Abstract
Regular consumption of fruits and vegetables is associated with reduced risk of age-related functional decline and chronic diseases such as cancer and cardiovascular disease. These effects are primarily attributed to phytochemicals, plant compounds with a wide range of biological activities and health benefits. Apples, the top contributor of fruit phenolics in American diets, have high antioxidant, antiproliferative and chemopreventive activity in vitro and in vivo. However, little is known about their effects on aging. The objectives of this study were to determine the effects of whole apple phytochemical extracts on lifespan, healthspan and resistance to various stresses in vivo using C. elegans as a model. The mean and maximum lifespan of animals treated with 2.5, 5 and 10 mg/ml whole apple extracts increased significantly in a dose-dependent manner by up to 39 and 25%, respectively. Healthspan also significantly improved as indicated by improved motility and reduced lipofuscin accumulation. Animals pre-treated with whole apple extracts were more resistant to stresses such as heat, UV radiation, paraquat-induced oxidative stress, and pathogenic infection, suggesting that cellular defense and immune system functions also improved. Our findings indicate that, in C. elegans, whole apple extracts slow aging, extend lifespan, improve healthspan, and enhance resistance to stress.
Keywords: Apple, Phytochemical, Antioxidant, Aging, Healthspan, Caenorhabditis elegans
1 Introduction
Age is a major risk factor for many chronic diseases. Epidemiological studies have consistently shown that regular consumption of fruits and vegetables is associated with a significantly reduced risk of developing such diseases, including various types of cancer, cardiovascular disease and diabetes (Willett, 1994; Joshipura et al. 2001; Lock et al. 2005). For example, Doll and Peto (1981) estimated that at least one-third of all cancers could be prevented by dietary modification. We, and others, have proposed that the health benefits of fruits and vegetables stem from additive and synergistic interactions of complex mixtures of phytochemicals, the bioactive non-nutrient plant compounds that have been linked to reducing the risk of major chronic diseases (Liu, 2003).
As an organism ages, it experiences a progressive deterioration of bodily functions over time, increasing both susceptibility to environmental challenge and risk of disease and death (Kirkwood, 2005). The nonparasitic nematode Caenorhabditis elegans is a popular model in aging research because these animals decline behaviorally and physiologically with age in a manner similar to that of higher mammals, including humans. As they age, animals move more slowly and exhibit sarcopenia, the progressive deterioration of muscle tissue. They become infertile. They accumulate oxidized proteins and lipofuscin, hallmarks of aging common to many species (Klass, 1977; Johnson, 2003). At the gene and protein level, C. elegans share up to 80% homology with human genes and a conserved protein network involved in aging (Braeckman & Vanfleteren, 2007; Bell et al. 2009). Besides being a biologically relevant aging model, these nematodes are easy to culture and have a short life cycle, allowing for rapid replication of experimental treatments. Moreover, standard assay conditions to study the pharmacology of drugs and interactions with genes have been described in this model organism (Rand & Johnson, 1995). Specifically, human pharmacological interventions ranging from vitamins and clinical drugs to antioxidant supplements and phytochemicals are known to extend lifespan or delay physiological aging in C. elegans (Collins et al. 2006; Lucanic et al. 2012). For example, anticonvulsant drugs: ethosuximide, trimethadione, and 3,3-diethyl-2-pyrrolidinone extend lifespan and delay age-related degenerative changes by modulating neuromuscular activity (Kornfeld & Evason, 2006). Similarly, extracts of Ginkgo biloba, components of green tea, and blueberry polyphenols, extend healthspan and lifespan, and enhance stress resistance through a variety of mechanisms (Wu et al. 2002; Zhang et al. 2009; Wilson et al. 2006; Gong et al. 2012). Despite these and other studies, however, surprisingly little is known about possible anti-aging effects of commonly consumed fruits and vegetables.
Apples are a popular, widely available and economically significant fruit. In the United States, they are the top contributor of phenolics, a major class of biologically significant phytochemicals associated with a wide range of bioactivities and health benefits both in vitro and in vivo (Boyer & Liu, 2004). Whole apple extracts have potent antioxidant effects and anti-proliferative activity against colon, liver and breast cancer cells in vitro in a dose-dependent manner (Eberhardt et al. 2000; Sun et al. 2002). In MCF-7 human breast cancer cells, these extracts inhibit activation of the transcription factor NFκB, thereby promoting resistance to anticancer chemotherapeutic drugs, and regulate the cell cycle by inducing G1 arrest and modulating the expression of key cell cycle proteins (Yoon & Liu, 2007; Sun & Liu, 2008). Previously, our group isolated new, structurally diverse compounds from the apple peels of Red Delicious apples, and showed that these compounds have potent antioxidant and antiproliferative activity against MCF-7 breast cancer cells and HepG2 liver cancer cells (He & Liu, 2007; 2008). Here, we explored the possibility that whole apple phytochemical extracts from these apples modulate specific biochemical and immunological biomarkers of health, which correlate with improved quality of life (healthspan) and increased lifespan in vivo. Specifically, our objectives were to determine the effects of whole apple phytochemical extracts on lifespan, healthspan and stress resistance in vivo using Caenorhabditis elegans as a model of aging.
2 Materials and Methods
2.1 Chemicals and Reagents
Acetone was purchased from Fischer Scientific (Pittsburgh, PA, USA). Sodium carbonate was purchased from Mallinckrodt Baker, Inc. (Phillipsburg, NJ, USA) and gallic acid from ICN Biomedical Inc. (Aurora, OH, USA). 5-fluoro-2-deoxyuridine (FUDR) and methyl viologen dichloride hydrate (paraquat) were purchased from Sigma-Aldrich (St. Louis, MO, USA).
2.2 Extraction of Apples
Fresh apples of the Red Delicious variety were purchased from Cornell Orchards in September, 2007 (Cornell University, Ithaca, NY, USA) and extracted using the method previously reported by our laboratory (Sun et al. 2002). Briefly, whole apples were sliced, blended in ice-chilled 80% acetone, homogenized and rotary evaporated under vacuum at 45°C until approximately 90% of the filtrate and all acetone had been evaporated (no solvent remained in the extract). The concentrate was re-suspended in water to a stock concentration of 100 mg/ml based on the dry weight of the extract, aliquoted and frozen at −80°C until use. Subsequent working concentrations were made from this master stock. These extracts have been characterized based on bioactivity-guided fractionation and structure identification using HR-MS, 1D and 2D NMR, and X-ray diffraction analysis using the methods we reported previously (He & Liu, 2007; 2008). Briefly, 29 compounds, including triterpenoids, flavonoids, organic acids and plant sterols were isolated from Red Delicious apple peels. Of the flavonoids, the major compounds were: quercetin-3-O-β-D-glucopyranoside (82.6%), quercetin-3-O-β-D-galactopyranoside (17.1%), quercetin 0.2%), (−)-catechin, (−)-epicatechin, and quercetin-3-O-α-L-arabinofuranoside (He & Liu, 2008). The major triterpenoids identified were: 2α-hydroxyursolic acid, 2α-hydroxy-3β-{[(2E)-3-phenyl-1-oxo-2-propenyl]oxy}olean-12-en-28-oic acid, and 3β-trans-p-coumaroyloxy-2α-hydroxyolean-12-en-28-oic acid, ursolic acid, 2α-hydroxyursolic acid, and 3β-trans-p-coumaroyloxy-2α-hydroxyolean-12-en-28-oic acid, and maslinic acid (He & Liu, 2007).
