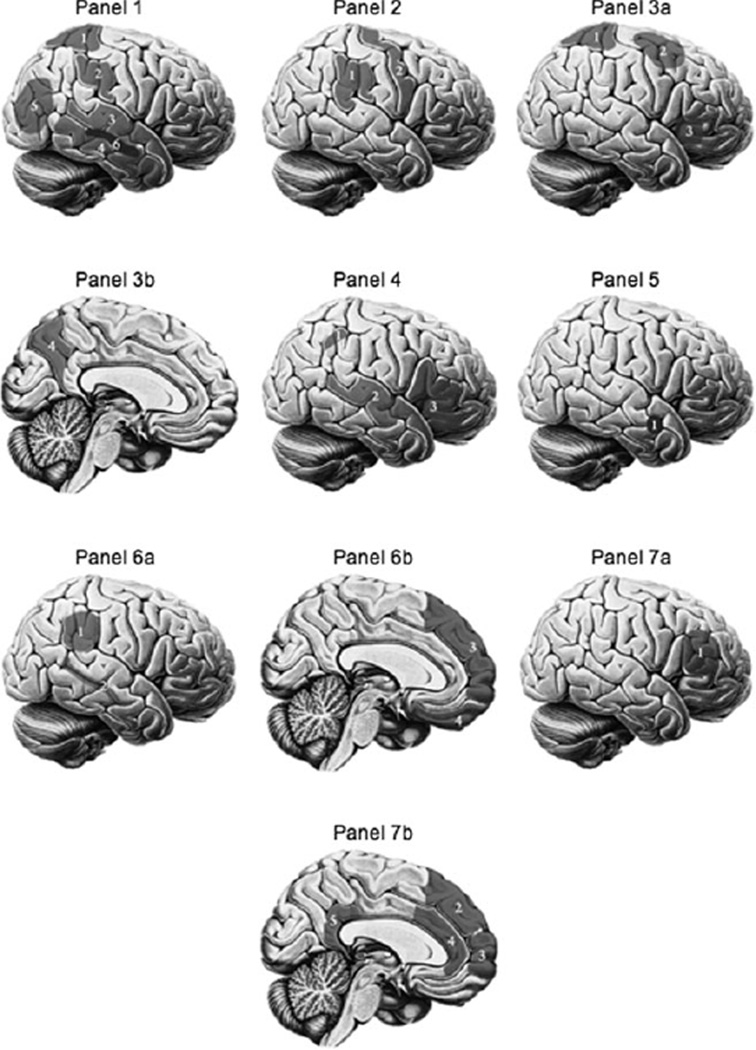
Figure 1.
(Panel 1) Regions discussed in the section Perception. 1, superior parietal lobule; 2, supramarginal gyrus; 3, superior temporal gyrus; 4, middle temporal gyrus; 5, lateral occipital cortex; 6, parahippocampal gyrus (not actually shown—the parahippocampal gyrus is a fairly medial structure but is shown on the lateral surface of the brain in Panel 1 for display purposes). (Panel 2) Regions discussed in the section Attention. 1, inferior parietal lobule; 2, precentral gyrus. (Panel 3) Regions on the (a) lateral and (b) medial surfaces dis- cussed in the section Number. 1, superior parietal lobule; 2, premotor association area; 3, Broca’s area; 4, precuneus. (Panel 4) Regions discussed in the section Language. 1, dorsal region of inferior parietal lobule; 2, superior temporal gyrus; 3, inferior frontal gyrus. (Panel 5) Region discussed in the section Inferring others’ emotions. 1, amygdala (not actually shown—the amygdala is not a cortical region, but is shown on the lateral surface of the brain in this Panel for display purposes). (Panel 6) Regions on the (a) lateral and (b) medial surfaces discussed in the section Attribution and belief inference. 1, temporoparietal junction; 2, superior temporal sulcus; 3, medial prefrontal cortex; 4, orbitofrontal cortex. (Panel 7) Regions on the (a) lateral and (b) medial surfaces dis- cussed in the section The self. 1, right middle frontal cortex; 2, dorsomedial prefrontal cortex; 3, ventromedial prefrontal cortex; 4, anterior cingulate cortex; 5, posterior cingulate cortex.
Of course, the perceptual processing differences in these two studies could feasibly have arisen from developmental, neurobiological, and genetic factors that are not specifically related to culture. A third study (Lin, Lin, & Han, 2008) helped to rule out this possibility by manipulating perceptual styles within subjects. Following previous behavioral experiments, a priming manipulation was used to instantiate different styles of self-construal. As predicted, priming participants with a Western, independent self-construal style led to greater activation of the lateral occipital cortex 1 (again, a region implicated in object processing) in response to local versus global visual targets. The opposite pattern (greater lateral occipital cortex response to global vs. local targets) appeared in the same participants following interdependent (Eastern) priming. By using priming techniques to elicit two well-characterized cultural styles of perception (see e.g. Markus & Kitayama, 1991; Nisbett & Miyamoto, 2005, for further details), in a within-subjects design, this study showed that cultural perceptual styles themselves relate to differences in perceptual processing in the lateral occipital cortex, strengthening the conclusions of Gutchess et al. (2006) and Goh et al. (2007).
Other work demonstrates that cultural effects on perception are not limited to differences in visual scene processing. For instance, cultural messages about the brand of a food product can dramatically influence gustatory perceptions of that product, as measured both by neural responses during consumption and by subjective taste preferences (McClure et al., 2004). Moreover, other researchers have used neuropsychological evidence to support the claim that culture and language shape perceptions of color (Davidoff, 2001). Taken together, these studies suggest that many components of perception are culturally flexible, and that cultural neuroscience may usefully contribute to scientific understanding of how and when complex social processes influence basic appraisals of the physical world.
Attention
Given that different cultures exhibit different preferred perceptual styles, one would expect that certain perceptual tasks would be easier for members of some cultures than for members of other cultures. At the neural level, one would therefore predict that attentional systems should be more strongly recruited for whichever kind of processing is culturally non-preferred. Thus, people from Eastern cultures should need to recruit top-down attentional resources in order to engage in a relatively unfamiliar, ‘Western’ perceptual style of local, context-independent visual processing, whereas people from Western cultures should require greater recruitment of attentional resources when making global, context-dependent judgments. Cognitive neuroscience research on the frontoparietal attentional network (Corbetta & Shulman, 2002; Gitelman et al., 1999) and cultural psychology research on context-dependent/ independent processing (Ji, Peng, & Nisbett, 2000; Kitayama, Duffy, Kawamura, & Larsen, 2003) were recently synthesized to provide a clear test of this hypothesis. As anticipated, East Asians recruited prefrontal and parietal attention regions (e.g. inferior parietal lobule, pre- central gyrus; Fig. 1 panel 2) for (culturally non-preferred) context-independent judgments more than for (culturally preferred) context-dependent judgments, whereas Americans showed the opposite pattern (Hedden, Ketay, Aron, Markus, & Gabrieli, 2008). The finding that less attentional processing is required for culturally preferred modes of attention fits with previous reports of reduced attentional activation in response to tasks with which one is well- practiced (Milham, Banich, Claus, & Cohen, 2003) and with well-supported cognitive models suggesting that automaticity increases with experience (Cohen, Servan- Schreiber, & McClelland, 1992).
Number
The ability to represent and combine number concepts is critical to the function of human cultures all across the world, underlying several basic cultural phenomena, such as economic exchange, hierarchy, and resource distribution. Still, the neural processes subserving basic numerical processes vary considerably across cultures (Ansari, 2008). One recent fMRI study examined Western English speakers and Eastern Chinese speakers performing various number- representation and calculation tasks involving Arabic numerals (Tang et al., 2006). Whereas Western participants preferentially activated regions of the left perisylvian cortex (e.g. Broca’s area) for mental calculation, Chinese participants tended to recruit a visuo-premotor association network (e.g. premotor association area) (see Fig. 1 panels 3a and 3b for regions discussed in this section). Parallel cross-cultural dissociations also occurred for number- comparison tasks and even for simply judging the orientation of numerals. Because all participants carried out these tasks using Arabic numerals, which have the same meaning and appearance across cultures, these findings do not reduce to differences in visual input or numerical representational systems. This study demonstrates that, although people from different cultures may receive equivalent inputs (‘4 + 4’) and provide equivalent outputs (‘8’), the underlying processes operating between these inputs and outputs may differ across cultures.
From what aspects of culture might these different computational strategies arise? One possible source is the culturally preferred methods of mathematical problem solving that schools explicitly teach. One study testing this possibility (Lee et al., 2007) explored two mathematical approaches taught in Singaporean schools: the ‘model method’ (in which children represent word problems by constructing diagrams of mathematical information) and the ‘symbol method’ (in which children transform word problems into equations using symbols). Compared with the model method, the symbol method increased activation in the precuneus and superior parietal lobules, despite equivalent behavioral performance for the two approaches (see also Sohn et al., 2004, for a similar study). The authors interpreted these results as suggesting that the symbolic method is more attentionally demanding (but not more effective) than the model method. Further research will likely help to reveal more completely the consequences of different culturally taught computation methods. Such research will also likely clarify extant behavioral findings—for instance, studies suggesting that expertise with the East Asian abacus provides advantages in visuospatial tasks (Hatano, Miyake, & Binks, 1977), but also vulnerabilities to certain kinds of distracters (Hatano, Amaiwa, & Shimizu, 1987). Emerging insights into how the mathematical approaches taught in schools influence the neural substrates of mathematical processing may help educators to develop more effective pedagogical strategies, perhaps improving education worldwide. Cultural neuroscience will be an important component of this mission.
Language
Language is the quintessential cultural experience and primary vehicle for communicating culture. Even in infancy, people’s perceptual systems differentially attune to the language(s) of the culture in which they are raised (Aslin, 1981; Cheour et al., 1998; Kuhl, Williams, Lacerda, Stevens, & Lindblom, 1992; Näätänen et al., 1997). Some of the neural regions involved in language processing appear to be relatively constant across cultures and languages, including the inferior frontal gyrus and left superior posterior temporal gyrus (Bolger, Perfetti, & Schneider, 2005). Other regions, however, apparently matter for processing some languages more than others. For example, whereas native Chinese speakers activate dorsal regions of the inferior parietal lobe when reading Chinese characters (Tan, Laird, Li, & Fox, 2005), native English speakers recruit the superior temporal gyrus for reading English words (Bolger et al., 2005) (Fig. 1 panel 4). Do such findings reflect differences in languages per se (e.g. differences in orthography) or cultural effects on language processing? One study supporting the former view (Siok, Perfetti, Jin, & Tan, 2004) observes that the fluent reading of Western alphabetic languages, such as English, requires relating visual forms to sounds, whereas reading logographic languages, such as Chinese, whose characters do not have specific phonetic analogues, relies more heavily on associations between visual forms and meanings. These orthographic differences demonstrably result in different neural structures being important for reading different languages (Siok et al., 2004). Thus, it seems likely that the findings of Tan et al. (2005) and Bolger et al. (2005) derive, at least in part, from differences in the relevant languages themselves.
In contrast, other findings related to language processing are more difficult to explain in terms of stimulus differences. Several studies suggest that language influences one’s perception of color categories (Davidoff, 2001). Thus, language, a key component of culture, can apparently shape non-linguistic thought to some extent (see also Boroditsky, 2001, on conceptions of time in Mandarin and English speakers). In sum, despite recent progress, the relationship between language and culture in the brain is not yet well understood and requires further exploration. Because language embodies culture, questions in this domain are likely to be complex, although not intractable.
Inferring others’ emotions
One recent and compelling behavioral finding in the emotion recognition literature is that, although some research has long suggested that certain emotional expressions are universally interpreted across cultures (Ekman, 1992), people seem to be better at correctly identifying the emotions of members of their own groups versus other groups (Elfenbein & Ambady, 2002; Markham & Wang, 1996). Relatively little is known, however, about how culture modulates the neural mechanisms underlying this ‘ingroup advantage’ in emotional recognition. In one relevant study (Chiao et al., 2008), American and Japanese participants viewed pictures of American and Japanese targets while undergoing fMRI scanning. Both groups of target stimuli included various emotional expressions (e.g. fear, happiness, anger) as well as neutral expressions. Fearful faces from one’s own cultural group elicited greater bilateral amygdala (Fig. 1 panel 5) activation than did fearful faces from the other cultural group (this result emerged for both American and Japanese participants). That no analogous effects occurred for other emotions may suggest that the rapid and accurate decoding of fear is particularly important within cultural groups. From an evolutionary perspective, heightened sensitivity to ingroup fear expressions serves important functions in coordinating group action in response to danger. Perhaps, less obviously (but consistent with the earlier section on attention), being particularly sensitive to fearful expressions within one’s ingroup may aid learning important cultural rules (e.g. ‘it scares people when you talk like that’; ‘those kinds of people are dangerous’). One challenge from this study is its silence on the question of whether the observed amygdala modulation reflects a ‘culture effect’, a ‘race effect’, or some combination.