2.3 Strains, Maintenance and Culturing of Nematodes
Strains used were: Bristol N2 (wild-type), daf-16(mgDf47) and age-1(hx546). All strains were obtained from the C. elegans Genetics Center (CGC) based at the University of Minnesota. Animals were maintained at 20°C on petri dishes containing Nematode Growth Medium (NGM) seeded with a live E. coli strain OP50 as the food source according to the general procedures outlined by Brenner (Brenner, 1974).
2.4 Lifespan Assay
Several gravid adult nematodes were placed on NGM plates seeded with E. coli strain OP50 and allowed to lay eggs at 20°C for approximately 6 hours to obtain a synchronous population. After 6 hours, the nematodes were removed and the plates were placed back at 20°C until the progeny reached young adulthood (about 72 hours). On day 0 of the experiment, these young adult nematodes were transferred to 35mm NGM petri dishes containing either no extracts or the appropriate doses of dissolved, whole apple extracts and 50 μM of 5-fluoro-2-deoxyuridine (FUDR) to prevent progeny production. Plates were then dried in a sterile hood, seeded with 100 L of 4-fold concentrated, saturated E. coli OP50 culture, and dried again. Animals were transferred every other day to fresh extracts or control plates until day 8 of adulthood. Animals were scored daily or every other day by gentle prodding with a platinum wire. Those animals that failed to move were scored as dead. Animals that exhibited bagging, exploded or crawled off the plates were censored. Statistical analyses were performed using SPSS statistical software Kaplan-Meier Survival function; p-values were obtained using the log-rank test. The experiment was repeated multiple times and a representative trial is shown. All experiments, except for the P. aeruginosa pathogen killing assay and heat shock treatment were performed at 20°C.
2.5 Healthspan Assays
Animals were treated with whole apple extracts or cultured on control plates as described under “Lifespan Assay” above.
2.5.1 Lipofuscin
Animals (N=18 per group) were treated with whole apple extracts and on day 8 of adulthood, mounted onto 2% agarose pads and immobilized in 20μM sodium azide. Slides were visualized using the Leica DM5000B Microscope (Bannockburn, IL), with the I3 cube filter (excitation 450/490, emission 510), and images were captured using a Hamamatsu ORCA-ER camera and OpenLab software. Image quantification of fluorescence intensity was done densitometrically by tracing around each animal’s intestine and determining mean pixel intensity using ImageJ freeware (NIH) (Rasband, 1997).
2.5.2 Motility
Animals were treated as described above. On days 12, 14, 16 and 18 of adulthood, animals were visualized using an Olympus SZ61 stereomicroscope (New York/New Jersey Scientific, Middlebush, NJ, USA). Motility classes were determined using the method reported by Golden et al., where ‘A’ animals move spontaneously and smoothly, leaving sinusoidal and symmetric tracks; ‘C’ animals only move the nose or tail when prodded with a platinum wire; and ‘B’ animals represent every behavioral class in between (Herndon et al. 2002; Golden et al. 2008). N≥47 animals for days 10 and 12, N≥25 for day 16.
2.6 Stress Resistance Assays
For all stress resistance assays, animals were transferred to 35mm NGM/OP50 plates with whole apple extracts or control plates at the young adult stage containing 50μM FUDR, then incubated for 2 days followed by exposure to the stressor on the third day of adulthood. All trials were repeated at least 2–3 times, and a representative trial is shown.
2.6.1 Heat Shock
Heat shock experiments were carried out according to the method of Lithgow and colleagues (Lithgow et al. 1995). Animals were incubated at 35°C on the third day of adulthood for 8 hours and monitored every 30 minutes thereafter until approximately 50% of the controls had died. Plates were removed from the incubator and scored for survival. Triplicate plates were used for each time point with N≥61 animals per group.
2.6.2 UV Irradiation
Animals were transferred to bacteria-free NGM plates on day three of adulthood and UV-irradiated at 1200J/m2 with a UV Stratalinker 2400 (Stratagene, La Jolla, CA, USA) equipped with five 254nm UV light bulbs (model 1800), each generating 15 watts. After UV irradiation, animals were transferred back to the standard NGM/OP50 plates without whole apple extracts and monitored daily for survival by gentle prodding with a platinum wire (Cypser & Johnson, 2002). N=66, 82, 79, 72 animals for 0, 2.5, 5 and 10 mg/ml groups, respectively.
2.6.3 Pseudomonas aeruginosa Infection
Animals were grown on NGM/OP50 plates at 25°C and transferred to plates with or without whole apple extracts for a period of two days. On the third day of adulthood, animals were shifted to modified NGM plates, prepared according to the method of Tan et al, containing P. aeruginosa strain PA14 at 25°C, and scored for survival every 8–13 hours (Tan et al. 1999). Plates were seeded with 10μL of P. aeruginosa, and allowed to dry overnight at 37°C and then at room temperature for another 24 hours. N=82, 80, 73 and 76 animals for 0, 2.5, 5 and 10 mg/ml groups, respectively.
2.6.4 Oxidative Stress
The oxidative stress paraquat assay on plates was performed using the methods described previously (Ishii et al. 1990; Gruber et al. 2007). On day three of adulthood, animals were transferred to freshly prepared NGM/OP50 plates, containing 10mM paraquat and scored as above. N≥54 animals per each group.
2.7 Brood Size
The N2 animals were grown on NGM/OP50 plates until the late larval stage, L4, and transferred to the control plate or plates with different concentrations of whole apple extracts, one animal per plate per concentration, with N=8 animals per group. Animals were then transferred every 24 hours to the fresh control or whole apple extracts plates until egg production had ceased. The total number of progeny that grew up from each animal was counted, and the number of progeny for each concentration was averaged (Li et al. 2008).
2.8 Statistical Analyses
Survival data were analyzed using SPSS version 16 for Windows (SPSS Inc., Chicago, IL, USA) Kaplan-Meier Survival function and log-rank test. All other analyses were done using Minitab statistical software (State College, PA, USA). Data for heat shock were analyzed using two-sample t-test (assuming equal variance); lipofuscin accumulation and brood size were analyzed using one-way ANOVA; motility classes were compared using logistic regression. Graphs were plotted with the SigmaPlot version 10 for Windows software (Systat Software Inc., San Jose, CA, USA). A p-value < 0.05 was considered to be statistically significant.