Attribution and belief inference
Determining how people make sense of others’ actions has long preoccupied social psychology. One of the bedrock findings of the field (sometimes called ‘the fundamental attribution error’) has been that people tend to explain others’ behavior as arising from dispositions (personality), while neglecting situational causality (Gilbert & Malone, 1995; Jones & Harris, 1967; Ross, 1977). Yet, recent evidence suggests that this ostensibly universal bias may be much more pronounced for Americans (who constituted the majority of participants in initial attribution research) than for members of other cultures. South and East Asians, for instance, give more weight to situational forces in explaining the causes of people’s actions (Nisbett, 2003). Therefore, whereas the neural correlates of dispositional attributions seem clear in American participants (with medial prefrontal cortex and superior temporal sulcus appearing to be the most important regions in such processes; Harris, Todorov, & Fiske, 2005; see Fig. 1 panels 6a and 6b for these and other regions covered in this section), a different pattern of results might emerge from an examination of other cultural populations.
Although more research is needed to fully substantiate these claims, the idea that the neural processes underlying causal attributions for others’ behavior might vary from culture to culture gains partial support from comparisons of different theory-of-mind tasks. One recent study (Kobayashi, Glover, & Temple, 2006) showed that whereas American and Japanese participants activated many of the same regions in response to thinking about others’ beliefs (including dorsal medial prefrontal cortex [mPFC] and bilateral temporo-parietal junction), other regions differentially activated for the two groups. For example, Japanese participants exhibited greater activation in orbitofrontal regions of the cortex for thinking about others’ beliefs than did American participants. The orbital frontal cortex has been implicated in general evaluation processes, as well as more specific social cognitive tasks, such as thinking about others’ feelings (Hynes, Baird, & Grafton, 2006). This may suggest a mode of thinking about others’ beliefs in Japanese culture that emphasizes greater attention to others’ feelings relative to American modes of belief inference, which may be more cognitive and emotionally distant.
The self
One of the first social-cultural topics to be explored in neuroscience was how people represent the self (Craik et al., 1999). Across a wide range of studies, including both Western (Kelley et al., 2002) and Eastern (Zhang et al., 2006) participants, an area of the ventral mPFC/ anterior cingulate cortex (ACC) activates more for thinking about the self compared with thinking about other people (see Fig. 1 panels 7a and 7b for regions discussed in this section). However, given cultural differences in self-other construal—particularly differences in Western independent views of the self as distinct from others and Eastern inter- dependent views of the self as fundamentally related to others (Markus & Kitayama, 1991)—one might expect cultural differences in self-other understanding to emerge at the level of the brain. To test this hypothesis, Westerners and Chinese participated in a study that included thinking about both the self and a close other (one’s mother) during fMRI scanning (Zhu, Zhang, Fan, & Han, 2007). Consistent with prior work, ventral mPFC (and perigenual ACC) responded preferentially to the self for all participants. However, thinking about one’s mother elicited preferential activation in the ventral mPFC only for the Chinese participants. This finding supports previous theoretical assertions (Markus & Kitayama, 1991) that Easterners view close others (and their relationships to those close others) as part of the self, whereas Westerners tend to conceive of the self as an independent entity. A recent study using cultural priming (Ng, Han, Mao, & Lai, 2010) provides evidence that this Eastern/Western difference is indeed a cultural one, rather than an artifact arising from a factor that covaries with culture (such as language or genetic factors).
The by-now-familiar Eastern-Western distinction is not the only cultural source of neural differences in conceptions of the self. Differences in self-representation also occur as a function of religion (arguably among the most important components of culture; Tillich, 1959). Given that religion deeply influences people’s understanding of themselves, different religious teachings may lead to the recruitment of different cognitive processes during self-referential thought. For example, to the extent that Christianity encourages people to judge themselves through the eyes of God (Ching, 1984), Christians might be expected to con- sider the self from a more distal vantage when making self-judgments. In a study consistent with this claim (Han et al., 2008), Christian and non-religious Chinese participants judged themselves and familiar others. Whereas self- referential processing was, as usual, associated with ventral mPFC for non-religious participants, it associated with dorsal mPFC for Christian participants. Moreover, this dorsal mPFC activity correlated with behavioral ratings of the importance of Jesus’ judgment in evaluating a person. Other researchers have reported ventral mPFC for judging the self from the first-person perspective and dorsal mPFC activity for judging the self from a third-person perspective (D’Argembeau et al., 2007). Thus, the dorsal mPFC activity observed in the religion study may, indeed, indicate judging the self partially from another (divine) perspective. A third study using a different (perhaps more literal) operationalization of self-perception provides further con- verging evidence for the claim that one’s culture influences the way in which one perceives the self. Sui and Han (2007) had participants view their own faces during fMRI scanning, a task that tends to elicit activity in the right middle frontal cortex (Sugiura et al., 2000). Priming Western, independent modes of self-construal versus Eastern, interdependent modes of self-construal in Chinese subjects increased right middle frontal activity when participants viewed pictures of their own versus others’ faces (Sui & Han, 2007), suggesting that the neural correlates of self-perception (like other kinds of perception) modulate as a function of different (primed) cultural modes of self- construal. Similarly, Chiao et al. (2010) found that, after being primed to think in an individualistic, ‘Western’ manner, bicultural individuals showed greater self-referential activation (mPFC and posterior cingulate) for general versus contextual self-judgments; conversely, bicultural individuals primed to think in a collectivistic, ‘Eastern’ manner showed greater self-referential activation for contextual versus general self-judgments. These findings are consistent with previous demonstrations (reviewed earlier) that Westerners tend to view the self as a stable independent entity whereas Easterners tend to construe the self in a more context-sensitive and relational manner. As with studies related to object perception and self-construal discussed earlier (Lin et al., 2008; Ng et al., 2010), the use of priming procedures in neural investigations of the self circumvents potential confounds, such as genetic population differences.
Social interaction and genes
Perhaps because of the difficulties involved in studying dynamic social behaviors through neuroimaging, neuro- scientific examination of cultural differences in behavior has, so far, focused on variation in neurotransmitter activity arising from genetic population differences. One finding has been that, relative to Westerners, Asian populations exhibit a high frequency of homozygosity for the short allele of the serotonin transporter gene-linked polymorphic region. Homozygosity for the short allele increases an individual’s risk for depression following stressful events (Caspi et al., 2003). The broad cultural differences in interdependent/ independent modes of self-construal in Asian versus Western cultures (Markus & Kitayama, 1991) may have arisen, in part, because of this genetic difference, which would cause members of Asian cultures to be more vulnerable to stressful and traumatic life experiences, and would therefore encourage close, harmonious family groups and strong social-support networks (Laland, 1993; Taylor et al., 2006; see also Fiske, 2009, for a more detailed instantiation of this argument).
This idea converges with suggestions that serotonin influences interdependence preferences (Zizzo, 2002). Genetic differences related to serotonin may help to explain the presence of elaborate politeness norms (which tend to promote harmony and prevent interpersonal trauma) observed in some cultures (Cohen, Vandello, Puente, & Rantilla, 1999). However, twin studies suggest that interdependence preferences do not themselves show large heritability coefficients (Zizzo, 2003). Thus, whereas genetic differences between populations may have promoted cultural preferences for interdependence / independence (and concomitant cultural norms), those cultural preferences may not have a strong genetic component per se.
Candidates for future research
Default network
Recently, a great deal of interest has centered on so-called ‘default’ neural activity—that is, the neural activity observed when participants lie passively inside a scanning environment in the absence of a particular task or stimulus (e.g. Buckner & Vincent, 2007; Mason et al., 2007; Raichle et al., 2001). Little agreement has emerged about what functional significance, if any, this default activity has. Notably, however, the regions observed during default or resting activity overlap strikingly with the regions observed in social-cognitive tasks (e.g. Mitchell, 2008). Building on such observations, the default network may be involved in the processing of social information—that is, maybe people lying in the scanner between experimenter-imposed tasks are thinking about the self and social others, either consciously or unconsciously (e.g. D’Argembeau et al., 2005; Gusnard, Akbudak, Shulman, & Raichle, 2001; Iacoboni et al., 2004). One study (D’Argembeau et al., 2005) used positron emission tomography (PET) to examine correlations between self-reported self-referential thought and default activity in ventral mPFC. As discussed in previous sections, ventral mPFC reliably subserves self-referential processes. Not only did overlapping ventral mPFC activations occur during rest and during explicitly directed self- referential thinking in this study, but activity in both of these conditions correlated with the amount of self- referential thought reported by participants (D’Argembeau et al., 2005).
Given the differences (reviewed above) in how Eastern and Western cultures construe the self, other people and social relationships (Markus & Kitayama, 1991; Triandis, Bontempo, Villareal, Asai, & Lucca, 1988), to the extent that default network activity does indeed contain a social- cognitive component, one would expect cultural differences in default activity (see earlier paragraphs on The self, and Attribution and belief inference). In contrast, to the extent that default network activity contains no specifically social component (Greicius, Srivastava, Reiss, & Menon, 2004), one might not expect such differences to emerge. Cross- cultural comparisons of resting state activity may therefore help to clarify the functional meaning of these activations.
Regulation and inhibition: Feelings, thoughts and actions
Cultures vary widely in how much their members self- monitor and express emotion (Mesquita & Frijda, 1992; Pennebaker, Rimé, & Blankenship, 1996). Complementing studies on the neural substrates of self-referential thought noted above, various experiments have begun to examine the neural pathways involved in controlling one’s feelings, thoughts and actions. Lateral and medial prefrontal regions have been implicated in the suppression of emotion (Ochsner, Bunge, Gross, & Gabrieli, 2002). These prefrontal regions appear to coordinate with cingulate control systems to regulate cortical (orbito-frontal cortex) and sub- cortical (amygdala) emotion-generative structures (Ochsner & Gross, 2005). Dorsolateral PFC appears to be important for exercising sustained control over thoughts and memories, whereas ACC may be more involved in transient aspects of thought control (Anderson et al., 2004; Mitchell et al., 2007; Wyland, Kelley, Macrae, Gordon, & Heatherton, 2003), such as registering discrepancies (Botvinick, Cohen, & Carter, 2004).
Regions involved in behavioral inhibition may vary considerably as a function of task: dosolateral prefrontal cortex, as well as sensorimotor cortex, supplementary motor area (SMA) and pre-SMA have all been implicated, with the motor areas apparently playing a particular role in inhibiting unwanted movements (Mostofsky et al., 2003). All of these studies, however, have used mostly Western participant populations. Insofar as cultures vary in their norms for exercising control over thoughts, feelings, and behaviors, one might expect cultural differences in the regions involved in self-regulation and inhibition tasks, or in the strength of connections between these regions. Such findings would lead to greater under- standing of the functional profiles of regulation and inhibition regions.
Prejudice and dehumanization
In cultures all over the world, people discriminate against one another on the basis of group membership (Cuddy et al., 2009; Sidanius & Pratto, 2001). However, this dis- crimination varies widely from culture to culture, both in terms of which groups are primarily the targets of discrimination and in terms of the content and intensity of stereo- types pertaining to each group (Cuddy et al., 2009). Such variation seems fitting, because social groups are cultural constructs. This is true even for groups with ostensibly biological bases, such as race. Just as the perception of color categories is, in part, culturally determined (David- off, 2001, above), so too are the categorical definitions of race—and certainly people’s reactions to race—culture dependent (Fredrickson, 2002; Jones, 1997; Sears, 1998).
A variety of socially defined categories elicit neural sig- natures of prejudice. In the USA, for example, drug addicts and homeless persons are among the least-esteemed members of society. These individuals are often dehumanized, one consequence of which is that that they fail to elicit the neural responses (dorsal mPFC) typically associated with perceiving other people and other people’s minds (Harris & Fiske, 2006). Other cultures may dehumanize different kinds of groups (e.g. ‘untouchable’ castes). Therefore, the neural markers of prejudice and dehumanization observed in US samples (e.g. reduced mPFC activity, increased insula and amygdala response; Hart et al., 2000; Krendl, Macrae, Kelley, Fugelsang, & Heatherton, 2006; Phelps et al., 2000), should occur in response to different groups across cultures. Given that short-term context moderates such neural responses to outgroups (Harris & Fiske, 2007; Wheeler & Fiske, 2005), long-term cultural context should do so as well. A more nuanced question may be how the qualitative nature of prejudice toward outgroups varies broadly across different cultures, and what combinations of neural processes might give rise to these different ‘flavors’ of prejudice.
Neural signatures of fundamental warmth and competence judgments
The Stereotype Content Model describes an arguably universal pair of dimensions that define social cognition. Warmth judgments answer the question of whether the other intends good or ill, and are associated with perceived trust- worthiness and friendliness. Competence judgments answer the question of the other’s ability to enact those intentions. Although a thorough review goes beyond our scope here (see Fiske, Cuddy, Glick, 2007), the dimensions usefully describe varieties of stereotypes across North American, European, and East Asian settings (Cuddy et al., 2009).