3 Results
3.1 Whole Apple Extracts Increase the Lifespan of Wild-type C. elegans
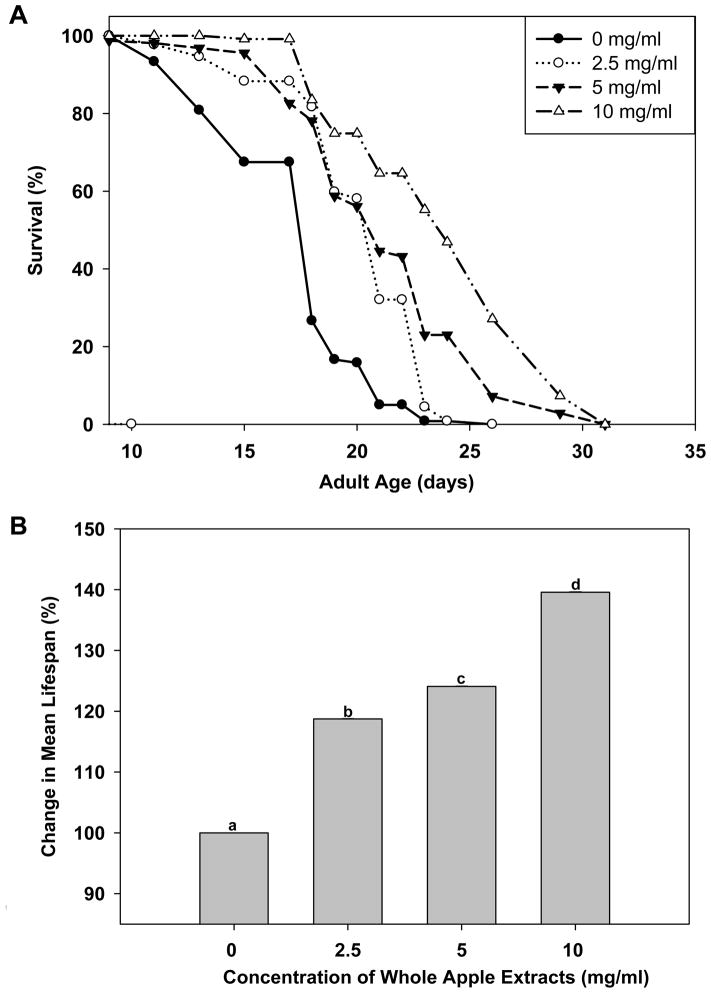
Wild-type adult C. elegans have a mean lifespan of 2–3 weeks at 20°C. Under our standard laboratory conditions, wild-type control animals lived an average of 17.23 ± .30 days (maximum of 24 days). After administering 2.5, 5 and 10 mg/ml whole apple extracts, starting at the young adult stage, mean lifespan increased to 20.46 ± .17 days (maximum of 24 days), 21.38 ± .35 days (maximum of 30 days) and 24.05 ± .53 days (maximum of 30 days), respectively, in a statistically significant, dose-dependent manner (p<0.05; Figure 1). These changes represent lifespan increases of 18.7, 24.1, and 39.6%, respectively, compared to the control group (Table 1).
Fig. 1.
Effect of whole apple extracts on the lifespan of C. elegans. Day 1 young adult wild-type animals (N ≥120 in each group) were treated without (0 mg/ml) or with a low (2.5 mg/ml), moderate (5 mg/ml) and high (10 mg/ml) dose of standardized whole apple extracts, which contained 170 ± 4.6 mg of phenolics per 100 g of apples. Survival was monitored starting on day 1 of adulthood. Nematodes, that were exposed to the whole apple extracts survived significantly longer than those that did not (p<0.05, log-rank test). The experiment was repeated multiple times and a representative trial is shown: a) Effect of apple extracts on the lifespan; and b) Percent increase in lifespan.
Table 1.
Effects of whole apple extracts on the mean lifespan of C. elegans.
| Concentration of Whole Apple Extracts (mg/ml) | N | Mean Lifespan* (days) | % of Control |
|---|---|---|---|
| 0 | 120 | 17.23±.30a** | 100.0 |
| 2.5 | 132 | 20.46±.17b | 118.7 |
| 5 | 158 | 21.38±35c | 124.1 |
| 10 | 120 | 24.05±.53d | 139.6 |
Mean ± SEM
Values with no letters in common within each column are significantly different (p < 0.01 for all pairwise comparisons).
3.2 Whole Apple Extracts Improve Motility and Attenuate Lipofuscin Accumulation
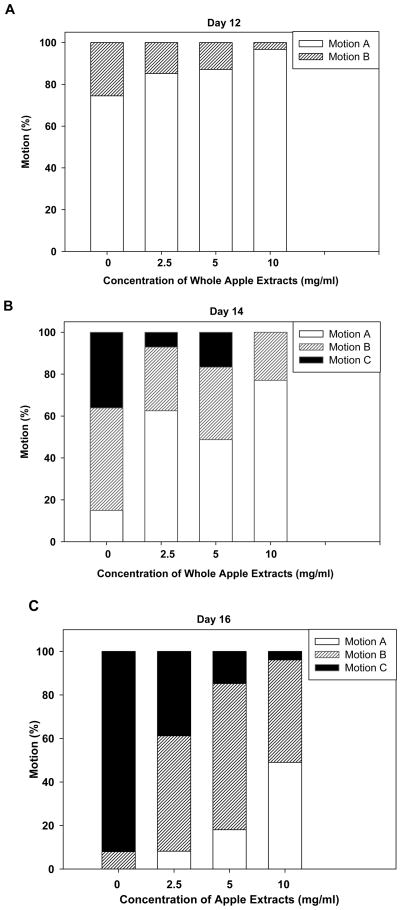
Next, we examined whether the increase in lifespan was accompanied by an overall improvement in health and vitality. We tested the motility of 12-, 14-, and 16-day-old animals treated with three concentrations of whole apple extracts. Motility was classified according to movement spontaneity on petri dishes: Class A animals moved spontaneously; class B animals required prodding to stimulate whole-body movement; and class C animals moved only their heads or tails in response to gentle prodding with a platinum wire (Herndon et al. 2002; Golden et al. 2008). The decline in motility on days 12, 14, and 16 was delayed significantly in a dose-dependent manner in animals treated with low (2.5 mg/ml), moderate (5 mg/ml), and high (10 mg/ml) concentrations of whole apple extracts (p≤0.01; Figures 2a–c). On day 12, most animals in all groups continued to move spontaneously, but there was already a small, significant difference in spontaneous (Class A) motility among the control and treatment groups (p<0.05; Figure 2a). By day 14, animals treated with low, moderate, and high doses of whole apple extracts had significantly (p<0.05) more high-motility (class A) individuals (63, 49, and 77%, respectively) than the control group (15%; Figure 2b). By day 16, the dose-dependent effects on motility were fully evident (Figure 2c), such that 92% of the control group would barely move their heads upon prodding with a platinum wire (class C), while 49% of the high-dose treatment group still moved spontaneously (class A). We continued to follow the animals that remained alive to day 18; interestingly, treatment groups still displayed highly significant dose-dependent differences in motility while most of the control animals had died (p<0.01, data not shown).
Fig. 2.
Effect of whole apple extracts on motility of a) day 12, b) day 14 and c) day 16 animals. Motility was classified into three groups: Motion A animals moved spontaneously; Motion B animals required prodding to stimulate movement; and Motion C animals only moved their heads in response to prodding. The decline in motility on days 12, 14, and 16 was significantly delayed in a dose-dependent manner in animals treated with 2.5, 5, and 10 mg/ml of apple extract. (N ≥ 47 animals per group for days 12 and 14; N ≥ 25 for day 16, p<0.05, logistic regression).
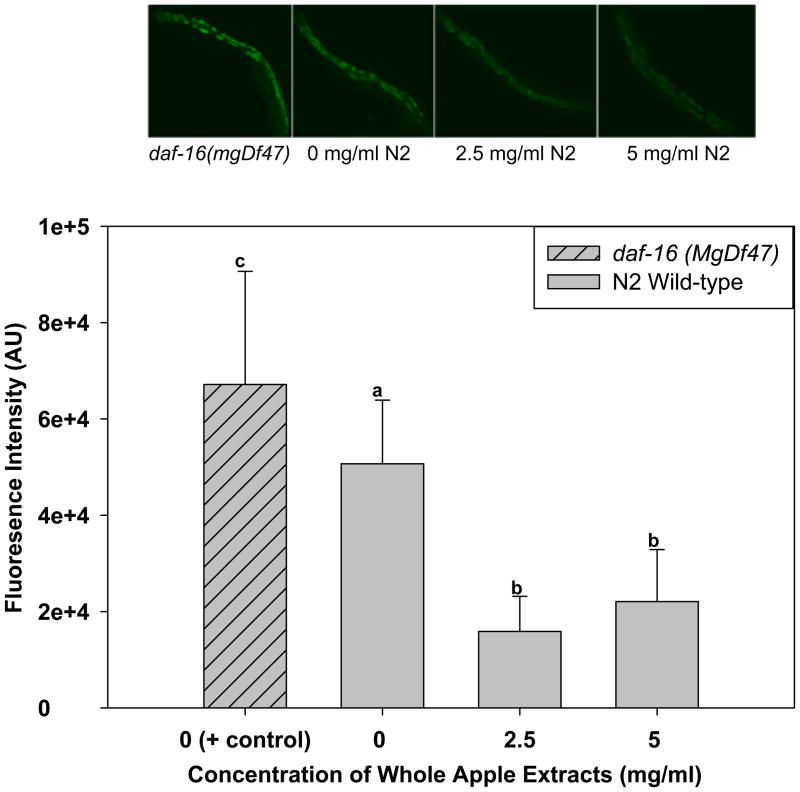
Lipofuscin is a byproduct of lysosomal degradation that accumulates with age in most organisms, including C. elegans (Clokey & Jacobson, 1986). Wild-type N2 animals treated with 2.5 and 5 mg/ml whole apple extracts accumulated only about half as much lipofuscin as the N2 control animals (p<0.05; Figure 3). Animals that have a mutation in daf-16, a gene encoding the forkhead transcription factor, age and accumulate lipofuscin at a faster rate than their wild-type N2 counterparts, and were used as a positive control. As expected, this group accumulated more lipofuscin after 8 days than did control N2 animals (p<0.05).
Fig. 3.
Effect of whole apple extracts on lipofuscin accumulation in C. elegans (N=18 animals per group). Wild-type N2 animals treated with 2.5 and 5 mg/ml apple extracts accumulated only about half as much lipofuscin as the N2 control worms. daf-16(mgDf47) mutant animals without extract treatment, which age at a faster rate, were used as a positive control. Bars with no letters in common are significantly different (p<0.05, One-way ANOVA).
3.3 Whole Apple Extracts Increase Resistance to Heat Stress, UV Irradiation and Pathogenic Infection
Increased lifespan often correlates with increased stress resistance (Johnson et al. 1996; Lithgow & Walker, 2002). We treated young adult C. elegans with doses of 0, 2.5, 5 and 10 mg/ml whole apple extracts for two days, and examined their response to a variety of chemical and environmental stressors, including heat shock, UV radiation, and the pathogen Pseudomonas aeruginosa.
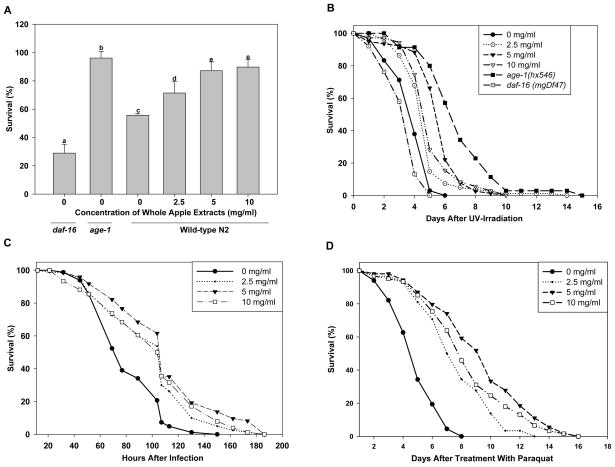
Animals were placed in a 35°C heat shock chamber and monitored until approximately half (55%) of the wild-type N2 controls had died (10.5 hours). At that time, 71.4, 87.1 and 89.73% of 2.5, 5 and 10 mg/ml apple-treated animals were still alive, respectively. Animals that have a mutation in age-1, the C. elegans PI3 kinase, are more resistant to heat stress, were used as a negative control and survived at a 96.13% rate; daf-16 animals, which are more sensitive to heat stress, were used as a positive control and had a 28.96% survival rate (p<0.05; Figure 4a)
Fig. 4.
Effect of pretreatment with whole apple extracts on resistance to stress in C. elegans. Animals were treated with various concentrations of whole apple extracts (0, 2.5, 5, or 10 mg/ml, in each group) as young adults for 2 days at 20°C and exposed to a variety of stressors on the third day of adulthood. Those that were pretreated with whole apple extracts, survived significantly longer after a) 35°C heat shock (N ≥ 61, bars with no letters in common are significantly different, p<0.05, two-sample t-test). b) UV irradiation at 1200 J/m2 (N ≥ 66, p<0.05, log-rank test). c) infection with P. aeruginosa (N ≥ 73, p<0.05, log-rank test). d) exposure to 10 mM paraquat (N ≥ 54 animals, p<0.05, log-rank test). Each experiment is representative of three independent trials. daf-16(mgDf47) and age-1(hx546) animals served as positive and negative controls, respectively.