Ongoing work searches for neural signatures of these dimensions. For example, in Western populations, amygdala activation correlates robustly with trustworthiness judgments (a core component of the warmth dimension), suggesting the need for vigilance to negative and extreme others (Said, Baron, & Todorov, 2009; cf. Fiske, 1980). The neural processes underlying appraisals of competence are, at present, less well understood; however, combinations of warmth and competence reliably predict important components of social perception, including the neural (and behavioral) signatures of dehumanization reviewed in the previous section (Harris & Fiske, 2006).
A cross-cultural effort to clarify the neural substrates of competence judgments would be tremendously useful in building toward a deeper understanding of this important and arguably universal dimension of social perception. Meanwhile, if the amygdala does, indeed, track with Westerners’ perceptions of warmth/trustworthiness, as present studies suggest, and if warmth/trustworthiness does, in fact, constitute a universal dimension of social perception, then similar results to those observed among Westerners should be obtained in other populations as well. This hypothesis awaits cross-cultural comparisons.
Conclusion
The past two decades have seen tremendous expansion in the use of neuroscience to study high-level social and cognitive processes, as well as cultural psychology, to under- stand human diversity (e.g. Fiske, 2000). The growth of social neuroscience has not been without its (sometimes justified) detractors. Although neuroscience is not the best tool for every job in psychology (for a detailed set of cautions, see Zhou & Cacioppo, 2010), neuroscience is particularly useful for determining when two apparently distinct mental operations, in fact, recruit the same under- lying processes—and, conversely, when two apparently similar operations occur by quite different neural processes. Given that one of the major goals of cultural psychology is to identify differences and similarities in human thought across populations, neuroscience would seem to have much to contribute to cultural psychology. In addition to helping us build a more complete picture of the relation- ships among culture, psychology, and biology, cultural neuroscience may, in time, yield other benefits, such as improved educational practices (see the paragraph Number, above), increased mutual understanding across cultures and more effective mental health care for people all across the world.
Footnotes
Neural activity was measured in terms of event related potentials (ERP) in this study. Although this method does not allow for any direct means of precise functional localization, the authors point to several studies suggesting that the specific signal under discussion here (P1 amplitude) arose from activity in the lateral occipital cortex, and that this activity is modulated by spatial attention in a manner consistent with the authors’ interpretation.
References
- Aguirre GK, D’Esposito M. Environmental knowledge is subserved by separable dorsal/ventral neural area. Journal of Neuroscience. 1997;17:2512–2518. doi: 10.1523/JNEUROSCI.17-07-02512.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson MC, Ochsner KN, Kuhl B, et al. Neural systems underlying the suppression of unwanted memories. Science. 2004;303(5655):232–235. doi: 10.1126/science.1089504. [DOI] [PubMed] [Google Scholar]
- Ansari D. Effects of development and enculturation on number representation in the brain. Nature Reviews Neuroscience. 2008;9(4):278–291. doi: 10.1038/nrn2334. [DOI] [PubMed] [Google Scholar]
- Aslin RN. Experiential influences and sensitive periods in perceptual development: A unified model. In: Aslin RN, Alberts JR, Petersen MR, editors. Development of Perception. Psychobiological Perspectives (Vol. 2): The Visual System. New York: Academic Press; 1981. pp. 45–93. [Google Scholar]
- Bolger DJ, Perfetti CA, Schneider W. Cross-cultural effect on the brain revisited: Universal structures plus writing system variation. Human Brain Mapping. 2005;25(1):92–104. doi: 10.1002/hbm.20124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boroditsky L. Does language shape thought?: Mandarin and English speakers’ conceptions of time. Cognitive Psychology. 2001;43(1):1–22. doi: 10.1006/cogp.2001.0748. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Cohen JD, Carter CS. Conflict monitoring and anterior cingulate cortex: An update. Trends in Cognitive Sciences. 2004;8(12):539–546. doi: 10.1016/j.tics.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Vincent JL. Unrest at rest: Default activity and spontaneous network correlations. Neuroimage. 2007;37(4):1091–1096. doi: 10.1016/j.neuroimage.2007.01.010. [DOI] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, et al. Influence of life stress on depression: Moderation by a polymorphism in the 5-HTT gene. Science. 2003;301(5631):386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Cheour M, Ceponiene R, Lehtokoski A, et al. Development of language-specific phoneme representations in the infant brain. Nature Neuroscience. 1998;1:351–353. doi: 10.1038/1561. [DOI] [PubMed] [Google Scholar]
- Chiao JY, Harada T, Komeda H, Li Z, Mano Y, Saito D, et al. Dynamic cultural influences on neural representations of the Self. Journal of Cognitive Neuroscience. 2010;22:1–11. doi: 10.1162/jocn.2009.21192. [DOI] [PubMed] [Google Scholar]
- Chiao JY, Iidaka T, Gordon HL, Nogawa J, Bar M, Aminoff E, et al. Cultural specificity in amygdala response to fear faces. Journal of Cognitive Neuroscience. 2008;20(12):2167–2174. doi: 10.1162/jocn.2008.20151. [DOI] [PubMed] [Google Scholar]
- Ching J. Paradigms of the self in Buddhism and Christianity. Buddhist-Christian Studies. 1984;4:31–50. [Google Scholar]
- Chomsky N. Aspects of the Theory of Syntax. Cambridge, MA: MIT Press; 1965. [Google Scholar]