In an analogous experiment, N2 animals pretreated with whole apple extracts survived significantly longer after UV irradiation than N2 control animals (p<0.05; Table 2, Figure 4b). Post-irradiation survival time for N2 animals pretreated with 2.5, 5 and 10 mg/ml whole apple extracts was increased by 22.6, 46.2, 61.5%, respectively. age-1 animals, which are also more resistant to UV stress than N2, were used as a negative control and survived 74.4% longer than the N2 controls. daf-16 animals, which are more sensitive to UV stress than N2, were used as a positive control and survived 12.8% less than the N2 controls.
Table 2.
Effect of pretreatment with whole apple extracts on resistance to UV irradiation in C. elegans.
| Concentration of Whole Apple Extracts (mg/ml) | N | Mean Lifespan* (days) | % of Control |
|---|---|---|---|
| 0 | 66 | 3.92±.15a** | 100.0 |
| 2.5 | 82 | 4.88±.18b | 122.6 |
| 5 | 79 | 5.70±.18c | 146.2 |
| 10 | 72 | 6.37±.18c,d | 161.5 |
| age-1 (neg control) | 36 | 6.89±.32d | 174.4 |
| daf-16 (pos cont) | 38 | 3.49±.19e | 87.2 |
Mean ± SEM
Values with no letters in common within each column are significantly different (p < 0.01, log-rank test).
N2 animals treated with whole apple extracts showed increased resistance to the pathogen Pseudomonas aeruginosa. After being transferred to pathogen-seeded plates on the third day of adulthood and monitored every 8–13 hours, control animals survived an average of 82 ± 2.6 hours, while animals treated with 2.5, 5 and 10 mg/ml of whole apple extracts had significantly increased mean lifespans of 100 ± 3.8, 111 ± 4.4 and 102 ± 4.4 hours, respectively (p<0.05; Table 3, Figure 4c).
Table 3.
Effect of pretreatment with whole apple extracts on resistance to infection by Pseudomonas aeruginosa in C. elegans.
| Concentration of Whole Apple Extracts (mg/ml) | N | Mean Lifespan* (hours) | % of Control |
|---|---|---|---|
| 0 | 82 | 82.0±2.62a** | 100.0 |
| 2.5 | 80 | 100.0±3.75b | 122.0 |
| 5 | 73 | 110.9±4.41c | 135.2 |
| 10 | 76 | 102.1±4.4c | 124.5 |
Mean ± SEM
Values with no letters in common within each column are significantly different (p < 0.05, log-rank test).
3.4 Whole Apple Extracts Increase Resistance to Oxidative Stress Induced By Paraquat
To test the antioxidant potential of whole apple extracts in vivo, we placed animals on NGM plates containing 10mM of the superoxide-generating chemical paraquat. While control animals survived an average of 4.97 ± 0.19 days (maximum of 7 days), those treated with 2.5, 5 and 10 mg/ml of whole apple extracts survived for an average of 7.71 ± 0.32 (maximum of 10 days), 9.41 ± 0.43 (maximum of 14 days) and 8.54 ± 0.39 days (maximum of 15 days), respectively. These represented statistically significant increases in mean life span of 55.1, 89.3 and 71.8% over the control (p<0.05; Table 4, Figure 4d).
Table 4.
Effect of pretreatment with whole apple extracts on resistance to chronic challenge with paraquat in C. elegans.
| Concentration of Whole Apple Extracts (mg/ml) | N | Mean Lifespan* (days) | % of Control |
|---|---|---|---|
| 0 | 67 | 4.97±.19a** | 100.0 |
| 2.5 | 58 | 7.71±.32b | 155.13 |
| 5 | 54 | 9.41±.43c | 189.3 |
| 10 | 61 | 8.54±.39c | 171.83 |
Mean ± SEM
Values with no letters in common within each column are significantly different (p < 0.05, log-rank test).
3.5 Whole Apple Extracts Do Not Effect Brood Size
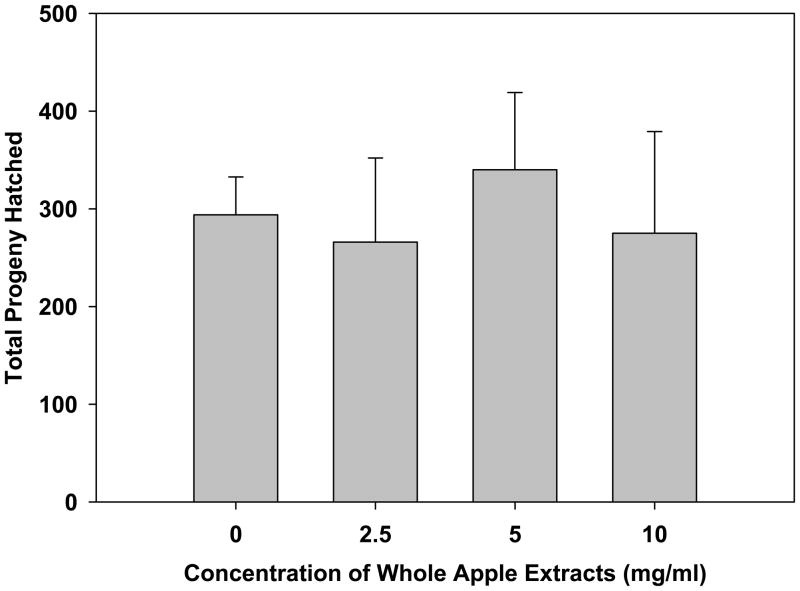
An increase in lifespan is often correlated with a decrease in fecundity. To test whether whole apple extracts adversely affect fecundity, we measured brood sizes of 8 N2 animals per each group: 0 (control), 2.5, 5 and 10 mg/ml. There were no significant differences between treatment groups and the control (p=0.321; Figure 5).
Fig. 5.
Effect of whole apple extracts on brood size of C. elegans (N=8 animals per group). There were no significant differences in the number of total progeny between whole apple extract-treated and untreated animals (p=.321, One-way ANOVA).
4 Discussion
Multiple studies have shown that compounds and extracts derived from plants can delay age-related decline and extend lifespan and healthspan across a variety of species. In the current study, we show for the first time that whole apple extracts extend lifespan and healthspan in a dose-dependent manner in vivo. Mean lifespan of C. elegans increased by up to 39% and maximum lifespan by up to 25%, when whole apple extracts were included in their diet. We also found corresponding improvements in several physiological and functional indicators of healthspan. Previous work from our group has shown that whole apple extracts have antioxidant and antiproliferative activity in vitro, and antitumor activity in vivo (Eberhardt et al. 2000; Sun et al. 2002; Liu et al. 2005). Our finding of anti-aging effects in vivo significantly broadens our knowledge of the biological effects of whole apple phytochemicals.