- Chua HF, Boland JE, Nisbett RE. Cultural variation in eye movements during scene perception. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(35):12629–12633. doi: 10.1073/pnas.0506162102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen D, Vandello J, Puente S, Rantilla A. ‘When you call me that, smile!’ How norms for politeness, interaction styles, and aggression work together in Southern Culture. Social Psychology Quarterly. 1999;62:257–275. [Google Scholar]
- Cohen JD, Servan-Schreiber D, McClelland JL. A parallel distributed processing approach to automaticity. The American Journal of Psychology. 1992;105:239–269. [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nature Reviews Neuroscience. 2002;3(3):201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Craik FIM, Moroz TM, Moscovitch M, et al. In search of the self: A positron emission tomography study. Psychological Science. 1999;10(1):26–34. [Google Scholar]
- Cuddy AJC, Fiske ST, Kwan VSY, et al. Stereotype content model across cultures: Towards universal similarities and some differences. British Journal of Social Psychology. 2009;48(1):1–33. doi: 10.1348/014466608X314935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Argembeau A, Collette F, Van der Linden M, Laurys S, Del Fiore G, Degueldre C, et al. Self-referential reflective activity and its relationship with rest: A PET study. Neuroimage. 2005;25:616–624. doi: 10.1016/j.neuroimage.2004.11.048. [DOI] [PubMed] [Google Scholar]
- D’Argembeau A, Ruby P, Collette F, Degueldre C, Balteau E, Luxen A, et al. Distinct regions of the medial prefrontal cortex are associated with self-referential processing and perspective taking. Journal of Cognitive Neuroscience. 2007;19(6):935–944. doi: 10.1162/jocn.2007.19.6.935. [DOI] [PubMed] [Google Scholar]
- Davidoff J. Language and perceptual categorization. Trends in Cognitive Sciences. 2001;5(9):382–387. doi: 10.1016/s1364-6613(00)01726-5. [DOI] [PubMed] [Google Scholar]
- Ekman P. An argument for basic emotions. Cognition and Emotion. 1992;6:169–200. [Google Scholar]
- Elfenbein HA, Ambady N. Is there an in-group advantage in emotion recognition? Psychological Bulletin. 2002;128(2):243–249. doi: 10.1037/0033-2909.128.2.243. [DOI] [PubMed] [Google Scholar]
- Fiske AP. Complementarity theory: Why human social capacities evolved to require cultural complements. Personality and Social Psychology Review. 2002;4:76–94. doi: 10.1207/S15327957PSPR0401_7. [DOI] [PubMed] [Google Scholar]
- Fiske ST. Attention and weight in person perception: The impact of negative and extreme behavior. Journal of Personality and Social Psychology. 1980;38:889–906. [Google Scholar]
- Fiske ST. Stereotyping, prejudice, and discrimination at the seam between the centuries: Evolution, culture, mind, and brain. European Journal of Social Psychology. 2000;30:299–322. [Google Scholar]
- Fiske ST. Cultural processes. In: Berntson GG, Cacioppo JT, editors. Handbook of Neuroscience for the Behavioral Sciences. New York: Wiley; 2009. pp. 985–1001. [Google Scholar]
- Fiske ST, Cuddy AJC, Glick P. Universal dimensions of social cognition: Warmth and competence. Trends in Cognitive Sciences. 2007;11:77–83. doi: 10.1016/j.tics.2006.11.005. [DOI] [PubMed] [Google Scholar]
- Fredrickson GM. Racism: A Short History. Princeton, NJ: Princeton University Press; 2002. [Google Scholar]
- Gilbert DT, Malone PS. The correspondence bias. Psychological Bulletin. 1995;117:21–38. doi: 10.1037/0033-2909.117.1.21. [DOI] [PubMed] [Google Scholar]
- Gitelman DR, Nobre AC, Parrish TB, et al. A large-scale distributed network for covert spatial attention: Further anatomical delineation based on stringent behavioral and cognitive controls. Brain. 1999;122(6):1093–1106. doi: 10.1093/brain/122.6.1093. [DOI] [PubMed] [Google Scholar]
- Goh JO, Chee MW, Tan JC, et al. Age and culture modulate object processing and object–scene binding in the ventral visual area. Cognitive, Affective, & Behavioral Neuroscience. 2007;7(1):44–52. doi: 10.3758/cabn.7.1.44. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Srivastava G, Reiss AL, Menon V. Default-mode network activity distinguishes Alzheimer’s disease from healthy aging: Evidence from functional MRI. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(13):4637–4642. doi: 10.1073/pnas.0308627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: Relation to a default mode of brain function. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:4259–4264. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutchess AH, Welsh RC, Boduroglu A, Park DC. Cultural differences in neural function associated with object processing. Cognitive, Affective, and Behavioral Neuroscience. 2006;6:102–109. doi: 10.3758/cabn.6.2.102. [DOI] [PubMed] [Google Scholar]
- Han S, Mao L, Gu X, Zhu Y, Ge J, Ma Y. Neural consequences of religious belief on self-referential processing. Social Neuroscience. 2008;3:1–15. doi: 10.1080/17470910701469681. [DOI] [PubMed] [Google Scholar]
- Han S, Northoff G. Culture-sensitive neural substrates of human cognition: A transcultural neuroimaging approach. Nature Reviews Neuroscience. 2008;9:646–654. doi: 10.1038/nrn2456. [DOI] [PubMed] [Google Scholar]
- Harris LT, Fiske ST. Dehumanizing the lowest of the low: Neuroimaging responses to extreme out-groups. Psychological Science. 2006;17(10):847–853. doi: 10.1111/j.1467-9280.2006.01793.x. [DOI] [PubMed] [Google Scholar]
- Harris LT, Fiske ST. Social groups that elicit disgust are differentially processed in mPFC. Social Cognitive and Affective Neuroscience. 2007;2:45–51. doi: 10.1093/scan/nsl037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris LT, Todorov A, Fiske ST. Attributions on the brain: Neuroimaging dispositional inferences, beyond theory of mind. Neuroimage. 2005;28(4):763–769. doi: 10.1016/j.neuroimage.2005.05.021. [DOI] [PubMed] [Google Scholar]
- Hart AJ, Whalen PJ, Shin LM, McInerney SC, Fischer H, Rauch SL. Differential response in the human amygdala to racial outgroup vs ingroup face stimuli. Neuroreport. 2000;11(11):2351–2355. doi: 10.1097/00001756-200008030-00004. [DOI] [PubMed] [Google Scholar]
- Hatano G, Miyake Y, Binks MG. Performance of expert abacus operators. Cognition. 1977;5(1):47–55. [Google Scholar]
- Hatano G, Amaiwa S, Shimizu K. Formation of a mental abacus for computation and its use as a memory device for digits: A developmental study. Developmental Psychology. 1987;23(6):832–838. [Google Scholar]