Specific apple fractions and phytochemicals have been shown to extend the mean lifespan of C. elegans and Drosophila melanogaster, and to suppress amyloid-beta protein aggregation in C. elegans (Sunagawa et al 2011; Peng et al. 2011; Toda et al 2011). For example, Sunagawa and coworkers showed that procyanidins from Fuji apples extended the mean lifespan of wild-type C. elegans by 12.1%, an effect that depended on SIR-2, a member of the sirtuin family of NAD+-dependent protein deacetylases. Likewise, apple polyphenols extended mean lifespan by 12.0% (Sunagawa et al. 2011). Peng and colleagues showed that polyphenols from the pomace of Red Fuji apples extended the mean lifespan of Drosophila by 10%, and that this effect was mediated at least in part through SOD, CAT, MTH, and Rpn11 activity (Peng et al. 2011). Other investigators demonstrated that quercetin and EGCG, two phytochemicals found in apples, extended the mean lifespan and healthspan of C. elegans (up to 15% for quercetin and either 0, 5 or 10% for EGCG depending on the dose and culturing conditions) (Brown et al. 2006; Kampkotter et al. 2008; Zhang et al. 2009). Here, we asked whether phytochemicals from whole apple extracts have anti-aging and stress-resistance activity and how this activity compares to that of single apple phytochemicals and fractions published previously. We hypothesized that biological activity would be enhanced due to synergistic interactions of multiple compounds across different phytochemical classes, and selected the Red Delicious apple variety based on its wide availability, popularity, and previously characterized antioxidant and biological activity both in vitro and in vivo. We find that the magnitude of mean lifespan extension (39%), is greater than single apple phytochemicals and apple fractions reported previously. Our findings suggest that interactions among various compounds are needed to elicit the maximum effect. This hypothesis is consistent with our own preliminary data, which show that quercetin-3-β-D-glucoside and 2-α-hydroxy-ursolic acid do not increase mean C. elegans lifespan as much as whole apple extracts (data not shown).
In addition to living longer, the animals in our study showed an improved healthspan. Animals treated with whole apple extract accumulated half as much lipofuscin as untreated animals (Figure 3). Treated animals also consistently showed more youthful and vigorous movement than control animals. The largest change was observed in post-reproductive, mid- to late-age day 14 and 16 animals. We continued to follow treated animals through day 18 (very late-age animals) even though more than half of the control animals had already died. The motility of many animals in apple-treated groups on day 18 resembled those of day 2–8 controls (classes ‘B’ and ‘A’) (data not shown).
We also tested the animals’ responses to various stressors. Previous studies have established a correlation between longer lifespan and resistance to stress in C. elegans and other animals, including mammals (Johnson et al. 1996; Lithgow & Walker, 2002; Gems & Partridge, 2008). After pretreatment with 2.5, 5 and 10 mg/ml doses of whole apple extracts for the first two days of adulthood, the animals were challenged with a battery of stressors including heat shock, UV radiation, paraquat-induced oxidative stress, and the pathogen P. aeruginosa.
Pretreatment with whole apple extracts greatly improved survival following heat shock. This result is consistent with other studies in C. elegans that showed enhancement of both thermotolerance and lifespan by plant-derived extracts (Wilson et al. 2006; Benedetti et al. 2008). In our study, whole apple phytochemicals may have affected stress-signaling pathways through activating heat shock proteins (hsps). Alternatively, these compounds may have themselves acted as chemical chaperones that stabilize protein conformation and promote a general cellular stress response (Benedetti et al. 2008). This may explain why animals exposed to whole apple extracts had significantly improved survival. UV light can damage DNA (e.g. cyclobutane pyrimidine dimers and 6-4 photoproducts) and accelerate aging (Rittie & Fisher, 2002; Clancy, 2008;). In our experiments, pretreatment with whole apple extracts significantly improved survival following UV irradiation. Phytochemicals found in apples have been previously shown to repair damaged DNA. For example, EGCG and quercetin can modulate DNA repair genes and mechanisms, including: H2AX, histone acetylation, ATM/ATR, GADD, Chk1/2, Cdc25c, p53, and KAP1 (Rajendran et al. 2010). Whole apple phytochemicals could have acted through these or other mechanisms to increase resistance to UV damage and extend lifespan. Aging is also associated with an increase in oxidative damage to DNA, proteins and lipids (Bokov et al. 2004). Previously, our group showed that whole apple extracts have antioxidant activity in vitro (Eberhardt et al. 2000). Here, we used a paraquat-induced oxidative stress model to examine whether similar effects can be observed in vivo. After pretreating animals with whole apple extracts for two days, we found that their survival was significantly improved after exposure to 10mM paraquat. This result suggests a possible antioxidant mechanism underlying the anti-aging effects of whole apple phytochemicals. Animals were also more resistant to the pathogen P. aeruginosa. Infection with this bacterium occurs by ingestion and, under normal conditions, P. aeruginosa is lethal within 1–3 days. Animals we pretreated with whole apple extracts survived up to 35.2% longer after infection, indicating that apple phytochemicals might improve immune response.
5 Conclusions
In conclusion, we have shown for the first time that whole apple phytochemical extracts increase the mean and maximum lifespan and confer multiplex stress resistance in vivo in the C. elegans aging model. Our results indicate that whole apple extracts also improve healthspan, as measured by reduced lipofuscin accumulation and improved motility. Animals pretreated with whole apple extracts are more resistant to heat, UV irradiation, and paraquat-induced oxidative stress, suggesting that cellular defense and immune system functions are also improved. Studies are underway to determine the contributions of individual phytochemicals and elucidate the mechanisms by which they may synergize to produce the anti-aging and other health benefits we observed.
Highlights.
Whole apple extracts increase the mean lifespan of C. elegans up to 39% in a dose-dependent manner.
Whole apple extracts improve healthspan as measured by motility and lipofuscin accumulation.
Whole apple extracts increased resistance to stress (e.g., heat shock, oxidative stress).
We suggest these benefits are due to synergistic interactions of multiple compounds across different phytochemical classes.
Acknowledgments
We thank Carolle Mok for technical assistance, Ben Hamilton, Atsushi Ebata, Nicole Liachko and Gizem Rizki for training and experimental troubleshooting, as well as Jason Neuswanger for critical editing of the manuscript.
Footnotes
Conflict of Interest Statement
Authors have no conflict of interest.
Publisher's Disclaimer: This is a PDF le of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bell R, Hubbard A, Chettier R, Chen D, Miller JP, Kapahi P, Tarnopolsky M, Sahasrabuhde S, Melov S, Hughes RE. A human protein interaction network shows conservation of aging processes between human and invertebrate species. PLoS Genetics. 2009;5:e1000414. doi: 10.1371/journal.pgen.1000414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedetti MG, Foster AL, Vantipalli MC, White MP, Sampayo JN, Gill MS, Olsen A, Lithgow GJ. Compounds that confer thermal stress resistance and extended lifespan. Experimental Gerontology. 2008;43:882–891. doi: 10.1016/j.exger.2008.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bokov A, Chaudhuri A, Richardson A. The role of oxidative damage and stress in aging. Mechanisms of Ageing and Development. 2004;125:811–826. doi: 10.1016/j.mad.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Boyer J, Liu RH. Apple phytochemicals and their health benefits. Nutrition Journal. 2004;3:5. doi: 10.1186/1475-2891-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braeckman BP, Vanfleteren JR. Genetic control of longevity in C. elegans. Experimental Gerontology. 2007;42:90–98. doi: 10.1016/j.exger.2006.04.010. [DOI] [PubMed] [Google Scholar]
- Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MK, Evans JL, Luo Y. Beneficial effects of natural antioxidants EGCG and alpha-lipoic acid on lifespan and age-dependent behavioral declines in Caenorhabditis elegans. Pharmacology Biochemistry and Behavior. 2006;85:620–628. doi: 10.1016/j.pbb.2006.10.017. [DOI] [PubMed] [Google Scholar]
- Clancy S. DNA damage & repair: mechanisms for maintaining DNA integrity. Nature Education. 2008;1(1) [Google Scholar]
- Clokey GV, Jacobson LA. The autofluorescent lipofuscin granules in the intestinal cells of Caenorhabditis elegans are secondary lysosomes. Mechanisms of Ageing and Development. 1986;35:79–94. doi: 10.1016/0047-6374(86)90068-0. [DOI] [PubMed] [Google Scholar]
- Collins JJ, Evason K, Kornfeld K. Pharmacology of delayed aging and extended lifespan of Caenorhabditis elegans. Experimental Gerontology. 2006;41:1032–1039. doi: 10.1016/j.exger.2006.06.038. [DOI] [PubMed] [Google Scholar]
- Cypser JR, Johnson TE. Multiple stressors in Caenorhabditis elegans induce stress hormesis and extended longevity. Journals of Gerontology Series A: Biological and Medical Sciences. 2002;57:109–114. doi: 10.1093/gerona/57.3.b109. [DOI] [PubMed] [Google Scholar]
- Doll R, Peto R. The causes of cancer: quantitative estimates of avoidable risks of cancer in the United States today. Journal of the National Cancer Institute. 1981;66:1191–1308. [PubMed] [Google Scholar]
- Eberhardt MV, Lee CY, Liu RH. Antioxidant activity of fresh apples. Nature. 2000;405:903–904. doi: 10.1038/35016151. [DOI] [PubMed] [Google Scholar]
- Gems D, Partridge L. Stress-response hormesis and aging: “That which Does Not Kill Us Makes Us Stronger”. Cellular Metabolism. 2008;7:200–203. doi: 10.1016/j.cmet.2008.01.001. [DOI] [PubMed] [Google Scholar]
- Golden TR, Hubbard A, Dando C, Herren MA, Melov S. Age-related behaviors have distinct transcriptional profiles in Caenorhabditis elegans. Aging Cell. 2008;7:850–865. doi: 10.1111/j.1474-9726.2008.00433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong Y, Luo Y, Huang J, Zhang J, Peng Y, Liu Z, Baolu Z. Theanine improves stress resistance in Caenorhabditis elegans. Journal of Functional Foods. 2012;4:988–993. [Google Scholar]
- Gruber J, Tang SY, Halliwell B. Evidence for a trade-off between survival and fitness caused by resveratrol treatment of Caenorhabditis elegans. Annals of the New York Academy of Sciences. 2007;1100:530–542. doi: 10.1196/annals.1395.059. [DOI] [PubMed] [Google Scholar]
- He X, Liu RH. Triterpenoids isolated from apple peels have potent antiproliferative activity and may be partially responsible for apple’s anticancer activity. Journal of Agricultural and Food Chemistry. 2007;55:4366–4370. doi: 10.1021/jf063563o. [DOI] [PubMed] [Google Scholar]
- He X, Liu RH. Phytochemicals of apple peels: isolation, structure elucidation, and their antiproliferative and antioxidant activities. Journal of Agricultural and Food Chemistry. 2008;56:9905–9910. doi: 10.1021/jf8015255. [DOI] [PubMed] [Google Scholar]
- Herndon LA, Schmeissner PJ, Dudaronek JM, Brown PA, Listner KM, Sakano Y, Paupard MC, Hall DH, Driscoll M. Stochastic and genetic factors influence tissue-specific decline in ageing C. elegans. Nature. 2002;419:808–814. doi: 10.1038/nature01135. [DOI] [PubMed] [Google Scholar]
- Ishii N, Takahashi K, Tomita S, Keino T, Honda S, Yoshino K, Suzuki K. A methyl viologen-sensitive mutant of the nematode Caenorhabditis elegans. Mutation Research. 1990;237:165–171. doi: 10.1016/0921-8734(90)90022-j. [DOI] [PubMed] [Google Scholar]
- Johnson TE, Lithgow GJ, Murakami S. Hypothesis: interventions that increase the response to stress offer the potential for effective life prolongation and increased health. Journals of Gerontology Series A: Biological and Medical Sciences. 1996;51:392–395. doi: 10.1093/gerona/51a.6.b392. [DOI] [PubMed] [Google Scholar]
- Johnson TE. Advantages and disadvantages of Caenorhabditis elegans for aging research. Experimental gerontology. 2003;38:1329–1332. doi: 10.1016/j.exger.2003.10.020. [DOI] [PubMed] [Google Scholar]
- Joshipura KJ, Hu FB, Manson JAE, Stampfer MJ, Rimm EB, Speizer FE, Colditz G, Ascherio A, Rosner B, Spiegelman D. The effect of fruit and vegetable intake on risk for coronary heart disease. Annals of Internal Medicine. 2001;134:1106–1114. doi: 10.7326/0003-4819-134-12-200106190-00010. [DOI] [PubMed] [Google Scholar]
- Kampkotter A, Timpel C, Zurawski RF, Ruhl S, Chovolou Y, Proksch P, Watjen W. Increase of stress resistance and lifespan of Caenorhabditis elegans by quercetin. Comparative Biochemistry and Physiology Part B Biochemistry and Molecular Biology. 2008;149:314–323. doi: 10.1016/j.cbpb.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Kirkwood TB. Understanding the odd science of aging. Cell. 2005;120:437–447. doi: 10.1016/j.cell.2005.01.027. [DOI] [PubMed] [Google Scholar]