- Hedden T, Ketay S, Aron A, Markus H, Gabrieli JDE. Cultural influences on neural substrates of attentional control. Psychological Science. 2008;19(1):12–17. doi: 10.1111/j.1467-9280.2008.02038.x. [DOI] [PubMed] [Google Scholar]
- Hynes CA, Baird AA, Grafton ST. Differential role of the orbital frontal lobe in emotional versus cognitive perspective-taking. Neuropsychologia. 2006;44(3):374–383. doi: 10.1016/j.neuropsychologia.2005.06.011. [DOI] [PubMed] [Google Scholar]
- Iacoboni M, Lieberman MD, Knowlton BJ, et al. Watching social interactions produces dorsomedial prefrontal and medial parietal BOLD fMRI signal increases compared to a resting baseline. Neuroimage. 2004;21(3):1167–1173. doi: 10.1016/j.neuroimage.2003.11.013. [DOI] [PubMed] [Google Scholar]
- Ji LJ, Peng K, Nisbett RE. Culture, control, and perception of relationships in the environment. Journal of Personality and Social Psychology. 2000;78(5):943–955. doi: 10.1037//0022-3514.78.5.943. [DOI] [PubMed] [Google Scholar]
- Jones EE, Harris VA. The attribution of attitudes. Journal of Experimental Social Psychology. 1967;3(1):1–24. [Google Scholar]
- Jones JM. Prejudice and Racism. 2nd edn. New York: McGraw-Hill; 1997. [Google Scholar]
- Kelley WM, Macrae CN, Wyland CL, Caglar S, Inati S, Heatherton TF. Finding the self? An event-related fMRI study. Journal of Cognitive Neuroscience. 2002;14(5):785–794. doi: 10.1162/08989290260138672. [DOI] [PubMed] [Google Scholar]
- Kitayama S, Duffy S, Kawamura T, Larsen JT. Perceiving an object and its context in different cultures: A cultural look at new look. Psychological Science. 2003;14(3):201–206. doi: 10.1111/1467-9280.02432. [DOI] [PubMed] [Google Scholar]
- Kobayashi C, Glover GH, Temple E. Cultural and linguistic influence on neural bases of ‘Theory of Mind’: An fMRI study with Japanese bilinguals. Brain and Language. 2006;98(2):210–220. doi: 10.1016/j.bandl.2006.04.013. [DOI] [PubMed] [Google Scholar]
- Krendl AC, Macrae CN, Kelley WM, Fugelsang JA, Heatherton TF. The good, the bad, and the ugly: An fMRI investigation of the functional anatomic correlates of stigma. Social Neuroscience. 2006;1(1):5–15. doi: 10.1080/17470910600670579. [DOI] [PubMed] [Google Scholar]
- Kuhl PK, Williams KA, Lacerda F, Stevens KN, Lindblom B. Linguistic experience alters phonetic perception in infants by 6 months of age. Science. 1992;255(5044):606–608. doi: 10.1126/science.1736364. [DOI] [PubMed] [Google Scholar]
- Laland KN. The mathematical modeling of human culture and its implications for psychology and the human sciences. British Journal of Psychology. 1993;84:145–169. doi: 10.1111/j.2044-8295.1993.tb02471.x. [DOI] [PubMed] [Google Scholar]
- Lee K, Lim ZY, Yeong SHM, Ng SF, Venkatraman V, Chee MWL. Strategic differences in algebraic problem solving: Neuroanatomical correlates. Brain Research. 2007;1155:163–171. doi: 10.1016/j.brainres.2007.04.040. [DOI] [PubMed] [Google Scholar]
- Lin Z, Lin Y, Han S. Self-construal priming modulates visual activity underlying global/local perception. Biological Psychology. 2008;77(1):93–97. doi: 10.1016/j.biopsycho.2007.08.002. [DOI] [PubMed] [Google Scholar]
- McClure SM, Li J, Tomlin D, Cypert KS, Montague LM, Motague PR. Neural correlates of behavioral preference for culturally familiar drink. Neuron. 2004;44(2):379–387. doi: 10.1016/j.neuron.2004.09.019. [DOI] [PubMed] [Google Scholar]
- Maguire EA, Gadian DG, Johnsrude IS, et al. Navigation related structural change in the hippocampi of taxi drivers. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:4398–4403. doi: 10.1073/pnas.070039597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markham R, Wang L. Recognition of emotion by Chinese and Australian children. Journal of Cross-Cultural Psychology. 1996;27(5):616–643. [Google Scholar]
- Markus HR, Kitayama S. Culture and the self: Implications for cognition, emotion, and motivation. Psychological Review. 1991;98(2):224–253. [Google Scholar]
- Martin A, Wiggs CL, Ungerleider LG, Haxby JV. Neural correlates of category-specific knowledge. Nature. 1996;379(6566):649–652. doi: 10.1038/379649a0. [DOI] [PubMed] [Google Scholar]
- Mason MF, Norton MI, Van Horn JD, Wegner DM, Grafton ST, Macrae CN. Wandering minds: The default network and stimulus-independent thought. Science. 2007;315(5810):393–395. doi: 10.1126/science.1131295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesquita B, Frijda NH. Cultural variations in emo- tions: A review. Psychological Bulletin. 1992;112(2):179–204. doi: 10.1037/0033-2909.112.2.179. [DOI] [PubMed] [Google Scholar]
- Mikhail J. Universal moral grammar: Theory, evidence and the future. Trends in Cognitive Sciences. 2007;11(4):143–152. doi: 10.1016/j.tics.2006.12.007. [DOI] [PubMed] [Google Scholar]
- Milham MP, Banich MT, Claus ED, Cohen NJ. Practice-related effects demonstrate complementary roles of anterior cingulate and prefrontal cortices in attentional control. Neuroimage. 2003;18(2):483–493. doi: 10.1016/s1053-8119(02)00050-2. [DOI] [PubMed] [Google Scholar]
- Mitchell JP. Contributions of functional neuroimaging to the study of social cognition. Current Directions in Psychological Science. 2008;17(2):142–146. [Google Scholar]
- Mitchell JP, Heatherton TF, Kelley WM, Wyland CL, Wegner DM, Neil Macrae C. Separating sustained from transient aspects of cognitive control during thought suppression. Psychological Science. 2007;18(4):292–297. doi: 10.1111/j.1467-9280.2007.01891.x. [DOI] [PubMed] [Google Scholar]
- Mostofsky SH, Schafer JGB, Abrams MT, et al. fMRI evidence that the neural basis of response inhibition is task-dependent. Cognitive Brain Research. 2003;17(2):419–430. doi: 10.1016/s0926-6410(03)00144-7. [DOI] [PubMed] [Google Scholar]
- Näätänen R, Lehtokoski A, Lennes M, et al. Language- specific phoneme representations revealed by electric and mag- netic brain responses. Nature. 1997;385(6615):432–434. doi: 10.1038/385432a0. [DOI] [PubMed] [Google Scholar]