- Klass M. Aging in the nematode Caenorhabditis elegans: major biological and environmental factors affecting life span. Mechanisms of Aging and Development. 1977;6:413–429. doi: 10.1016/0047-6374(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Kornfeld K, Evason K. Effects of anticonvulsant drugs on life span. Archives of Neurology. 2006;63:491–496. doi: 10.1001/archneur.63.4.491. [DOI] [PubMed] [Google Scholar]
- Li J, Ebata A, Dong Y, Rizki G, Iwata T, Lee SS. Caenorhabditis elegans HCF-1 functions in longevity maintenance as a DAF-16 regulator. PLoS Biology. 2008;6:e233. doi: 10.1371/journal.pbio.0060233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lithgow GJ, White TM, Melov S, Johnson TE. Thermotolerance and extended life-span conferred by single-gene mutations and induced by thermal stress. Proceedings of the National Academy of Sciences. 1995;92:7540–7544. doi: 10.1073/pnas.92.16.7540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lithgow GJ, Walker GA. Stress resistance as a determinate of C. elegans lifespan. Mechanisms of Ageing and Development. 2002;123:765–771. doi: 10.1016/s0047-6374(01)00422-5. [DOI] [PubMed] [Google Scholar]
- Liu RH. Health benefits of fruit and vegetables are from additive and synergistic combinations of phytochemicals. American Journal of Clinical Nutrition. 2003;78:517S–520S. doi: 10.1093/ajcn/78.3.517S. [DOI] [PubMed] [Google Scholar]
- Liu RH, Liu J, Chen B. Apples prevent mammary tumors in rats. Journal of Agricultural and Food Chemistry. 2005;53:2341–2343. doi: 10.1021/jf058010c. [DOI] [PubMed] [Google Scholar]
- Lock K, Pomerleau J, Causer L, Altmann DR, McKee M. The global burden of disease attributable to low consumption of fruit and vegetables: implications for the global strategy on diet. Bulletin of the World Health Organization. 2005;83:100–108. [PMC free article] [PubMed] [Google Scholar]
- Lucanic M, Lithgow GJ, Alavez S. Pharmacological lifespan extension of invertebrates. Ageing Research Reviews. 2013;12:445–458. doi: 10.1016/j.arr.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng C, Chan HY, Huang Y, Yu H, Chen ZY. Apple polyphenols extend the mean lifespan of Drosophila melanogaster. Journal of Agricultural and Food Chemistry. 2011;59:2097–2106. doi: 10.1021/jf1046267. [DOI] [PubMed] [Google Scholar]
- Rajendran P, Ho E, Williams DE, Dashwood RH. Dietary phytochemicals, HDAC inhibition, and DNA damage/repair defects in cancer cells. Clinical Epigenetics. 2011;3:4. doi: 10.1186/1868-7083-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rand JB, Johnson CD, Rand JB, Johnson CD. Genetic pharmacology: interactions between drugs and gene products in Caenorhabditis elegans. Methods in Cell Biology. 1995;48:187–204. doi: 10.1016/s0091-679x(08)61388-6. [DOI] [PubMed] [Google Scholar]
- Rasband WS. ImageJ. U. S. National Institutes of Health; Bethesda Maryland, USA: 1997–2009. http://rsb.info.nih.gov/ij/ [Google Scholar]
- Rittié L, Fisher GJ. UV-light-induced signal cascades and skin aging. Ageing Research Reviews. 2002;1:705–720. doi: 10.1016/s1568-1637(02)00024-7. [DOI] [PubMed] [Google Scholar]
- Sun J, Chu YF, Wu X, Liu RH. Antioxidant and antiproliferative activities of common fruits. Journal of Agricultural and Food Chemistry. 2002;50:7449–7454. doi: 10.1021/jf0207530. [DOI] [PubMed] [Google Scholar]
- Sun J, Liu RH. Apple phytochemical extracts inhibit proliferation of estrogen-dependent and estrogen-independent human breast cancer cells through cell cycle modulation. Journal of Agricultural and Food Chemistry. 2008;56:11661–11667. doi: 10.1021/jf8021223. [DOI] [PubMed] [Google Scholar]
- Sunagawa T, Shimizu T, Kanda T, Sami M, Shirasawa T. Procyanidins from apples (Malus pumila Mill.) extend the lifespan of Caenorhabditis elegans. Planta Medica. 2011;77:122–127. doi: 10.1055/s-0030-1250204. [DOI] [PubMed] [Google Scholar]
- Tan MW, Mahajan-Miklos S, Ausubel FM. Killing of Caenorhabditis elegans by Pseudomonas aeruginosa used to model mammalian bacterial pathogenesis. Proceedings of the National Academy of Sciences. 1999;96:715–720. doi: 10.1073/pnas.96.2.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toda T, Sunagawa T, Kanda T, Tagashira M, Shirasawa T, Shimizu T. Apple Procyanidins suppress amyloid B-protein aggregation. Biochemistry Research International. 2011 doi: 10.1155/2011/784698. Article ID 784698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiegant FA, Surinova S, Ytsma E, Langelaar-Makkinje M, Wikman G, Post JA. Plant adaptogens increase lifespan and stress resistance in C. elegans. Biogerontology. 2009;10:27–42. doi: 10.1007/s10522-008-9151-9. [DOI] [PubMed] [Google Scholar]
- Willett WC. Diet and health: what should we eat? Science. 1994;264:532–537. doi: 10.1126/science.8160011. [DOI] [PubMed] [Google Scholar]
- Wilson MA, Shukitt-Hale B, Kalt W, Ingram DK, Joseph JA. Blueberry polyphenols increase lifespan and thermotolerance in Caenorhabditis elegans. Aging Cell. 2006;5:59–68. doi: 10.1111/j.1474-9726.2006.00192.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe KL, Liu RH. Apple peels as a value-added food ingredient. Journal of Agricultural and Food Chemistry. 2003;51:1676–1683. doi: 10.1021/jf025916z. [DOI] [PubMed] [Google Scholar]
- Wu Z, Smith JV, Paramasivam V, Butko P, Khan I, JRC, Luo Y. Ginkgo biloba extract EGb 761 increases stress resistance and extends life span of Caenorhabditis elegans. Cellular and Molecular Biology. 2002;48:725–731. [PubMed] [Google Scholar]
- Yoon H, Liu RH. Effect of selected phytochemicals and apple extracts on NF-kappaB activation in human breast cancer MCF-7 cells. Journal of Agricultural and Food Chemistry. 2007;55:3167–73. doi: 10.1021/jf0632379. [DOI] [PubMed] [Google Scholar]
- Yoon H, Liu RH. Effect of 2alpha-hydroxyursolic acid on NF-kappaB activation induced by TNF-alpha in human breast cancer MCF-7 cells. Journal of Agricultural and Food Chemistry. 2008;56:8412–8417. doi: 10.1021/jf8012844. [DOI] [PubMed] [Google Scholar]
- Zhang L, Jie G, Zhang J, Zhao B. Significant longevity-extending effects of EGCG on Caenorhabditis elegans under stress. Free Radical Biology and Medicine. 2009;46:414–21. doi: 10.1016/j.freeradbiomed.2008.10.041. [DOI] [PubMed] [Google Scholar]