- Ng SH, Han S, Mao L, Lai JCL. Dynamic bicultural brains: fMRI study of their flexible neural representation of self and significant others in response to culture primes. Asian Journal of Social Psychology. 2010;13(2):83–91. [Google Scholar]
- Nisbett RE. The Geography of Thought: How Asians and Westerners Think Differently … and Why. New York: Free Press; 2003. [Google Scholar]
- Nisbett RE, Miyamoto Y. The influence of culture: Holistic versus analytic perception. Trends in Cognitive Sciences. 2005;9(10):467–473. doi: 10.1016/j.tics.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Bunge SA, Gross JJ, Gabrieli JDE. Rethinking feelings: An fMRI study of the cognitive regulation of emotion. Journal of Cognitive Neuroscience. 2002;14(8):1215–1229. doi: 10.1162/089892902760807212. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. The cognitive control of emotion. Trends in Cognitive Sciences. 2005;9(5):242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Pennebaker JW, Rimé B, Blankenship VE. Stereotypes of emotional expressiveness of northerners and southerners: A cross-cultural test of Montesquieu’s hypotheses. Journal of Personality and Social Psychology. 1996;70(2):372–380. doi: 10.1037//0022-3514.70.2.372. [DOI] [PubMed] [Google Scholar]
- Phelps EA, O’Connor KJ, Cunningham WA, Funayama ES, Gatenby JC, Gore JC, et al. Performance on indirect measures of race evaluation predicts amygdala activation. Journal of Cognitive Neuroscience. 2000;12:729–738. doi: 10.1162/089892900562552. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(2):676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross L. The intuitive psychologist and his shortcomings: Distortions in the attribution process. In: Berkowitz L, editor. Advances in Experimental Social Psychology. Vol. 10. New York: Academic Press; 1977. pp. 174–221. [Google Scholar]
- Said CP, Baron SG, Todorov A. Nonlinear amygdala response to face trustworthiness: Contributions of high and low spatial frequency information. Journal of Cognitive Neuroscience. 2009;21:519–528. doi: 10.1162/jocn.2009.21041. [DOI] [PubMed] [Google Scholar]
- Sears DO. Racism and politics in the United States. Confronting racism: The problem and the response. In: Eberhardt JL, Fiske ST, Susan T, editors. Confronting racism: The problem and the response. Thousand Oaks, CA: Sage; 1998. pp. 76–100. [Google Scholar]
- Sidanius J, Pratto F. Social Dominance: An Intergroup Theory of Social Hierarchy and Oppression. Cambridge: Cambridge University Press; 2001. [Google Scholar]
- Siok WT, Perfetti CA, Jin Z, Tan LH. Biological abnormality of impaired reading is constrained by culture. Nature. 2004;431(7004):71–76. doi: 10.1038/nature02865. [DOI] [PubMed] [Google Scholar]
- Sohn MH, Goode A, Koedinger KR, et al. Behavioral equivalence, but not neural equivalence—neural evidence of alternative strategies in mathematical thinking. Nature Neuroscience. 2004;7:1193–1194. doi: 10.1038/nn1337. [DOI] [PubMed] [Google Scholar]
- Sommer T, Rose M, Weiller C, Büchel C. Contributions of occipital, parietal, and parahippocampal cortex to encoding of object-location association. Neuropsychologia. 2005;43:732–743. doi: 10.1016/j.neuropsychologia.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Sugiura M, Kawashima R, Nakamura K, et al. Passive and active recognition of one’s own face. Neuroimage. 2000;11(1):36–48. doi: 10.1006/nimg.1999.0519. [DOI] [PubMed] [Google Scholar]
- Sui J, Han S. Self-construal priming modulates neural substrates of self-awareness. Psychological Science. 2007;18(10):861–866. doi: 10.1111/j.1467-9280.2007.01992.x. [DOI] [PubMed] [Google Scholar]
- Tan LH, Laird AR, Li K, Fox PT. Neuroanatomical correlates of phonological processing of Chinese characters and alphabetic words: A meta-analysis. Human Brain Mapping. 2005;25(1):83–91. doi: 10.1002/hbm.20134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y, Zhang W, Chen K, et al. Arithmetic processing in the brain shaped by cultures. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(28):10775–10780. doi: 10.1073/pnas.0604416103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SE, Way BM, Welch WT, Hilmert CJ, Lehman BJ, Eisenberger NI. Early family environment, current adversity, the serotonin transporter promoter polymorphism, and depressive symptomatology. Biological Psychiatry. 2006;60(7):671–676. doi: 10.1016/j.biopsych.2006.04.019. [DOI] [PubMed] [Google Scholar]
- Tillich P. Theology of Culture. Oxford: Oxford University Press; 1959. [Google Scholar]
- Triandis HC, Bontempo R, Villareal MJ, Asai M, Lucca N. Individualism and collectivism: Cross-cultural perspectives on self-ingroup relationships. Journal of Personality and Social Psychology. 1988;54(2):323–338. [Google Scholar]
- Ungerleider LG. Functional brain imaging studies of cortical mechanisms for memory. Science. 1995;270:769–775. doi: 10.1126/science.270.5237.769. [DOI] [PubMed] [Google Scholar]
- Wheeler ME, Fiske ST. Social-cognitive goals affect amygdala and stereotype activation. Psychological Science. 2005;16(1):56–63. doi: 10.1111/j.0956-7976.2005.00780.x. [DOI] [PubMed] [Google Scholar]
- Wyland CL, Kelley WM, Macrae CN, Gordon HL, Heatherton TF. Neural correlates of thought suppression. Neuropsychologia. 2003;41(14):1863–1867. doi: 10.1016/j.neuropsychologia.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Zhang L, Zhou T, Zhang J, Liu Z, Fan J, Zhu Y. In search of the Chinese self: An fMRI study. Science in China Series C: Life Sciences. 2006;49(1):89–96. doi: 10.1007/s11427-004-5105-x. [DOI] [PubMed] [Google Scholar]
- Zhou H, Cacioppo J. Culture and the brain: Opportunities and obstacles. Asian Journal of Social Psychology. 2010;13(2):59–71. [Google Scholar]
- Zhu Y, Zhang L, Fan J, Han S. Neural basis of cultural influence on self-representation. Neuroimage. 2007;34(3):1310–1316. doi: 10.1016/j.neuroimage.2006.08.047. [DOI] [PubMed] [Google Scholar]
- Zizzo DJ. Between utility and cognition: The neurobiology of relative position. Journal of Economic Behavior and Organization. 2002;48:71–91. [Google Scholar]
- Zizzo DJ. Empirical evidence on interdependent preferences: Nature or nurture? Cambridge Journal of Economics. 2003;27(6):867–880. [Google Scholar]